An Experimental Design Approach for Producing Curcumin-Loaded Solid Lipid Nanoparticles
Abstract
1. Introduction
2. Results
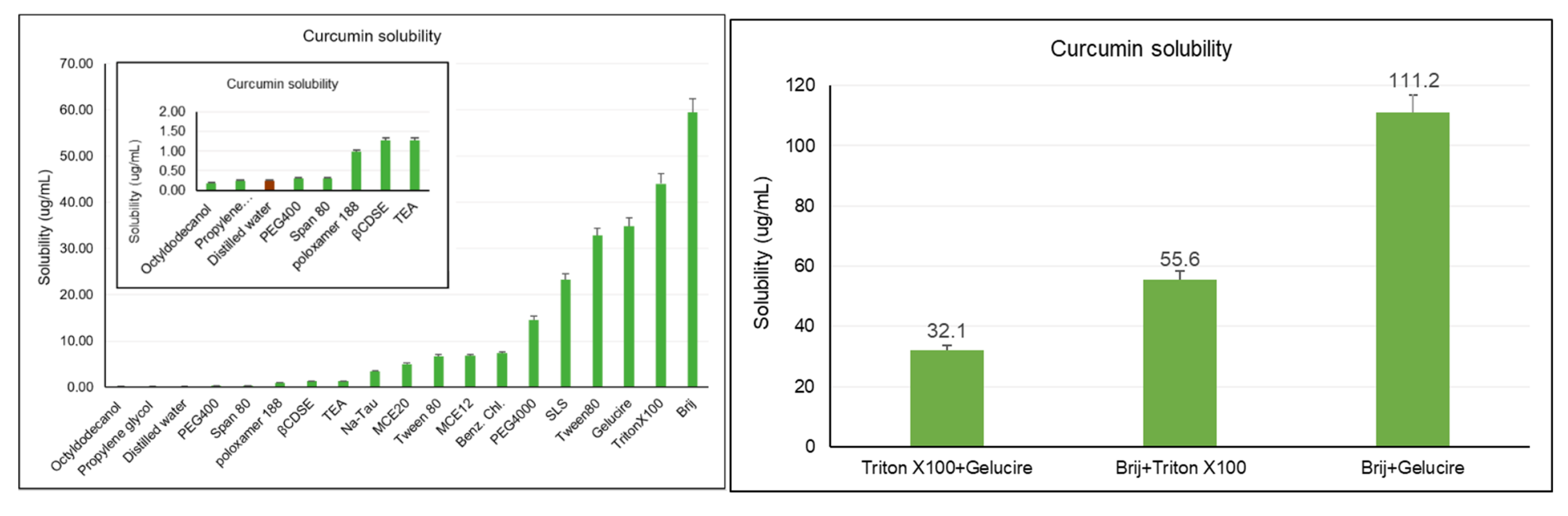
2.1. Saturation Solubility Study
2.2. Preliminary Study
2.3. Experimental Design
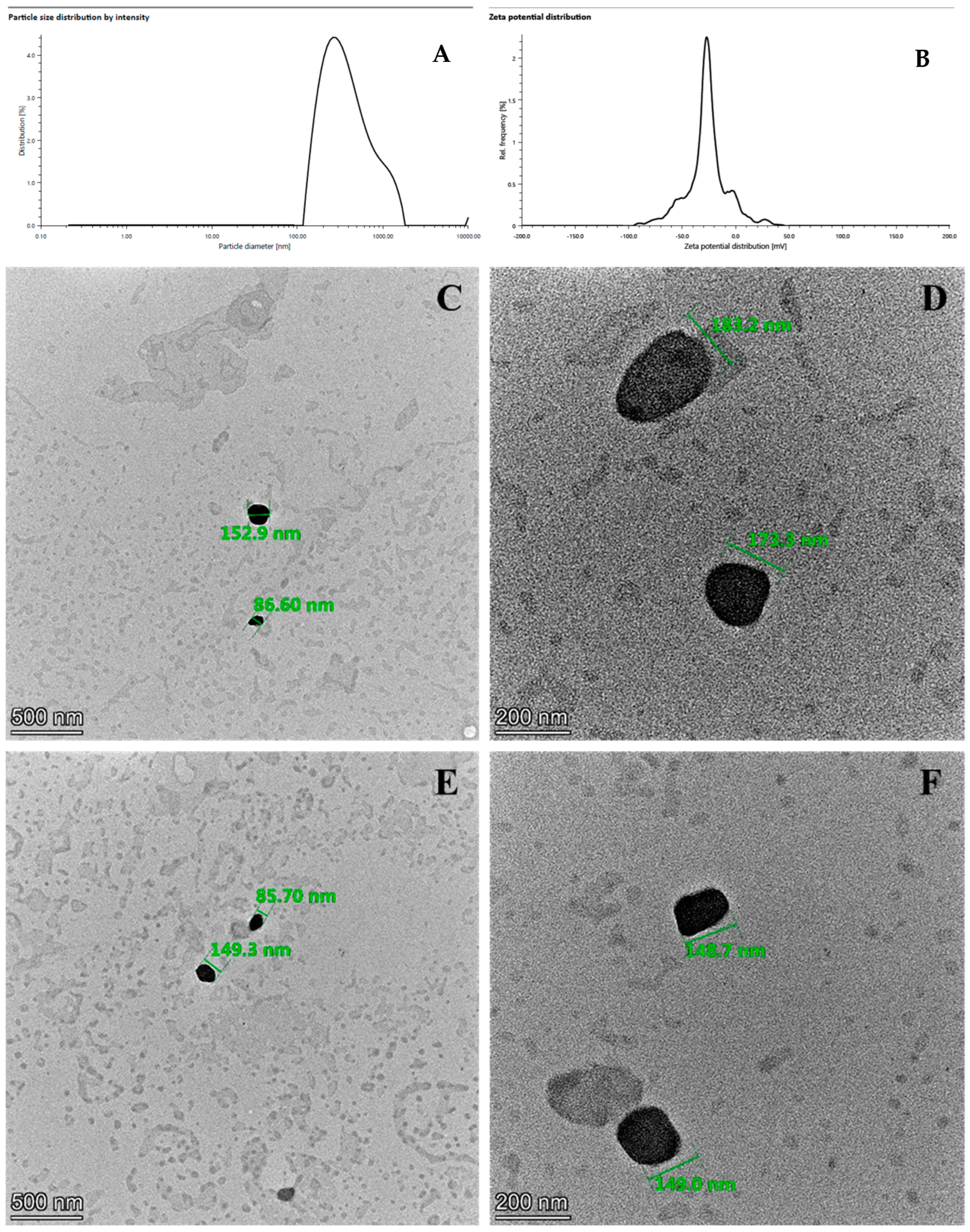
2.4. Morphological Analysis
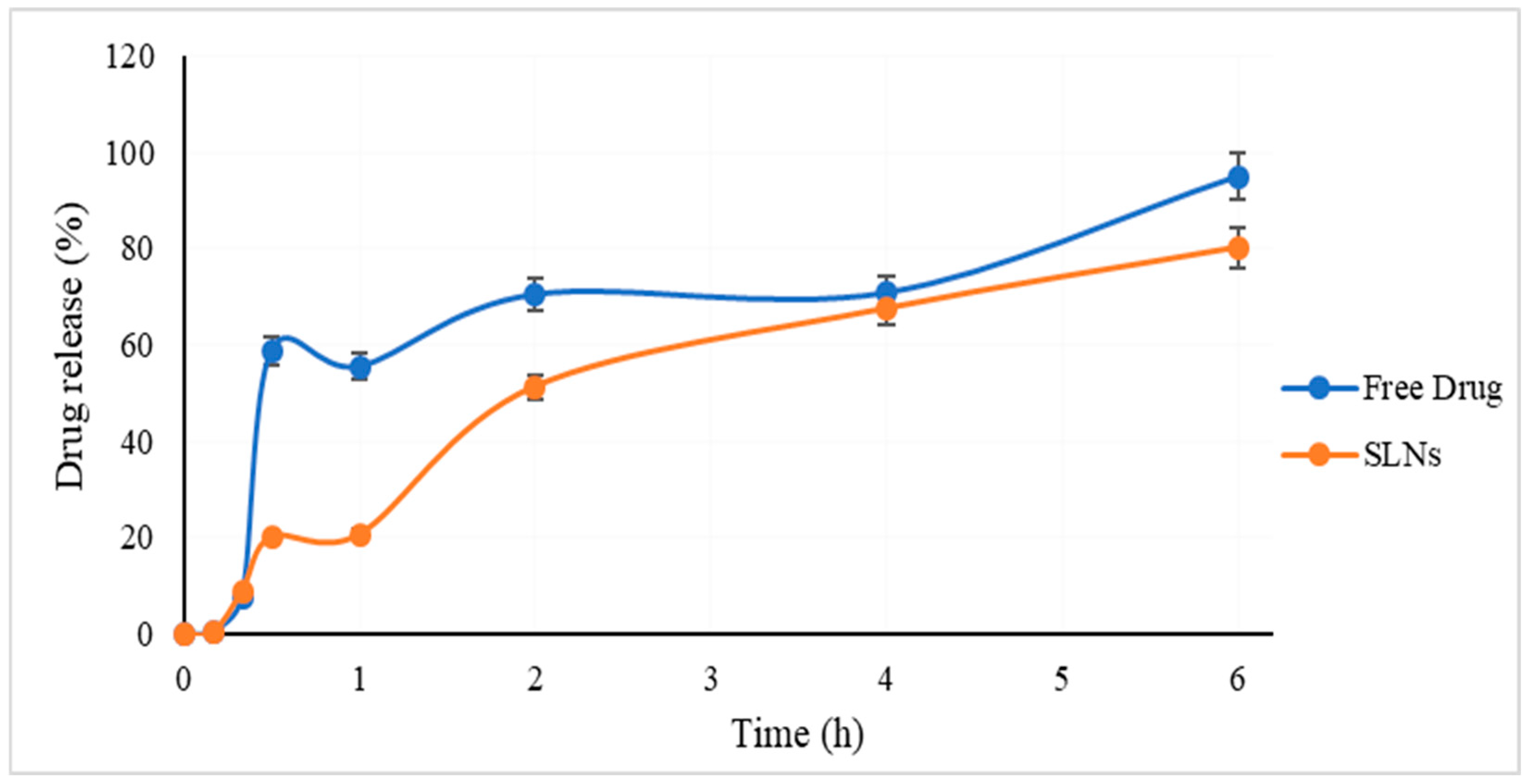
2.5. In Vitro Release Study
2.6. Short-Term Stability
2.7. Cell Viability
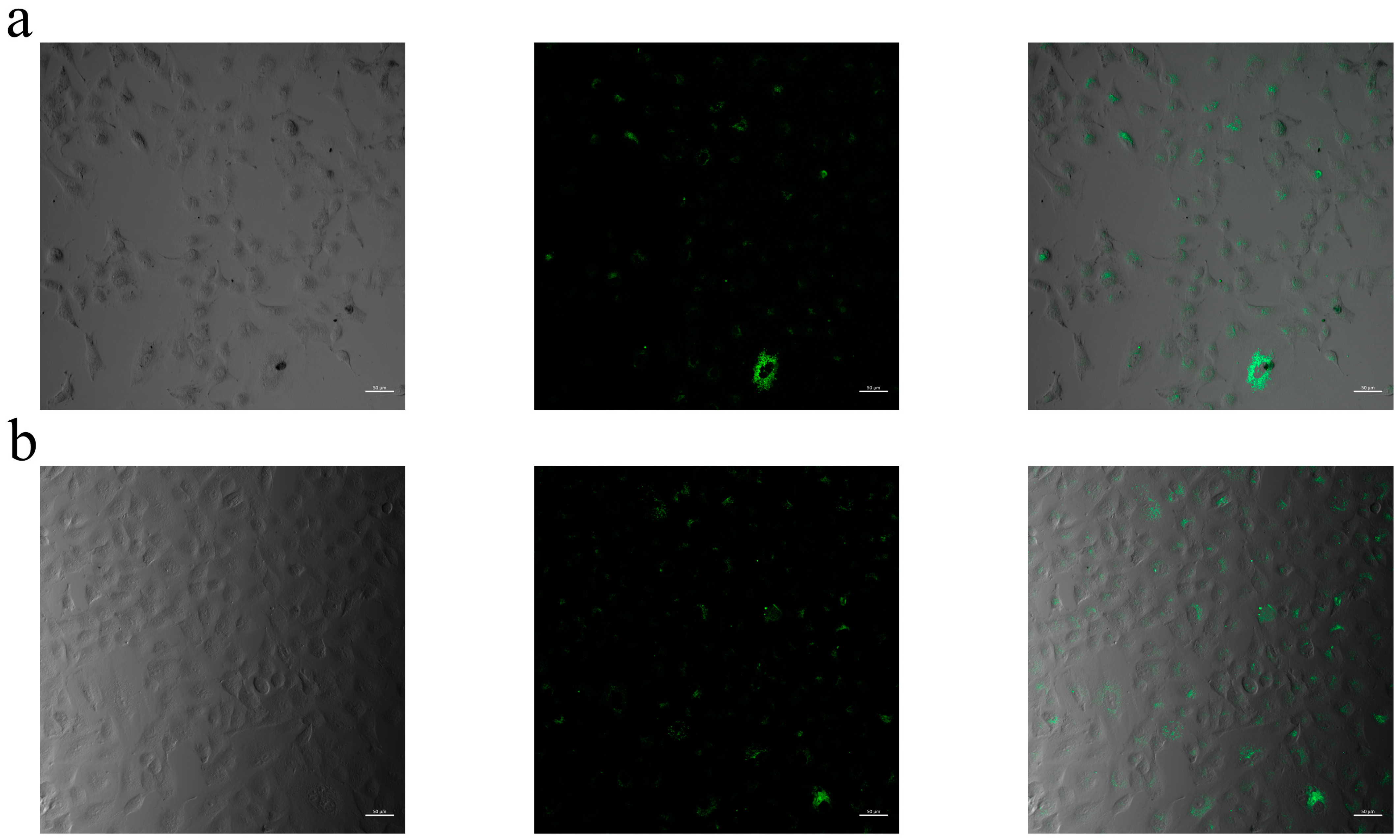
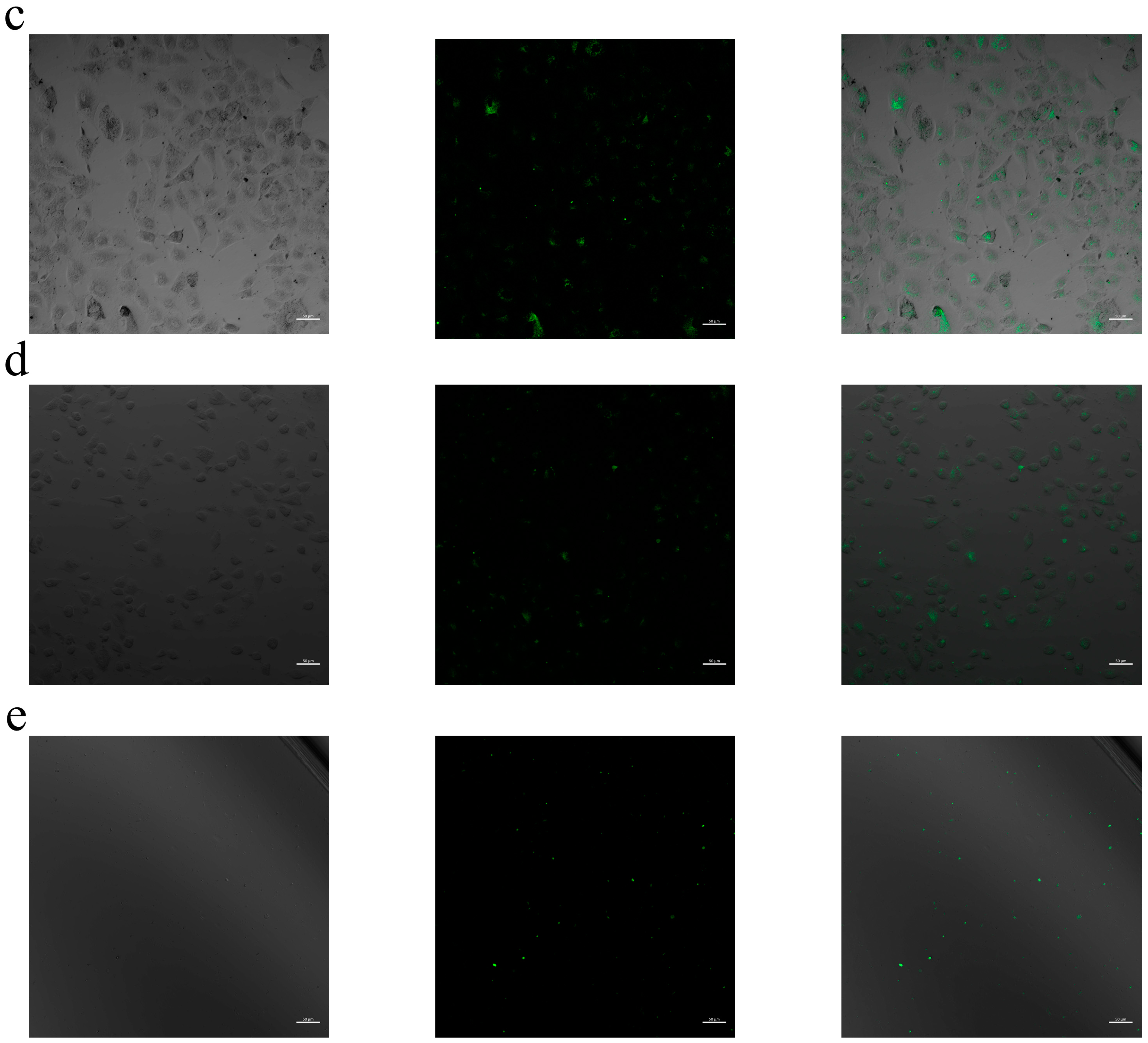
2.8. Cellular Internalization
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination Method for Curcumin
4.3. Saturation Solubility Study
4.4. Determination of Parameters: Preliminary Study
4.5. Preparation of SLNs
4.6. Design of Experiments
4.7. Particle Size and Zeta Potential Measurements
4.8. Encapsulation Efficiency
4.9. Morphological Analysis
4.10. In Vitro Release Study
4.11. Short-Term Stability
4.12. In Vitro Cell Viability Assay
4.13. Confocal Laser Scanning Microscopy (CLSM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLN | Solid lipid nanoparticle |
| TEA | Triethanol amine |
| Benz. Ch. | Benzalkonium chloride |
| Na-Tau | Sodium taurocholate |
| SLS | Sodium lauryl sulfate |
| βCDSE | β cyclodextrine sulfobutyl ethers |
| PG | Propylene glycol |
| OD | Octyldodecanol |
| MCE12 | Macrogol cetostrearyl ether 12 |
| MCE20 | Macrogol setotrearil ether 20 |
| DoE | Design of experiment |
| CCD | Central composite design |
| 2D | 2-dimensional |
| 3D | 3-dimensional |
| PS | Particle diameter |
| PI | Polydispersity index |
| EE | Encapsulation efficiency |
| CLSM | Confocal Laser Scanning Microscopy |
| HLB | Hydrophilic–lipophilic balance |
References
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent developments in formulation design for improving oral bioavailability of curcumin: A review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Jaber, N.; Al-Remawi, M.; Abdel-Rahem, R.A. Novel Chitosan Beeswax Matrix for Gastro-Retentive Delivery of Curcumin: A Promising Adjuvant Therapy for Helicobacter Infection. J. Pharm. Innov. 2024, 19, 16. [Google Scholar] [CrossRef]
- Amekyeh, H.; Alkhader, E.; Sabra, R.; Billa, N. Prospects of Curcumin Nanoformulations in Cancer Management. Molecules 2022, 27, 361. [Google Scholar] [CrossRef]
- Nogami, S.; Minoura, K.; Kiminami, N.; Kitaura, Y.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Stabilizing effect of the cyclodextrins additive to spray-dried particles of curcumin/polyvinylpyrrolidone on the supersaturated state of curcumin. Adv. Powder Technol. 2021, 32, 1750–1756. [Google Scholar] [CrossRef]
- Kumar, R. Nanotechnology based approaches to enhance aqueous solubility and bioavailability of griseofulvin: A literature survey. J. Drug Deliv. Sci. Technol. 2019, 53, 101221. [Google Scholar] [CrossRef]
- Alkhafaji, E.; Dmour, I.; Al-Essa, M.K.; Alshaer, W.; Aljaberi, A.; Khalil, E.A.; Taha, M.O. Preparation of novel shell-ionotropically crosslinked micelles based on hexadecylamine and tripolyphosphate for cancer drug delivery. Pharm. Dev. Technol. 2024, 29, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; Jain, D.K. Nanotechnology Based Approaches for Enhancing Oral Bioavailability of Poorly Water Soluble Antihypertensive Drugs. Scientifica 2016, 2016, 8525679. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619. [Google Scholar] [CrossRef]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to Improve the Solubility of Curcumin from Turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Yet, B.; Nemutlu, E.; Çaylı, Y.A.; Eroğlu, H.; Öner, L. Celecoxib Nanoformulations with Enhanced Solubility, Dissolution Rate, and Oral Bioavailability: Experimental Approaches over In Vitro/In Vivo Evaluation. Pharmaceutics 2023, 15, 363. [Google Scholar] [CrossRef]
- Merchant, H.A.; Shoaib, H.M.; Tazeen, J.; Yousuf, R.I. Once-daily tablet formulation and in vitro release evaluation of cefpodoxime using hydroxypropyl methylcellulose: A technical note. AAPS PharmSciTech 2006, 7, E178–E183. [Google Scholar] [CrossRef]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, Characterization, and In vitro Evaluation of Curcumin- and Resveratrol-Loaded Solid Lipid Nanoparticles. AAPS PharmSciTech 2019, 20, 145. [Google Scholar] [CrossRef]
- Das, A.; Parta, A.; Mitra, R.K. Modulation of anionic reverse micellar interface with non-ionic surfactants can regulate enzyme activity within the micellar waterpool. Colloid Polym. Sci. 2016, 294, 715–726. [Google Scholar] [CrossRef]
- Shinde, U.K.; Suryawanshi, D.G.; Amin, P.D. Development of Gelucire® 48/16 and TPGS Mixed Micelles and Its Pellet Formulation by Extrusion Spheronization Technique for Dissolution Rate Enhancement of Curcumin. AAPS PharmSciTech 2021, 22, 182. [Google Scholar] [CrossRef]
- Li, C.-Z.; Chang, H.-M.; Hsu, W.-L.; Venkatesan, P.; Lin, M.H.; Lai, P.-S. Curcumin-Loaded Oil-Free Self-Assembled Micelles Inhibit the Influenza A Virus Activity and the Solidification of Curcumin-Loaded Micelles for Pharmaceutical Applications. Pharmaceutics 2022, 14, 2422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.M.K.; Craig, D.Q.M.; Day, R.M. Design of Experiment Approach to Modeling the Effects of Formulation and Drug Loading on the Structure and Properties of Therapeutic Nanogels. Mol. Pharm. 2022, 19, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar]
- Chae, J.; Choi, Y.; Hong, J.; Kim, N.; Kim, J.; Lee, H.-Y.; Choi, J. Anticancer and Antibacterial Properties of Curcumin-Loaded Mannosylated Solid Lipid Nanoparticles for the Treatment of Lung Diseases. ACS Appl. Bio Mater. 2024, 7, 2175–2185. [Google Scholar]
- Demirbolat, G.M.; Coskun, G.P.; Erdogan, O.; Cevik, O. Long Chain Fatty Acids Can Form Aggregates and Affect The Membrane Integrity. Colloids Surf. B Biointerfaces 2021, 204, 111795. [Google Scholar] [CrossRef]
- Sharma, K.; Agrawal, S.S.; Gupta, M. Development and Validation of UV spectrophotometric method for the estimation of Curcumin in Bulk Drug and Pharmaceutical Dosage Forms. Int. J. Drug Dev. Res. 2012, 4, 375–380. [Google Scholar]
- İnal, T.G.; Caylı, Y.A.; Izat, N.; ÖNER, L.; Sahin, S. Effect of particle size and surfactant on the solubility, permeability and dissolution characteristics of deferasirox. J. Res. Pharm. 2019, 23, 851–859. [Google Scholar] [CrossRef]
- Garg, T.K. An Approach for Improvement of the Water Solubility of Gliclazide in Solid Dispersion with PEG 4000. Int. J. Pharm. Sci. Res. 2011, 2, 1600. [Google Scholar]
- Shahgaldian, P.; Da Silva, E.; Coleman, A.W.; Rather, B.; Zaworotko, M.J. Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): A detailed study of preparation and stability parameters. Int. J. Pharm. 2003, 253, 23–38. [Google Scholar] [CrossRef]
- Kazakova, O.; Lipkovska, N.; Barvinchenko, V. Keto-enol tautomerism of curcumin in the preparation of nanobiocomposites with fumed silica, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 277, 121287. [Google Scholar] [CrossRef]
- Yerlikaya, F.; Arslan, A.; Arabacı, B.; Gencer, P.; Nemutlu, E. Application of Plackett-Burman Design for Development and Evaluation of a Betamethasone Suspension for Injection Formulation Tt–Bir Betametazon Enjeksiyonluk Süspansiyon Formülasyonunun Geliştirilmesi Ve Değerlendirilmesi İçin Plackett-Burman Tasarimin. J. Fac. Pharm. Ankara Univ. 2023, 47, 477–489. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Aktas, E.; Coskun, G.P.; Erdogan, O.; Cevik, O. New Approach to Formulate Methotrexate-Loaded Niosomes: In Vitro Characterization and Cellular Effectiveness. J. Pharm. Innov. 2022, 17, 622–637. [Google Scholar] [CrossRef]
- Diwan, R.; Ravi, P.R.; Pathare, N.S.; Aggarwal, V. Pharmacodynamic, pharmacokinetic and physical characterization of cilnidipine loaded solid lipid nanoparticles for oral delivery optimized using the principles of design of experiments. Colloids Surf. B Biointerfaces 2020, 193, 111073. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Erdoğan, Ö.; Coşkun, G.P.; Çevik, Ö. PEG4000 modified liposomes enhance the solubility of quercetin and improve the liposome functionality: In vitro characterization and the cellular efficacy. Turk. J. Chem. 2022, 46, 1011–1023. [Google Scholar]
- Su, Q.; Pan, J.; Wang, C.; Zhang, M.; Cui, H.; Zhao, X. Curcumin and Baicalin Co-Loaded Nanoliposomes for Synergistic Treatment of Non-Small Cell Lung Cancer. Pharmaceutics 2024, 16, 973. [Google Scholar] [CrossRef]



| Run | Critical Factors | Response Variables | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | PS (nm) | ZP (mV) | EE% | DL% | |
| 1 | −1 | −1 | −1 | 287.9 ± 9.8 | −17.3 ± 1 | 29.66 ± 1.92 | 0.15 ± 0.01 |
| 2 | −1 | 1 | −1 | 201.7 ± 13.5 | −15.9 ± 0.9 | 10.73 ± 2.06 | 0.05 ± 0.01 |
| 3 | 1 | −1 | −1 | 236.9 ± 8.8 | −18.3 ± 0.4 | 19.38 ± 1.53 | 0.29 ± 0.02 |
| 4 | 1 | 1 | −1 | 398.0 ± 27.4 | −10.7 ± 0.9 | 13.28 ± 1.58 | 0.20 ± 0.02 |
| 5 | −1 | −1 | 1 | 424.9 ± 14.8 | −36.1 ± 2.1 | 10.07 ± 2.07 | 0.05 ± 0.01 |
| 6 | −1 | 1 | 1 | 340.4 ± 13.8 | −16.7 ± 0.8 | 28.02 ± 1.93 | 0.14 ± 0.01 |
| 7 | 1 | −1 | 1 | 656.2 ± 21.0 | −39.0 ± 2.6 | 23.02 ± 1.05 | 0.34 ± 0.02 |
| 8 | 1 | 1 | 1 | 512.3 ± 33.4 | −31.8 ± 1.4 | 23.95 ± 1.47 | 0.36 ± 0.02 |
| 9 | 0 | −1 | 0 | 929.5 ± 50.9 | −31.5 ± 2.4 | 24.85 ± 1.26 | 0.25 ± 0.01 |
| 10 | 0 | 1 | 0 | 999.6 ± 51.2 | −30.2 ± 1.1 | 21.74 ± 1.29 | 0.22 ± 0.01 |
| 11 | −1 | 0 | 0 | 780.0 ± 33.2 | −30.6 ± 1.1 | 24.90 ± 0.60 | 0.12 ± 0.01 |
| 12 | 1 | 0 | 0 | 509.2 ± 27.3 | −21.9 ± 1.6 | 28.36 ± 1.01 | 0.42 ± 0.02 |
| 13 | 0 | 0 | −1 | 364.8 ± 9.7 | −24.1 ± 1.1 | 28.11 ± 1.24 | 0.28 ± 0.01 |
| 14 | 0 | 0 | 1 | 507.2 ± 20.7 | −22.5 ± 1.1 | 28.76 ± 1.23 | 0.29 ± 0.01 |
| 15 | 0 | 0 | 0 | 354.1 ± 44.5 | −17.8 ± 4.1 | 34.31 ± 1.19 | 0.34 ± 0.01 |
| 16 | 0 | 0 | 0 | 356.3 ± 10.8 | −23.7 ± 1.0 | 33.33 ± 1.20 | 0.33 ± 0.01 |
| 17 | 0 | 0 | 0 | 339.5 ± 7.4 | −25.2 ± 1.4 | 20.60 ± 1.30 | 0.21 ± 0.01 |
| Coefficients | |||||
|---|---|---|---|---|---|
| ZP (mV) | PS (nm) | EE% | DL% | ||
| bo | Constant | −25.097 ** | 603.585 ** | 29.361 ** | 0.295 ** |
| b1 | Curcumin (mg) | 3.690 | −8.340 | −0.926 | −0.011 |
| b2 | Surfactant (%) | −0.510 | 27.770 | 0.461 | 0.110 ** |
| b3 | Stirring (rpm) | −5.980 * | 95.170 | 1.266 | 0.021 |
| b1 × b1 | Cur × Cur | −3.605 | 220.752 | −6.027 | −0.061 |
| b2 × b2 | Sur × Sur | 0.995 | −99.198 | −2.692 | −0.026 |
| b3 × b3 | Stir × Stir | 3.945 | −307.798 | −0.887 | −0.011 |
| b1 × b2 | Cur × Sur | −0.750 | 23.488 | −0.524 | −0.008 |
| b1 × b3 | Cur × Stir | 2.200 | −37.913 | 5.489 * | 0.038 |
| b2 × b3 | Sur × Stir | −2.775 | 32.238 | 2.076 | 0.028 |
| PS (nm) | ZP (mV) | EE (%) | DL (%) | |
|---|---|---|---|---|
| Optimum formulation | 389.31 ± 9.95 | −27.50 ± 0.80 | 28.95 ± 4.36 | 0.27 ± 0.03 |
| Calculated | 369.66 | −30.85 | 28.28 | 0.41 |
| Drug | r2 | e | Intercept | |
|---|---|---|---|---|
| 0th-order kinetics | Free | 0.658 | 3 | 20.723 |
| Loaded | 0.915 | 7.080 | ||
| 1st-order kinetics | Free | 0.325 | 88 | 2.243 |
| Loaded | 0.430 | 1.592 | ||
| Higuchi | Free | 0.799 | 70 | 2.534 |
| Loaded | 0.962 | −8.108 | ||
| Korsmeyer–Peppas | Free | 0.842 | 87 | 2.089 |
| Loaded | 0.743 | 1.248 |
| Independent Factors | Level Used | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| X1: The amount of curcumin (mg) | 2.5 | 5 | 7.5 |
| X2: The ratio of surfactants (Brij–Gelucire) | 400:0 | 200:200 | 300:100 |
| X3: The stirring speed (rpm) | 500 | 700 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saka, O.M.; Aygüler, C.İ.; Özdemir, N.S.; Sürücü, B.; Çakırlı, E.; Nemutlu, E.; Demirbolat, G.M. An Experimental Design Approach for Producing Curcumin-Loaded Solid Lipid Nanoparticles. Pharmaceuticals 2025, 18, 470. https://doi.org/10.3390/ph18040470
Saka OM, Aygüler Cİ, Özdemir NS, Sürücü B, Çakırlı E, Nemutlu E, Demirbolat GM. An Experimental Design Approach for Producing Curcumin-Loaded Solid Lipid Nanoparticles. Pharmaceuticals. 2025; 18(4):470. https://doi.org/10.3390/ph18040470
Chicago/Turabian StyleSaka, Ongun Mehmet, Cemre İrem Aygüler, Neval Sevinç Özdemir, Bilge Sürücü, Egemen Çakırlı, Emirhan Nemutlu, and Gülen Melike Demirbolat. 2025. "An Experimental Design Approach for Producing Curcumin-Loaded Solid Lipid Nanoparticles" Pharmaceuticals 18, no. 4: 470. https://doi.org/10.3390/ph18040470
APA StyleSaka, O. M., Aygüler, C. İ., Özdemir, N. S., Sürücü, B., Çakırlı, E., Nemutlu, E., & Demirbolat, G. M. (2025). An Experimental Design Approach for Producing Curcumin-Loaded Solid Lipid Nanoparticles. Pharmaceuticals, 18(4), 470. https://doi.org/10.3390/ph18040470








