Advances in Lipid-Polymer Hybrid Nanoparticles: Design Strategies, Functionalization, Oncological and Non-Oncological Clinical Prospects
Abstract
1. Introduction
2. Design and Fabrication
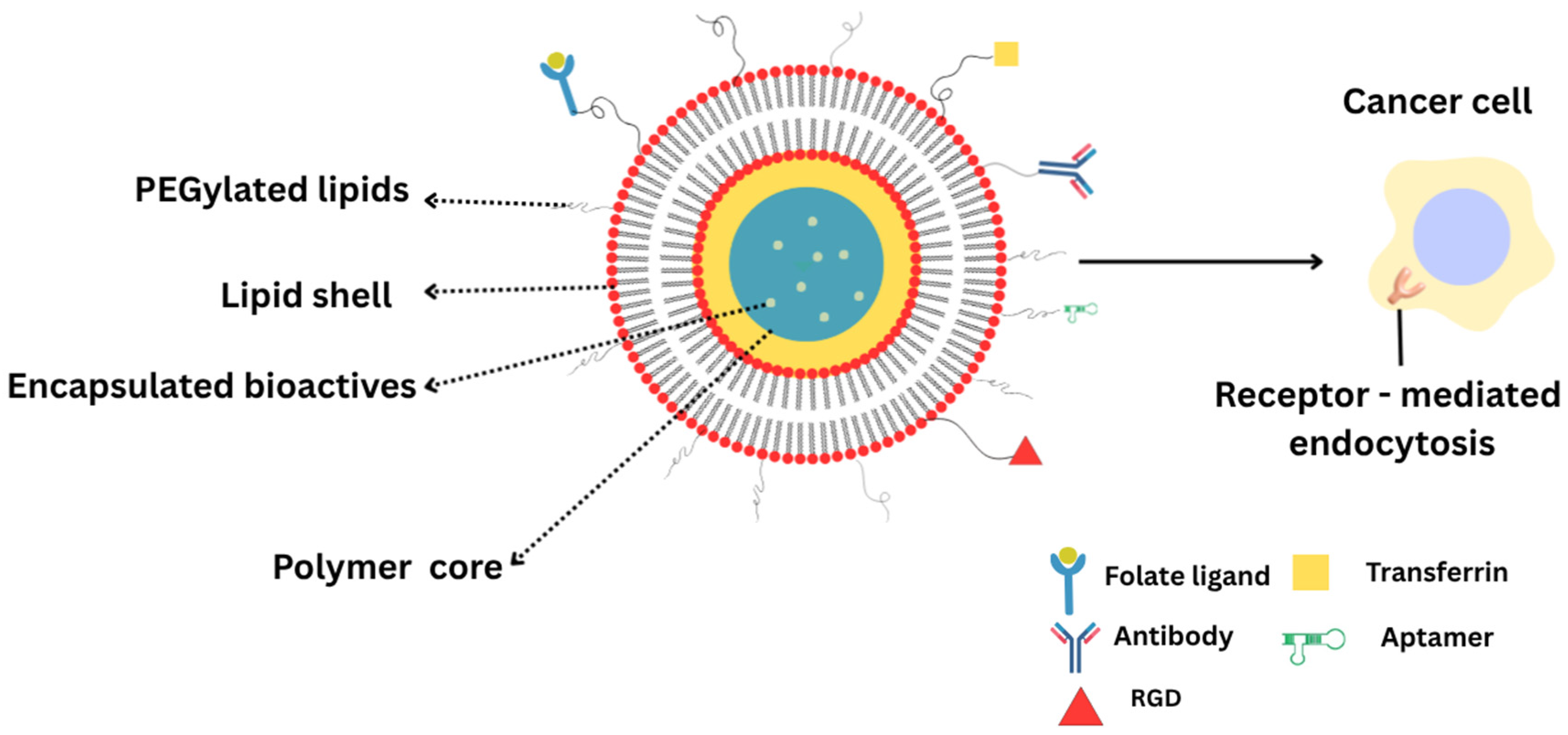
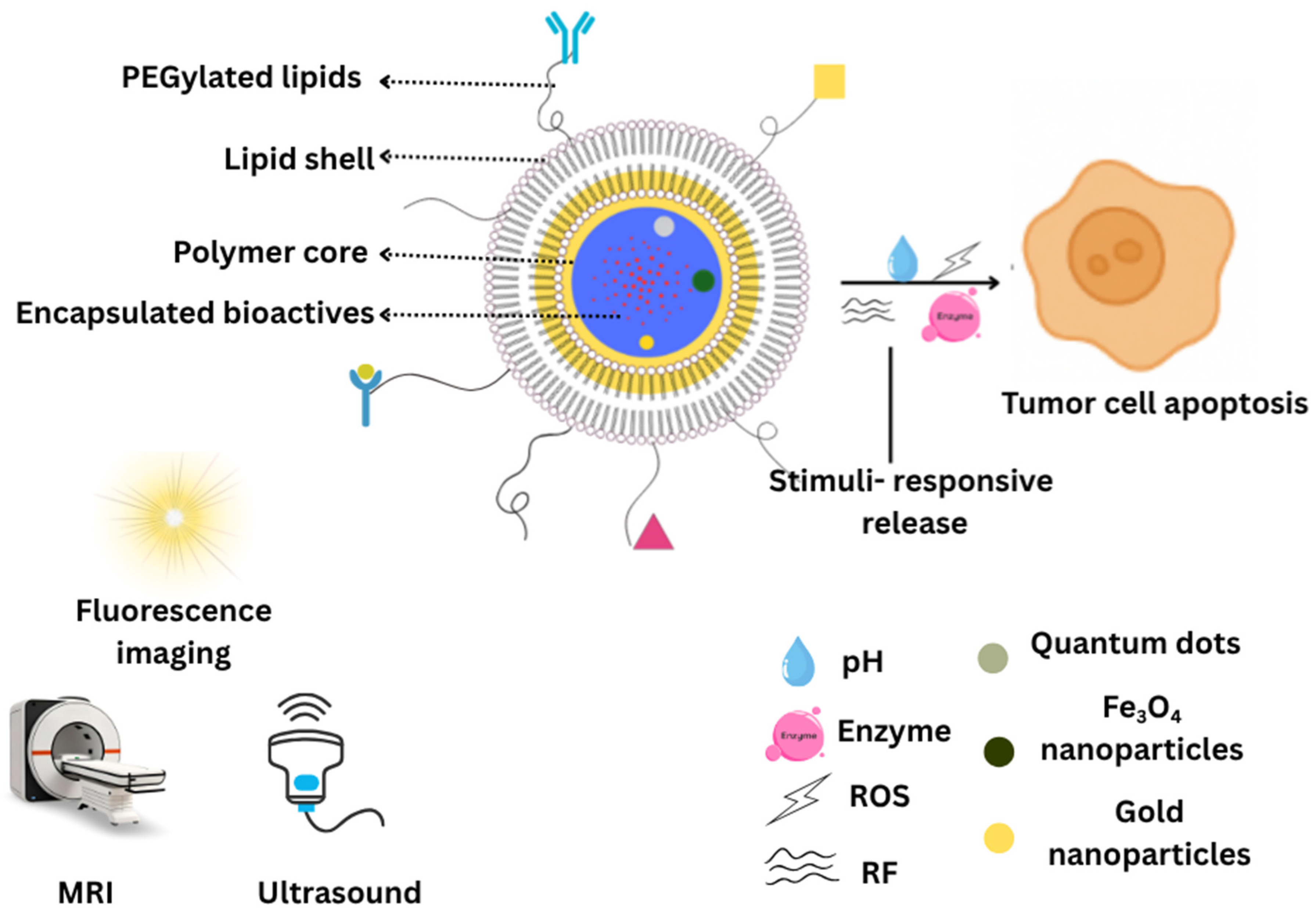
2.1. Structural Features of LPHNPs
2.1.1. Core–Shell Architecture
2.1.2. Bio-Mimetic Coatings
2.1.3. Polymer-Caged Nanobins
2.2. Materials
2.3. Methods of Preparation
2.3.1. Self-Assembly Nanoprecipitation
2.3.2. Emulsification-Solvent Evaporation
2.3.3. Microfluidic Technique
2.4. Quality by Design (QbD) Considerations in LPHNP Development
3. Functionalization Strategies for Targeted Therapy
3.1. Folate Ligands
3.2. iRGD and Tumor-Penetrating Peptides
3.3. Transferrin, Aptamer, and Antibody-Conjugated Systems
4. Therapeutic Applications
4.1. Oncological
4.1.1. Phytochemicals and Natural Bioactives
4.1.2. Co-Delivery of Drugs and Phytochemicals
4.1.3. Gene and siRNA
4.1.4. Pulmonary
4.2. Non-Oncological
4.2.1. Ocular
4.2.2. Topical
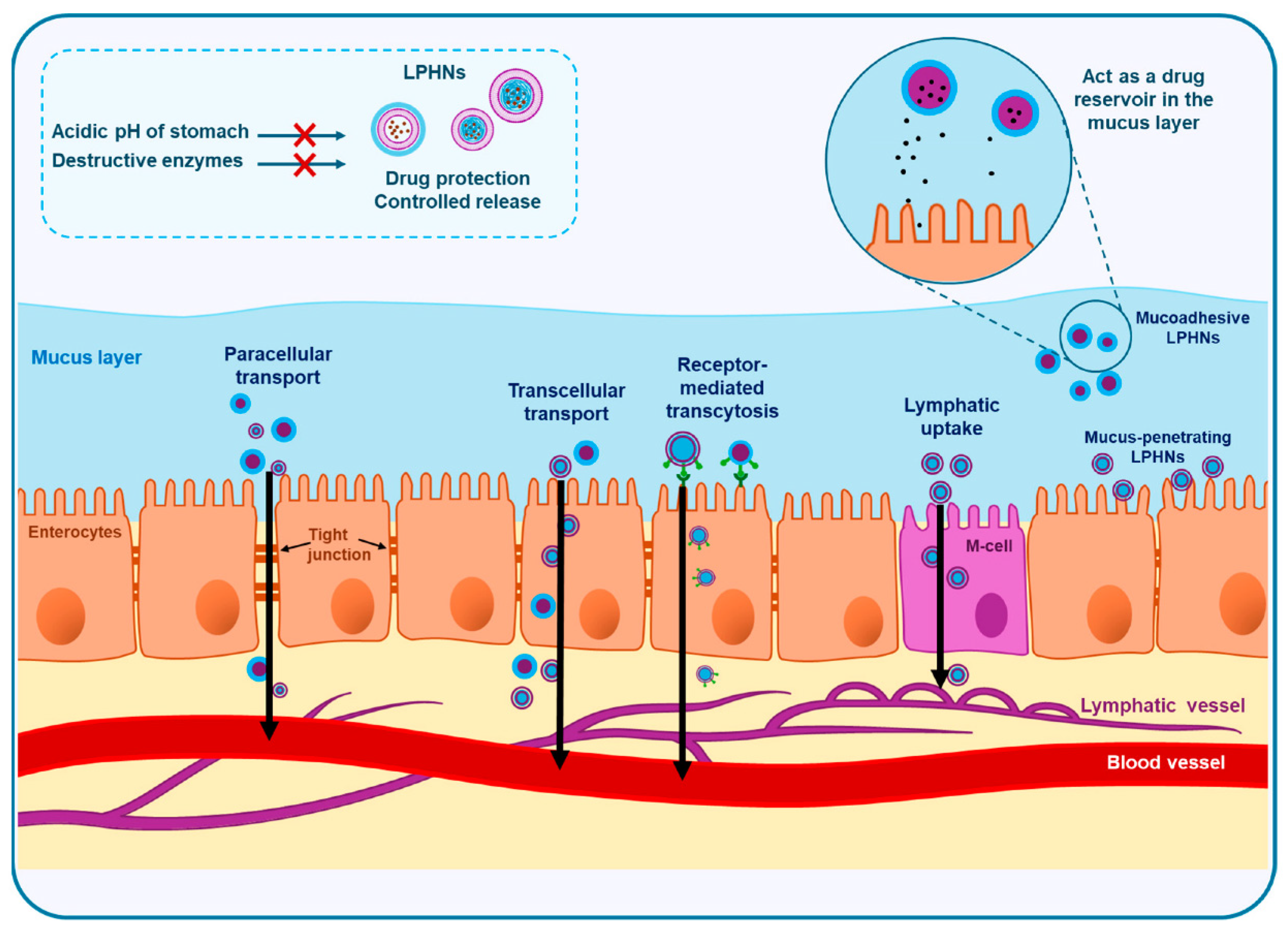
4.2.3. Oral and Nanophytomedicine
4.2.4. Theranostics and Imaging
5. Translation Challenges
5.1. Patents and Clinical Trials
5.2. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CPT | Camptothecin |
| CMAs | Critical material attributes |
| CQAs | Critical quality attributes |
| CUR | Curcumin |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
| EPR | Enhanced permeability and retention |
| t½ | Half-life |
| HCPT | Hydroxycamptothecin |
| LPHNPs | Lipid-polymer hybrid nanoparticles |
| mRN | Messenger RNA |
| PEG | Polyethylene glycol |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| QbD | Quality by design |
| QTPP | Quality target product profile |
| RBC | Red blood cell |
| siRNA | Small interfering RNA |
| TNBC | Triple-negative breast cancer |
References
- Zhu, Y.; Shen, R.; Vuong, I.; Reynolds, R.A.; Shears, M.J.; Yao, Z.C.; Hu, Y.; Cho, W.J.; Kong, J.; Reddy, S.K.; et al. Multi-step screening of DNA/lipid nanoparticles and co-delivery with siRNA to enhance and prolong gene expression. Nat. Commun. 2022, 13, 4282. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.C.; Chowdhury, A.; Hossain, M.M.; Hossain, M.K. Applications, drawbacks, and future scope of nanoparticle-based polymer composites. In Nanoparticle-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–275. [Google Scholar]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Gorain, B.; Al-Dhubiab, B.E.; Nair, A.; Kesharwani, P.; Pandey, M.; Choudhury, H. Multivesicular liposome: A lipid-based drug delivery system for efficient drug delivery. Curr. Pharm. Des. 2021, 27, 4404–4415. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Pawar, S.; Rauf, M.A.; Abdelhady, H.; Iyer, A.K. Tau-targeting nanoparticles for treatment of Alzheimer’s disease. Exploration 2025, 5, 20230137. [Google Scholar] [CrossRef]
- Chauhan, N.; Vasava, P.; Khan, S.L.; Siddiqui, F.A.; Islam, F.; Chopra, H.; Emran, T.B. Ethosomes: A novel drug carrier. Ann. Med. Surg. 2022, 82, 104595. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A review of the state of the art. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Hasegawa, M.; Ayano, E.; Maitani, Y.; Kanazawa, H. Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection. Int. J. Mol. Sci. 2019, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, Y.J.; Im, G.B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic nanoparticles applied as functional therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in polymeric micelles for drug delivery applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Al-Dhubiab, B.E. 6—Dendrimers: An effective drug delivery and therapeutic approach. In Design and Applications of Theranostic Nanomedicines; Ray, S., Nayak, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 125–142. [Google Scholar]
- Saeed, M.; Haq, R.S.U.; Ahmed, S.; Siddiqui, F.; Yi, J. Recent advances in carbon nanotubes, graphene and carbon fibers-based microwave absorbers. J. Alloys Compd. 2024, 970, 172625. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, Applications, and Prospects of Graphene Quantum Dots: A Comprehensive Review. Small 2022, 18, e2102683. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Attimarad, M.; Nair, A.B. Nanosuspension Innovations: Expanding Horizons in Drug Delivery Techniques. Pharmaceutics 2025, 17, 136. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Shah, J.; Nair, A.B. Innovations in Nanoemulsion Technology: Enhancing Drug Delivery for Oral, Parenteral, and Ophthalmic Applications. Pharmaceutics 2024, 16, 1333. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.; Hilles, A.R.; Kumar, M.; Ansari, M.D.; Mahmood, S. Lipid-polymer hybrid nanoparticles: A cutting-edge frontier in breast cancer treatment strategies. Nanomedicine 2025, 20, 1775–1798. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.A.; Ramadan, E.; Kristó, K.; Regdon, G., Jr.; Sovány, T. Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery. Pharmaceutics 2025, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, S.; Lamberti, G.; Barba, A.A. Polymer-Lipid Pharmaceutical Nanocarriers: Innovations by New Formulations and Production Technologies. Pharmaceutics 2021, 13, 198. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Tanna, V.; Vora, A.; Shah, P.; Nair, A.B.; Shah, J.; Sawarkar, S.P. PLGA Nanoparticles Based Mucoadhesive Nasal In Situ Gel for Enhanced Brain Delivery of Topiramate. AAPS PharmSciTech 2024, 25, 205. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric Lipid Hybrid Nanoparticles (PLNs) as Emerging Drug Delivery Platform-A Comprehensive Review of Their Properties, Preparation Methods, and Therapeutic Applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Godara, S.; Lather, V.; Kirthanashri, S.V.; Awasthi, R.; Pandita, D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110576. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, X.; Xu, Y.; Liu, D.; He, H.; Wang, Y.; Tang, X. Hydroxycamptothecin (HCPT)-loaded PEGlated lipid-polymer hybrid nanoparticles for effective delivery of HCPT: QbD-based development and evaluation. Drug Deliv. Transl. Res. 2022, 12, 306–324. [Google Scholar] [CrossRef]
- Anwer, M.K.; Ali, E.A.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Saqr, A.A.; Ansari, M.N.; Aboudzadeh, M.A. Development of Sustained Release Baricitinib Loaded Lipid-Polymer Hybrid Nanoparticles with Improved Oral Bioavailability. Molecules 2021, 27, 168. [Google Scholar] [CrossRef]
- Markowski, A.; Jaromin, A.; Migdał, P.; Olczak, E.; Zygmunt, A.; Zaremba-Czogalla, M.; Pawlik, K.; Gubernator, J. Design and Development of a New Type of Hybrid PLGA/Lipid Nanoparticle as an Ursolic Acid Delivery System against Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 5536. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Liu, C.; Zhang, X.; Zhang, X.; Chen, X.; Kou, Y.; Mao, S. Chitosan based polymer-lipid hybrid nanoparticles for oral delivery of enoxaparin. Int. J. Pharm. 2018, 547, 499–505. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ouyang, Y.; Zhang, L.; Li, J.; Wang, S. Blood Cell Membrane-Coated Nanomaterials as a Versatile Biomimetic Nanoplatform for Antitumor Applications. Nanomaterials 2024, 14, 1757. [Google Scholar] [CrossRef] [PubMed]
- Magallanes-Puebla, A.; López-Marín, L.M. Lipid bilayer-coated nanoparticles: Mimetism for biomedical applications. Mundo Nano Rev. Interdiscip. Nanociencias Nanotecnología 2023, 16, 1e–15e. [Google Scholar] [CrossRef]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells 2019, 8, 881. [Google Scholar] [CrossRef]
- Li, M.; Fang, H.; Liu, Q.; Gai, Y.; Yuan, L.; Wang, S.; Li, H.; Hou, Y.; Gao, M.; Lan, X. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater. Sci. 2020, 8, 1802–1814. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Song, Q.; Wu, T.; Zhuang, X.; Bao, Y.; Kong, M.; Qi, Y.; Tan, S.; Zhang, Z. Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma. ACS Nano 2015, 9, 6918–6933. [Google Scholar] [CrossRef]
- Hong, B.J.; Iscen, A.; Chipre, A.J.; Li, M.M.; Lee, O.S.; Leonard, J.N.; Schatz, G.C.; Nguyen, S.T. Highly Stable, Ultrasmall Polymer-Grafted Nanobins (usPGNs) with Stimuli-Responsive Capability. J. Phys. Chem. Lett. 2018, 9, 1133–1139. [Google Scholar] [CrossRef]
- Alam Khan, S.; Jawaid Akhtar, M. Structural modification and strategies for the enhanced doxorubicin drug delivery. Bioorg. Chem. 2022, 120, 105599. [Google Scholar] [CrossRef] [PubMed]
- Aldhubiab, B.; Almuqbil, R.M.; Nair, A.B. Harnessing the Power of Nanocarriers to Exploit the Tumor Microenvironment for Enhanced Cancer Therapy. Pharmaceuticals 2025, 18, 746. [Google Scholar] [CrossRef] [PubMed]
- Efimova, A.; Mulashkin, F.; Rudenskaya, G.; Evtushenko, E.; Orlov, V.; Melik-Nubarov, N.; Krivtsov, G.; Yaroslavov, A. Biodegradable electrostatic complexes of chitosan cationic microparticles and anionic liposomes. Polym. Sci. Ser. B 2018, 60, 84–90. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, G.; Zhao, Z.; Xue, F.; Gu, Y.; Chen, C.; Zhang, Y. 7,8-Dihydroxyflavone nano-liposomes decorated by crosslinked and glycosylated lactoferrin: Storage stability, antioxidant activity, in vitro release, gastrointestinal digestion and transport in Caco-2 cell monolayers. J. Funct. Foods 2020, 65, 103742. [Google Scholar] [CrossRef]
- Simões, M.G.; Hugo, A.; Gómez-Zavaglia, A.; Simões, P.N.; Alves, P. Formulation and Characterization of Stimuli-Responsive Lecithin-Based Liposome Complexes with Poly(acrylic acid)/Poly(N,N-dimethylaminoethyl methacrylate) and Pluronic® Copolymers for Controlled Drug Delivery. Pharmaceutics 2022, 14, 735. [Google Scholar] [CrossRef]
- Mallick, A.M.; Biswas, A.; Mishra, S.; Jadhav, S.; Chakraborty, K.; Tripathi, A.; Mukherjee, A.; Roy, R.S. Engineered vitamin E-tethered non-immunogenic facial lipopeptide for developing improved siRNA based combination therapy against metastatic breast cancer. Chem. Sci. 2023, 14, 7842–7866. [Google Scholar] [CrossRef]
- Ghosh, B.; McCarley, R.L. Redox-responsive liposomes aimed at nitroreductase for contents release. J. Liposome Res. 2025, 35, 290–299. [Google Scholar] [CrossRef]
- Li, Y.; Tao, T.; Xiong, Y.; Guo, W.; Liang, Y. Multifunctional PLGA nanosystems: Enabling integrated diagnostic and therapeutic strategies. Front. Pharmacol. 2025, 16, 1670397. [Google Scholar] [CrossRef]
- Soomherun, N.; Kreua-Ongarjnukool, N.; Niyomthai, S.T.; Chumnanvej, S. Lipid-Polymer Hybrid Nanoparticles Synthesized via Lipid-Based Surface Engineering for a robust drug delivery platform. Colloids Surf. B Biointerfaces 2024, 237, 113858. [Google Scholar] [CrossRef]
- Scopel, R.; Falcão, M.A.; Cappellari, A.R.; Morrone, F.B.; Guterres, S.S.; Cassel, E.; Kasko, A.M.; Vargas, R.M. Lipid-polymer hybrid nanoparticles as a targeted drug delivery system for melanoma treatment. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 127–138. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Z.; Li, C.; Zhou, W.; Shaw, J.P.; Baguley, B.C.; Liu, J.; Zhang, W. Optimization of Weight Ratio for DSPE-PEG/TPGS Hybrid Micelles to Improve Drug Retention and Tumor Penetration. Pharm. Res. 2018, 35, 13. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kiwada, H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int. J. Pharm. 2008, 354, 56–62. [Google Scholar] [CrossRef]
- Moreadith, R.W.; Kim, W.; Smith, K.; Yoon, K.; Weimer, R.; Fang, Z. Overcoming the PEG dilemma with Poly(2-ethyl-2-oxazoline) lipids in lipid nanoparticle formulations. Eur. Polym. J. 2025, 241, 114392. [Google Scholar] [CrossRef]
- Chen, J.; Su, M.; Xu, C.; Cao, Z.; Yang, X.; Wang, J. Cationic lipid-polymer hybrid nanoparticle drives in situ generation and lymphatic navigation of tumor antigens to prime systemic antitumor immunity. Nano Today 2024, 57, 102335. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, T.; Yang, Z.; Zhang, Y.; Lei, J.; Zhao, P. Self-Assembled Folic Acid-Targeted Pectin-Multi-Arm Polyethylene Glycol Nanoparticles for Tumor Intracellular Chemotherapy. ACS Omega 2021, 6, 1223–1234. [Google Scholar] [CrossRef]
- Salel, S.; Iyisan, B. Polymer-lipid hybrid nanoparticles as potential lipophilic anticancer drug carriers. Discov. Nano 2023, 18, 114. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, Y.; Wang, B.; Wang, Q.; Cai, T.; Cai, Y. Strategies for Preparing Different Types of Lipid Polymer Hybrid Nanoparticles in Targeted Tumor Therapy. Curr. Pharm. Des. 2021, 27, 2274–2288. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Care, A.; Deng, W. Targeted polymer lipid hybrid nanoparticles for in-vitro siRNA therapy in triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2024, 98, 105911. [Google Scholar] [CrossRef]
- Kambar, N.; Leal, C. Microfluidic synthesis of multilayered lipid-polymer hybrid nanoparticles for the formulation of low solubility drugs. Soft Matter 2023, 19, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- González-García, D.; Tapia, O.; Évora, C.; García-García, P.; Delgado, A. Conventional and microfluidic methods: Design and optimization of lipid-polymeric hybrid nanoparticles for gene therapy. Drug Deliv. Transl. Res. 2025, 15, 908–924. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Yadav, R.B.; Kushwaha, K.; Yadav, S.; Sharma, S.; Agrawal, U. Lipid-polymer hybrid nanoparticles: Development & statistical optimization of norfloxacin for topical drug delivery system. Bioact. Mater. 2017, 2, 269–280. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): In vitro and in vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Mahvelati, F.; Malaekeh-Nikouei, B.; Hashemi, E.; Oroojalian, F.; Hashemi, M. Bromelain Loaded Lipid-Polymer Hybrid Nanoparticles for Oral Delivery: Formulation and Characterization. Appl. Biochem. Biotechnol. 2022, 194, 3733–3748. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Panda, D.S.; Khalid, M.; Singh, L.; Quazi, A.M. Development of Lipid Polymer Hybrid Nanoparticles of Abietic Acid: Optimization, In-Vitro and Preclinical Evaluation. AAPS PharmSciTech 2024, 25, 145. [Google Scholar] [CrossRef]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in vitro evaluation of core-shell type lipid-polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef]
- Raman, S.; Mahmood, S.; Rahman, A. A review on lipid-polymer hybrid nanoparticles and preparation with recent update. Mater. Sci. Forum 2020, 981, 322–327. [Google Scholar] [CrossRef]
- Saravanakumar, S.M.; Cicek, P.V. Microfluidic Mixing: A Physics-Oriented Review. Micromachines 2023, 14, 1827. [Google Scholar] [CrossRef]
- Pascolo, G.; Bekchanov, B.; Soliman, M.A.N.; Spessot, E.; Ali, H.; Qutachi, O.; Abdi, M.; Tirella, A.; Elsawy, M.A. Microfluidics Enabling the Controlled Manufacturing of Peptide and Protein Micro and Nano Biomaterials for Biomedical Applications. Biomacromolecules 2025, 26, 3903–3928. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Li, W.; Correia, A.; Khan, M.M.; Rahim, M.A.; Santos, H.A. Microfluidic fabrication and characterization of Sorafenib-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery. Int. J. Pharm. 2020, 581, 119275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, e1904673. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, B.; Ye, L.; Hu, S.; Li, B.; Yang, Y.; Jia, X.; Feng, L.; Xiong, Z. Timescale dependent homogeneous assembly of lipid-polymer hybrid nanoparticles in laminar mixing for enhanced lung cancer treatment. Chem. Eng. J. 2024, 498, 155625. [Google Scholar] [CrossRef]
- Rouco, H.; García-García, P.; Évora, C.; Díaz-Rodríguez, P.; Delgado, A. Screening strategies for surface modification of lipid-polymer hybrid nanoparticles. Int. J. Pharm. 2022, 624, 121973. [Google Scholar] [CrossRef]
- Abdelkarim, M.; Abostait, A.; Czitrom, S.; McColman, S.; Ahmed, I.; Wong, C.C.W.; Bogojevic, D.; Abdelgawad, M.; Labouta, H.I. Hydrodynamic focusing to synthesize lipid-based nanoparticles: Computational and experimental analysis of chip design and formulation parameters. J. Control. Release 2025, 387, 114192. [Google Scholar] [CrossRef]
- Torres-Flores, G.; Nazende, G.T.; Emre, T.A. Preparation of Fenofibrate loaded Eudragit L100 nanoparticles by nanoprecipitation method. Mater. Today Proc. 2019, 13, 428–435. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities. Lab Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Cerdá, S.L.; Fontana, F.; Wang, S.; Correia, A.; Molinaro, G.; Tello, R.P.; Hirvonen, J.; Celia, C.; Barreto, G.; Santos, H.A. Development of siRNA and Budesonide Dual-Loaded Hybrid Lipid–Polymer Nanoparticles by Microfluidics Technology as a Platform for Dual Drug Delivery to Macrophages: An In Vitro Mechanistic Study. Adv. Ther. 2023, 6, 2300048. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid-Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Gameiro, M.; Mano, J.F.; Gaspar, V.M. Emerging lipid–polymer hybrid nanoparticles for genome editing. Polym. Chem. 2024, 15, 3436–3468. [Google Scholar] [CrossRef]
- de Araujo, M.M.; Borgheti-Cardoso, L.N.; Praça, F.G.; Marcato, P.D.; Bentley, M. Solid Lipid-Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies. J. Funct. Biomater. 2023, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Ottonelli, I.; Baraldi, C.; Ruozi, B.; Vandelli, M.A.; Tosi, G.; Duskey, J.T. Advantages and challenges of polymer-lipid hybrid nanoparticles for the delivery of biotech drugs. Nanomedicine 2025, 20, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- ICH. Pharmaceutical Development. Q8(2R); ICH Harmonised Tripartite Guideline: Geneva, Switzerland, 2009. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Dalal, R.; Shah, J.; Gorain, B.; Choudhury, H.; Jacob, S.; Mehta, T.A.; Shah, H.; Nair, A.B. Development and Optimization of Asenapine Sublingual Film Using QbD Approach. AAPS PharmSciTech 2021, 22, 244. [Google Scholar] [CrossRef]
- Karia, P.; Ayre, A.; Khan, A. Critical Review of Risk Assessment Tools in Pharmaceutical Quality by Design. Indian J. Pharm. Educ. Res. 2024, 58, s1145–s1155. [Google Scholar] [CrossRef]
- Çoban, Ö.; Demirtaş, H.; Kaya-Yasar, Y.; Engin, S.; Yıldırım, S.; Morsali, M.R. Formulation Optimization and in Vitro–in Vivo Evaluation of Alpha Lipoic Acid-Loaded Lipid–Polymer Hybrid Nanoparticles Via Design of Experiments. J. Pharm. Innov. 2025, 20, 50. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on Q10 Pharmaceutical Quality System; availability. Notice. Fed. Regist. 2009, 74, 15990–15991. [Google Scholar]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Mohanty, A.; Uthaman, S.; Park, I.K. Utilization of Polymer-Lipid Hybrid Nanoparticles for Targeted Anti-Cancer Therapy. Molecules 2020, 25, 4377. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sofias, A.M.; Kiessling, F.; Lammers, T. Nanoparticle Delivery to Tumours: From EPR and ATR Mechanisms to Clinical Impact. Nat. Rev. Bioeng. 2024, 2, 714–716. [Google Scholar] [CrossRef]
- Vagena, I.-A.; Malapani, C.; Gatou, M.-A.; Lagopati, N.; Pavlatou, E.A. Enhancement of EPR effect for passive tumor targeting: Current status and future perspectives. Appl. Sci. 2025, 15, 3189. [Google Scholar] [CrossRef]
- Dong, S.; Ashley, J.; Teng, L. Drug Delivery Strategies That Target Tumors. In Drug Delivery to Tumors: Recent Strategies and Techniques; Springer: Berlin/Heidelberg, Germany, 2025; pp. 61–88. [Google Scholar]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Wang, X.; Song, F.; Hu, J.; Li, L. Insights into intercellular receptor-ligand binding kinetics in cell communication. Front. Bioeng. Biotechnol. 2022, 10, 953353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Li, M.; Wang, X. Optimizing ligand-receptor binding thermodynamics and kinetics: The role of terahertz wave modulation in molecular recognition. Fundam. Res. 2024, in press. [CrossRef]
- Chen, Y.Q.; Xue, M.D.; Li, J.L.; Huo, D.; Ding, H.M.; Ma, Y. Uncovering the Importance of Ligand Mobility on Cellular Uptake of Nanoparticles: Insights from Experimental, Computational, and Theoretical Investigations. ACS Nano 2024, 18, 6463–6476. [Google Scholar] [CrossRef]
- Schorr, K.; Beck, S.; Zimmer, O.; Baumann, F.; Keller, M.; Witzgall, R.; Goepferich, A. The quantity of ligand-receptor interactions between nanoparticles and target cells. Nanoscale Horiz. 2025, 10, 803–823. [Google Scholar] [CrossRef]
- Wu, B.; Yu, P.; Cui, C.; Wu, M.; Zhang, Y.; Liu, L.; Wang, C.X.; Zhuo, R.X.; Huang, S.W. Folate-containing reduction-sensitive lipid-polymer hybrid nanoparticles for targeted delivery of doxorubicin. Biomater. Sci. 2015, 3, 655–664. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Pan, J.; Liu, B.; Feng, S.S. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials 2010, 31, 330–338. [Google Scholar] [CrossRef]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-modified, indocyanine green-loaded lipid-polymer hybrid nanoparticles for targeted delivery of cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Yang, X.; Jia, M.; Li, Y.; Huang, Y.; Lin, J.; Wu, S.; Hou, Z. Mitomycin C-soybean phosphatidylcholine complex-loaded self-assembled PEG-lipid-PLA hybrid nanoparticles for targeted drug delivery and dual-controlled drug release. Mol. Pharm. 2014, 11, 2915–2927. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, D.; Wu, F.; Guo, L.; He, G.; Ouyang, L.; Song, X.; Huang, W.; Li, X. Discovery and in vivo evaluation of novel RGD-modified lipid-polymer hybrid nanoparticles for targeted drug delivery. Int. J. Mol. Sci. 2014, 15, 17565–17576. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, J.; Fu, C.; Xie, X.; Peng, F.; You, J.; Tang, H.; Wang, Z.; Li, P.; Chen, J. iRGD-modified lipid-polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int. J. Nanomed. 2017, 12, 4147–4162. [Google Scholar] [CrossRef] [PubMed]
- Sakpakdeejaroen, I.; Muanrit, P.; Panthong, S.; Ruangnoo, S. Alpha-Mangostin-Loaded Transferrin-Conjugated Lipid-Polymer Hybrid Nanoparticles: Development and Characterization for Tumor-Targeted Delivery. Sci. World J. 2022, 2022, 9217268. [Google Scholar] [CrossRef]
- Pula, W.; Ganugula, R.; Esposito, E.; Ravi Kumar, M.N.V.; Arora, M. Engineered urolithin A-laden functional polymer-lipid hybrid nanoparticles prevent cisplatin-induced proximal tubular injury in vitro. Eur. J. Pharm. Biopharm. 2024, 200, 114334. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Zhu, C.; Xiang, C. Anti prostate cancer therapy: Aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar] [CrossRef]
- Simard, P.; Leroux, J.C. In vivo evaluation of pH-sensitive polymer-based immunoliposomes targeting the CD33 antigen. Mol. Pharm. 2010, 7, 1098–1107. [Google Scholar] [CrossRef]
- Hu, C.M.; Kaushal, S.; Tran Cao, H.S.; Aryal, S.; Sartor, M.; Esener, S.; Bouvet, M.; Zhang, L. Half-antibody functionalized lipid-polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells. Mol. Pharm. 2010, 7, 914–920. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, Z.; Wang, B.; Chen, G.; Zhang, Y.; Deng, H.; Tang, Z.; Mao, J.; Wang, L. Combination Chemotherapy of Lung Cancer—Co-Delivery of Docetaxel Prodrug and Cisplatin Using Aptamer-Decorated Lipid-Polymer Hybrid Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 2249–2261. [Google Scholar] [CrossRef]
- Forrester, J.; Davidson, C.G.; Blair, M.; Donlon, L.; McLoughlin, D.M.; Obiora, C.R.; Stockdale, H.; Thomas, B.; Nutman, M.; Brockbank, S.; et al. Low-Cost Microfluidic Mixers: Are They up to the Task? Pharmaceutics 2025, 17, 566. [Google Scholar] [CrossRef]
- Hengelbrock, A.; Schmidt, A.; Strube, J. Formulation of nucleic acids by encapsulation in lipid nanoparticles for continuous production of mRNA. Processes 2023, 11, 1718. [Google Scholar] [CrossRef]
- Jadon, R.S.; Sharma, M. Docetaxel-loaded lipid-polymer hybrid nanoparticles for breast cancer therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Bin Jumah, M.N.; Rizwanullah, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S. Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments. Polymers 2022, 14, 3705. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhuang, Y.; Wang, P.; Zou, T.; Lan, M.; Li, L.; Liu, F.; Cai, T.; Cai, Y. Polymeric Lipid Hybrid Nanoparticles as a Delivery System Enhance the Antitumor Effect of Emodin in Vitro and in Vivo. J. Pharm. Sci. 2021, 110, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.M.; Fayed, M.A.A.; Helal, D.; Ahmed, K.A. Assessment of the hepatoprotective effect of developed lipid-polymer hybrid nanoparticles (LPHNPs) encapsulating naturally extracted β-Sitosterol against CCl4 induced hepatotoxicity in rats. Sci. Rep. 2019, 9, 19779. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Wu, Y.; Li, W.; Hu, Y.; Zhao, G.; Fu, C.; Fu, S.; Zou, L. Lipid-Polymer Hybrid Nanoparticles for Oral Delivery of Tartary Buckwheat Flavonoids. J. Agric. Food Chem. 2018, 66, 4923–4932. [Google Scholar] [CrossRef]
- Dave, R.; Shah, U.; Patel, A.; Patel, R.; Patel, M. Unlocking the potential of novel hybrid lipid polymer nanoparticles in redefining camptothecin delivery: Achieving controlled release, enhanced cytotoxicity, and improved pharmacokinetics for colorectal cancer therapy. J. Drug Deliv. Sci. Technol. 2025, 105, 106644. [Google Scholar] [CrossRef]
- Raysing, S.D.; Gorle, A.P. Development, characterization, and in vitro cytotoxicity of resveratrol-loaded lipid polymer hybrid nanocarriers (LPHN) in glioblastoma multiforme cells. Colloid Polym. Sci. 2025, 303, 1501–1518. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm Romero, K.; Quirós, M.I.; Vargas Huertas, F.; Vega-Baudrit, J.R.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and In Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles. Polymers 2021, 13, 4207. [Google Scholar] [CrossRef]
- Fang, D.L.; Chen, Y.; Xu, B.; Ren, K.; He, Z.Y.; He, L.L.; Lei, Y.; Fan, C.M.; Song, X.R. Development of lipid-shell and polymer core nanoparticles with water-soluble salidroside for anti-cancer therapy. Int. J. Mol. Sci. 2014, 15, 3373–3388. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Rao, R.; Nair, A.B. Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications. Pharmaceutics 2025, 17, 464. [Google Scholar] [CrossRef]
- Jacob, S.; Rao, R.; Gorain, B.; Boddu, S.H.S.; Nair, A.B. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects. Pharmaceutics 2025, 17, 1079. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Sarma, H.; Dutta, A.; Bharali, A.; Rahman, S.S.; Baruah, S.; Biswas, N.; Sahu, B.P. pH sensitive lipid polymeric hybrid nanoparticle (LPHNP) of paclitaxel and curcumin for targeted delivery in breast cancer. Drug Dev. Ind. Pharm. 2024, 50, 856–864. [Google Scholar] [CrossRef]
- Afshari, A.R.; Sanati, M.; Kesharwani, P.; Sahebkar, A. Recent Advances in Curcumin-Based Combination Nanomedicines for Cancer Therapy. J. Funct. Biomater. 2023, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M.I. Co-Delivery of Curcumin and Cisplatin to Enhance Cytotoxicity of Cisplatin Using Lipid-Chitosan Hybrid Nanoparticles. Int. J. Nanomed. 2020, 15, 2207–2217. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, X.; Liu, S.; Tian, C.; Wang, H. Targeted nanomedicine for prostate cancer therapy: Docetaxel and curcumin co-encapsulated lipid-polymer hybrid nanoparticles for the enhanced anti-tumor activity in vitro and in vivo. Drug Deliv. 2016, 23, 1757–1762. [Google Scholar] [CrossRef]
- Li, C.; Ge, X.; Wang, L. Construction and comparison of different nanocarriers for co-delivery of cisplatin and curcumin: A synergistic combination nanotherapy for cervical cancer. Biomed. Pharmacother. 2017, 86, 628–636. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Akhtar, M.J.; Khan, S.A. Role of Natural Phytoconstituents as a Potential Bioenhancer of Anti-Cancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents. Processes 2024, 12, 2060. [Google Scholar] [CrossRef]
- Pourali, P.; Alowaidi, J.S.A.; Beyramabdi, P.; Hosseini Torshiz, G.; Taslim, T.; Ghahramani, S.; Homayouni Tabrizi, M. Synergistic anticancer, inflammatory, and antioxidant effect of piperine and chrysin combination loaded on lipid-polymer hybrid nanoparticles. Polym. Bull. 2025, 82, 11791–11814. [Google Scholar] [CrossRef]
- Scherman, D.; Rousseau, A.; Bigey, P.; Escriou, V. Genetic pharmacology: Progresses in siRNA delivery and therapeutic applications. Gene Ther. 2017, 24, 151–156. [Google Scholar] [CrossRef]
- Asadi, H.; Rostamizadeh, K.; Esmaeilzadeh, A.; Khodaei, M.; Fathi, M. Novel lipid-polymer hybrid nanoparticles for siRNA delivery and IGF-1R gene silencing in breast cancer cells. J. Drug Deliv. Sci. Technol. 2018, 48, 96–105. [Google Scholar]
- Zhu, X.; Xu, Y.; Solis, L.M.; Tao, W.; Wang, L.; Behrens, C.; Xu, X.; Zhao, L.; Liu, D.; Wu, J.; et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl. Acad. Sci. USA 2015, 112, 7779–7784. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, Y.; Zhou, K.; Chen, P.; Guo, H.; Chen, L.; Ding, J.; Song, T.; Shi, J. Optimized fluorodendrimer-incorporated hybrid lipid-polymer nanoparticles for efficient siRNA delivery. Biomater. Sci. 2020, 8, 758–762. [Google Scholar] [CrossRef]
- Aljabbari, A.; Lokras, A.G.; Kirkensgaard, J.J.K.; Rades, T.; Franzyk, H.; Thakur, A.; Zhang, Y.; Foged, C. Elucidating the nanostructure of small interfering RNA-loaded lipidoid-polymer hybrid nanoparticles. J. Colloid Interface Sci. 2023, 633, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Bobak, T.R.; Bohrmann, L.; Geczy, R.; Sekar, S.; Sathyanarayanan, G.; Kutter, J.P.; Franzyk, H.; Foged, C.; Saatchi, K. Pulmonary delivery of siRNA-loaded lipid-polymer hybrid nanoparticles: Effect of nanoparticle size. OpenNano 2023, 13, 100180. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy. Bioengineering 2024, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.R.; Sun, D. Mechanism of pH-sensitive Amphiphilic Endosomal Escape of Ionizable Lipid Nanoparticles for Cytosolic Nucleic Acid Delivery. Pharm. Res. 2025, 42, 1065–1077. [Google Scholar] [CrossRef]
- Das, R.; Medeiros, J.; Krishna, J.; Munugoti, S.; Dutta, R.; Devarajan, A.; Ghosh, A.; Thayumanavan, S. Multicheckpoint Cellular Targeting of siRNAs Using Antibody-Directed Lipid-Polymer Hybrid Nanoparticles. Bioconjug. Chem. 2025, 36, 1527–1540. [Google Scholar] [CrossRef]
- Kubiatowicz, L.J.; Mohapatra, A.; Krishnan, N.; Fang, R.H.; Zhang, L. mRNA nanomedicine: Design and recent applications. Exploration 2022, 2, 20210217. [Google Scholar] [CrossRef]
- Su, X.; Fricke, J.; Kavanagh, D.G.; Irvine, D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 2011, 8, 774–787. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cabibbo, M.; Scialabba, C.; Giammona, G.; Cavallaro, G. Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast. Biomacromolecules 2022, 23, 3439–3451. [Google Scholar] [CrossRef]
- Conte, G.; Costabile, G.; Baldassi, D.; Rondelli, V.; Bassi, R.; Colombo, D.; Linardos, G.; Fiscarelli, E.V.; Sorrentino, R.; Miro, A.; et al. Hybrid Lipid/Polymer Nanoparticles to Tackle the Cystic Fibrosis Mucus Barrier in siRNA Delivery to the Lungs: Does PEGylation Make the Difference? ACS Appl. Mater. Interfaces 2022, 14, 7565–7578. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, S.N.; Ayoko, G.A.; Richard, D.; Wang, T.; Islam, N. Inhaled Ivermectin-Loaded Lipid Polymer Hybrid Nanoparticles: Development and Characterization. Pharmaceutics 2024, 16, 1061. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Mohammadi, S.; Vaezi, Z.; Naderi-Manesh, H. Improvement of anti-biofilm activities via co-delivery of curcumin and gentamicin in lipid-polymer hybrid nanoparticle. J. Biomater. Sci. Polym. Ed. 2022, 33, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Parra-Ortiz, E.; Wan, F.; Cañadas, O.; Garcia-Alvarez, B.; Thakur, A.; Franzyk, H.; Pérez-Gil, J.; Malmsten, M.; Foged, C. Insights into the mechanisms of interaction between inhalable lipid-polymer hybrid nanoparticles and pulmonary surfactant. J. Colloid Interface Sci. 2023, 633, 511–525. [Google Scholar] [CrossRef]
- Xu, Y.; Cañadas, O.; Alonso, A.; Franzyk, H.; Thakur, A.; Pérez-Gil, J.; Foged, C. Effect of lipid-polymer hybrid nanoparticles on the biophysical function and lateral structure of pulmonary surfactant: Mechanistic in vitro studies. J. Colloid Interface Sci. 2023, 654, 1111–1123. [Google Scholar] [CrossRef]
- Kaviarasi, B.; Rajana, N.; Pooja, Y.S.; Rajalakshmi, A.N.; Singh, S.B.; Mehra, N.K. Investigating the effectiveness of Difluprednate-Loaded core-shell lipid-polymeric hybrid nanoparticles for ocular delivery. Int. J. Pharm. 2023, 640, 123006. [Google Scholar] [CrossRef]
- Diebold, Y.; Jarrín, M.; Sáez, V.; Carvalho, E.L.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef]
- Jiang, M.; Gan, L.; Zhu, C.; Dong, Y.; Liu, J.; Gan, Y. Cationic core-shell liponanoparticles for ocular gene delivery. Biomaterials 2012, 33, 7621–7630. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Zhao, Y.; Chen, D.; Zhu, C.; Liu, J.; Gan, Y. Hyaluronan-modified core-shell liponanoparticles targeting CD44-positive retinal pigment epithelium cells via intravitreal injection. Biomaterials 2013, 34, 5978–5987. [Google Scholar] [CrossRef]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Gupta, A.; Kafetzis, K.N.; Tagalakis, A.D.; Yu-Wai-Man, C. RNA therapeutics in ophthalmology–translation to clinical trials. Exp. Eye Res. 2021, 205, 108482. [Google Scholar] [CrossRef]
- Dave, V.; Kushwaha, K.; Yadav, R.B.; Agrawal, U. Hybrid nanoparticles for the topical delivery of norfloxacin for the effective treatment of bacterial infection produced after burn. J. Microencapsul. 2017, 34, 351–365. [Google Scholar] [CrossRef]
- Mohammed, K.H.A.; Rasslan, F.; Abd El-Fattah, M.A.; Shawky, S.; Amin, O.M.; Eassa, H.A. Treating Burn Infections With Topical Delivery of Positively Charged Norfloxacin-Loaded Lipid-Polymer Hybrid Nanoparticles. Recent Adv. Drug Deliv. Formul. 2025, 19, 142–155. [Google Scholar] [CrossRef]
- Jian, Y.; Zheng, Q.; Hu, S.; Jian, Y. siRNA nanodelivery systems in the treatment of skin Diseases: Research progress and clinical translation prospects. Eur. J. Pharmacol. 2025, 1005, 178075. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Katare, O.P. Nano-engineered lipid-polymer hybrid nanoparticles of fusidic acid: An investigative study on dermatokinetics profile and MRSA-infected burn wound model. Drug Deliv. Transl. Res. 2019, 9, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Meraj Anjum, M.; Kanoujia, J.; Parashar, P.; Arya, M.; Yadav, A.K.; Saraf, S.A. Evaluation of a polymer-lipid-polymer system utilising hybrid nanoparticles of dapsone as a novel antiacne agent. Curr. Drug Ther. 2016, 11, 86–100. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Elmowafy, M.; Shalaby, K.; Alzarea, S.I.; Massoud, D.; Kassem, A.M.; Ibrahim, M.F. Hydrocortisone-Loaded Lipid-Polymer Hybrid Nanoparticles for Controlled Topical Delivery: Formulation Design Optimization and In Vitro and In Vivo Appraisal. ACS Omega 2023, 8, 18714–18725. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.R.; Marepally, S.; Patel, A.R.; Voshavar, C.; Chaudhuri, A.; Singh, M. Topical delivery of anti-TNFα siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J. Control. Release 2013, 170, 51–63. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Mujtaba, M.A.; Khalid, M.; Panda, D.S.; Singh, L.; Alsaidan, O.A.; Quazi, A.M. Development of Sesamol Loaded-Polymer-Lipid Blend Nanoparticles: Statistical Optimization, In-Vitro, and Preclinical Assessment. J. Pharm. Innov. 2025, 20, 119. [Google Scholar] [CrossRef]
- Szymczak, J.; Cielecka-Piontek, J. Fisetin-In Search of Better Bioavailability-From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 4158. [Google Scholar] [CrossRef]
- Awadeen, R.H.; Boughdady, M.F.; Zaghloul, R.A.; Elsaed, W.M.; Abu, H., II; Meshali, M.M. Formulation of lipid polymer hybrid nanoparticles of the phytochemical Fisetin and its in vivo assessment against severe acute pancreatitis. Sci. Rep. 2023, 13, 19110. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef]
- Wang, W.; Shang, S.; Wang, Y.; Xu, B. Utilization of nanomaterials in MRI contrast agents and their role in therapy guided by imaging. Front. Bioeng. Biotechnol. 2024, 12, 1484577. [Google Scholar] [CrossRef]
- Colaco, V.; Roy, A.A.; Naik, G.A.R.R.; Mondal, A.; Mutalik, S.; Dhas, N. Advancement in lipid-based nanocomposites for theranostic applications in lung carcinoma treatment. OpenNano 2024, 15, 100199. [Google Scholar] [CrossRef]
- Luo, H.; Gao, S. Recent advances in fluorescence imaging-guided photothermal therapy and photodynamic therapy for cancer: From near-infrared-I to near-infrared-II. J. Control. Release 2023, 362, 425–445. [Google Scholar] [CrossRef]
- Phafat, B.; Bhattacharya, S. Quantum Dots as Theranostic Agents: Recent Advancements, Surface Modifications, and Future Applications. Mini Rev. Med. Chem. 2023, 23, 1257–1272. [Google Scholar] [CrossRef]
- Mhlanga, N.; Mphuthi, N.; Van der Walt, H.; Nyembe, S.; Mokhena, T.; Sikhwivhilu, L. Nanostructures and nanoparticles as medical diagnostic imaging contrast agents: A review. Mater. Today Chem. 2024, 40, 102233. [Google Scholar] [CrossRef]
- Mieszawska, A.J.; Gianella, A.; Cormode, D.P.; Zhao, Y.; Meijerink, A.; Langer, R.; Farokhzad, O.C.; Fayad, Z.A.; Mulder, W.J. Engineering of lipid-coated PLGA nanoparticles with a tunable payload of diagnostically active nanocrystals for medical imaging. Chem. Commun. 2012, 48, 5835–5837. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Huang, L.; Li, Z.; Zhang, W.; Wang, Y.; Shen, Y.; Wang, J.; Lu, W. Multifunctional ROS-Responsive and TME-Modulated Lipid-Polymer Hybrid Nanoparticles for Enhanced Tumor Penetration. Int. J. Nanomed. 2022, 17, 5883–5897. [Google Scholar] [CrossRef]
- Kong, S.D.; Sartor, M.; Hu, C.M.; Zhang, W.; Zhang, L.; Jin, S. Magnetic field activated lipid-polymer hybrid nanoparticles for stimuli-responsive drug release. Acta Biomater. 2013, 9, 5447–5452. [Google Scholar] [CrossRef]
- Fernandes, C.; Jathar, M.; Sawant, B.K.S.; Warde, T. Scale-up of nanoparticle manufacturing process. In Pharmaceutical Process Engineering and Scale-Up Principles; Springer: Berlin/Heidelberg, Germany, 2023; pp. 173–203. [Google Scholar]
- Costa, C.; Padrela, L. Progress on drug nanoparticle manufacturing: Exploring the adaptability of batch bottom-up approaches to continuous manufacturing. J. Drug Deliv. Sci. Technol. 2025, 111, 107120. [Google Scholar] [CrossRef]
- Muhammad, Q.; Jang, Y.; Kang, S.H.; Moon, J.; Kim, W.J.; Park, H. Modulation of immune responses with nanoparticles and reduction of their immunotoxicity. Biomater. Sci. 2020, 8, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Arabiyat, S.A. Prospectus and Concerns of Immunomodulatory Nanotechnologies and Nanoparticles Biocompatibility and Toxicity. In Nanotechnology Based Microbicides and Immune Stimulators; Springer: Berlin/Heidelberg, Germany, 2025; pp. 165–189. [Google Scholar]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified mRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhang, C.; Zhang, X.; Yan, J.; Zeng, C.; Talebian, F.; Lynch, K.; Zhao, W.; Hou, X.; Du, S.; et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 2022, 345, 306–313. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y. A Review of pH-Responsive Organic-Inorganic Hybrid Nanoparticles for RNAi-Based Therapeutics. Macromol. Biosci. 2021, 21, e2100183. [Google Scholar] [CrossRef]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- Salah Othman, R.; Zarei, S.; Rezaei Haghighat, H.; Afshar Taromi, A.; Khonakdar, H.A. Recent Advances in Smart Polymeric Micelles for Targeted Drug Delivery. Polym. Adv. Technol. 2025, 36, e70180. [Google Scholar] [CrossRef]
- Guo, H.; Mi, P. Polymer-drug and polymer-protein conjugated nanocarriers: Design, drug delivery, imaging, therapy, and clinical applications. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1988. [Google Scholar] [CrossRef]

| Nanocarrier | Representative Particle Size/Typical Drug Loading | Typical Characteristics | Key Advantages | Major Limitations | Representative Examples | Clinical Stages | References |
|---|---|---|---|---|---|---|---|
| Vesicular carriers | 50–300 nm/1–10% | Bilayered vesicles (e.g., liposomes, niosomes) with aqueous core | Biocompatible, encapsulates hydrophilic and lipophilic drugs, surface-modifiable | Physical/chemical instability, short circulation time, high production cost | Liposomes, niosomes, ethosomes, transfersomes, transethosomes, invasomes, cubosomes | Approved clinical trials | [10,11,12,13,14] |
| Polymeric nanoparticles | 100–1000 nm/5–20% | Biodegradable polymer-based solid particles (100–1000 nm) | Intracellular release, controlled drug release, high stability, scalable production | Low cell interaction, poor delivery efficiency, potential for burst release, toxic monomer accumulation risks, scalability issues | Polylactic-co-glycolic acid (PLGA) nanoparticles, chitosan nanoparticles | Approved formulations and clinical research | [15] |
| Inorganic nanoparticles | 10–100 nm/variable | Metal/metal oxide-based particles (e.g., gold, silica, iron oxide) | High imaging and diagnostic potential, magnetic/photothermal properties | Cytotoxicity, long-term biocompatibility concerns | Gold nanoparticles, iron oxide nanoparticles, silica nanoparticles | Preclinical and some clinical investigation | [16,17] |
| Polymeric micelles | 20–100 nm/1–5% | Self-assembled amphiphilic block copolymers forming core–shell structures | Solubilize hydrophobic drugs, passive and active targeting possible | Low drug loading for hydrophilic drugs, instability in circulation | Polyethylene glycol (PEG)-polylactic acid (PLA) micelles, PCL-based micelles | FDA-approved clinical trials | [18] |
| Dendrimers | <10 nm/high | Highly branched 3D polymers with tunable surface groups | High drug loading, functionalizable, penetrates biological barriers | Complex synthesis, potential toxicity, limited clinical use | PAMAM dendrimers, polylysine dendrimers | Preclinical and early clinical | [19] |
| Carbon nanotubes/graphene | 1–50 nm diameter/variable | One-dimensional carbon structures with high surface area | Exceptional mechanical strength, cellular uptake, potential for gene/drug delivery | Toxicity concerns, difficulty in excretion, regulatory challenges | Single-walled carbon nanotubes, graphene oxide | Preclinical | [20] |
| Quantum dots | 2–10 nm/variable | Semiconductor nanocrystals with size-dependent fluorescence | Excellent optical properties for imaging and tracking | Heavy metal content, photobleaching, toxicity concerns | CdSe/ZnS quantum dots, InP quantum dots | Preclinical; limited clinical use | [21] |
| Lipid-based nanosystems | 50–300 nm/1–5% | Solid lipid nanoparticles, nanostructured lipid carriers | Biocompatible, suitable for both hydrophilic and hydrophobic drugs | Low drug loading in the lipid matrix, lipid oxidation risk, drug expulsion during storage, tendency to aggregate, high instability in biological fluids | Solid lipid nanoparticles, nanostructured lipid carriers | Some marketed, ongoing clinical trials | [22] |
| Nanosuspensions | 200–1000 nm/nearly 100% drug | Surfactant-stabilized colloidal dispersions of pure drug particles, usually with a particle size of less than 1 µm | Improves solubility and dissolution of poorly soluble drugs, suitable for parenteral and oral delivery | Physical instability (aggregation, sedimentation), high energy input required | Nanocrystals | Several approved formulations | [23] |
| Nanoemulsions | 20–200 nm/low | Thermodynamically unstable systems of two immiscible liquids stabilized by surfactants; droplet size typically 20–200 nm | Enhances solubility and absorption of hydrophobic drugs, suitable for oral, topical, and parenteral routes | Thermodynamic instability, sensitivity to environmental conditions, limited drug loading for hydrophilic drugs | Microemulsions (o/w or w/o) | Approved products and clinical trials | [24] |
| Lipid-polymer hybrid nanoparticles | 50–200 nm/5–15% | Core–shell nanostructures comprising a biodegradable polymeric core surrounded by a lipid layer | High stability and controlled release; enhanced cellular uptake and pharmacokinetics; potential for co-delivery of hydrophilic and hydrophobic drugs | Complex formulation and scale-up; possible phase separation, stability challenges under physiological conditions. | PEG-PLGA/1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) hybrid systems | Preclinical and clinical research | [25] |
| Category | Examples | Approved Drug Products | Role | Advantages | Disadvantages | Applications |
|---|---|---|---|---|---|---|
| Lipids | Phosphatidylcholine, cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine | Doxil® (liposomal doxorubicin), AmBisome® (liposomal amphotericin B) | Form lipid shell; enhance stability; improve drug encapsulation | Biocompatible, flexible bilayer formation supports hydrophobic and hydrophilic drugs | May require stabilizers; prone to oxidation and hydrolysis | Drug delivery systems, sustained release formulations |
| Polymers | Polylactic-co-glycolic acid, PLA, polycaprolactone, chitosan, PEG-modified polymers | Lupron Depot® (PLGA-based), Somatuline® depot | Provide structural core; control drug release; ensure stability | Biodegradable, tunable degradation rate, mechanical strength | May induce burst release; hydrophobic core limits hydrophilic drug loading | Cancer therapy, protein/peptide delivery, vaccines |
| PEGylated lipids | DSPE-N-[methoxy(PEG)], 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(PEG)], 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(PEG)] | Comirnaty® (Pfizer–BioNTech mRNA vaccine), Spikevax® (Moderna mRNA vaccine) | Provide stealth properties; reduce opsonization; extend circulation time | Increases half-life, prevents aggregation, improves biodistribution | May reduce cellular uptake (PEG dilemma) | Long-circulating drug carriers, tumor targeting |
| Charged lipids | 1,2-Dioleoyl-3-trimethylammonium-propane, dioleoyl phosphatidic acid | Approved mRNA vaccines (Comirnaty®, Spikevax®) | Facilitate electrostatic interaction with nucleic acids; assist self-assembly | Enhance nucleic acid loading, promote endosomal escape | Can be cytotoxic at high concentrations | Gene delivery, mRNA vaccines |
| Surfactants/stabilizers | Poloxamer 188, poloxamer 407, polysorbate 80 | Taxol® (contains Polysorbate 80), Taxotere® | Prevent aggregation; improve dispersion; stabilize nanoparticles | Enhance solubility, reproducibility in preparation | Possible hypersensitivity reactions; surfactant residues | Emulsification, nanoprecipitation, drug solubilization |
| Targeting ligand conjugates | Folic acid- PEG, arginine-glycine-aspartic acid peptide-PEG, antibody-PEG conjugates | Mylotarg® (CD33-targeted ADC), Enhertu® (HER2-targeted ADC) | Enable active targeting to receptors; improve cellular uptake | Increased specificity, reduced off-target effects | Complex synthesis; high cost | Tumor-targeted therapy, receptor-mediated delivery |
| Name | Preparation Method | Composition (Lipid/Polymer) | Medical Conditions | Therapeutic Application | In Vivo Model | Route of Administration | Comparative Control | Key Observations | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Ursolic acid | Nanoprecipitation | PLGA Resomer RG 503 H, PLGA, soy phosphatidylcholine, phospholipon 90G, dimethyldioctadecyl-ammonium (bromide salt), DSPE-PEG 2000 | Oncological | Pancreatic ductal adenocarcinoma | Xenograft mouse model (AsPC-1, BxPC-3) | Intravenous | Free ursolic acid | Nanocarriers demonstrated excellent physicochemical and biological characteristics-IC50 below 20 µM, particle size around 150 nm, encapsulation efficiency up to 70%, and high stability. Cytotoxicity assays on AsPC-1 and BxPC-3 cells, hemolysis testing, and TEM imaging confirmed their activity and safety. | [34] |
| Thymoquinone | Single-step nanoprecipitation method | Chitosan, phospholipon 90G | Oncological | Breast cancer | Tumor-bearing mice; oral pharmacokinetic model | Oral | Free thymoquinone | Optimized thymoquinone-loaded nanoparicles exhibited favorable properties, including particle size < 200 nm, polydispersity index (PDI) < 0.25, entrapment efficiency > 85%, and zeta potential > 25 mV. They demonstrated strong stability, sustained drug release for up to 48 h, and high mucin-binding efficiency (>70%). In vitro and ex vivo studies showed significantly improved anti-breast cancer activity in MDA-MB-231 and MCF-7 cell lines, intestinal permeation, and oral bioavailability (4.74-fold higher) compared to free phytochemical. | [121] |
| Emodin | Nanoprecipitation method | PLGA copolymer, soybean lecithin, DSPE-PEG 2000 | Oncological | Breast cancer | Breast tumor xenograft mouse model | Intravenous | Free emodin | Nanoparticles had an average particle size of 122.7 ± 1.79 nm and entrapment efficiency of 72.8%. In comparison to free emodin, emodin-loaded nanoparticles showed enhanced cytotoxicity against MCF-7 breast cancer cells by increasing drug uptake and promoting early apoptosis. The elevated Bax/Bcl-2 ratio confirmed apoptosis induction as the primary anticancer mechanism. In vivo, emodin-nanoparticles inhibited tumor growth by over 60%, likely due to improved passive targeting at the tumor site. | [122] |
| Abietic acid | Microinjection technique | Chitosan, cholesterol | Non-oncological | Antioxidant and anti-inflammatory | Rodent inflammatory model; ex vivo gut permeation | Oral | Pure abietic acid | Optimized nanoparticles demonstrated particle size of 384.5 ± 6.36 nm, PDI of 0.376, zeta potential of 23.0 mV, and encapsulation efficiency of 80.01 ± 1.89%. Hybrid nanoparticles enhanced ex vivo gut permeation by 2.49-fold, attributed to lipid and surfactant components. The formulation showed markedly higher antioxidant and anti-inflammatory activities (21.51 ± 2.23% swelling vs. 46.51 ± 1.74% for pure phytochemical. | [70] |
| β-Sitosterol | Single-step nanoprecipitation method | PLGA Resomer RG 503 H, PLGA, DSPE-PEG 2000 | Non-oncological | Hepatoprotective | Carbon tetrachloride induced hepatotoxicity rat model | Oral | Free β-Sitosterol | In a CCl4-induced hepatotoxicity rat model, β-Sitosterol-LPHNPs (400 mg/kg) effectively normalized ALT, AST, MDA, CAT, bilirubin, and albumin levels without inhibiting CYP2E1 activity. Histological and immunohistochemical analyses confirmed preservation of normal liver architecture and reduced cleaved caspase-3 expression, indicating a strong hepatoprotective effect of the formulation. | [123] |
| Tartary buckwheat (TBFs) extracts | Single-step solvent evaporation with ultrasound | DSPE-N-[methoxy PEG-2000]), poly(D,L-lactide-co-glycolide, MW = 5000, lactide: glycolide ratio 50:50), egg lecithin, cholesterol | Non-oncological | Immuno-modulator | Immuno-suppressed mouse model | Oral | Free TBFs extract | Optimized formulation demonstrated high encapsulation efficiency (96.4 ± 1.1%), uniform nanosize (61.25 ± 1.83 nm), spherical morphology, and excellent stability. Compared to free TBFs, TBFs/LPHNPs showed stronger antioxidant and anti-inflammatory activities in RAW 264.7 macrophages and improved intestinal absorption through enhanced Caco-2 transmembrane transport. In vivo studies further confirmed an enhanced immune response in immunosuppressed mice. | [124] |
| Drug/Phytochemical | LPHNP Composition | Application and Model | Quantitative Outcomes | Reference |
|---|---|---|---|---|
| Curcumin + Paclitaxel | PLGA-core/lipid shell, chitosan-coated | Breast cancer (in vitro); pharmacokinetic evaluation in rats | IC50 reduced from 480.06 to 282.97 µg/mL; AUC increased 3.8-fold (CUR) increased 6.6-fold (paclitaxel) | [134] |
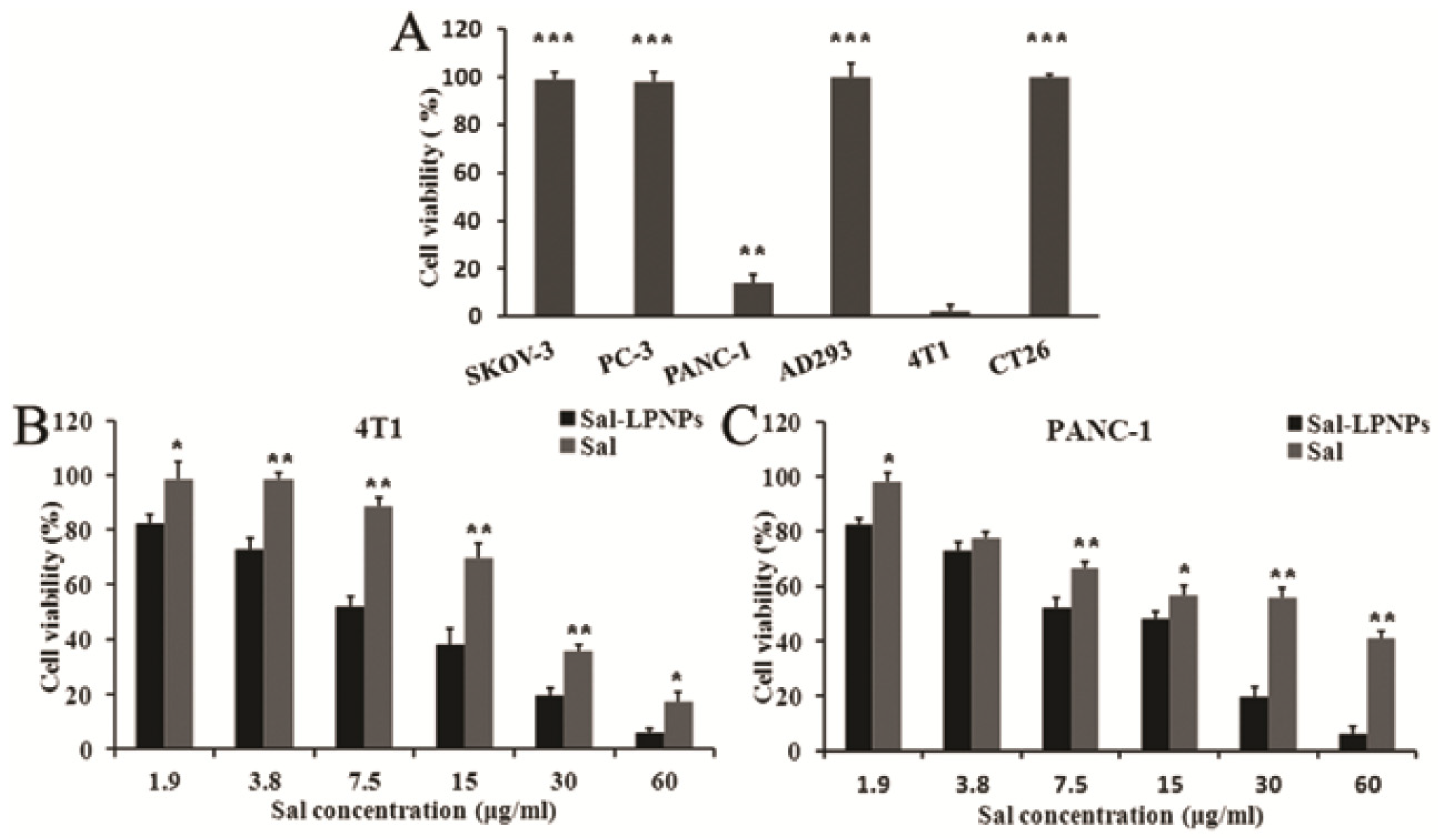
| Salidroside | PLGA-PEG-PLGA, lecithin/cholesterol | Pancreatic cancer (in vitro); PANC-1, 4T1 cells | IC50: 9.54 (PANC-1), 8.23 (4T1) µg/mL | [129] |
| Chrysin + Piperine | Chitosan-lecithin hybrid | Pancreatic cancer (in vitro); PANC cells; HFF normal cells | IC50: 14 µg/mL (PANC); >500 µg/mL (HFF) | [142] |
| Docetaxel + Curcumin | Polymer-lipid hybrid | Prostate cancer (in vitro); PC-3 prostate tumor xenograft | Significant tumor inhibition; docetaxel-CUR-LPHNPs group exhibited the highest tumor inhibition rate (82.5%), followed by docetaxel-CUR-nanoparticles (62.1%) and docetaxel-LPHNPs (45.2%). | [137] |
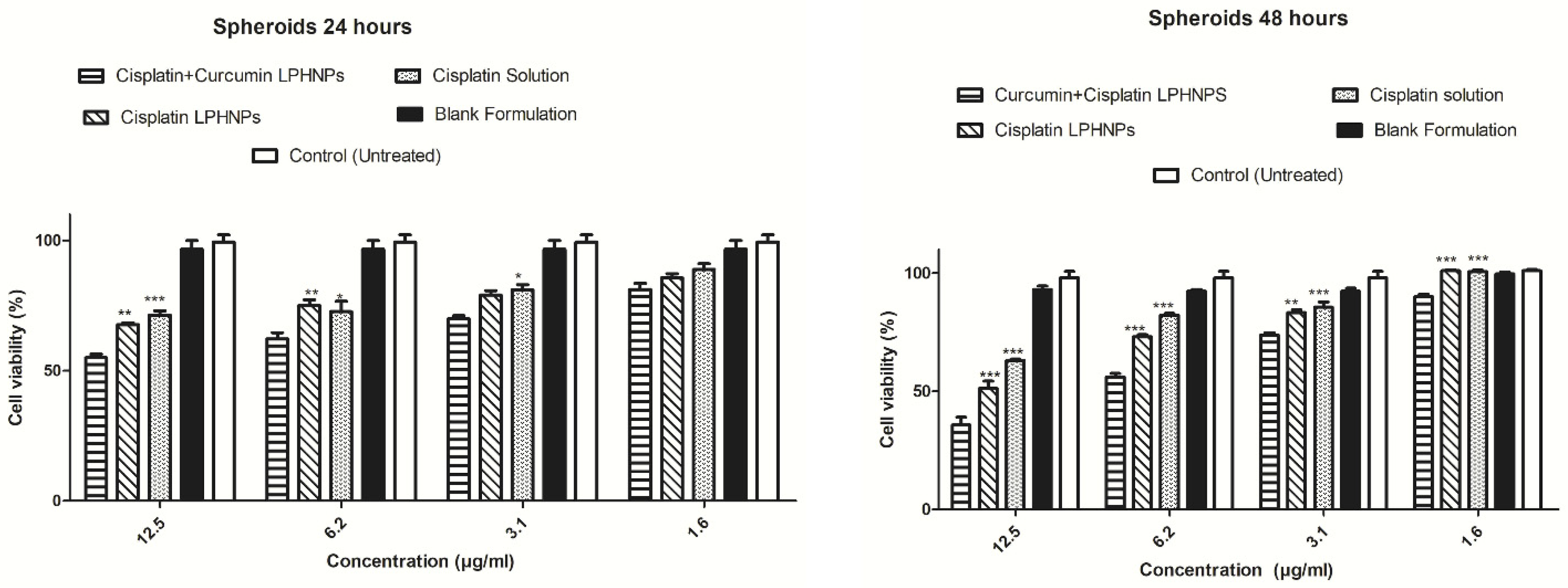
| Cisplatin + Curcumin | Hybrid dual-delivery | Cervical cancer (in vitro); HeLa/HUVEC; cervical tumor mice | Synergistic cytotoxicity; superior in vivo tumor suppression when compared with coloaded polymeric nanoparticles | [138] |
| Hydroxycamptothecin | LPHNPs via modified solvent evaporation method utilizing PLGA, DSPE-PEG2000 and lecithin | Breast cancer (in vitro) MCF-7 cells; liver carcinoma (in vitro) HepG2 cells; in vivo pharmacokinetics | LPNPs exhibited lower IC50 and reduced cell viability; 3× higher bioavailability compared to drug solution in rats | [32] |
| Enoxaparin | Oral chitosan–lipid hybrid nanoparticles via self-assembly method using glyceryl monooleate | Bioavailability enhancement via in vivo anticoagulant activity in rats | Bioavailability increased 5-fold compared to enoxaparin solution. Nanoparticles with a glyceryl monooleate/chitosan ratio of 0.2 showed oral bioavailability ~10% | [35] |
| Factor VII siRNA or luciferase-encoding mRNA | Parallelized microfluidic device | Gene silencing in mice | A 4-fold increase in hepatic gene silencing and a 5-fold enhancement in luciferase expression | [176] |
| Application ID | Publication Date | Title | Patent Status | Summary of Invention |
|---|---|---|---|---|
| 202021056479 | 25 December 2020 | Design and development of lipid-polymer hybrid nanoparticles for combinatorial drug delivery | Pending | The invention describes the development of lipid-polymer hybrid nanoparticles that address challenges in cancer therapy by offering stability, biocompatibility, and tunable surface properties for targeted delivery and controlled release. |
| 17228224 | 12 April 2021 | Dual-targeting lipid-polymer hybrid nanoparticles | Pending | The invention discloses dual-targeting polymer-lipid hybrid nanoparticles made of a lipid shell functionalized with a targeting moiety and a polymeric core containing a heme oxygenase-1 inhibitor. |
| 2021101545 | 26 March 2021 | Method for formation of lipid-polymer hybrid nanoparticles for combinatorial vincristine sulfate and lomustine drug delivery | Granted | The disclosure describes lipid-polymer hybrid nanoparticles capable of co-encapsulating vincristine sulfate and lomustine, with tunable surface properties for targeted delivery and controlled release. |
| 202211651315.3 | 22 December 2022 | Application of polymer lipid hybrid nanoparticles as immunologic adjuvant and immune preparation | Granted | Formulation composed of biodegradable amphiphilic block copolymers and lipids. When combined with immunopotentiators, nanoparticles significantly enhance humoral and cellular immune responses. |
| 23710967 | 23 February 2023 | Polymer-lipid hybrid nanoparticles comprising a lipid and a block copolymer as well as methods of making and uses thereof | Pending | Formulation designed to encapsulate protein or polynucleotide antigens, making them useful as vaccines, pharmaceuticals, targeted delivery systems, and non-viral nucleotide carriers. |
| 202310252352.5 | 15 March 2023 | Application of emodin polymer lipid hybrid nanoparticles | Pending | Formulation to improve breast cancer therapy by targeting tumor sites and releasing emodin to inhibit the IL-6/JAK2/STAT3 pathway, thereby overcoming drug resistance. |
| 202341017235 | 15 March 2023 | Polymeric lipid hybrid nanoparticles for controlled release system by the nanoprecipitation method | Pending | Formulation prepared using PLGA as the core and lecithin-PEG 2000 as the lipid shell for myocardial infarction therapy to improve amlodipine’s pharmacokinetics and solubility. |
| 202410758382.8 | 13 June 2024 | Polymer for mRNA delivery, lipid/polymer hybrid nanoparticle using same, and preparation method and application thereof | Granted | A cationic poly(β-amino ester) polymer is developed for effective mRNA delivery, formulated into lipid-polymer hybrid nanoparticles with auxiliary lipids like DOTAP. |
| WO/2024/252406 | 12 December 2024 | Lipid-polymer hybrid nanoparticles | Pending | Formulation consisting of a biodegradable polymeric core and a lipid shell, where most of the drug adheres to the inner lipid layer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Varkey, N.R.; Boddu, S.H.S.; Gorain, B.; Rao, R.; Nair, A.B. Advances in Lipid-Polymer Hybrid Nanoparticles: Design Strategies, Functionalization, Oncological and Non-Oncological Clinical Prospects. Pharmaceuticals 2025, 18, 1772. https://doi.org/10.3390/ph18121772
Jacob S, Varkey NR, Boddu SHS, Gorain B, Rao R, Nair AB. Advances in Lipid-Polymer Hybrid Nanoparticles: Design Strategies, Functionalization, Oncological and Non-Oncological Clinical Prospects. Pharmaceuticals. 2025; 18(12):1772. https://doi.org/10.3390/ph18121772
Chicago/Turabian StyleJacob, Shery, Namitha Raichel Varkey, Sai H. S. Boddu, Bapi Gorain, Rekha Rao, and Anroop B. Nair. 2025. "Advances in Lipid-Polymer Hybrid Nanoparticles: Design Strategies, Functionalization, Oncological and Non-Oncological Clinical Prospects" Pharmaceuticals 18, no. 12: 1772. https://doi.org/10.3390/ph18121772
APA StyleJacob, S., Varkey, N. R., Boddu, S. H. S., Gorain, B., Rao, R., & Nair, A. B. (2025). Advances in Lipid-Polymer Hybrid Nanoparticles: Design Strategies, Functionalization, Oncological and Non-Oncological Clinical Prospects. Pharmaceuticals, 18(12), 1772. https://doi.org/10.3390/ph18121772







