PDE-4 Inhibition in Sarcoidosis Patients: A Retrospective Single-Center Analysis of 51 Patients
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Clinical Outcomes During Follow-Up
2.3. Lung Function Parameters, BMI and Serological Parameters
2.4. Subgroup Analyses
2.5. Adverse Effects
3. Discussion
4. Materials and Methods
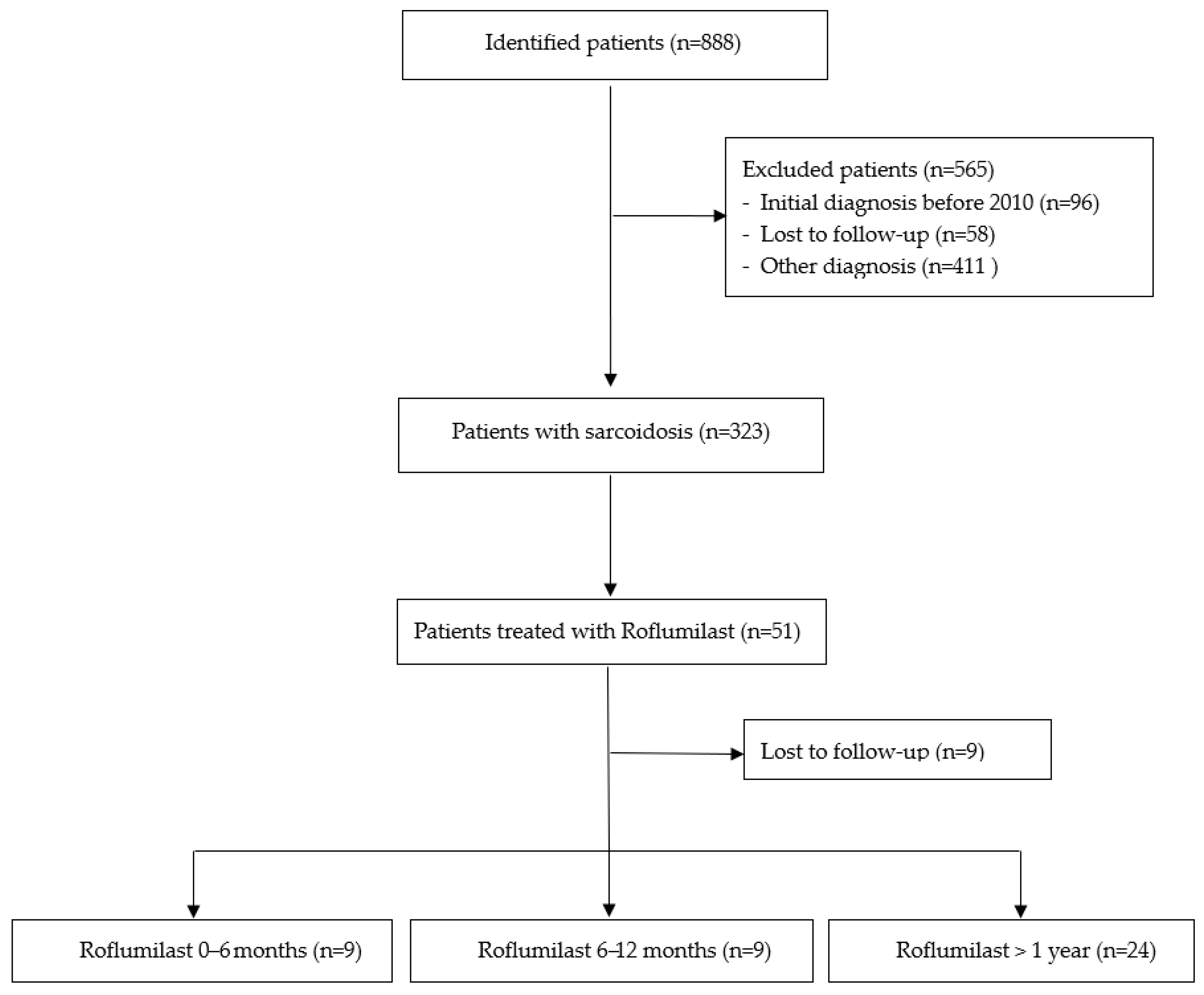
4.1. Patient Recruitment
4.2. Inclusion and Exclusion Criteria
4.3. Treatment Duration, Dosing, and Concomitant Therapies
4.4. Therapy Escalation and De-Escalation
4.5. Disease Progression
4.6. Subgroup Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| COPD | Chronic obstructive pulmonary disease |
| DLCO | Diffusing Capacity of the Lungs for Carbon Monoxide |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| IPF | Idiopathic pulmonary fibrosis |
| OCS | Oral corticosteroid |
| PDE-4 | Phosphodiesterase 4 |
| PPF | Progressive pulmonary fibrosis |
| sIL2R | Soluble Interleukin-2 Receptor |
| suppl | Supplementary material |
References
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef]
- Baughman, R.P.; Valeyre, D.; Korsten, P.; Mathioudakis, A.G.; Wuyts, W.A.; Wells, A.; Rottoli, P.; Nunes, H.; Lower, E.E.; Judson, M.A.; et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur. Respir. J. 2021, 58, 2004079. [Google Scholar] [CrossRef] [PubMed]
- Skowasch, D.; Bonella, F.; Buschulte, K.; Kneidinger, N.; Korsten, P.; Kreuter, M.; Müller-Quernheim, J.; Pfeifer, M.; Prasse, A.; Quadder, B.; et al. Therapeutic Pathways in SarcoidosisA Position Paper of the German Society of Respiratory Medicine (DGP). Pneumologie 2024, 78, 151–166. [Google Scholar]
- Frye, B.C.; Schupp, J.C.; Köhler, T.C.; Voll, R.E.; Müller-Quernheim, J. Sarcoidosis. Z. Rheumatol. 2016, 75, 389–401. [Google Scholar]
- Kahlmann, V.; Janssen Bonás, M.; Moor, C.C.; Grutters, J.C.; Mostard, R.L.M.; van Rijswijk, H.N.A.J.; van der Maten, J.; Marges, E.R.; Moonen, L.A.A.; Overbeek, M.J.; et al. First-Line Treatment of Pulmonary Sarcoidosis with Prednisone or Methotrexate. N. Engl. J. Med. 2025, 393, 231–242. [Google Scholar] [CrossRef]
- Khan, N.A.; Donatelli, C.V.; Tonelli, A.R.; Wiesen, J.; Neto, M.L.R.; Sahoo, D.; Culver, D.A. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir. Med. 2017, 132, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Obi, O.N.; Saketkoo, L.A.; Maier, L.A.; Baughman, R.P. Developmental drugs for sarcoidosis. J. Autoimmun. 2024, 149, 103179. [Google Scholar] [CrossRef]
- Culver, D.A.; Aryal, S.; Barney, J.; Hsia, C.C.; James, W.E.; Maier, L.A.; Marts, L.T.; Obi, O.N.; Sporn, P.H.; Sweiss, N.J.; et al. Efzofitimod for the Treatment of Pulmonary Sarcoidosis. Chest 2022, 163, 881. [Google Scholar] [CrossRef]
- Obi, O.N.; Baughman, R.P.; Crouser, E.D.; Julian, M.W.; Locke, L.W.; Chandrasekaran, A.; Ramesh, P.; Kinnersley, N.; Niranjan, V.; Culver, D.A.; et al. Therapeutic Doses of Efzofitimod Demonstrate Efficacy in Pulmonary Sarcoidosis. ERJ Open Res. 2024, 11, 00536–2024. [Google Scholar] [CrossRef]
- Leffers, H.C.B.; Baslund, B.; Lindhardsen, J.; Krintel, S.B.; Graudal, N. Abatacept and tofacitinib in refractory sarcoidosis: Drug survival, safety, and treatment response. Clin. Exp. Rheumatol. 2024, 42, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Bechman, K.; Biddle, K.; Miracle, A.; He, K.; Gibson, M.; Russell, M.D.; Walsh, S.; Brex, P.; Patel, A.S.; Myall, K.J.; et al. Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis. Thorax 2025, 80, 702–710. [Google Scholar] [CrossRef] [PubMed]
- 2024 GOLD Report—Global Initiative for Chronic Obstructive Lung Disease—GOLD. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 27 January 2025).
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of Sarcoidosis and Its Management. N. Engl. J. Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Mavropoulos, A.; Bogdanos, D.P. Phosphodiesterase 4 Inhibitors in Immune-mediated Diseases: Mode of Action, Clinical Applications, Current and Future Perspectives. Curr. Med. Chem. 2017, 24, 3054–3067. [Google Scholar] [CrossRef]
- Richeldi, L.; Azuma, A.; Cottin, V.; Hesslinger, C.; Stowasser, S.; Valenzuela, C.; Wijsenbeek, M.S.; Zoz, D.F.; Voss, F.; Maher, T.M. Trial of a Preferential Phosphodiesterase 4B Inhibitor for Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2022, 386, 135. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Assassi, S.; Azuma, A.; Cottin, V.; Hoffmann-Vold, A.-M.; Kreuter, M.; Oldham, J.M.; Richeldi, L.; Valenzuela, C.; Wijsenbeek, M.S.; et al. Nerandomilast in Patients with Progressive Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2203–2214. [Google Scholar] [CrossRef]
- Baughman, R.P.; Judson, M.A.; Culver, D.A.; Birring, S.S.; Parambil, J.; Zeigler, J.; Lower, E.E. Roflumilast (Daliresp®) to reduce acute pulmonary events in fibrotic sarcoidosis: A multi-center, double blind, placebo controlled, randomized clinical trial. Sarcoidosis Vasc. Diffus. Lung Dis. 2021, 38, e2021035. [Google Scholar]
- Baughman, R.P.; Lower, E.E.; Gibson, K. Pulmonary manifestations of sarcoidosis. Presse Med. 2012, 41 Pt 2, e289–e302. [Google Scholar] [CrossRef]
- Baughman, R.P.; Nunes, H.; Sweiss, N.J.; Lower, E.E. Established and experimental medical therapy of pulmonary sarcoidosis. Eur. Respir. J. 2013, 41, 1424–1438. [Google Scholar] [CrossRef]
- Baughman, R.P.; Judson, M.A.; Ingledue, R.; Craft, N.L.; Lower, E.E. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch. Dermatol. 2012, 148, 262–264. [Google Scholar] [CrossRef]
- Zabel, P.; Entzian, P.; Dalhoff, K.; Schlaak, M. Pentoxifylline in treatment of sarcoidosis. Am. J. Respir. Crit. Care Med. 1997, 155, 1665–1669. [Google Scholar] [CrossRef]
- Hernandez, J.B.; Chang, C.; LeBlanc, M.; Grimm, D.; Le Lay, J.; Kaestner, K.H.; Zheng, Y.; Montminy, M. The CREB/CRTC2 pathway modulates autoimmune disease by promoting Th17 differentiation. Nat. Commun. 2015, 6, 7216. [Google Scholar] [CrossRef] [PubMed]
- Toumpanakis, D.; Karagiannis, K.; Paredi, P.; Bikov, A.; Bonifazi, M.; Lota, H.K.; Kalsi, H.; Minelli, C.; Dikaios, N.; Kastis, G.A.; et al. Contribution of Peripheral Airways Dysfunction to Poor Quality of Life in Sarcoidosis. Chest 2025, 168, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Jouanjan, L.; Terschluse, C.; Feineis, M.; Wirtz, S.; Soriano, D.; Kuss, P.; Wachenfeld, E.; Bamberg, F.; Schlett, C.L.; et al. Morphological CT phenotypes in Pulmonary Sarcoidosis at diagnosis: Splitting and Lumping based on predictors of clinical progression. ERJ Open Res. 2025, in press.

| Roflumilast Treatment ≥ 6 Months (n = 33) | Roflumilast Treatment < 6 Months (n = 18) | Mann–Whitney U-Test/Fisher’s test p-Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age at diagnosis (mean/range) | 45.5 (26–62) | 51.3 (27–70) | 0.1 |
| Sex (female/male) % | 16/17 48 | 7/11 39 | 0.6 |
| BMI (kg/m2) (mean/range) | 31.3 (19–47.7) | 29.4 (21.3–47.7) | 0.3 |
| Smoking (active/ex-smokers) | 4/8 | 1/12 | 0.6 |
| PY (mean) | 12.3 | 14.5 | |
| Other organ involvement: | |||
| Neurologic | 1 | 1 | 1 |
| Eyes | 3 | 2 | 1 |
| Heart | 1 | 1 | 1 |
| Liver | 2 | 1 | 1 |
| Spleen | 4 | 2 | 1 |
| Bone | 3 | 0 | 0.5 |
| Skin | 8 | 3 | 0.7 |
| Initial immunosuppression | |||
| Corticosteroids (N/%) mg/day (SD) | 19 (58) 15.8 (11.6) | 9 (50) 10.5 (8.1) | 0.8 0.6 |
| Azathioprine (N/%) | 4 (12) | 2 (11) | 1 |
| Methotrexate (N/%) | 1 (3) | 0 (0) | 1 |
| Mycophenolate (N/%) | 1 (3) | 0 (0) | 1 |
| Abatacept (N/%) | 0 (0) | 1 (5) | 0.4 |
| Body plethysmography | |||
| Tiffeneau Index < 0.7 (-n/%) | 8 (24%) | 4 (22%) | 1 |
| FEV1% (mean/SD) | 81.5 (21.1) | 75.6 (17.8) | 0.4 |
| FVC% (mean/SD) | 88.1 (20.1) | 82.5 (19.5) | 0.3 |
| TLC% (mean/SD) | 81.1 (40.3) | 76.1 (36.8) | 0.4 |
| DLCO% (mean/SD) | 75.6 (18.5) | 71.3 (16.7–94) | 0.2 |
| Biochemical markers | |||
| sIL2R (mean/SD) | 784.5 (549.5) | 538.6 (230.8) | 0.3 |
| Neopterine (mean/SD) | 14.3 (7.7) | 15.3 (5.8) | 0.9 |
| Ambulatory Visits | With Roflumilast | w/o Roflumilast | Odds Ratio (95% CI) | p-Value (Fisher’s Exact) |
|---|---|---|---|---|
| N | 142 | 172 | ||
| Disease progression | ||||
| FEV1 decrease > 10% of mean value (N/%) | 6 (4%) | 31 (18%) | 0.20 (0.08–0.50) | p < 0.01 |
| Progressive disease (N/%) | 15 (11%) | 41 (24%) | 0.38 (0.19–0.72) | p < 0.01 |
| New organ involvement (N/%) | 3 (2%) | 9 (5%) | 0.39; (0.10–1.74) | p = 0.24 |
| Ambulatory Visits with Corticosteroid Therapy | With Roflumilast | w/o Roflumilast | Odds Ratio (95% CI) | p-Value (Fisher’s Exact) |
|---|---|---|---|---|
| N | 97 | 144 | ||
| Therapy escalation (N/%) | 49 (51%) | 100 (69%) | 0.45; (0.26–0.76) | p < 0.01 |
| Increase in OCS dose (N/%) | 13 (13%) | 25 (17%) | 0.73; (0.34–1.54) | p = 0.47 |
| Corticosteroid Pulse therapy (N/%) | 5 (5%) | 22 (15%) | 0.30; (0.11–0.83) | p < 0.05 |
| Additional 2nd/3rd line treatment (N/%) | 31 (32%) | 53 (37%) | 0.81; (0.47–1.39) | p = 0.49 |
| Therapy de-escalation (N/%) | 43 (44%) | 59 (41%) | 0.79; (0.68–1.93) | p = 0.69 |
| Reduction in steroid dose (N/%) | 35 (36%) | 49 (34%) | 1.15; (0.62–2.11) | p = 0.78 |
| Steroid discontinued (N/%) | 6 (6%) | 8 (6%) | 1.14; (0.37–3.44) | p = 1.00 |
| Additional 2nd/3rd line treatment discontinued (N/%) | 2 (2%) | 2 (1%) | 1.52; (0.21–10.99) | p = 1.00 |
| Patient Number | |
|---|---|
| Side effects: | |
| Gastrointestinal disorders | 7/51 |
| Circulatory disorders | 2/51 |
| Anxiety | 1/51 |
| Sleeping disorders | 2/51 |
| Others: | |
| Alternative drug | 1/51 |
| All medications discontinued | 4/51 |
| Not specified | 10/51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feineis, M.E.; Terschluse, C.; Jouanjan, L.; Soriano, D.; Agarwal, P.; Schupp, J.; Müller-Quernheim, J.; Stolz, D.; Frye, B.C. PDE-4 Inhibition in Sarcoidosis Patients: A Retrospective Single-Center Analysis of 51 Patients. Pharmaceuticals 2025, 18, 1729. https://doi.org/10.3390/ph18111729
Feineis ME, Terschluse C, Jouanjan L, Soriano D, Agarwal P, Schupp J, Müller-Quernheim J, Stolz D, Frye BC. PDE-4 Inhibition in Sarcoidosis Patients: A Retrospective Single-Center Analysis of 51 Patients. Pharmaceuticals. 2025; 18(11):1729. https://doi.org/10.3390/ph18111729
Chicago/Turabian StyleFeineis, Martin Elias, Charlott Terschluse, Louis Jouanjan, Daniel Soriano, Prerana Agarwal, Jonas Schupp, Joachim Müller-Quernheim, Daiana Stolz, and Björn Christian Frye. 2025. "PDE-4 Inhibition in Sarcoidosis Patients: A Retrospective Single-Center Analysis of 51 Patients" Pharmaceuticals 18, no. 11: 1729. https://doi.org/10.3390/ph18111729
APA StyleFeineis, M. E., Terschluse, C., Jouanjan, L., Soriano, D., Agarwal, P., Schupp, J., Müller-Quernheim, J., Stolz, D., & Frye, B. C. (2025). PDE-4 Inhibition in Sarcoidosis Patients: A Retrospective Single-Center Analysis of 51 Patients. Pharmaceuticals, 18(11), 1729. https://doi.org/10.3390/ph18111729







