Further In Vitro and Ex Vivo Pharmacological and Kinetic Characterizations of CCF219B: A Positive Allosteric Modulator of the α1A-Adrenergic Receptor
Abstract
1. Introduction
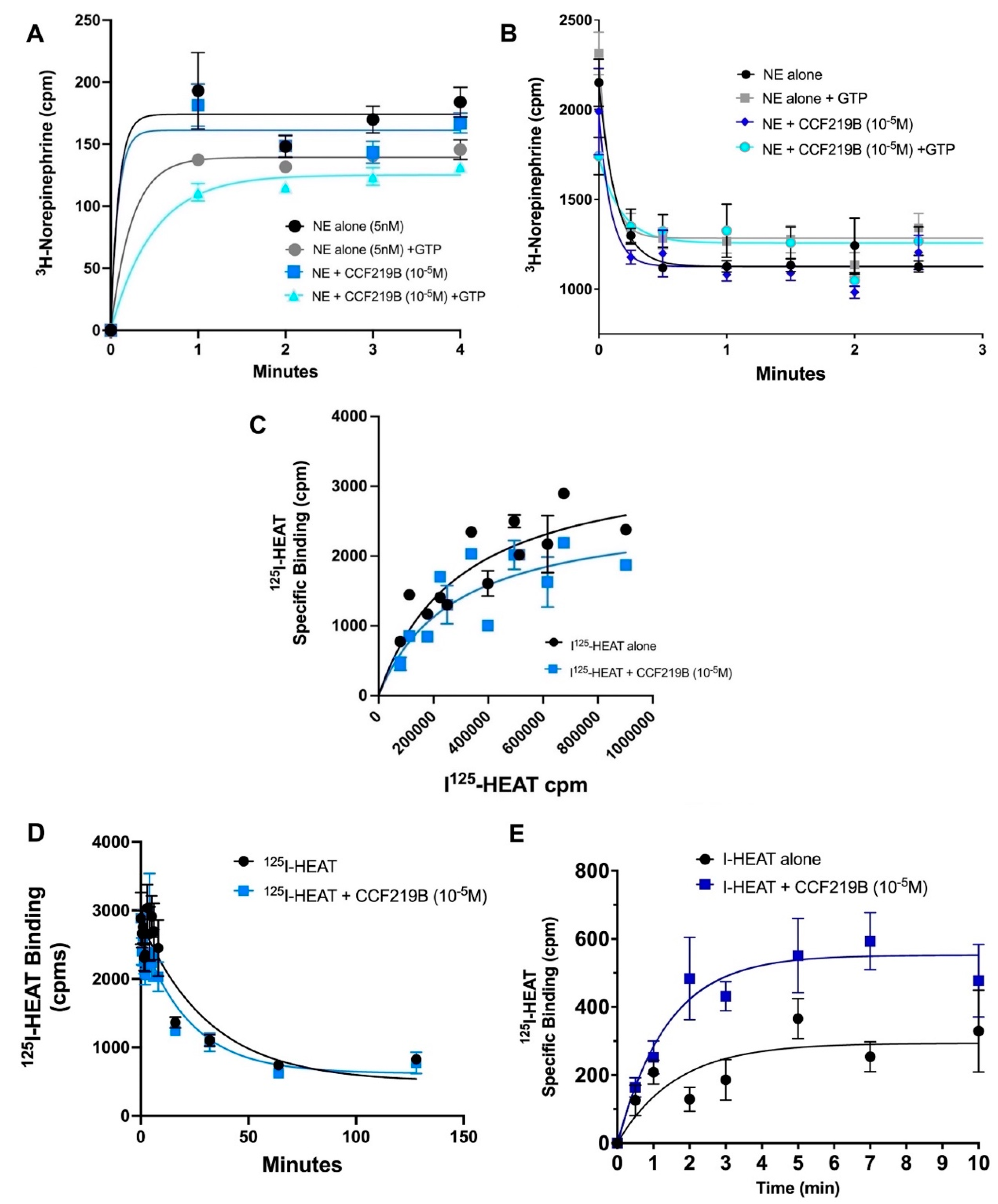
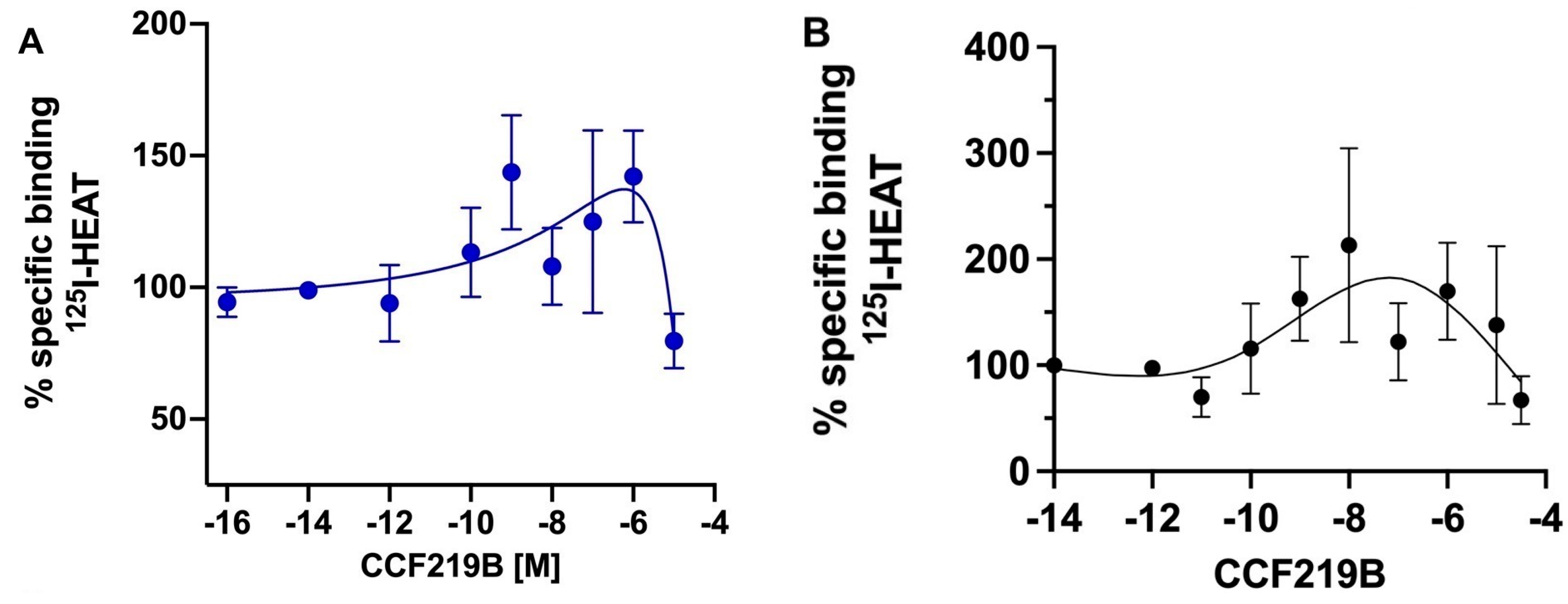
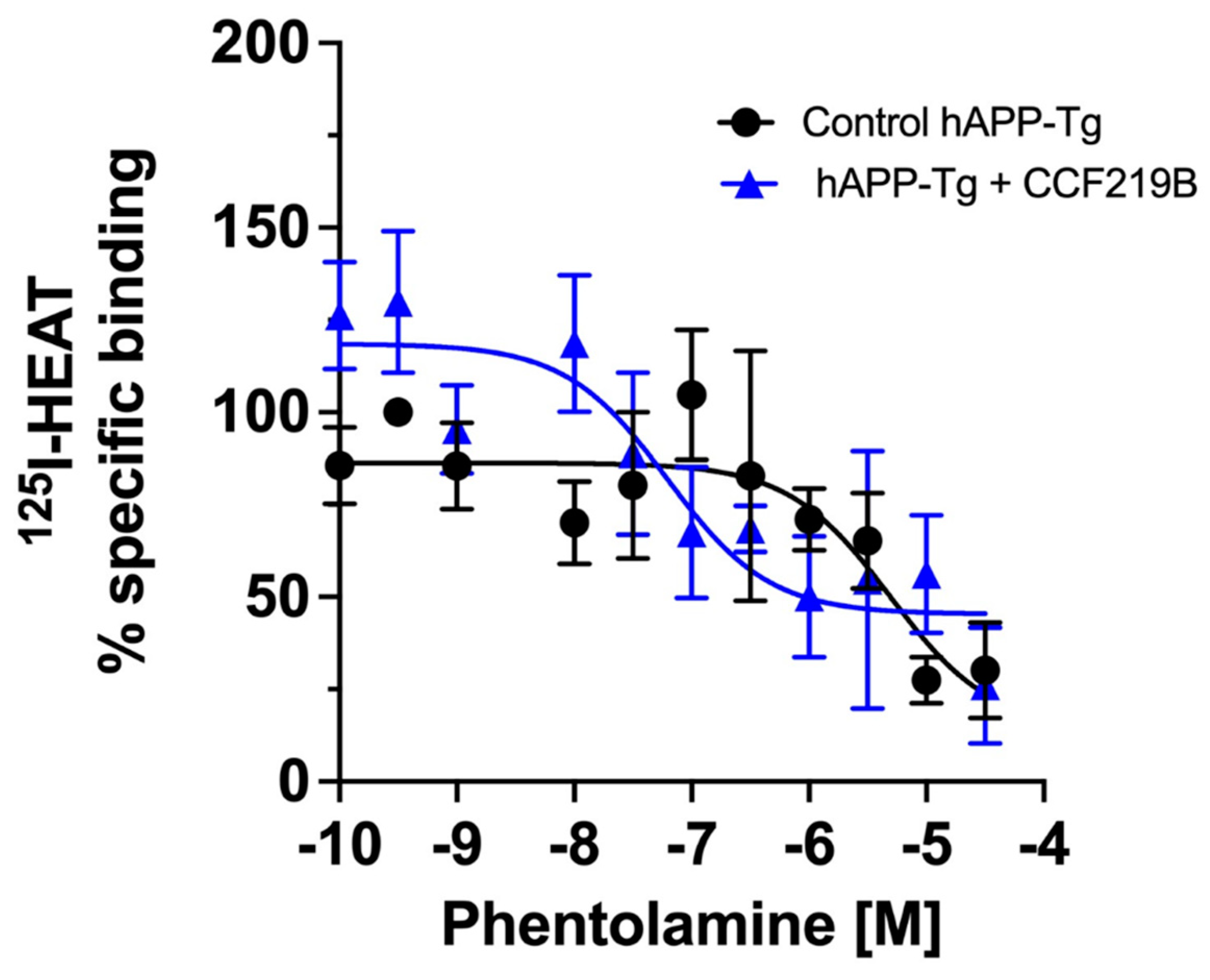
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Compounds
4.3. MAPK Assays
4.4. Mouse Brain Membrane Preparation
4.5. Radioligand-Binding Studies
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perez, D.M. α1-Adrenergic Receptors in Neurotransmission, Synaptic Plasticity, and Cognition. Front. Pharmacol. 2020, 11, 581098. [Google Scholar] [CrossRef]
- Gottfries, C.-G.; Adolfsson, R.; Aquilonius, S.-M.; Carlsson, A.; Eckernas, S.-A.; Nordberg, A.; Oreland, L.; Svennerholm, L.; Wiberg, Å.; Winblad, B. Biochemical changes in Dementia disorders of Alzheimer type (AD/SDAT). Neurobiol. Aging 1983, 4, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Chalermpalanupap, T.; Kinkead, B.; Hu, W.T.; Kummer, M.P.; Hammerschmidt, T.; Heneka, M.T.; Weinshenker, D.; I Levey, A. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Gavrilyuk, V.; Polak, P.E.; Vasser, R.; Zhao, J.; Heneka, M.T.; Feinstein, D.L. Noradrenaline deficiency in brain increases β-amyloid plaque burden in an animal model of Alzheimer’s disease. Neurobiol. Aging 2007, 28, 1206–1214. [Google Scholar] [CrossRef]
- Gannon, M.; Che, P.; Chen, Y.; Jiao, K.; Roberson, E.D.; Wang, Q. Noradrenergic dysfunction in Alzheimer’s disease. Front. Neurosci. 2015, 9, 220. [Google Scholar] [CrossRef]
- Ponnusamy, R.; McNerney, M.W.; Moghadam, S.; Salehi, A. Assessing disease-modifying effects of norepinephrine in Down syndrome and Alzheimer’s disease. Brain Res. 2019, 1702, 3–11. [Google Scholar] [CrossRef]
- Kuo, H.-I.; Qi, F.-X.; Paulus, W.; Kuo, M.-F.; A Nitsche, M. Noradrenergic Enhancement of Motor Learning, Attention, and Working Memory in Humans. Int. J. Neuropsychopharmacol. 2021, 24, 490–498. [Google Scholar] [CrossRef]
- Doze, V.A.; Papay, R.S.; Goldenstein, B.L.; Gupta, M.K.; Collette, K.M.; Nelson, B.W.; Lyons, M.J.; Davis, B.A.; Luger, E.J.; Wood, S.G.; et al. Long-Term α1A-Adrenergic Receptor Stimulation Improves Synaptic Plasticity, Cognitive Function, Mood, and Longevity. Mol. Pharmacol. 2011, 80, 747–758. [Google Scholar] [CrossRef]
- Szot, P.; White, S.; Greenup, J.; Leverenz, J.; Peskind, E.; Raskind, M. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: Evidence of compensatory changes. Neuroscience 2007, 146, 471–480. [Google Scholar] [CrossRef]
- Hong, C.-J.; Wang, Y.-C.; Liu, T.-Y.; Liu, H.-C.; Tsai, S.-J. A study of alpha-adrenoceptor gene polymorphisms and Alzheimer disease. J. Neural Transm. 2001, 108, 445–450. [Google Scholar] [CrossRef]
- Papay, R.S.; Macdonald, J.D.; Stauffer, S.R.; Perez, D.M. Characterization of a novel positive allosteric modulator of the α1A-Adrenergic receptor. Curr. Res. Pharmacol. Drug Discov. 2022, 4, 100142. [Google Scholar] [CrossRef] [PubMed]
- Papay, R.S.; Stauffer, S.R.; Perez, D.M. A PAM of the α1A-Adrenergic receptor rescues biomarker, long-term potentiation, and cognitive deficits in Alzheimer’s disease mouse models without effects on blood pressure. Curr. Res. Pharmacol. Drug Discov. 2023, 5, 100160. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A. High Blood Pressure and Microinfarcts: A Link Between Vascular Risk Factors, Dementia, and Clinical Alzheimer’s Disease. J. Am. Geriatr. Soc. 2009, 57, 2146–2147. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Li, X.-C.; Kandel, E.R. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell 1994, 79, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.M.; Shen, A.; Xu, B.; Jovanovic, A.; de Chabot, J.; Zhang, J.; Xiang, Y.K. Arrestin-dependent nuclear export of phosphodiesterase 4D promotes GPCR-induced nuclear cAMP signaling required for learning and memory. Sci. Signal. 2023, 16, eade3380. [Google Scholar] [CrossRef]
- Indrigo, M.; Morella, I.; Orellana, D.; D’Isa, R.; Papale, A.; Parra, R.; Gurgone, A.; Lecca, D.; Cavaccini, A.; Tigaret, C.M.; et al. Nuclear ERK1/2 signaling potentiation enhances neuroprotection and cognition via Importinα1/KPNA2. EMBO Mol. Med. 2023, 15, e15984. [Google Scholar] [CrossRef]
- Ye, X.; Shao, S.; Wang, Y.; Su, W. Ginsenoside Rg2 alleviates neurovascular damage in 3xTg-AD mice with Alzheimer’s disease through the MAPK-ERK pathway. J. Chem. Neuroanat. 2023, 133, 102346. [Google Scholar] [CrossRef]
- Perez-Aso, M.; Segura, V.; Montó, F.; Barettino, D.; Noguera, M.; Milligan, G.; D’Ocon, P. The three α1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 2322–2333. [Google Scholar] [CrossRef]
- Stickle, D.; Barber, R. Estimation of the kinetic constants for binding of epinephrine to β-adrenergic receptors of the S49 cell. Biochem. Pharmacol. 1991, 42, 1069–1077. [Google Scholar] [CrossRef]
- Contreras, M.L.; Wolfe, B.B.; Molinoff, P.B. Thermodynamic properties of agonist interactions with the beta adrenergic receptor-coupled adenylate cyclase system. II. Agonist binding to soluble beta adrenergic receptors. J. Pharmacol. Exp. Ther. 1986, 237, 165–172. [Google Scholar]
- Goate, A.; Chartier-Harlin, M.-C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L.; et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.R.; Rice, H.C.; Srikanth, P.; Callahan, D.G.; Shin, T.; Benjamin, L.N.P.; Walsh, D.M.; Selkoe, D.J.; Young-Pearse, T.L. The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 2014, 23, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, I.; Filipkowski, R.; Priller, C.; Ris, L.; Neyton, J.; Croes, S.; Terwel, D.; Gysemans, M.; Devijver, H.; Borghgraef, P.; et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol. Aging 2007, 30, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.; Creese, I. Characterization of alpha-1-adrenergic receptor subtypes in rat-brain—A reevaluation of [3H]WB4104 and [3H]prazosin binding. Mol. Pharmacol. 1986, 29, 321–330. [Google Scholar] [CrossRef]
- Hulme, E.C.; Trevethick, M.A. Ligand binding assays at equilibrium: Validation and interpretation. Br. J. Pharmacol. 2010, 161, 1219–1237. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Andrew, S.M.; Hogarth, M.P.; Pietersz, G.A.; McKenzie, I.F. The effect of temperature on the binding kinetics and equilibrium constants of monoclonal antibodies to cell surface antigens. Mol. Immunol. 2002, 27, 327–333. [Google Scholar] [CrossRef]
- Vega, S.; Abian, O.; Velazquez-Campoy, A. On the link between conformational changes, ligand binding and heat capacity. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 868–878. [Google Scholar] [CrossRef]
- Perez, D.M. Current Developments on the Role of α1-Adrenergic Receptors in Cognition, Cardioprotection, and Metabolism. Front. Cell Dev. Biol. 2021, 9, 652152. [Google Scholar] [CrossRef]
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart–Brain Axis. J. Am. Hear. Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef]
- Maity, S.; Chandanathil, M.; Millis, R.M.; Connor, S.A. Norepinephrine stabilizes translation-dependent, homosynaptic long-term potentiation through mechanisms requiring the cAMP sensor Epac, mTOR and MAPK. Eur. J. Neurosci. 2020, 52, 3679–3688. [Google Scholar] [CrossRef]
- Maity, S.; Rah, S.; Sonenberg, N.; Gkogkas, C.G.; Nguyen, P.V. Norepinephrine triggers metaplasticity of LTP by increasing translation of specific mRNAs. Learn. Mem. 2015, 22, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Slosky, L.M.; Caron, M.G.; Barak, L.S. Biased Allosteric Modulators: New Frontiers in GPCR Drug Discovery. Trends Pharmacol. Sci. 2021, 42, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, A.; Kenakin, T. G Protein-Coupled Receptor Allosterism and Complexing. Pharmacol. Rev. 2002, 54, 323–374. [Google Scholar] [CrossRef]
- DeVree, B.T.; Mahoney, J.P.; Vélez-Ruiz, G.A.; Rasmussen, S.G.F.; Kuszak, A.J.; Edwald, E.; Fung, J.-J.; Manglik, A.; Masureel, M.; Du, Y.; et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature 2016, 535, 182–186. [Google Scholar] [CrossRef]
- Huang, S.K.; Pandey, A.; Tran, D.P.; Villanueva, N.L.; Kitao, A.; Sunahara, R.K.; Sljoka, A.; Prosser, R.S. Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling. Cell 2021, 184, 1884–1894.e14. [Google Scholar] [CrossRef]
- Frei, J.N.; Broadhurst, R.W.; Bostock, M.J.; Solt, A.; Jones, A.J.Y.; Gabriel, F.; Tandale, A.; Shrestha, B.; Nietlispach, D. Conformational plasticity of ligand-bound and ternary GPCR complexes studied by 19F NMR of the β1-adrenergic receptor. Nat. Commun. 2020, 11, 669. [Google Scholar] [CrossRef]
- Toyoda, Y.; Zhu, A.; Kong, F.; Shan, S.; Zhao, J.; Wang, N.; Sun, X.; Zhang, L.; Yan, C.; Kobilka, B.K.; et al. Structural basis of α1A-adrenergic receptor activation and recognition by an extracellular nanobody. Nat. Commun. 2023, 14, 3655. [Google Scholar] [CrossRef]
- Kruse, A.C.; Ring, A.M.; Manglik, A.; Hu, J.; Hu, K.; Eitel, K.; Hübner, H.; Pardon, E.; Valant, C.; Sexton, P.M.; et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 2013, 504, 101–106. [Google Scholar] [CrossRef]
- Rokosh, D.G.; Simpson, P.C. Knockout of the α1A/C-adrenergic receptor subtype: The α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc. Natl. Acad. Sci. USA 2002, 99, 9474–9479. [Google Scholar] [CrossRef]
- Szot, P.; White, S.S.; Greenup, J.L.; Leverenz, J.B.; Peskind, E.R.; Raskind, M.A. α1-Adrenoreceptor in human hippocampus: Binding and receptor subtype mRNA expression. Mol. Brain Res. 2005, 139, 367–371. [Google Scholar] [CrossRef]
- Papay, R.; Gaivin, R.; Jha, A.; Mccune, D.F.; Mcgrath, J.C.; Rodrigo, M.C.; Simpson, P.C.; Doze, V.A.; Perez, D.M. Localization of the mouse α1A-adrenergic receptor (AR) in the brain: α1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J. Comp. Neurol. 2006, 497, 209–222. [Google Scholar] [CrossRef]
- Clark, D.A.; Arranz, M.J.; Mata, I.; Lopéz-Ilundain, J.; Pérez-Nievas, F.; Kerwin, R.W. Polymorphisms in the Promoter Region of the Alpha1A-Adrenoceptor Gene Are Associated with Schizophrenia/Schizoaffective Disorder in a Spanish Isolate Population. Biol. Psychiatry 2005, 58, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Knauber, J.; Müller, W.E. Subchronic treatment with prazosin improves passive avoidance learning in aged mice: Possible relationships to α1 -receptor up-regulation. J. Neural Transm. 2000, 107, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Rokosh, D.G.; Stewart, A.F.; Chang, K.C.; Bailey, B.A.; Karliner, J.S.; Camacho, S.A.; Long, C.S.; Simpson, P.C. α1-Adrenergic Receptor Subtype mRNAs Are Differentially Regulated by α1-Adrenergic and Other Hypertrophic Stimuli in Cardiac Myocytes in Culture and in Vivo. J. Biol. Chem. 1996, 271, 5839–5843. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; A Ross, S.; Rorabaugh, B.R.; Chalothorn, D.; Yun, J.; Gonzalez-Cabrera, P.J.; McCune, D.F.; Piascik, M.T.; Perez, D.M. The α1B-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovasc. Res. 2003, 60, 598–607. [Google Scholar] [CrossRef][Green Version]
- Kunieda, T.; Zuscik, M.J.; Boongird, A.; Perez, D.M.; Lüders, H.O.; Najm, I.M. Systemic Overexpression of the α1B-Adrenergic Receptor in Mice: An Animal Model of Epilepsy. Epilepsia 2002, 43, 1324–1329. [Google Scholar] [CrossRef]
- Paladini, C.A.; Fiorillo, C.D.; Morikawa, H.; Williams, J.T. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat. Neurosci. 2001, 4, 275–281. [Google Scholar] [CrossRef]
- Battaglia, G.; Fornai, F.; Busceti, C.L.; Lembo, G.; Nicoletti, F.; De Blasi, A. Alpha1B adrenergic receptor knockout mice are protected against methamphetamine toxicity. J. Neurochem. 2004, 86, 413–421. [Google Scholar] [CrossRef][Green Version]
- Papay, R.; Zuscik, M.J.; Ross, S.A.; Yun, J.; McCune, D.F.; Gonzalez-Cabrera, P.; Gaivin, R.; Macklin, W.B.; Drazba, J.; Perez, D.M. Mice expressing the α1B-adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation. J. Neurochem. 2002, 83, 623–634. [Google Scholar] [CrossRef]
- Zuscik, M.J.; Sands, S.; Ross, S.A.; Waugh, D.J.J.; Gaivin, R.J.; Morilak, D.; Perez, D.M. Overexpression of the α1B-adrenergic receptor causes apoptotic neurodegeneration: Multiple system atrophy. Nat. Med. 2000, 6, 1388–1394. [Google Scholar] [CrossRef]
- Collette, K.M.; Zhou, X.D.; Amoth, H.M.; Lyons, M.J.; Papay, R.S.; Sens, D.A.; Perez, D.M.; Doze, V.A. Long-term α1B-adrenergic receptor activation shortens lifespan, while α1A-adrenergic receptor stimulation prolongs lifespan in association with decreased cancer incidence. AGE 2014, 36, 9675. [Google Scholar] [CrossRef]
- Pizzanelli, C.; Lazzeri, G.; Fulceri, F.; Giorgi, F.S.; Pasquali, L.; Cifelli, G.; Murri, L.; Fornai, F. Lack of α1b-adrenergic receptor protects against epileptic seizures. Epilepsia 2009, 50, 59–64. [Google Scholar] [CrossRef]
- Bekdash, R.A. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef] [PubMed]
- Celej, M.S.; Montich, G.G.; Fidelio, G.D. Protein stability induced by ligand binding correlates with changes in protein flexibility. Protein Sci. 2003, 12, 1496–1506. [Google Scholar] [CrossRef]
- May, L.T.; Lin, Y.; Sexton, P.M.; Christopoulos, A. Regulation of M2 Muscarinic Acetylcholine Receptor Expression and Signaling by Prolonged Exposure to Allosteric Modulators. J. Pharmacol. Exp. Ther. 2005, 312, 382–390. [Google Scholar] [CrossRef]
- Lane, J.R.; Abdul-Ridha, A.; Canals, M. Regulation of G Protein-Coupled Receptors by Allosteric Ligands. ACS Chem. Neurosci. 2013, 4, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Mos, I.; Jacobsen, S.E.; Foster, S.R.; Bräuner-Osborne, H. Calcium-Sensing Receptor Internalization Is β-Arrestin–Dependent and Modulated by Allosteric Ligands. Mol. Pharmacol. 2019, 96, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Breitwieser, G.E. Rescue of Calcium-sensing Receptor Mutants by Allosteric Modulators Reveals a Conformational Checkpoint in Receptor Biogenesis. J. Biol. Chem. 2007, 282, 9517–9525. [Google Scholar] [CrossRef]
- Leach, K.; Wen, A.; Cook, A.E.; Sexton, P.M.; Conigrave, A.D.; Christopoulos, A. Impact of Clinically Relevant Mutations on the Pharmacoregulation and Signaling Bias of the Calcium-Sensing Receptor by Positive and Negative Allosteric Modulators. Endocrinology 2013, 154, 1105–1116. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papay, R.S.; Perez, D.M. Further In Vitro and Ex Vivo Pharmacological and Kinetic Characterizations of CCF219B: A Positive Allosteric Modulator of the α1A-Adrenergic Receptor. Pharmaceuticals 2025, 18, 476. https://doi.org/10.3390/ph18040476
Papay RS, Perez DM. Further In Vitro and Ex Vivo Pharmacological and Kinetic Characterizations of CCF219B: A Positive Allosteric Modulator of the α1A-Adrenergic Receptor. Pharmaceuticals. 2025; 18(4):476. https://doi.org/10.3390/ph18040476
Chicago/Turabian StylePapay, Robert S., and Dianne M. Perez. 2025. "Further In Vitro and Ex Vivo Pharmacological and Kinetic Characterizations of CCF219B: A Positive Allosteric Modulator of the α1A-Adrenergic Receptor" Pharmaceuticals 18, no. 4: 476. https://doi.org/10.3390/ph18040476
APA StylePapay, R. S., & Perez, D. M. (2025). Further In Vitro and Ex Vivo Pharmacological and Kinetic Characterizations of CCF219B: A Positive Allosteric Modulator of the α1A-Adrenergic Receptor. Pharmaceuticals, 18(4), 476. https://doi.org/10.3390/ph18040476







