Synthesis and Reactivity of Oligo(ethylene glycol)-Tethered Morita–Baylis–Hillman Dimers in the Formation of Macrocyclic Structures Showing Remarkable Cytotoxicity
Abstract
1. Introduction
2. Results and Discussion
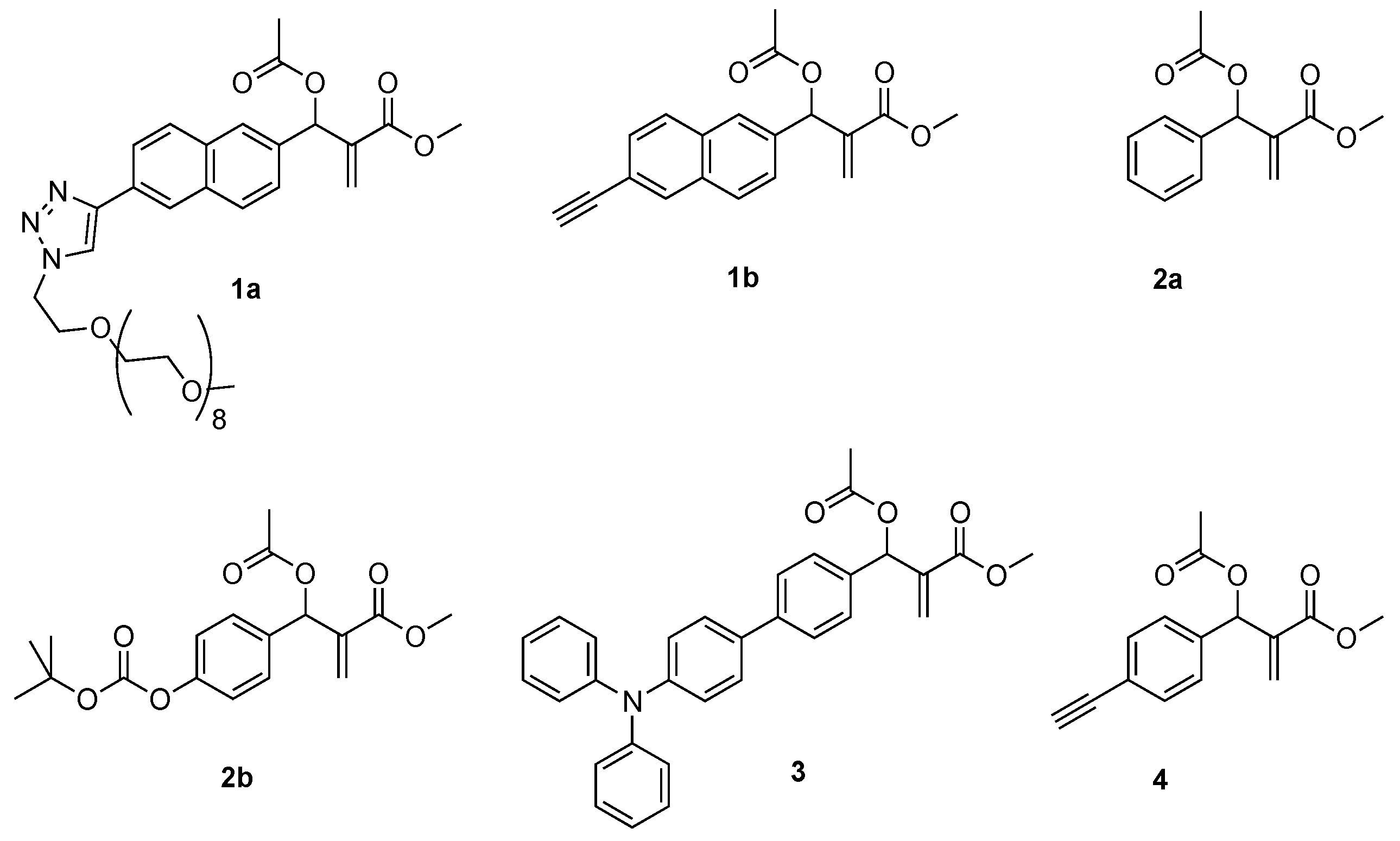
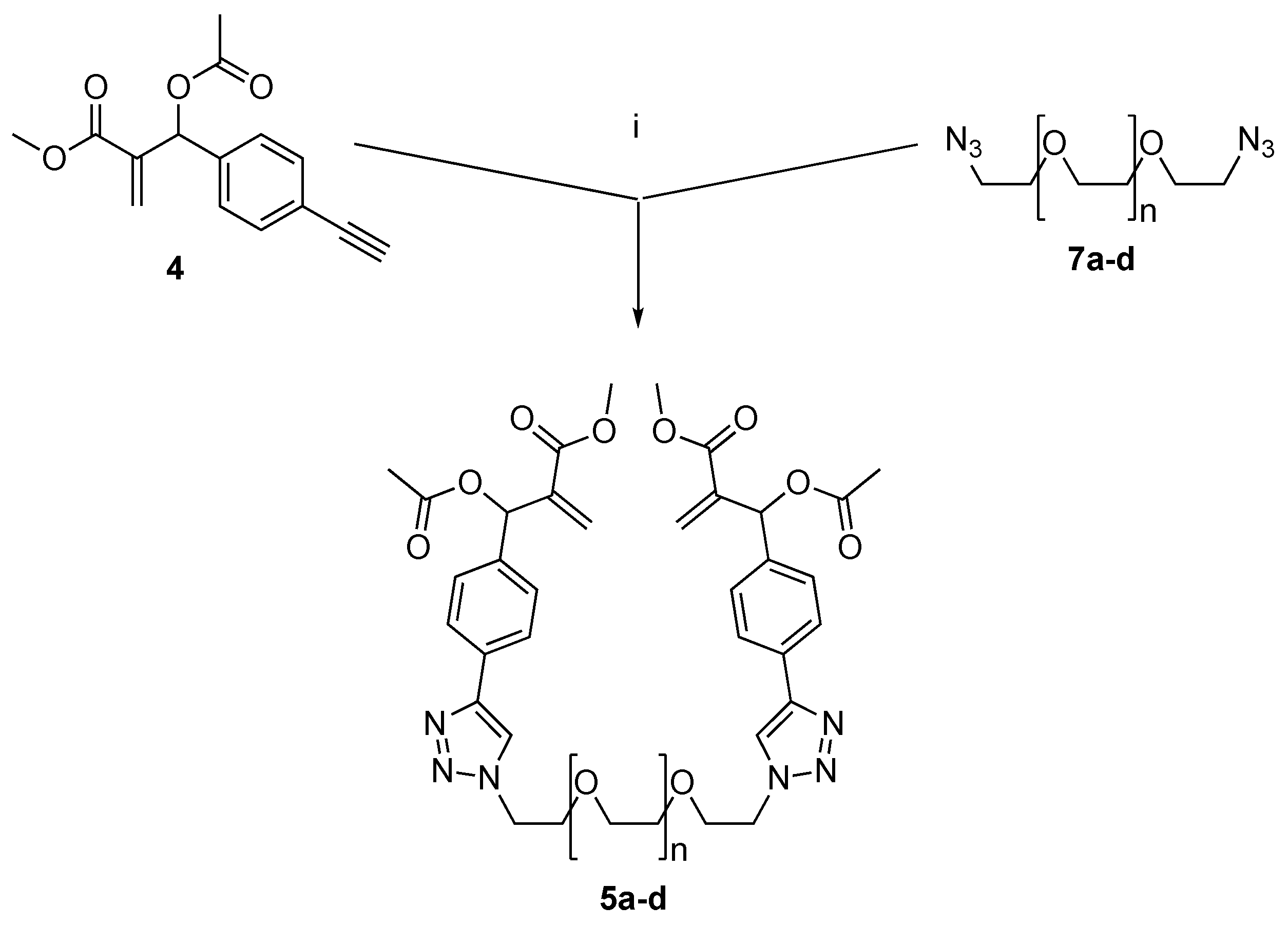
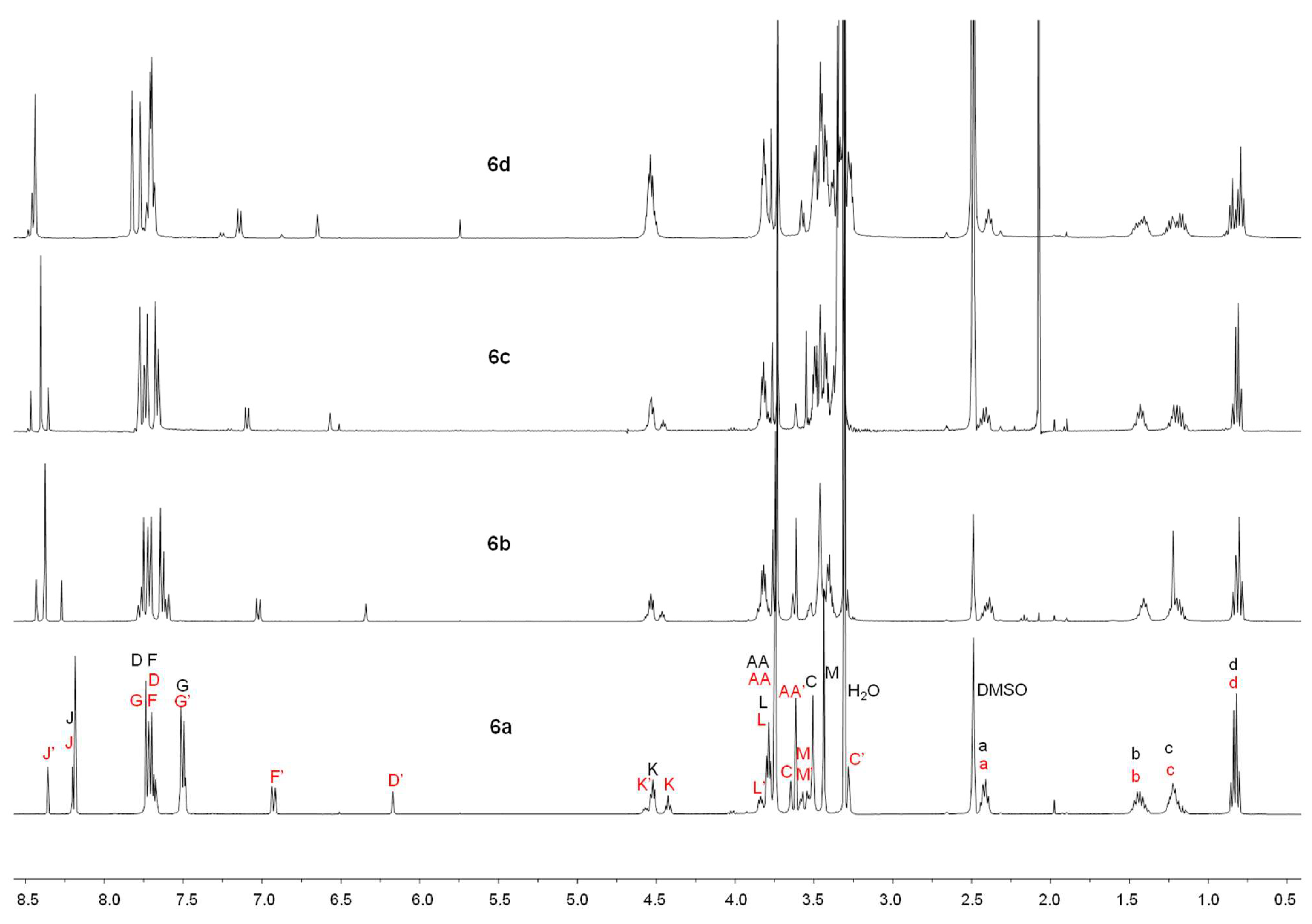
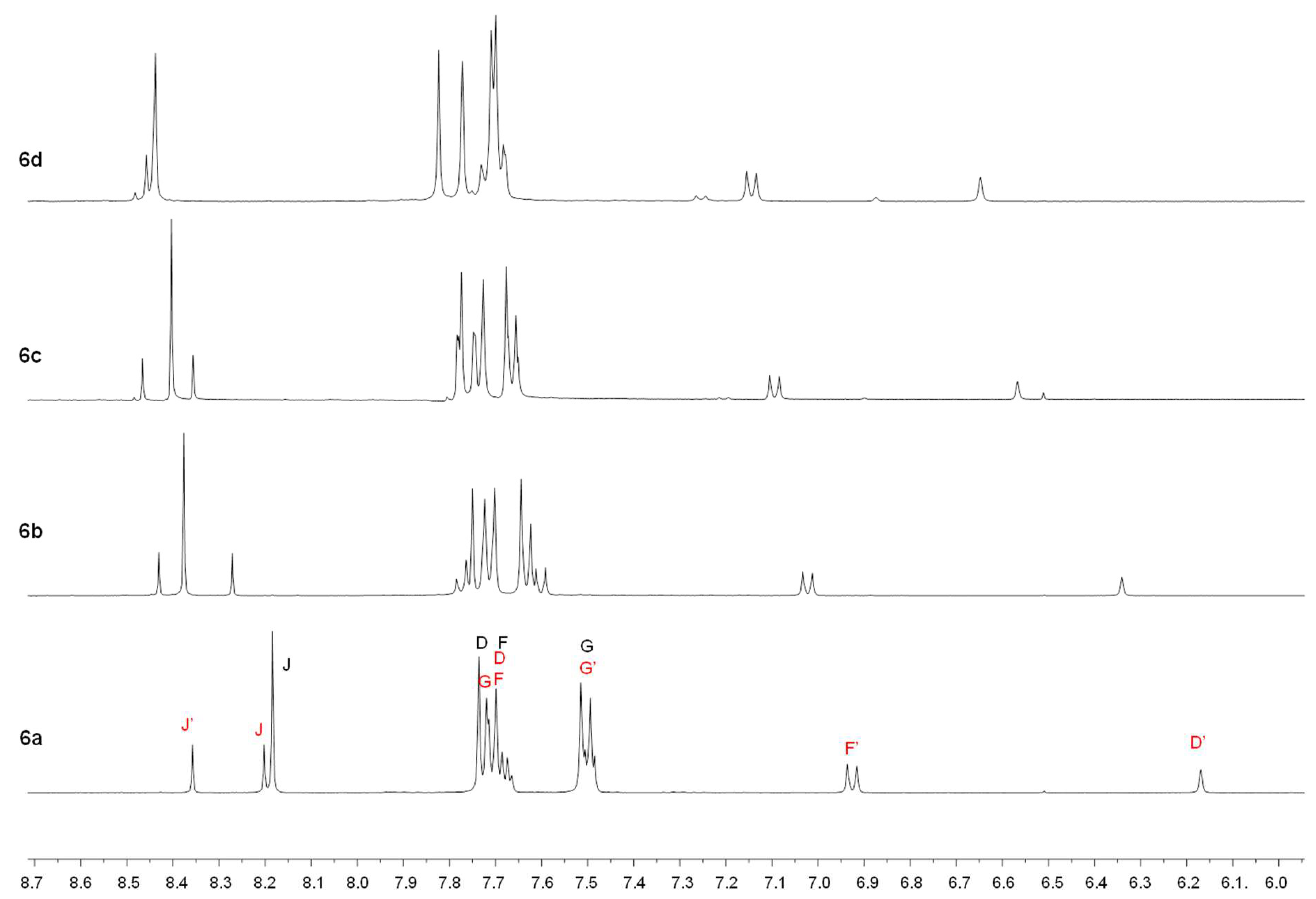
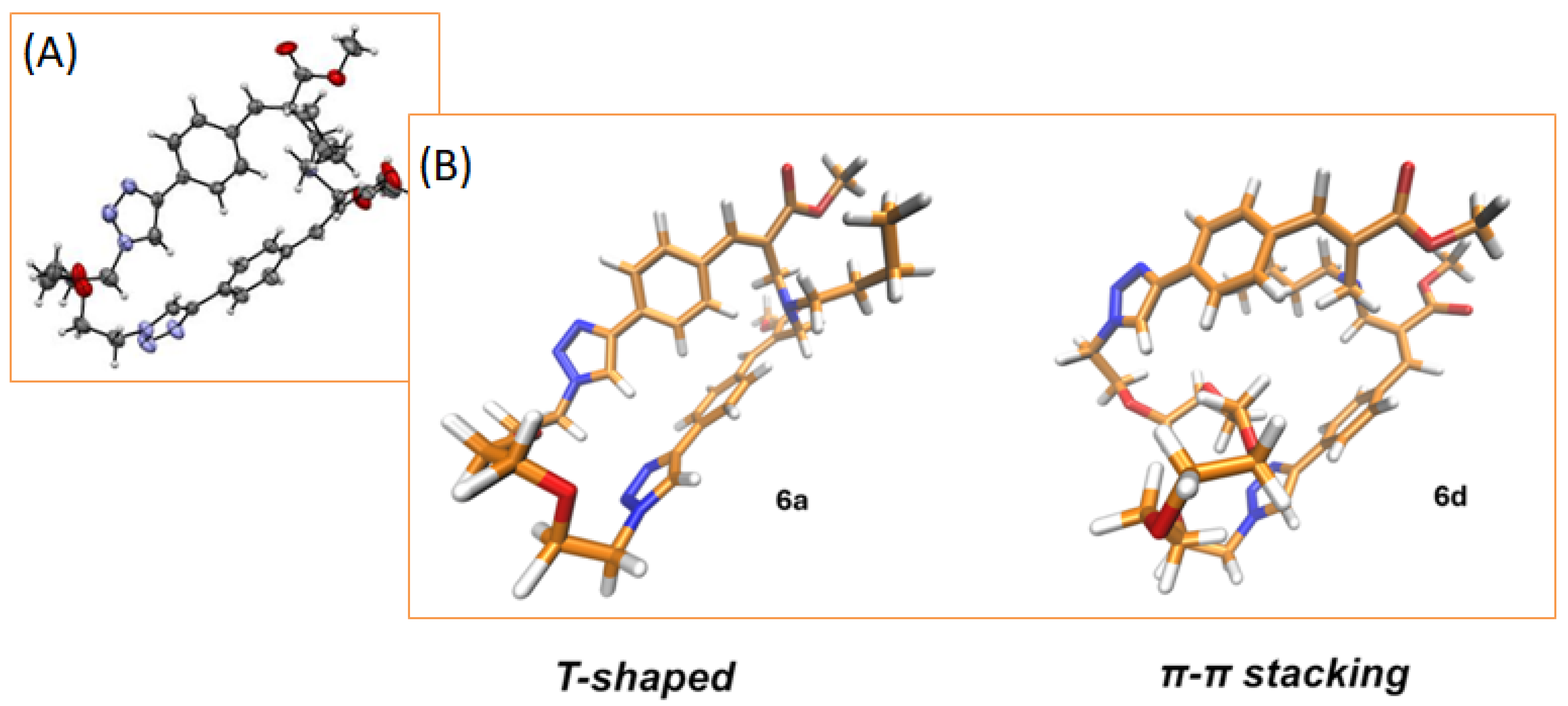
2.1. Synthesis
2.2. Biological Evaluation of Macrocycle Derivatives 6a-d: In Vitro Cytotoxicity Assay
3. Conclusions
4. Experimental Section
4.1. Synthesis
4.2. Biological Evaluation of Macrocycle Derivatives 6a-d
4.2.1. Materials
4.2.2. In Vitro Cytotoxicity Assay
4.2.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar]
- Pedersen, C.J. The Discovery of Crown Ethers (Noble Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar]
- Bradshaw, J.S.; Izatt, R.M. Crown Ethers: The Search for Selective Ion Ligating Agents. Acc. Chem. Res. 1997, 30, 338–345. [Google Scholar] [CrossRef]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar]
- Nicoli, F.; Baroncini, M.; Silvi, S.; Groppi, J.; Credi, A. Direct synthetic routes to functionalised crown ethers. Org. Chem. Front. 2021, 8, 5531–5549. [Google Scholar] [PubMed]
- Ullah, F.; Khan, T.A.; Iltaf, J.; Anwar, S.; Khan, M.F.; Khan, M.R.; Ullah, S.; Fayyaz ur Rehman, M.; Mustaqeem, M.; Kotwica-Mojzych, K.; et al. Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Appl. Sci. 2022, 12, 1102. [Google Scholar] [CrossRef]
- Gokel, G.W.; Ferdani, R.; Liu, J.; Pajewski, R.; Shabany, H.; Uetrecht, P. Hydraphile Channels: Models for Transmembrane, Cation-Conducting Transporters. Chem. A Eur. J. 2001, 7, 33–39. [Google Scholar]
- Leevy, W.M.; Gokel, M.R.; Hughes-Strange, G.B.; Schlesinger, P.H.; Gokel, G.W. Structure and medium effects on hydraphile synthetic ion channel toxicity to the bacterium E. coli. New J. Chem. 2005, 29, 205–209. [Google Scholar]
- Leevy, W.M.; Gammon, S.T.; Levchenko, T.; Daranciang, D.D.; Murillo, O.; Torchilin, V.; Piwnica-Worms, D.; Huettner, J.E.; Gokel, G.W. Structure–activity relationships, kinetics, selectivity, and mechanistic studies of synthetic hydraphile channels in bacterial and mammalian cells. Org. Biomol. Chem. 2005, 3, 3544–3550. [Google Scholar] [CrossRef][Green Version]
- Cai, M.-Y.; Arenaz, P. Antimutagenic effect of crown ethers on heavy metal-induced sister chromatid exchanges. Mutagenesis 1998, 13, 27–32. [Google Scholar]
- Su, Z.; Ran, X.; Leitch, J.J.; Schwan, A.L.; Faragher, R.; Lipkowski, J. How Valinomycin Ionophores Enter and Transport K+ across Model Lipid Bilayer Membranes. Langmuir 2019, 35, 16935–16943. [Google Scholar]
- Huang, S.; Liu, Y.; Liu, W.-Q.; Neubauer, P.; Li, J. The Nonribosomal Peptide Valinomycin: From Discovery to Bioactivity and Biosynthesis. Microorganisms 2021, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, M.; Kralj, M.; Supek, F.; Frkanec, L.; Piantanida, I.; Šmuc, T.; Tušek-Božić, L. Antitumor Potential of Crown Ethers: Structure−Activity Relationships, Cell Cycle Disturbances, and Cell Death Studies of a Series of Ionophores. J. Med. Chem. 2007, 50, 1007–1018. [Google Scholar]
- Roy, A.; Talukdar, P. Recent Advances in Bioactive Artificial Ionophores. ChemBioChem 2021, 22, 2925–2940. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Feng, H.; Zhong, W.; Dong, H.; Shen, Y.; Yu, B.; Cong, H. Design of crown ether based micelles and their anti-tumor properties by perturbing potassium ion homeostasis. Mater. Des. 2021, 211, 110159. [Google Scholar] [CrossRef]
- Gad, S.C.; Reilly, C.; Siino, K.; Gavigan, F.A.; Witz, G. Thirteen Cationic I0n0ph0res: Their Acute Toxicity, Neurobehavioral and Membrane Effects. Drug Chem. Toxicol. 1985, 8, 451–468. [Google Scholar]
- Guberović, I.; Marjanović, M.; Mioč, M.; Ester, K.; Martin-Kleiner, I.; Šumanovac Ramljak, T.; Mlinarić-Majerski, K.; Kralj, M. Crown ethers reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Sci. Rep. 2018, 8, 14467. [Google Scholar]
- Kralj, M.; Tušek-Božić, L.; Frkanec, L. Biomedical Potentials of Crown Ethers: Prospective Antitumor Agents. ChemMedChem 2008, 3, 1478–1492. [Google Scholar]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.-M.; Wang, D. Click Chemistry, A Powerful Tool for Pharmaceutical Sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar]
- Saady, A.; Goldup, S.M. Triazole formation and the click concept in the synthesis of interlocked molecules. Chem 2023, 9, 2110–2127. [Google Scholar]
- Khodadadi Yazdi, M.; Sajadi, S.M.; Seidi, F.; Rabiee, N.; Fatahi, Y.; Rabiee, M.; Dominic, C.D.M.; Zarrintaj, P.; Formela, K.; Saeb, M.R.; et al. Clickable polysaccharides for biomedical applications: A comprehensive review. Prog. Polym. Sci. 2022, 133, 101590. [Google Scholar]
- Basavaiah, D.; Naganaboina, R.T. The Baylis–Hillman reaction: A new continent in organic chemistry—our philosophy, vision and over three decades of research. New J. Chem. 2018, 42, 14036–14066. [Google Scholar]
- Juma, W.P.; Nyoni, D.; Brady, D.; Bode, M.L. The Application of Biocatalysis in the Preparation and Resolution of Morita-Baylis-Hillman Adducts and Their Derivatives. ChemBioChem 2022, 23, e202100527. [Google Scholar]
- Pellissier, H. Recent developments in the asymmetric organocatalytic Morita−Baylis−Hillman reaction. Tetrahedron 2017, 73, 2831–2861. [Google Scholar]
- Razzano, V.; Paolino, M.; Reale, A.; Giuliani, G.; Artusi, R.; Caselli, G.; Visintin, M.; Makovec, F.; Donati, A.; Villafiorita-Monteleone, F.; et al. Development of Imidazole-Reactive Molecules Leading to a New Aggregation-Induced Emission Fluorophore Based on the Cinnamic Scaffold. ACS Omega 2017, 2, 5453–5459. [Google Scholar]
- Razzano, V.; Paolino, M.; Reale, A.; Giuliani, G.; Donati, A.; Giorgi, G.; Artusi, R.; Caselli, G.; Visintin, M.; Makovec, F.; et al. Poly-histidine grafting leading to fishbone-like architectures. RSC Adv. 2018, 8, 8638–8656. [Google Scholar]
- Paolino, M.; Visintin, M.; Margotti, E.; Visentini, M.; Salvini, L.; Reale, A.; Razzano, V.; Giuliani, G.; Caselli, G.; Tavanti, F.; et al. Functionalization of protein hexahistidine tags by functional nanoreactors. New J. Chem. 2019, 43, 17946–17953. [Google Scholar]
- Paolino, M.; Reale, A.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Caselli, G.; Visintin, M.; Makovec, F.; Scialabba, C.; et al. Nanoreactors for the multi-functionalization of poly-histidine fragments. New J. Chem. 2019, 43, 6834–6837. [Google Scholar]
- Tassone, G.; Paolino, M.; Pozzi, C.; Reale, A.; Salvini, L.; Giorgi, G.; Orlandini, M.; Galvagni, F.; Mangani, S.; Yang, X.; et al. Xanthopsin-Like Systems via Site-Specific Click-Functionalization of a Retinoic Acid Binding Protein. ChemBioChem 2022, 23, e202100449. [Google Scholar]
- Saletti, M.; Paolino, M.; Venditti, J.; Bonechi, C.; Giuliani, G.; Boccia, A.; Botta, C.; Cappelli, A. Synthesis, photophysical and photochemical features of a Morita-Baylis-Hillman adduct derivative bearing a triphenylamine moiety. Dye. Pigment. 2023, 219, 111571. [Google Scholar] [CrossRef]
- Saletti, M.; Venditti, J.; Paolino, M.; Zacchei, A.; Giuliani, G.; Giorgi, G.; Bonechi, C.; Donati, A.; Cappelli, A. A tri(ethylene glycol)-tethered Morita–Baylis–Hillman dimer in the formation of macrocyclic crown ether-paracyclophane hybrid structures. RSC Adv. 2023, 13, 35773–35780. [Google Scholar] [CrossRef] [PubMed]
- Saletti, M.; Paolino, M.; Venditti, J.; Bonechi, C.; Giuliani, G.; Lamponi, S.; Tassone, G.; Boccia, A.; Botta, C.; Blancafort, L.; et al. A Facile Access to Green Fluorescent Albumin Derivatives. ChemBioChem 2024, 25, e202300862. [Google Scholar] [CrossRef] [PubMed]
- Lami, M.; Barneschi, L.; Saletti, M.; Olivucci, M.; Cappelli, A.; Paolino, M. Preparation of Light-responsive Unnatural RNA Bases via a Chromogenic Morita-Baylis-Hillman Adduct Path. ChemPhotoChem 2024, 8, e202400093. [Google Scholar] [CrossRef]
- Venditti, J.; Saletti, M.; Paolino, M.; Contena, S.; Bonechi, C.; Giuliani, G.; Giorgi, G.; Boccia, A.C.; Botta, C.; Blancafort, L.; et al. Reactivity of a Morita–Baylis–Hillman Adduct Derivative Bearing a Triphenylamine Moiety with Lysine Models. Chem. Asian J. 2024, 19, e202400617. [Google Scholar] [CrossRef]
- Paolino, M.; Tassone, G.; Governa, P.; Saletti, M.; Lami, M.; Carletti, R.; Sacchetta, F.; Pozzi, C.; Orlandini, M.; Manetti, F.; et al. Morita–Baylis–Hillman Adduct Chemistry as a Tool for the Design of Lysine-Targeted Covalent Ligands. ACS Med. Chem. Lett. 2025, 16, 397–405. [Google Scholar] [CrossRef]
- Payne, C.M.; Baltos, J.-A.; Langiu, M.; Sinh Lu, C.; Tyndall, J.D.A.; Gregory, K.J.; May, L.T.; Vernall, A.J. Development of Putative Bivalent Dicovalent Ligands for the Adenosine A1 Receptor. ChemBioChem 2024, 25, e202400242. [Google Scholar] [CrossRef]
- Bonger, K.M.; van den Berg, R.J.B.H.N.; Heitman, L.H.; IJzerman, A.P.; Oosterom, J.; Timmers, C.M.; Overkleeft, H.S.; van der Marel, G.A. Synthesis and evaluation of homo-bivalent GnRHR ligands. Bioorg. Med. Chem. 2007, 15, 4841–4856. [Google Scholar] [CrossRef]
- Goswami, L.N.; Houston, Z.H.; Sarma, S.J.; Jalisatgi, S.S.; Hawthorne, M.F. Efficient synthesis of diverse heterobifunctionalized clickable oligo(ethylene glycol) linkers: Potential applications in bioconjugation and targeted drug delivery. Org. Biomol. Chem. 2013, 11, 1116–1126. [Google Scholar] [CrossRef]
- van Mourik, T.; Bühl, M.; Gaigeot, M.-P. Density functional theory across chemistry, physics and biology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20120488. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 2016.
- Mennucci, B. Polarizable continuum model. WIREs Comput. Mol. Sci. 2012, 2, 386–404. [Google Scholar]
- Brandenburg, J.G.; Grimme, S. Dispersion Corrected Hartree–Fock and Density Functional Theory for Organic Crystal Structure Prediction BT—Prediction and Calculation of Crystal Structures: Methods and Applications; Atahan-Evrenk, S., Aspuru-Guzik, A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–23. ISBN 978-3-319-05774-3. [Google Scholar]
- Reuter, K.; Maas, R.G.M.; Reuter, A.; Kilgenstein, F.; Asfaha, Y.; von Hänisch, C. Synthesis of heteroatomic bridged paracyclophanes. Dalt. Trans. 2017, 46, 4530–4541. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, L.N.; Purgatorio, R.; Beloglazkin, A.A.; Tafeenko, V.A.; Reza, R.G.; Levickaya, D.D.; Sblano, S.; Boccarelli, A.; de Candia, M.; Catto, M.; et al. Chemical and Biological Evaluation of Novel 1H-Chromeno[3,2-c]pyridine Derivatives as MAO Inhibitors Endowed with Potential Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 7724. [Google Scholar] [CrossRef] [PubMed]
- Majellaro, M.; Stefanachi, A.; Tardia, P.; Vicenti, C.; Boccarelli, A.; Pannunzio, A.; Campanella, F.; Coluccia, M.; Denora, N.; Leonetti, F.; et al. Investigating Structural Requirements for the Antiproliferative Activity of Biphenyl Nicotinamides. ChemMedChem 2017, 12, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Nevskaya, A.A.; Matveeva, M.D.; Borisova, T.N.; Niso, M.; Colabufo, N.A.; Boccarelli, A.; Purgatorio, R.; de Candia, M.; Cellamare, S.; Voskressensky, L.G.; et al. A New Class of 1-Aryl-5,6-dihydropyrrolo[2,1-a]isoquinoline Derivatives as Reversers of P-Glycoprotein-Mediated Multidrug Resistance in Tumor Cells. ChemMedChem 2018, 13, 1588–1596. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of Biologically Synthesized Silver Nanoparticles in MDA-MB-231 Human Breast Cancer Cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar]
- Bashi, M.; Madanchi, H.; Yousefi, B. Investigation of cytotoxic effect and action mechanism of a synthetic peptide derivative of rabbit cathelicidin against MDA-MB-231 breast cancer cell line. Sci. Rep. 2024, 14, 13497. [Google Scholar]
- Zheng, M.; Priebe, W.; Walch, E.T.; Roth, K.G.; Han, M.; Tang, C.-H.; Lee, S.; Poindexter, N.J.; Fokt, I.; Grimm, E.A. WP760, a melanoma selective drug. Cancer Chemother. Pharmacol. 2007, 60, 625–633. [Google Scholar]
- Carrasquel-Ursulaez, W.; Reeves, R.D.; Dehghany, M.; Jones, C.; Schomaker, J.M.; Chanda, B. Re-evaluation of the mechanism of cytotoxicity of dialkylated lariat ether compounds. RSC Adv. 2020, 10, 40391–40394. [Google Scholar]
- Schneider, T.; Brüssow, N.; Yuvanc, A.; Budisa, N. Synthesis of New Aza- and Thia-Crown Ether Based Amino Acids. ChemistrySelect 2020, 5, 2854–2857. [Google Scholar] [CrossRef]
- Awasthi, A.; Kumar, N.; Mishra, A.; Ravi, R.; Dalal, A.; Shankar, S.; Chandra, R. Noscapine–Amino Acid Conjugates Suppress the Progression of Cancer Cells. ACS Pharmacol. Transl. Sci. 2022, 5, 1292–1304. [Google Scholar] [PubMed]
- Maeng, H.-J.; Kim, E.-S.; Chough, C.; Joung, M.; Lim, J.W.; Shim, C.-K.; Shim, W.-S. Addition of amino acid moieties to lapatinib increases the anti-cancer effect via amino acid transporters. Biopharm. Drug Dispos. 2014, 35, 60–69. [Google Scholar]
- Heh, E.; Allen, J.; Ramirez, F.; Lovasz, D.; Fernandez, L.; Hogg, T.; Riva, H.; Holland, N.; Chacon, J. Peptide Drug Conjugates and Their Role in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 829. [Google Scholar] [CrossRef]
- Hou, L.; Hou, Y.; Liang, Y.; Chen, B.; Zhang, X.; Wang, Y.; Zhou, K.; Zhong, T.; Long, B.; Pang, W.; et al. Anti-tumor effects of P-LPK-CPT, a peptide-camptothecin conjugate, in colorectal cancer. Commun. Biol. 2022, 5, 1248. [Google Scholar]
- Dean, T.T.; Jelú-Reyes, J.; Allen, A.C.; Moore, T.W. Peptide–Drug Conjugates: An Emerging Direction for the Next Generation of Peptide Therapeutics. J. Med. Chem. 2024, 67, 1641–1661. [Google Scholar] [PubMed]
- Wang, M.; Rakesh, K.P.; Leng, J.; Fang, W.-Y.; Ravindar, L.; Channe Gowda, D.; Qin, H.-L. Amino acids/peptides conjugated heterocycles: A tool for the recent development of novel therapeutic agents. Bioorg. Chem. 2018, 76, 113–129. [Google Scholar] [PubMed]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S.; Gunassekaran, G.R.; Lee, S.-M.; Yoon, J.-W.; Lee, Y.-K.; Lee, B. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Bethesda: Rockville, Maryland, 2004. [Google Scholar]
- Lamponi, S. Preliminary In Vitro Cytotoxicity, Mutagenicity and Antitumoral Activity Evaluation of Graphene Flake and Aqueous Graphene Paste. Life 2022, 12, 242. [Google Scholar] [CrossRef]
- Ross, A.; Willson, V.L. One-Way Anova BT—Basic and Advanced Statistical Tests: Writing Results Sections and Creating Tables and Figures; Ross, A., Willson, V.L., Eds.; SensePublishers: Rotterdam, The Netherlands, 2017; pp. 21–24. ISBN 978-94-6351-086-8. [Google Scholar]




| Compounds | IC50 (µM) | |
|---|---|---|
| MDA-MB-231 Cells | A375 Cells | |
| Doxorubicin | 1.5 | 0.5 |
| (E,Z)-6a | 0.8 | 0.8 |
| (E,E)-6a | 1.0 | 0.8 |
| 6b | 1.0 | 0.8 |
| 6c | 0.8 | 0.8 |
| 6d | 1.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, M.; Saletti, M.; Venditti, J.; Zacchei, A.; Donati, A.; Bonechi, C.; Giuliani, G.; Lamponi, S.; Cappelli, A. Synthesis and Reactivity of Oligo(ethylene glycol)-Tethered Morita–Baylis–Hillman Dimers in the Formation of Macrocyclic Structures Showing Remarkable Cytotoxicity. Pharmaceuticals 2025, 18, 473. https://doi.org/10.3390/ph18040473
Paolino M, Saletti M, Venditti J, Zacchei A, Donati A, Bonechi C, Giuliani G, Lamponi S, Cappelli A. Synthesis and Reactivity of Oligo(ethylene glycol)-Tethered Morita–Baylis–Hillman Dimers in the Formation of Macrocyclic Structures Showing Remarkable Cytotoxicity. Pharmaceuticals. 2025; 18(4):473. https://doi.org/10.3390/ph18040473
Chicago/Turabian StylePaolino, Marco, Mario Saletti, Jacopo Venditti, Arianna Zacchei, Alessandro Donati, Claudia Bonechi, Germano Giuliani, Stefania Lamponi, and Andrea Cappelli. 2025. "Synthesis and Reactivity of Oligo(ethylene glycol)-Tethered Morita–Baylis–Hillman Dimers in the Formation of Macrocyclic Structures Showing Remarkable Cytotoxicity" Pharmaceuticals 18, no. 4: 473. https://doi.org/10.3390/ph18040473
APA StylePaolino, M., Saletti, M., Venditti, J., Zacchei, A., Donati, A., Bonechi, C., Giuliani, G., Lamponi, S., & Cappelli, A. (2025). Synthesis and Reactivity of Oligo(ethylene glycol)-Tethered Morita–Baylis–Hillman Dimers in the Formation of Macrocyclic Structures Showing Remarkable Cytotoxicity. Pharmaceuticals, 18(4), 473. https://doi.org/10.3390/ph18040473







