AHR Agonist ITE Boosted PD1 Antibody’s Effects by Inhibiting Myeloid-Derived Cells Suppressive Cells in an Orthotopic Mouse Glioma Model
Abstract
1. Introduction
2. Results
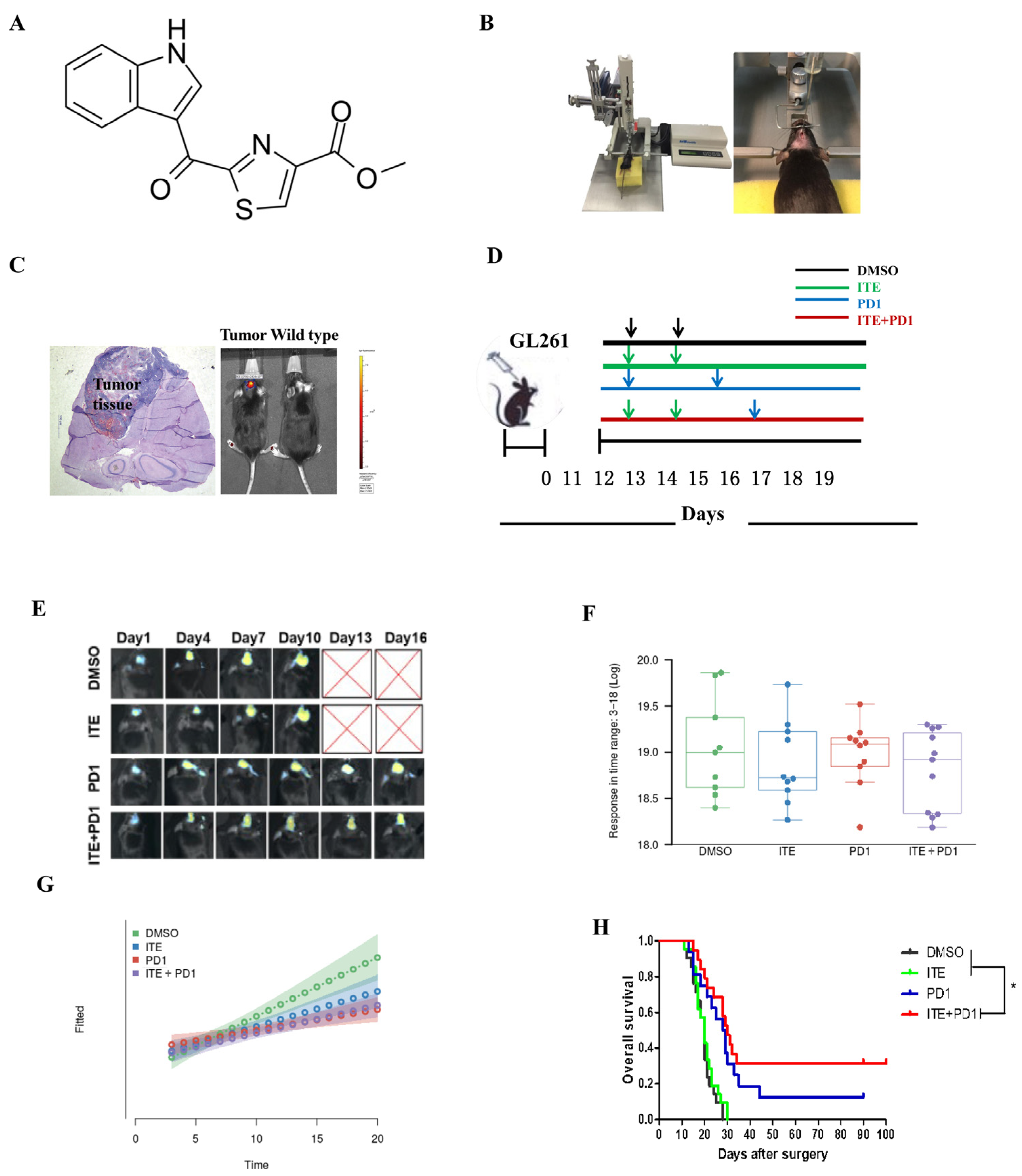
2.1. ITE Synergizes with PD1 Antibody to Suppress Tumor Growth and Extend Animal Survival
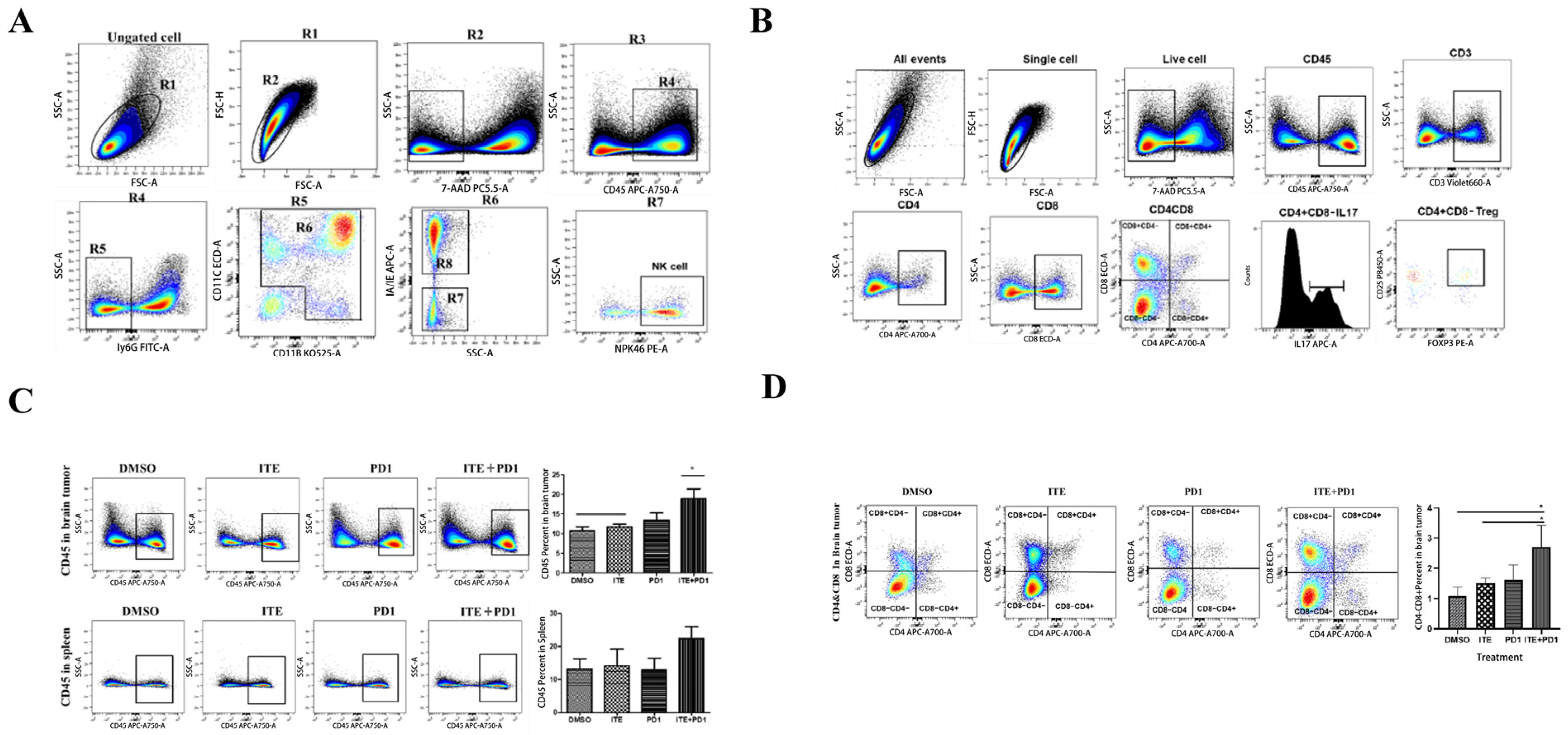
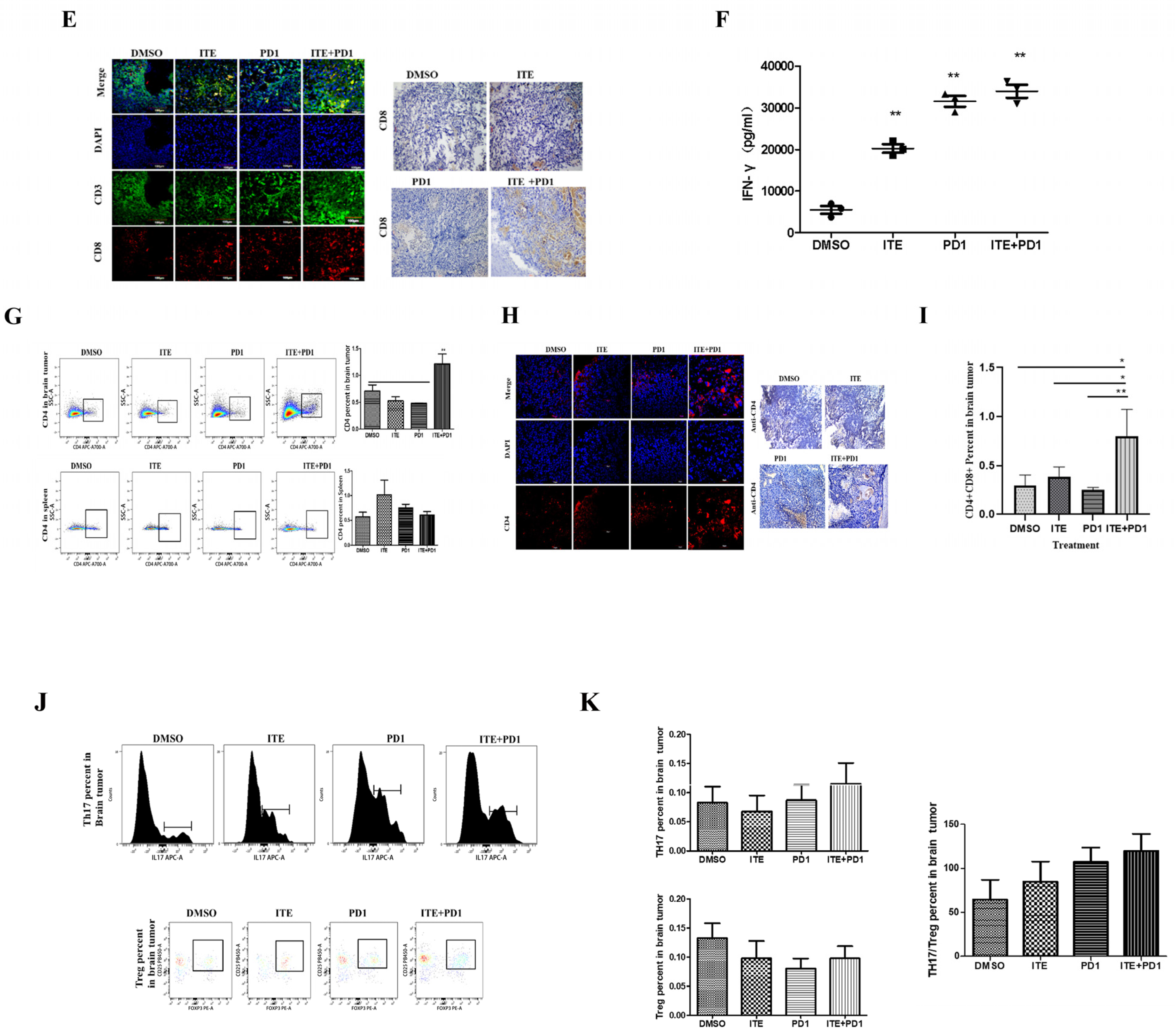
2.2. ITE Synergized with PD1 Antibody to Increase Cytotoxic Immune Cells Infiltration in Glioma
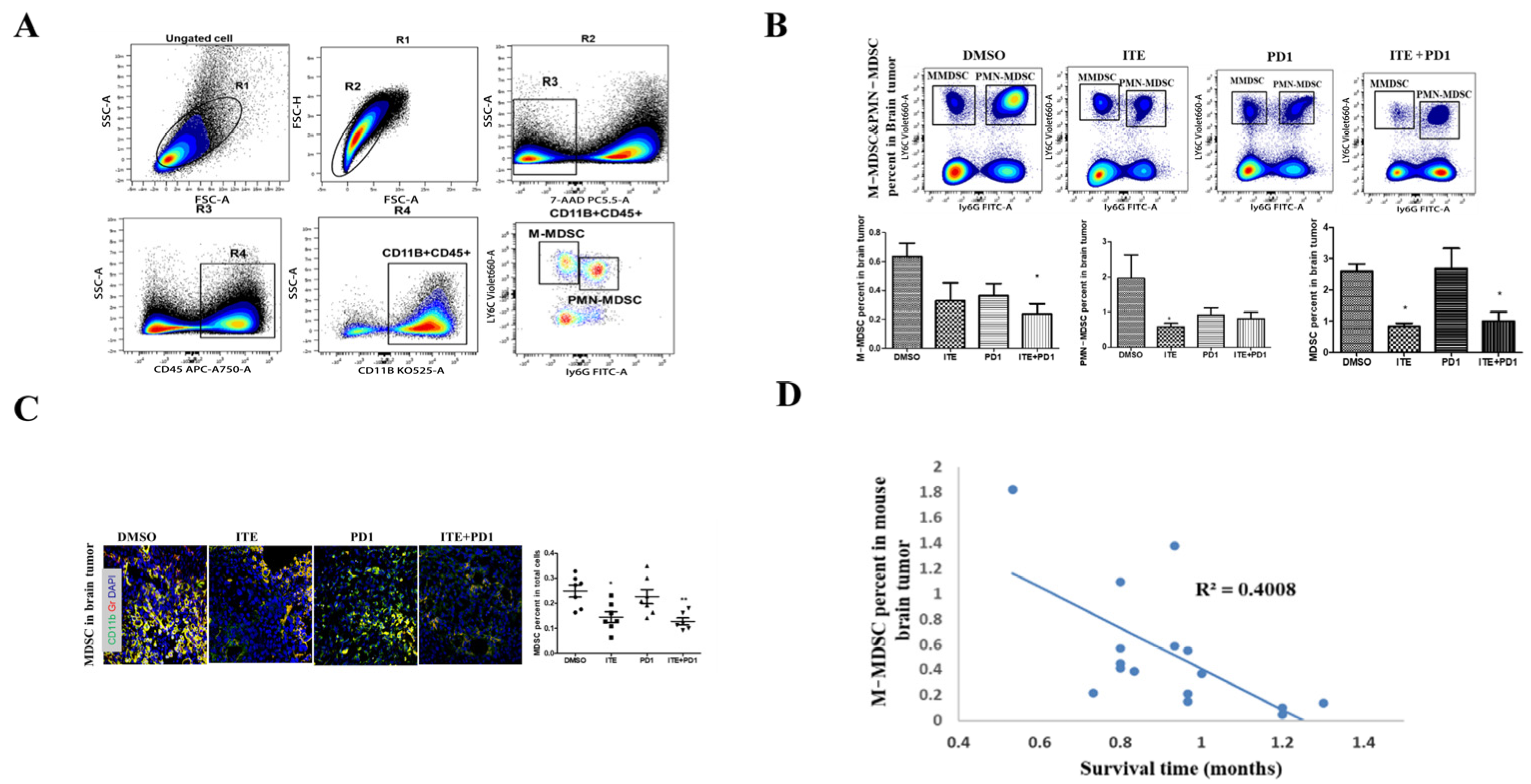
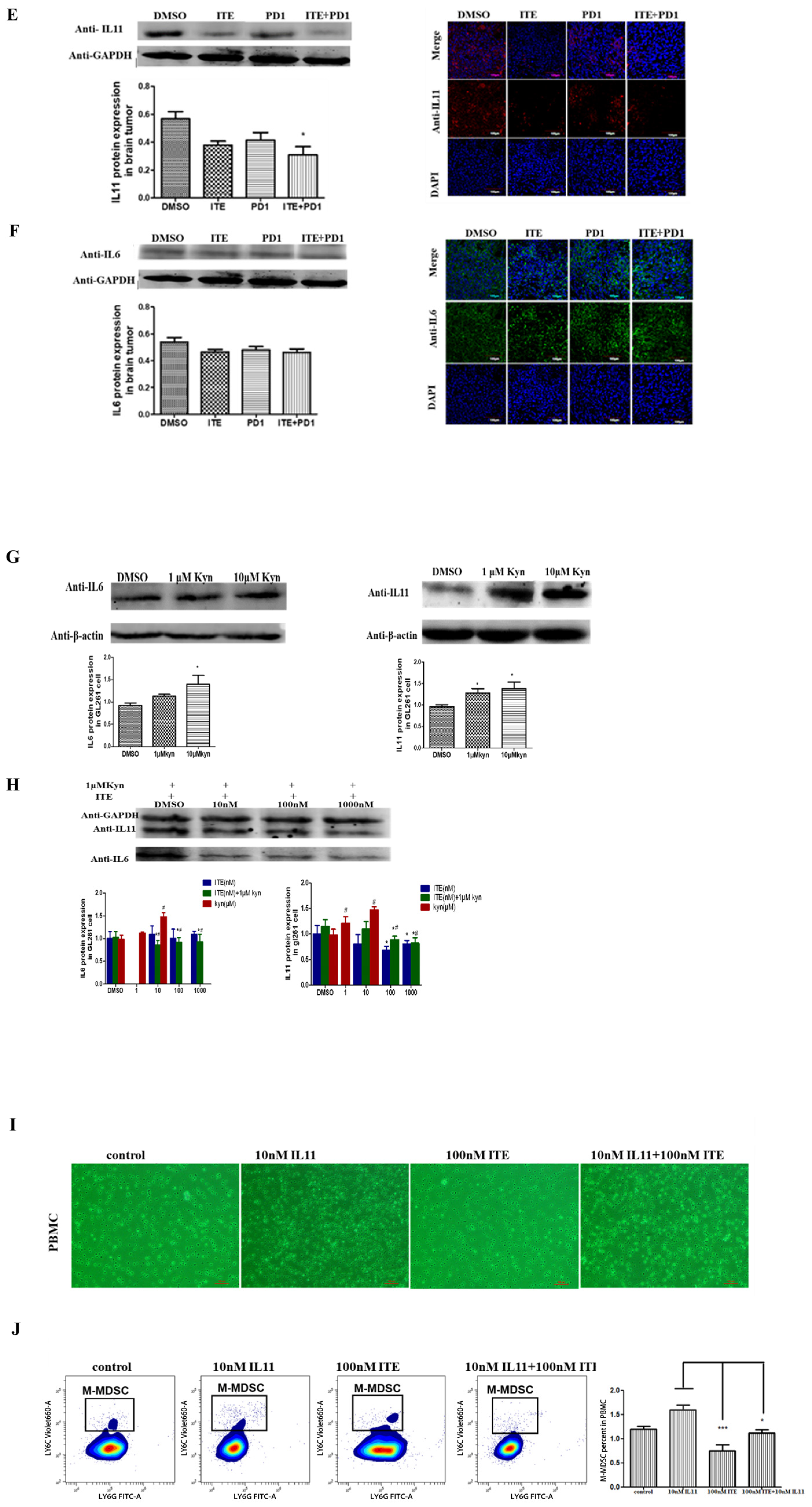
2.3. ITE Inhibited MDSC Infiltration in the Brain Tumor
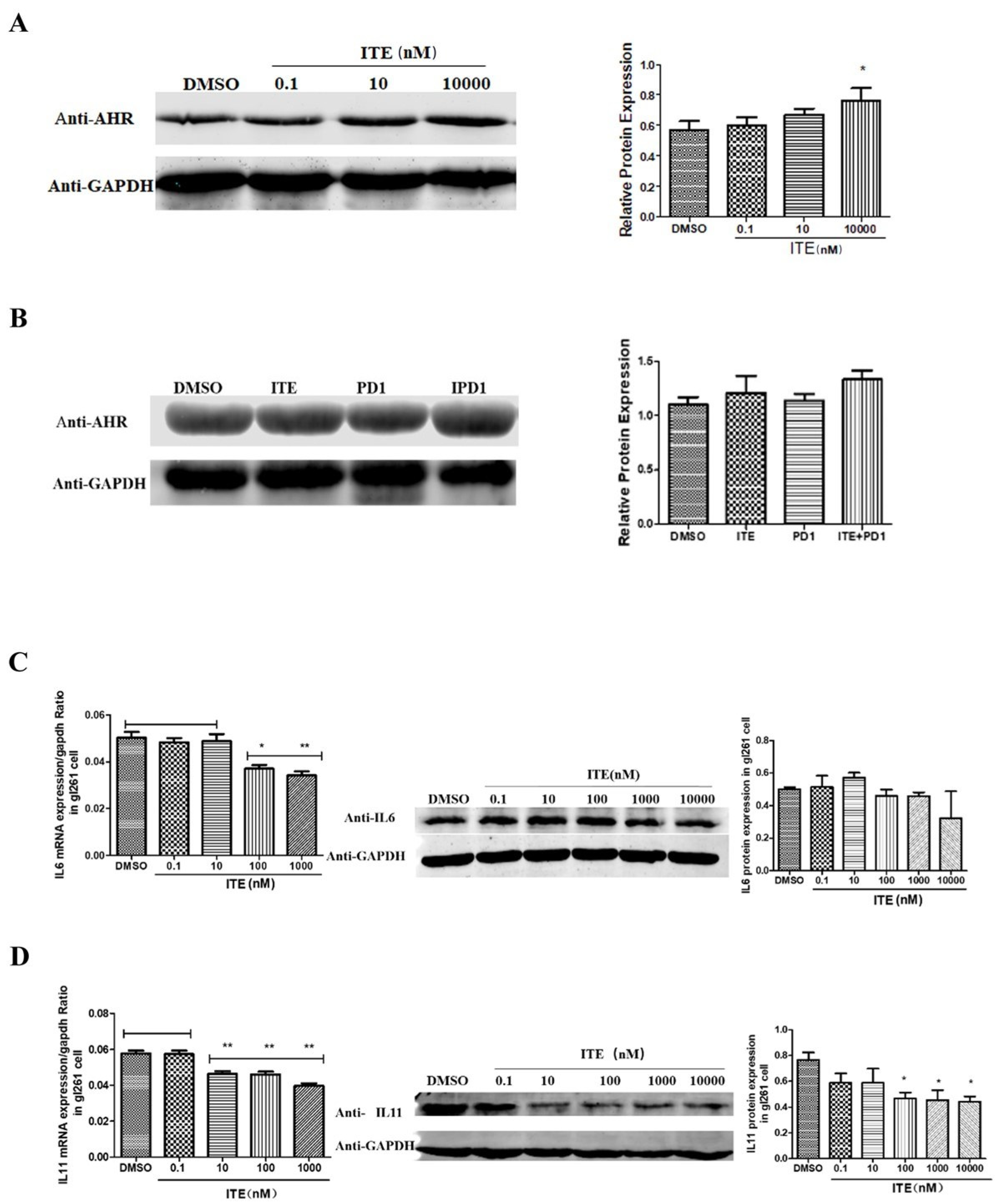
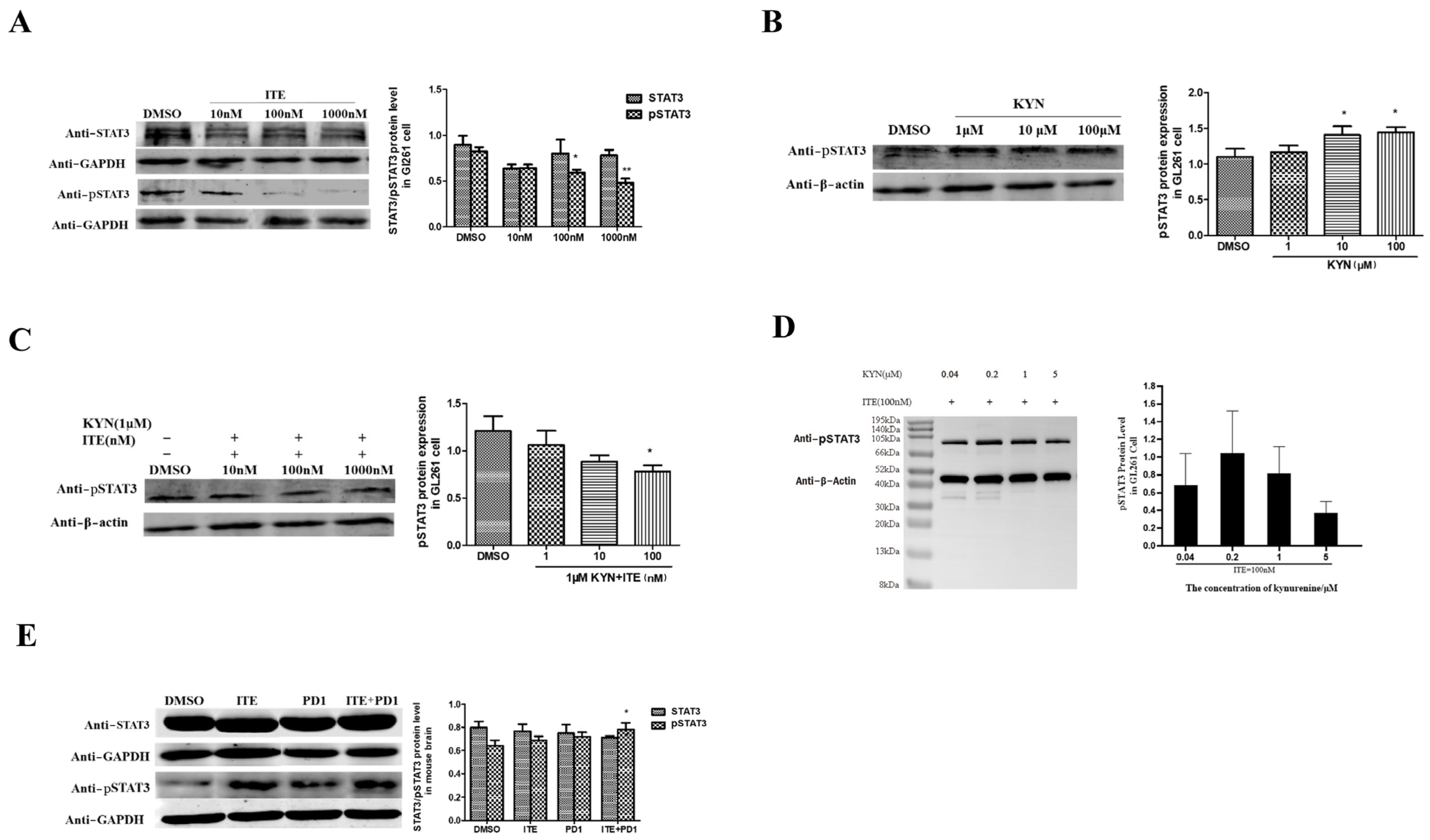
2.4. Kynurenine Induced STAT3, IL11 Expression Was Suppressed by ITE
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Animals and Reagents
4.2. Mouse Orthotopic Glioma Model
4.3. Drug Treatment
4.4. Animal Imaging
4.5. Preparation of Single-Cell Suspension
4.6. Flow Cytometry
4.7. Immunohistochemistry (IHC)
4.8. Immunofluorescence
4.9. RT-PCR
4.10. Western-Blot
4.11. Mouse Peripheral Blood Mononuclear Cell Collection
- (1)
- Anesthetize the mice with 3% chloral hydrate, take 700 µL of whole blood from the mouse heart inject an anticoagulation tube containing heparin sodium, and mix well after adding the same amount of Hank’s solution;
- (2)
- Add 1/2 blood volume of the peripheral blood mononuclear cell separation solution (Solarbio #P6340) to the centrifuge tube, suck the mouse’s whole blood with a pipette, then slowly inject it into the liquid surface of the stratification solution along the tube wall;
- (3)
- Put it in a centrifuge at 1500 rpm for 10 min. It can be seen that most of the albuginea-like mononuclear cells are suspended at the interface between the plasma and the stratified fluid;
- (4)
- Suck the white membrane cell layer with a straw, transfer it to a new centrifuge, add the same amount of Hank’s solution, mix the liquid, put it in a horizontal centrifuge at 1000 rpm for 10 min, and discard the supernatant;
- (5)
- Repeat step 4 twice;
- (6)
- Add RPMI-1640 complete medium (1% penicillin-streptomycin solution + 10% FBS) to the pellet, mix well, transfer it to a 5 mL cell culture flask with complete medium, and cultivate it in a CO2 incubator.
4.12. Statistical Analysis
4.13. Institutional Review Board Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamborero, D.; Rubio-Perez, C.; Muinos, F.; Sabarinathan, R.; Piulats, J.M.; Muntasell, A.; Dienstmann, R.; Lopez-Bigas, N.; Gonzalez-Perez, A. A Pan-cancer Landscape of Interactions between Solid Tumors and Infiltrating Immune Cell Populations. Clin. Cancer Res. 2018, 24, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Roszman, T.; Elliott, L.; Brooks, W. Modulation of T-Cell Function by Gliomas. Immunol. Today 1991, 12, 370–374. [Google Scholar] [CrossRef]
- Bloch, O.; Crane, C.A.; Kaur, R.; Safaee, M.; Rutkowski, M.J.; Parsa, A.T. Gliomas Promote Immunosuppression Through Induction of B7-H1 Expression in Tumor-Associated Macrophages. Clin. Cancer Res. 2013, 19, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Peterson, T.E.; Balgeman, A.; Chen, S.; Zhang, L.; Renner, D.N.; Johnson, A.J.; Parney, I.F. Increasing Glioma-Associated Monocytes Leads to Increased Intratumoral and Systemic Myeloid-Derived Suppressor Cells in a Murine Model. Neuro Oncol. 2015, 17, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Pakawat, C.; Harrison, F.S.; Karolina, W.; Elsamadicy, A.A.; Cui, X.; Peter, F. Imst-11. Downregulation Of Sphingosine-1-Phosphate Receptor Type 1 Mediates Bone Marrow T-Cell Sequestration in Patients and Mice with Glioblastoma. Neuro Oncol. 2016, 18 (Suppl. S6), i88. [Google Scholar]
- Kren, L.; Slaby, O.; Mucková, K.; Lzicarova, E.; Sova, M.; Vybihal, V.; Svoboda, T.; Fadrus, P.; Lakomy, R.; Vanhara, P.; et al. Expression of Immune-Modulatory Molecules HLA-G And HLA-E By Tumor Cells in Glioblastomas: An Unexpected Prognostic Significance? Neuropathology 2011, 31, 129–134. [Google Scholar] [CrossRef]
- Belting, M.; Wittrup, A. Nanotubes, Exosomes, And Nucleic Acid-Binding Peptides Provide Novel Mechanisms of Intercellular Communication in Eukaryotic Cells: Implications in Health and Disease. J. Cell Biol. 2008, 183, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.A.; Han, S.J.; Barry, J.J.; Ahn, B.J.; Lanier, L.L.; Parsa, A.T. TGF-Beta Downregulates the Activating Receptor NKG2D On NK Cells And CD8+ T Cells in Glioma Patients. Neuro Oncol. 2010, 12, 7–13. [Google Scholar] [CrossRef]
- Sordillo, P.P.; Sordillo, L.A.; Helson, L. The Kynurenine Pathway: A Primary Resistance Mechanism in Patients with Glioblastoma. Anticancer Res. 2017, 37, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Liu, H.J.; McKenzie, G.; Witting, P.K.; Stasch, J.-P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine Is an Endothelium-Derived Relaxing Factor Produced During Inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, R.B.; Zamarin, D.; Munn, D.H.; Wolchok, J.D.; Allison, J.P. Indoleamine 2,3-Dioxygenase Is a Critical Resistance Mechanism in Antitumor T Cell Immunotherapy Targeting CTLA-4. J. Exp. Med. 2013, 210, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Filley, A.C.; Henriquez, M.; Dey, M. Recurrent Glioma Clinical Trial, Checkmate-143: The Game is Not over Yet. Oncotarget 2017, 8, 91779–91794. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Dey, M.; Lauing, K.L.; Gritsina, G.; Kaur, R.; Lukas, R.V.; Nicholas, M.K.; Rademaker, A.W.; Dostal, C.R.; McCusker, R.H.; et al. The Kynurenine to Tryptophan Ratio as A Prognostic Tool for Glioblastoma Patients Enrolling in Immunotherapy. J. Clin. Neurosci. 2015, 22, 1964–1968. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Zhai, L.; Lenzen, A.; Lauing, K.L.; Qian, J.; Scholtens, D.M.; Gritsina, G.; Sun, X.; Liu, Y.; Yu, F.; et al. IDO1 Inhibition Synergizes with Radiation and PD-1 Blockade to Durably Increase Survival Against Advanced Glioblastoma. Clin. Cancer Res. 2018, 24, 2559–2573. [Google Scholar] [CrossRef] [PubMed]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Trump, S.; Lenz, C.; Dührsen, U.; Eils, R.; Becher, B.; Scheffler, M.; Seiffert, M.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270. [Google Scholar] [CrossRef]
- Nothdurft, S.; Thumser-Henner, C.; Breitenbücher, F.; Okimoto, R.A.; Dorsch, M.; Opitz, C.A.; Sadik, A.; Esser, C.; Hölzel, M.; Asthana, S.; et al. Functional screening identifies aryl hydrocarbon receptor as suppressor of lung cancer metastasis. Oncogenesis 2020, 9, 102. [Google Scholar] [CrossRef]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.-H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of The AHR Restricts a Treg-Macrophage Suppressive Axis Induced By L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef]
- Quintana, F.J.; Murugaiyan, G.; Farez, M.F.; Mitsdoerffer, M.; Tukpah, A.M.; Burns, E.J.; Weiner, H.L. An Endogenous Aryl Hydrocarbon Receptor Ligand Acts on Dendritic Cells and T Cells to Suppress Experimental Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. USA 2010, 107, 20768–20773. [Google Scholar] [CrossRef] [PubMed]
- Nugent, L.F.; Shi, G.; Vistica, B.P.; Ogbeifun, O.; Hinshaw, S.J.H.; Gery, I. ITE, A Novel Endogenous Nontoxic Aryl Hydrocarbon Receptor Ligand, Efficiently Suppresses EAU And T-Cell-Mediated Immunity. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7463–7469. [Google Scholar] [CrossRef]
- Kenison, J.E.; Jhaveri, A.; Li, Z.; Khadse, N.; Tjon, E.; Tezza, S.; Nowakowska, D.; Plasencia, A.; Stanton, V.P., Jr.; Sherr, D.H.; et al. Tolerogenic Nanoparticles Suppress Central Nervous System Inflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 32017–32028. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C.; et al. Effector T-Cell Infiltration Positively Impacts Survival of Glioblastoma Patients and is Impaired by Tumor-Derived TGF-Β. Clin. Cancer Res. 2011, 17, 4296–4308. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Tihan, T.; Han, S.J.; Wrensch, M.R.; Wiencke, J.; Sughrue, M.E.; Parsa, A.T. CD8+ T-Cell Infiltrate in Newly Diagnosed Glioblastoma Is Associated with Long-Term Survival. J. Clin. Neurosci. 2010, 17, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Schad, S.E.; Chow, A.; Mangarin, L.; Pan, H.; Zhang, J.; Ceglia, N.; Caushi, J.X.; Malandro, N.; Zappasodi, R.; Gigoux, M.; et al. Tumor-induced double positive T cells display distinct lineage commitment mechanisms and functions. J. Exp. Med. 2022, 219, e20212169. [Google Scholar] [CrossRef]
- Nascimbeni, M.; Shin, E.C.; Chiriboga, L.; Kleiner, D.E.; Rehermann, B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 2004, 104, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Clénet, M.L.; Gagnon, F.; Moratalla, A.C.; Viel, E.C.; Arbour, N. Peripheral human CD4+CD8+ T lymphocytes exhibit a memory phenotype and enhanced responses to IL-2, IL-7 and IL-15. Sci. Rep. 2017, 7, 11612. [Google Scholar] [CrossRef] [PubMed]
- Kerkvliet, N.I.; Shepherd, D.M.; Baecher-Steppan, L. T Lymphocytes Are Direct, Aryl Hydrocarbon Receptor (Ahr)-Dependent Targets of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD): Ahr Expression in Both CD4+ And CD8+ T Cells Is Necessary for Full Suppression of a Cytotoxic T Lymphocyte Response by TCDD. Toxicol. Appl. Pharmacol. 2002, 185, 146–152. [Google Scholar] [CrossRef]
- Munn, D.H.; Shafizadeh, E.; Attwood, J.T.; Bondarev, I.; Pashine, A.; Mellor, A.L. Inhibition of T Cell Proliferation by Macrophage Tryptophan Catabolism. J. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, H.; Wan, N.; Wang, T.; Bertera, S.; Trucco, M.; Dai, Z. Suppression of Memory CD8 T Cell Generation and Function by Tryptophan Catabolism. J. Immunol. 2007, 178, 4260–4266. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction Between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Alban, T.J.; Alvarado, A.G.; Sorensen, M.D.; Bayik, D.; Volovetz, J.; Serbinowski, E.; Mulkearns-Hubert, E.E.; Sinyuk, M.; Hale, J.S.; Onzi, G.R.; et al. Global Immune Fingerprinting in Glioblastoma Patient Peripheral Blood Reveals Immune-Suppression Signatures Associated with Prognosis. JCI Insight 2018, 3, e122264. [Google Scholar] [CrossRef]
- Natsume, A.; Tsujimura, K.; Mizuno, M.; Takahashi, T.; Yoshida, J. IFN-β Gene Therapy Induces Systemic Antitumor Immunity Against Malignant Glioma. J. Neurooncol. 2000, 47, 117–124. [Google Scholar] [CrossRef]
- Samoto, K.; Perng, G.C.; Ehtesham, M.; Liu, Y.; Wechsler, S.L.; Nesburn, A.B.; Black, K.L.; Yu, J.S. A Herpes Simplex Virus Type 1 Mutant Deleted for Gamma34.5 And LAT Kills Glioma Cells In Vitro and Is Inhibited for In Vivo Reactivation. Cancer Gene Ther. 2001, 8, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Neamah, W.H.; Busbee, P.B.; Alghetaa, H.; Abdulla, O.A.; Nagarkatti, M.; Nagarkatti, P. Ahr Activation Leads to Alterations in the Gut Microbiome with Consequent Effect on Induction of Myeloid Derived Suppressor Cells in A CXCR2-Dependent Manner. Int. J. Mol. 2020, 21, 9613. [Google Scholar] [CrossRef] [PubMed]
- Kenison, J.E.; Wang, Z.; Yang, K.; Snyder, M.; Quintana, F.J.; Sherr, D.H. The Aryl Hydrocarbon Receptor Suppresses Immunity TO Oral Squamous Cell Carcinoma Through Immune Checkpoint Regulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012692118. [Google Scholar] [CrossRef] [PubMed]
- Mastio, J.; Condamine, T.; Dominguez, G.; Kossenkov, A.V.; Donthireddy, L.; Veglia, F.; Lin, C.; Wang, F.; Fu, S.; Zhou, J.; et al. Identification of Monocyte-Like Precursors of Granulocytes in Cancer as a Mechanism for Accumulation of PMN-Mdscs. J. Exp. Med. 2019, 216, 2150–2169. [Google Scholar] [CrossRef] [PubMed]
- Elpek, K.G.; Cremasco, V.; Shen, H.; Harvey, C.J.; Wucherpfennig, K.W.; Goldstein, D.R.; Monach, P.A.; Turley, S.J. The Tumor Microenvironment Shapes Lineage, Transcriptional, and Functional Diversity of Infiltrating Myeloid Cells. Cancer Immunol. Res. 2014, 2, 655–667. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef]
- Kerzee, J.K.; Ramos, K.S. Constitutive and inducible expression of Cyp1a1 and Cyp1b1 in vascular smooth muscle cells: Role of the Ahr bHLH/PAS transcription factor. Circ. Res. 2001, 89, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Baloyan, D.; Chisanga, D.; Shi, W.; Brien, M.O.; Afshar-Sterle, S.; Alorro, M.; Pang, L.; Williams, D.S.; Parslow, A.C.; et al. Host IL11 Signaling Suppresses CD4+ T Cell-Mediated Antitumor Responses to Colon Cancer in Mice. Cancer Immunol. Res. 2021, 9, 735–747. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, Y.; Zhang, C.; Li, J.; Huang, H.P.; Zhu, Y.; Deng, G.X.; Cheng, J.H.; Lin, Y.X.; Shi, Z.M.; et al. Pharmacologic inhibition of IL11/STAT3 signaling increases MHC-I expression and T cell infiltration. J. Transl. Med. 2023, 21, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.Y.; Sandanaraj, E.; Chong, Y.K.; Lim, S.W.; Koh, L.W.H.; Ng, W.H.; Tan, N.S.; Tan, P.; Ang, B.T.; Tang, C. A STAT3-based gene signature stratifies glioma patients for targeted therapy. Nat. Commun. 2019, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.K.; Pennington, J.M.; Bisson, W.H.; Kolluri, S.K.; Kerkvliet, N.I. TCDD, FICZ, and Other High Affinity Ahr Ligands Dose-Dependently Determine the Fate of CD4+ T Cell Differentiation. Toxicol. Sci. 2018, 161, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Abron, J.D.; Singh, N.P.; Mishra, M.K.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. An Endogenous Aryl Hydrocarbon Receptor Ligand, ITE, Induces Regulatory T Cells and Ameliorates Experimental Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, 220–230. [Google Scholar] [CrossRef]
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor? Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.N. The X-Files in Immunity: Sex-Based Differences Predispose Immune Responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Harbo, H.F.; Gold, R.; Tintore, M. Sex and Gender Issues in Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Maglione, A.; Rolla, S.; Mercanti, S.F.; Cutrupi, S.; Clerico, M. The Adaptive Immune System in Multiple Sclerosis: An Estrogen-Mediated Point of View. Cells 2019, 8, 1280. [Google Scholar] [CrossRef]
- Gargaro, M.; Scalisi, G.; Manni, G.; Mondanelli, G.; Grohmann, U.; Fallarino, F. The Landscape of Ahr Regulators and Coregulators to Fine-Tune Ahr Functions. Int. J. Mol. Sci. 2021, 22, 757. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jin, U.H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (Sahrms). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef] [PubMed]
- Daniela, D.; Ballarotto, M.; Gargaro, M.; López-Cara, L.C.; Fallarino, F.; Macchiarulo, A. Targeting Aryl hydrocarbon receptor for next-generation immunotherapies: Selective modulators (SAhRMs) versus rapidly metabolized ligands (RMAhRLs). Eur. J. Med. Chem. Chim. Ther. 2020, 185, 111842. [Google Scholar]
- Hoffman, T.E.; Acerbo, E.R.; Carranza, K.F.; Gilberto, V.S.; Wallis, L.E.; Hanneman, W.H. Ultrasensitivity Dynamics of Diverse Aryl Hydrocarbon Receptor Modulators in A Hepatoma Cell Line. Arch. Toxicol. 2019, 93, 635–647. [Google Scholar] [CrossRef]
- Wu, J.B.; Shi, C.; Chu, G.C.; Xu, Q.; Zhang, Y.; Li, Q.; Yu, J.S.; Zhau, H.E.; Chung, L.W.K. Near-Infrared Fluorescence Heptamethine Carbocyanine Dyes Mediate Imaging and Targeted Drug Delivery for Human Brain Tumor. Biomaterials 2015, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Brand |
|---|---|
| CD8 | Abcam (Shanghai, China) |
| CD4 | Abcam |
| STAT3 | Abclonal (Wuhan, China) |
| STAT3 phosphorylation | SAB (Shanghai, China) |
| LY6G | CST (Shanghai, China) |
| Gr | CST |
| IL6 | Abclonal |
| IL11 | Abclonal |
| β-actin | Protein-tech (Wuhan, China) |
| GAPDH | Protein-tech |
| a-tubμlin | Protein-tech |
| Goat anti-mouse fluorescent secondary antibody-488 | Abcam |
| Goat anti-mouse fluorescent secondary antibody-633 | Abcam |
| Goat anti-rabbit fluorescent secondary antibody | Abcam |
| Goat anti-mouse fluorescent secondary antibody-800W | Li-COR Bioscience (Lincoln, NE, USA) |
| Goat anti-rabbit fluorescent secondary antibody-800W | Li-COR Bioscience |
| 7-AAD | Bio-Legend (Shanghai, China) |
| CD45 | Bio-Legend |
| CD3 | Bio-Legend |
| CD4 | Bio-Legend |
| CD8 | Bio-Legend |
| CD25 | Bio-Legend |
| IL17A | BD (Shanghai, China) |
| FOXP3 | BD |
| LY6G | Bio-Legend |
| CD11C | Bio-Legend |
| IA/IE | Bio-Legend |
| LY6C | Bio-Legend |
| NPK46 | Bio-Legend |
| CD11B | Bio-Legend |
| CD24 | Bio-Legend |
| CD64 | Bio-Legend |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PTK2 (human) | ACATTATTGGCCACTGTGGATGAG | GGCCAGTTTCATCTTGTTGATGAG |
| HPRT (human) | TGACACTGGCAAAACAATGCA | GGTCCTTTTCACCAGCAAGCT |
| MYH9 (human) | ATCTCGTGCTATCCGCCAAG | GTTGTACGGCTCCAACAGGA |
| Myh9 (mouse) | ACGCCAAGACGGTGAAGAAT | CTTGGCGGATAGCACGAGAT |
| COCL1 (human) | TCTGCGACAACGGCAAGGTG | GACGCCGGTGGTTTCTTGGT |
| ITGA5 (human) | GCCTGTGGAGTACAAGTCCTT | AATTCGGGTGAAGTTATCTGTGG |
| ITGB5 (human) | CAGGTGGAGGACTATCCTGTG | GTGCCGTGTAGGAGAAAGGAG |
| Vav3 (mouse) | TTACACGAAGATGAGTGCAAATG | CAACACTGGATAGGACTTTATTCATC |
| VAV3 (human) | ACGGACCAATGGACTGCG | TTCTGCCCTGCCAAAACA |
| Gapdh (mouse) | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT |
| GAPDH (human) | TCATTGAGCCCTTCCACAATG | GGTGTGAACCACGAGAAATATGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Zhao, L.; Ma, Y.; Shu, Q.; Sun, H.; Lu, J.; Meng, F.; Wan, F. AHR Agonist ITE Boosted PD1 Antibody’s Effects by Inhibiting Myeloid-Derived Cells Suppressive Cells in an Orthotopic Mouse Glioma Model. Pharmaceuticals 2025, 18, 471. https://doi.org/10.3390/ph18040471
Gong P, Zhao L, Ma Y, Shu Q, Sun H, Lu J, Meng F, Wan F. AHR Agonist ITE Boosted PD1 Antibody’s Effects by Inhibiting Myeloid-Derived Cells Suppressive Cells in an Orthotopic Mouse Glioma Model. Pharmaceuticals. 2025; 18(4):471. https://doi.org/10.3390/ph18040471
Chicago/Turabian StyleGong, Pei, Lijiao Zhao, Yunlong Ma, Qiuting Shu, Hui Sun, Jing Lu, Fanhua Meng, and Fang Wan. 2025. "AHR Agonist ITE Boosted PD1 Antibody’s Effects by Inhibiting Myeloid-Derived Cells Suppressive Cells in an Orthotopic Mouse Glioma Model" Pharmaceuticals 18, no. 4: 471. https://doi.org/10.3390/ph18040471
APA StyleGong, P., Zhao, L., Ma, Y., Shu, Q., Sun, H., Lu, J., Meng, F., & Wan, F. (2025). AHR Agonist ITE Boosted PD1 Antibody’s Effects by Inhibiting Myeloid-Derived Cells Suppressive Cells in an Orthotopic Mouse Glioma Model. Pharmaceuticals, 18(4), 471. https://doi.org/10.3390/ph18040471






