Comprehensive Chemometric and Chromatographic Investigation of Lipophilicity of Biologically Active Androstane-3-Oxime Derivatives Across Diverse UHPLC Systems
Abstract
1. Introduction
2. Results
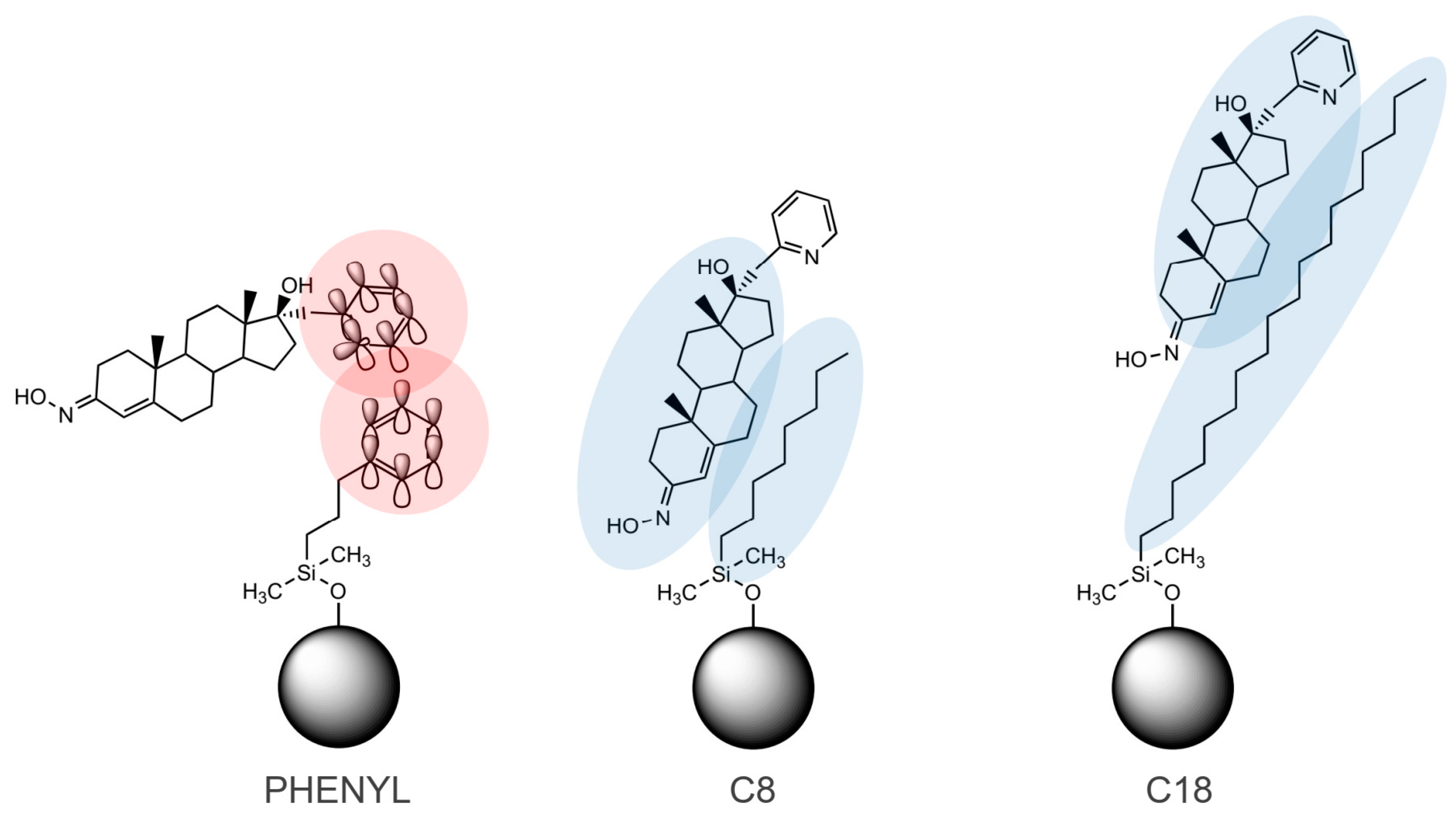
2.1. Lipophilicity Determination on C18 Column
2.2. Lipophilicity Determination on C8 Column
2.3. Lipophilicity Determination on Phenyl Column
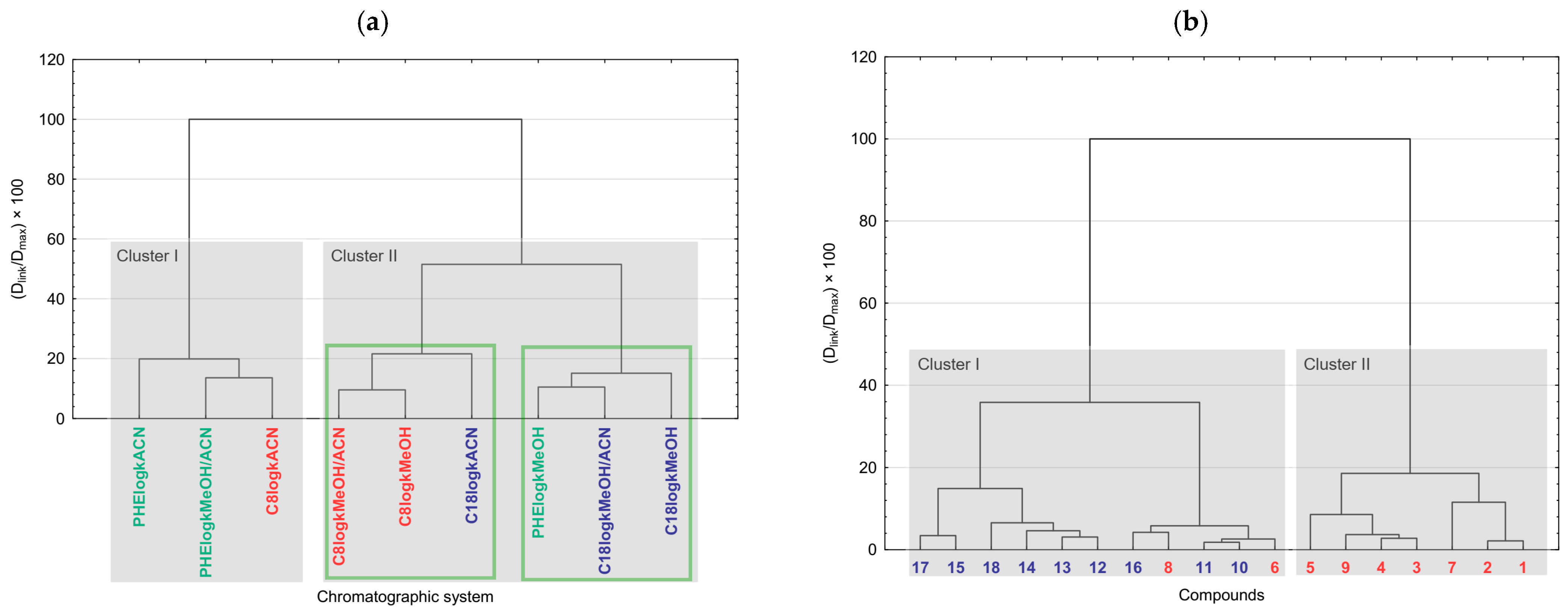
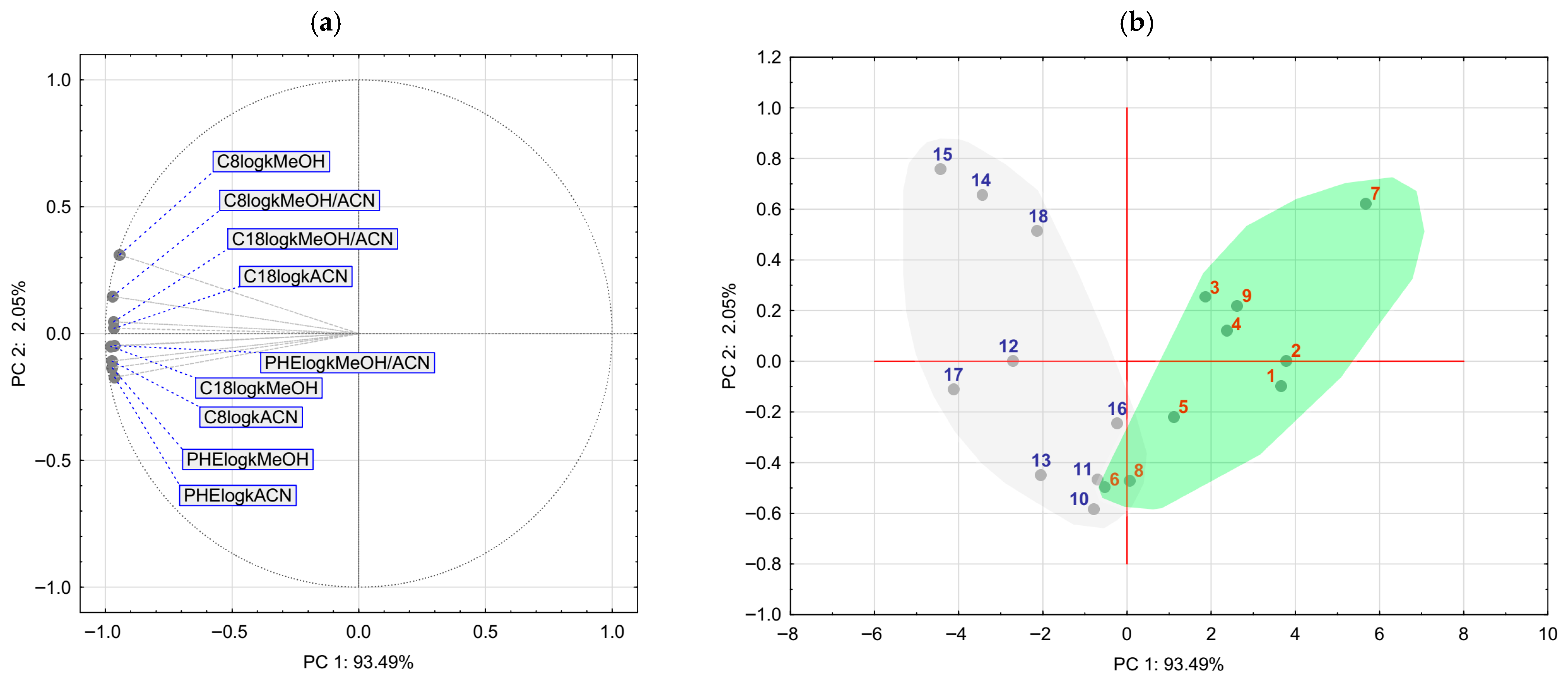
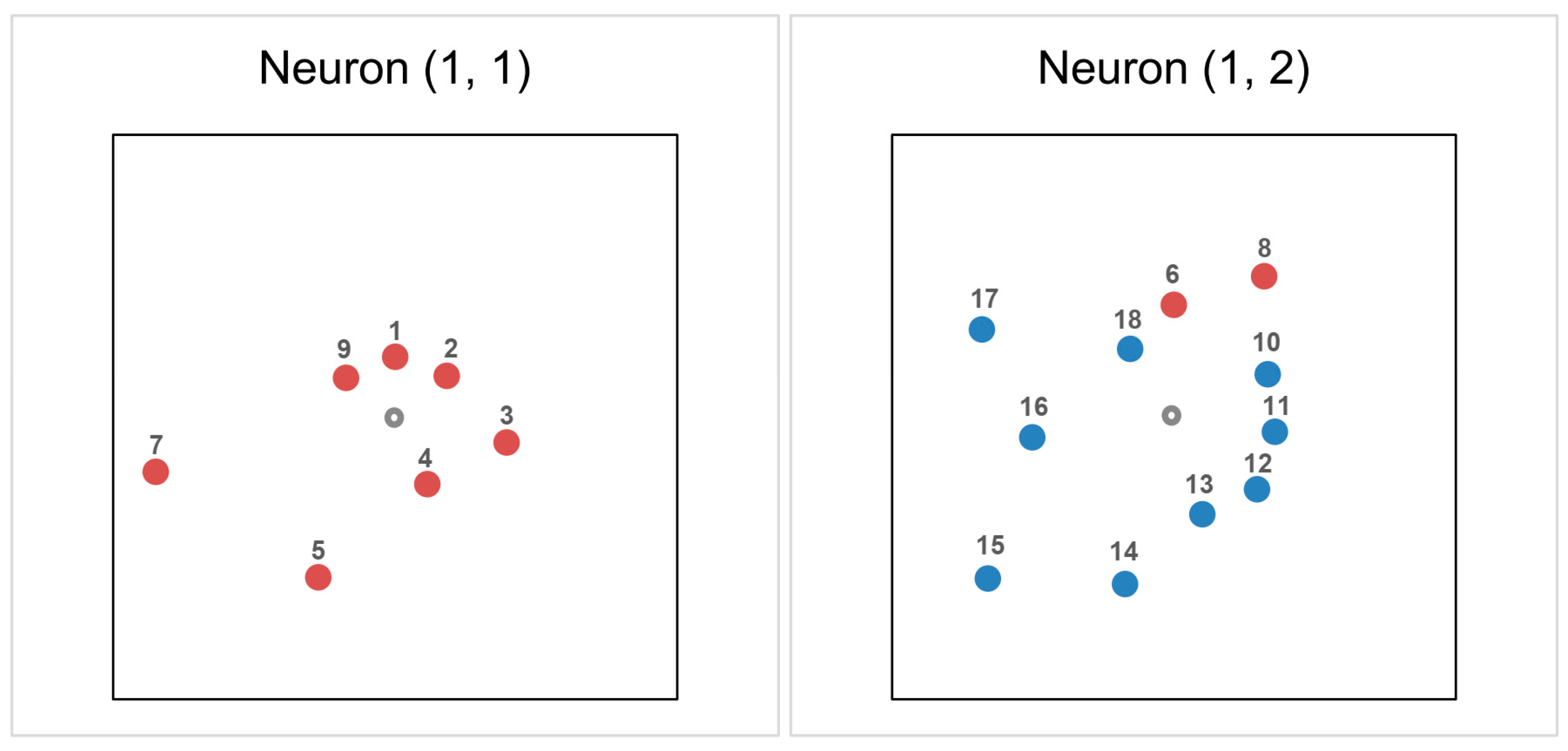
2.4. Chemometrics of the Experimental Lipophilicity Data
3. Discussion
4. Materials and Methods
4.1. Androstane-3-Oxime Derivatives
4.2. UHPLC Chromatographic Analysis
- (1)
- Mobile phase A: methanol/water (protic modifier);
- (2)
- Mobile phase B: methanol/acetonitrile/water (combination of protic and aprotic modifiers);
- (3)
- Mobile phase C: acetonitrile/water (aprotic modifier).
4.3. In Silico Lipophilicity Descriptors
4.4. Chemometric Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RP | Reversed-Phase |
| UHPLC | Ultra-High Performance Liquid Chromatography |
| PRT | Pattern Recognition Techniques |
| CANN | Clustering by Artificial Neural Networks |
| HCA | Hierarchical Cluster Analysis |
| PCA | Principal Component Analysis |
| MeOH | Methanol |
| ACN | Acetonitrile |
References
- Huo, S.; Wu, J.; He, X.; Pan, L.; Du, J. Two New Cytotoxic Steroidal Alkaloids from Sarcococca Hookeriana. Molecules 2019, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Dai, Z.; Xie, T.Z.; Xiao, X.; Liu, Y.P.; Zhao, L.X.; Yang, X.W.; Luo, X.D. Cytotoxic androstane derivatives from Sarcococca ruscifolia. Fitoterapia 2020, 144, 104604. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.H.; Sekar, M.; Fuloria, S.; Begum, M.Y.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Chidambaram, K.; Subramaniyan, V.; Sathasivam, K.V.; et al. Chemistry, Biosynthesis and Pharmacology of Sarsasapogenin: A Potential Natural Steroid Molecule for New Drug Design, Development and Therapy. Molecules 2022, 27, 2032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Li, L.; Shen, N.; Li, X.Y.; Bao, N.M.; Yang, X.X.; Qiu, B.; Chen, Z.; Gu, W.; et al. β-Ecdysterone and Sarsasapogenin, first isolated from Polygonatum kingianum, reveal its therapeutic potential in MAFLD via 16S rRNA sequencing and metabolomics. J. Agric. Food Res. 2025, 23, 102236. [Google Scholar] [CrossRef]
- An, R.F.; Wu, K.T.; Pan, J.; Zhang, W.J.; Qin, H.Y.; Li, X.R.; Liu, W.; Huang, X.F. Design, synthesis and cytotoxic activity of novel lipophilic cationic derivatives of diosgenin and sarsasapogenin. Bioorganic Med. Chem. Lett. 2025, 119, 130094. [Google Scholar] [CrossRef] [PubMed]
- Ajduković, J.J.; Djurendić, E.A.; Petri, E.T.; Klisurić, O.R.; Celić, A.S.; Sakač, M.N.; Jakimov, D.S.; Gaši, K.M. 17(E)-picolinylidene androstane derivatives as potential inhibitors of prostate cancer cell growth: Antiproliferative activity and molecular docking studies. Bioorg. Med. Chem. 2013, 21, 7257–7266. [Google Scholar] [CrossRef] [PubMed]
- Dzichenka, Y.; Shapira, M.; Yantsevich, A.; Cherkesova, T.; Grbović, L.; Savić, M.; Usanov, S.; Jovanović-Šanta, S. Modified bile acids and androstanes-Novel promising inhibitors of human cytochrome P450 17A1. J. Steroid Biochem. Mol. Biol. 2021, 205, 105777. [Google Scholar] [CrossRef] [PubMed]
- Šestić, T.L.; Bekić, S.S.; Marinović, M.A.; Kvasnicová, M.; Štenclová, T.; Rárová, L.; Ćelić, A.S.; Petri, E.T.; Ajduković, J.J.; Strnad, M.; et al. Heterocyclic androstane derivatives targeting hormone-related cancers: Synthesis, bioactivity and docking studies. Eur. J. Med. Chem. 2025, 296, 117850. [Google Scholar] [CrossRef] [PubMed]
- Vinš, P.; Černý, I.; Mikšátková, P.; Drašar, P. Synthesis of 5α-androstane-3α,17β-diol 17-O-glucuronide histaminyl conjugate for immunoassays. Steroids 2016, 109, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Savić, M.P.; Škorić, D.Đ.; Kuzminac, I.Z.; Jakimov, D.S.; Kojić, V.V.; Rárová, L.; Strnad, M.; Djurendić, E.A. New A-homo lactam D-homo lactone androstane derivative: Synthesis and evaluation of cytotoxic and anti-inflammatory activities in vitro. Steroids 2020, 157, 108596. [Google Scholar] [CrossRef] [PubMed]
- Kulmány, Á.E.; Herman, B.E.; Zupkó, I.; Sinreih, M.; Rižner, T.L.; Savić, M.; Oklješa, A.; Nikolić, A.; Nagy, V.; Ocsovszki, I.; et al. Heterocyclic androstane and estrane d-ring modified steroids: Microwave-assisted synthesis, steroid-converting enzyme inhibition, apoptosis induction, and effects on genes encoding estrogen inactivating enzymes. J. Steroid Biochem. Mol. Biol. 2021, 214, 105997. [Google Scholar] [CrossRef] [PubMed]
- Ajduković, J.J.; Jakimov, D.S.; Rárová, L.; Strnad, M.; Dzichenka, Y.U.; Usanov, S.; Škorić, D.Ð.; Jovanović-Šanta, S.S.; Sakač, M.N. Novel alkylaminoethyl derivatives of androstane 3-oximes as anticancer candidates: Synthesis and evaluation of cytotoxic effect. RSC Adv. 2021, 11, 37449–37461. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, S.Z.; Podunavac-Kuzmanović, S.O.; Jevrić, L.R.; Jovanov, P.T.; Djurendić, E.A.; Ajduković, J.J. Comprehensive QSRR modeling as a starting point in characterization and further development of anticancer drugs based on 17α-picolyl and 17(E)-picolinylidene androstane structures. Eur. J. Pharm. Sci. 2016, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, S.; Karadžić Banjac, M.; Anojčić, J.; Podunavac-Kuzmanović, S.; Jevrić, L.; Nikolić, A.; Savić, M.; Kuzminac, I. Chemometrics of anisotropic lipophilicity of anticancer androstane derivatives determined by reversed-phase ultra high performance liquid chromatography with polar aprotic and protic modifiers. J. Chromatogr. A 2022, 1673, 463197. [Google Scholar] [CrossRef] [PubMed]
- Ciura, K. Modeling of small molecule’s affinity to phospholipids using IAM-HPLC and QSRR approach enhanced by similarity-based machine algorithms. J. Chromatogr. A 2024, 1714, 464549. [Google Scholar] [CrossRef] [PubMed]
- Kaliszan, R. QSRR: Quantitative Structure-(Chromatographic) Retention Relationship. Chem. Rev. 2007, 107, 3212–3246. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, S.; Karadžić Banjac, M.; Podunavac-Kuzmanović, S.; Ajduković, J.; Salaković, B.; Rárova, L.; Đorđević, M.; Ivanov, M. Local QSAR modeling of cytotoxic activity of newly designed androstane 3-oximes towards malignant melanoma cells. J. Mol. Struct. 2023, 1283, 135272. [Google Scholar] [CrossRef]
- Benhaim, D.; Grushka, E. Characterization of the GEMINI C18TM Column: Lipophilicity Measurement and LSER. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2198–2218. [Google Scholar] [CrossRef]
- Cecil, T.; Bautista, J.; Collinson, M.M.; Rutan, S.C. Preparation and characterization of stationary phase gradients on C8 liquid chromatography columns. J. Chromatogr. A 2024, 1727, 464974. [Google Scholar] [CrossRef] [PubMed]
- Nasal, A.; Siluk, D.; Kaliszan, R. Chromatographic Retention Parameters in Medicinal Chemistry and Molecular Pharmacology. Curr. Med. Chem. 2003, 10, 381–426. [Google Scholar] [CrossRef] [PubMed]
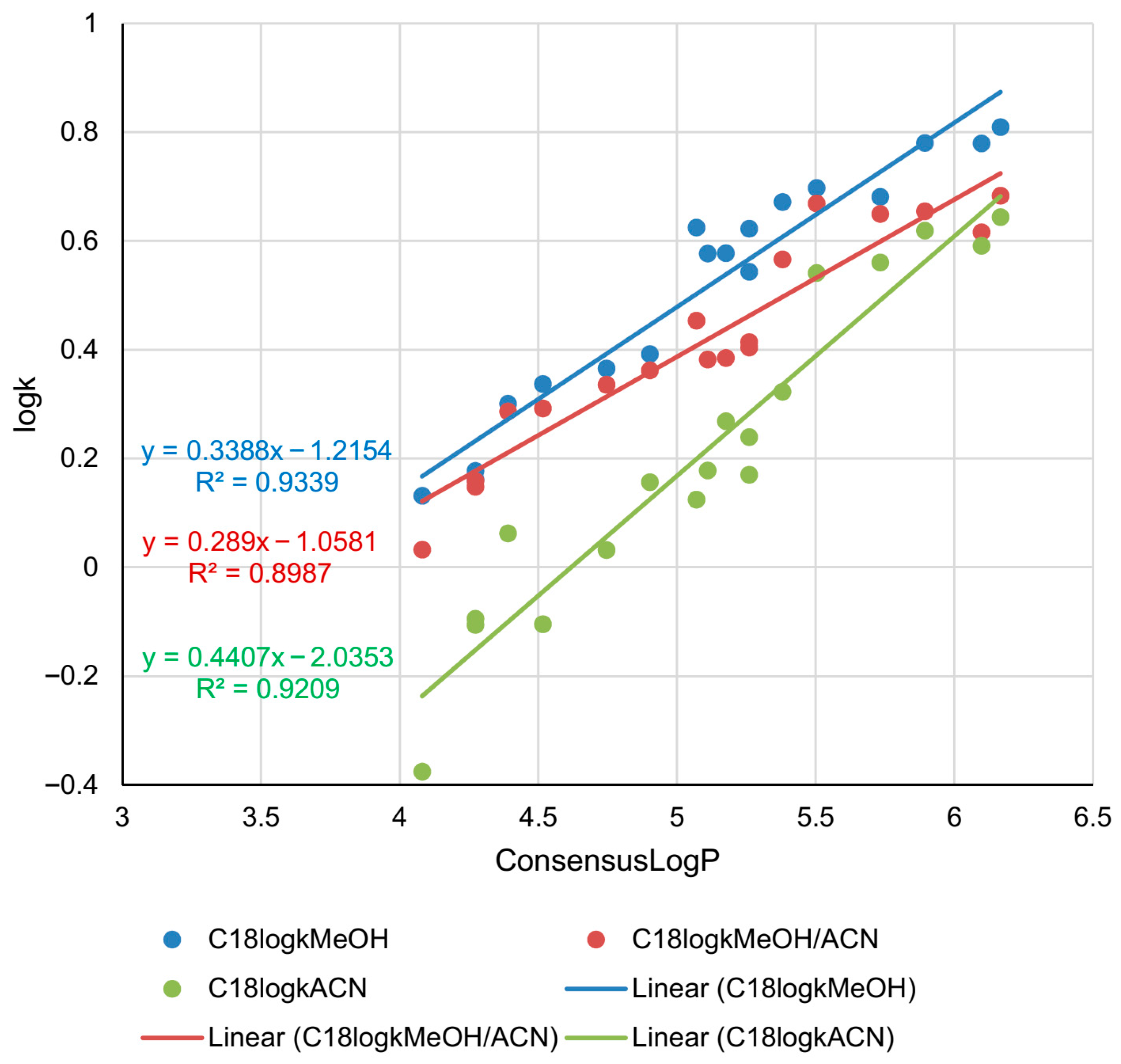
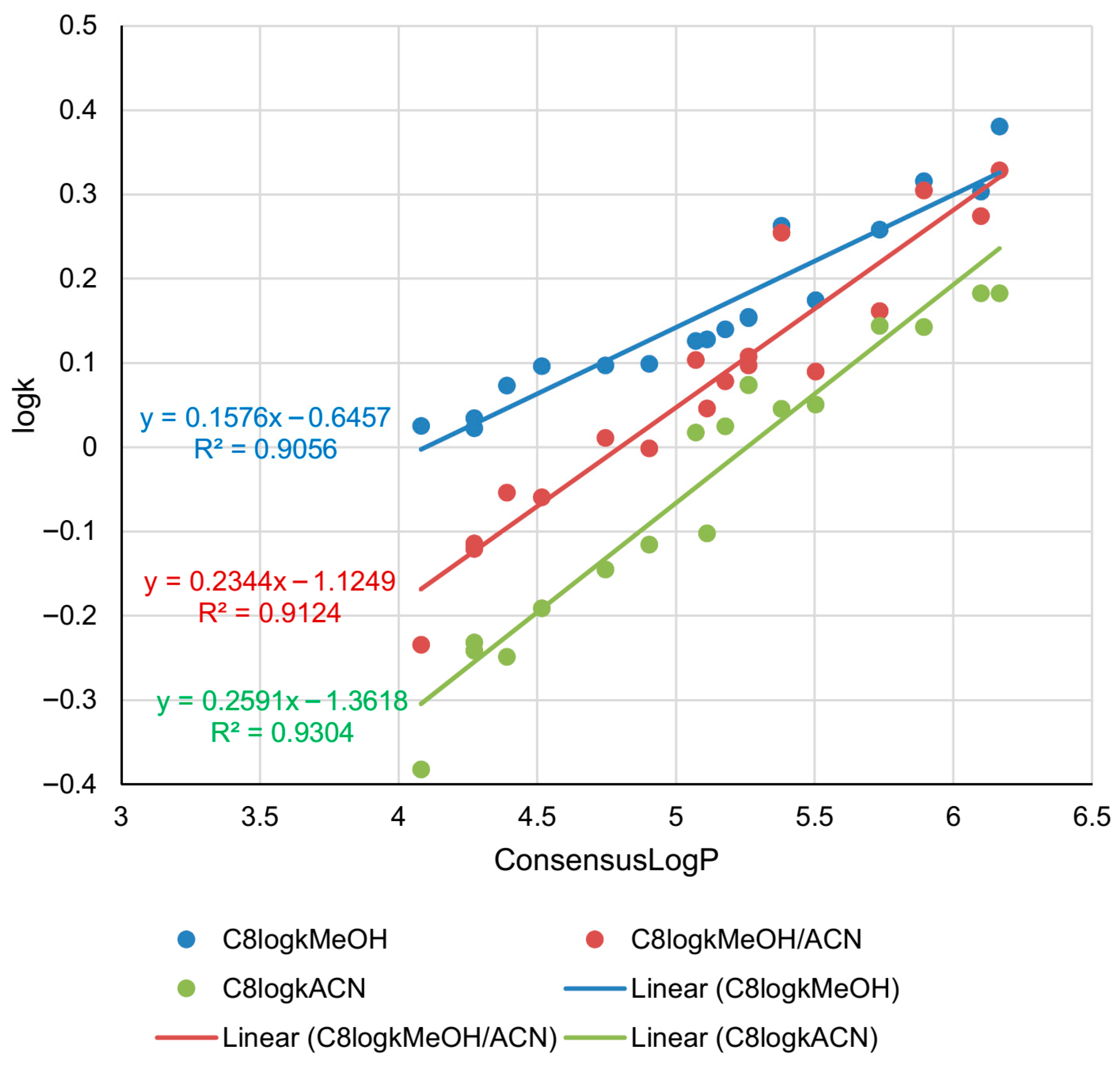
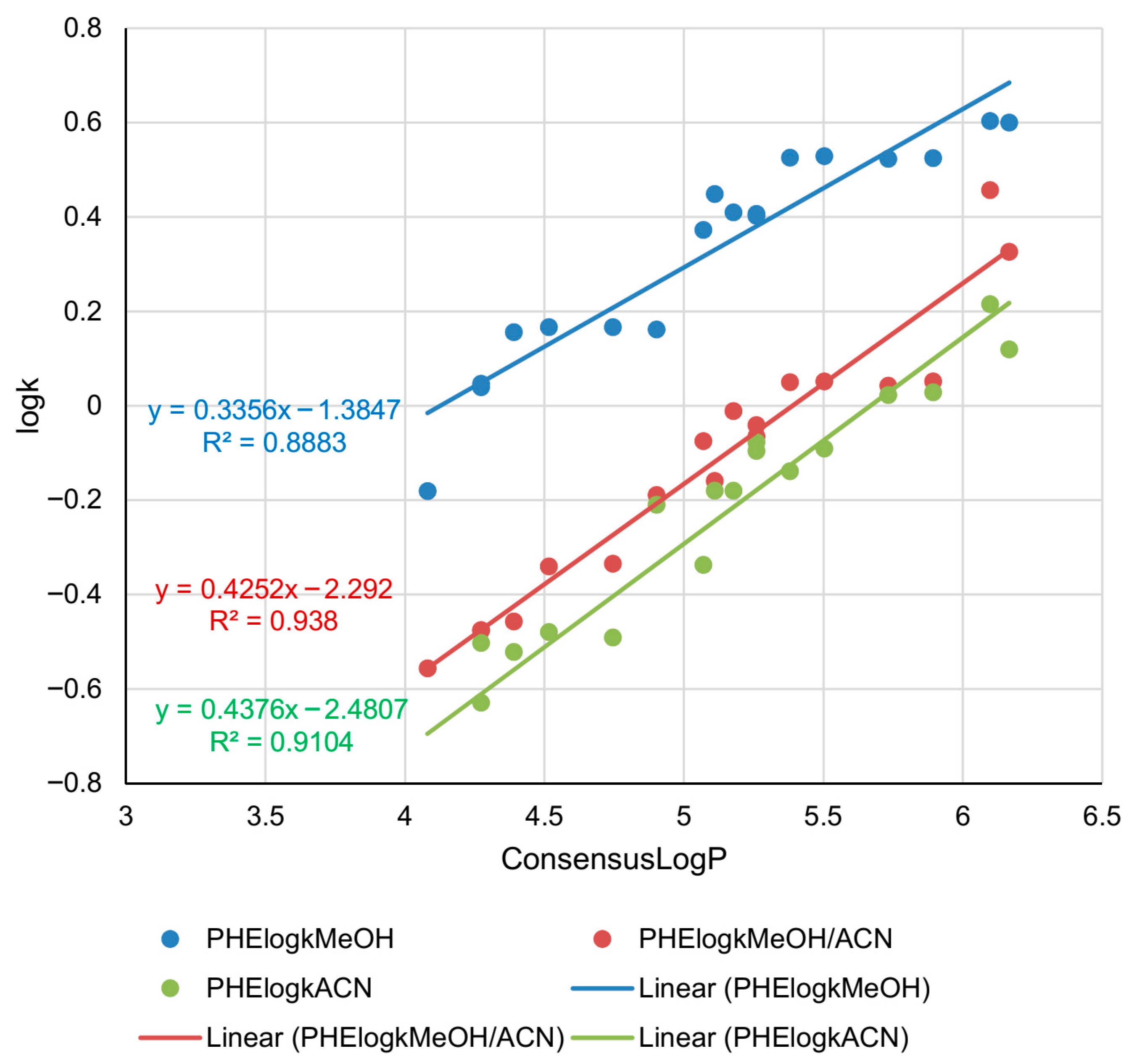
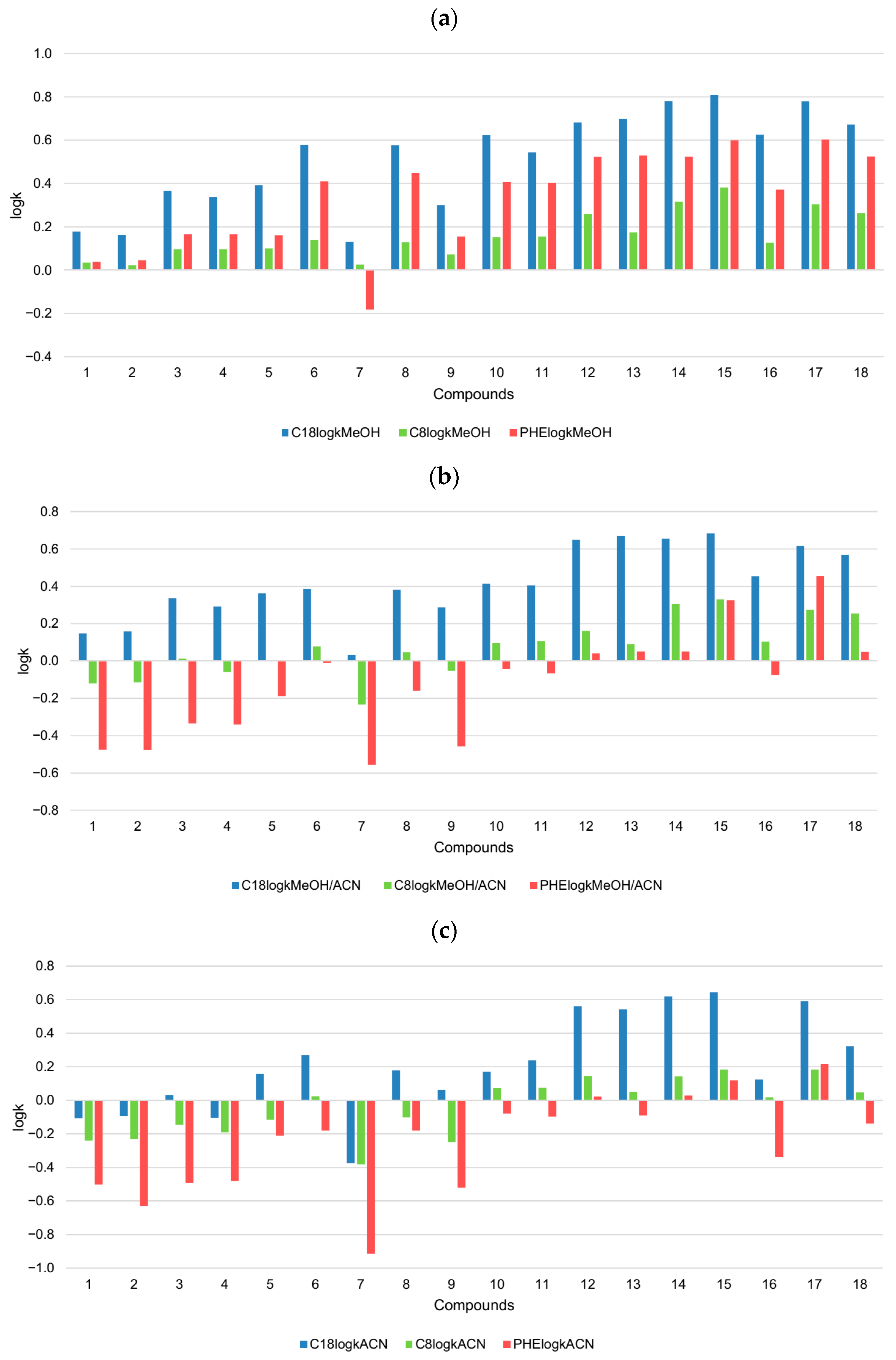
| Compound | C18logkMeOH | C18logkMeOH/ACN | C18logkACN | C8logkMeOH | C8logkMeOH/ACN | C8logkACN | PHElogkMeOH | PHElogkMeOH/ACN | PHElogkACN |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.178 | 0.148 | −0.106 | 0.035 | −0.121 | −0.241 | 0.039 | −0.475 | −0.502 |
| 2 | 0.162 | 0.159 | −0.095 | 0.023 | −0.114 | −0.231 | 0.046 | −0.476 | −0.629 |
| 3 | 0.366 | 0.336 | 0.032 | 0.097 | 0.011 | −0.145 | 0.166 | −0.335 | −0.491 |
| 4 | 0.338 | 0.292 | −0.104 | 0.097 | −0.059 | −0.191 | 0.166 | −0.341 | −0.480 |
| 5 | 0.392 | 0.362 | 0.157 | 0.099 | −0.001 | −0.116 | 0.161 | −0.190 | −0.210 |
| 6 | 0.578 | 0.385 | 0.269 | 0.140 | 0.079 | 0.025 | 0.409 | −0.011 | −0.180 |
| 7 | 0.132 | 0.033 | −0.376 | 0.025 | −0.234 | −0.382 | −0.181 | −0.557 | −0.915 |
| 8 | 0.577 | 0.383 | 0.178 | 0.128 | 0.046 | −0.102 | 0.448 | −0.160 | −0.180 |
| 9 | 0.301 | 0.287 | 0.062 | 0.073 | −0.054 | −0.248 | 0.155 | −0.457 | −0.522 |
| 10 | 0.623 | 0.414 | 0.171 | 0.154 | 0.097 | 0.074 | 0.406 | −0.041 | −0.078 |
| 11 | 0.544 | 0.405 | 0.239 | 0.155 | 0.108 | 0.074 | 0.402 | −0.065 | −0.096 |
| 12 | 0.681 | 0.650 | 0.561 | 0.258 | 0.162 | 0.144 | 0.522 | 0.042 | 0.022 |
| 13 | 0.698 | 0.669 | 0.542 | 0.175 | 0.090 | 0.051 | 0.529 | 0.051 | −0.091 |
| 14 | 0.780 | 0.655 | 0.619 | 0.316 | 0.305 | 0.143 | 0.524 | 0.051 | 0.028 |
| 15 | 0.810 | 0.683 | 0.644 | 0.381 | 0.329 | 0.183 | 0.599 | 0.326 | 0.119 |
| 16 | 0.625 | 0.453 | 0.125 | 0.126 | 0.104 | 0.018 | 0.373 | −0.075 | −0.337 |
| 17 | 0.780 | 0.616 | 0.591 | 0.304 | 0.275 | 0.183 | 0.603 | 0.456 | 0.215 |
| 18 | 0.672 | 0.566 | 0.323 | 0.263 | 0.255 | 0.046 | 0.525 | 0.050 | −0.139 |
| Compound | WLOGP | VGlogP | KLOPlogP | PHYSlogP | WGlogP | logPCD | ConsensusLogP |
|---|---|---|---|---|---|---|---|
| 1 | 5.150 | 3.500 | 4.020 | 4.555 | 4.201 | 4.210 | 4.273 |
| 2 | 5.150 | 3.500 | 4.020 | 4.555 | 4.201 | 4.210 | 4.273 |
| 3 | 5.410 | 4.722 | 4.224 | 4.754 | 4.572 | 4.790 | 4.745 |
| 4 | 5.640 | 4.449 | 3.819 | 4.394 | 4.212 | 4.580 | 4.516 |
| 5 | 5.790 | 4.845 | 4.305 | 4.909 | 4.697 | 4.870 | 4.903 |
| 6 | 5.800 | 5.118 | 4.694 | 5.222 | 5.028 | 5.200 | 5.177 |
| 7 | 4.640 | 4.054 | 3.686 | 4.087 | 3.948 | 4.070 | 4.081 |
| 8 | 6.030 | 5.082 | 4.534 | 5.008 | 4.862 | 5.150 | 5.111 |
| 9 | 5.250 | 4.396 | 3.806 | 4.279 | 4.141 | 4.470 | 4.390 |
| 10 | 6.150 | 4.900 | 4.733 | 5.563 | 5.176 | 5.040 | 5.260 |
| 11 | 6.150 | 4.900 | 4.733 | 5.563 | 5.176 | 5.040 | 5.260 |
| 12 | 6.410 | 6.121 | 4.938 | 5.762 | 5.547 | 5.620 | 5.733 |
| 13 | 6.640 | 5.848 | 4.533 | 5.402 | 5.187 | 5.410 | 5.503 |
| 14 | 6.800 | 6.244 | 5.019 | 5.917 | 5.672 | 5.710 | 5.894 |
| 15 | 6.800 | 6.517 | 5.407 | 6.230 | 6.003 | 6.040 | 6.166 |
| 16 | 5.640 | 5.453 | 4.400 | 5.095 | 4.923 | 4.910 | 5.070 |
| 17 | 7.030 | 6.480 | 5.247 | 6.016 | 5.837 | 5.980 | 6.098 |
| 18 | 6.250 | 5.796 | 4.520 | 5.288 | 5.116 | 5.310 | 5.380 |
| Compound | Set | Neuron No. | Position | Activations |
|---|---|---|---|---|
| 1 | Train | 1 | (1, 1) | 0.257 |
| 2 | Train | 1 | (1, 1) | 0.281 |
| 3 | Validation | 1 | (1, 1) | 0.486 |
| 4 | Train | 1 | (1, 1) | 0.316 |
| 5 | Validation | 1 | (1, 1) | 0.756 |
| 6 | Train | 2 | (1, 2) | 0.476 |
| 7 | Test | 1 | (1, 1) | 1.045 |
| 8 | Train | 2 | (1, 2) | 0.712 |
| 9 | Train | 1 | (1, 1) | 0.267 |
| 10 | Train | 2 | (1, 2) | 0.438 |
| 11 | Train | 2 | (1, 2) | 0.434 |
| 12 | Train | 2 | (1, 2) | 0.468 |
| 13 | Train | 2 | (1, 2) | 0.430 |
| 14 | Train | 2 | (1, 2) | 0.739 |
| 15 | Test | 2 | (1, 2) | 1.049 |
| 16 | Train | 2 | (1, 2) | 0.609 |
| 17 | Train | 2 | (1, 2) | 0.899 |
| 18 | Train | 2 | (1, 2) | 0.345 |
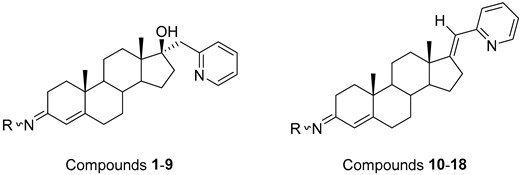 | ||
|---|---|---|
| Compounds of GROUP A (17-Picolyl Series) | R | Compounds of GROUP B (17-Picolynilidene Series) |
| 1 | 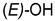 | 10 |
| 2 | 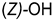 | 11 |
| 3 | 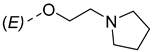 | 12 |
| 4 | 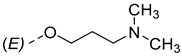 | 13 |
| 5 | 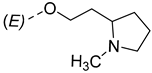 | 14 |
| 6 | 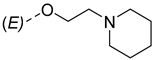 | 15 |
| 7 | 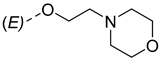 | 16 |
| 8 | 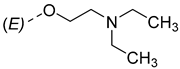 | 17 |
| 9 | 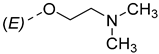 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, S.Z.; Karadžić Banjac, M.Ž.; Podunavac-Kuzmanović, S.O.; Anojčić, J.S.; Ajduković, J.J. Comprehensive Chemometric and Chromatographic Investigation of Lipophilicity of Biologically Active Androstane-3-Oxime Derivatives Across Diverse UHPLC Systems. Pharmaceuticals 2025, 18, 1778. https://doi.org/10.3390/ph18121778
Kovačević SZ, Karadžić Banjac MŽ, Podunavac-Kuzmanović SO, Anojčić JS, Ajduković JJ. Comprehensive Chemometric and Chromatographic Investigation of Lipophilicity of Biologically Active Androstane-3-Oxime Derivatives Across Diverse UHPLC Systems. Pharmaceuticals. 2025; 18(12):1778. https://doi.org/10.3390/ph18121778
Chicago/Turabian StyleKovačević, Strahinja Z., Milica Ž. Karadžić Banjac, Sanja O. Podunavac-Kuzmanović, Jasmina S. Anojčić, and Jovana J. Ajduković. 2025. "Comprehensive Chemometric and Chromatographic Investigation of Lipophilicity of Biologically Active Androstane-3-Oxime Derivatives Across Diverse UHPLC Systems" Pharmaceuticals 18, no. 12: 1778. https://doi.org/10.3390/ph18121778
APA StyleKovačević, S. Z., Karadžić Banjac, M. Ž., Podunavac-Kuzmanović, S. O., Anojčić, J. S., & Ajduković, J. J. (2025). Comprehensive Chemometric and Chromatographic Investigation of Lipophilicity of Biologically Active Androstane-3-Oxime Derivatives Across Diverse UHPLC Systems. Pharmaceuticals, 18(12), 1778. https://doi.org/10.3390/ph18121778











