The Age-Related Efficacy of Dimethyl Fumarate in Naive Versus Switcher Multiple Sclerosis Patients: A Multicenter Population-Based Study
Abstract
1. Introduction
2. Results
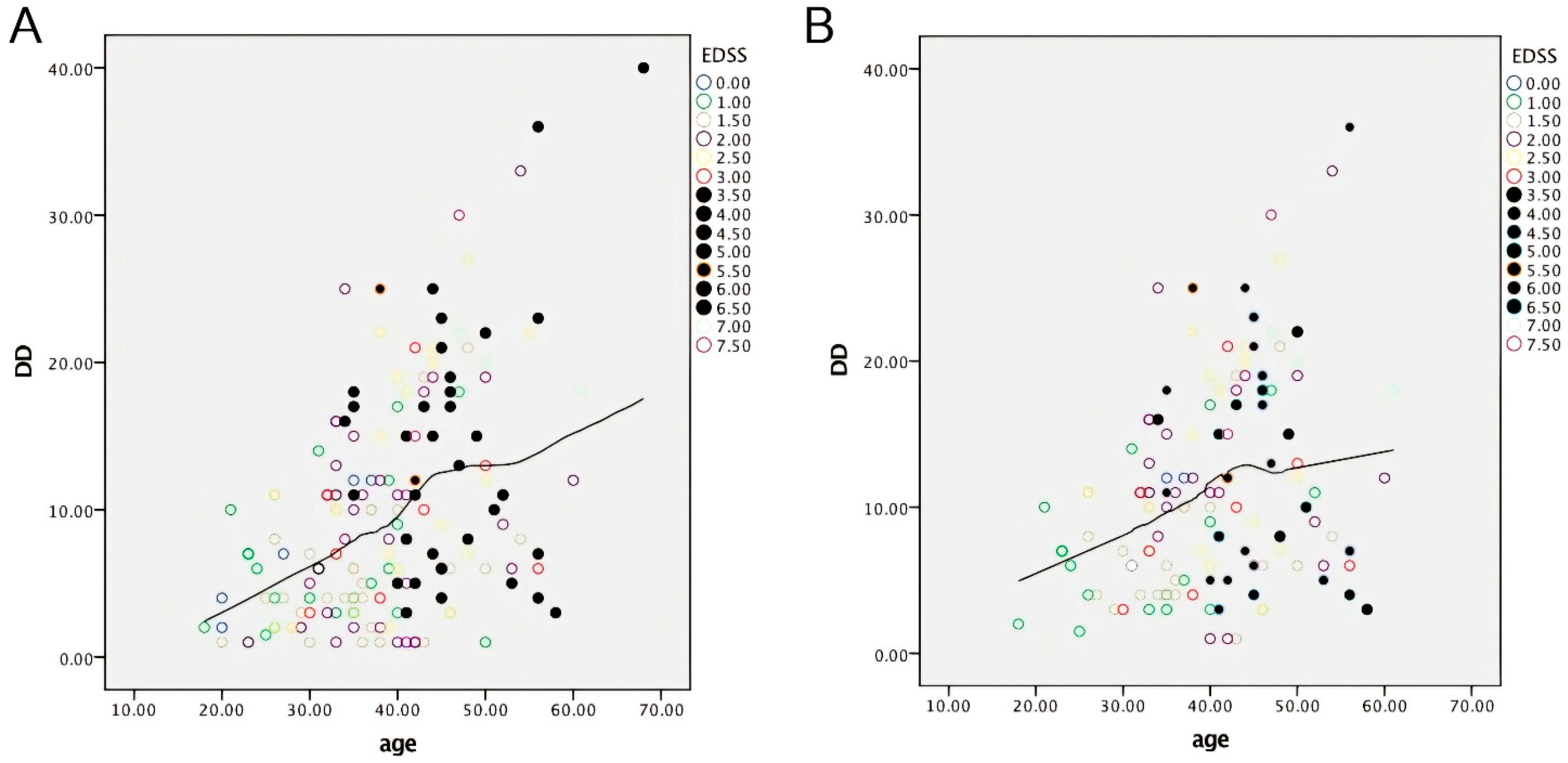
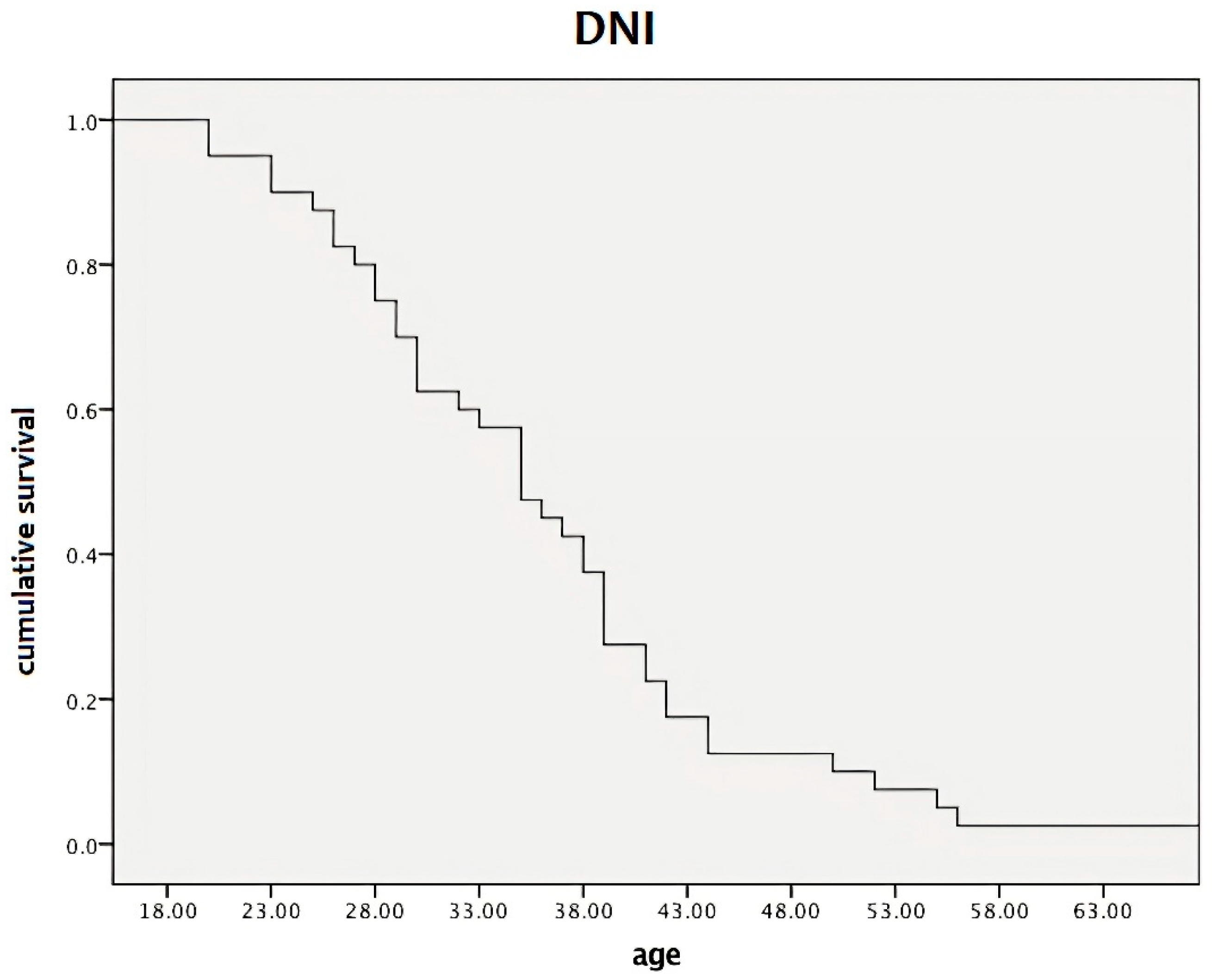
2.1. Data from Descriptive Statistics
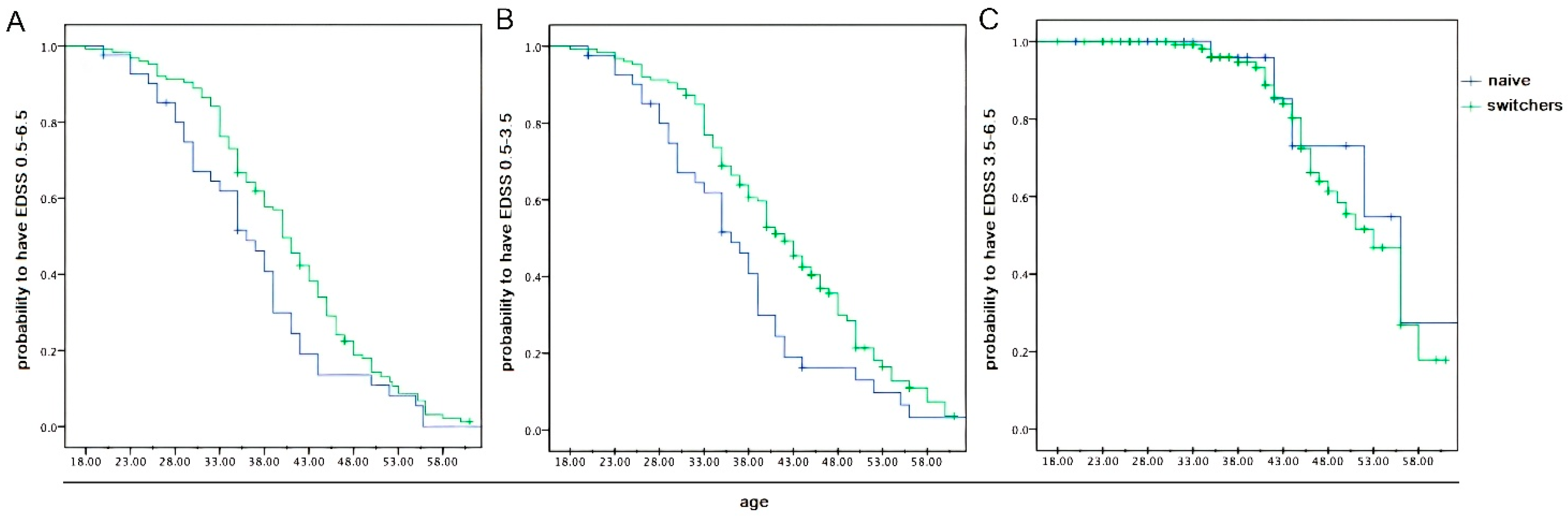
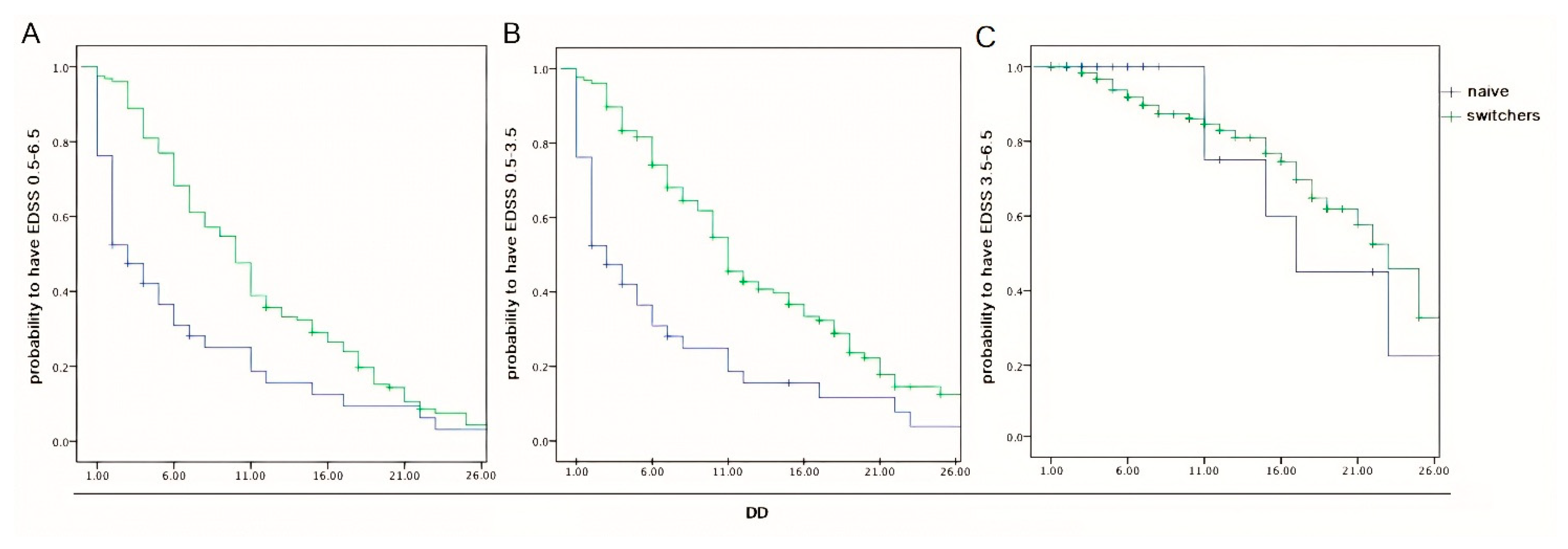
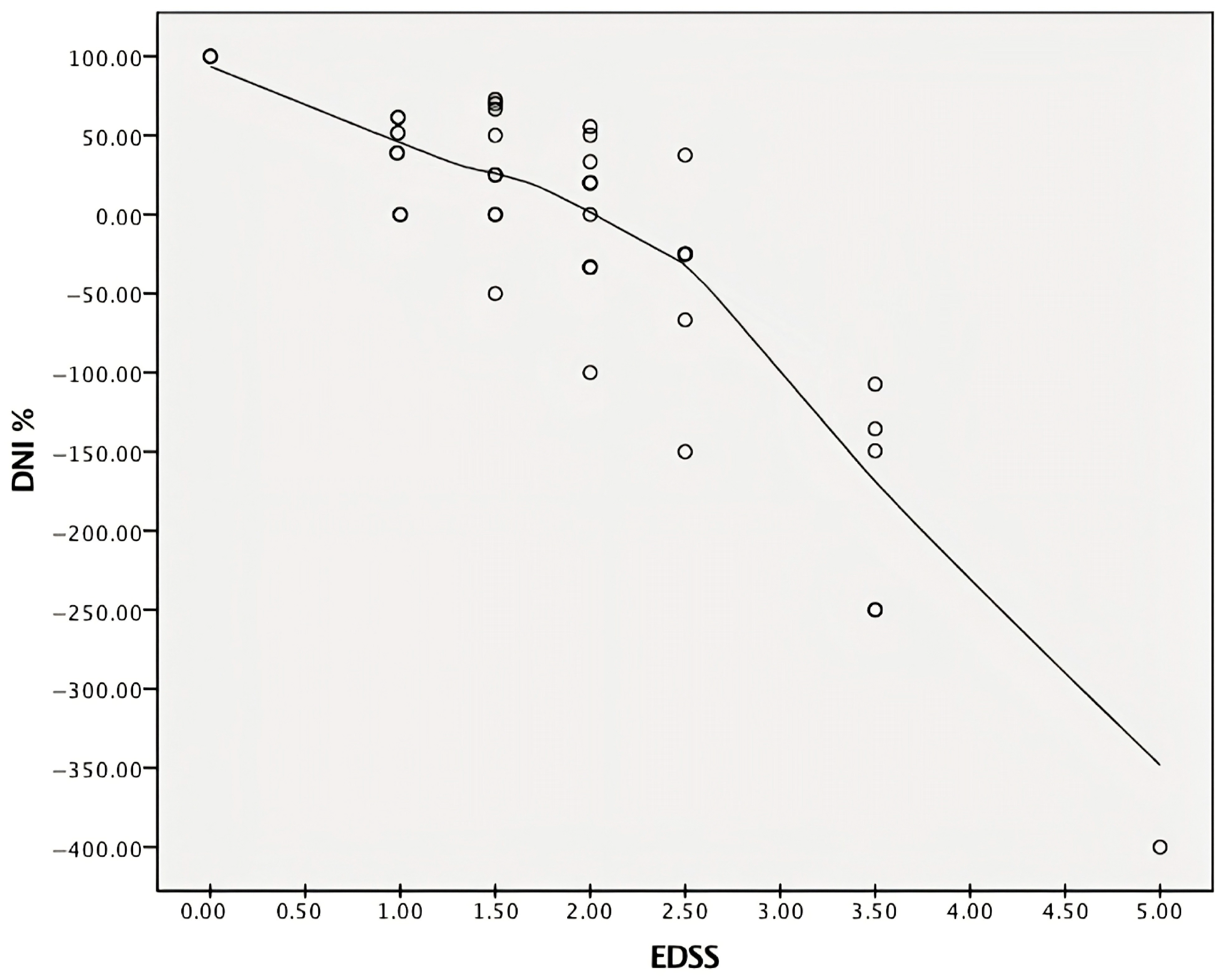
2.2. Data from Inferential Statistics
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARR | annual relapse rate |
| BBB | blood–brain barrier |
| CDP | confirmed disability progression |
| CNS | central nervous system |
| DD | disease duration |
| DMF | dimethyl fumarate |
| DMTs | disease-modifying therapies |
| DNI | differential neurological impairment |
| EDSS | expanded disability status scale |
| LL | lesion load |
| MS | multiple sclerosis |
| TinT | time in therapy |
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Bö, L.; Mörk, S.; Chang, A. Pathogenesis of tissue injury in MS lesions. J. Neuroimmunol. 1999, 98, 49–56. [Google Scholar] [CrossRef]
- Libbey, J.E.; McCoy, L.L.; Fujinami, R.S. Molecular mimicry in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 127–147. [Google Scholar]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Brieva, L.; Calles, C.; Landete, L.; Oreja-Guevara, C. Current challenges in secondary progressive multiple sclerosis: Diagnosis, activity detection and treatment. Front. Immunol. 2025, 16, 1543649. [Google Scholar] [CrossRef]
- Giovannoni, G.; Popescu, V.; Wuerfel, J.; Hellwig, K.; Iacobaeus, E.; Jensen, M.B.; García-Domínguez, J.M.; Sousa, L.; De Rossi, N.; Hupperts, R.; et al. Smouldering multiple sclerosis: The ‘real MS’. Ther. Adv. Neurol. Disord. 2022, 15, 17562864211066751. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Amato, M.P.; Avolio, C.; Gallo, P.; Gasperini, C.; Inglese, M.; Marfia, G.A.; Patti, F. Towards a biological view of multiple sclerosis from early subtle to clinical progression: An expert opinion. J. Neurol. 2025, 272, 179. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Trojano, M. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382–5394. [Google Scholar] [CrossRef]
- De Masi, R.; Orlando, S.; De Donno, A. The Age-Related Efficacy of Dimethyl Fumarate and Natalizumab in the Real-World Management of Multiple Sclerosis. Pharmaceuticals 2021, 14, 81. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.C. Multiple Sclerosis and Aging: The Dynamics of Demyelination and Remyelination. ASN Neuro. 2022, 14, 17590914221118502. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Barkhof, F.; Ciccarelli, O.; Cossarizza, A.; De Stefano, N.; Gasperini, C.; Geraldes, R.; Granziera, C.; Haider, L.; et al. The ageing central nervous system in multiple sclerosis: The imaging perspective. Brain 2024, 147, 3665–3680. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef]
- De Masi, R.; Orlando, S. GANAB and N-Glycans Substrates Are Relevant in Human Physiology, Polycystic Pathology and Multiple Sclerosis: A Review. Int. J. Mol. Sci. 2022, 23, 7373. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Define Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Confirm Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef]
- Gold, R.; Arnold, D.L.; Bar-Or, A.; Hutchinson, M.; Kappos, L.; Havrdova, E.; MacManus, D.G.; Yousry, T.A.; Pozzilli, C.; Selmaj, K.; et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult. Scler. 2017, 23, 253–265. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Amezcua, L.; Mao-Draayer, Y.; Vargas, W.S.; Farber, R.; Schaefer, S.; Branco, F.; England, S.M.; Belviso, N.; Lewin, J.B.; Mendoza, J.P.; et al. Endorse Study Investigators. Efficacy of Dimethyl Fumarate in Young Adults with Relapsing-Remitting Multiple Sclerosis: Analysis of the DEFINE, CONFIRM, and ENDORSE Studies. Neurol. Ther. 2023, 12, 883–897. [Google Scholar] [CrossRef]
- Hua, L.H.; Bar-Or, A.; Cohan, S.L.; Lublin, F.D.; Coyle, P.K.; Cree, B.A.; Meng, X.; Su, W.; Cox, G.M.; Fox, R.J. Effects of baseline age and disease duration on the efficacy and safety of siponimod in patients with active SPMS: Post hoc analyses from the EXPAND study. Mult. Scler. Relat. Disord. 2023, 75, 104766. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Ohashi, T.; Onizuka, Y.; Hiramatsu, K.; Hase, M.; Yun, J.; Matta, A.; Torii, S. Efficacy and safety of dimethyl fumarate in treatment-naïve Japanese patients with multiple sclerosis: Interim analysis of the randomized placebo-controlled study. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319852727. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Thakolwiboon, S.; Mills, E.A.; Yang, J.; Doty, J.; Belkin, M.I.; Cho, T.; Schultz, C.; Mao-Draayer, Y. Immunosenescence and multiple sclerosis: Inflammaging for prognosis and therapeutic consideration. Front. Aging 2023, 4, 1234572. [Google Scholar] [CrossRef]
- Dema, M.; Eixarch, H.; Villar, L.M.; Montalban, X.; Espejo, C. Immunosenescence in multiple sclerosis: The identification of new therapeutic targets. Autoimmun. Rev. 2021, 20, 102893. [Google Scholar] [CrossRef]
- Bolton, C.; Smith, P.A. The influence and impact of ageing and immunosenescence (ISC) on adaptive immunity during multiple sclerosis (MS) and the animal counterpart experimental autoimmune encephalomyelitis (EAE). Ageing Res. Rev. 2018, 41, 64–81. [Google Scholar] [CrossRef]
- Weideman, A.M.; Tapia-Maltos, M.A.; Johnson, K.; Greenwood, M.; Bielekova, B. Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Front. Neurol. 2017, 8, 577. [Google Scholar] [CrossRef]
- De Masi, R.; Orlando, S. IFI35 as a biomolecular marker of neuroinflammation and treatment response in multiple sclerosis. Life Sci. 2020, 259, 118233. [Google Scholar] [CrossRef] [PubMed]
- De Masi, R.; Orlando, S.; Bagordo, F.; Grassi, T. IFP35 Is a Relevant Factor in Innate Immunity, Multiple Sclerosis, and Other Chronic Inflammatory Diseases: A Review. Biology 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; Bass, B.; Rice, G.P.; Noseworthy, J.; Carriere, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study. 2. Predictive value of the early clinical course. Brain 1989, 112, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S.; Adeleine, P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain 2003, 126, 770–782. [Google Scholar] [CrossRef]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef]
- Kusznir Vitturi, B. Refining outcome measures in multiple sclerosis clinical trials: A call to action. Mult. Scler. Relat. Disord. 2025, 100, 106558. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
| Naive (n = 74) Mean ± SD (95% CI) | Switchers (n = 95) Mean ± SD (95% CI) | p | |
|---|---|---|---|
| Female-to-male sex ratio | 1.9:1.2 | 1.8:1.1 | 0.416 |
| Age (years) | 35.5 ± 10.3 (32.5–38.8) | 40.2 ± 8.7 (38.6–41.7) | 0.005 |
| Age at onset (years) | 29.0 ± 5.4 (26.7–31.1) | 29.6 ± 4.5 (25.8–30.9) | 0.566 |
| Disease Duration (years) | 5.9 ± 2.7 (3.8–8.4) | 11.2 ± 7.1 (10.0–12.5) | <0.001 |
| Time in Therapy (years) | 5.8 ± 2.1 (3.7–7.9) | 6.1 ± 2.5 (3.6–8.8) | 0.432 |
| Expanded Disability Status Scale score | 1.8 ± 1.0 (1.5–2.2) | 2.7 ± 1.7 (2.4–3.0) | <0.001 |
| ARRpre | 0.87 ± 0.56 (0.7–1.0) | 0.48 ± 0.28 (0.35–0.51) | <0.001 |
| ARRpost | 0.01 ± 0.0 | 0.11 ± 0.0 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Masi, R.; Orlando, S.; Greco, A.; Costa, M.C. The Age-Related Efficacy of Dimethyl Fumarate in Naive Versus Switcher Multiple Sclerosis Patients: A Multicenter Population-Based Study. Pharmaceuticals 2025, 18, 1730. https://doi.org/10.3390/ph18111730
De Masi R, Orlando S, Greco A, Costa MC. The Age-Related Efficacy of Dimethyl Fumarate in Naive Versus Switcher Multiple Sclerosis Patients: A Multicenter Population-Based Study. Pharmaceuticals. 2025; 18(11):1730. https://doi.org/10.3390/ph18111730
Chicago/Turabian StyleDe Masi, Roberto, Stefania Orlando, Assunta Greco, and Maria Carmela Costa. 2025. "The Age-Related Efficacy of Dimethyl Fumarate in Naive Versus Switcher Multiple Sclerosis Patients: A Multicenter Population-Based Study" Pharmaceuticals 18, no. 11: 1730. https://doi.org/10.3390/ph18111730
APA StyleDe Masi, R., Orlando, S., Greco, A., & Costa, M. C. (2025). The Age-Related Efficacy of Dimethyl Fumarate in Naive Versus Switcher Multiple Sclerosis Patients: A Multicenter Population-Based Study. Pharmaceuticals, 18(11), 1730. https://doi.org/10.3390/ph18111730





