Evaluation of Antiproliferative Activity and Molecular Modeling Studies of Some Novel Benzimidazolone-Bridged Hybrid Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
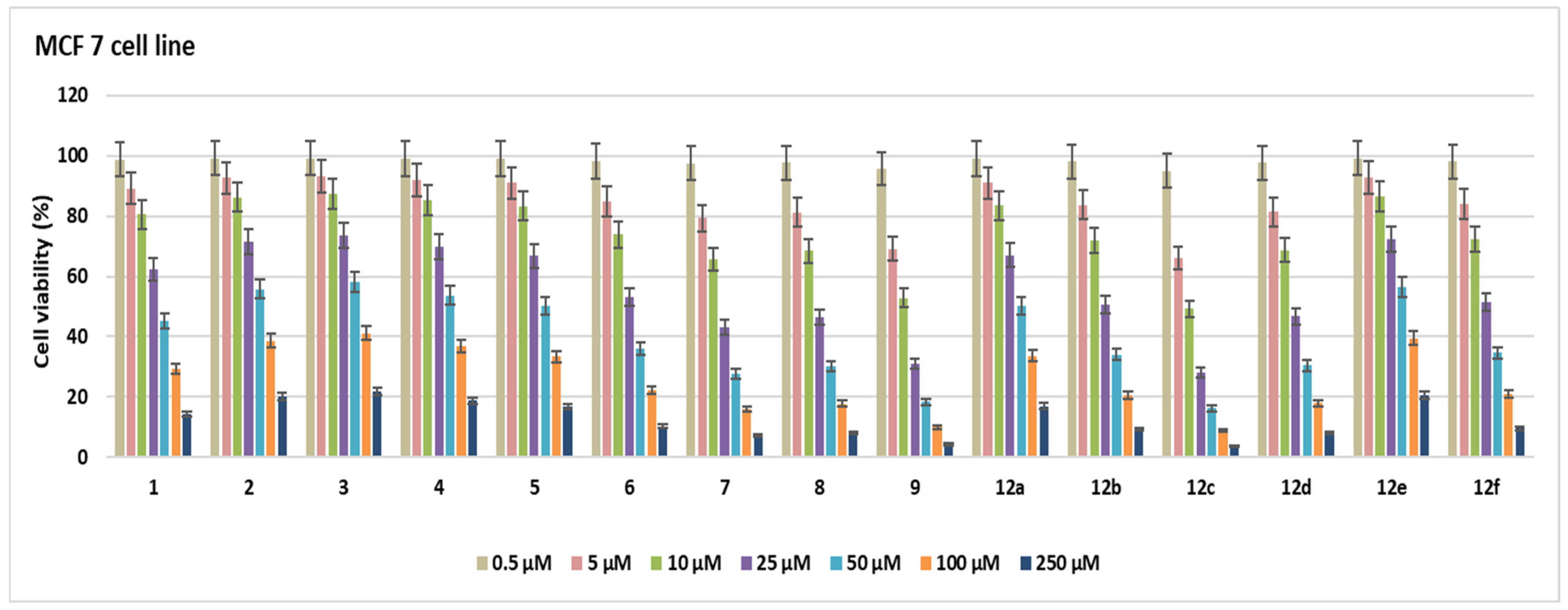
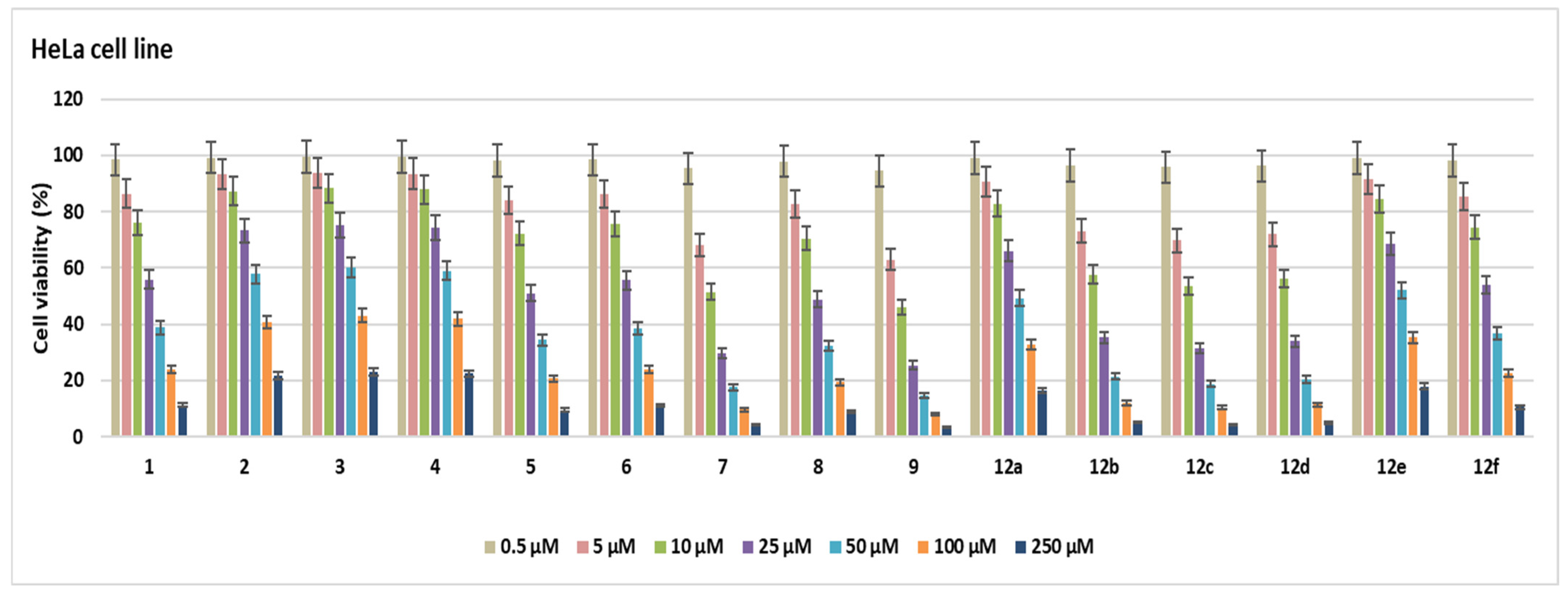
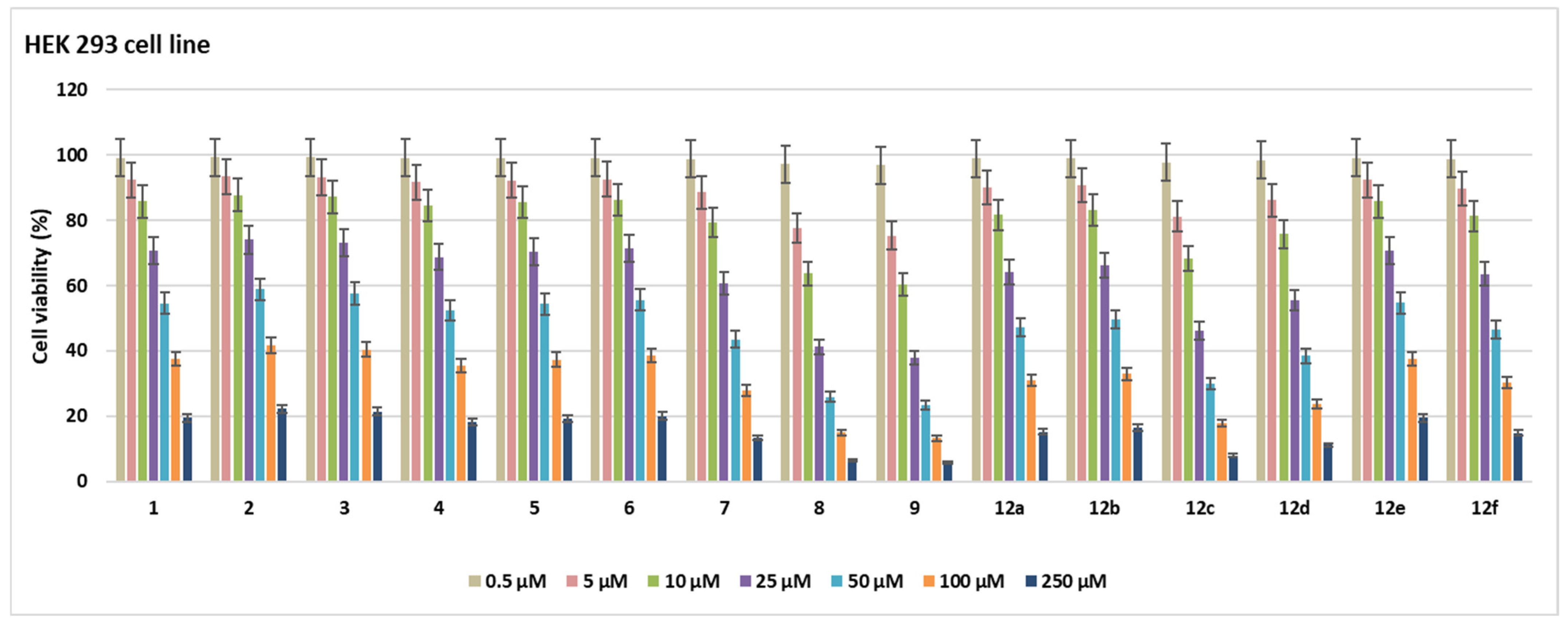
2.2. Cell Viability
2.3. Biochemical Analyses
2.3.1. LDH Assay
2.3.2. Total Oxidative Stress (TOS)
2.3.3. Glutathione Redox Imbalance (GSH/GSSG)
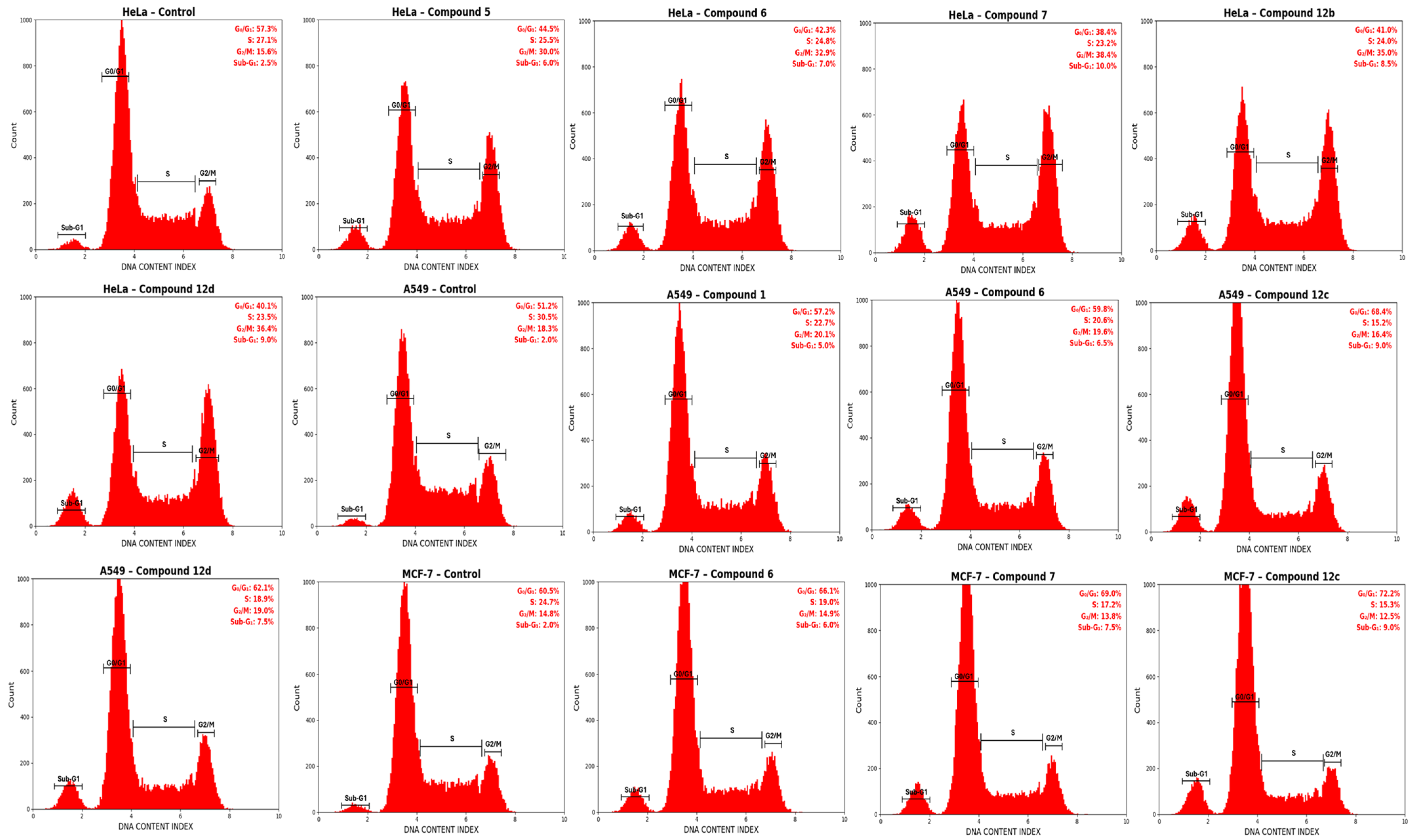
2.4. Cell Cycle Analysis
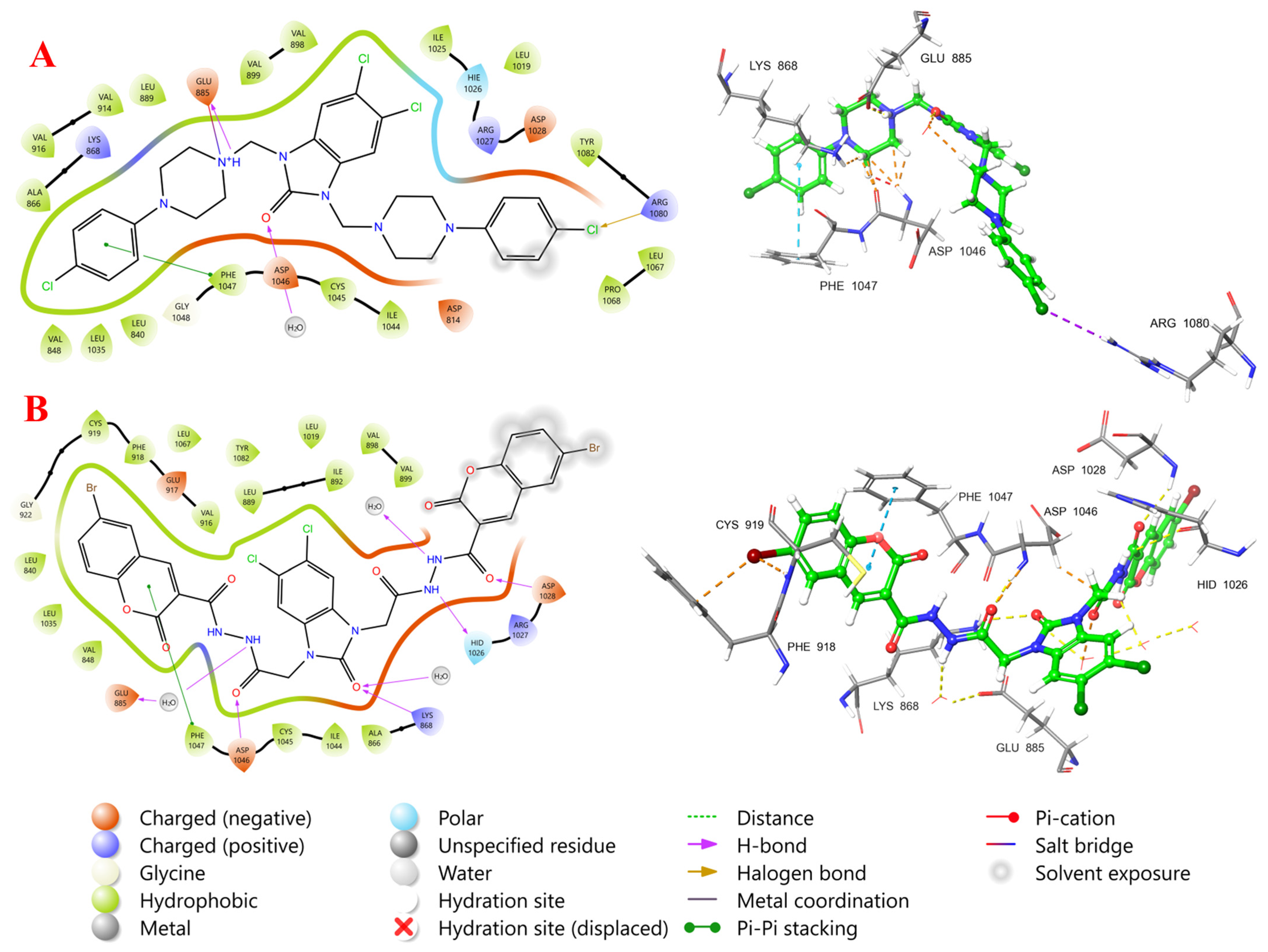
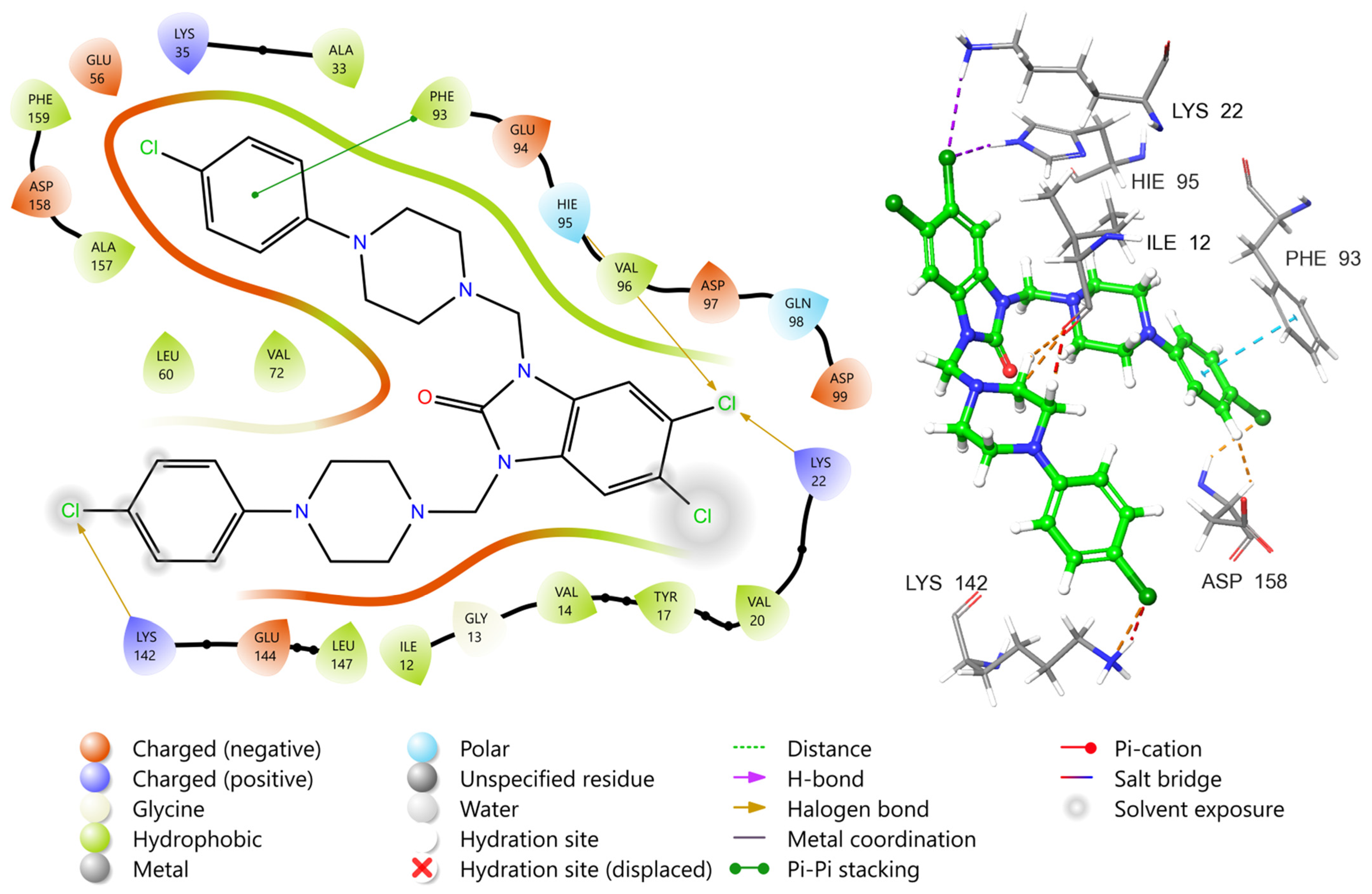
2.5. Molecular Docking Study
2.6. ADMET Predictions
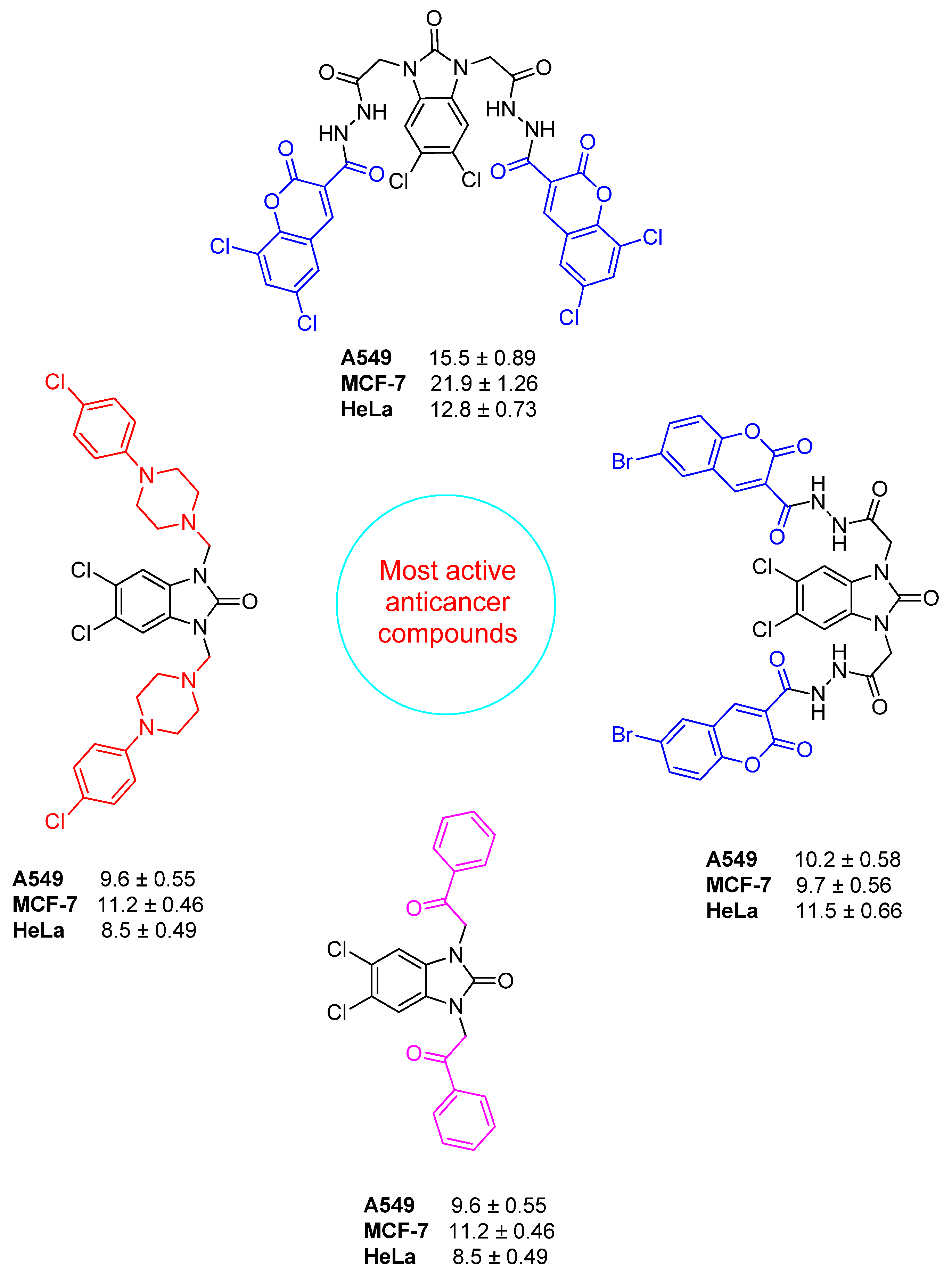
2.7. Structure-Activity Relationships (SAR Study)
3. Material and Methods
3.1. Chemistry
3.1.1. Synthesis of 5,6-Dichloro-1,3-dihydro-2H-benzimidazol-2-one (1)
3.1.2. Synthesis of Diethyl 2,2′-(5,6-Dichloro-2-oxo-1H-benzimidazole-1,3(2H)-diyl)diacetate (2)
3.1.3. Synthesis of 2,2′-(5,6-Dichloro-2-oxo-1H-benzimidazole-1,3(2H)-diyl)diacetohydrazide (3)
3.1.4. Synthesis of Compounds 4 and 5
3.1.5. Synthesis of 2,2′-(5,6-Dichloro-2-oxo-1H-benzo[d]imidazole-1,3(2H)-diyl)diacetic Acid (6)
3.1.6. Synthesis of 5,6-Dichloro-1,3-bis((5-mercapto-1,3,4-oxadiazol-2-yl)methyl)-1H-benzo[d]imidazol-2(3H)-one (7)
3.1.7. Synthesis of 5,6-Dichloro-1,3-bis(2-oxo-2-phenylethyl)-1,3-dihydro-2H-benzimidazol-2-one (8)
3.1.8. Synthesis of 5,6-Dichloro-1,3-bis((4-(4-chlorophenyl)piperazin-1-yl)methyl)-1H-benzo[d]imidazol-2(3H)-one (9)
3.1.9. Synthesis of Compounds 10a–f
3.1.10. Synthesis of Compounds 11a–f
3.1.11. Synthesis of Compounds 12a–f
3.2. Anticancer Activity
3.2.1. Cell Viability
3.2.2. Selectivity Index (SI) Analysis
3.3. Biochemical Analyses
3.3.1. LDH Assay
3.3.2. TOS Levels
3.3.3. Determination of Intracellular GSH/GSSG Ratio
3.4. Cell Cycle Analysis
3.5. Molecular Docking Protocol
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Meer, D.J.; Kramer, I.; van Maaren, M.C.; van Diest, P.J.; Linn, S.C.; Maduro, J.H.; JA Strobbe, L.; Siesling, S.; Schmidt, M.K.; Voogd, A.C. Comprehensive Trends in Incidence, Treatment, Survival and Mortality of First Primary Invasive Breast Cancer Stratified by Age, Stage and Receptor Subtype in the Netherlands between 1989 and 2017. Int. J. Cancer 2021, 148, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global Patterns of Breast Cancer Incidence and Mortality: A Population-based Cancer Registry Data Analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Al-Mathkuri, T.S.F.; Al-Jubori, H.M.S.; Majed, A.A.; Moker, M.H.; Santali, E.; Alnajjar, R. Synthesis, Breast Cancer Activity, Molecular Docking and Dynamic Simulation of 1,4-Dihydropyridine Derivatives. J. Mol. Struct. 2025, 1321, 139704. [Google Scholar] [CrossRef]
- Velihina, Y.; Gesese, R.; Zhirnov, V.; Kobzar, O.; Bui, B.; Pilyo, S.; Vovk, A.; Shen, H.-Y.; Brovarets, V. Design, Synthesis and Evaluation of the Anti-Breast Cancer Activity of 1,3-Oxazolo[4,5-d]Pyrimidine and 1,3-Oxazolo[5,4-d]Pyrimidine Derivatives. RSC Med. Chem. 2023, 14, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya Thandra, K.; Barsouk, A.; Saginala, K.; Sukumar Aluru, J.; Barsouk, A. Epidemiology of Lung Cancer. Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Smolarz, B.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Kołaciński, R.; Romanowicz, H. Lung Cancer—Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). Int. J. Mol. Sci. 2025, 26, 2049. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Dakal, T.C.; Dhabhai, B.; Pant, A.; Moar, K.; Chaudhary, K.; Yadav, V.; Ranga, V.; Sharma, N.K.; Kumar, A.; Maurya, P.K.; et al. Oncogenes and Tumor Suppressor Genes: Functions and Roles in Cancers. Medcomm 2024, 5, e582. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, B. Decoding Tumor Angiogenesis for Therapeutic Advancements: Mechanistic Insights. Biomedicines 2024, 12, 827. [Google Scholar] [CrossRef]
- Sobczyńska-Rak, A.; Żylińska, B.; Nowicka, B.; Rak, E.; Rzepka, T. Role and Mechanisms of Angiogenesis in Tumours. Biology 2025, 14, 756. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, X.; Duan, R.; Zhang, H. Characteristics of HPV Infection in Women Cervical and Anal with HIV-Infected and the Advances in Epidemiological Research. Infect. Drug Resist. 2025, 18, 1785–1791. [Google Scholar] [CrossRef]
- Hamid, M.K.I.; Hasneen, S.; Lima, A.K.; Shawon, S.R.; Shahriar, M.; Anjum, R. Cervical Cancer Trends, HPV Vaccine Utilization, and Screening in Low- and Lower-Middle-Income Countries: An Updated Review. Ther. Adv. Vaccines Immunother. 2025, 13, 25151355251356646. [Google Scholar] [CrossRef]
- WHO. Cervical Cancer; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Abbas, M.A.; Hameed, S.; Farman, M.; Kressler, J.; Mahmood, N. Conjugates of Degraded and Oxidized Hydroxyethyl Starch and Sulfonylureas: Synthesis, Characterization, and in Vivo Antidiabetic Activity. Bioconjug. Chem. 2015, 26, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rode, M.; Karale, B.; Rindhe, S. Synthesis and Biological Activities of Some Benzimidazolone Derivatives. Indian J. Pharm. Sci. 2015, 77, 230–236. [Google Scholar] [CrossRef]
- Pribut, N.; Basson, A.E.; van Otterlo, W.A.L.; Liotta, D.C.; Pelly, S.C. Aryl Substituted Benzimidazolones as Potent HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors. ACS Med. Chem. Lett. 2019, 10, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-L.; Wang, X.A.; Civiello, R.L.; Trehan, A.K.; Pearce, B.C.; Yin, Z.; Combrink, K.D.; Gulgeze, H.B.; Zhang, Y.; Kadow, K.F.; et al. Respiratory Syncytial Virus Fusion Inhibitors. Part 3: Water-Soluble Benzimidazol-2-One Derivatives with Antiviral Activity in Vivo. Bioorganic Med. Chem. Lett. 2006, 16, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Bhatt, N.; Bhatt, P.; Joshi, H.D. Synthesis and Pharmacological Evaluation of Novel 1-(Piperidin-4-Yl)-1H-Benzo[d]Imidazol-2(3H)-One Derivatives as Potential Antimicrobial Agents. Med. Chem. Res. 2014, 23, 2133–2139. [Google Scholar] [CrossRef]
- Brogden, R.N.; Carmine, A.A.; Heel, R.C.; Speight, T.M.; Avery, G.S. Domperidone. Drugs 1982, 24, 360–400. [Google Scholar] [CrossRef]
- Richards, D.M.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Oxatomide. Drugs 1984, 27, 210–231. [Google Scholar] [CrossRef]
- Schwarz, C.; Hartung, B.; Leucht, S. Benperidol for Schizophrenia. Cochrane Database Syst. Rev. 2005, 2010, CD003083. [Google Scholar] [CrossRef]
- Pinder, R.M.; Brogden, R.N.; Sawyer, P.R.; Speight, T.M.; Spencer, R.; Avery, G.S. Pimozide. Drugs 1976, 12, 81–131. [Google Scholar] [CrossRef] [PubMed]
- Townsend, B.R.; Malka, S.T.; Di Paola, S.G.; Nisly, A.E.; Gilbert, B.W. DRopEridol for Abdominal Pain in the Emergency Department for Morphine Equivalent Reduction. The DREAMER Study. Am. J. Emerg. Med. 2025, 90, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Jambagi, V.B.; Barigidad, N.N.; Laxmeshwar, S.S.; Devaru, V. Synthesis, Structure-Activity Relationship of Iodinated-4-Aryloxymethyl-Coumarins as Potential Anti-Cancer and Anti-Mycobacterial Agents. Eur. J. Med. Chem. 2014, 74, 225–233. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Mendonça Nogueira, T.C.; de Souza, M.V.N. Quinoxaline Nucleus: A Promising Scaffold in Anti-Cancer Drug Discovery. Anticancer Agents Med. Chem. 2016, 16, 1339–1352. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Wu, Z.-H.; Guo, J.-W.; Huang, M.-J.; You, Y.-Z.; Liu, H.-M.; Huang, L.-H. Synthesis and Anti-Gastric Cancer Activity Evaluation of Novel Triazole Nucleobase Analogues Containing Steroidal/Coumarin/Quinoline Moieties. Eur. J. Med. Chem. 2019, 181, 111520. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Ashour, A.E. Design, Synthesis and Biological Evaluation of Novel Quinazoline Derivatives as Potential Anti-Cancer Agents. J. Enzym. Inhib. Med. Chem. 2012, 27, 541–545. [Google Scholar] [CrossRef]
- Ioannou, N.; Dalgleish, A.G.; Seddon, A.M.; Mackintosh, D.; Guertler, U.; Solca, F.; Modjtahedi, H. Anti-Tumour Activity of Afatinib, an Irreversible ErbB Family Blocker, in Human Pancreatic Tumour Cells. Br. J. Cancer 2011, 105, 1554–1562. [Google Scholar] [CrossRef]
- Abood, R.G.; Abdulhussein, H.A.; Abbas, S.; Majed, A.A.; Al-Khafagi, A.A.; Adil, A.; Alsalim, T.A. Anti-Breast Cancer Potential of New Indole Derivatives: Synthesis, in-Silico Study, and Cytotoxicity Evaluation on MCF-7 Cells. J. Mol. Struct. 2025, 1326, 141176. [Google Scholar] [CrossRef]
- Dos Santos, F.A.; Pereira, M.C.; de Oliveira, T.B.; Mendonça Junior, F.J.B.; de do Lima, M.C.A.; da Pitta, M.G.R.; da Pitta, I.R.; de Melo Rêgo, M.J.B.; da Rocha Pitta, M.G. Anticancer Properties of Thiophene Derivatives in Breast Cancer MCF-7 Cells. Anticancer Drugs 2018, 29, 157–166. [Google Scholar] [CrossRef]
- Kulabaş, N.; Tatar, E.; Bingöl Özakpınar, Ö.; Özsavcı, D.; Pannecouque, C.; De Clercq, E.; Küçükgüzel, İ. Synthesis and Antiproliferative Evaluation of Novel 2-(4H-1,2,4-Triazole-3-Ylthio)Acetamide Derivatives as Inducers of Apoptosis in Cancer Cells. Eur. J. Med. Chem. 2016, 121, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Krzysztof, L.Z.; Konrad, M.; Marta, S.; Joanna, W.; Angelika, L.B.; Berta, F.; Agata, P.; Tomasz, P. Synthesis and In Vitro Antiproliferative Activity of Thiazole-Based Nitrogen Mustards: The Hydrogen Bonding Interaction between Model Systems and Nucleobases. Anti-Cancer Agents Med. Chem. 2014, 14, 1271–1281. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Patterson, T.A.; Owens, M.A. Coumarin-Based Benzopyranone Derivatives Induced Apoptosis in Human Lung (A549) Cancer Cells. Anticancer Res. 2012, 32, 4271–4276. [Google Scholar]
- Plech, T.; Kaproń, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Search for Factors Affecting Antibacterial Activity and Toxicity of 1,2,4-Triazole-Ciprofloxacin Hybrids. Eur. J. Med. Chem. 2015, 97, 94–103. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.; Rossi, M.; Bolognesi, M. Molecular Hybridization Approach Is a Tool Which Arises to Design New Pharmacophores in Drug Discovery. Curr. Top. Med. Chem. 2019, 19, 1829–1852. [Google Scholar]
- Menteşe, E.; Güzel, Y.Ü.; Akyüz, G.; Karaali, N.Ü. Synthesis of Novel Quinazolinone-Triheterocyclic Hybrides as Dual Inhibition of Urease and Ache. J. Iran. Chem. Soc. 2024, 21, 2425–2431. [Google Scholar] [CrossRef]
- Sandhu, S.; Bansal, Y.; Silakari, O.; Bansal, G. Coumarin Hybrids as Novel Therapeutic Agents. Bioorganic Med. Chem. 2014, 22, 3806–3814. [Google Scholar] [CrossRef]
- Çalişkan, N.; Menteşe, E.; Yilmaz, F.; Ilhan, M.S. Synthesis and Anticancer Activities of Amide-Bridged Coumarin–Quinazolinone Hybrid Compounds. Russ. J. Org. Chem. 2024, 60, 918–926. [Google Scholar] [CrossRef]
- Menteşe, E.; Yılmaz, F.; Menteşe, M.; Beriş, F.Ş.; Emirik, M. Developing Effective Antimicrobial Agents: Synthesis and Molecular Docking Study of Ciprofloxacin-Benzimidazole Hybrids. ChemistrySelect 2024, 9, e202303173. [Google Scholar] [CrossRef]
- Çalışkan, N.; Menteşe, E.; Yılmaz, F.; İlhan, S.; Emirik, M. Synthesis and Anticancer Evaluation of Some Glycine Conjugated Hybrid Compounds Containing Coumarin, Thiophene and Quinazoline Moieties. Pharmaceuticals 2025, 18, 1627–1645. [Google Scholar] [CrossRef] [PubMed]
- Keser, M.; Menteşe, E.; Ilhan, S.; Emirik, M.; Atmaca, H. Design, Synthesis, and Molecular Docking of Triazole-Coumarin Hybrids as Potent Breast Cancer Inhibitors Targeting Cell Cycle and Apoptosis. Chem. Biodivers. 2025; early view. [Google Scholar] [CrossRef] [PubMed]
- Karaali, N.Ü.; Akyüz, G.; Uluçay, B.; Emirik, M. Aromatic Carbothioamide-, Triazole-, Thiadiazole-, and Thiazolidinone-Derived Benzimidazoles: Synthesis, Characterization, Urease and AChE Inhibition Properties, and Docking Study. J. Heterocycl. Chem. 2025; early view. [Google Scholar] [CrossRef]
- Karaali, N.Ü. Design, Synthesis, and Urease Inhibition Activities of Some Bis-Coumarin-Perimidine Hybrid Compounds. Russ. J. Org. Chem. 2024, 60, S78–S84. [Google Scholar] [CrossRef]
- Menteşe, E.; Yılmaz, F.; Menteşe, M.; Beriş, F.Ş. Design, Synthesis, and Structure–Activity Relationship of Some New Ciprofloxacin Hybrids as Antibacterial Agents. J. Heterocycl. Chem. 2025, 62, 1473–1482. [Google Scholar] [CrossRef]
- Yilmaz, F. Green Synthesis and Biological Evaluation of Some 1,2,4-Triazol-3-Ones. Russ. J. Org. Chem. 2024, 60, 513–521. [Google Scholar] [CrossRef]
- Yilmaz, F. Microwave-Assisted Synthesis and Investigation of Urease Inhibitory Activities of Some 1,2,4-Triazol-3-Ones Containing Salicyl and Isatin Moieties. Russ. J. Gen. Chem. 2024, 94, 2018–2022. [Google Scholar] [CrossRef]
- Soltan, O.M.; Shoman, M.E.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. Molecular Hybrids: A Five-Year Survey on Structures of Multiple Targeted Hybrids of Protein Kinase Inhibitors for Cancer Therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-Containing Hybrids and Their Anticancer Activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid Molecules with a Dual Mode of Action: Dream or Reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; Le Couter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor Angiogenesis: Past, Present and the near Future. Carcinogenesis 2000, 21, 505–515. [Google Scholar] [CrossRef]
- Łukasik, P.; Załuski, M.; Gutowska, I. Cyclin-Dependent Kinases (CDK) and Their Role in Diseases Development–Review. Int. J. Mol. Sci. 2021, 22, 2935. [Google Scholar] [CrossRef]
- Freeman-Cook, K.; Hoffman, R.L.; Miller, N.; Almaden, J.; Chionis, J.; Zhang, Q.; Eisele, K.; Liu, C.; Zhang, C.; Huser, N.; et al. Expanding Control of the Tumor Cell Cycle with a CDK2/4/6 Inhibitor. Cancer Cell 2021, 39, 1404–1421.e11. [Google Scholar] [CrossRef]
- Güven, O.; Menteşe, E.; Emirik, M.; Sökmen, B.B.; Akyüz, G. Benzimidazolone-Piperazine/Triazole/Thiadiazole/Furan/Thiophene Conjugates: Synthesis, in Vitro Urease Inhibition, and in Silico Molecular Docking Studies. Arch. Pharm. 2023, 356, 2300336. [Google Scholar] [CrossRef] [PubMed]
- Menteşe, E.; Güven, O.; Çalışkan, N.; Baltaş, N. Synthesis and Biological Evaluation of Benzimidazolone Bridged Triheterocyclic Compounds. J. Heterocycl. Chem. 2021, 58, 1259–1267. [Google Scholar] [CrossRef]
- Liu, W.; Lau, F.; Liu, K.; Wood, H.B.; Zhou, G.; Chen, Y.; Li, Y.; Akiyama, T.E.; Castriota, G.; Einstein, M.; et al. Benzimidazolones: A New Class of Selective Peroxisome Proliferator-Activated Receptor γ (PPARγ) Modulators. J. Med. Chem. 2011, 54, 8541–8554. [Google Scholar] [CrossRef] [PubMed]
- Anichina, K.; Popova-Daskalova, G.; Vuchev, D.; Guncheva, M.; Yancheva, D.; Georgiev, N. Synthesis and In Vitro Biological Studies of Heterocyclic Benzimidazole Derivatives as Potential Therapeutics for Trichinellosis. Appl. Sci. 2025, 15, 6758–6773. [Google Scholar] [CrossRef]
- Wei, S.; Wu, W.; Ji, Z. Synthesis and Antibacterial Activities of 1-Alkyl-3-Methacryloyl (Acryloyl) of Benzimidazolone (Thione) Derivatives. Int. J. Mol. Sci. 2012, 13, 4819–4830. [Google Scholar] [CrossRef]
- Kahveci, B.; Yilmaz, F.; Menteşe, E.; Özil, M.; Karaoǧlu, S.A. Microwave-Assisted Synthesis of Some Novel Benzimidazole Derivatives Containing Imine Function and Evaluation of Their Antimicrobial Activity. J. Heterocycl. Chem. 2014, 51, 982–990. [Google Scholar] [CrossRef]
- Galić, N.; Perić, B.; Kojić-Prodić, B.; Cimerman, Z. Structural and Spectroscopic Characteristics of Aroylhydrazones Derived from Nicotinic Acid Hydrazide. J. Mol. Struct. 2001, 559, 187–194. [Google Scholar] [CrossRef]
- Bektaş, H.; Demirbaş, A.; Demirbaş, N.; Karaoğlu, Ş.A. Synthesis of Some New Biheterocyclic Triazole Derivatives and Evaluation of Their Antimicrobial Activity. Turk. J. Chem. 2010, 34, 165–180. [Google Scholar] [CrossRef]
- Rando, D.G.; Sato, D.N.; Siqueira, L.; Malvezzi, A.; Leite, C.Q.F.; do_Amaral, A.T.; Ferreira, E.I.; Tavares, L.C. Potential Tuberculostatic Agents. Topliss Application on Benzoic Acid [(5-Nitro-Thiophen-2-Yl)-Methylene]-Hydrazide Series. Bioorganic Med. Chem. 2002, 10, 557–560. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Exploration of Structural Requirements for the Inhibition of VEGFR-2 Tyrosine Kinase: Binding Site Analysis of Type II, ‘DFG-out’ Inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 5712–5727. [Google Scholar] [CrossRef]
- Al-Sanea, M.M.; Chilingaryan, G.; Abelyan, N.; Sargsyan, A.; Hovhannisyan, S.; Gasparyan, H.; Gevorgyan, S.; Albogami, S.; Ghoneim, M.M.; Farag, A.K.; et al. Identification of Novel Potential VEGFR-2 Inhibitors Using a Combination of Computational Methods for Drug Discovery. Life 2021, 11, 1070. [Google Scholar] [CrossRef]
- Takaki, T.; Echalier, A.; Brown, N.R.; Hunt, T.; Endicott, J.A.; Noble, M.E.M. The Structure of CDK4/Cyclin D3 Has Implications for Models of CDK Activation. Proc. Natl. Acad. Sci. USA 2009, 106, 4171–4176. [Google Scholar]
- Güven, O.; Menteşe, E.; Bilgin Sökmen, B.; Emirik, M.; Akyüz, G. Benzimidazolone Conjugated Biscoumarins: Synthesis, Molecular Docking Studies, Urease, Lipase, and Acetylcholinesterase Inhibitory Activities. J. Mol. Struct. 2025, 1338, 142362. [Google Scholar] [CrossRef]
- Ahmad, Y.; Habib, M.S.; Mohammady, A.; Bakhtiari, B.; Shamsi, S.A. Quinoxaline Derivatives. X. Novel Rearrangement of Certain Quinoxaline N-Oxides to 6-Benzimidazolinones. J. Org. Chem. 1968, 33, 201–205. [Google Scholar] [CrossRef]
- Yilmaz, F.; Menteşe, E.; Sökmen, B.B. Synthesis and Biological Evaluation of Some 1,3-Benzoxazol-2(H)-One Hybrid Molecules as Potential Antioxidant and Urease Inhibitors. J. Heterocycl. Chem. 2021, 58, 260–269. [Google Scholar] [CrossRef]
- Atmaca, H.; Ilhan, S.; Çamli Pulat, Ç.; Dundar, B.A.; Zora, M. Evaluation of Novel Spiro-Pyrrolopyridazine Derivatives as Anticancer Compounds: In Vitro Selective Cytotoxicity, Induction of Apoptosis, EGFR Inhibitory Activity, and Molecular Docking Analysis. ACS Omega 2024, 9, 23713–23723. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release, version 2021-4; Maestro, Schrödinger, LLC: New York, NY, USA, 2021.
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular Conformations, Interactions, and Properties Associated with Drug Efficiency and Clinical Performance among VEGFR TK Inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, S.I.; Pelletier, L.A.; Espada, A.; Gutiérrez, J.; Sanfeliciano, S.M.G.; Rauch, C.T.; Ganado, M.P.; Baquero, C.; Zapatero, E.; Zhang, A.; et al. Crystal Structure of Active CDK4-Cyclin D and Mechanistic Basis for Abemaciclib Efficacy. npj Breast Cancer 2022, 8, 126. [Google Scholar] [CrossRef]
- Schrödinger Release, version 2018-1; Induced Fit Docking Protocol; Glide, Schrödinger, LLC: New York, NY, USA; Prime, Schrödinger, LLC: New York, NY, USA, 2016.
- Emirik, M. Potential Therapeutic Effect of Turmeric Contents against SARS-CoV-2 Compared with Experimental COVID-19 Therapies: In Silico Study. J. Biomol. Struct. Dyn. 2022, 40, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
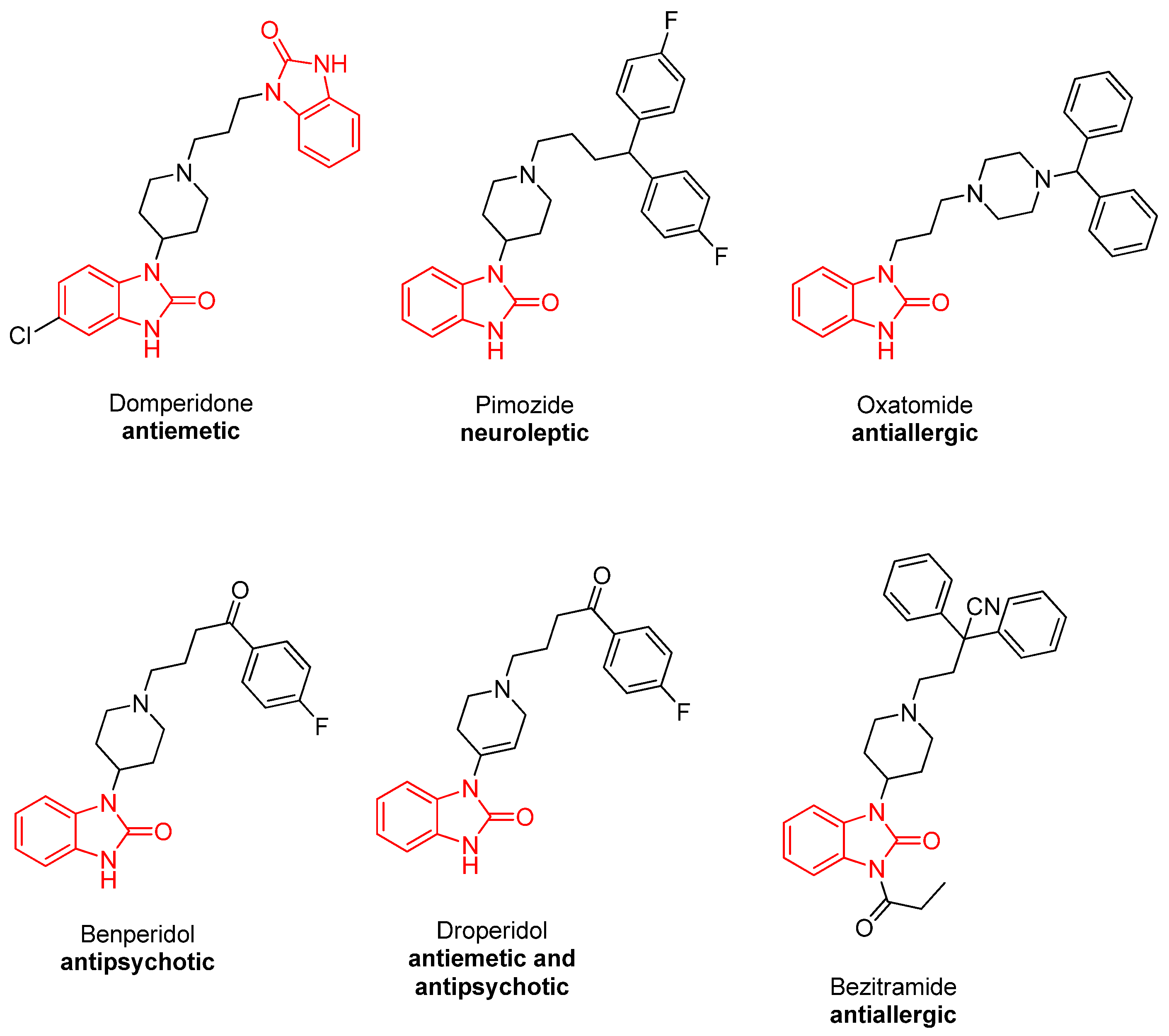





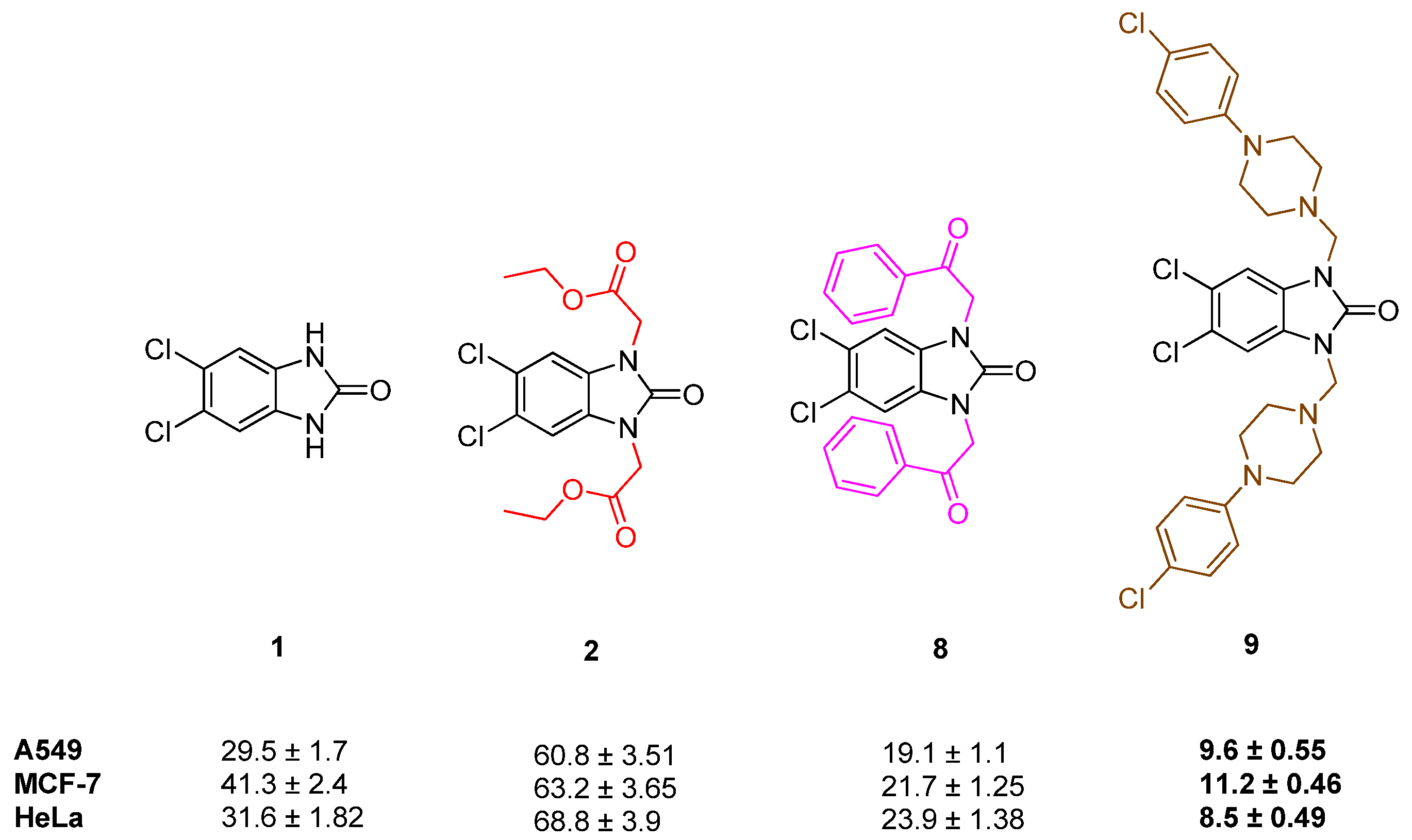
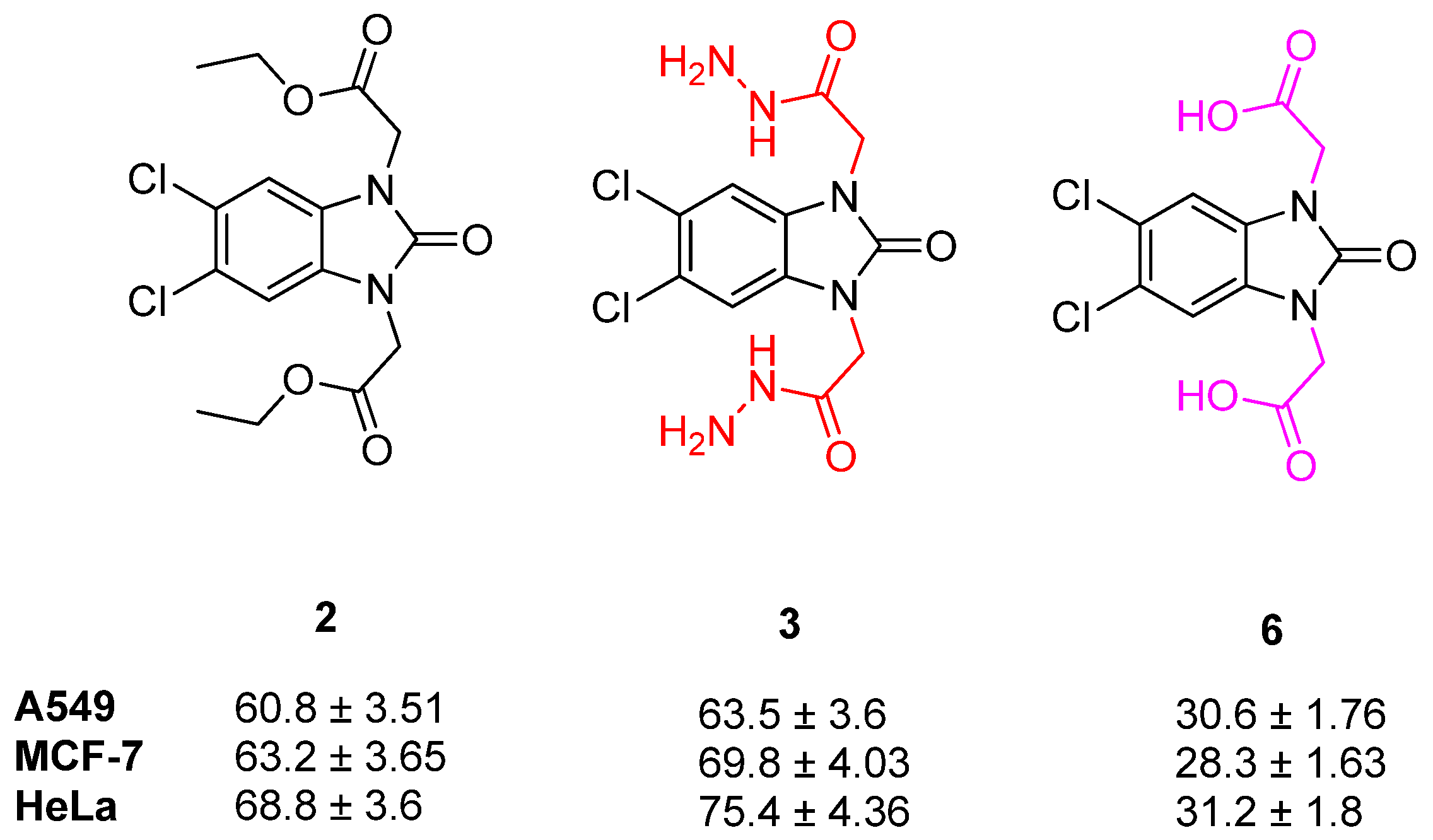
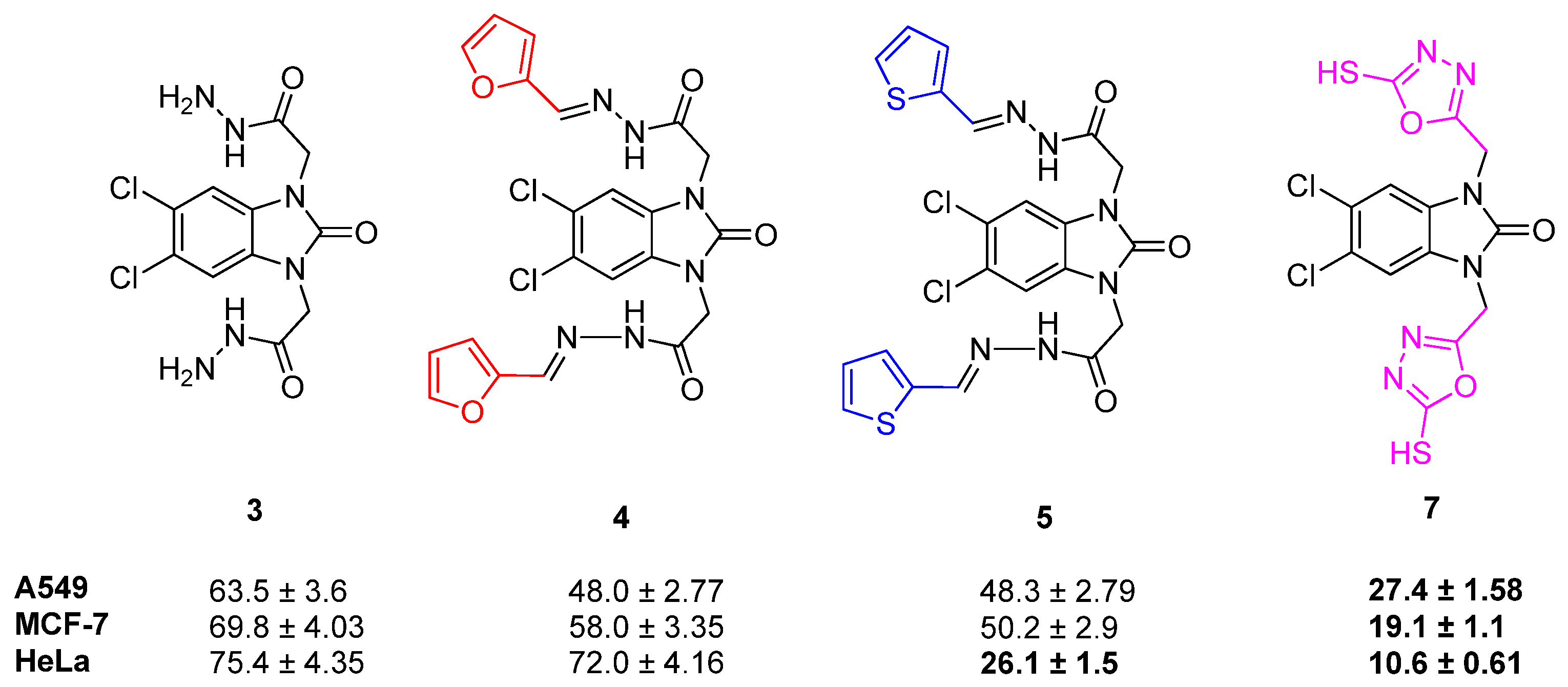

| Compound | A549 IC50 Values (µM ± SD)/SI | MCF-7 IC50 Values (µM ± SD)/SI | HeLa IC50 Values (µM ± SD)/SI | HEK 293 IC50 Values (µM ± SD) |
|---|---|---|---|---|
| 1 | 29.5 ± 1.7/2.03 | 41.3 ± 2.4/1.46 | 31.6 ± 1.82/1.9 | 60.1 ± 3.47 |
| 2 | 60.8 ± 3.51/1.17 | 63.2 ± 3.65/1.13 | 68.8 ± 3.9/1.04 | 71.3 ± 4.12 |
| 3 | 63.5 ± 3.6/1.06 | 69.8 ± 4.03/0.97 | 75.4 ± 4.35/0.89 | 67.9 ± 3.9 |
| 4 | 48.0 ± 2.77/1.15 | 58.0 ± 3.35/0.95 | 72.0 ± 4.16/0.76 | 55.0 ± 3.17 |
| 5 | 48.3 ± 2.79/1.22 | 50.2 ± 2.9/1.18 | 26.1 ± 1.5/2.27 | 59.4 ± 3.43 |
| 6 | 30.6 ± 1.76/2.04 | 28.3 ± 1.63/2.21 | 31.2 ± 1.8/2 | 62.7 ± 3.62 |
| 7 | 27.4 ± 1.58/1.4 | 19.1 ± 1.1/2.01 | 10.6 ± 0.61/3.63 | 38.5 ± 2.2 |
| 8 | 19.1 ± 1.1/0.91 | 21.7 ± 1.25/0.8 | 23.9 ± 1.38/0.73 | 17.5 ± 1.01 |
| 9 | 9.6 ± 0.55/1.58 | 11.2 ± 0.46/1.35 | 8.5 ± 0.49/1.78 | 15.2 ± 0.87 |
| 12a | 21.7 ± 1.25/2.05 | 50.8 ± 2.93/0.87 | 48.5 ± 2.8/0.91 | 44.6 ± 2.57 |
| 12b | 35.2 ± 2/1.39 | 25.8 ± 1.5/1.9 | 13.6 ± 0.78/3.61 | 49.1 ± 2.83 |
| 12c | 10.2 ± 0.58/2.01 | 9.7 ± 0.56/2.21 | 11.5 ± 0.66/1.86 | 21.5 ± 1.24 |
| 12d | 15.5 ± 0.89/2.01 | 21.9 ± 1.26/1.42 | 12.8 ± 0.73/2.4 | 31.2 ± 1.8 |
| 12e | 52.8 ± 3/1.14 | 65.1 ± 3.76/0.92 | 54.3 ± 3.13/1.1 | 60.2 ± 3.47 |
| 12f | 23.6 ± 1.3/1.84 | 26.4 ± 1.52/1.64 | 29.1 ± 1.26/1.49 | 43.5 ± 2.51 |
| Doxorubicin | 4.3 ± 0.2/1.20 | 6.4 ± 0.37/0.77 | 3.4 ± 0.19/1.54 | 5.2 ± 0.3 |
| Cell Line | Group | LDH (µU/mL) | TOS (µmol H2O2 eq/L) | GSH/GSSG (nmol/mg Protein) |
|---|---|---|---|---|
| HeLa | Control | 6.2 ± 0.8 | 2.1 ± 0.3 | 31.2 ± 2.4 |
| H2O2 | 41.5 ± 3.2 a | 10.4 ± 0.7 a | 6.8 ± 0.9 a | |
| 5 | 24.8 ± 2.1 a,b | 6.9 ± 0.5 a,b | 11.8 ± 1.3 a,b | |
| 6 | 27.3 ± 2.5 a,b | 7.8 ± 0.6 a,b | 10.6 ± 1.1 a,b | |
| 7 | 30.9 ± 2.7 a,b | 8.6 ± 0.7 a,b | 9.4 ± 1.0 a,b | |
| 12b | 28.5 ± 2.3 a,b | 8.1 ± 0.6 a,b | 10.1 ± 1.1 a,b | |
| 12d | 29.7 ± 2.4 a,b | 8.4 ± 0.6 a,b | 9.7 ± 1.0 a,b | |
| A549 | Control | 5.9 ± 0.6 | 2.0 ± 0.2 | 30.4 ± 2.1 |
| H2O2 | 40.7 ± 3.0 a | 10.5 ± 0.8 a | 7.1 ± 0.9 a | |
| 1 | 19.8 ± 1.7 a,b | 6.1 ± 0.4 a,b | 13.5 ± 1.3 a,b | |
| 6 | 24.5 ± 2.0 a,b | 7.3 ± 0.5 a,b | 11.2 ± 1.0 a,b | |
| 12c | 30.4 ± 2.6 a,b | 8.5 ± 0.7 a,b | 9.5 ± 0.8 a,b | |
| 12d | 27.3 ± 2.2 a,b | 7.9 ± 0.6 a,b | 10.1 ± 0.9 a,b | |
| MCF-7 | Control | 5.4 ± 0.6 | 1.9 ± 0.2 | 29.8 ± 2.3 |
| H2O2 | 39.5 ± 2.7 a | 10.1 ± 0.7 a | 7.4 ± 0.9 a | |
| 6 | 22.7 ± 1.8 a,b | 6.9 ± 0.5 a,b | 12.1 ± 1.2 a,b | |
| 7 | 25.3 ± 2.0 a,b | 7.6 ± 0.6 a,b | 11.3 ± 1.0 a,b | |
| 12c | 28.8 ± 2.2 a,b | 8.3 ± 0.6 a,b | 10.2 ± 1.0 a,b |
| Compound | VEGFR2 | CDK4 | Compound | VEGFR2 | CDK4 |
|---|---|---|---|---|---|
| 1 | −9.34 | −5.73 | 12a | −10.05 | −6.96 |
| 2 | −6.32 | −6.31 | 12b | −8.63 | −8.62 |
| 3 | −11.44 | −9.55 | 12c | −13.44 | −6.96 |
| 4 | −11.59 | −8.70 | 12d | −11.84 | −6.53 |
| 5 | −12.93 | −8.01 | 12e | −8.63 | −4.10 |
| 6 | −12.17 | −7.62 | 12f | −11.37 | −6.72 |
| 7 | −10.50 | −8.63 | Sorafenib | −14.66 | - |
| 8 | −9.88 | −7.33 | Abemaciclib | - | −13.60 |
| 9 | −12.92 | −10.02 |
| Compound | QPlogHERG | QPPCaco | QPlogBB | QPlogKhsa | HOA% |
|---|---|---|---|---|---|
| 1 | −3.60 | 610.14 | −0.16 | −0.33 | 87.54 |
| 2 | −4.63 | 758.73 | −0.65 | −0.09 | 100.00 |
| 3 | −4.28 | 17.69 | −2.19 | −0.63 | 35.22 |
| 4 | −5.23 | 583.52 | −0.89 | 0.22 | 74.24 |
| 5 | −6.27 | 656.37 | −0.83 | 0.62 | 82.95 |
| 6 | −0.30 | 4.62 | −1.34 | −0.67 | 50.78 |
| 7 | −4.96 | 369.76 | −0.57 | −0.06 | 92.11 |
| 8 | −6.97 | 1556.63 | −0.37 | 0.40 | 100.00 |
| 9 | −8.33 | 291.27 | 1.36 | 1.26 | 83.64 |
| 12a | −8.37 | 7.39 | −3.87 | 0.31 | 38.34 |
| 12b | −8.15 | 5.33 | −3.94 | 0.54 | 40.92 |
| 12c | −8.18 | 5.34 | −3.94 | 0.59 | 41.86 |
| 12d | −7.99 | 5.33 | −3.79 | 0.80 | 34.23 |
| 12e | −8.05 | 4.92 | −4.44 | 0.25 | 34.87 |
| 12f | −8.26 | 9.28 | −4.63 | 0.98 | 40.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güven, O.; Menteşe, E.; Yılmaz, F.; Güner, A.; Emirik, M.; Çalışkan, N. Evaluation of Antiproliferative Activity and Molecular Modeling Studies of Some Novel Benzimidazolone-Bridged Hybrid Compounds. Pharmaceuticals 2025, 18, 1899. https://doi.org/10.3390/ph18121899
Güven O, Menteşe E, Yılmaz F, Güner A, Emirik M, Çalışkan N. Evaluation of Antiproliferative Activity and Molecular Modeling Studies of Some Novel Benzimidazolone-Bridged Hybrid Compounds. Pharmaceuticals. 2025; 18(12):1899. https://doi.org/10.3390/ph18121899
Chicago/Turabian StyleGüven, Okan, Emre Menteşe, Fatih Yılmaz, Adem Güner, Mustafa Emirik, and Nedime Çalışkan. 2025. "Evaluation of Antiproliferative Activity and Molecular Modeling Studies of Some Novel Benzimidazolone-Bridged Hybrid Compounds" Pharmaceuticals 18, no. 12: 1899. https://doi.org/10.3390/ph18121899
APA StyleGüven, O., Menteşe, E., Yılmaz, F., Güner, A., Emirik, M., & Çalışkan, N. (2025). Evaluation of Antiproliferative Activity and Molecular Modeling Studies of Some Novel Benzimidazolone-Bridged Hybrid Compounds. Pharmaceuticals, 18(12), 1899. https://doi.org/10.3390/ph18121899





