Lignin-Based Nanocarrier for Simultaneous Delivery of 131I and SN-38 in the Combined Treatment of Solid Tumors by a Nanobrachytherapy Approach
Abstract
:1. Introduction
2. Results and Discussion
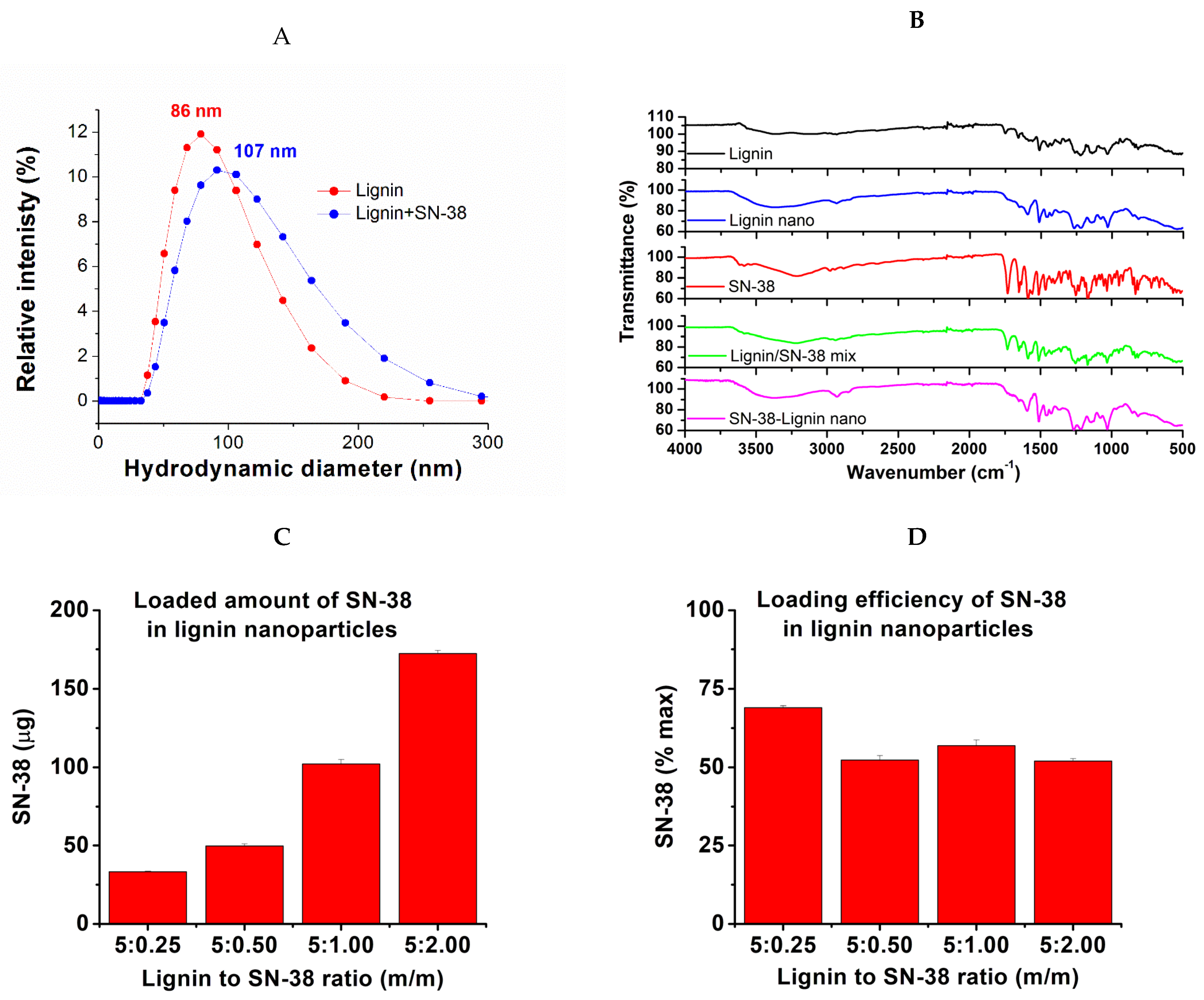
2.1. The Nanoparticle Preparation and SN-38 Loading
2.2. The Radiolabeling
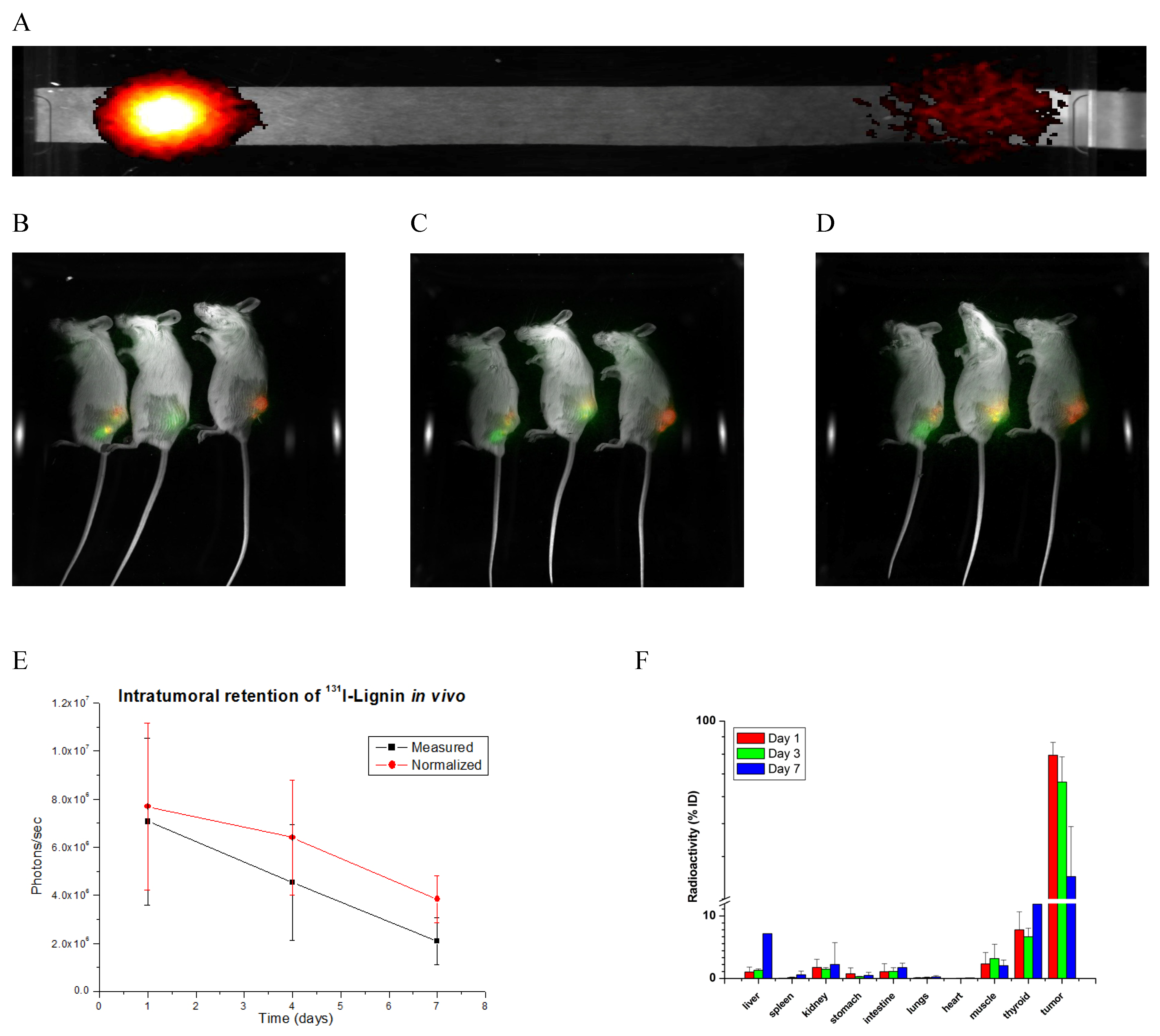
2.3. Biodistribution
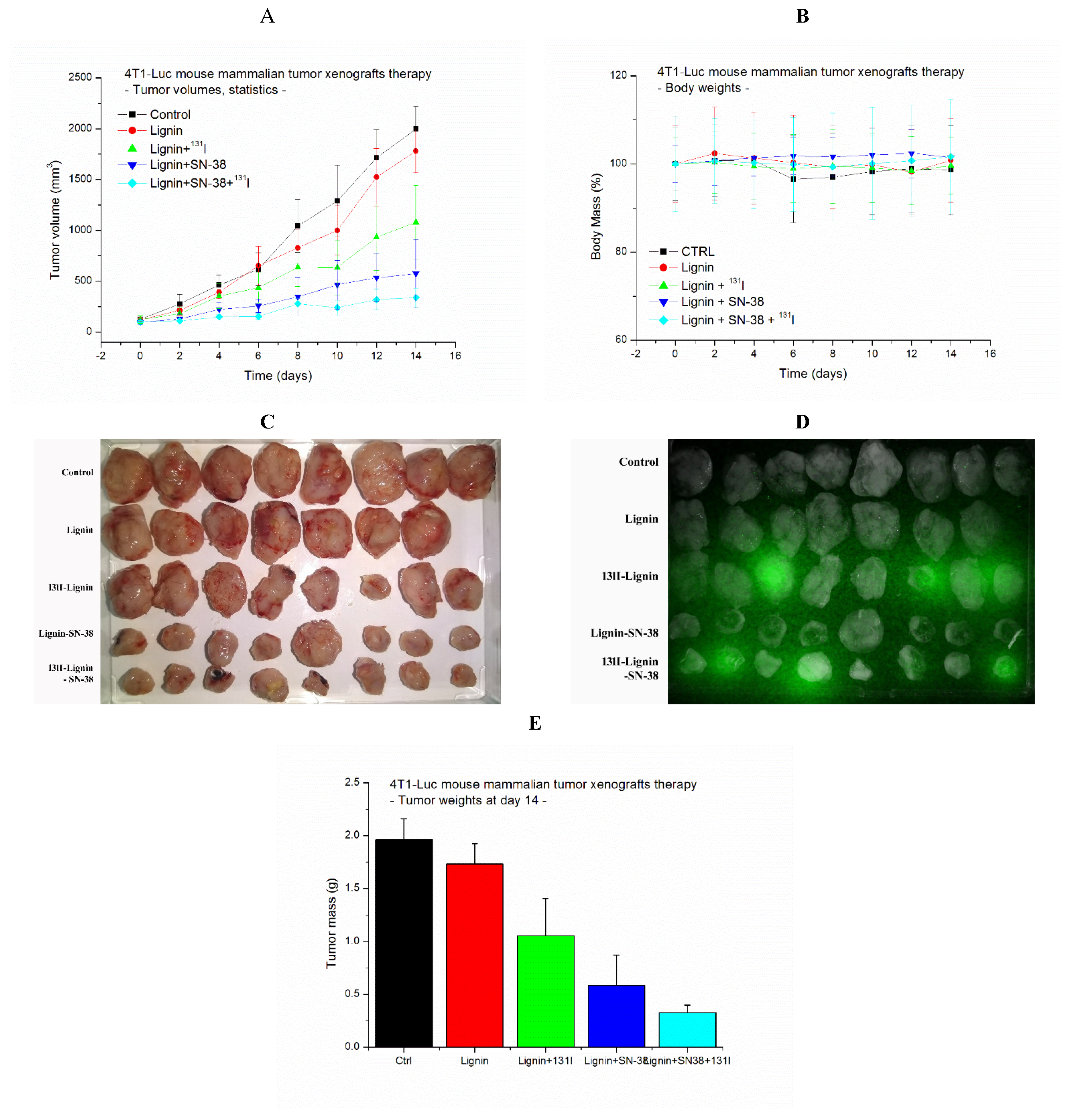
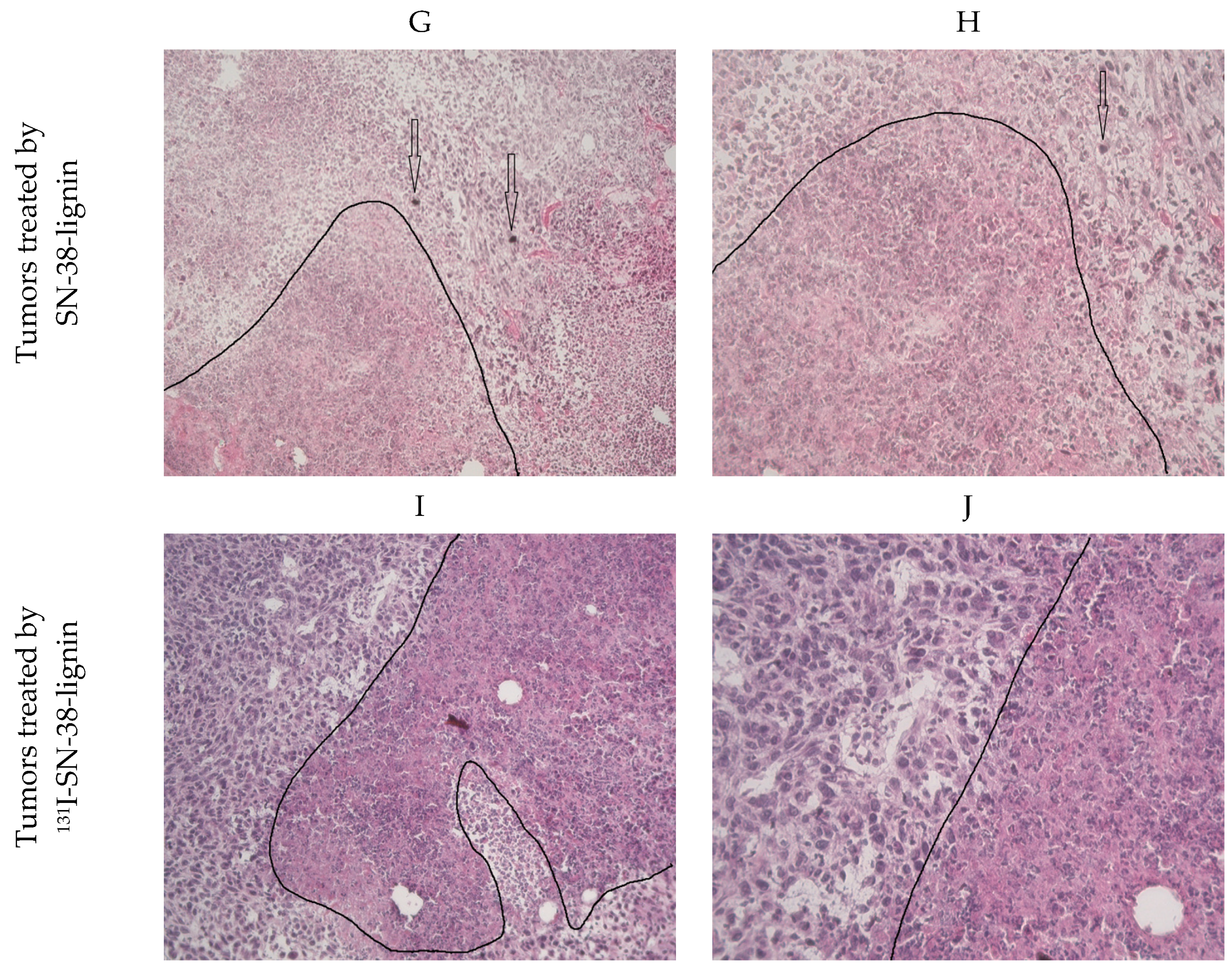
2.4. Antitumor Effect
3. Material and Methods
3.1. Preparation and Analysis of the Nanoparticles
3.2. HPLC Analysis
3.3. The Radiolabeling
3.4. The Tumors Establishment
3.5. The Nanomaterial In Vivo Localization, Retention, and Tissue Distribution
3.6. The Antitumor Effects Determination
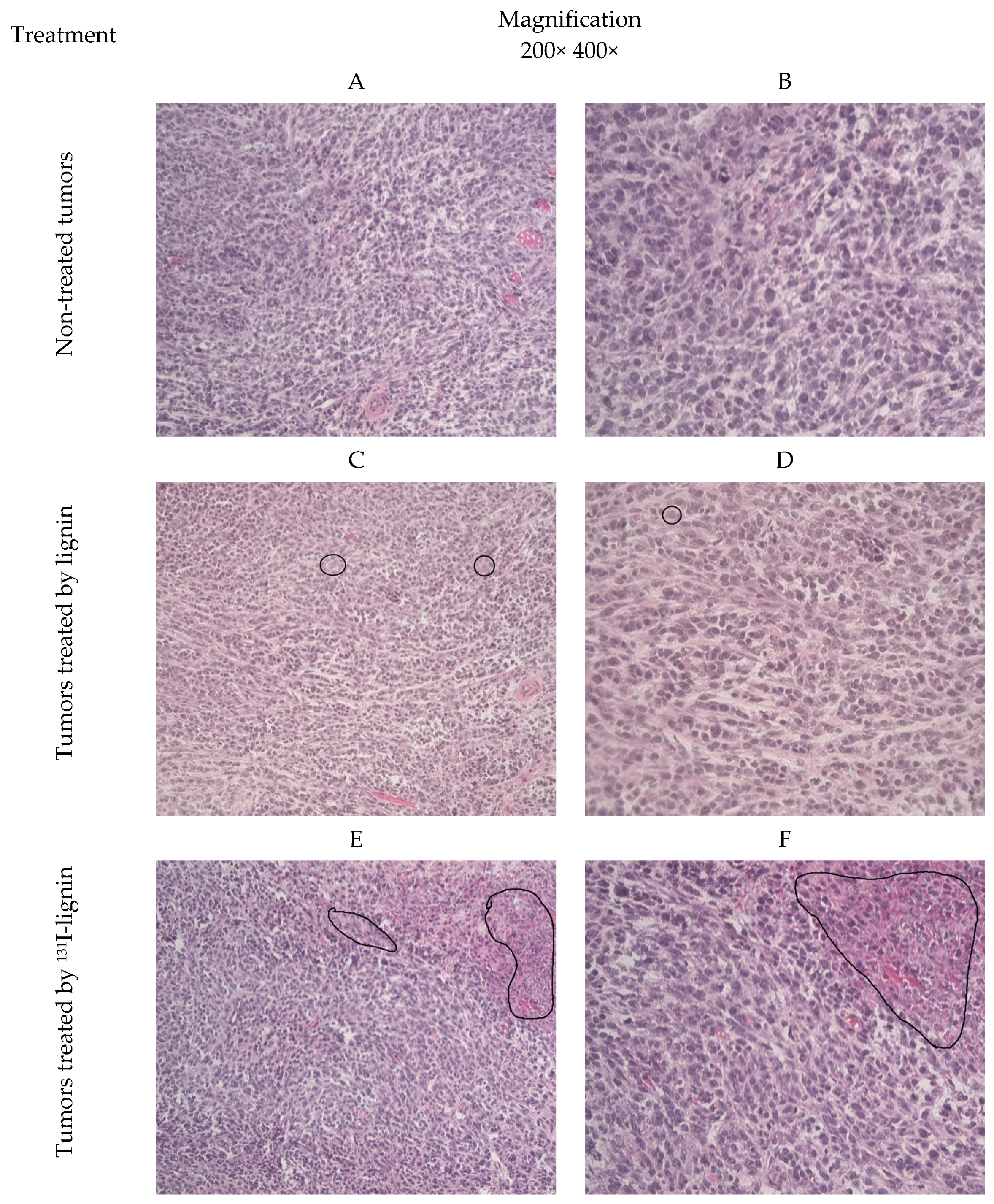
3.7. Histopathology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organisation, W.H. WHO Statistics—Cancer Incidence. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 August 2024).
- Alard, E.; Butnariu, A.-B.; Grillo, M.; Kirkham, C.; Zinovkin, D.A.; Newnham, L.; Macciochi, J.; Pranjol, M.Z. Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets. Cancers 2020, 12, 1826. [Google Scholar] [CrossRef] [PubMed]
- Lohmueller, J.; Finn, O.J. Current modalities in cancer immunotherapy: Immunomodulatory antibodies, CARs and vaccines. Pharmacol. Ther. 2017, 178, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. CAR-T “the living drugs”, immune checkpoint inhibitors, and precision medicine: A new era of cancer therapy. J. Hematol. Oncol. 2019, 12, 113. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Yoshie, O.; Kitahata, K.; Kamei, M.; Hara, Y.; Nakayama, T. Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers 2021, 13, 2495. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ning, Q.; Tang, S.S. Recent Advances and Next Breakthrough in Immunotherapy for Cancer Treatment. J. Immunol. Res. 2022, 2022, 8052212. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, A.; Herbst, F.; Fraietta, J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022, 21, 78. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv324. [Google Scholar] [CrossRef]
- Lai, X.; Friedman, A. Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model. PLoS ONE 2017, 12, e0178479. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhao, J.; Huang, Y.; Jin, R.; Tang, Z.; Wang, P.; Song, X.; Zhu, H.; Yang, Z.; Yu, N. A Novel Long-Circulating DOX Liposome: Formulation and Pharmacokinetics Studies. Pharm. Nanotechnol. 2020, 8, 391–398. [Google Scholar] [CrossRef]
- Joo, W.D.; Visintin, I.; Mor, G. Targeted cancer therapy—Are the days of systemic chemotherapy numbered? Maturitas 2013, 76, 308–314. [Google Scholar] [CrossRef]
- Jia, Y.; Sheng, Z.; Hu, D.; Yan, F.; Zhu, M.; Gao, G.; Wang, P.; Liu, X.; Wang, X.; Zheng, H. Highly penetrative liposome nanomedicine generated by a biomimetic strategy for enhanced cancer chemotherapy. Biomater. Sci. 2018, 6, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guérin, F.; Dumas, I.; Bossi, A.; Morice, P.; Viswanathan, A.N.; et al. Brachytherapy: An overview for clinicians. CA A Cancer J. Clin. 2019, 69, 386–401. [Google Scholar] [CrossRef]
- Otter, S.J.; Stewart, A.J.; Devlin, P.M. Modern Brachytherapy. Hematol./Oncol. Clin. N. Am. 2019, 33, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lee, S.J.; Hsu, J.C.; Chakraborty, S.; Chakravarty, R.; Cai, W. Cancer Brachytherapy at the Nanoscale: An Emerging Paradigm. Chem. Biomed. Imaging 2024, 2, 4–26. [Google Scholar] [CrossRef]
- Vukadinović, A.; Milanović, Z.; Ognjanović, M.; Janković, D.; Radović, M.; Mirković, M.; Karageorgou, M.-A.; Bouziotis, P.; Erić, S.; Vranješ-Đurić, S.; et al. 90Y-CA/SPIONs for dual magnetic hyperthermia-radionuclide nanobrachytherapy of solid tumours. Nanotechnology 2022, 33, 405102. [Google Scholar] [CrossRef] [PubMed]
- Stanković, D.; Radović, M.; Stanković, A.; Mirković, M.; Vukadinović, A.; Mijović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; et al. Synthesis, Characterization, and Therapeutic Efficacy of 177Lu-DMSA@SPIONs in Nanobrachytherapy of Solid Tumors. Pharmaceutics 2023, 15, 1943. [Google Scholar] [CrossRef]
- Schaal, J.L.; Liu, W.; Chilkoti, A.; Li, X.; Mastria, E.; Zalutsky, M.R. Abstract 1809: Next-generation brachytherapy: A preclinical study of a thermally stabilized biopolymer gel for delivering intratumoral radionuclide therapy in a pancreatic tumor mouse model. Cancer Res. 2015, 75, 1809. [Google Scholar] [CrossRef]
- Daruich de Souza, C.; Nogueira, B.R.; Zeituni, C.A.; Rostelato, M.E.C.M. Chapter 15—Radioactive nanoparticles and their biomedical application in nanobrachytherapy. In Nanoparticle Therapeutics; Kesharwani, P., Singh, K.K., Eds.; Academic Press: New York, NY, USA, 2022; pp. 529–560. [Google Scholar]
- Stanković, A.; Mihailović, J.; Mirković, M.; Radović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; Mijović, M.; Vranješ-Đurić, S.; et al. Aminosilanized flower-structured superparamagnetic iron oxide nanoparticles coupled to 131I-labeled CC49 antibody for combined radionuclide and hyperthermia therapy of cancer. Int. J. Pharm. 2020, 587, 119628. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Jabir, N.R.; Anwar, K.; Firoz, C.K.; Oves, M.; Kamal, M.A.; Tabrez, S. An overview on the current status of cancer nanomedicines. Curr. Med. Res. Opin. 2018, 34, 911–921. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; AbuelEzz, N.Z.; Radwan, R.A.; Mohamed, S.A. Nanoparticles in nanomedicine: A comprehensive updated review on current status, challenges and emerging opportunities. J. Microencapsul. 2021, 38, 414–436. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. ChemMedChem 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Degl’Innocenti, A.; Belenli Gümüş, M.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef]
- Sadhukha, T.; Niu, L.; Wiedmann, T.S.; Panyam, J. Effective elimination of cancer stem cells by magnetic hyperthermia. Mol. Pharm. 2013, 10, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef]
- Pathak, N.; Singh, P.; Singh, P.K.; Sharma, S.; Singh, R.P.; Gupta, A.; Mishra, R.; Mishra, V.K.; Tripathi, M. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front. Nutr. 2022, 9, 963413. [Google Scholar] [CrossRef] [PubMed]
- Hoan, N.X.; Anh, L.T.; Ha, H.T.; Cuong, D.X. Antioxidant Activities, Anticancer Activity, Physico-Chemistry Characteristics, and Acute Toxicity of Alginate/Lignin Polymer. Molecules 2023, 28, 5181. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, L.; Bag, J.; Seetha; Mittal, D.; Leekha, A.; Mishra, H.; Mishra, M.; Verma, A.K.; Mishra, P.K.; Ekielski, A.; et al. Assessing the potential of lignin nanoparticles as drug carrier: Synthesis, cytotoxicity and genotoxicity studies. Int. J. Biol. Macromol. 2020, 152, 786–802. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Wu, Z.; Zhan, P.; Zhang, L.; Wei, Q.; Wang, F.; Shao, L. Construction of Microporous Lignin-Based Hypercross-Linked Polymers with High Surface Areas for Enhanced Iodine Capture. ACS Appl. Polym. Mater. 2021, 3, 2178–2188. [Google Scholar] [CrossRef]
- Abdullah, T.; İlyasoğlu, G.; Memić, A. Designing Lignin-Based Biomaterials as Carriers of Bioactive Molecules. Pharmaceutics 2023, 15, 1114. [Google Scholar] [CrossRef]
- Ndlovu, N.L.; Mdlalose, W.B.; Ntsendwana, B.; Moyo, T. Evaluation of Advanced Nanomaterials for Cancer Diagnosis and Treatment. Pharmaceutics 2024, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, Z.; Ji, X.; Zhang, F. Fabrication Mechanisms of Lignin Nanoparticles and Their Ultraviolet Protection Ability in PVA Composite Film. Polymers 2022, 14, 4196. [Google Scholar] [CrossRef]
- Verdini, F.; Gaudino, E.C.; Canova, E.; Tabasso, S.; Behbahani, P.J.; Cravotto, G. Lignin as a Natural Carrier for the Efficient Delivery of Bioactive Compounds: From Waste to Health. Molecules 2022, 27, 3598. [Google Scholar] [CrossRef]
- Yu, M.; Guo, Y.; Wang, X.; Zhu, H.; Li, W.; Zhou, J. Lignin-based electrospinning nanofibers for reversible iodine capture and potential applications. Int. J. Biol. Macromol. 2022, 208, 782–793. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-Based Nanoparticles: A Review on Their Preparations and Applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef]
- Nan, N.; Hu, W.; Wang, J. Lignin-Based Porous Biomaterials for Medical and Pharmaceutical Applications. Biomedicines 2022, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Yiamsawas, D.; Beckers, S.J.; Lu, H.; Landfester, K.; Wurm, F.R. Morphology-Controlled Synthesis of Lignin Nanocarriers for Drug Delivery and Carbon Materials. ACS Biomater. Sci. Eng. 2017, 3, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, H.G.; Yang, Y.L.; Yoon, Y.; Kim, S.; Oh, E. Multifunctional Drug Delivery System Using Starch-Alginate Beads for Controlled Release. Biol. Pharm. Bull. 2005, 28, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Liu, D.; Shao, L.; Sheng, Z.; Liu, N.; Wu, Z.; Luo, W.; Zhan, P.; Zhang, L. Simple and scalable preparation of lignin based porous carbon coated nano-clay composites and their efficient removal for the diversified iodine. Int. J. Biol. Macromol. 2024, 270, 132091. [Google Scholar] [CrossRef] [PubMed]
- Morsali, M.; Moreno, A.; Loukovitou, A.; Pylypchuk, I.; Sipponen, M.H. Stabilized Lignin Nanoparticles for Versatile Hybrid and Functional Nanomaterials. Biomacromolecules 2022, 23, 4597–4606. [Google Scholar] [CrossRef]
- Erfani Jazi, M.; Narayanan, G.; Aghabozorgi, F.; Farajidizaji, B.; Aghaei, A.; Kamyabi, M.A.; Navarathna, C.M.; Mlsna, T.E. Structure, chemistry and physicochemistry of lignin for material functionalization. SN Appl. Sci. 2019, 1, 1094. [Google Scholar] [CrossRef]
- Grappa, R.; Venezia, V.; Silvestri, B.; Costantini, A.; Luciani, G. Synthesis of Lignin Nanoparticles: Top-Down and Bottom-Up Approaches. Med. Sci. Forum 2024, 25, 3. [Google Scholar] [CrossRef]
- Imlimthan, S.; Correia, A.; Figueiredo, P.; Lintinen, K.; Balasubramanian, V.; Airaksinen, A.J.; Kostiainen, M.A.; Santos, H.A.; Sarparanta, M. Systematic in vitro biocompatibility studies of multimodal cellulose nanocrystal and lignin nanoparticles. J. Biomed. Mater. Res. Part A 2020, 108, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Maitz, S.; Schlemmer, W.; Hobisch, M.A.; Hobisch, J.; Kienberger, M. Preparation and Characterization of a Water-Soluble Kraft Lignin. Adv. Sustain. Syst. 2020, 4, 2000052. [Google Scholar] [CrossRef]
- Katahira, R.; Elder, T.J.; Beckham, G.T. A brief introduction to lignin structure. In Lignin Valorization. Emerging Approaches, 1st ed.; Beckham, G.T., Ed.; The Royal Society of Chemistry: Croydon, London, UK, 2018. [Google Scholar]
- Ma, Z.; Han, Y.; Qi, J.; Qu, Z.; Wang, X. High iodine adsorption by lignin-based hierarchically porous flower-like carbon nanosheets. Ind. Crop. Prod. 2021, 169, 113649. [Google Scholar] [CrossRef]
- Armand, J.P.; Ducreux, M.; Mahjoubi, M.; Abigerges, D.; Bugat, R.; Chabot, G.; Herait, P.; de Forni, M.; Rougier, P. CPT-11 (irinotecan) in the treatment of colorectal cancer. Eur. J. Cancer 1995, 31A, 1283–1287. [Google Scholar] [CrossRef]
- Rougier, P.; Bugat, R. CPT-11 in the treatment of colorectal cancer: Clinical efficacy and safety profile. Semin. Oncol. 1996, 23, 34–41. [Google Scholar] [PubMed]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Takagi, T.; Saotome, T. Chemotherapy with irinotecan (CPT-11), a topoisomerase-I inhibitor, for refractory and relapsed non-Hodgkin’s lymphoma. Leuk. Lymphoma 2001, 42, 577–586. [Google Scholar] [CrossRef]
- Von Hoff, D. Future directions for clinical research with CPT-11 (irinotecan). Eur. J. Cancer 1996, 32A (Suppl. S3), S9–S12. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan—Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Eckardt, J.; Ramlau, R. Recent advances with topotecan in the treatment of lung cancer. Oncologist 2007, 12, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Song, I.C.; Ko, Y.B.; Lee, H.J. The combination of cisplatin and topotecan as a second-line treatment for patients with advanced/recurrent uterine cervix cancer. Medicine 2018, 97, e0340. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, B.; Zhang, T.; Xu, Y.; Liu, H.; Lv, T.; Zhang, F.; Zhan, P.; Song, Y. Irinotecan, topotecan, paclitaxel or docetaxel for second-line treatment of small cell lung cancer: A single-center retrospective study of efficiency comparation and prognosis analysis. Transl. Lung Cancer Res. 2019, 8, 829–837. [Google Scholar] [CrossRef]
- Prijovich, Z.M.; Burnouf, P.-A.; Roffler, S.R. Versatile online SPE–HPLC method for the analysis of Irinotecan and its clinically relevant metabolites in biomaterials. J. Sep. Sci. 2014, 37, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.B.; Liu, H.Y.; Lv, Y.Y.; Liu, X.F.; Guo, Y.; Sun, C.K.; Xu, L. Enhanced in vitro antitumor efficacy and strong anti-cell-migration activity of a hydroxycamptothecin-encapsulated magnetic nanovehicle. Chemistry 2012, 18, 14037–14046. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.-B.; Wang, Y.; Guo, Y.; Xu, L. Integrin αVβ3-Targeted Magnetic Nanohybrids with Enhanced Antitumor Efficacy, Cell Cycle Arrest Ability, and Encouraging Anti-Cell-Migration Activity. ACS Appl. Mater. Interfaces 2014, 6, 16643–16652. [Google Scholar] [CrossRef]
- Lin, H.C.; Chuang, C.H.; Cheng, M.H.; Lin, Y.C.; Fang, Y.P. High Potency of SN-38-Loaded Bovine Serum Albumin Nanoparticles Against Triple-Negative Breast Cancer. Pharmaceutics 2019, 11, 569. [Google Scholar] [CrossRef]
- Jiang, Z.; Fu, Y.; Shen, H. Development of Intratumoral Drug Delivery Based Strategies for Antitumor Therapy. Drug Des. Devel Ther. 2024, 18, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.S.; Kim, J.; Lim, D.K.; Kim, D.H.; Jeon, S.I.; Kim, K. Recent Studies and Progress in the Intratumoral Administration of Nano-Sized Drug Delivery Systems. Nanomaterials 2023, 13, 2225. [Google Scholar] [CrossRef] [PubMed]
- De Lombaerde, E.; De Wever, O.; De Geest, B.G. Delivery routes matter: Safety and efficacy of intratumoral immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188526. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Pressnall, M.M.; Lu, R.; Huayamares, S.G.; Griffin, J.D.; Groer, C.; DeKosky, B.J.; Forrest, M.L.; Berkland, C.J. Human intratumoral therapy: Linking drug properties and tumor transport of drugs in clinical trials. J. Control. Release 2020, 326, 203–221. [Google Scholar] [CrossRef]
- Chang, H.P.; Le, H.K.; Shah, D.K. Pharmacokinetics and Pharmacodynamics of Antibody-Drug Conjugates Administered via Subcutaneous and Intratumoral Routes. Pharmaceutics 2023, 15, 1132. [Google Scholar] [CrossRef]
- Che, Y.; Yang, Y.; Suo, J.; Chen, C.; Wang, X. Intratumoral Injection of a Human Papillomavirus Therapeutic Vaccine-Induced Strong Anti-TC-1-Grafted Tumor Activity in Mice. Cancer Manag. Res. 2021, 13, 7339–7354. [Google Scholar] [CrossRef] [PubMed]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukadinović, A.; Ognjanović, M.; Mijović, M.; Warren, B.; Erić, S.; Prijović, Ž. Lignin-Based Nanocarrier for Simultaneous Delivery of 131I and SN-38 in the Combined Treatment of Solid Tumors by a Nanobrachytherapy Approach. Pharmaceuticals 2025, 18, 177. https://doi.org/10.3390/ph18020177
Vukadinović A, Ognjanović M, Mijović M, Warren B, Erić S, Prijović Ž. Lignin-Based Nanocarrier for Simultaneous Delivery of 131I and SN-38 in the Combined Treatment of Solid Tumors by a Nanobrachytherapy Approach. Pharmaceuticals. 2025; 18(2):177. https://doi.org/10.3390/ph18020177
Chicago/Turabian StyleVukadinović, Aleksandar, Miloš Ognjanović, Milica Mijović, Bryce Warren, Slavica Erić, and Željko Prijović. 2025. "Lignin-Based Nanocarrier for Simultaneous Delivery of 131I and SN-38 in the Combined Treatment of Solid Tumors by a Nanobrachytherapy Approach" Pharmaceuticals 18, no. 2: 177. https://doi.org/10.3390/ph18020177
APA StyleVukadinović, A., Ognjanović, M., Mijović, M., Warren, B., Erić, S., & Prijović, Ž. (2025). Lignin-Based Nanocarrier for Simultaneous Delivery of 131I and SN-38 in the Combined Treatment of Solid Tumors by a Nanobrachytherapy Approach. Pharmaceuticals, 18(2), 177. https://doi.org/10.3390/ph18020177






