Updated Advances on Drugs and Bone-Targeting Nanoparticles for Osteoporosis Therapy: Carrier Materials, Modification, Function Mechanism, and Applications—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection and Data Collection Process
3. Results and Discussion
3.1. Database Search and Included Studies
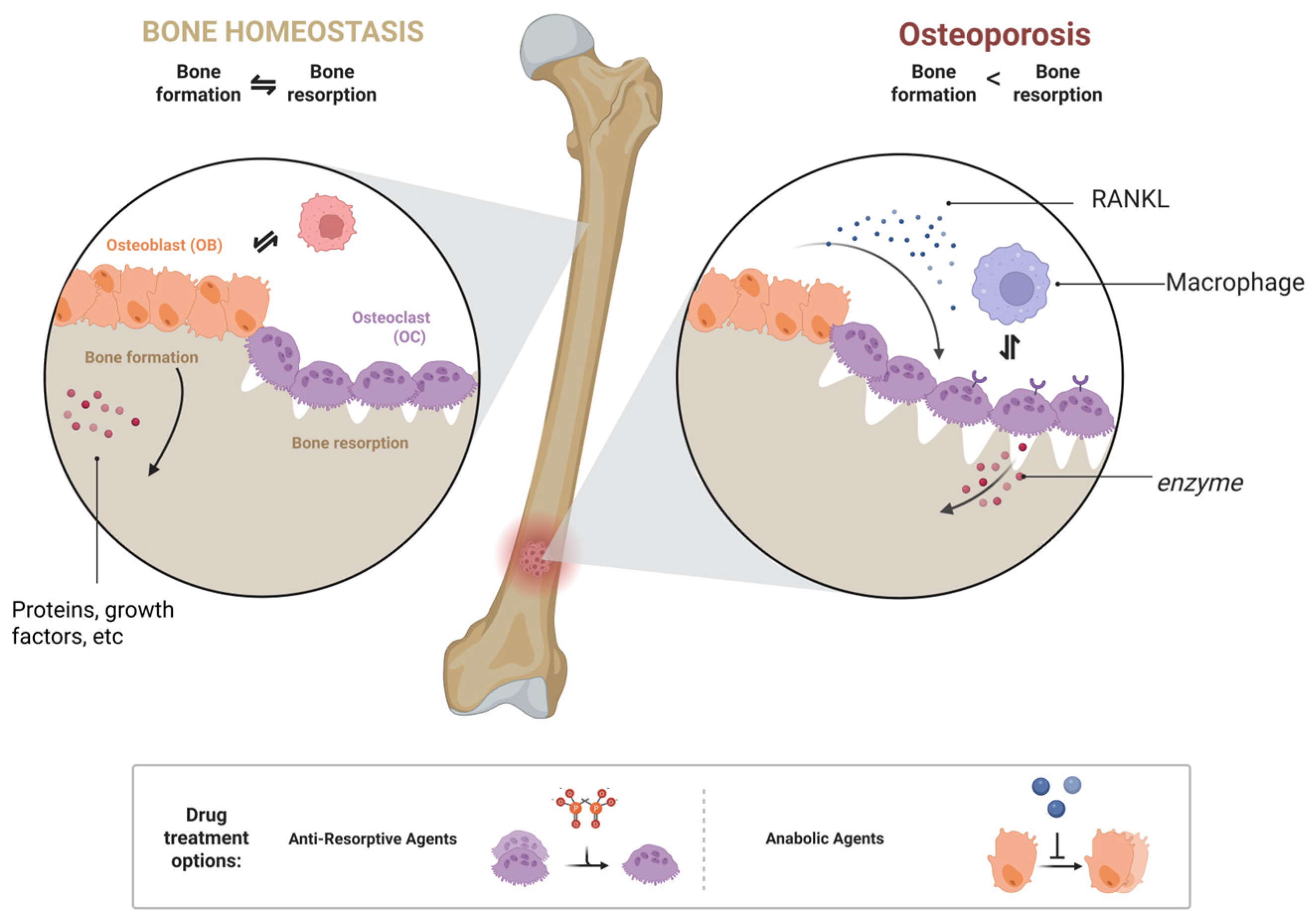
3.2. The Pathogenesis of Osteoporosis
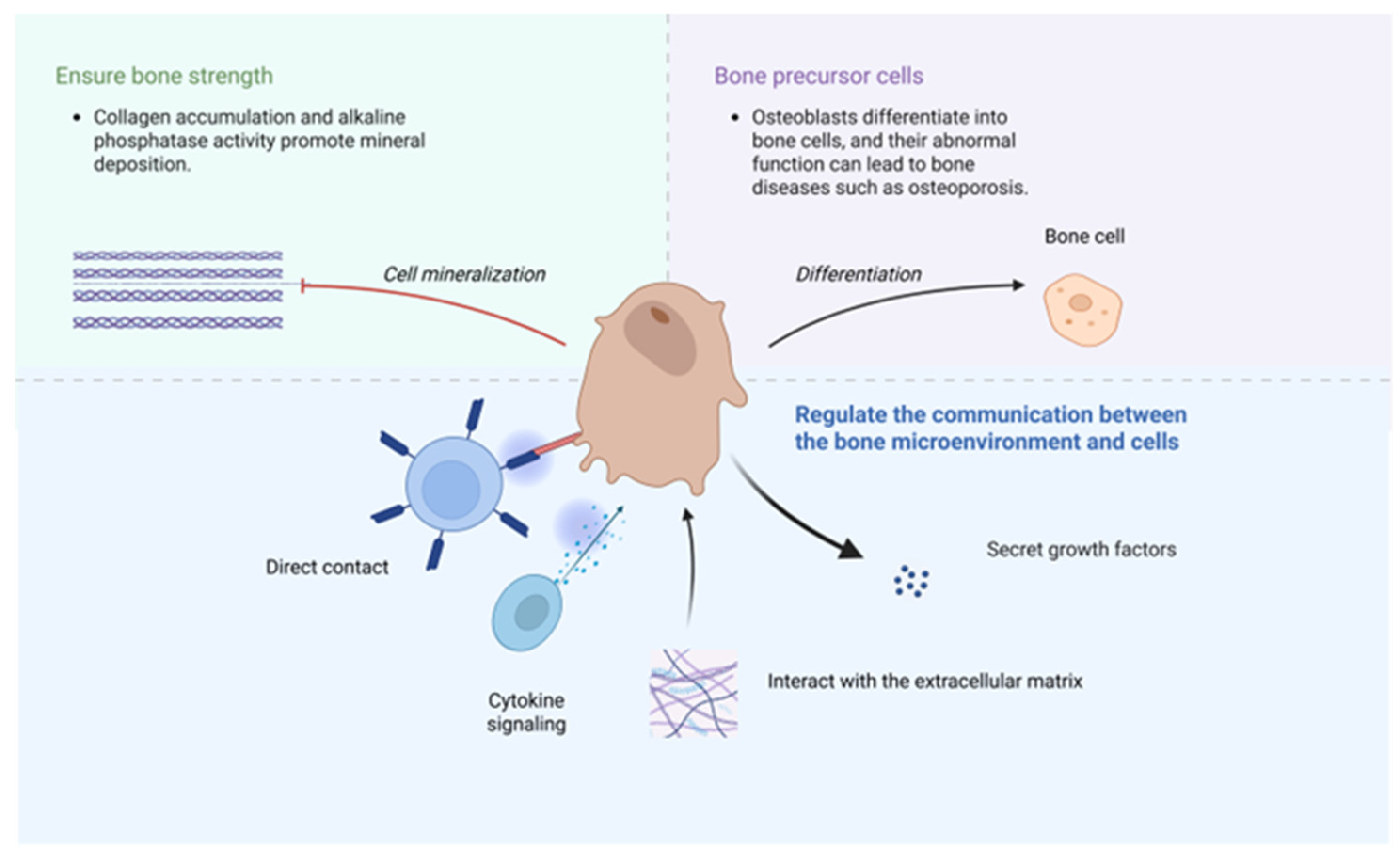
3.3. The Role of Osteoblasts and Osteoclasts in Bone
3.4. Current Development Status of Existing Anti-Osteoporosis Drugs
3.4.1. Anti-Resorptive Agents
3.4.2. Anabolic Agents
3.4.3. Other Drugs
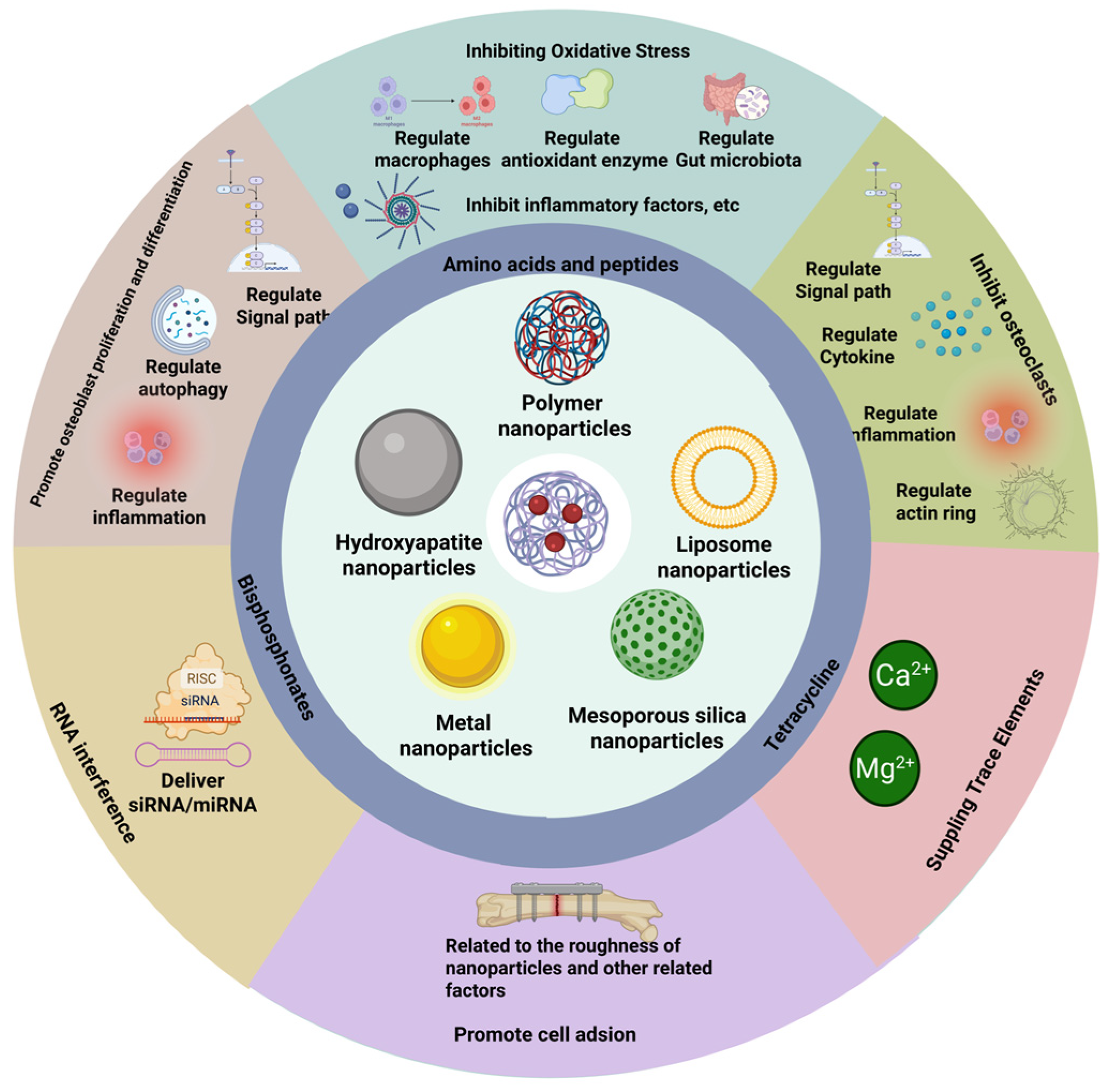
3.5. Bone Nanoparticles
3.6. Carrier Materials
3.6.1. Polymer
3.6.2. Liposomes
3.6.3. Metal Nanoparticles
3.6.4. Hydroxyapatite
3.6.5. Other Carrier Materials
3.7. Modified Materials
3.7.1. Bisphosphonates
3.7.2. Tetracycline
3.7.3. Amino Acids and Oligopeptides
3.7.4. Surface Modifications
3.7.5. Other Finishing Materials
3.8. Synthesis Methods of Nanoparticles
3.8.1. Emulsion Solvent Evaporation Method
3.8.2. Self-Assembly Method
3.8.3. Green Synthesis Method
3.8.4. Other Synthesis Methods
3.9. Targeted Group Connection Method
3.9.1. Covalent Binding Method
3.9.2. Electrostatic Adsorption Method
3.10. Bone-Targeting Nanoparticles: Acting Mechanisms on Osteoporosis
3.10.1. Bone-Targeting Nanoparticles for Inhibiting Oxidative Stress in Osteoblasts
3.10.2. Nanoparticles That Promote Osteoblast Proliferation and Differentiation
3.10.3. Anti-Resorptive Therapy
3.10.4. Nanoparticles Based on RNA Interference
3.10.5. Bone-Targeting Nanoparticles for Supplying Trace Elements
3.10.6. Enhanced Osteoblast Adhesion
3.10.7. Other Mechanisms
3.11. Clinical Application Predicaments
3.11.1. Safety Issues
3.11.2. Regulatory Issues
3.11.3. Cost Issues
3.12. Future Development Trends
3.12.1. Multi-Target or Intelligent Responsive Nanoparticles
3.12.2. Multi-Mechanism Synergistic Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| PDGF | Platelet-Derived Growth Factor |
| BMPs | Bone Morphogenetic Proteins |
| IGFs | Insulin-Like Growth Factors |
| RANKL | Receptor Activator of Nuclear Factor-Kappa B Ligand |
| MOF | major osteoporotic fractures |
| PTH | Parathyroid hormone |
| LS | Lumbar Spine |
| TH | Thoracic Spine |
| TR | Trochanter |
| FN | Femoral Neck |
| Hip | Hip Joint |
| VF | Vertebral Fracture |
| NVF | Non-Vertebral Fracture |
| HF | Hip fractures |
| PAD | Pamam dendrimer |
| HCCP | Hexachlorocyclotriphosphazene |
| Cur | Curcumin |
| PLGA | Polylactic-glycolic acid copolymer |
| NPs | Nanoparticles |
| CCK-8 | Cell Counting Kit-8 |
| Ovariectomized | OXV |
| OXV | Ovariectomized |
| CS | Chitosan |
| PEG | Polyethylene Glycol |
| BMD | Bone mineral density |
| BV/TV | Bone volume fraction |
| MOF | Metal–organic framework |
| Tb.N | Trabecular bone count |
| AuNPs | Gold nanoparticles |
| MSC | Mesenchymal stem cells |
| HAP | Hydroxyapatite |
| HANP | Hydroxyapatite nanoparticles |
| SCT | Salmon calcitonin |
| TC | Tetracycline |
| Asp | Aspartic Acid |
| Ser | Serine |
| Gly | Glycine |
| Arg | Arginine |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
| MAL | Maleimide |
| ROS | Reactive oxygen species |
| BMMs | Bone marrow-derived macrophages |
| CD | Cyclodextrin |
| mEVs | Milk-derived extracellular vesicles |
| CCMV | Cowpea chlorotic mottle virus |
| EDTA | Ethylene diamine tetraacetic acid |
References
- Anam, A.K.; Insogna, K. Update on Osteoporosis Screening and Management. Med. Clin. N. Am. 2021, 105, 1117–1134. [Google Scholar] [CrossRef]
- Subarajan, P.; Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: A Review of Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2024, 53, 497–512. [Google Scholar] [CrossRef]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Miller, P.D. Management of severe osteoporosis. Expert Opin. Pharmacother. 2016, 17, 473–488. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Q.; An, R.; Zhang, H.M.; Shen, J.Y.; Qin, X.Y.; Han, X.L.; Li, J.; Li, X.W.; Gao, X.M.; et al. Anti-osteoporosis effect of Semen Cuscutae in ovariectomized mice through inhibition of bone resorption by osteoclasts. J. Ethnopharmacol. 2022, 285, 114834. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]
- Wen, C.; Xu, X.; Zhang, Y.; Xia, J.; Liang, Y.; Xu, L. Bone Targeting Nanoparticles for the Treatment of Osteoporosis. Int. J. Nanomed. 2024, 19, 1363–1383. [Google Scholar] [CrossRef]
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef]
- Zeghoud, S.; Ben Amor, I.; Alnazza Alhamad, A.; Darwish, L.; Hemmami, H. Osteoporosis therapy using nanoparticles: A review. Ann. Med. Surg. 2024, 86, 284–291. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.S.; Kumar, V.; Das, S.L. Classification of Osteoporosis. Indian J. Orthop. 2023, 57, 49–54. [Google Scholar] [CrossRef]
- Dabas, A.; Malhotra, R.; Kumar, R.; Khadgawat, R. Idiopathic juvenile osteoporosis in a child: A four-year follow-up with review of literature. J. Pediatr. Endocrinol. Metab. 2021, 34, 1487–1490. [Google Scholar] [CrossRef]
- Chotiyarnwong, P.; McCloskey, E.V.; Harvey, N.C.; Lorentzon, M.; Prieto-Alhambra, D.; Abrahamsen, B.; Adachi, J.D.; Borgström, F.; Bruyere, O.; Carey, J.J.; et al. Is it time to consider population screening for fracture risk in postmenopausal women? A position paper from the International Osteoporosis Foundation Epidemiology/Quality of Life Working Group. Arch. Osteoporos. 2022, 17, 87. [Google Scholar] [CrossRef]
- Blake, J.; Cosman, F.A.; Lewiecki, E.M.; McClung, M.R.; Pinkerton, J.; Shapiro, M. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Gavali, S.; Gupta, M.K.; Daswani, B.; Wani, M.R.; Sirdeshmukh, R.; Khatkhatay, M.I. Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1498–1507. [Google Scholar] [CrossRef]
- Norton, A.; Thieu, K.; Baumann, C.W.; Lowe, D.A.; Mansky, K.C. Estrogen regulation of myokines that enhance osteoclast differentiation and activity. Sci. Rep. 2022, 12, 15900. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y.; Xue, K.; Li, J.; Son, G.; Wang, J.; Qian, W.; Wang, S.; Zheng, J.; Yang, C.; et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 2023, 61, 102649. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, R.; Hao, S.; Luo, P.; Wang, P.; Almatari, Y.; Guo, L.; Guo, L. Up-regulation of SIRT1 induced by 17beta-estradiol promotes autophagy and inhibits apoptosis in osteoblasts. Aging 2021, 13, 23652–23671. [Google Scholar] [CrossRef]
- Wade, E.; Mulholland, K.; Shaw, I.; Cundy, T.; Robertson, S. Idiopathic juvenile osteoporosis-a polygenic disorder? JBMR Plus 2024, 8, ziae099. [Google Scholar] [CrossRef] [PubMed]
- DeShields, S.C.; Cunningham, T.D. Comparison of osteoporosis in US adults with type 1 and type 2 diabetes mellitus. J. Endocrinol. Investig. 2018, 41, 1051–1060. [Google Scholar] [CrossRef]
- Ali, D.; Tencerova, M.; Figeac, F.; Kassem, M.; Jafari, A. The pathophysiology of osteoporosis in obesity and type 2 diabetes in aging women and men: The mechanisms and roles of increased bone marrow adiposity. Front. Endocrinol. 2022, 13, 981487. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Voor, M.J.; Tyagi, N. Exercise-Linked Skeletal Irisin Ameliorates Diabetes-Associated Osteoporosis by Inhibiting the Oxidative Damage-Dependent miR-150-FNDC5/Pyroptosis Axis. Diabetes 2022, 71, 2777–2792. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Qian, B.; Li, P. Potential Metabolic Pathways Involved in Osteoporosis and Evaluation of Fracture Risk in Individuals with Diabetes. Biomed. Res. Int. 2024, 2024, 6640796. [Google Scholar] [CrossRef]
- Zhao, L.; Du, W.; Zhao, D.; Ji, X.; Huang, Y.; Pang, Y.; Guo, K.; Yin, X. Catalpol Protects Against High Glucose-Induced Bone Loss by Regulating Osteoblast Function. Front. Pharmacol. 2021, 12, 626621. [Google Scholar] [CrossRef] [PubMed]
- Leanza, G.; Cannata, F.; Faraj, M.; Pedone, C.; Viola, V.; Tramontana, F.; Pellegrini, N.; Vadalà, G.; Piccoli, A.; Strollo, R.; et al. Bone canonical Wnt signaling is downregulated in type 2 diabetes and associates with higher advanced glycation end-products (AGEs) content and reduced bone strength. eLife 2024, 12, RP90437. [Google Scholar] [CrossRef]
- Poleboina, S.; Sheth, V.G.; Sharma, N.; Sihota, P.; Kumar, N.; Tikoo, K. Selenium nanoparticles stimulate osteoblast differentiation via BMP-2/MAPKs/β-catenin pathway in diabetic osteoporosis. Nanomedicine 2022, 17, 607–625. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Jiang, Y.; Wan, M.; Jiao, B.; Liao, X.; Rao, S.; Hong, C.; Yang, Q.; Zhu, Y.; et al. Brain-derived extracellular vesicles promote bone-fat imbalance in Alzheimer’s disease. Int. J. Biol. Sci. 2023, 19, 2409–2427. [Google Scholar] [CrossRef]
- Wang, L.T.; Chen, L.R.; Chen, K.H. Hormone-Related and Drug-Induced Osteoporosis: A Cellular and Molecular Overview. Int. J. Mol. Sci. 2023, 24, 5814. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Naciu, A.M.; Yavropoulou, M.P.; Paccou, J. Risk and management of osteoporosis due to inhaled, epidural, intra-articular or topical glucocorticoids. Jt. Bone Spine 2023, 90, 105604. [Google Scholar] [CrossRef]
- Schepper, J.D.; Collins, F.; Rios-Arce, N.D.; Kang, H.J.; Schaefer, L.; Gardinier, J.D.; Raghuvanshi, R.; Quinn, R.A.; Britton, R.; Parameswaran, N.; et al. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J. Bone Miner. Res. 2020, 35, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, X.; He, C. Glucocorticoid-induced autophagy and apoptosis in bone. Apoptosis 2020, 25, 157–168. [Google Scholar] [CrossRef]
- Gado, M.; Baschant, U.; Hofbauer, L.C.; Henneicke, H. Bad to the Bone: The Effects of Therapeutic Glucocorticoids on Osteoblasts and Osteocytes. Front. Endocrinol. 2022, 13, 835720. [Google Scholar] [CrossRef]
- Tominami, K.; Kanetaka, H.; Sasaki, S.; Mokudai, T.; Kaneko, T.; Niwano, Y. Cold atmospheric plasma enhances osteoblast differentiation. PLoS ONE 2017, 12, e0180507. [Google Scholar] [CrossRef]
- Mroczek, J.; Pikula, S.; Suski, S.; Weremiejczyk, L.; Biesaga, M.; Strzelecka-Kiliszek, A. Apigenin Modulates AnxA6- and TNAP-Mediated Osteoblast Mineralization. Int. J. Mol. Sci. 2022, 23, 13179. [Google Scholar] [CrossRef]
- Neag, G.; Finlay, M.; Naylor, A.J. The Cellular Choreography of Osteoblast Angiotropism in Bone Development and Homeostasis. Int. J. Mol. Sci. 2021, 22, 7253. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef]
- Xue, C.; Luo, H.; Wang, L.; Deng, Q.; Kui, W.; Da, W.; Chen, L.; Liu, S.; Xue, Y.; Yang, J.; et al. Aconine attenuates osteoclast-mediated bone resorption and ferroptosis to improve osteoporosis via inhibiting NF-κB signaling. Front. Endocrinol. 2023, 14, 1234563. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases. Front. Immunol. 2021, 12, 664871. [Google Scholar] [CrossRef]
- Chiba, K.; Okazaki, N.; Kurogi, A.; Watanabe, T.; Mori, A.; Suzuki, N.; Adachi, K.; Era, M.; Yokota, K.; Inoue, T.; et al. Randomized controlled trial of daily teriparatide, weekly high-dose teriparatide, or bisphosphonate in patients with postmenopausal osteoporosis: The TERABIT study. Bone 2022, 160, 116416. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.L.; Yuan, Z.F.; Li, Y.; Ai, J.; Xu, H.; Sun, J.C.; Feng, S.Q. Alendronate prevents glucocorticoid-induced osteoporosis in patients with rheumatic diseases: A meta-analysis. Medicine 2016, 95, e3990. [Google Scholar] [CrossRef]
- Beaudart, C.; Demonceau, C.; Sabico, S.; Veronese, N.; Cooper, C.; Harvey, N.; Fuggle, N.; Bruyère, O.; Rizzoli, R.; Reginster, J.Y. Efficacy of osteoporosis pharmacological treatments in men: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2023, 35, 1789–1806. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Park, S.Y.; Lee, S.H.; Park, J.H.; Suh, S.W. Comparison of Denosumab and Zoledronic Acid in Postmenopausal Women with Osteoporosis: Bone Mineral Density (BMD) and Trabecular Bone Score (TBS). J. Korean Med. Sci. 2022, 37, e68. [Google Scholar] [CrossRef]
- Ha, J.; Kim, J.; Jeong, C.; Lee, J.; Lim, Y.; Baek, K.H. Effects of denosumab and zoledronic acid on postmenopausal osteoporosis, bone density, and fat-free mass. Arch. Osteoporos. 2025, 20, 17. [Google Scholar] [CrossRef]
- Moshi, M.R.; Nicolopoulos, K.; Stringer, D.; Ma, N.; Jenal, M.; Vreugdenburg, T. The Clinical Effectiveness of Denosumab (Prolia®) for the Treatment of Osteoporosis in Postmenopausal Women, Compared to Bisphosphonates, Selective Estrogen Receptor Modulators (SERM), and Placebo: A Systematic Review and Network Meta-Analysis. Calcif. Tissue Int. 2023, 112, 631–646. [Google Scholar] [CrossRef]
- Kobayakawa, T.; Miyazaki, A.; Saito, M.; Suzuki, T.; Takahashi, J.; Nakamura, Y. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci. Rep. 2021, 11, 11801. [Google Scholar] [CrossRef]
- Rhee, Y.; Chang, D.G.; Ha, J.; Kim, S.; Lee, Y.; Jo, E.; Koh, J.M. Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea. Endocrinol. Metab. 2022, 37, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pérez, A.; Marin, F.; Eriksen, E.F.; Kendler, D.L.; Krege, J.H.; Delgado-Rodríguez, M. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and meta-analysis. Bone 2019, 120, 1–8. [Google Scholar] [CrossRef]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.; Hu, M.Y.; Harris, A.G.; et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef]
- Cohen, A.; Shiau, S.; Nair, N.; Recker, R.R.; Lappe, J.M.; Dempster, D.W.; Nickolas, T.L.; Zhou, H.; Agarwal, S.; Kamanda-Kosseh, M.; et al. Effect of Teriparatide on Bone Remodeling and Density in Premenopausal Idiopathic Osteoporosis: A Phase II Trial. J. Clin. Endocrinol. Metab. 2020, 105, e3540–e3556. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Pavo, I.; Alam, J.; Disch, D.P.; Schuster, D.; Harris, J.M.; Krege, J.H. Teriparatide in patients with osteoporosis and type 2 diabetes. Bone 2016, 91, 152–158. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, G.; Song, J.; Kong, X.; Zhang, W.; Meng, C. Comparative Efficacy of Alendronate upon Vertebral Bone Mineral Density and Fracture Rates in East Asians Versus Non-East Asians with Postmenopausal Osteoporosis: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2018, 50, 738–746. [Google Scholar] [CrossRef]
- Curtis, J.R.; Arora, T.; Liu, Y.; Lin, T.C.; Spangler, L.; Brunetti, V.C.; Stad, R.K.; McDermott, M.; Bradbury, B.D.; Kim, M. Comparative effectiveness of denosumab vs alendronate among postmenopausal women with osteoporosis. J. Bone Miner. Res. 2024, 39, 826–834. [Google Scholar] [CrossRef]
- Tutaworn, T.; Nieves, J.W.; Wang, Z.; Levin, J.E.; Yoo, J.E.; Lane, J.M. Bone loss after denosumab discontinuation is prevented by alendronate and zoledronic acid but not risedronate: A retrospective study. Osteoporos. Int. 2023, 34, 573–584. [Google Scholar] [CrossRef]
- Ebina, K.; Etani, Y.; Noguchi, T.; Nakata, K.; Okada, S. Clinical effects of teriparatide, abaloparatide, and romosozumab in postmenopausal osteoporosis. J. Bone Miner. Metab. 2025, 43, 3–9. [Google Scholar] [CrossRef]
- Hartz, M.C.; Johannessen, F.B.; Harsløf, T.; Langdahl, B.L. The Effectiveness and Safety of Romosozumab and Teriparatide in Postmenopausal Women with Osteoporosis. J. Clin. Endocrinol. Metab. 2025, 110, e1640–e1652. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.K.; Clarke, B.L. Abaloparatide: Recombinant human PTHrP (1-34) anabolic therapy for osteoporosis. Maturitas 2017, 97, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Pal, S.; Chattopadhyay, N. Abaloparatide, the second generation osteoanabolic drug: Molecular mechanisms underlying its advantages over the first-in-class teriparatide. Biochem. Pharmacol. 2019, 166, 185–191. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef]
- Li, C.; Lin, X.; Lin, Q.; Lin, Y.; Lin, H. Jiangu granules ameliorate postmenopausal osteoporosis via rectifying bone homeostasis imbalance: A network pharmacology analysis based on multi-omics validation. Phytomedicine 2024, 122, 155137. [Google Scholar] [CrossRef]
- Zhang, N.D.; Han, T.; Huang, B.K.; Rahman, K.; Jiang, Y.P.; Xu, H.T.; Qin, L.P.; Xin, H.L.; Zhang, Q.Y.; Li, Y.M. Traditional Chinese medicine formulas for the treatment of osteoporosis: Implication for antiosteoporotic drug discovery. J. Ethnopharmacol. 2016, 189, 61–80. [Google Scholar] [CrossRef]
- Chi, K.; Yan, J.; Zhu, Y.; Wu, J. Efficacy and safety of oral traditional Chinese medicine combined with conventional anti-osteoporosis drugs for osteoporosis and fractures: A meta-analysis of randomized controlled trials. Medicine 2023, 102, e36634. [Google Scholar] [CrossRef]
- Hou, W.; Chen, S.; Zhu, C.; Gu, Y.; Zhu, L.; Zhou, Z. Associations between smoke exposure and osteoporosis or osteopenia in a US NHANES population of elderly individuals. Front. Endocrinol. 2023, 14, 1074574. [Google Scholar] [CrossRef]
- Linhares, D.G.; Borba-Pinheiro, C.J.; Castro, J.B.P.; Santos, A.; Santos, L.L.D.; Cordeiro, L.S.; Drigo, A.J.; Nunes, R.A.M.; Vale, R.G.S. Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14195. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Wang, Y.; Xu, W.; Chen, H.; Xu, J.; Luo, S.; Zhang, Y.; Zhao, D.; Hu, J. The efficacy and safety of denosumab in postmenopausal women with osteoporosis previously treated with bisphosphonates: A review. J. Orthop. Transl. 2020, 22, 7–13. [Google Scholar] [CrossRef]
- Nordqvist, J.; Engdahl, C.; Scheffler, J.M.; Gupta, P.; Gustafsson, K.L.; Lagerquist, M.K.; Carlsten, H.; Islander, U. A tissue-selective estrogen complex as treatment of osteoporosis in experimental lupus. Lupus 2022, 31, 143–154. [Google Scholar] [CrossRef]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Singh, S.; Dutta, S.; Khasbage, S.; Kumar, T.; Sachin, J.; Sharma, J.; Varthya, S.B. A systematic review and meta-analysis of efficacy and safety of Romosozumab in postmenopausal osteoporosis. Osteoporos. Int. 2022, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhou, L.; Li, J.; Liang, B.; Zhou, L.; Xue, F.; Jiang, L.; Hong, W. An acid-responsive bone-targeting nanoplatform loaded with curcumin balances osteogenic and osteoclastic functions. Regen. Biomater. 2025, 12, rbaf028. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.T.; Yang, Y.; Yang, Z.; Wang, X.; Zhang, P.; Lv, L.W.; Liu, Y.; Liu, Y.S.; Zhou, Y.S. Aptamer-immobilized bone-targeting nanoparticles in situ reduce sclerostin for osteoporosis treatment. Nano Today 2022, 45, 101529. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Sezlev Bilecen, D.; Rodriguez-Cabello, J.C.; Uludag, H.; Hasirci, V. Construction of a PLGA based, targeted siRNA delivery system for treatment of osteoporosis. J. Biomater. Sci. Polym. Ed. 2017, 28, 1859–1873. [Google Scholar] [CrossRef]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-Based Biocompatible Nanoparticles for Next-Generation Tolerogenic Vaccines against Autoimmune Disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Boltnarova, B.; Kubackova, J.; Skoda, J.; Stefela, A.; Smekalova, M.; Svacinova, P.; Pavkova, I.; Dittrich, M.; Scherman, D.; Zbytovska, J.; et al. PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials 2021, 11, 749. [Google Scholar] [CrossRef]
- Zhao, Z.; Deng, Y.; Deng, Y.; Chen, Z.; Zhou, Z. Synthesis and Evaluation of Bone Targeting PLGA Nanoparticles Loaded with Components of Traditional Chinese Medicine Formulas. Recent Pat. Nanotechnol. 2023, 18, 33–44. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkheshen, S.A.; Shamma, R.N.; Amer, M.S.; Elhelw, R.; El-Kayal, M. Injectable systems of chitosan in situ forming composite gel incorporating linezolid-loaded biodegradable nanoparticles for long-term treatment of bone infections. Drug Deliv. Transl. Res. 2024, 14, 80–102. [Google Scholar] [CrossRef]
- Tihan, G.T.; Zgarian, R.G.; Berteanu, E.; Ionita, D.; Totea, G.; Iordachel, C.; Tatia, R.; Prodana, M.; Demetrescu, I. Alkaline Phosphatase Immobilization on New Chitosan Membranes with Mg2+ for Biomedical Applications. Mar. Drugs 2018, 16, 287. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Trivedi, R.; Vairamani, M.; Selvamurugan, N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohydr. Polym. 2019, 216, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhao, J.; Lee, Y.Y.; Chen, J.; Wang, Y. Chitosan-based bone-targeted nanoparticles delivery of cyclolinopeptide J for the synergistic treatment of osteoporosis. Int. J. Biol. Macromol. 2025, 304, 140884. [Google Scholar] [CrossRef]
- Wang, C.; Hou, W.; Guo, X.; Li, J.; Hu, T.; Qiu, M.; Liu, S.; Mo, X.; Liu, X. Two-phase electrospinning to incorporate growth factors loaded chitosan nanoparticles into electrospun fibrous scaffolds for bioactivity retention and cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 507–515. [Google Scholar] [CrossRef]
- Li, H.; Ji, Q.X.; Chen, X.M.; Sun, Y.; Xu, Q.C.; Deng, P.P.; Hu, F.; Yang, J.J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. Part A 2017, 105, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Rong, H.; Liang, J.; Mao, C.; Li, Z.; Dai, Z.; Li, D.; Guo, W.; Chen, S.; Wang, Z.; et al. Chitosan modified with PAP as a promising delivery system for melatonin in the treatment of osteoporosis: Targeting the divalent metal transporter 1. J. Biol. Eng. 2024, 18, 27. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-León, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid nanoparticles for siRNA delivery in cancer treatment. J. Control. Release 2023, 361, 130–146. [Google Scholar] [CrossRef]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, J.; Rong, L.; Yang, H.; Wang, B.; Lu, S.; Zhang, L.; Li, F.; Yang, S.; Wang, Z.; et al. Osteoblast-specific down-regulation of NLRP3 inflammasome by aptamer-functionalized liposome nanoparticles improves bone quality in postmenopausal osteoporosis rats. Theranostics 2024, 14, 3945–3962. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, J.; Jiang, Z.; Guo, Q.; Zhang, Z.; Li, J.; Hu, Y.; Wang, L. Zoledronate combined metal-organic frameworks for bone-targeting and drugs deliveries. Sci. Rep. 2022, 12, 12290. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Chen, Z.; Mo, M.; Xie, J.; Zhou, X.; Wu, Y.; Yang, Q.; Zheng, M.; Hu, X.; et al. Proangiogenic effect and underlying mechanism of holmium oxide nanoparticles: A new biomaterial for tissue engineering. J. Nanobiotechnol. 2024, 22, 357. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Gao, H.; Li, T.; Cao, X.; Zhong, S.; Wang, Y. miR-29b-Loaded Gold Nanoparticles Targeting to the Endoplasmic Reticulum for Synergistic Promotion of Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 19217–19227. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Dai, Q.; Tan, J.; Dou, C.; Luo, F. Gold-nanosphere mitigates osteoporosis through regulating TMAO metabolism in a gut microbiota-dependent manner. J. Nanobiotechnol. 2023, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Heo, D.N.; Kim, H.J.; Ko, W.K.; Lee, S.J.; Heo, M.; Bang, J.B.; Lee, J.B.; Hwang, D.S.; Do, S.H.; et al. Inhibition of Osteoclast Differentiation and Bone Resorption by Bisphosphonate-conjugated Gold Nanoparticles. Sci. Rep. 2016, 6, 27336. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, Z.; Hu, W.; Tang, S.; Zhou, X.; Dong, S. Biosynthesized Silver Nanoparticles Inhibit Osteoclastogenesis by Suppressing NF-κB Signaling Pathways. Adv. Biol. 2024, 8, e2300355. [Google Scholar] [CrossRef]
- Liang, W.; Ding, P.; Li, G.; Lu, E.; Zhao, Z. Hydroxyapatite Nanoparticles Facilitate Osteoblast Differentiation and Bone Formation Within Sagittal Suture During Expansion in Rats. Drug Des. Dev. Ther. 2021, 15, 905–917. [Google Scholar] [CrossRef]
- Kadu, K.; Kowshik, M.; Ramanan, S.R. Tailoring of hydroxyapatite nanoparticle surfaces of varying morphologies to facilitate counterion diffusion and subsequent protein denaturation. Biophys. Chem. 2023, 296, 106979. [Google Scholar] [CrossRef]
- De Lama-Odría, M.D.C.; Valle, L.J.D.; Puiggalí, J. Lanthanides-Substituted Hydroxyapatite for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 3446. [Google Scholar] [CrossRef]
- da Silva de Barros, A.O.; Ricci-Junior, E.; Alencar, L.M.R.; Fechine, P.B.A.; Andrade Neto, D.M.; Bouskela, E.; Santos-Oliveira, R. High doses of hydroxyapatite nanoparticle (nHAP) impairs microcirculation in vivo. Colloids Surf. B Biointerfaces 2024, 233, 113601. [Google Scholar] [CrossRef]
- Kotak, D.J.; Devarajan, P.V. Bone targeted delivery of salmon calcitonin hydroxyapatite nanoparticles for sublingual osteoporosis therapy (SLOT). Nanomedicine 2020, 24, 102153. [Google Scholar] [CrossRef]
- Gautam, N.; Sharma, P.; Chaudhary, A.; Sahu, S.; Vohora, D.; Mishra, M.; Dutta, D.; Singh, M.; Talegaonkar, S. Investigating the osteogenic potential of bone-targeted daidzein loaded hydroxyapatite nanoparticles for postmenopausal osteoporosis: Pharmacodynamic, biochemical, and genotoxicity evaluations. J. Drug Target. 2025, 33, 1575–1590. [Google Scholar] [CrossRef]
- Mora-Raimundo, P.; Lozano, D.; Benito, M.; Mulero, F.; Manzano, M.; Vallet-Regí, M. Osteoporosis Remission and New Bone Formation with Mesoporous Silica Nanoparticles. Adv. Sci. 2021, 8, e2101107. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, P.; Meng, Y.; Lin, T.; Zhang, Z.; Shu, H.; Ma, J.; Cohen Stuart, M.; Gao, Y.; Wang, J.; et al. Rational polyelectrolyte nanoparticles endow preosteoclast-targeted siRNA transfection for anabolic therapy of osteoporosis. Sci. Adv. 2023, 9, eade7379. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Dong, Z.; Gu, C.; Mao, D.; Chen, J.; Luo, L.; Huang, Y.; Xiao, J.; Li, Z.; et al. Metal-polyDNA nanoparticles reconstruct osteoporotic microenvironment for enhanced osteoporosis treatment. Sci. Adv. 2023, 9, eadf3329. [Google Scholar] [CrossRef]
- Rudnick-Glick, S.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Targeted drug delivery of near IR fluorescent doxorubicin-conjugated poly(ethylene glycol) bisphosphonate nanoparticles for diagnosis and therapy of primary and metastatic bone cancer in a mouse model. J. Nanobiotechnol. 2016, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Panniello, A.; Comparelli, R.; Arduino, I.; Fanizza, E.; Iacobazzi, R.M.; Perrone, M.G.; Striccoli, M.; Curri, M.L.; Scilimati, A.; et al. Luminescent Alendronic Acid-Conjugated Micellar Nanostructures for Potential Application in the Bone-Targeted Delivery of Cholecalciferol. Molecules 2024, 29, 2367. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, Y.; Zhong, X.; Fan, C.; Liu, Z.; Chen, X.; Luo, Z.; Wu, J.; Tima, S.; Zhang, Z.; et al. Alendronate-functionalized polymeric micelles target icaritin to bone for mitigating osteoporosis in a rat model. J. Control. Release 2024, 376, 37–51. [Google Scholar] [CrossRef]
- Song, J.; Cui, N.; Mao, X.; Huang, Q.; Lee, E.S.; Jiang, H. Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface-A First-Principles Insight. Materials 2022, 15, 797. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.J.; Hathaway-Schrader, J.D.; Lubker, R.; Davies, C.; Novince, C.M. Tetracyclines and bone: Unclear actions with potentially lasting effects. Bone 2022, 159, 116377. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Behets, G.; Viaene, L.; Bammens, B.; Claes, K.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D.; D’Haese, P.C.; et al. Static histomorphometry allows for a diagnosis of bone turnover in renal osteodystrophy in the absence of tetracycline labels. Bone 2021, 152, 116066. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Xie, C.; Zhao, Y. Design, synthesis and evaluation of liposomes modified with dendritic aspartic acid for bone-specific targeting. Chem. Phys. Lipids 2020, 226, 104832. [Google Scholar] [CrossRef]
- Nirwan, N.; Anjaneyulu, Y.P.; Sultana, Y.; Vohora, D. Development of linagliptin-loaded liposomes using aspartic acid conjugate for bone-targeted delivery to combat osteoporosis. J. Drug Target. 2025, 33, 1014–1025. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, Y.; Cao, Y.; Ding, Y.; Cai, J.; Yang, T.; Zhou, X.; Wu, Q.; Li, D.; Liu, Q.; et al. Bone-targeting engineered milk-derived extracellular vesicles for MRI-assisted therapy of osteoporosis. Regen. Biomater. 2024, 11, rbae112. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Nishizawa, K.; Mishiro, K.; Effendi, N.; Fuchigami, T.; Munekane, M.; Wakabayashi, H.; Kinuya, S. Synthesis and Evaluation of Radiogallium Labeled Bone-Imaging Probes Using Oligo-γ-Carboxy Glutamic Acid Peptides as Carriers to Bone. Mol. Pharm. 2024, 21, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Lee, C.Y.; Lin, S.Y.; Kang, L.; Fu, Y.C.; Chen, C.H.; Wang, C.K. Bone-Targeting Nanoparticles of a Dendritic (Aspartic acid)3-Functionalized PEG-PLGA Biopolymer Encapsulating Simvastatin for the Treatment of Osteoporosis in Rat Models. Int. J. Mol. Sci. 2022, 23, 10530. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.L.; Hu, Q.; Liu, M.S.; Peng, T.; Xu, W.F.; Huang, Q.H.; Li, B.; Liao, X.L. Arg-Gly-Asp Peptide-Functionalized Superparamagnetic γ-Fe2O3 Nanoparticles Enhance Osteoblast Migration In Vitro. J. Nanosci. Nanotechnol. 2020, 20, 6173–6179. [Google Scholar] [CrossRef]
- Kopac, T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Gu, P.; Cai, G.; Yang, Y.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles containing Angelica sinensis polysaccharide promote dendritic cells activation and associated molecular mechanisms. Int. J. Biol. Macromol. 2022, 207, 559–569. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, F.; Zhao, X.; Wang, L.; Wu, W.; Zu, C.; Wu, M. Improving the skin penetration and antifebrile activity of ibuprofen by preparing nanoparticles using emulsion solvent evaporation method. Eur. J. Pharm. Sci. 2018, 114, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, B.R.; Siddiqui, E.A.; Syed, A.S.; Velhal, S.M.; Ahmad, A.; Bandivdekar, A.B.; Devarajan, P.V. Nevirapine Loaded Core Shell Gold Nanoparticles by Double Emulsion Solvent Evaporation: In vitro and In vivo Evaluation. Curr. Drug Deliv. 2016, 13, 1071–1083. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Wu, Y.; Li, X.; Zhou, Y.; Wang, Z.; Liang, H.; Ding, F.; Hong, S.; Steinmetz, N.F.; et al. Molecularly Stimuli-Responsive Self-Assembled Peptide Nanoparticles for Targeted Imaging and Therapy. ACS Nano 2023, 17, 8004–8025. [Google Scholar] [CrossRef]
- Tang, Y.; Rajendran, P.; Veeraraghavan, V.P.; Hussain, S.; Balakrishna, J.P.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; Rengarajan, T.; Mohan, S.K. Osteogenic differentiation and mineralization potential of zinc oxide nanoparticles from Scutellaria baicalensis on human osteoblast-like MG-63 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111656. [Google Scholar] [CrossRef]
- Zheng, L.; Zhuang, Z.; Li, Y.; Shi, T.; Fu, K.; Yan, W.; Zhang, L.; Wang, P.; Li, L.; Jiang, Q. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioact. Mater. 2022, 14, 250–261. [Google Scholar] [CrossRef]
- Weng, Z.; Ye, J.; Cai, C.; Liu, Z.; Liu, Y.; Xu, Y.; Yuan, J.; Zhang, W.; Liu, L.; Jiang, J.; et al. Inflammatory microenvironment regulation and osteogenesis promotion by bone-targeting calcium and magnesium repletion nanoplatform for osteoporosis therapy. J. Nanobiotechnol. 2024, 22, 314. [Google Scholar] [CrossRef]
- Xiang, K.; Xiao, Z.; Jing, Z.; Li, Y.; Li, M.; Su, Z.; Huang, Z.; Wu, T.; He, P.; Zhang, Y.; et al. An Iron Balance Dual-Drive Strategy (IBDS) Promotes Bone Regeneration in Smokers by Regulating Mitochondrial Iron Homeostasis. Adv. Mater. 2025, 37, e2501933. [Google Scholar] [CrossRef]
- Xue, P.; Chang, Z.; Chen, H.; Xi, H.; Tan, X.; He, S.; Qiao, H.; Jiang, X.; Liu, X.; Du, B. Macrophage membrane (MMs) camouflaged near-infrared (NIR) responsive bone defect area targeting nanocarrier delivery system (BTNDS) for rapid repair: Promoting osteogenesis via phototherapy and modulating immunity. J. Nanobiotechnol. 2024, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhao, Y.; Cheng, R.; Liu, Y.; Guo, S.; Sun, L.; Guo, Y.; Hao, F.; Zhao, B. Mussel-inspired immunomodulatory and osteoinductive dual-functional hydroxyapatite nanoplatform for promoting bone regeneration. J. Nanobiotechnol. 2024, 22, 320. [Google Scholar] [CrossRef]
- Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials 2023, 13, 692. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Hu, T.; Shao, D.; Huang, S.; Xie, Y.; Zheng, X. Zn-doped MnO2 nanocoating with enhanced catalase-mimetic activity and cytocompatibility protects pre-osteoblasts against H2O2-induced oxidative stress. Colloids Surf. B Biointerfaces 2021, 202, 111666. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Bai, R.; Wang, Z.; Qin, Y.; Wang, J.; Wei, Y.; Zhao, R.; Nie, G.; Han, B. Colon-targeted engineered postbiotics nanoparticles alleviate osteoporosis through the gut-bone axis. Nat. Commun. 2024, 15, 10893. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lv, X.; Zhao, M.; Zhang, P.; Ren, X.; Mei, X. Encapsulation of green tea polyphenol by pH responsive, antibacterial, alginate microgels used for minimally invasive treatment of bone infection. Colloids Surf. B Biointerfaces 2018, 170, 648–655. [Google Scholar] [CrossRef]
- Rasool, N.; Negi, D.; Singh, Y. Thiol-Functionalized, Antioxidant, and Osteogenic Mesoporous Silica Nanoparticles for Osteoporosis. ACS Biomater. Sci. Eng. 2023, 9, 3535–3545. [Google Scholar] [CrossRef]
- Oh, W.T.; Yang, Y.S.; Xie, J.; Ma, H.; Kim, J.M.; Park, K.H.; Oh, D.S.; Park-Min, K.H.; Greenblatt, M.B.; Gao, G.; et al. WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects. Mol. Ther. 2023, 31, 435–453. [Google Scholar] [CrossRef]
- Lee, S.Y.; An, H.J.; Kim, J.M.; Sung, M.J.; Kim, D.K.; Kim, H.K.; Oh, J.; Jeong, H.Y.; Lee, Y.H.; Yang, T.; et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res. Ther. 2021, 12, 589. [Google Scholar] [CrossRef]
- Arya, P.N.; Saranya, I.; Selvamurugan, N. Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation. World J. Stem Cells 2024, 16, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Jia, T.T.; Feng, Y.; Liu, S.Y.; Zhang, W.J.; Zhang, D.J.; Xu, X. Hyperlipidemia Impairs Osseointegration via the ROS/Wnt/β-Catenin Pathway. J. Dent. Res. 2021, 100, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Hao, Z.; Yao, P.; Bai, J.; Chen, H.; Wu, X.; Zhong, Y.; Xue, D. Synthetic nanoparticles functionalized with cell membrane-mimicking, bone-targeting, and ROS-controlled release agents for osteoporosis treatment. J. Control. Release 2025, 378, 306–319. [Google Scholar] [CrossRef]
- Elghareeb, M.M.; Elshopakey, G.E.; Elkhooly, T.A.; Salama, B.; Samy, A.; Bazer, F.W.; Elmetwally, M.A.; Almutairi, M.H.; Aleya, L.; Abdel-Daim, M.M.; et al. Estradiol and zinc-doped nano hydroxyapatite as therapeutic agents in the prevention of osteoporosis; oxidative stress status, inflammation, bone turnover, bone mineral density, and histological alterations in ovariectomized rats. Front. Physiol. 2022, 13, 989487. [Google Scholar] [CrossRef]
- Peng, J.; Liu, R.; Xu, J.; Yao, Y.; Li, B.; Chen, D.; Chang, Z.; Zhao, R.; Feng, Y.; Hou, R.; et al. Acid-responsive aggregated carrot-derived nanoantioxidants alleviate oxidative stress and restore osteoblast activity. J. Nanobiotechnol. 2025, 23, 206. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.; Guo, J.; Wang, Q.; Cao, J.; Wang, H.; Li, G.; Mao, J.; Zou, X.; Chen, D.; et al. Nano-Hydroxyapatite Modulates Osteoblast Differentiation Through Autophagy Induction via mTOR Signaling Pathway. J. Biomed. Nanotechnol. 2019, 15, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Wang, Z.; Liu, B.; Zhu, S.; Zhu, L.; Peng, B. Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention. Theranostics 2019, 9, 5183–5199. [Google Scholar] [CrossRef]
- Gong, S.; Lang, S.; Wang, Y.; Li, X.; Tian, A.; Ma, J.; Ma, X. pH-Responsive Mesoporous Silica Nanoparticles Loaded with Naringin for Targeted Osteoclast Inhibition and Bone Regeneration. Int. J. Nanomed. 2024, 19, 6337–6358. [Google Scholar] [CrossRef]
- Arnst, J.; Jing, Z.; Cohen, C.; Ha, S.W.; Viggeswarapu, M.; Beck, G.R., Jr. Bioactive silica nanoparticles target autophagy, NF-κB, and MAPK pathways to inhibit osteoclastogenesis. Biomaterials 2023, 301, 122238. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Sobierajska, P.; Roecken, M.; Kornicka-Garbowska, K.; Kępska, M.; Idczak, R.; Nedelec, J.M.; Wiglusz, R.J. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J. Nanobiotechnol. 2020, 18, 33. [Google Scholar] [CrossRef]
- Tao, H.; Ge, G.; Liang, X.; Zhang, W.; Sun, H.; Li, M.; Geng, D. ROS signaling cascades: Dual regulations for osteoclast and osteoblast. Acta Biochim. Biophys. Sin. 2020, 52, 1055–1062. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Mei, J.; Shao, D.; Zhou, F.; Qiao, H.; Liang, Y.; Li, K.; Tang, T. Cerium Oxide Nanoparticles Regulate Osteoclast Differentiation Bidirectionally by Modulating the Cellular Production of Reactive Oxygen Species. Int. J. Nanomed. 2020, 15, 6355–6372. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Q.; Gu, C.; Li, M.; Chen, K.; Chen, P.; Tang, Z.; Liu, X.; Pan, H.; Liu, Z.; et al. Smart Nanosacrificial Layer on the Bone Surface Prevents Osteoporosis through Acid-Base Neutralization Regulated Biocascade Effects. J. Am. Chem. Soc. 2020, 142, 17543–17556. [Google Scholar] [CrossRef]
- Lin, W.; Hu, S.; Li, K.; Shi, Y.; Pan, C.; Xu, Z.; Li, D.; Wang, H.; Li, B.; Chen, H. Breaking Osteoclast-Acid Vicious Cycle to Rescue Osteoporosis via an Acid Responsive Organic Framework-Based Neutralizing and Gene Editing Platform. Small 2024, 20, e2307595. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Ko, W.K.; Kim, S.J.; Han, I.B.; Hong, J.B.; Sheen, S.H.; Sohn, S. Inhibitory Effects of Gold and Silver Nanoparticles on the Differentiation into Osteoclasts In Vitro. Pharmaceutics 2021, 13, 462. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xin, H.; Cai, B.; Wang, L.; Lv, Q.; Hou, Y.; Liu, F.; Dai, T.; Kong, L. RNA interference-based osteoanabolic therapy for osteoporosis by a bone-formation surface targeting delivery system. Biochem. Biophys. Res. Commun. 2022, 601, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Delvin, E. Micro-RNA: A Future Approach to Personalized Diagnosis of Bone Diseases. Calcif. Tissue Int. 2023, 112, 271–287. [Google Scholar] [CrossRef]
- Ko, N.Y.; Chen, L.R.; Chen, K.H. The Role of Micro RNA and Long-Non-Coding RNA in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 4886. [Google Scholar] [CrossRef]
- Yao, J.; Xin, R.; Zhao, C.; Yu, C. MicroRNAs in osteoblast differentiation and fracture healing: From pathogenesis to therapeutic implication. Injury 2024, 55, 111410. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, S.A.; Kang, Y.H.; Hwa, S.M.; Koh, E.B.; Hwang, S.C.; Oh, S.H.; Byun, J.H. Zinc Sulfate Stimulates Osteogenic Phenotypes in Periosteum-Derived Cells and Co-Cultures of Periosteum-Derived Cells and THP-1 Cells. Life 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.T.; Deng, Z.L.; Yu, K.X. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Zhou, X.; Moussa, F.M.; Mankoci, S.; Ustriyana, P.; Zhang, N.; Abdelmagid, S.; Molenda, J.; Murphy, W.L.; Safadi, F.F.; Sahai, N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016, 39, 192–202. [Google Scholar] [CrossRef]
- Ungai-Salánki, R.; Haty, E.; Gerecsei, T.; Francz, B.; Béres, B.; Sztilkovics, M.; Székács, I.; Szabó, B.; Horvath, R. Single-cell adhesion strength and contact density drops in the M phase of cancer cells. Sci. Rep. 2021, 11, 18500. [Google Scholar] [CrossRef]
- Obata, A.; Ogasawara, T.; Kasuga, T. Combinatorial effects of inorganic ions on adhesion and proliferation of osteoblast-like cells. J. Biomed. Mater. Res. A 2019, 107, 1042–1051. [Google Scholar] [CrossRef]
- Jing, C.; Li, B.; Tan, H.; Zhang, C.; Liang, H.; Na, H.; Chen, S.; Liu, C.; Zhao, L. Alendronate-Decorated Nanoparticles as Bone-Targeted Alendronate Carriers for Potential Osteoporosis Treatment. ACS Appl. Bio Mater. 2021, 4, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Zhu, D.; Li, Z.; Wang, Z.; Li, J.; Mei, X.; Xu, W.; Cheng, K.; Zhong, B. Nanoparticles functionalized with stem cell secretome and CXCR4-overexpressing endothelial membrane for targeted osteoporosis therapy. J. Nanobiotechnol. 2022, 20, 35. [Google Scholar] [CrossRef]
- Ma, S.; Xu, S.; Li, M.; Du, Y.; Tian, G.; Deng, J.; Zhang, W.; Wei, P.; Zhao, B.; Zhang, X.; et al. A Bone Targeting Nanoparticle Loaded OGP to Restore Bone Homeostasis for Osteoporosis Therapy. Adv. Healthc. Mater. 2023, 12, e2300560. [Google Scholar] [CrossRef]
- Briffault, E.; Reyes, R.; Garcia-Garcia, P.; Rouco, H.; Diaz-Gomez, L.; Arnau, M.R.; Evora, C.; Diaz-Rodriguez, P.; Delgado, A. SFRP1-Silencing GapmeR-Loaded Lipid-Polymer Hybrid Nanoparticles for Bone Regeneration in Osteoporosis: Effect of Dosing and Targeting Strategy. Int. J. Nanomed. 2024, 19, 12171–12188. [Google Scholar] [CrossRef]
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S.; et al. Osteoblast-Targeting-Peptide Modified Nanoparticle for siRNA/microRNA Delivery. ACS Nano 2016, 10, 5759–5768. [Google Scholar] [CrossRef]
- Chen, Z.H.; Du, D.Y.; Fu, Y.F.; Wu, J.J.; Guo, D.Y.; Li, Y.Y.; Chen, M.N.; Yuan, Z.D.; Zhang, K.W.; Zhang, Z.Y.; et al. Citric acid-modified pH-sensitive bone-targeted delivery of estrogen for the treatment of postmenopausal osteoporosis. Mater. Today Bio 2023, 22, 100747. [Google Scholar] [CrossRef]
- Xia, D.; Qian, Q.; Wang, S.; Dong, X.; Liu, Y. Alendronate Functionalized Bone-Targeting Pomolic Acid Liposomes Restore Bone Homeostasis for Osteoporosis Treatment. Int. J. Nanomed. 2024, 19, 7983–7996. [Google Scholar] [CrossRef]
- Salave, S.; Shinde, S.D.; Rana, D.; Sahu, B.; Kumar, H.; Patel, R.; Benival, D.; Kommineni, N. Peptide Engraftment on PEGylated Nanoliposomes for Bone Specific Delivery of PTH (1-34) in Osteoporosis. Pharmaceutics 2023, 15, 608. [Google Scholar] [CrossRef]
- Xing, X.; Tang, Q.; Zou, J.; Huang, H.; Yang, J.; Gao, X.; Xu, X.; Ma, S.; Li, M.; Liang, C.; et al. Bone-targeted delivery of senolytics to eliminate senescent cells increases bone formation in senile osteoporosis. Acta Biomater. 2023, 157, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, H.; Wang, Q.; Zhai, F.; Wang, J.; Huang, C.; Cui, L. Studies of a novel bone-targeted nano drug delivery system with a HAP core-PSI coating structure for tanshinol injection. J. Drug Target. 2023, 31, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Disha, C.; Vasireddi, R.; Razdan, R.; Mahapatra, D.R. Risedronate/zinc-hydroxyapatite based nanomedicine for osteoporosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Li, J.; He, J.; Luo, F.; Yu, T.; Dai, Q.; Chen, Y.; Xu, J.; Yang, X.; Dong, S. Bone-targeted pH-responsive cerium nanoparticles for anabolic therapy in osteoporosis. Bioact. Mater. 2021, 6, 4697–4706. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, K.K.; Park, K.W.; Kim, M.; Lee, C.S. Genetically Modified Ferritin Nanoparticles with Bone-Targeting Peptides for Bone Imaging. Int. J. Mol. Sci. 2021, 22, 4854. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Singh, G.; Barnwal, R.P.; Sharma, T.; Singh, B. QbD-driven development and characterization of superparamagnetic iron oxide nanoparticles (SPIONS) of a bone-targeting peptide for early detection of osteoporosis. Int. J. Pharm. 2024, 654, 123936. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.J.; Shi, J.; Lan, H.; Guo, Y.; Fu, D. Ångstrom-scale gold particles loaded with alendronate via alpha-lipoic acid alleviate bone loss in osteoporotic mice. J. Nanobiotechnol. 2024, 22, 212. [Google Scholar] [CrossRef]
- Lee, C.S.; Fan, J.; Hwang, H.S.; Kim, S.; Chen, C.; Kang, M.; Aghaloo, T.; James, A.W.; Lee, M. Bone-Targeting Exosome Mimetics Engineered by Bioorthogonal Surface Functionalization for Bone Tissue Engineering. Nano Lett. 2023, 23, 1202–1210. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, H.; Hu, A.; Liu, R.; Zeng, X.; Wang, H. Nanoparticles Targeting Delivery Antagomir-483-5p to Bone Marrow Mesenchymal Stem Cells Treat Osteoporosis by Increasing Bone Formation. Curr. Stem Cell Res. Ther. 2023, 18, 115–126. [Google Scholar] [CrossRef]
- Cao, S.; Li, Y.; Shen, L.; Shao, B.; Yu, L.; Li, J.; Yuan, Q. Functionalized Virus Nanoparticles Alleviates Osteoporosis via Targeting the Function of RANK-Specific Motifs. ACS Appl. Mater. Interfaces 2023, 15, 32272–32280. [Google Scholar] [CrossRef]
- Cheng, C.; Xing, Z.; Hu, Q.; Kong, N.; Liao, C.; Xu, S.; Zhang, J.; Kang, F.; Zhu, X. A bone-targeting near-infrared luminescence nanocarrier facilitates alpha-ketoglutarate efficacy enhancement for osteoporosis therapy. Acta Biomater. 2024, 173, 442–456. [Google Scholar] [CrossRef]
- Pallardy, M.J.; Turbica, I.; Biola-Vidamment, A. Why the Immune System Should Be Concerned by Nanomaterials? Front. Immunol. 2017, 8, 544. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, E.; Tukaj, C.; Radomski, M.W.; Inkielewicz-Stepniak, I. Molecular Mechanism of Silver Nanoparticles-Induced Human Osteoblast Cell Death: Protective Effect of Inducible Nitric Oxide Synthase Inhibitor. PLoS ONE 2016, 11, e0164137. [Google Scholar] [CrossRef]
- Müller, L.K.; Simon, J.; Rosenauer, C.; Mailänder, V.; Morsbach, S.; Landfester, K. The Transferability from Animal Models to Humans: Challenges Regarding Aggregation and Protein Corona Formation of Nanoparticles. Biomacromolecules 2018, 19, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Mangla, B.; Kumar, P.; Javed, S.; Pathan, T.; Ahsan, W.; Aggarwal, G. Regulating nanomedicines: Challenges, opportunities, and the path forward. Nanomedicine 2025, 20, 1911–1927. [Google Scholar] [CrossRef] [PubMed]
- Metselaar, J.M.; Lammers, T. Challenges in nanomedicine clinical translation. Drug Deliv. Transl. Res. 2020, 10, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, B.; Tang, Y.; Shen, A.; Lin, Y.; Zhao, X.; Li, J.; Monteiro, M.J.; Gu, W. Bone targeted nano-drug and nano-delivery. Bone Res. 2024, 12, 51. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, J.; Fu, Y.; Pan, H.; He, H.; Gan, Q.; Liu, C. Smart Biomaterials for Articular Cartilage Repair and Regeneration. Adv. Funct. Mater. 2023, 33, 2212561. [Google Scholar] [CrossRef]
- Lu, S.; Cao, J.; Song, Z.; Gong, F.; Yang, P.; Ge, J.; He, Y.; Han, Z.; Hou, G.; Zhang, Z.; et al. Pyroptosis-responsive microspheres modulate the inflammatory microenvironment to retard osteoporosis in female mice. Nat. Commun. 2025, 16, 8127. [Google Scholar] [CrossRef] [PubMed]

| Electronic Database | Search and Terms |
|---|---|
| Web of Science PubMed | #1((Osteoblast) OR (Osteoclast)) OR (Osteoporosis) #2 (nanoparticles) AND (bone) |
| #3 ((((((((Bisphosphonates) OR (Alendronic)) OR (Zoledronic)) OR (Teriparatide)) OR (Osteoporosis)) OR (Anabolic Agents)) OR (Anti-Resorptive Agents)) OR (Abaloparatide)) AND (Osteoporosis) #4((((targeted) OR (targeting)) OR (responsive)) AND (((Nanoparticles) OR (Nanometer particle)) OR (Nano formulation))) AND (bone) |
| Drug Name | Drug Category | Mechanism of Action | Effect and Function | Site of Action a | Effect Size | Reference |
|---|---|---|---|---|---|---|
| Bisphosphonates | bisphosphonates | Anti-Resorptive | Increase bone density | LS | 6.8%↑ | [40] |
| Alendronate | Bisphosphonates | Anti-Resorptive | Increase bone density | LS, TH, TR | 3.7%↑, 2.1%↑, 1.7↑% | [41] |
| Alendronate | Bisphosphonates | Anti-Resorptive | Increase bone density | LS, TH, FN | 5.2%↑, 2.3%↑, 2.5%↑ | [42] |
| Zoledronic acid | Bisphosphonates | Anti-Resorptive | Increase bone density, etc. | LS, TH, FN | 6.1%↑, 3.1%↑, 3.9%↑ | [43] |
| Zoledronic acid | Bisphosphonates | Anti-Resorptive | Increase bone density, etc. | LS, TH | 7.1%↑, 4.4%↑ | [44] |
| Zoledronic acid | Bisphosphonates | Anti-Resorptive | Increase bone density, etc. | LS, TH, FN | 6.1%↑, 3.8%↑, 3.1%↑ | [42] |
| Denosumab | RANKL inhibitor | Anti-Resorptive | Increase bone density, etc. | LS | 7.7%↑ | [45] |
| Denosumab | RANKL inhibitor | Anti-Resorptive | Increase bone density, etc. | LS | 7.2%↑ | [46] |
| Denosumab | RANKL inhibitor | Anti-Resorptive | Increase bone density, etc. | LS, TH | 9.7%↑, 5.1%↑ | [44] |
| Denosumab | RANKL inhibitor | Anti-Resorptive | Increase bone density, etc. | LS, TH, FN | 7.3%↑, 3.6%↑, 3.2%↑ | [47] |
| Denosumab | RANKL inhibitor | Anti-Resorptive | Increase bone density | LS, TH, FN | 5.8%↑, 2.3%↑, 2.1%↑ | [42] |
| Teriparatide | PTH | Anabolic Agents | Increase bone density, etc. | LS | 12.0%↑ | [40] |
| Teriparatide | PTH | Anabolic Agents | Reduce the risk of fractures | HF | 56.0%↓ | [48] |
| Teriparatide | PTH | Anabolic Agents | Reduce the risk of fractures | VF | 80.0%↓ | [49] |
| Teriparatide | PTH | Anabolic Agents | Increase bone density | LS | 5.5%↑ | [50] |
| Teriparatide | PTH | Anabolic Agents | Reduce the risk of fractures | NVF | 53.0%↓(T2D) 43.0%↓(non-T2D) | [51] |
| Teriparatide | PTH | Anabolic Agents | Increase bone density | LS, FN | 8.2%↑, 1.3%↑ | [42] |
| Abaloparatide | PTH | Anabolic Agents | Reduce the risk of fractures | VF, NVF | 86.0%↓, 43.0%↓ | [49] |
| Abaloparatide | PTH | Anabolic Agents | Increase bone density | LS, TH, FN | 11.3%↑, 3.9%↑,4.0%↑ | [42] |
| Romosozumab | Monoclonal antibody | Dual effect | Increase bone density | LS, TH, FN | 12.1%↑, 2.5%↑, 2.2%↑ | [42] |
| Carrier Material | Targeted Ligand | Other Substance | Target Type | Targeted Verification Method | Effect and Function | Reference |
|---|---|---|---|---|---|---|
| PLGA, CS, CD | Alendronate | - | Active targeting | HAP affinity | Continuously release drugs and precisely target the bone matrix | [159] |
| PLGA-TK-PEG, BMSCM | DSPE-PEG-ALN | SS-31 | Active targeting | Biological distribution | Antioxidant stress | [135] |
| PLGA | Tetracycline | Astragaloside A Icariin Notoginsenoside R1 | Active targeting | HAP affinity, biological distribution | Osteoblast proliferation and differentiation | [76] |
| PEG-PLGA | (Aspartic acid)3 | Simvastatin | Active targeting | Biological distribution | Osteoblast proliferation and differentiation | [113] |
| PLGA | HMEC membranes overexpressing CXCR4 | Sec | Active targeting | Biological distribution | Proliferation and differentiation of osteoblasts, inhibition of osteoclasts | [160] |
| PLGA | Alendronate | Osteogenic peptide | Active targeting | Biological distribution, HAP affinity | Osteoblast proliferation and differentiation | [161] |
| Lipids, PLGA | APT, Alendronate | SFRP1 silent GapmeR | Active targeting | - | Osteoblast proliferation and differentiation | [162] |
| Chitosan | Asp-8 | Cyclic peptide J | Active targeting | Biological distribution, HAP affinity | Osteoblast proliferation and differentiation | [80] |
| Polymer micelles | Alendronate | Icaritin | Active targeting | Biological distribution, HAP affinity | Osteoblast proliferation and differentiation | [105] |
| Polyurethane (PU) nano-micelles | Bone-targeted peptide (SDSSD) | SiRNA/miRNA | Active targeting | Biological distribution | RNAi therapy | [163] |
| Polymer micelles | Citric acid | Estrogen | Active targeting | Biological distribution, HAP affinity | Inhibit osteoclasts | [164] |
| Liposome | Adapter | SiRNA | Active targeting | Biological distribution | Osteoblast proliferation and differentiation | [87] |
| Liposome | Alendronate | Pomolic acid | Active targeting | Biological distribution | Inhibit osteoclasts | [165] |
| Liposome | Bone-targeted peptide (SDSSD) | PTH (1–34) | Active targeting | HAP affinity | Targeted drug delivery | [166] |
| Liposome | Asp-8 | PTX | Active targeting | Biological distribution, HAP affinity | Targeted drug delivery | [109] |
| Liposome | Bone affinity peptide (DSS)6 | Quercetin | Active targeting | Biological distribution | Eliminate senescent cells, etc. | [167] |
| Hydroxyapatite | Alendronate sodium | Tanshinol | Active targeting | Biological distribution | Targeted drug delivery | [168] |
| Zinc hydroxyapatite | Risedronate | - | Active targeting | - | Provide trace elements | [169] |
| Hydroxyapatite | - | salmon calcitonin | Passive targeting | - | Targeted drug delivery | [98] |
| Cerium oxide | Alendronate sodium | - | Active targeting | - | Inhibition of osteoclasts | [170] |
| Ferritin | Asp-6 | - | Active targeting | Biological distribution, HAP affinity | Bone imaging | [171] |
| ZIF-8, PVP | Zoledronate | DOX, BSA, SiRNA | Active targeting | Biological distribution | Targeted drug delivery | [88] |
| Paramagnetic iron oxide | (-D-Asp-)8 | - | Active targeting | Biological distribution | Diagnosis of osteoporosis | [172] |
| Gold | Alendronate | - | Active targeting | Biological distribution | Osteoblast proliferation and differentiation | [173] |
| CM-NH2-PAA | Alendronate sodium | - | Active targeting | Biological distribution, HAP affinity | Deliver trace elements and resist oxidative stress | [122] |
| Mesoporous silica | Alendronate | SiRNA, Osteostatin | Active targeting | HAP affinity | Proliferation and differentiation of osteoblasts, inhibition of osteoclasts | [100] |
| Exosome mimetics | Alendronate | - | Active targeting | Biological distribution, HAP affinity | Cell adhesion, etc. | [174] |
| mEVs | (AspSerSer, DSS)6 | SRT2104 | Active targeting | Biological distribution | Proliferation and differentiation of osteoblasts, inhibition of osteoclasts | [111] |
| mEVs | (AspSerSer, DSS)6 | Antagomir-483-5p | Active targeting | - | Promote osteogenic differentiation, etc. | [175] |
| CCMV | Peptide RM | - | Active targeting | Inhibition of osteoclasts | [176] | |
| mSiO2, β-NaYF4 | EDTA | α-ketoglutarate | Active targeting | Biological distribution | Proliferation and differentiation of osteoblasts, inhibition of osteoclasts | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Xu, Y.; Zhou, S.; Liu, J.; Zhang, M.; Zhang, B.; Chen, H. Updated Advances on Drugs and Bone-Targeting Nanoparticles for Osteoporosis Therapy: Carrier Materials, Modification, Function Mechanism, and Applications—A Systematic Review. Pharmaceuticals 2025, 18, 1809. https://doi.org/10.3390/ph18121809
Lin Y, Xu Y, Zhou S, Liu J, Zhang M, Zhang B, Chen H. Updated Advances on Drugs and Bone-Targeting Nanoparticles for Osteoporosis Therapy: Carrier Materials, Modification, Function Mechanism, and Applications—A Systematic Review. Pharmaceuticals. 2025; 18(12):1809. https://doi.org/10.3390/ph18121809
Chicago/Turabian StyleLin, Yehao, Yidong Xu, Siyue Zhou, Junyu Liu, Min Zhang, Baoxin Zhang, and Haixia Chen. 2025. "Updated Advances on Drugs and Bone-Targeting Nanoparticles for Osteoporosis Therapy: Carrier Materials, Modification, Function Mechanism, and Applications—A Systematic Review" Pharmaceuticals 18, no. 12: 1809. https://doi.org/10.3390/ph18121809
APA StyleLin, Y., Xu, Y., Zhou, S., Liu, J., Zhang, M., Zhang, B., & Chen, H. (2025). Updated Advances on Drugs and Bone-Targeting Nanoparticles for Osteoporosis Therapy: Carrier Materials, Modification, Function Mechanism, and Applications—A Systematic Review. Pharmaceuticals, 18(12), 1809. https://doi.org/10.3390/ph18121809








