BTLA: An Emerging Immune Checkpoint Target in Cancer Immunotherapy
Abstract
1. Introduction
1.1. Overview of Immune Checkpoint Molecules in Cancer Immunotherapy
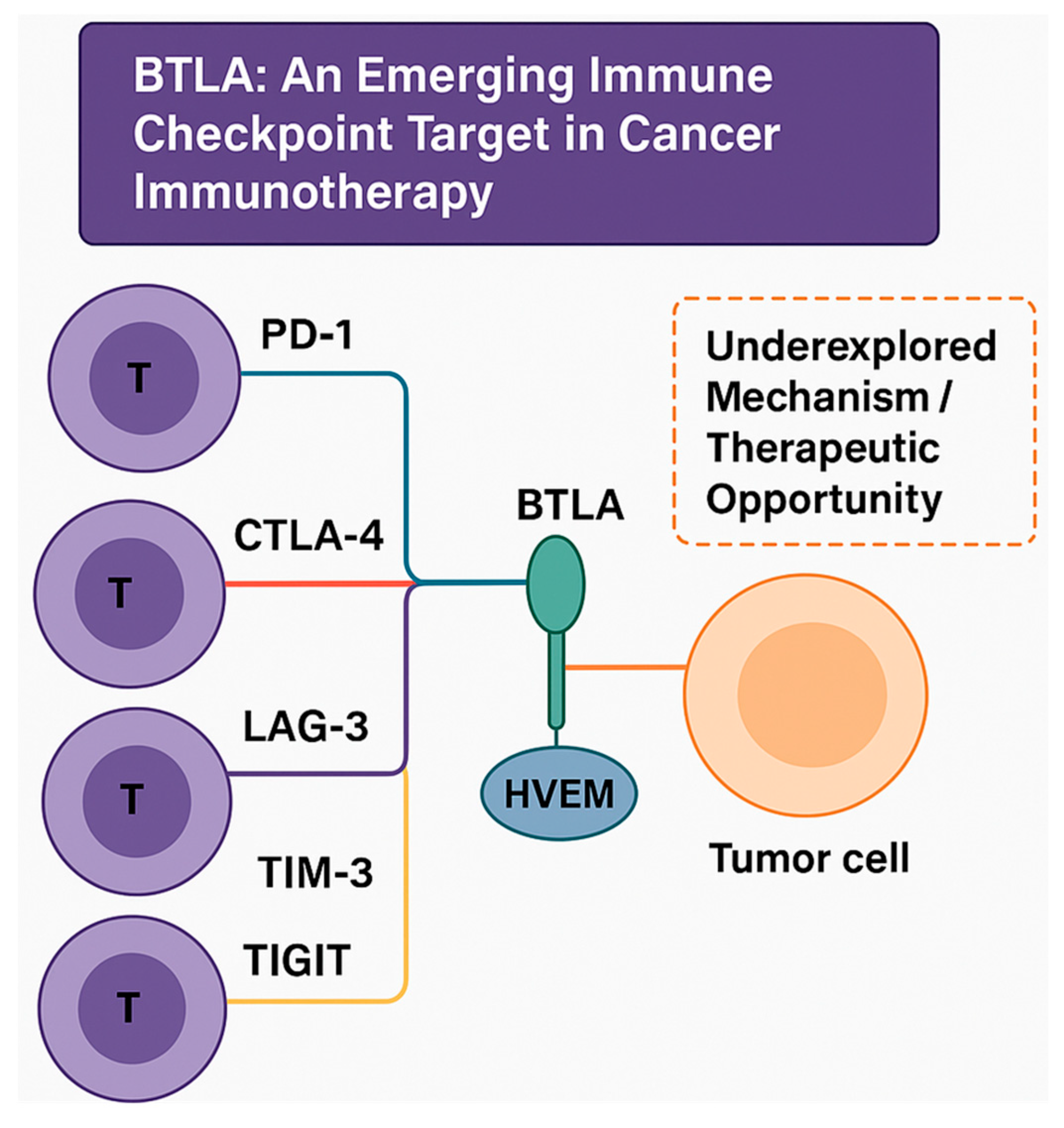
1.2. Brief Introduction to BTLA and Its Role in Immune Regulation
1.3. Importance of BTLA in Tumor Immunology and Immunotherapy
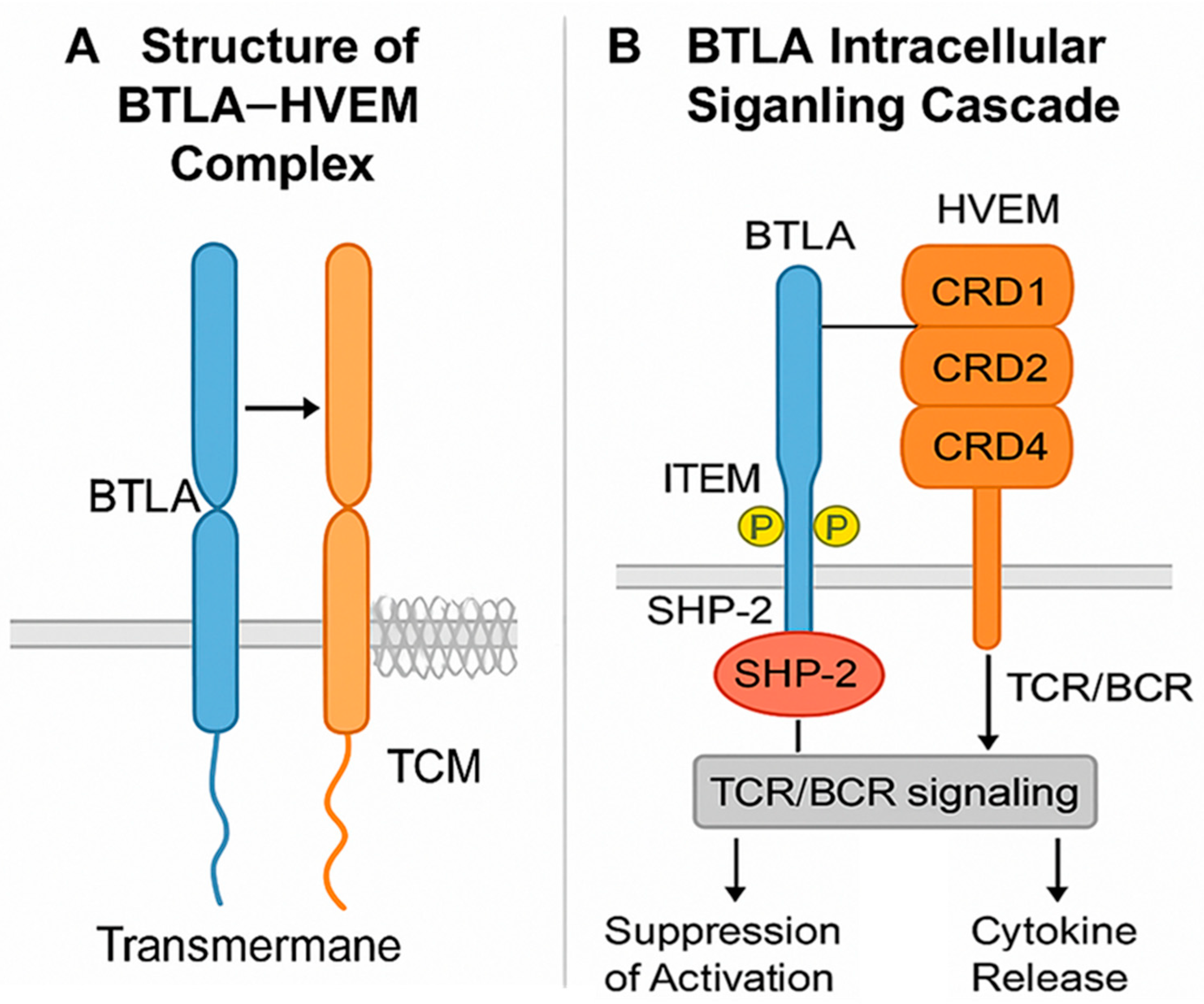
2. Molecular Structure and Signaling Pathway of BTLA
2.1. Structure of BTLA and Its Ligands HVEM
2.2. BTLA Expression on Immune Cells (B, T, and NK Cells) and BTLA-Mediated Immune Suppression
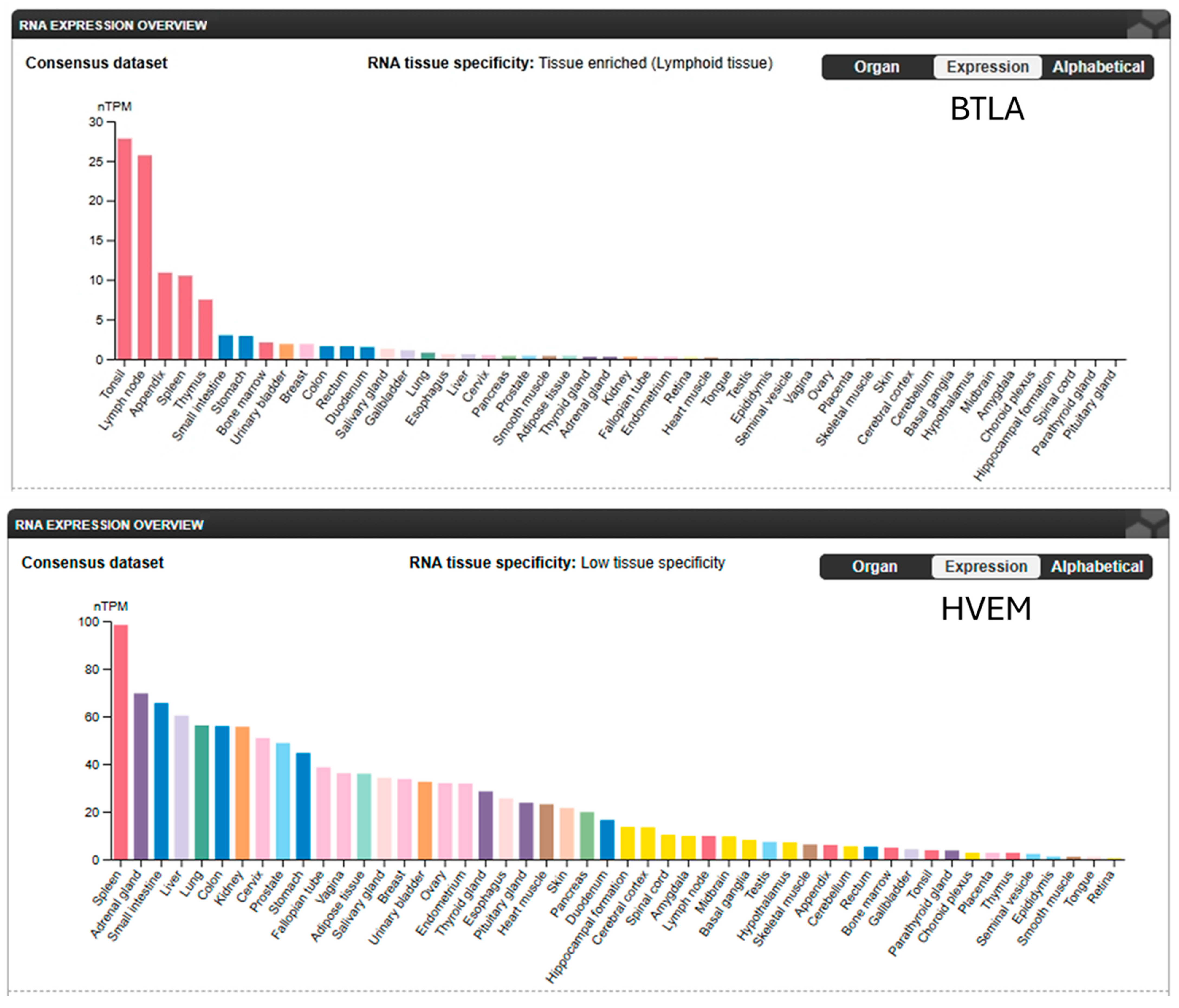
3. BTLA in the Tumor Microenvironment
3.1. BTLA Expression in Different Tumor Types
3.2. Role of BTLA in Tumor Immune Evasion
3.3. Effects of BTLA Signaling on TILs and Tregs
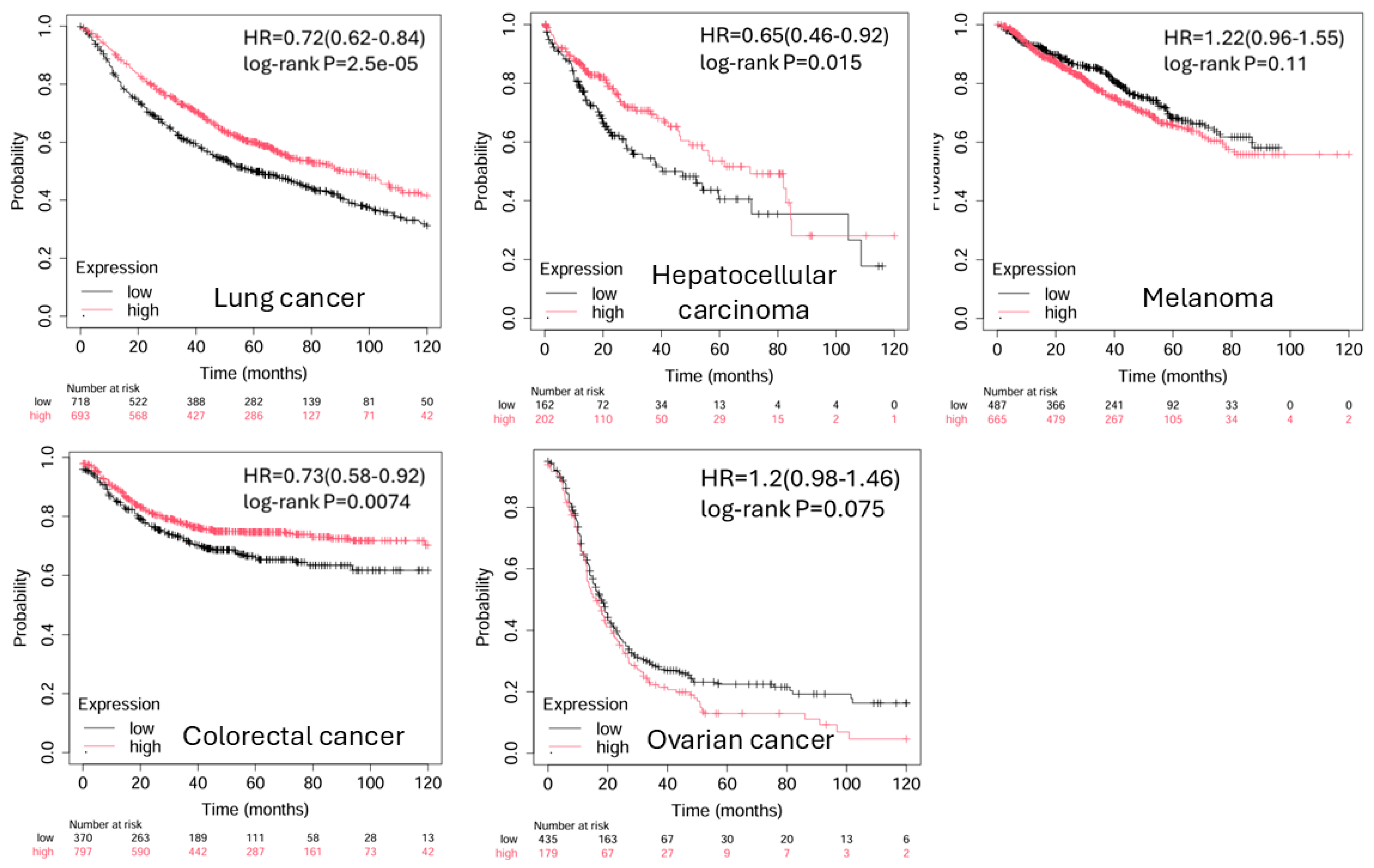
4. BTLA in Cancer Progression and Prognosis
Correlation Between BTLA Expression and Tumor Progression
5. BTLA as a Target for Immunotherapy
5.1. Overview of Current ICI Targeting BTLA
5.2. Advances in BTLA Blockade and Combination Immunotherapy
5.3. Potential Side Effects and Toxicity Concerns with BTLA Blockade
6. BTLA and Tumor Immunity: Beyond T Cells
6.1. BTLA’s Role in Modulating B Cell Responses in Cancer
6.2. Impact on NK Cells and Other Immune Cell Subsets
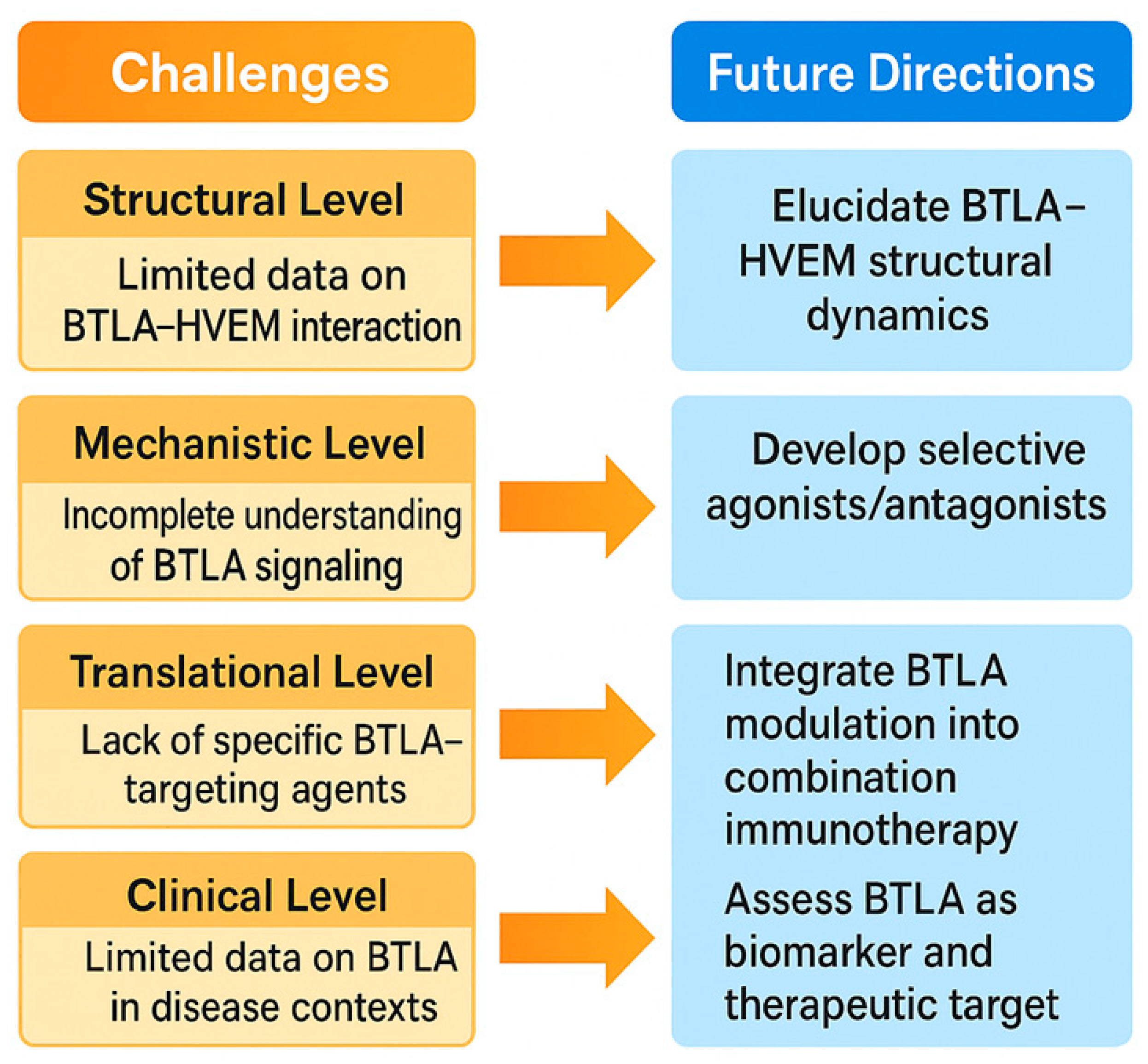
7. Challenges and Future Directions in BTLA Research
7.1. Current Limitations and Biomarker Needs for BTLA-Targeted Immunotherapy
7.2. Innovation in BTLA Modulation and Prospects for Personalized Medicine
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Zhu, H.X.; Yao, Y.; Bian, Z.H.; Zheng, Y.J.; Li, L.; Moutsopoulos, H.M.; Gershwin, M.E.; Lian, Z.X. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J. Autoimmun. 2019, 104, 102333. [Google Scholar] [CrossRef]
- Mejia-Guarnizo, L.V.; Monroy-Camacho, P.S.; Turizo-Smith, A.D.; Rodriguez-Garcia, J.A. The role of immune checkpoints in antitumor response: A potential antitumor immunotherapy. Front. Immunol. 2023, 14, 1298571. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Granda-Díaz, R.; Martínez-Pérez, A.; Aguilar-García, C.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Beyond the anti-PD-1/PD-L1 era: Promising role of the BTLA/HVEM axis as a future target for cancer immunotherapy. Mol. Cancer 2023, 22, 142. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; He, M.; Zhang, G.L.; Zhao, Q. Distinct Changes of BTLA and HVEM Expressions in Circulating CD4+ and CD8+ T Cells in Hepatocellular Carcinoma Patients. J. Immunol. Res. 2018, 2018, 4561571. [Google Scholar] [CrossRef]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X.; et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Parvez, A.; Choudhary, F.; Mudgal, P.; Khan, R.; Qureshi, K.A.; Farooqi, H.; Aspatwar, A. PD-1 and PD-L1: Architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front. Immunol. 2023, 14, 1296341. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Girigoswami, A.; Girigoswami, K. Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes 2023, 14, 1370. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Quirk, S.K.; Shure, A.K.; Agrawal, D.K. Immune-mediated adverse events of anticytotoxic T lymphocyte-associated antigen 4 antibody therapy in metastatic melanoma. Transl. Res. J. Lab. Clin. Med. 2015, 166, 412–424. [Google Scholar] [CrossRef]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Wojciechowicz, K.; Spodzieja, M.; Lisowska, K.A.; Wardowska, A. The role of the BTLA-HVEM complex in the pathogenesis of autoimmune diseases. Cell. Immunol. 2022, 376, 104532. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zheng, Y.; Cai, P.; Zheng, Z. The Role of B and T Lymphocyte Attenuator in Respiratory System Diseases. Front. Immunol. 2021, 12, 635623. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Hedges, D.; Tan, A.C.; Rodriguez, P.; Sukrithan, V.; Ratan, A.; McCarter, M.D.; Carpten, J.; Colman, H.; Ikeguchi, A.P.; et al. Differences in Co-Expression of T Cell Co-Inhibitory and Co-Stimulatory Molecules with PD-1 Across Different Human Cancers. J. Oncol. Res. Ther. 2024, 9, 10224. [Google Scholar] [CrossRef]
- Andrzejczak, A.; Karabon, L. BTLA biology in cancer: From bench discoveries to clinical potentials. Biomark. Res. 2024, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef] [PubMed]
- del Rio, M.L.; Lucas, C.L.; Buhler, L.; Rayat, G.; Rodriguez-Barbosa, J.I. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J. Leukoc. Biol. 2010, 87, 223–235. [Google Scholar] [CrossRef]
- Cai, G.; Freeman, G.J. The CD160, BTLA, LIGHT/HVEM pathway: A bidirectional switch regulating T-cell activation. Immunol. Rev. 2009, 229, 244–258. [Google Scholar] [CrossRef]
- Jones, A.; Bourque, J.; Kuehm, L.; Opejin, A.; Teague, R.M.; Gross, C.; Hawiger, D. Immunomodulatory Functions of BTLA and HVEM Govern Induction of Extrathymic Regulatory T Cells and Tolerance by Dendritic Cells. Immunity 2016, 45, 1066–1077. [Google Scholar] [CrossRef]
- Arifin, M.Z.; Leitner, J.; Egan, D.; Waidhofer-Sollner, P.; Kolch, W.; Zhernovkov, V.; Steinberger, P. BTLA and PD-1 signals attenuate TCR-mediated transcriptomic changes. iScience 2024, 27, 110253. [Google Scholar] [CrossRef] [PubMed]
- Kadekar, D.; Agerholm, R.; Vinals, M.T.; Rizk, J.; Bekiaris, V. The immune checkpoint receptor associated phosphatases SHP-1 and SHP-2 are not required for gammadeltaT17 cell development, activation, or skin inflammation. Eur. J. Immunol. 2020, 50, 873–879. [Google Scholar] [CrossRef]
- Xu, X.; Hou, B.; Fulzele, A.; Masubuchi, T.; Zhao, Y.; Wu, Z.; Hu, Y.; Jiang, Y.; Ma, Y.; Wang, H.; et al. PD-1 and BTLA regulate T cell signaling differentially and only partially through SHP1 and SHP2. J. Cell Biol. 2020, 219, e201905085. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.H.; Li, N. Immune evasion in cancer: Mechanisms and cutting-edge therapeutic approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef]
- Yu, L.; Sun, M.; Zhang, Q.; Zhou, Q.; Wang, Y. Harnessing the immune system by targeting immune checkpoints: Providing new hope for Oncotherapy. Front. Immunol. 2022, 13, 982026. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Liu, K.; Xiong, H. Roles of BTLA in Immunity and Immune Disorders. Front. Immunol. 2021, 12, 654960. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Z.Z.; Zhong, N.N.; Cao, L.M.; Liu, B.; Bu, L.L. Charting new frontiers: Co-inhibitory immune checkpoint proteins in therapeutics, biomarkers, and drug delivery systems in cancer care. Transl. Oncol. 2023, 38, 101794. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Mao, R.; Su, Z.; Zhang, J. BTLA/HVEM Signaling: Milestones in Research and Role in Chronic Hepatitis B Virus Infection. Front. Immunol. 2019, 10, 617. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Obeid, R.A.; Fadhil, A.A.; Amir, A.A.; Adhab, Z.H.; Jabouri, E.A.; Ahmad, I.; Alshahrani, M.Y. BTLA and HVEM: Emerging players in the tumor microenvironment and cancer progression. Cytokine 2023, 172, 156412. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Cui, G.; Yu, L.; Zhang, X. BTLA Expression in Stage I-III Non-Small-Cell Lung Cancer and Its Correlation with PD-1/PD-L1 and Clinical Outcomes. OncoTargets Ther. 2020, 13, 215–224. [Google Scholar] [CrossRef]
- Wojciechowicz, K.; Spodzieja, M.; Wardowska, A. The BTLA-HVEM complex—The future of cancer immunotherapy. Eur. J. Med. Chem. 2024, 268, 116231. [Google Scholar] [CrossRef]
- Drewniak-Switalska, M.; Fortuna, P.; Krzystek-Korpacka, M. Negative Immune Checkpoint Inhibitors. Pharmaceutics 2025, 17, 713. [Google Scholar] [CrossRef]
- Liu, W.; Garrett, S.C.; Fedorov, E.V.; Ramagopal, U.A.; Garforth, S.J.; Bonanno, J.B.; Almo, S.C. Structural Basis of CD160:HVEM Recognition. Structure 2019, 27, 1286–1295.e1284. [Google Scholar] [CrossRef]
- Patsoukis, N.; Duke-Cohan, J.S.; Chaudhri, A.; Aksoylar, H.I.; Wang, Q.; Council, A.; Berg, A.; Freeman, G.J.; Boussiotis, V.A. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun. Biol. 2020, 3, 128. [Google Scholar] [CrossRef]
- Gavrieli, M.; Watanabe, N.; Loftin, S.K.; Murphy, T.L.; Murphy, K.M. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. Biophys. Res. Commun. 2003, 312, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Masubuchi, T.; Cai, Q.; Zhao, Y.; Hui, E. Molecular features underlying differential SHP1/SHP2 binding of immune checkpoint receptors. eLife 2021, 10, e74276. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.; Hawiger, D. The BTLA-HVEM-CD5 Immunoregulatory Axis-An Instructive Mechanism Governing pTreg Cell Differentiation. Front. Immunol. 2019, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, A.; Balkhi, S.; Vivona, V.; Mortara, L. Key immune cells and their crosstalk in the tumor microenvironment of bladder cancer: Insights for innovative therapies. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002304. [Google Scholar] [CrossRef]
- He, Y.; Vlaming, M.; van Meerten, T.; Bremer, E. The Implementation of TNFRSF Co-Stimulatory Domains in CAR-T Cells for Optimal Functional Activity. Cancers 2022, 14, 299. [Google Scholar] [CrossRef]
- Ware, C.F.; Sedy, J.R. TNF Superfamily Networks: Bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14). Curr. Opin. Immunol. 2011, 23, 627–631. [Google Scholar] [CrossRef]
- Yao, Z.; Zeng, Y.; Liu, C.; Jin, H.; Wang, H.; Zhang, Y.; Ding, C.; Chen, G.; Wu, D. Focusing on CD8+ T-cell phenotypes: Improving solid tumor therapy. J. Exp. Clin. Cancer Res. CR 2024, 43, 266. [Google Scholar] [CrossRef]
- Melique, S.; Yang, C.; Lesourne, R. Negative times negative equals positive, THEMIS sets the rule on thymic selection and peripheral T cell responses. Biomed. J. 2022, 45, 334–346. [Google Scholar] [CrossRef]
- Haymaker, C.; Wu, R.; Bernatchez, C.; Radvanyi, L. PD-1 and BTLA and CD8+ T-cell “exhaustion” in cancer: “Exercising” an alternative viewpoint. Oncoimmunology 2012, 1, 735–738. [Google Scholar] [CrossRef]
- del Rio, M.L.; Kaye, J.; Rodriguez-Barbosa, J.I. Detection of protein on BTLAlow cells and in vivo antibody-mediated down-modulation of BTLA on lymphoid and myeloid cells of C57BL/6 and BALB/c BTLA allelic variants. Immunobiology 2010, 215, 570–578. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Payer, Á.R.; González-García, E.; López-Soto, A.; Gonzalez, S. BTLA/HVEM Axis Induces NK Cell Immunosuppression and Poor Outcome in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 1766. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Zeng, Y.; Qu, Q.; Shen, D.; Mu, C.; Lei, W.; Su, M.; Mao, J.; Gao, L.; et al. B and T lymphocyte attenuator (BTLA) and PD-1 pathway dual blockade promotes antitumor immune responses by reversing CD8+ T-cell exhaustion in non-small cell lung cancer. Front. Immunol. 2025, 16, 1553042. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shayan, G.; Avery, L.; Jie, H.B.; Gildener-Leapman, N.; Schmitt, N.; Lu, B.F.; Kane, L.P.; Ferris, R.L. Tumor-infiltrating Tim-3+ T cells proliferate avidly except when PD-1 is co-expressed: Evidence for intracellular cross talk. Oncoimmunology 2016, 5, e1200778. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, Z.L.; He, M.; Gao, Z.; Kuang, D.M. BTLA identifies dysfunctional PD-1-expressing CD4+ T cells in human hepatocellular carcinoma. Oncoimmunology 2016, 5, e1254855. [Google Scholar] [CrossRef]
- Ritthipichai, K.; Haymaker, C.L.; Martinez, M.; Aschenbrenner, A.; Yi, X.; Zhang, M.; Kale, C.; Vence, L.M.; Roszik, J.; Hailemichael, Y.; et al. Multifaceted Role of BTLA in the Control of CD8+ T-cell Fate after Antigen Encounter. Clin. Cancer Res. 2017, 23, 6151–6164. [Google Scholar] [CrossRef]
- Song, J.; Wu, L. Friend or Foe: Prognostic and Immunotherapy Roles of BTLA in Colorectal Cancer. Front. Mol. Biosci. 2020, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Lan, X.; Meng, Y.; Guo, X.; Guo, Y.; Zhao, L.; Chen, X.; Liu, A. BTLA marks a less cytotoxic T-cell subset in diffuse large B-cell lymphoma with high expression of checkpoints. Exp. Hematol. 2018, 60, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lin, H.W.; Chien, C.L.; Lai, Y.L.; Sun, W.Z.; Chen, C.A.; Cheng, W.F. BTLA blockade enhances Cancer therapy by inhibiting IL-6/IL-10-induced CD19(high) B lymphocytes. J. Immunother. Cancer 2019, 7, 313. [Google Scholar] [CrossRef]
- Fanale, D.; Brando, C.; Corsini, L.R.; Cutaia, S.; Di Donna, M.C.; Randazzo, U.; Filorizzo, C.; Lisanti, C.; Magrin, L.; Gurrera, V.; et al. Low plasma PD-L1 levels, early tumor onset and absence of peritoneal carcinomatosis improve prognosis of women with advanced high-grade serous ovarian cancer. BMC Cancer 2023, 23, 437. [Google Scholar] [CrossRef]
- Shi, H.; Li, L.; Zhou, L.; Hong, C. Development and evaluation of an ovarian cancer prognostic model based on adaptive immune-related genes. Medicine 2025, 104, e42030. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, C.J.; Drake, C.G. Molecular pathways: Coexpression of immune checkpoint molecules: Signaling pathways and implications for cancer immunotherapy. Clin. Cancer Res. 2013, 19, 4917–4924. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Yu, Y.; Wang, Q.; Yang, R.; Xia, B.; Li, C.; Lv, D.; Yi, T.; Han, L.; et al. Phase I/II Study of Tifcemalimab, an Anti-B- and T-lymphocyte Attenuator Antibody, in Combination with Toripalimab in Previously Treated Advanced Lung Cancer. Clin. Cancer Res. 2025, 31, 2926–2934. [Google Scholar] [CrossRef]
- Liu, F.; Chen, H.; Wu, S.; Zhu, C.; Zhang, M.; Rui, W.; Zhou, D.; Wang, Y.; Lin, X.; Zhao, X.; et al. Neoepitope BTLAP267L-specific TCR-T cell immunotherapy unlocks precision treatment for hepatocellular carcinoma. Cancer Biol. Med. 2025, 22, 20240434. [Google Scholar] [CrossRef]
- Li, X.; Qu, X.; Li, S.; Lin, K.; Yao, N.; Wang, N.; Shi, Y. Development of a Novel CD8+ T Cell-Associated Signature for Prognostic Assessment in Hepatocellular Carcinoma. Cancer Control J. Moffitt Cancer Cent. 2024, 31, 10732748241270583. [Google Scholar] [CrossRef]
- Haymaker, C.L.; Wu, R.C.; Ritthipichai, K.; Bernatchez, C.; Forget, M.A.; Chen, J.Q.; Liu, H.; Wang, E.; Marincola, F.; Hwu, P.; et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology 2015, 4, e1014246. [Google Scholar] [CrossRef]
- Derre, L.; Rivals, J.P.; Jandus, C.; Pastor, S.; Rimoldi, D.; Romero, P.; Michielin, O.; Olive, D.; Speiser, D.E. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J. Clin. Investig. 2010, 120, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Song, J.; Chen, B.; Qi, Y.; Jiang, W.; Li, H.; Zheng, D.; Wang, Y.; Zhang, X.; Liu, H. Exploration of the Prognostic and Immunotherapeutic Value of B and T Lymphocyte Attenuator in Skin Cutaneous Melanoma. Front. Oncol. 2020, 10, 592811. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, R.I.; Gheorghe, A.S.; Zob, D.L.; Stanculeanu, D.L. The Importance of Predictive Biomarkers and Their Correlation with the Response to Immunotherapy in Solid Tumors-Impact on Clinical Practice. Biomedicines 2024, 12, 2146. [Google Scholar] [CrossRef] [PubMed]
- Willsmore, Z.N.; Harris, R.J.; Crescioli, S.; Hussein, K.; Kakkassery, H.; Thapa, D.; Cheung, A.; Chauhan, J.; Bax, H.J.; Chenoweth, A.; et al. B Cells in Patients with Melanoma: Implications for Treatment with Checkpoint Inhibitor Antibodies. Front. Immunol. 2020, 11, 622442. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.J.; Peng, X.F.; Deng, L.; Wang, Y.; Li, J.J.; Guo, D.L.; Niu, X.H. Anti-PD-1/PD-L1 therapy for colorectal cancer: Clinical implications and future considerations. Transl. Oncol. 2024, 40, 101851. [Google Scholar] [CrossRef]
- Housseau, F.; Llosa, N.J. Immune checkpoint blockade in microsatellite instable colorectal cancers: Back to the clinic. Oncoimmunology 2015, 4, e1008858. [Google Scholar] [CrossRef]
- Takahara, T.; Nakamura, S.; Tsuzuki, T.; Satou, A. The Immunology of DLBCL. Cancers 2023, 15, 835. [Google Scholar] [CrossRef]
- Amin, A.D.; Peters, T.L.; Li, L.; Rajan, S.S.; Choudhari, R.; Puvvada, S.D.; Schatz, J.H. Diffuse large B-cell lymphoma: Can genomics improve treatment options for a curable cancer? Cold Spring Harb. Mol. Case Stud. 2017, 3, a001719. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Martinez-Perez, A.; Gonzalez-Rodriguez, A.P.; Payer, A.R.; Gonzalez-Garcia, E.; Aguilar-Garcia, C.; Gonzalez-Rodriguez, S.; Lopez-Soto, A.; Garcia-Torre, A.; et al. BTLA dysregulation correlates with poor outcome and diminished T cell-mediated antitumor responses in chronic lymphocytic leukemia. Cancer Immunol. Immunother. CII 2023, 72, 2529–2539. [Google Scholar] [CrossRef]
- Zhang, J.-A.; Lu, Y.-B.; Wang, W.-D.; Liu, G.-B.; Chen, C.; Shen, L.; Luo, H.-L.; Xu, H.; Peng, Y.; Luo, H.; et al. BTLA-Expressing Dendritic Cells in Patients with Tuberculosis Exhibit Reduced Production of IL-12/IFN-α and Increased Production of IL-4 and TGF-β, Favoring Th2 and Foxp3+ Treg Polarization. Front. Immunol. 2020, 11, 518. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wu, J.; Zheng, X. Immunological Mechanisms and Effects of Bacterial Infections in Acute-on-Chronic Liver Failure. Cells 2025, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Stienne, C.; Virgen-Slane, R.; Elmen, L.; Veny, M.; Huang, S.; Nguyen, J.; Chappell, E.; Balmert, M.O.; Shui, J.W.; Hurchla, M.A.; et al. Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa. Cell Rep. 2022, 38, 110553. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Medikonda, R.; Saleh, L.; Kim, T.; Pant, A.; Srivastava, S.; Kim, Y.H.; Jackson, C.; Tong, L.; Routkevitch, D.; et al. Combination checkpoint therapy with anti-PD-1 and anti-BTLA results in a synergistic therapeutic effect against murine glioblastoma. Oncoimmunology 2021, 10, 1956142. [Google Scholar] [CrossRef] [PubMed]
- Tojjari, A.; Saeed, A.; Sadeghipour, A.; Kurzrock, R.; Cavalcante, L. Overcoming Immune Checkpoint Therapy Resistance with SHP2 Inhibition in Cancer and Immune Cells: A Review of the Literature and Novel Combinatorial Approaches. Cancers 2023, 15, 5384. [Google Scholar] [CrossRef]
- Yu, X.; Yang, F.; Shen, Z.; Zhang, Y.; Sun, J.; Qiu, C.; Zheng, Y.; Zhao, W.; Yuan, S.; Zeng, D.; et al. BTLA contributes to acute-on-chronic liver failure infection and mortality through CD4+ T-cell exhaustion. Nat. Commun. 2024, 15, 1835. [Google Scholar] [CrossRef]
- Aubergeon, L.; Sawaf, M.; Felten, R.; Gottenberg, J.E.; Dumortier, H.; Monneaux, F. High BTLA Expression Likely Contributes to Contraction of the Regulatory T Cell Subset in Lupus Disease. Front. Immunol. 2021, 12, 767099. [Google Scholar] [CrossRef]
- Huarte, E.; Jun, S.; Rynda-Apple, A.; Golden, S.; Jackiw, L.; Hoffman, C.; Maddaloni, M.; Pascual, D.W. Regulatory T Cell Dysfunction Acquiesces to BTLA+ Regulatory B Cells Subsequent to Oral Intervention in Experimental Autoimmune Encephalomyelitis. J. Immunol. 2016, 196, 5036–5046. [Google Scholar] [CrossRef]
- Archilla-Ortega, A.; Domuro, C.; Martin-Liberal, J.; Munoz, P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J. Exp. Clin. Cancer Res. CR 2022, 41, 62. [Google Scholar] [CrossRef]
- Murphy, T.L.; Murphy, K.M. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu. Rev. Immunol. 2010, 28, 389–411. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Miao, Y.; Yin, C.; Tang, L.; Zhang, C.; Yu, P.; Han, Q.; Ma, Y.; Li, S.; et al. BTLA promoter hypomethylation correlates with enhanced immune cell infiltration, favorable prognosis, and immunotherapy response in melanoma. J. Immunother. Cancer 2025, 13, e009841. [Google Scholar] [CrossRef]
- Jain, M.; Jadhav, I.M.; Dangat, S.V.; Singuru, S.R.; Sethi, G.; Yuba, E.; Gupta, R.K. Overcoming the novel glycan-lectin checkpoints in tumor microenvironments for the success of the cross-presentation-based immunotherapy. Biomater. Sci. 2025, 13, 3447–3497. [Google Scholar] [CrossRef]
- Nishizaki, D.; Choi, S.; Pandya, C.; Lee, S.; Pabla, S.; DePietro, P.; Jensen, T.J.; Kurzrock, R.; Kato, S. Pan-Cancer Landscape of B- and T-Lymphocyte Attenuator: Implications for Potential Immunotherapy Combinations. JCO Precis. Oncol. 2025, 9, e2500304. [Google Scholar] [CrossRef] [PubMed]
- Anzengruber, F.; Ignatova, D.; Schlaepfer, T.; Chang, Y.T.; French, L.E.; Pascolo, S.; Contassot, E.; Bobrowicz, M.; Hoetzenecker, W.; Guenova, E. Divergent LAG-3 versus BTLA, TIGIT, and FCRL3 expression in Sezary syndrome. Leuk. Lymphoma 2019, 60, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Guruprasad, P.; Carturan, A.; Zhang, Y.; Cho, J.H.; Kumashie, K.G.; Patel, R.P.; Kim, K.H.; Lee, J.S.; Lee, Y.; Kim, J.H.; et al. The BTLA-HVEM axis restricts CAR T cell efficacy in cancer. Nat. Immunol. 2024, 25, 1020–1032. [Google Scholar] [CrossRef]
- Tang, P.; Shen, X.; Gao, J.; Zhang, J.; Feng, Y.; Zhang, J.; Huang, Z.; Wang, X. Distinct characteristics of BTLA/HVEM axis expression on Tregs and its impact on the expansion and attributes of Tregs in patients with active pulmonary tuberculosis. Front. Cell. Infect. Microbiol. 2024, 14, 1437207. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Li, S.; Gao, H.; Nanding, A.; Quan, L.; Yang, C.; Ding, S.; Xue, Y. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. OncoTargets Ther. 2017, 10, 919–926. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, Y.; He, Z.; Yu, Y.; Wu, L.; Zhao, M.; Yu, Y.; Zhang, W.; Zhang, M.; Liu, A.; et al. 644P Tifcemalimab combined with toripalimab and docetaxel as 2nd line treatment for immunotherapy-treated squamous cell non-small cell lung cancer (Sq-NSCLC) patients: A phase Ib/II study. Ann. Oncol. 2024, 35, S1645. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Xia, T.; Li, J.; Yao, W.; Zhang, Q.; Han, Z.; Wang, J.; Cao, Z.; Hu, J.; et al. Perioperative the BTLA inhibitor (tifcemalimab) combined with toripalimab and chemotherapy for resectable locally advanced thoracic esophageal squamous cell carcinoma trial (BT-NICE trial): A prospective, single-arm, exploratory study. Front. Immunol. 2025, 16, 1542877. [Google Scholar] [CrossRef]
- Song, Y.; Ma, J.; Zhang, H.; Xie, Y.; Peng, Z.; Shuang, Y.; Li, F.; Li, Y.; Yang, H.; Zou, L.; et al. Tifcemalimab as monotherapy or in combination with toripalimab in patients with relapsed/refractory lymphoma: A Phase I trial. Nat. Commun. 2025, 16, 4559. [Google Scholar] [CrossRef]
- Muench, G.; Hare, E.; Soroosh, P.; Parmley, S.; Lizzul, P.; Dahl, M. 50585 ANB032, a B and T Cell Lymphocyte Attenuator (BTLA) Checkpoint Receptor Agonist, Modulates Dendritic Cell (DC) Maturation and Function: A Novel Mechanism Addressing Atopic Dermatitis Pathophysiology. J. Am. Acad. Dermatol. 2024, 91, AB17. [Google Scholar] [CrossRef]
- Lewis, D.; Winner, P.; Saper, J.; Ness, S.; Polverejan, E.; Wang, S.; Kurland, C.L.; Nye, J.; Yuen, E.; Eerdekens, M.; et al. Phase 2, Randomized, Double Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of ANB032 in the Treatment of Subjects with Moderate to Severe Atopic Dermatitis. In Proceedings of the 5th Inflammatory Skin Disease Summit, Vienna, Austria, 15–18 November 2023. [Google Scholar]
- A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Two-Arm, Phase 2 Clinical Trial to Evaluate the Efficacy and Safety of LY3361237 as a Treatment for Adults with At Least Moderately Active Systemic Lupus Erythematosus. 2021. Available online: https://clinicaltrials.gov/study/NCT05123586 (accessed on 1 November 2025).
- De Miguel, M.; Landa Magdalena, A.; Moreno Garcia, V.; Gambardella, V.; Menis, J.; Daniele, G.; El-Khoueiry, A.B.; Spira, A.I.; Gravina, A.; Olszanski, A.J.; et al. 1006P Phase I dose escalation study of HFB200603, a best-in-class BTLA antagonist monoclonal antibody (mAb), in monotherapy and in combination with the anti-PD-1 mAb tislelizumab (TIS) in adult patients (pts) with advanced solid tumors. Ann. Oncol. 2024, 35, S684. [Google Scholar] [CrossRef]
- A Phase 1a/1b, Open-Label, Multi-Center, Dose Escalation and Expansion Study of HFB200603 (Anti-BTLA Antibody) as a Single Agent and in Combination with Tislelizumab (Anti-PD-1 Antibody) in Adult Patients with Advanced Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT05789069 (accessed on 1 November 2025).
- Battin, C.; Leitner, J.; Waidhofer-Sollner, P.; Grabmeier-Pfistershammer, K.; Olive, D.; Steinberger, P. BTLA inhibition has a dominant role in the cis-complex of BTLA and HVEM. Front. Immunol. 2022, 13, 956694. [Google Scholar] [CrossRef] [PubMed]
- A Randomized, Double-Blind, Placebo-Controlled, Multi-Regional Phase III Clinical Study of Toripalimab Alone or in Combination with Tifcemalimab (JS004/TAB004) as Consolidation Therapy in Patients with Limited-Stage Small Cell Lung Cancer Without Disease Progression Following Chemoradiotherapy. 2023. Available online: https://clinicaltrials.gov/study/NCT06095583 (accessed on 1 November 2025).
- A Phase Ib/II Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Initial Efficacy of Recombinant Humanized Anti-BTLA Monoclonal Antibody (JS004) Injection Combined with Toripalimab and with Standard Chemotherapy in Patients with Advanced Lung Cancer. 2022. Available online: https://clinicaltrials.gov/study/NCT05664971 (accessed on 1 November 2025).
- Toripalimab and JS004 Combined with Platinum-Based Chemotherapy for Relapsed and Extensive-Stage Small Cell Lung Cancer: A Single-Center, Randomized Trial. 2024. Available online: https://clinicaltrials.gov/study/NCT06648200 (accessed on 1 November 2025).
- Exploratory Clinical Study of Low-Dose Radiotherapy Combined with Concurrent Chemotherapy, Toripalimab and Tifcemalimab in First-Line Treatment of Extensive-Stage Small Cell Lung Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT06732258 (accessed on 1 November 2025).
- A Single Center, Prospective, Randomized Controlled, Second-Line Clinical Study on the Combination of Toripalimab and JS004 in the Treatment for Recurrent and Metastatic Clear Cell Renal Cell Carcinoma. 2024. Available online: https://www.clinicaltrials.gov/study/NCT06690697 (accessed on 1 November 2025).
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, F.; Pirzada, R.H.; Ahmad, B.; Choi, B.; Choi, S. Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. Int. J. Mol. Sci. 2024, 25, 7666. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.H.; Cho, S.; Shin, Y.M.; Choi, J.M. Emerging role of bystander T cell activation in autoimmune diseases. BMB Rep. 2022, 55, 57–64. [Google Scholar] [CrossRef]
- Rezazadeh-Gavgani, E.; Majidazar, R.; Lotfinejad, P.; Kazemi, T.; Shamekh, A. Immune Checkpoint Molecules: A Review on Pathways and Immunotherapy Implications. Immun. Inflamm. Dis. 2025, 13, e70196. [Google Scholar] [CrossRef]
- Parlati, L.; Sakka, M.; Retbi, A.; Bouam, S.; Hassani, L.; Meritet, J.F.; Rufat, P.; Bonnefont-Rousselot, D.; Batista, R.; Terris, B.; et al. Burden of grade 3 or 4 liver injury associated with immune checkpoint inhibitors. JHEP Rep. Innov. Hepatol. 2023, 5, 100880. [Google Scholar] [CrossRef]
- Bounab, Y.; Getahun, A.; Cambier, J.C.; Daeron, M. Phosphatase regulation of immunoreceptor signaling in T cells, B cells and mast cells. Curr. Opin. Immunol. 2013, 25, 313–320. [Google Scholar] [CrossRef]
- Baldanzi, G. Immune Checkpoint Receptors Signaling in T Cells. Int. J. Mol. Sci. 2022, 23, 3529. [Google Scholar] [CrossRef]
- Flores-Borja, F.; Blair, P. Mechanisms of induction of regulatory B cells in the tumour microenvironment and their contribution to immunosuppression and pro-tumour responses. Clin. Exp. Immunol. 2022, 209, 33–45. [Google Scholar] [CrossRef]
- Catalan, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillon, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Mandal, G.; Pradhan, S. B cell responses and antibody-based therapeutic perspectives in human cancers. Cancer Rep. 2024, 7, e2056. [Google Scholar] [CrossRef]
- Han, B.; Mao, F.Y.; Zhao, Y.L.; Lv, Y.P.; Teng, Y.S.; Duan, M.; Chen, W.; Cheng, P.; Wang, T.T.; Liang, Z.Y.; et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 Expression on Circulating NK Cells Is Associated with Tumor Progression in Human Gastric Cancer. J. Immunol. Res. 2018, 2018, 6248590. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon gamma and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Yang, X.R.; Guo, M.F.; Pan, Z.F.; Shang, M.; Qiu, M.J.; Wu, J.Y.; Jia, J.; Liang, Y.L.; Zheng, W.T.; et al. Up-regulation of BTLA expression in myeloid dendritic cells associated with the treatment outcome of neonatal sepsis. Mol. Immunol. 2021, 134, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Bonavida, B.; Chouaib, S. Resistance to anticancer immunity in cancer patients: Potential strategies to reverse resistance. Ann. Oncol. 2017, 28, 457–467. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, M.; Ning, Y.; Mao, G.; Li, X.; Deng, Q.; Chen, X.; Zuo, D.; Zhao, X.; Xie, E.; et al. Development of a bispecific antibody targeting PD-L1 and TIGIT with optimal cytotoxicity. Sci. Rep. 2022, 12, 18011. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Bae, J.; Li, H.; Sun, Z.; Moore, C.; Hsu, E.; Han, C.; Qiao, J.; Fu, Y.X. Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells. Nat. Biomed. Eng. 2021, 5, 1261–1273. [Google Scholar] [CrossRef]
- Kungwankiattichai, S.; Desai, A.; Koppinger, S.; Maziarz, R.T. Immune-related adverse events associated with cancer immunotherapy. Med 2025, 6, 100800. [Google Scholar] [CrossRef]
- Ibis, B.; Aliazis, K.; Cao, C.; Yenyuwadee, S.; Boussiotis, V.A. Immune-related adverse effects of checkpoint immunotherapy and implications for the treatment of patients with cancer and autoimmune diseases. Front. Immunol. 2023, 14, 1197364. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Ramachandra, M. Small Molecule Agents Targeting PD-1 Checkpoint Pathway for Cancer Immunotherapy: Mechanisms of Action and Other Considerations for Their Advanced Development. Front. Immunol. 2022, 13, 752065. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, P.G.; Ramachandra, M. Small-Molecule Immune Checkpoint Inhibitors Targeting PD-1/PD-L1 and Other Emerging Checkpoint Pathways. BioDrugs 2018, 32, 481–497. [Google Scholar] [CrossRef] [PubMed]
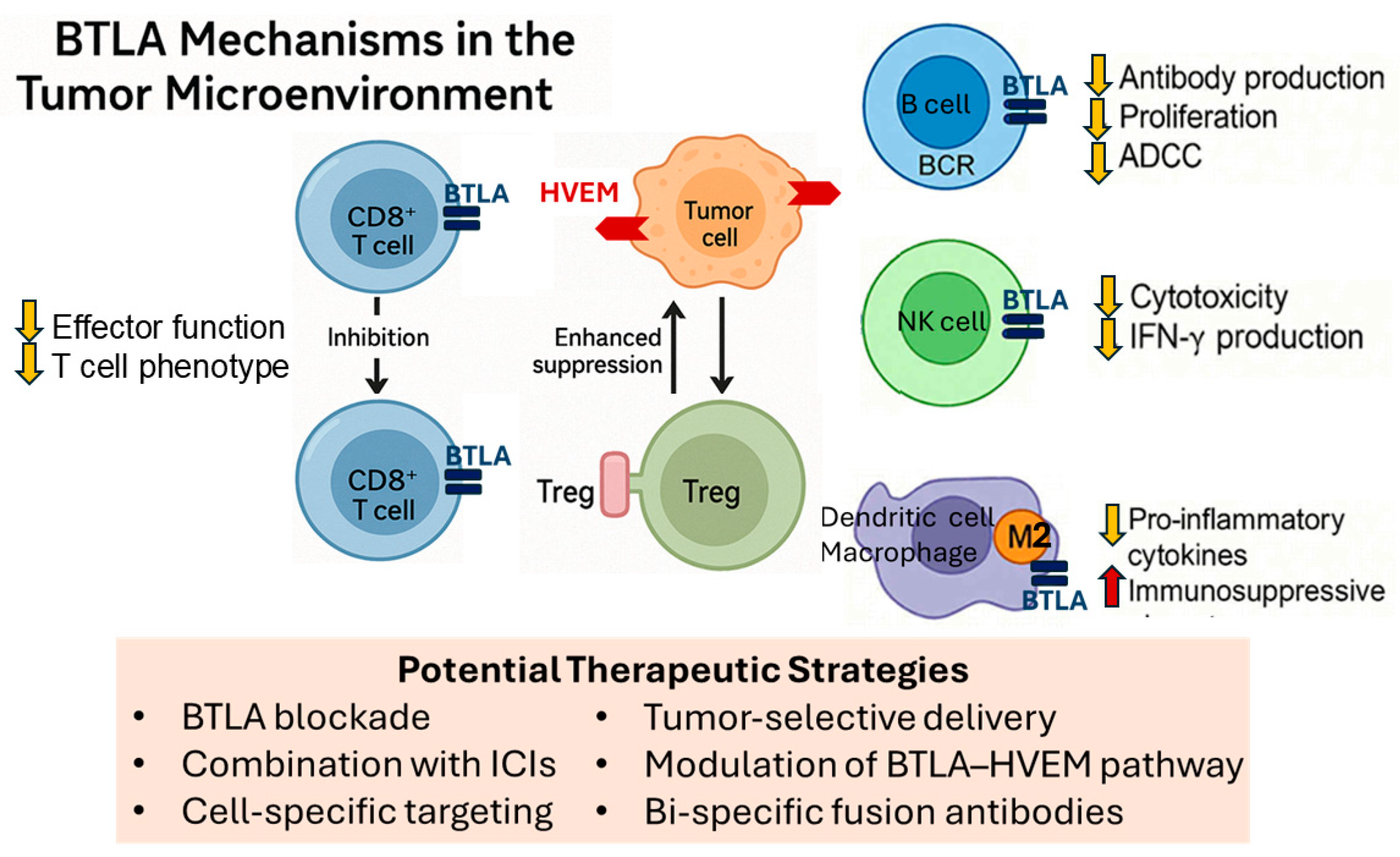
| Cancer Type | BTLA Expression Pattern | Clinical Prognostic Significance | References |
|---|---|---|---|
| Non-small cell lung cancer (NSCLC) | Highly expressed in TILs; associated with immunosuppression and advanced disease stage | Reduced overall survival (OS) and progression-free survival (PFS) | [31,49,59] |
| Hepatocellular carcinoma (HCC) | Correlates with vascular invasion and higher tumor grade | Associated with shorter overall and disease-free survival (DFS) | [5,51,60,61] |
| Melanoma | Linked to immunotherapy resistance and disease relapse | High expressers respond poorly to PD-1 monotherapy | [12,18,44,52,62,63,64,65,66] |
| Colorectal cancer (CRC) | Upregulated in MSS subtype; indicative of immune exclusion phenotype | Correlates with poor immunotherapy response and lower OS | [53,67,68] |
| Diffuse large B cell lymphoma (DLBCL) | Expressed on T and B cells; associated with poor-risk subtypes and lower survival | Associated with inferior event-free survival (EFS) | [54,69,70] |
| Chronic lymphocytic leukemia (CLL) | Co-expressed with exhaustion markers; linked to treatment resistance | Linked to poor response to immunotherapy and disease progression | [4,46,71] |
| Epithelial ovarian cancer (EOC) | Detected in cancerous tissues and plasma of EOC patients | High expression levels correlate with poor outcomes | [55,56,57] |
| Agent | Molecular Type | Mechanism of Action | Development | Target Indications | Refs. |
|---|---|---|---|---|---|
| Tifcemalimab (JS004/TAB004, Junshi Biosciences, Shanghai, China) | Recombinant humanized IgG4κ mAb | BTLA antagonist- blocks the BTLA/HVEM interaction to restore T cell activation | Phase 2/3 ongoing in solid tumors and lymphoma (mono- and combo-therapy with toripalimab) | Relapsed/refractory lymphoma, NSCLC, SCLC, ESCC, solid tumors, autoimmune exploration | [59,89,90,91] |
| ANB032 (AnaptysBio; San Diego, CA, USA) | Human IgG4 non-depleting mA | BTLA agonist- enhances BTLA inhibitory signaling to suppress inflammation without blocking HVEM | Phase 2b (AD trial completed; did not meet primary endpoint) | Atopic dermatitis, inflammatory and autoimmune diseases | [92,93] |
| LY3361237 (Venanprubart, Eli Lilly and Company, Indianapolis, IN, USA) | Human IgG4 mAb | BTLA agonist-activates BTLA to down-modulate autoreactive T/B cells | Phase 2 in progress (SLE and primary Sjögren’s syndrome) | Systemic lupus erythematosus, primary Sjögren’s syndrome | [94] |
| HFB200603 (HiFiBiO Therapeutics, Cambridge, MA, USA) | Humanized IgG1 mAb | BTLA antagonist- blocks BTLA/HVEM signaling to relieve tumor-induced immunosuppression | Phase 1 (monotherapy ± tislelizumab; ESMO 2024 update) | Solid tumors, immuno-oncology combinations | [95,96] |
| Agent | Phase/NCT | Indication and Population | Study Design and Regimen | Key Efficacy Findings | Ref. |
|---|---|---|---|---|---|
| Tifcemalimab | Phase I (NCT04477772) | Relapsed/refractory lymphoma (esp. cHL post-PD-(L)1) | Dose escalation and expansion; monotherapy and in combination with toripalimab (anti-PD-1) | cHL cohort: ORR 37%, median PFS 13.1 mo; durable responses in PD-(L)1-refractory cases | [91] |
| Tifcemalimab + Toripalimab | Phase I/II (NCT05000684) | Advanced or previously treated NSCLC/SCLC | Multi-cohort combination immunotherapy | NSCLC: ORR 4.3%, DCR 47.8%, mPFS 1.5 mo, mOS 18.9 mo; SCLC: ORR 35%, DCR 55%, mPFS 2.8 mo, mOS 12.3 mo | [59] |
| Tifcemalimab + Toripalimab | Phase I/II (NCT05000684) | Refractory extensive-stage SCLC | Combination therapy after prior systemic therapy | Reported promising antitumor activity (ORR 26.3%, DCR 57.9%) | [59] |
| Tifcemalimab + Toripalimab + Docetaxel | Phase Ib/II | Second-line squamous NSCLC after immunotherapy | Triple combination (BTLA+ PD-1+ chemotherapy) | Early data: 6- and 9-month OS rates 85.3%, 70.1% | [89] |
| Peri-operative ESCC (BT-NICE) -tifcemalimab + toripalimab ± chemo | Phase II (NCT06588335) | Resectable esophageal squamous cell carcinoma | Neoadjuvant tifcemalimab + toripalimab + chemo → surgery; adjuvant tifcemalimab + toripalimab (± chemo/RT) | Ongoing; endpoints: pCR rate, DFS, OS | [90] |
| Tifcemalimab + Toripalimab (Consolidation) | Phase III (NCT06095583) | Limited-stage SCLC post-concurrent chemoradiotherapy, no progression | Double-blind, placebo-controlled, 3-arm trial: toripalimab ± tifcemalimab vs. placebo | Ongoing; evaluating PFS/OS | [98] |
| Tifcemalimab + Toripalimab ± Chemotherapy | Phase II (NCT05664971) | Advanced or metastatic NSCLC previously treated with immunotherapy ± chemotherapy | tifcemalimab + toripalimab ± platinum-based chemotherapy | Ongoing; primary endpoints: ORR and safety | [99] |
| Tifcemalimab + Toripalimab | Phase II (NCT06648200) | Limited-stage SCLC following concurrent chemoradiotherapy | Prospective, multicenter trial, as consolidation therapy post-CRT | Ongoing; endpoints: PFS/OS; designed to precede the confirmatory Phase III trial (NCT06095583) | [100] |
| Tifcemalimab + Toripalimab ± Chemo/Radiotherapy | Phase II (NCT06732258) | Advanced or locally advanced solid tumors (exploratory) | Investigator-initiated, open-label basket trial testing BTLA + PD-1 blockade ± standard chemo/radiotherapy | Recruiting; no efficacy data yet; aims to identify tumor types most responsive to dual blockade | [101] |
| Tifcemalimab + Toripalimab | Phase II (NCT06690697) | Clear-cell renal cell carcinoma | Randomized single-center trial examining combined therapy vs. control | Ongoing (no efficacy data yet) | [102] |
| HFB200603 (HiFiBiO) | Phase I (NCT05789069) | Advanced solid tumors | Monotherapy and in combination with tislelizumab (PD-1) | Early poster: disease stabilization and preliminary responses across doses | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-C.; Lee, W.-C.; Tai, Y.-J.; Chiang, Y.-C. BTLA: An Emerging Immune Checkpoint Target in Cancer Immunotherapy. Pharmaceuticals 2025, 18, 1784. https://doi.org/10.3390/ph18121784
Chang M-C, Lee W-C, Tai Y-J, Chiang Y-C. BTLA: An Emerging Immune Checkpoint Target in Cancer Immunotherapy. Pharmaceuticals. 2025; 18(12):1784. https://doi.org/10.3390/ph18121784
Chicago/Turabian StyleChang, Ming-Cheng, Wan-Chi Lee, Yi-Jou Tai, and Ying-Cheng Chiang. 2025. "BTLA: An Emerging Immune Checkpoint Target in Cancer Immunotherapy" Pharmaceuticals 18, no. 12: 1784. https://doi.org/10.3390/ph18121784
APA StyleChang, M.-C., Lee, W.-C., Tai, Y.-J., & Chiang, Y.-C. (2025). BTLA: An Emerging Immune Checkpoint Target in Cancer Immunotherapy. Pharmaceuticals, 18(12), 1784. https://doi.org/10.3390/ph18121784






