Comprehensive Studies on the Regulation of Type 2 Diabetes by Cucurbitane-Type Triterpenoids in Momordica charantia L.: Insights from Network Pharmacology and Molecular Docking and Dynamics
Abstract
1. Introduction
2. Results
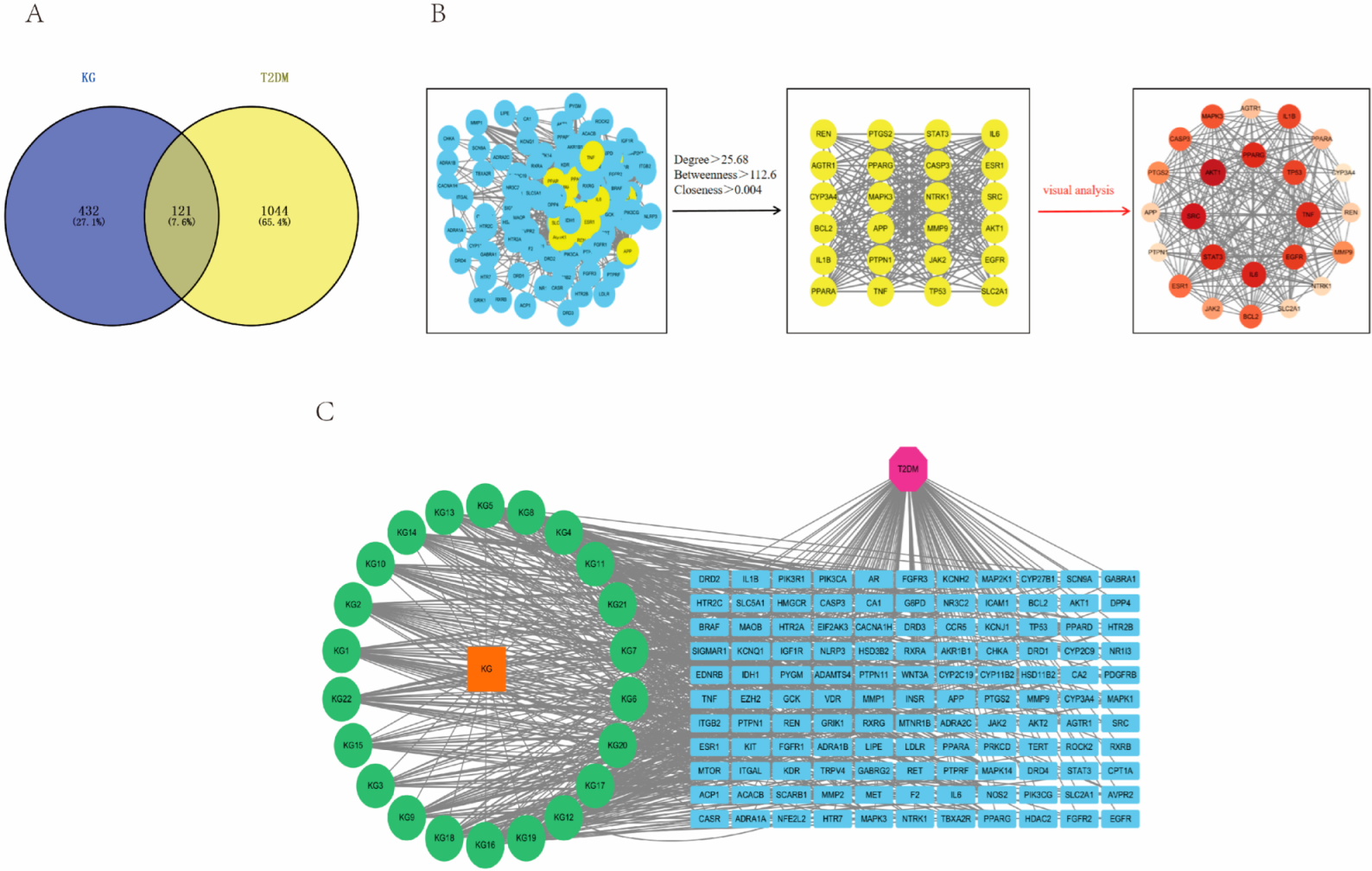
2.1. Components and Targets
2.2. Target Screening and PPI Network Analysis
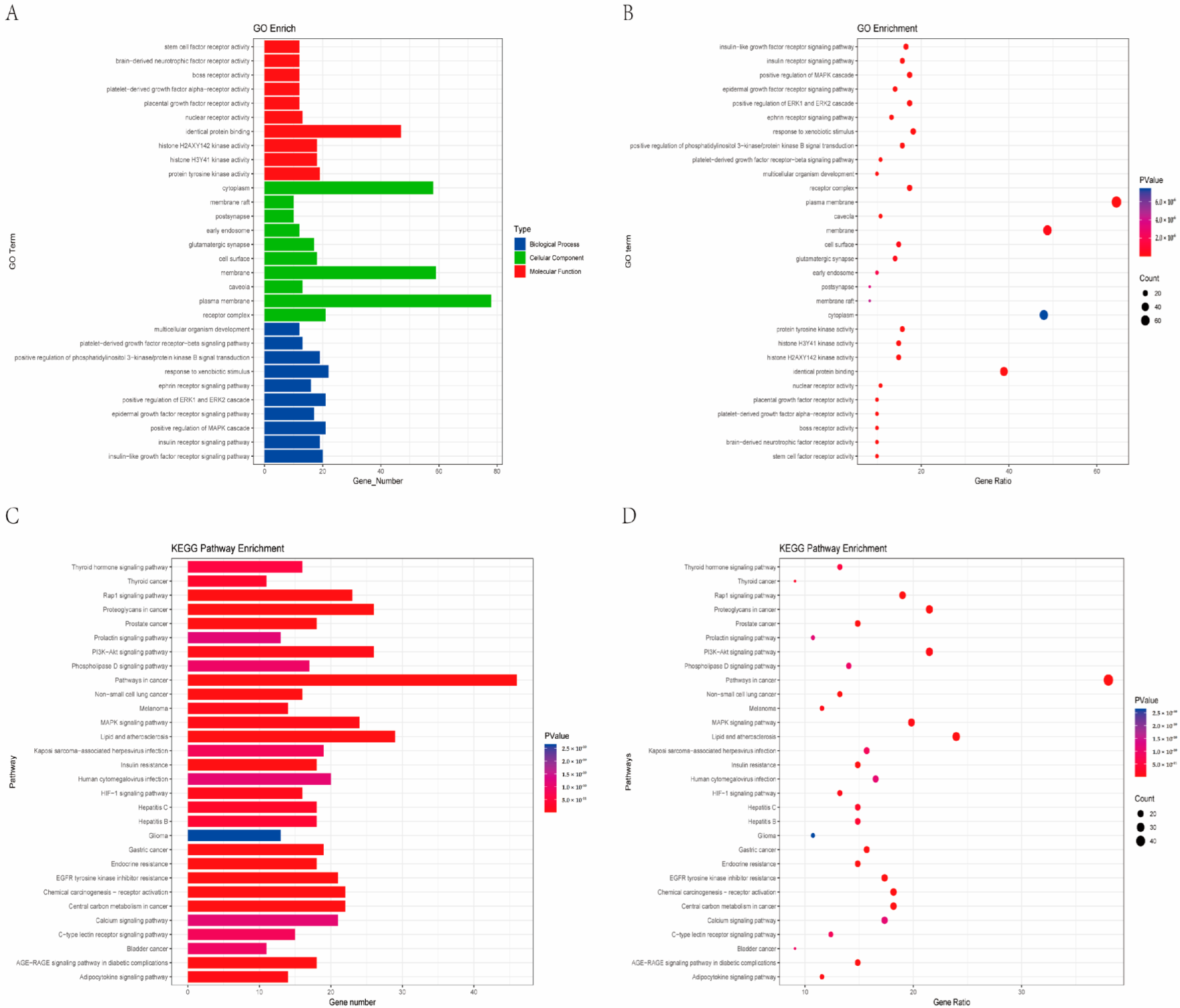
2.3. Functional Enrichment Analysis of Key Targets
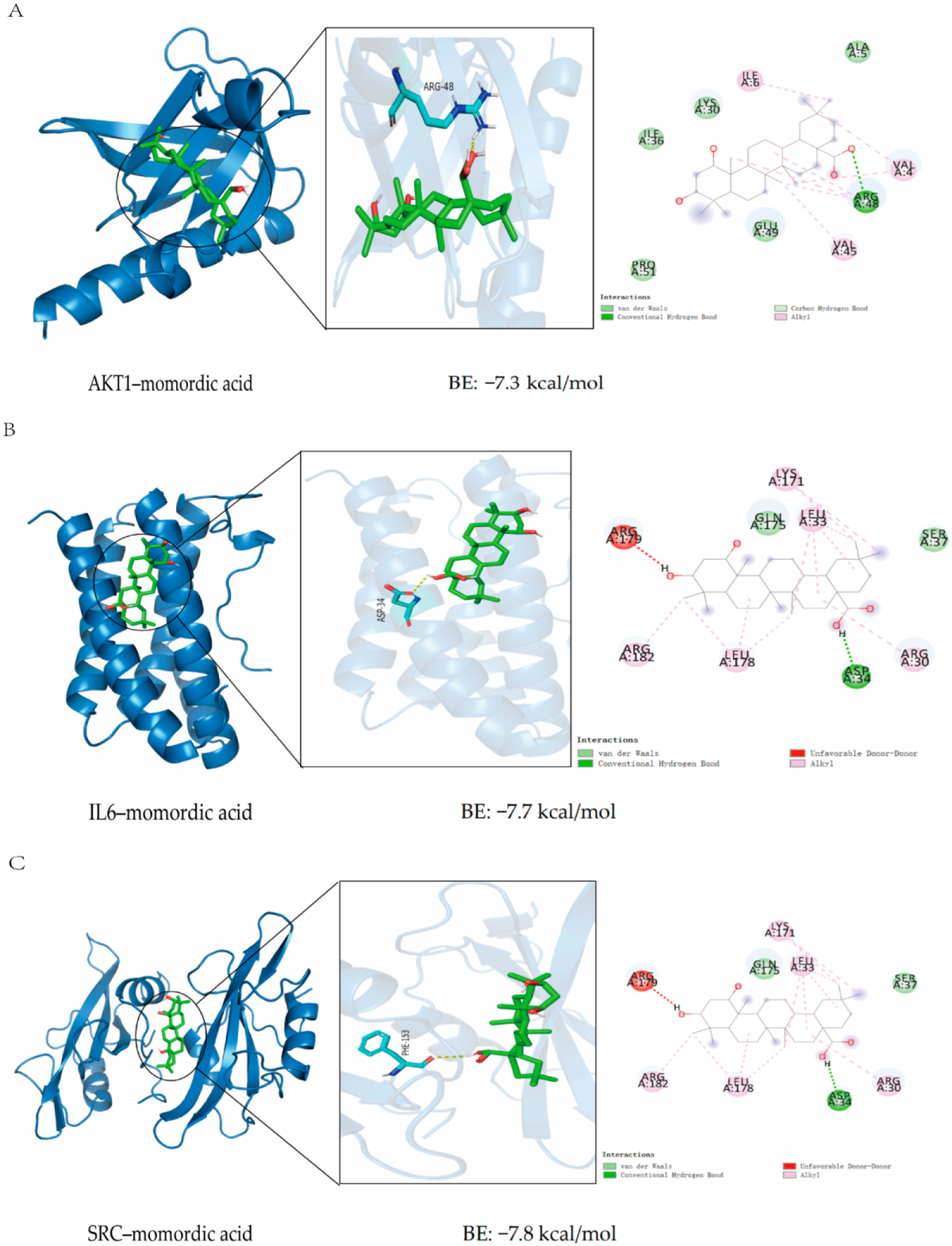
2.4. Molecular Docking Verification
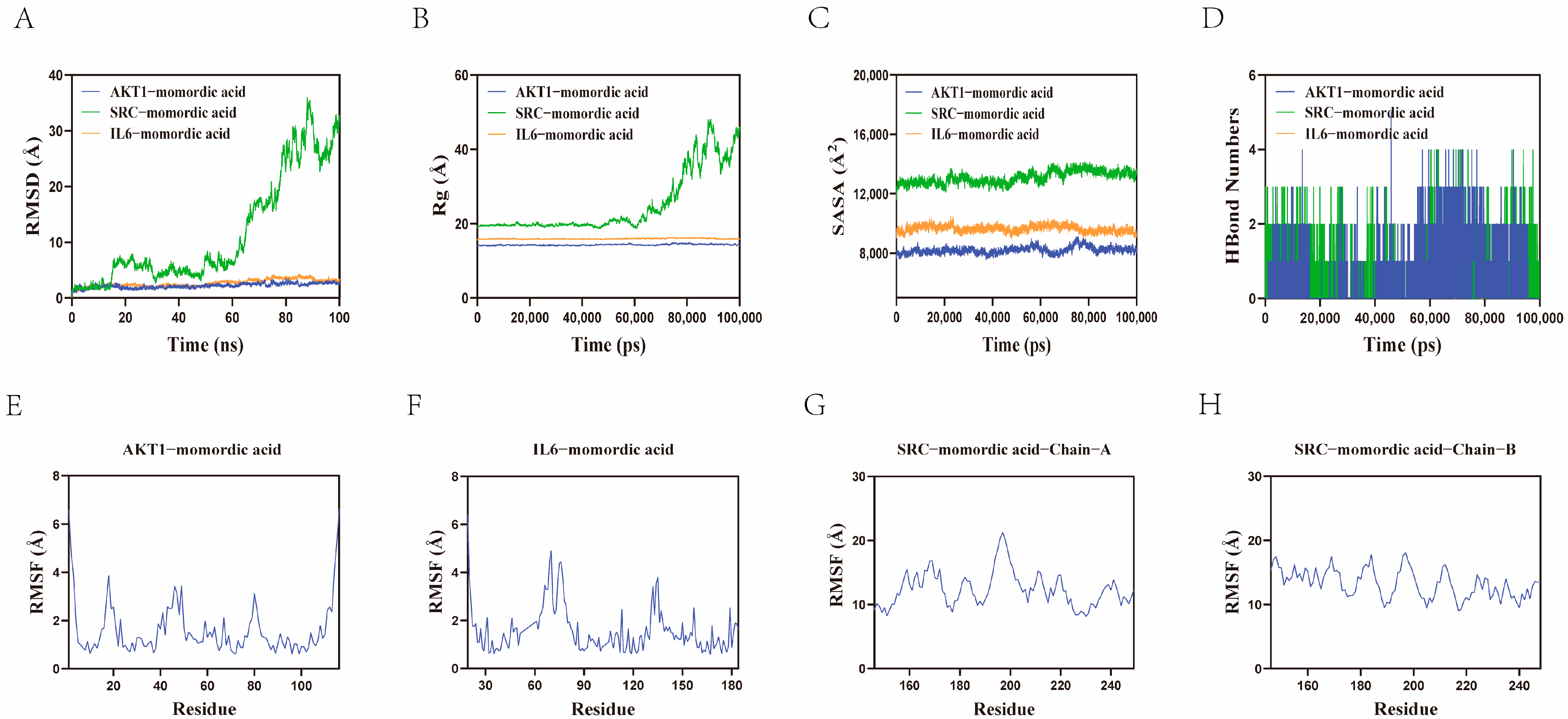
2.5. Molecular Dynamics Simulation and Binding Free Energy Calculation
3. Discussion
4. Materials and Methods
4.1. Collection of Component Targets
4.2. Collection of Disease Targets
4.3. Construction of PPI and CTD Networks
4.4. GO and KEGG Enrichment Analysis
4.5. Molecular Docking
4.6. Molecular Dynamics Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M.charantia | Momordica charantia L. |
| T2DM | type 2 diabetes mellitus |
| PPI | protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PME | Particle Mesh Ewald |
| BPs | Biological Processes |
| CCs | Cellular Components |
| MFs | Molecular Functions |
References
- Zimmet, P.Z. Diabetes and Its Drivers: The Largest Epidemic in Human History? Clin. Diabetes Endocrinol. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.P.; Saeedi, S.; Karuranga, M.; Pinkepank, K.; Ogurtsova, B.B.; Duncan, C.; Stein, A.; Basit, J.C.N.; Chan, J.C.; Mbanya, M.E.; et al. Idf Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pr. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lin, Z.; Zheng, Q.; Liao, M.; Li, H.; Feng, H.; Chen, H.; Zhang, Y.; Chen, X.; Liang, D. Major Bitter-Tasting Compounds from the Dichloromethane Fraction of Bitter Gourd (Fruit of Momordica charantia L.) Extract and Their Precursors. J. Agric. Food Chem. 2024, 72, 22237–22249. [Google Scholar] [CrossRef] [PubMed]
- Nuchtavorn, N.; Leanpolchareanchai, J.; Visansirikul, S.; Bunsupa, S. Optimization of Magnetic and Paper-Based Molecularly Imprinted Polymers for Selective Extraction of Charantin in Momordica Charantia. Int. J. Mol. Sci. 2023, 24, 7870. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Zhang, C.; Yan, S.; Wei, W.; Wang, X.; Deng, X.; Qian, H.; Lin, H.; Huang, W. Design, Synthesis and Biological Evaluation of Novel Peptide Mc2 Analogues from Momordica Charantia as Potential Anti-Diabetic Agents. Org. Biomol. Chem. 2015, 13, 4551–4561. [Google Scholar] [CrossRef]
- Zhan, K.; Ji, X.; Luo, L. Recent Progress in Research on Momordica Charantia Polysaccharides: Extraction, Purification, Structural Characteristics and Bioactivities. Chem. Biol. Technol. Agric. 2023, 10, 1. [Google Scholar] [CrossRef]
- Kao, P.-F.; Cheng, C.-H.; Cheng, T.-H.; Liu, J.-C.; Sung, L.-C. Therapeutic Potential of Momordicine I from Momordica Charantia: Cardiovascular Benefits and Mechanisms. Int. J. Mol. Sci. 2024, 25, 10518. [Google Scholar] [CrossRef]
- Poovitha, S.; Parani, M. In Vitro and in Vivo A-Amylase and A-Glucosidase Inhibiting Activities of the Protein Extracts from Two Varieties of Bitter Gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16, S16. [Google Scholar] [CrossRef]
- Singh, J.; Cumming, E.; Manoharan, G.; Kalasz, H.; Adeghate, E. Medicinal Chemistry of the Anti-Diabetic Effects of Momordica Charantia: Active Constituents and Modes of Actions. Open Med. Chem. J. 2011, 5 (Suppl. S2), 70–77. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of Antidiabetic Effects of Saponins and Polysaccharides from Momordica Charantia L. In Stz-Induced Type 2 Diabetic Mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Ashry, F.E.Z.Z.E.; El Maraghy, N.N.; Fahmy, A. Studies on the Antidiabetic Activities of Momordica Charantia Fruit Juice in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2017, 55, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.L.; Kasali, F.M.; Deyno, S.; Mtewa, A.; Nagendrappa, P.B.; Tolo, C.U.; Ogwang, P.E.; Sesaazi, D. Momordica Charantia L. Lowers Elevated Glycaemia in Type 2 Diabetes Mellitus Patients: Systematic Review and Meta-Analysis. J. Ethnopharmacol. 2019, 231, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, G.P.; Sinha, R.; Chapagain, K.; Maskey, R.; Pandey, D.R. Effects of Momordica Charantia (Karela/Bitterguord) in Type 2 Diabetic Patients Taking Allopathic Drugs: A Pilot Study. Kathmandu Univ. Med. J. 2021, 19, 243–247. [Google Scholar] [CrossRef]
- Keller, A.C.; Ma, J.; Kavalier, A.; He, K.; Brillantes, A.-M.B.; Kennelly, E.J. Saponins from the Traditional Medicinal Plant Momordica Charantia Stimulate Insulin Secretion in Vitro. Phytomedicine 2011, 19, 32–37. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Yu, J.; Tan, Y.; Guo, P.; Wu, C. The Gut Microbiota Confers the Lipid-Lowering Effect of Bitter Melon (Momordica charantia L.) in High-Fat Diet (Hfd)-Induced Hyperlipidemic Mice. Biomed. Pharmacother. 2020, 131, 110667. [Google Scholar] [CrossRef]
- Shivanagoudra, S.R.; Perera, W.H.; Perez, J.L.; Athrey, G.; Sun, Y.; Wu, C.S.; Jayaprakasha, G.K.; Patil, B.S. In Vitro and in Silico Elucidation of Antidiabetic and Anti-Inflammatory Activities of Bioactive Compounds from Momordica charantia L. Bioorganic Med. Chem. 2019, 27, 3097–3109. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Xie, M.; Ouyang, C.; Ding, X.; Zhang, H.; Dong, W.; Xiong, Y.; Tang, X. Momordicine I Alleviates Isoproterenol-Induced Cardiomyocyte Hypertrophy through Suppression of Pla2g6 and Dgk-Z. Korean J. Physiol. Pharmacol. 2023, 27, 75–84. [Google Scholar] [CrossRef]
- Limtrakul, P.; Pitchakarn, P.; Suzuki, S.; Kuguacin, J. A Triterpenoid from Momordica Charantia Linn: A Comprehensive Review of Anticarcinogenic Properties. Carcinogenesis 2013, 275, 55532. [Google Scholar]
- Chen, J.-C.; Liu, W.-Q.; Lu, L.; Qiu, M.-H.; Zheng, Y.-T.; Yang, L.-M.; Zhang, X.-M.; Zhou, L.; Li, Z.-R. Kuguacins F–S, Cucurbitane Triterpenoids from Momordica charantia. Phytochemistry 2009, 70, 133–140. [Google Scholar] [CrossRef]
- Akihisa, T.; Higo, N.; Tokuda, H.; Ukiya, M.; Akazawa, H.; Tochigi, Y.; Kimura, Y.; Suzuki, T.; Nishino, H. Cucurbitane-Type Triterpenoids from the Fruits of Momordica charantia and Their Cancer Chemopreventive Effects. J. Nat. Prod. 2007, 70, 1233–1239. [Google Scholar] [CrossRef]
- Chou, M.-C.; Lee, Y.-J.; Wang, Y.-T.; Cheng, S.-Y.; Cheng, H.-L. Cytotoxic and Anti-Inflammatory Triterpenoids in the Vines and Leaves of Momordica charantia. Int. J. Mol. Sci. 2022, 23, 1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Lu, L.; Zhang, X.M.; Zhou, L.; Li, Z.R.; Qiu, M.H. Eight New Cucurbitane Glycosides, Kuguaglycosides a – H, from the Root of Momordica Charantia L. Helv. Chim. Acta 2008, 91, 920–929. [Google Scholar] [CrossRef]
- Chen, J.; Tian, R.; Qiu, M.; Lu, L.; Zheng, Y.; Zhang, Z. Trinorcucurbitane and Cucurbitane Triterpenoids from the Roots of Momordica charantia. Phytochemistry 2008, 69, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Murakami, T.; Nakamura, J.; Kobayashi, H.; Matsuda, H.; Yoshikawa, M. Structures of New Cucurbitane-Type Triterpenes and Glycosides, Karavilagenins and Karavilosides, from the Dried Fruit of Momordica charantia L. In Sri Lanka. Chem. Pharm. Bull. 2006, 54, 1545–1550. [Google Scholar] [CrossRef]
- An, B.C.; Kwak, S.H.; Ahn, J.Y.; Won, H.Y.; Kim, T.H.; Ryu, Y.; Chung, M.J. Identification of Bioactive Substances Derived from the Probiotic-Induced Bioconversion of Lagerstroemia Speciosa Pers. Leaf Extract That Have Beneficial Effects on Diabetes and Obesity. Microorganisms 2024, 12, 1848. [Google Scholar] [CrossRef]
- Huang, H.T.; Zhang, L.J.; Huang, H.C.; Hwang, S.Y.; Wu, C.L.; Lin, Y.C.; Liaw, C.C.; Cheng, Y.; Susan, L. Morris-Natschke, Chung-Yi Huang, Kuo-Hsiung Lee, and Yao-Haur Kuo. Cucurbitane-Type Triterpenoids from the Vines of Momordica Charantia and Their Anti-Inflammatory Activities. J. Nat. Prod. 2020, 83, 1400–1408. [Google Scholar] [CrossRef]
- Nettey-Oppong, E.E.; Muhammad, R.; Ali, A.; Jeong, H.-W.; Seok, Y.-S.; Kim, S.-W.; Choi, S.H. The Impact of Temperature and Pressure on the Structural Stability of Solvated Solid-State Conformations of Bombyx Mori Silk Fibroins: Insights from Molecular Dynamics Simulations. Materials 2024, 17, 5686. [Google Scholar] [CrossRef]
- Feng, M.C.; Luo, F.; Huang, L.J.; Li, K.; Chen, Z.M.; Li, H.; Yao, C.; Qin, B.; Chen, G. Rheum Palmatum L. And Salvia Miltiorrhiza Bge. Alleviates Acute Pancreatitis by Regulating Th17 Cell Differentiation: An Integrated Network Pharmacology Analysis, Molecular Dynamics Simulation and Experimental Validation. Chin. J. Integr. Med. 2023, 30, 408–420. [Google Scholar] [CrossRef]
- Hajdin, C.E.; Ding, F.; Dokholyan, N.V.; Weeks, K.M. On the Significance of an Rna Tertiary Structure Prediction. Rna 2010, 16, 1340–1349. [Google Scholar] [CrossRef]
- Wang, B.-L.; Kou, S.-B.; Lin, Z.-Y.; Shi, J.-H. Investigation on the Binding Behavior between Bsa and Lenvatinib with the Help of Various Spectroscopic and in Silico Methods. J. Mol. Struct. 2020, 1204, 127521. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Zhao, R.; Wang, C.; Wang, C.; Zhang, T. Study on Simultaneous Binding of Resveratrol and Curcumin to Β-Lactoglobulin: Multi-Spectroscopic, Molecular Docking and Molecular Dynamics Simulation Approaches. Food Hydrocoll. 2022, 124, 107331. [Google Scholar] [CrossRef]
- Habibian-Dehkordi, S.; Farhadian, S.; Ghasemi, M.; Evini, M. Insight into the Binding Behavior, Structure, and Thermal Stability Properties of Β-Lactoglobulin/Amoxicillin Complex in a Neutral Environment. Food Hydrocoll. 2022, 133, 107830. [Google Scholar] [CrossRef]
- Jiang, B.; Ji, M.; Liu, W.; Chen, L.; Cai, Z.; Zhao, Y.; Bi, X. Antidiabetic Activities of a Cucurbitane-Type Triterpenoid Compound from Momordica charantia in Alloxan-Induced Diabetic Mice. Mol. Med. Rep. 2016, 14, 4865–4872. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica Charantia, a Nutraceutical Approach for Inflammatory Related Diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Taheri, R.; Mokhtari, Y.; Yousefi, A.; Bashash, D. The Pi3k/Akt Signaling Axis and Type 2 Diabetes Mellitus (T2dm): From Mechanistic Insights into Possible Therapeutic Targets. Cell Biol. Int. 2024, 48, 1049–1068. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yoon, S.-Y.; Baek, J.; Kim, S.J.; Yu, J.S.; Kang, H.; Kang, K.S.; Chung, S.J.; Kim, K.H. Metabolite Profile of Cucurbitane-Type Triterpenoids of Bitter Melon (Fruit of Momordica Charantia) and Their Inhibitory Activity against Protein Tyrosine Phosphatases Relevant to Insulin Resistance. J. Agric. Food Chem. 2021, 69, 1816–1830. [Google Scholar] [CrossRef]
- Htwe, T.N. Metabolic Risk Markers in Insulin Resistance and Non-Insulin Resistance Type 2 Diabetes Mellitus. SOJ Diabetes Endocrinol. Care 2021, 1, 2. [Google Scholar] [CrossRef]
- Geyer, N. Targeting the Hedgehog and Pi3k/Akt/Mtor Signaling Pathways in Rhabdomyosarcoma. Doctoral Thesis, Georg-August Universität, Göttingen, Germany, 2018. [Google Scholar]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota During the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, 3. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Yang, G.; Ho, C.T.; Li, S. Momordica Charantia: A Popular Health-Promoting Vegetable with Multifunctionality. Food Funct. 2017, 8, 1749–1762. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Baggioni, A. Berberine and Its Role in Chronic Disease. Anti-Inflamm. Nutraceuticals Chronic Dis. 2016, 928, 27–45. [Google Scholar]
- Shih, C.C.; Shlau, M.T.; Lin, C.H.; Wu, J.B. Momordica Charantia Ameliorates Insulin Resistance and Dyslipidemia with Altered Hepatic Glucose Production and Fatty Acid Synthesis and Ampk Phosphorylation in High-Fat-Fed Mice. Phytother. Res. 2013, 28, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tuan, N.Q.; Park, M.; Quan, K.T.; Oh, J.; Heo, K.; Na, M.; Myung, C. Cucurbitane Triterpenoids from the Fruits of Momordica Charantia Improve Insulin Sensitivity and Glucose Homeostasis in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, 7. [Google Scholar] [CrossRef]
- Jiang, S.J. Berberine Inhibits Hepatic Gluconeogenesisviathe Lkb1-Ampk-Torc2 Signaling Pathway in Streptozotocin-Induced Diabetic Rats. World J. Gastroenterol. 2015, 21, 25. [Google Scholar] [CrossRef]
- Liu, X.; Yin, M.; Dong, J.; Mao, G.; Min, W.; Kuang, Z.; Yang, P.; Liu, L.; Zhang, N.; Deng, H. Tubeimoside-1 Induces Tfeb-Dependent Lysosomal Degradation of Pd-L1 and Promotes Antitumor Immunity by Targeting Mtor. Acta Pharm. Sin. B 2021, 11, 3134–3149. [Google Scholar] [CrossRef]
- Rivera, R.G., Jr.; Regidor, P.J.S.; Ruamero, E.C.; Santos, C.D.R.D.; Gomez, C.B.; Allanigue, E.J.V.; Salinas, M.V. Applying Network Pharmacology and Molecular Docking in the Screening for Molecular Mechanisms of Ampalaya (Momordica charantia L.) and Banaba (Lagerstroemia speciosa L.) against Type 2 Diabetes Mellitus. Acta Med. Philipp. 2024, 58, 108–124. [Google Scholar]
- Varadi, M.; Tsenkov, M.; Velankar, S. Challenges in Bridging the Gap between Protein Structure Prediction and Functional Interpretation. Proteins Struct. Funct. Bioinform. 2023, 93, 400–410. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swissadme: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Yang, Y.; Gu, L.; Singla, R.K. Network Pharmacology and Molecular Docking to Explore the Mechanism of Kangxian Decoction for Epilepsy. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Dan, W.; He, Q.; Guo, J.; Dai, S.; Hui, X.; Meng, P.; Cao, Q.; Yun, W.; Guo, X. Exploring the Biological Mechanism of Huang Yam in Treating Tumors and Preventing Antitumor Drug-Induced Cardiotoxicity Using Network Pharmacology and Molecular Docking Technology. Evid.-Based Complement. Altern. Med. 2021, 2021, 3333878. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. Swisstargetprediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Putri, A.F.; Utomo, D.H.; Tunjung WA, S.; Putri, W.A. Analysis of the Anti-Alzheimer Potential of Bioactive Compounds from Citrus Hystrix Dc. Peel, Leaf, and Essential Oil by Network Pharmacology. Heliyon 2024, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. Thorn, Ryan Whaley, and Teri E. Klein. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.; et al. Drugbank 6.0: The Drugbank Knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. Omim.Org: Online Mendelian Inheritance in Man (Omim®), an Online Catalog of Human Genes and Genetic Disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The Genecards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Shi, S.; Liu, J.; Zhang, F.; Li, X.; Liu, X.; Hu, G.; Dong, Y. Exploration of Pharmacological Mechanisms of Dapagliflozin against Type 2 Diabetes Mellitus through Pi3k-Akt Signaling Pathway Based on Network Pharmacology Analysis and Deep Learning Technology. Curr. Comput.-Aided Drug Des. 2025, 21, 452–465. [Google Scholar] [CrossRef]
- Chandramohan, N.; Kiran, M.; Nagarajaram, H.A. Dissection of Hubs and Bottlenecks in a Protein-Protein Interaction Network. Comput. Biol. Chem. 2023, 102, 107802. [Google Scholar]
- Tuo, Y.; Lu, X.; Tao, F.; Tukhvatshin, M.; Xiang, F.; Wang, X.; Shi, Y.; Lin, J.; Hu, Y. The Potential Mechanisms of Catechins in Tea for Anti-Hypertension: An Integration of Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Foods 2024, 13, 2685. [Google Scholar] [CrossRef]
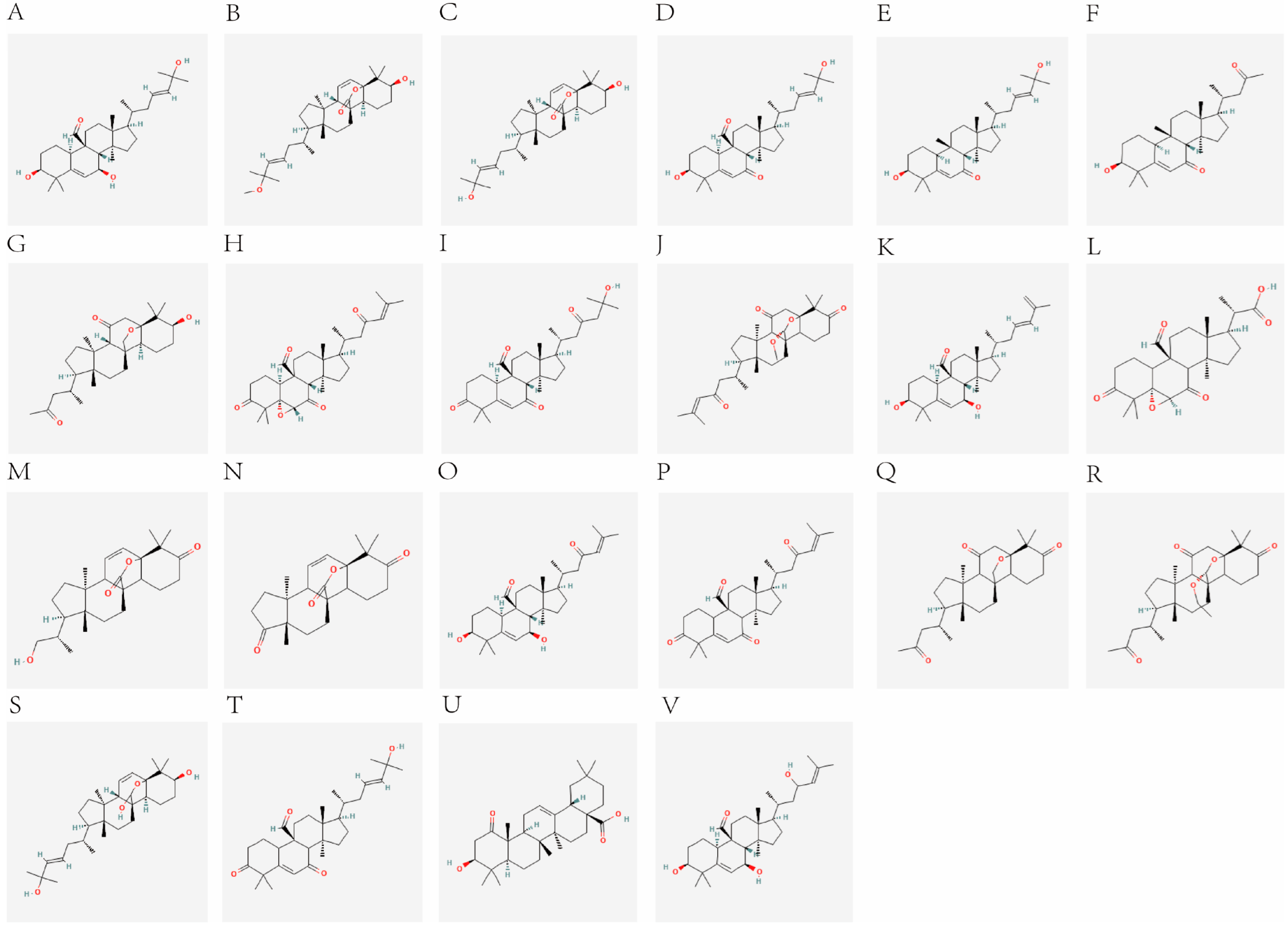
| NO. | Compound Name | SMILES | References |
|---|---|---|---|
| A | Momordicine I | C[C@H](CC(C=C(C)C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2[C@H](C=C4[C@H]3CC[C@@H](C4(C)C)O)O)C=O)C)C | [7,17] |
| B | Kuguacin J | C[C@H](C/C=C/C(=C)C)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2[C@H](C=C4[C@H]3CC[C@@H](C4(C)C)O)O)C=O)C)C | [18] |
| C | Kuguacin R | C[C@H](C/C=C/C(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@]34[C@H]2C=C[C@]5([C@H]3CC[C@@H](C5(C)C)O)OC4O)C)C | [19] |
| D | Kuguacin N | C[C@H](CC(=O)C=C(C)C)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2[C@H](C=C4[C@H]3CC[C@@H](C4(C)C)O)O)C=O)C)C | [19] |
| E | 25-O-methylkaravilagenin D | C[C@H](C/C=C/C(C)(C)OC)[C@H]1CC[C@@]2([C@@]1(CC[C@]34[C@H]2C=C[C@]5([C@H]3CC[C@@H](C5(C)C)O)OC4=O)C)C | [20] |
| F | 3, 7, 25-Trihydroxycucurbita-5, 23-dien-19-al | C[C@H](C/C=C/C(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2[C@H](C=C4[C@H]3CC[C@@H](C4(C)C)O)O)C=O)C)C | [21] |
| G | Karavilagenin D | C[C@H](C/C=C/C(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@]34[C@H]2C=C[C@]5([C@H]3CC[C@@H](C5(C)C)O)OC4=O)C)C | [22,23,24] |
| H | momordic acid | C[C@@]12CC[C@@H]3[C@@]([C@H]1CC=C4[C@]2(CC[C@@]5([C@H]4CC(CC5)(C)C)C(=O)O)C)(C(=O)C[C@@H](C3(C)C)O)C | [25] |
| I | Kuguacin A | C[C@H](C/C=C/C(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2C(=O)C=C4[C@H]3CC[C@@H](C4(C)C)O)C=O)C)C | [22,23] |
| J | Kuguacin B | C[C@H](C/C=C/C(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2C(=O)C=C4[C@H]3CC[C@@H](C4(C)C)O)C)C)C | [22,23] |
| K | Kuguacin C | C[C@H](CC(=O)C)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2C(=O)C=C4[C@H]3CC[C@@H](C4(C)C)O)C)C)C | [22,23] |
| L | Kuguacin E | C[C@H](CC(=O)C)[C@H]1CC[C@@]2([C@@]1(CC[C@]34[C@H]2C(=O)C[C@]5([C@H]3CC[C@@H](C5(C)C)O)OC4)C)C | [22,23] |
| M | Kuguacin F | C[C@H](CC(=O)C=C(C)C)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2C(=O)[C@H]4[C@@]5([C@H]3CCC(=O)C5(C)C)O4)C=O)C)C | [19,22] |
| N | Kuguacin H | C[C@H](CC(=O)CC(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3([C@H]2C(=O)C=C4[C@H]3CCC(=O)C4(C)C)C=O)C)C | [19,22,26] |
| O | Kuguacin I | C[C@H](CC(=O)C=C(C)C)[C@H]1CC[C@@]2([C@@]1(CC[C@]34C2C(=O)C[C@]5(C3CCC(=O)C5(C)C)O[C@H]4OC)C)C | [19,26] |
| P | Kuguacin K | C[C@@H]([C@H]1CC[C@@]2([C@@]1(CC[C@@]3(C2C(=O)[C@@H]4[C@@]5(C3CCC(=O)C5(C)C)O4)C=O)C)C)C(=O)O | [19,26] |
| Q | Kuguacin L | C[C@H](CO)[C@H]1CC[C@@]2([C@@]1(CC[C@]34C2C=C[C@]5(C3CCC(=O)C5(C)C)OC4=O)C)C | [19,26] |
| R | Kuguacin M | C[C@@]12CCC(=O)[C@]1(CC[C@]34C2C=C[C@]5(C3CCC(=O)C5(C)C)OC4=O)C | [19,26] |
| S | Kuguacin O | C[C@H](CC(=O)C=C(C)C)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3(C2C(=O)C=C4C3CCC(=O)C4(C)C)C=O)C)C | [19] |
| T | Kuguacin P | C[C@H](CC(=O)C)[C@H]1CC[C@@]2([C@@]1(CC[C@]34C2C(=O)C[C@]5(C3CCC(=O)C5(C)C)OC4)C)C | [19] |
| U | Kuguacin Q | CCO[C@H]1[C@@]23CC[C@@]4([C@H](CC[C@]4(C2C(=O)C[C@]5(C3CCC(=O)C5(C)C)O1)C)[C@H](C)CC(=O)C)C | [19] |
| V | Kuguacin S | C[C@H](C/C=C/C(C)(C)O)[C@H]1CC[C@@]2([C@@]1(CC[C@@]3(C2C(=O)C=C4C3CCC(=O)C4(C)C)C=O)C)C | [19] |
| Compound Name | Affinity (kcal/mol) | ||
|---|---|---|---|
| AKT1 | IL6 | SRC | |
| 25-O-methylkaravilagenin D | −6.53 | −7.30 | −7.40 |
| Kuguacin J | −6.27 | −6.73 | −7.23 |
| Kuguacin S | −6.27 | −7.33 | −7.53 |
| momordic acid | −7.30 | −7.70 | −7.80 |
| Momordicine I | −6.53 | −7.30 | −7.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Li, P.; Pang, Z. Comprehensive Studies on the Regulation of Type 2 Diabetes by Cucurbitane-Type Triterpenoids in Momordica charantia L.: Insights from Network Pharmacology and Molecular Docking and Dynamics. Pharmaceuticals 2025, 18, 474. https://doi.org/10.3390/ph18040474
Niu Y, Li P, Pang Z. Comprehensive Studies on the Regulation of Type 2 Diabetes by Cucurbitane-Type Triterpenoids in Momordica charantia L.: Insights from Network Pharmacology and Molecular Docking and Dynamics. Pharmaceuticals. 2025; 18(4):474. https://doi.org/10.3390/ph18040474
Chicago/Turabian StyleNiu, Yang, Peihang Li, and Zongran Pang. 2025. "Comprehensive Studies on the Regulation of Type 2 Diabetes by Cucurbitane-Type Triterpenoids in Momordica charantia L.: Insights from Network Pharmacology and Molecular Docking and Dynamics" Pharmaceuticals 18, no. 4: 474. https://doi.org/10.3390/ph18040474
APA StyleNiu, Y., Li, P., & Pang, Z. (2025). Comprehensive Studies on the Regulation of Type 2 Diabetes by Cucurbitane-Type Triterpenoids in Momordica charantia L.: Insights from Network Pharmacology and Molecular Docking and Dynamics. Pharmaceuticals, 18(4), 474. https://doi.org/10.3390/ph18040474





