Advancing Photodynamic Cancer Therapy with Smart Light-Responsive Lipid and Polymeric Nanocarriers: Evidence from a Meta-Analysis of Efficacy and Pharmacokinetics
Abstract
1. Introduction
2. Methods
2.1. Data Mining
2.2. Inclusion Data and Criteria
2.3. Meta Analysis
3. Results
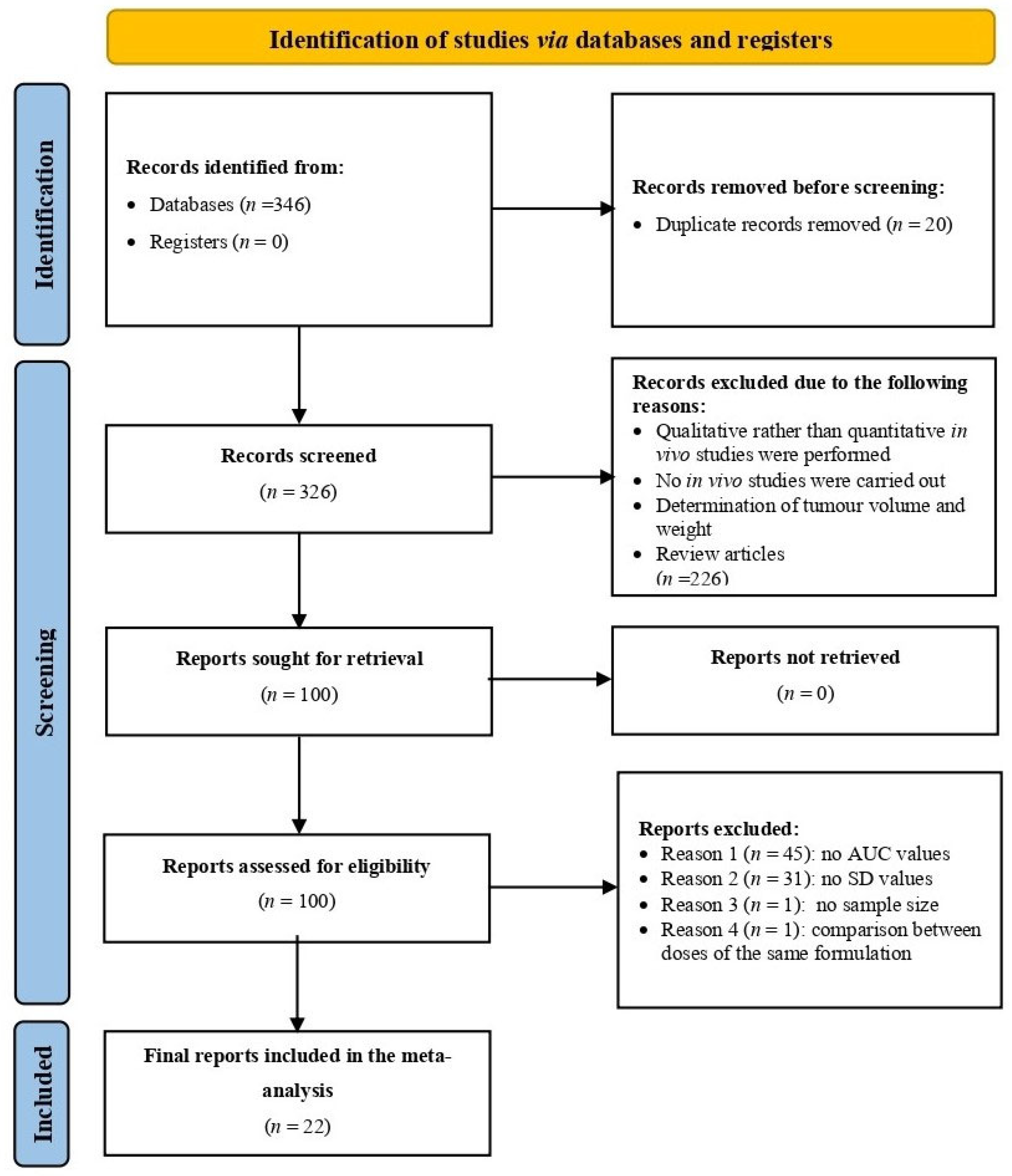
3.1. Overview of Included Studies
3.2. Pooled Effect Size
3.3. Study Weights
3.4. Heterogeneity of Effects
3.5. Sensitivity Analysis
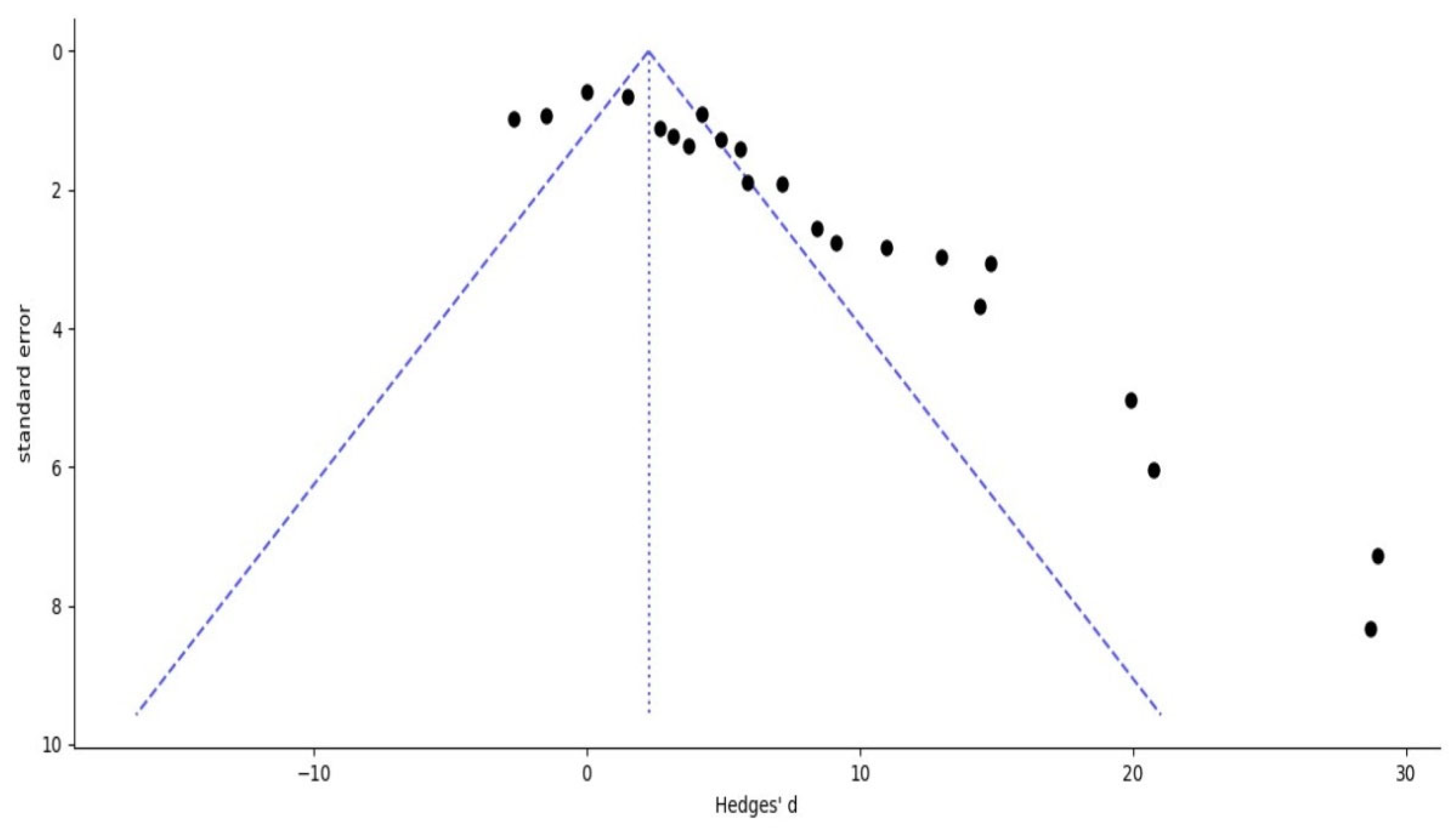
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| •OH | Hydroxyl radicals |
| 1O2 | Singlet oxygen |
| AUC | Area under the curve |
| CI | Confidence interval |
| DL | DerSimonian–Laird |
| EMA | European Medicines Agency |
| EPR | Enhanced permeability and retention |
| GRAS | Generally Recognized as Safe |
| LP-NCs | Lipid and polymeric nanocarriers |
| NIR | Near Infrared |
| O2•− | Superoxide anions |
| PDT | Photodynamic therapy |
| PS | Photosensitizer |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RES | Reticuloendothelial system |
| RevMan | Review Manager |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SE | Standard error |
| SMD | Standardized mean difference |
| US FDA | US Food and Drug Administration |
| UV | Ultraviolet |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Chen, J.; Hu, S.; Sun, M.; Shi, J.; Zhang, H.; Yu, H.; Yang, Z. Recent Advances and Clinical Translation of Liposomal Delivery Systems in Cancer Therapy. Eur. J. Pharm. Sci. 2024, 193, 106688. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, M.B.; Kuderer, N.M.; Desai, A.; Bergerot, C.; Lyman, G.H. Mitigating Long-Term and Delayed Adverse Events Associated with Cancer Treatment: Implications for Survivorship. Nat. Rev. Clin. Oncol. 2023, 20, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Kintzel, P.E.; Chase, S.L.; Schultz, L.M.; O’Rourke, T.J. Increased Risk of Metabolic Syndrome, Diabetes Mellitus, and Cardiovascular Disease in Men Receiving Androgen Deprivation Therapy for Prostate Cancer. Pharmacotherapy 2008, 28, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Saraswathy, M.; Gong, S. Different Strategies to Overcome Multidrug Resistance in Cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Kalscheuer, S.M.; Panyam, J. Exploiting Nanotechnology to Overcome Tumor Drug Resistance: Challenges and Opportunities. Adv. Drug Deliv. Rev. 2013, 65, 1731–1747. [Google Scholar] [CrossRef]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Essiet Imeh, A.; Li, W. Polymeric Nanoparticles—Promising Carriers for Cancer Therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef]
- Amrahli, M.; Centelles, M.; Cressey, P.; Prusevicius, M.; Gedroyc, W.; Xu, X.Y.; So, P.-W.; Wright, M.; Thanou, M. MR-Labelled Liposomes and Focused Ultrasound for Spatiotemporally Controlled Drug Release in Triple Negative Breast Cancers in Mice. Nanotheranostics 2021, 5, 125–142. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, N.A.; Pandya, S.K.; Kirodian, G.B.; Sanath, S. Liposomal Drug Delivery System from Laboratory to Clinic. J. Postgrad. Med. 2005, 51 (Suppl. 1), S5–S15. [Google Scholar] [PubMed]
- Bozzuto, G.; Molinari, A. Nanoparticles as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological Status of Nanoparticles: What We Know and What We Don’t Know. Chem. Biol. Interact. 2018, 295, 1–12. [Google Scholar] [CrossRef]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An Overview of Liposome Lyophilization and Its Future Potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yeh, M.-K. Clinical Development of Liposome-Based Drugs: Formulation, Characterization, and Therapeutic Efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- England, C.G.; Ng, C.F.; van Berkel, V.; Frieboes, H.B. A Review of Pharmacological Treatment Options for Lung Cancer: Emphasis on Novel Nanotherapeutics and Associated Toxicity. Curr. Drug Targets 2015, 16, 1057–1087. [Google Scholar] [CrossRef]
- Liu, D.; Qiao, S.; Cheng, B.; Li, D.; Chen, J.; Wu, Q.; Pan, H.; Pan, W. Enhanced Oral Delivery of Curcumin via Vitamin E TPGS Modified Nanodiamonds: A Comparative Study on the Efficacy of Non-Covalent and Covalent Conjugated Strategies. AAPS PharmSciTech 2020, 21, 187. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of Tumor Lethality of ROS in Photodynamic Therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring Photosensitive ROS for Advanced Photodynamic Therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, C.; Jin, B.; Sun, T.; Sun, K.; Wang, S.; Fan, Z. Advances in Smart Nanotechnology-Supported Photodynamic Therapy for Cancer. Cell Death Discov. 2024, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, M.; Chen, Y.; Pan, W.; Zhou, P.; Wan, X.; Li, N.; Tang, B. A COF-Based Nanoplatform for Highly Efficient Cancer Diagnosis, Photodynamic Therapy and Prognosis. Chem. Sci. 2020, 11, 6882–6888. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, P.; Jiang, D.; Yang, G.; Xue, Y.; Tang, Z.; Zhang, M.; Wang, H.; Jiang, X.; Wu, Y.; et al. In Situ Catalytic Reaction for Solving the Aggregation of Hydrophobic Photosensitizers in Tumor. ACS Appl. Mater. Interfaces 2020, 12, 5624–5632. [Google Scholar] [CrossRef]
- Li, J.; Wang, A.; Zhao, L.; Dong, Q.; Wang, M.; Xu, H.; Yan, X.; Bai, S. Self-Assembly of Monomeric Hydrophobic Photosensitizers with Short Peptides Forming Photodynamic Nanoparticles with Real-Time Tracking Property and without the Need of Release in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 28420–28427. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Lin, C.; Ortega, J.; Lovell, J.F. Porphyrin-Phospholipid Liposomes with Tunable Leakiness. J. Control. Release 2015, 220, 484–494. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Yang, Y.; Zhang, H.; Mei, X. Photo-Responsive and NGR-Mediated Multifunctional Nanostructured Lipid Carrier for Tumor-Specific Therapy. J. Pharm. Sci. 2015, 104, 1328–1339. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Deng, Y.; Sun, H.; Ke, X.; Ci, T. Near-Infrared Light Triggered Drug Delivery System for Higher Efficacy of Combined Chemo-Photothermal Treatment. Acta Biomater. 2017, 51, 374–392. [Google Scholar] [CrossRef]
- Li, H.-F.; Wu, C.; Xia, M.; Zhao, H.; Zhao, M.-X.; Hou, J.; Li, R.; Wei, L.; Zhang, L. Targeted and Controlled Drug Delivery Using a Temperature and Ultra-Violet Responsive Liposome with Excellent Breast Cancer Suppressing Ability. RSC Adv. 2015, 5, 27630–27639. [Google Scholar] [CrossRef]
- Tong, Q.; Xu, J.; Wu, A.; Zhang, C.; Yang, A.; Zhang, S.; Lin, H.; Lu, W. Pheophorbide A-Mediated Photodynamic Therapy Potentiates Checkpoint Blockade Therapy of Tumor with Low PD-L1 Expression. Pharmaceutics 2022, 14, 2513. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, B.; Li, J.; Wang, Z.; Xu, Z.; Wang, Y.; Zhou, T.; Huang, R.; Ye, J.; Zhang, H.; et al. Transforming Enemy into Friend” Strategy-Based Stimuli Responsive Dual-Drug Liposomes for Synergistic Chemo-Photodynamic Therapy. Chem. Eng. J. 2024, 487, 150526. [Google Scholar] [CrossRef]
- Ren, G.; Li, Y.; Ping, C.; Duan, D.; Li, N.; Tang, J.; Wang, R.; Guo, W.; Niu, X.; Ji, Q.; et al. Docetaxel Prodrug and Hematoporphyrin Co-Assembled Nanoparticles for Anti-Tumor Combination of Chemotherapy and Photodynamic Therapy. Drug Deliv. 2022, 29, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, J.H.; Nguyen Cao, T.G.; Kang, S.J.; Jeong, K.; Kang, H.C.; Kwon, Y.J.; Rhee, W.J.; Ko, Y.T.; Shim, M.S. Extracellular Vesicles with High Dual Drug Loading for Safe and Efficient Combination Chemo-Phototherapy. Biomater. Sci. 2022, 10, 2817–2830. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, Q.; Zhang, J.; Zhang, P.; Zhou, X.; Yalamarty, S.S.K.; Liang, X.; Liu, Y.; Zheng, Q.; Gao, J. Self-Assembled Dual-Targeted Epirubicin-Hybrid Polydopamine Nanoparticles for Combined Chemo-Photothermal Therapy of Triple-Negative Breast Cancer. Int. J. Nanomed. 2020, 15, 6791–6811. [Google Scholar] [CrossRef]
- Wan, J.; Ren, L.; Li, X.; He, S.; Fu, Y.; Xu, P.; Meng, F.; Xian, S.; Pu, K.; Wang, H. Photoactivatable Nanoagonists Chemically Programmed for Pharmacokinetic Tuning and in Situ Cancer Vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2210385120. [Google Scholar] [CrossRef]
- Wang, F.; Li, N.; Wang, W.; Ma, L.; Sun, Y.; Wang, H.; Zhan, J.; Yu, D. A Multifunctional, Highly Biocompatible, and Double-Triggering Caramelized Nanotheranostic System Loaded with Fe3O4 and DOX for Combined Chemo-Photothermal Therapy and Real-Time Magnetic Resonance Imaging Monitoring of Triple Negative Breast Cancer. Int. J. Nanomed. 2023, 18, 881–897. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.; Ji, Q.; Zhang, H.; Zhou, M.; Wang, Y.; Zhang, S.; Sun, J.; He, Z.; Luo, C. Modular Prodrug-Engineered Oxygen Nano-Tank with Outstanding Nanoassembly Performance, High Oxygen Loading, and Closed-Loop Tumor Hypoxia Relief. Adv. Sci. 2024, 11, e2405583. [Google Scholar] [CrossRef]
- Bai, X.; Lin, Y.; Gong, L.; Duan, J.; Sun, X.; Wang, C.; Liu, Z.; Jiang, J.; Zhou, X.; Zhou, M.; et al. Nanoparticles That Target the Mitochondria of Tumor Cells to Restore Oxygen Supply for Photodynamic Therapy: Design and Preclinical Validation against Breast Cancer. J. Control. Release 2023, 362, 356–370. [Google Scholar] [CrossRef]
- Chen, L.; Xu, R.; Ding, Y.; Wang, C.; Zhang, S.; Sun, Z.; Chen, Y.; Mi, Y.; Gao, M.; Ma, X.; et al. Intelligent Triggering of Nanomicelles Based on a ROS-Activated Anticancer Prodrug and Photodynamic Therapy (PDT)-Synergistic Therapy for Lung Cancers. Eur. J. Med. Chem. 2022, 241, 114622. [Google Scholar] [CrossRef]
- Du, J.; Liu, X.; Hou, Z.; Liu, X.; Yao, J.; Cheng, X.; Wang, X.; Tang, R. Acid-Sensitive Polymeric Prodrug Micelles for Achieving Enhanced Chemo-Photodynamic Therapy. J. Drug Deliv. Sci. Technol. 2022, 74, 103514. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Bian, J.; Zhang, X.; Fu, Y.; Li, Z.; Wei, S.; Xu, Z.; Liu, X.; Liu, Z.; et al. Ag/Pd Bimetal Nanozyme with Enhanced Catalytic and Photothermal Effects for ROS/Hyperthermia/Chemotherapy Triple-Modality Antitumor Therapy. Chem. Eng. J. 2020, 397, 125438. [Google Scholar] [CrossRef]
- Qu, H.; Li, L.; Chen, H.; Tang, M.; Cheng, W.; Lin, T.-Y.; Li, L.; Li, B.; Xue, X. Drug-Drug Conjugates Self-Assembled Nanomedicines Triggered Photo-/Immuno- Therapy for Synergistic Cancer Treatments. J. Control. Release 2023, 363, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Soman, S.; Kulkarni, S.; John, J.; Vineeth, P.; Ahmad, S.F.; George, S.D.; Nandakumar, K.; Mutalik, S. Transferrin-Conjugated UiO-66 Metal Organic Frameworks Loaded with Doxorubicin and Indocyanine Green: A Multimodal Nanoplatform for Chemo-Photothermal-Photodynamic Approach in Cancer Management. Int. J. Pharm. 2024, 665, 124665. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, Y.; Yan, H.; Li, D.; Zhao, Z.; Chen, X.; Yang, X.; Liu, X. Oxidation-Sensitive Polymeric Nanocarrier-Mediated Cascade PDT Chemotherapy for Synergistic Cancer Therapy and Potentiated Checkpoint Blockade Immunotherapy. Chem. Eng. J. 2021, 404, 126481. [Google Scholar] [CrossRef]
- Ya, Z.; Guo, S.; Li, Y.; Zhu, M.; Zhang, L.; Zong, Y.; Wan, M. Focused Acoustic Vortex-Mediated Sonochemotherapy for the Amplification of Immunogenic Cell Death Combined with Checkpoint Blockade to Potentiate Cancer Immunotherapy. Biomaterials 2023, 301, 122278. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, T.; Qiu, W.; Liang, M.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. Bioresponsive Prodrug Nanogel-Based Polycondensate Strategy Deepens Tumor Penetration and Potentiates Oxidative Stress. Chem. Eng. J. 2021, 420, 127657. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Xin, X.; Huo, P.; Zhang, Y.; Chen, H.; Feng, N.; Feng, Q.; Zhang, Z. Dual-Modal Imaging-Guided Theranostic Nanocarriers Based on 2-Methoxyestradiol and Indocyanine Green. Int. J. Pharm. 2021, 592, 120098. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M. Chapter 23: Including Variants on Randomized Trials. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1; The Cochrane Collaboration: Chichester, UK, 2020. [Google Scholar]
- Hathout, R.M. Do Polymeric Nanoparticles Really Enhance the Bioavailability of Oral Drugs? A Quantitative Answer Using Meta-Analysis. Gels 2022, 8, 119. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules 2024, 29, 636. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
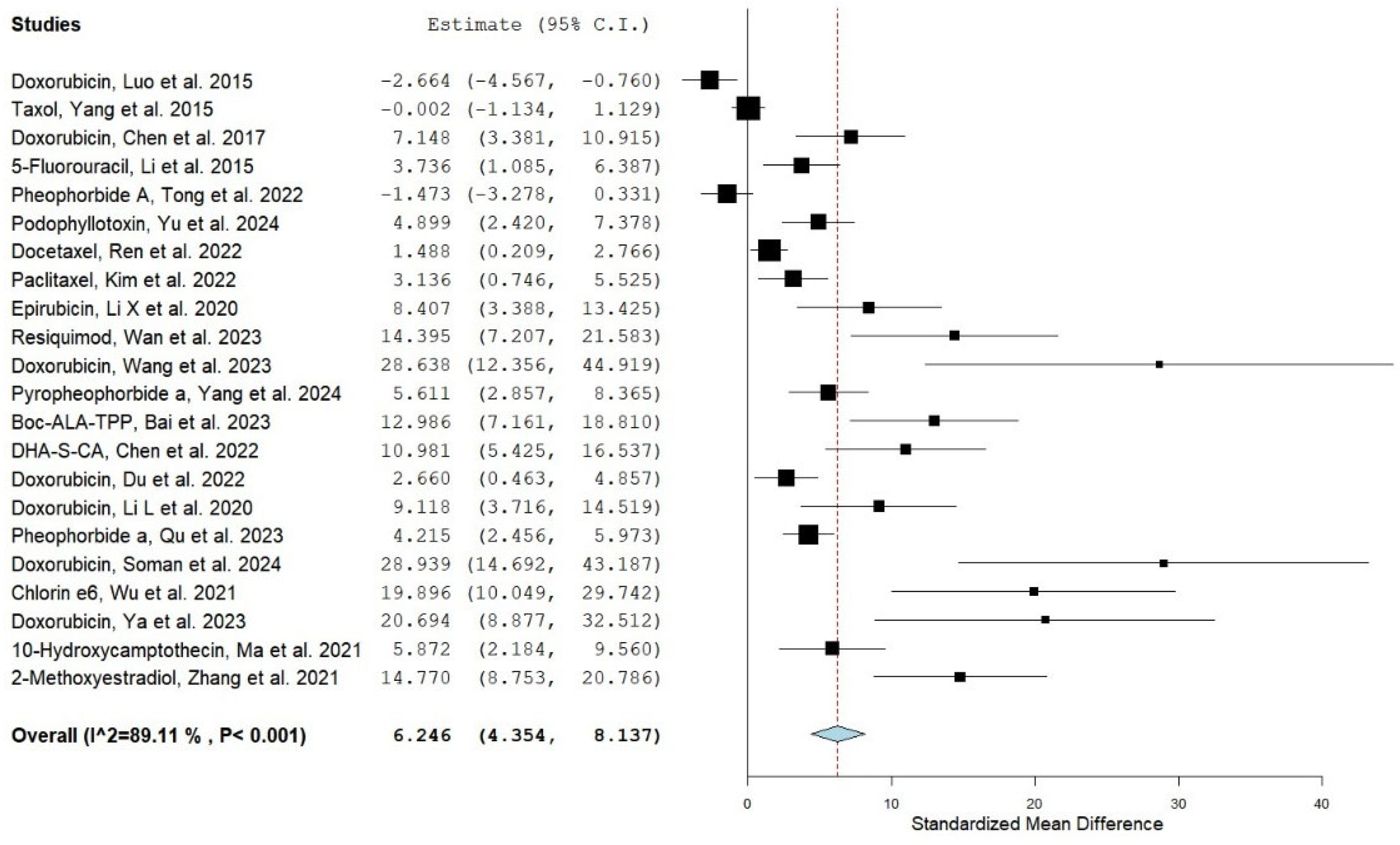

| No. | Drug/Study/Reference | Year | Group A Nanocarrier Drug Mean AUC (µg·h/mL) | Group B Conventional Drug Mean AUC (µg·h/mL) | Group A AUC-SD | Group B AUC-SD | Group A No. of Animals | Group B No. of Animals | SMD | UPPER CI | Lower CI | Type of Nanocarrier System |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Doxorubicin/ Luo et al., 2015 [26] | 2015 | 415 | 1075 | 57 | 299 | 4 | 4 | −2.664 | −4.567 | −0.76 | HPPH-Liposomes |
| 2 | Taxol/ Yang et al., 2015 [27] | 2015 | 44.087 | 44.105 | 7.104 | 7.388 | 6 | 6 | −0.002 | −1.134 | 1.129 | psCPP/NGR-NLC |
| 3 | Doxorubicin/ Chen et al., 2017 [28] | 2017 | 101.16 | 7.43 | 16.09 | 0.76 | 4 | 4 | 7.148 | 3.381 | 10.915 | PCH-DI |
| 4 | 5-Fuorouracil (ADR)/ Li et al., 2015 [29] | 2015 | 787.12 | 455.17 | 94.01 | 34.85 | 3 | 3 | 3.736 | 1.085 | 6.387 | Irradiated Liposomes |
| 5 * | Pheophorbide A (PA)/ Tong et al., 2022 [30] | 2022 | 241.24 * | 257.9 * | 11.47 | 5.59 | 3 | 3 | −1.473 | −3.278 | 0.331 | cRGD–PaNPs–IgG |
| 6 ** | Podophyllotoxin (PPT)/ Yu et al., 2024 [31] | 2024 | 20.99 ** | 5.25 ** | 3.91 | 1.24 | 5 | 5 | 4.899 | 2.42 | 7.378 | PPT LPs |
| 7 | Docetaxel (DTX/ Ren et al., 2022 [32] | 2022 | 0.00901694 | 0.00472472 | 0.00358859 | 0.00113927 | 6 | 6 | 1.488 | 0.209 | 2.766 | DSD/HP NPs |
| 8 * | Paclitaxel (PTX)/ Kim et al., 2022 [33] | 2022 | 6.01 * | 0.77 * | 1.86 | 0.31 | 3 | 3 | 3.136 | 0.746 | 5.525 | EV(ICG/PTX) |
| 9 | Epirubicin (EPI)/ Li X et al., 2020 [34] | 2020 | 3.7713 | 0.7505 | 0.3569 | 0.1924 | 3 | 3 | 8.407 | 3.388 | 13.425 | E/PCF-NPs |
| 10 | Resiquimod (RESQ)/ Wan et al., 2023 [35] | 2023 | 0.00002079 | 0.00000049 | 0.00000173 | 0.00000009 | 4 | 4 | 14.395 | 7.207 | 21.583 | PCL8-TK-NA |
| 11 | Doxorubicin/ Wang et al., 2023 [36] | 2023 | 3.30021 | 1.42947 | 0.0566 | 0.04722 | 3 | 3 | 28.638 | 12.356 | 44.919 | Fe3O4/DOX@CNSs |
| 12 *** | pyropheophorbide a (PPa)/ Yang et al., 2024 [37] | 2024 | 13.007 *** | 6.201 *** | 1.324 | 0.803 | 5 | 5 | 5.611 | 2.857 | 8.365 | HSSPAO Nas |
| 13 | BAT/ Bai et al., 2023 [38] | 2023 | 1331.684 | 703.43 | 58.034 | 21.135 | 5 | 5 | 12.986 | 7.161 | 18.81 | BAT-NPs |
| 14 | (DHA-S-CA)/ Chen et al., 2022 [39] | 2022 | 22.90669 | 10.47263 | 1.22544 | 0.65818 | 4 | 4 | 10.981 | 5.425 | 16.537 | IR808/DHA-S-CA NMs |
| 15 | Doxorubicin/ Du et al., 2022 [40] | 2022 | 61.625 | 26.485 | 12.34 | 8.36 | 3 | 3 | 2.66 | 0.463 | 4.857 | Hemin/Dox-M |
| 16 | Doxorubicin/ Li L et al., 2020 [41] | 2020 | 74.54 | 25.55 | 5.532 | 2.481 | 3 | 3 | 9.118 | 3.716 | 14.519 | Agpd@BSA/DOX |
| 17 | Pyropheophorbide-a (PA)/ Qu et al., 2023 [42] | 2023 | 262.8 | 93.26 | 53.38 | 6.51 | 8 | 8 | 4.215 | 2.456 | 5.973 | PARE NP |
| 18 | Doxorubicin/ Soman et al., 2024 [43] | 2024 | 2.75523 | 0.99553 | 0.04504 | 0.05959 | 4 | 4 | 28.939 | 14.692 | 43.187 | UIO-DOX |
| 19 | Chlorin e6 (Ce6)/ Wu et al., 2021 [44] | 2021 | 0.34151 | 0.1127 | 0.01261 | 0.00637 | 4 | 4 | 19.896 | 10.049 | 29.742 | NCCe6/NCDTXL |
| 20 | Doxorubicin/ Ya et al., 2023 [45] | 2023 | 14.152 | 1.299333333 | 0.6945 | 0.09383333 | 3 | 3 | 20.694 | 8.877 | 32.512 | CDLM |
| 21 | HCPT/ Ma et al., 2021 [46] | 2021 | 273.73 | 157.13 | 20.32 | 9.44 | 3 | 3 | 5.872 | 2.184 | 9.56 | DPH NGs |
| 22 | 2-ME/ Zhang et al., 2021 [47] | 2021 | 7.16666667 | 1.361666667 | 0.255 | 0.445 | 6 | 6 | 14.77 | 8.753 | 20.786 | cRGDyk-2-ME@ICGP-TSL |
| Drug Name | Study Author | Study Weight (%) |
|---|---|---|
| Doxorubicin | Luo et al., 2015 [26] | 5.99 |
| Taxol | Yang et al., 2015 [27] | 6.24 |
| Doxorubicin | Chen et al., 2017 [28] | 5.09 |
| 5-Fluorouracil | Li et al., 2015 [29] | 5.67 |
| Pheophorbide A | Tong et al., 2022 [30] | 6.03 |
| Podophyllotoxin | Yu et al., 2024 [31] | 5.75 |
| Docetaxel | Ren et al., 2022 [32] | 6.20 |
| Paclitaxel | Kim et al., 2022 [33] | 5.79 |
| Epirubicin | Li X et al., 2020 [34] | 4.40 |
| Resiquimod | Wan et al., 2023 [35] | 3.32 |
| Doxorubicin | Wang et al., 2023 [36] | 1.11 |
| Pyropheophorbide a | Yang et al., 2024 [37] | 5.62 |
| Boc-ALA-TPP | Bai et al., 2023 [38] | 3.98 |
| DHA-S-CA | Chen et al., 2022 [39] | 4.12 |
| Doxorubicin | Du et al., 2022 [40] | 5.88 |
| Doxorubicin, | Li L et al., 2020 [41] | 4.20 |
| Pheophorbide a | Qu et al., 2023 [42] | 6.05 |
| Doxorubicin | Soman et al., 2024 [43] | 1.38 |
| Chlorin e6 | Wu et al., 2021 [44] | 2.34 |
| Doxorubicin | Ya et al., 2023 [45] | 1.83 |
| 10-Hydroxycamptothecin | Ma et al., 2021 [46] | 5.14 |
| 2-Methoxyestradiol | Zhang et al., 2021 [47] | 3.88 |
| Drug/Study | Estimate | Lower Bound | Upper Bound | Std. Error | p-Value |
|---|---|---|---|---|---|
| Overall | 6.246 | 4.354 | 8.137 | 0.965 | <0.001 |
| Doxorubicin/ Luo et al., 2015 [26] | 6.727 | 4.830 | 8.623 | 0.968 | <0.001 |
| Taxol/ Yang et al., 2015 [27] | 6.819 | 4.761 | 8.878 | 1.050 | <0.001 |
| Doxorubicin/ Chen et al., 2017 [28] | 6.188 | 4.251 | 8.124 | 0.988 | <0.001 |
| 5-Fluorouracil/ Li et al., 2015 [29] | 6.460 | 4.472 | 8.447 | 1.014 | <0.001 |
| Pheophorbide A/ Tong et al., 2022 [30] | 6.746 | 4.791 | 8.701 | 0.998 | <0.001 |
| Podophyllotoxin/ Yu et al., 2024 [31] | 6.376 | 4.397 | 8.354 | 1.009 | <0.001 |
| Docetaxel/ Ren et al., 2022 [32] | 6.805 | 4.689 | 8.922 | 1.080 | <0.001 |
| Paclitaxel/ Kim et al., 2022 [33] | 6.519 | 4.518 | 8.520 | 1.021 | <0.001 |
| Epirubicin/ Li X et al., 2020 [34] | 6.128 | 4.204 | 8.053 | 0.982 | <0.001 |
| Resiquimod/ Wan et al., 2023 [35] | 5.904 | 4.020 | 7.787 | 0.961 | <0.001 |
| Doxorubicin/ Wang et al., 2023 [36] | 5.937 | 4.075 | 7.799 | 0.950 | <0.001 |
| Pyropheophorbide a/ Yang et al., 2024 [37] | 6.307 | 4.345 | 8.269 | 1.001 | <0.001 |
| Boc-ALA-TPP/ Bai et al., 2023 [38] | 5.891 | 4.009 | 7.774 | 0.961 | <0.001 |
| DHA-S-CA/ Chen et al., 2022 [39] | 5.994 | 4.092 | 7.896 | 0.970 | <0.001 |
| Doxorubicin/ Du et al., 2022 [40] | 6.569 | 4.556 | 8.581 | 1.027 | <0.001 |
| Doxorubicin/ Li L et al., 2020 [41] | 6.097 | 4.178 | 8.015 | 0.979 | <0.001 |
| Pheophorbide a/ Qu et al., 2023 [42] | 6.489 | 4.467 | 8.512 | 1.032 | <0.001 |
| Doxorubicin/ Soman et al., 2024 [43] | 5.851 | 4.002 | 7.701 | 0.944 | <0.001 |
| Chlorin e6/ Wu et al., 2021 [44] | 5.848 | 3.983 | 7.713 | 0.952 | <0.001 |
| Doxorubicin/ Ya et al., 2023 [45] | 5.925 | 4.052 | 7.798 | 0.956 | <0.001 |
| 10-Hydroxycamptothecin/ Ma et al., 2021 [46] | 6.279 | 4.328 | 8.230 | 0.995 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agiba, A.M.; El-Gazar, R.A.; Mekkawy, M.A.; Elsayyad, N.; ElShagea, H.N.; Segura-Medina, P.; Hamed, R.R. Advancing Photodynamic Cancer Therapy with Smart Light-Responsive Lipid and Polymeric Nanocarriers: Evidence from a Meta-Analysis of Efficacy and Pharmacokinetics. Pharmaceuticals 2025, 18, 1796. https://doi.org/10.3390/ph18121796
Agiba AM, El-Gazar RA, Mekkawy MA, Elsayyad N, ElShagea HN, Segura-Medina P, Hamed RR. Advancing Photodynamic Cancer Therapy with Smart Light-Responsive Lipid and Polymeric Nanocarriers: Evidence from a Meta-Analysis of Efficacy and Pharmacokinetics. Pharmaceuticals. 2025; 18(12):1796. https://doi.org/10.3390/ph18121796
Chicago/Turabian StyleAgiba, Ahmed M., Rabab A. El-Gazar, Mohamed A. Mekkawy, Nihal Elsayyad, Hala N. ElShagea, Patricia Segura-Medina, and Raghda Rabe Hamed. 2025. "Advancing Photodynamic Cancer Therapy with Smart Light-Responsive Lipid and Polymeric Nanocarriers: Evidence from a Meta-Analysis of Efficacy and Pharmacokinetics" Pharmaceuticals 18, no. 12: 1796. https://doi.org/10.3390/ph18121796
APA StyleAgiba, A. M., El-Gazar, R. A., Mekkawy, M. A., Elsayyad, N., ElShagea, H. N., Segura-Medina, P., & Hamed, R. R. (2025). Advancing Photodynamic Cancer Therapy with Smart Light-Responsive Lipid and Polymeric Nanocarriers: Evidence from a Meta-Analysis of Efficacy and Pharmacokinetics. Pharmaceuticals, 18(12), 1796. https://doi.org/10.3390/ph18121796







