Potential Implications of Body Mass Composition Changes in Heart Failure Patients in the Era of SGLT2i, GLP-1 RA, and GIP/GLP-1 RA
Abstract
1. Methods
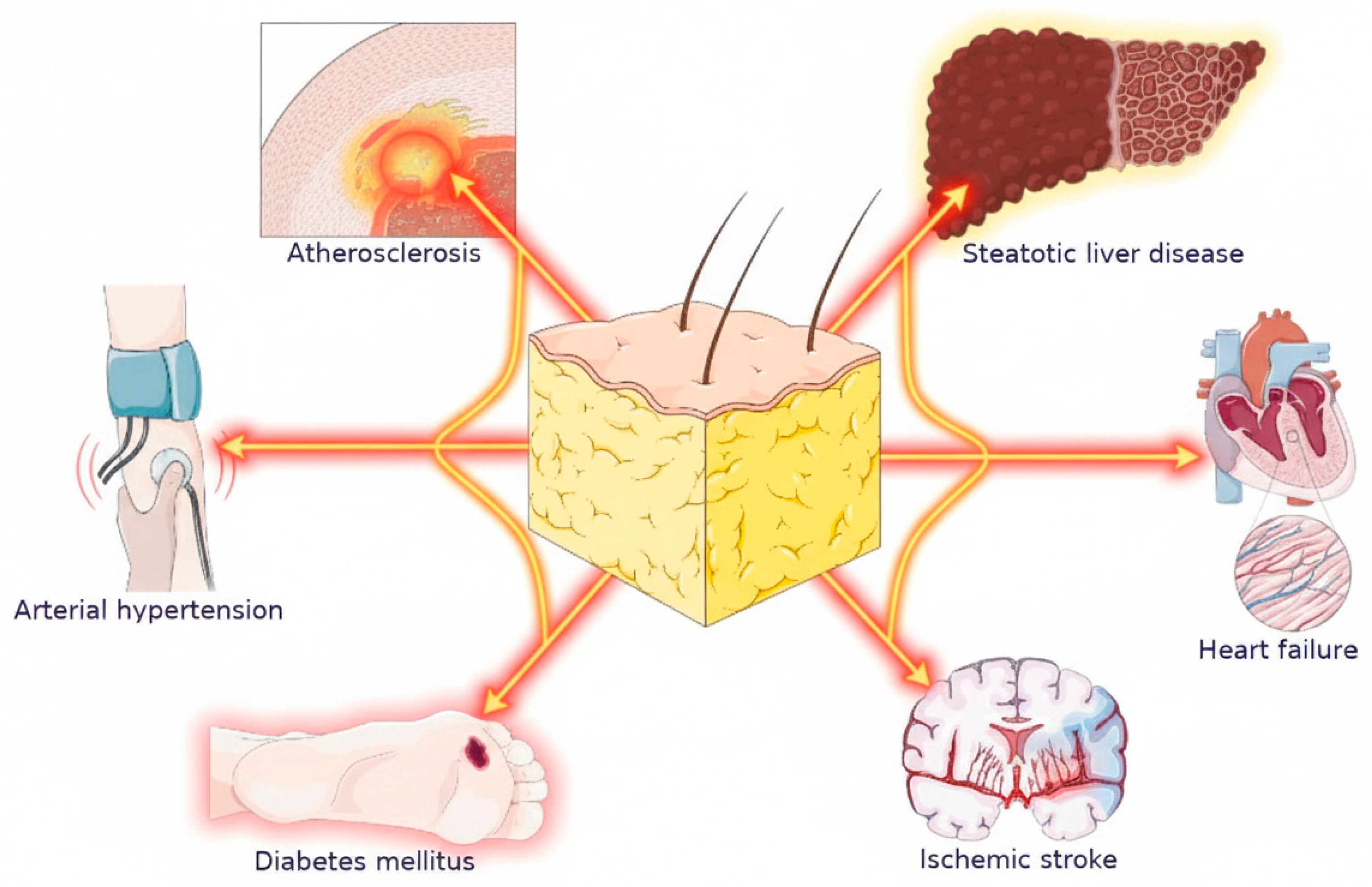
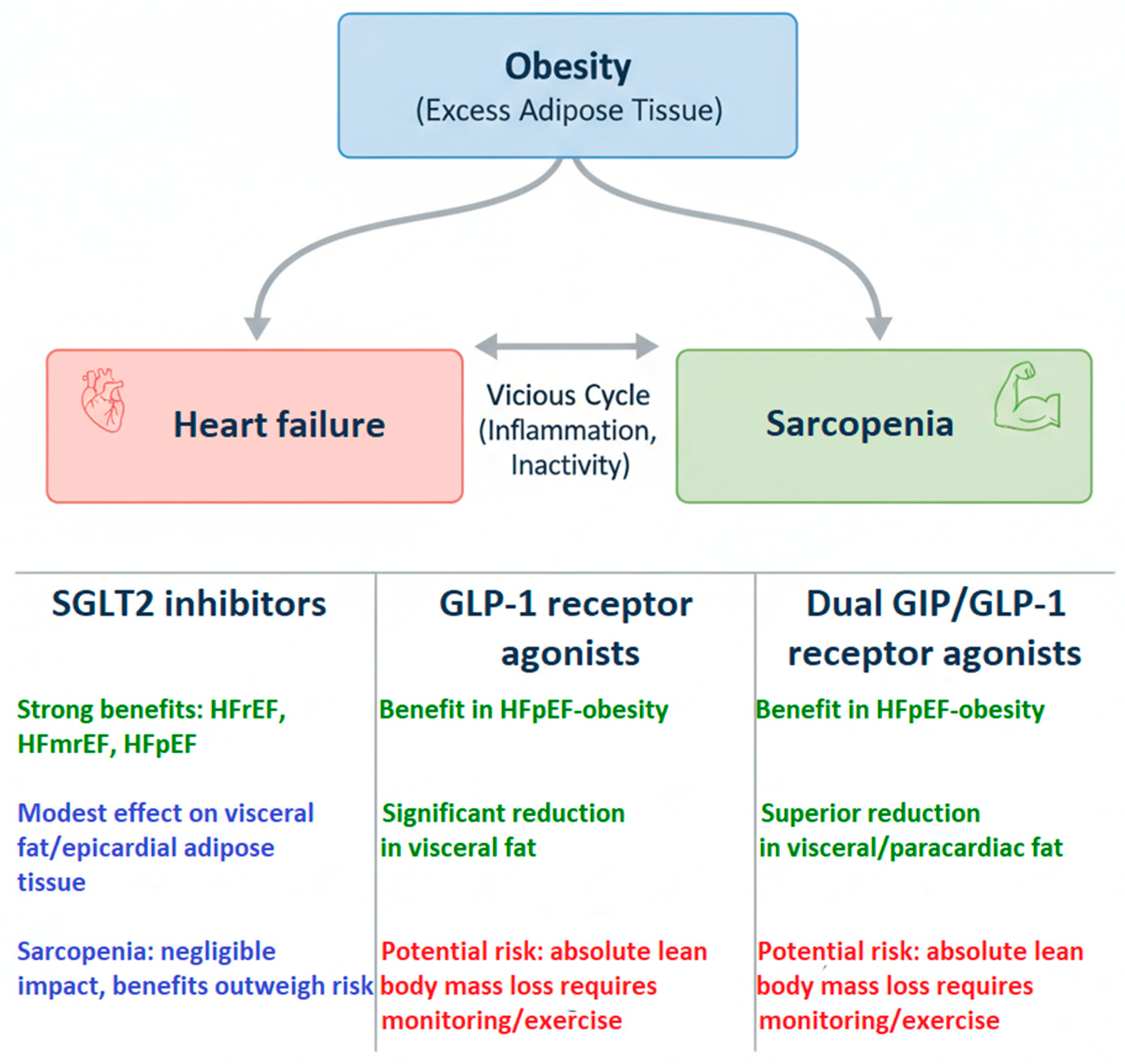
2. Obesity and Heart Failure
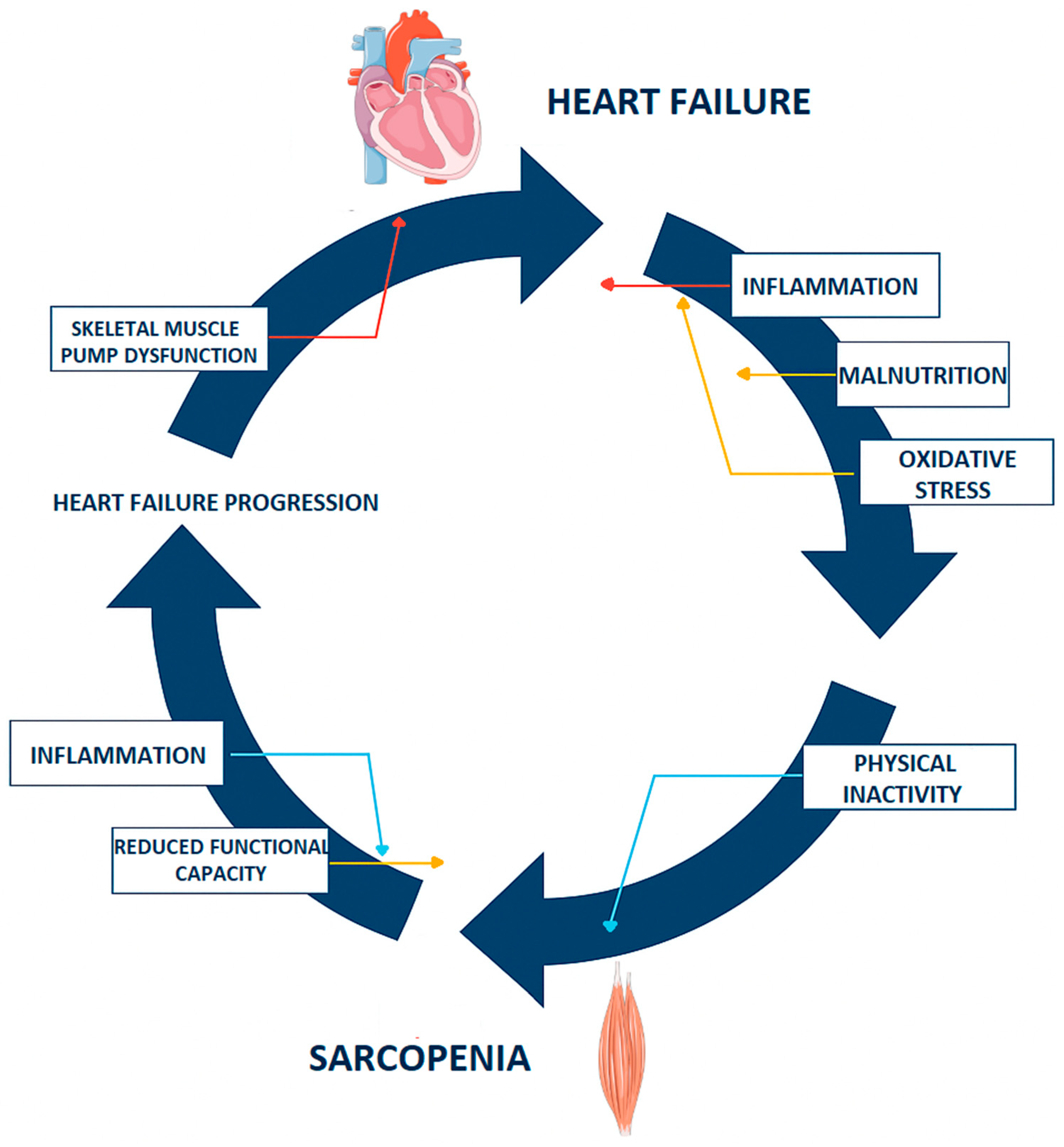
3. Sarcopenia in Heart Failure
4. Pharmacotherapy of Heart Failure in the Context of Obesity
5. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors
6. Glucagon-like Peptide-1 Receptor (GLP-1R) Agonists
7. Glucagon-like Peptide-1 and Gastric Inhibitory Polypeptide (GLP-1/GIP) Receptor Agonists
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Federation. World Obesity Atlas. 2025. Available online: https://data.worldobesity.org/publications/?cat=23 (accessed on 31 August 2025).
- Ebong, I.A.; Goff, D.C., Jr.; Rodriguez, C.J.; Chen, H.; Bertoni, A.G. Mechanisms of heart failure in obesity. Obes. Res. Clin. Pract. 2014, 8, e540–e548. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Kohan, L.; Holland, E.; Keeley, E.C.; Mazimba, S. Obesity paradox in heart failure: A heavy matter. ESC Heart Fail. 2016, 3, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kindel, T.L.; Strande, J.L. Bariatric surgery as a treatment for heart failure: Review of the literature and potential mechanisms. Surg. Obes. Relat. Dis. 2018, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zhai, A.; Haddad, H. The impact of obesity on heart failure. Curr. Opin. Cardiol. 2017, 32, 196–202. [Google Scholar] [CrossRef]
- Karwi, Q.; Zhang, L.; Altamimi, T.; Wagg, C.; Patel, V.; Uddin, G.; Joerg, A.R.; Padwal, R.S.; Johnstone, D.E.; Sharma, A.; et al. Weight loss enhances cardiac energy metabolism and function in heart failure associated with obesity. Diabetes Obes. Metab. 2019, 21, 1944–1955. [Google Scholar] [CrossRef]
- Sundström, J.; Bruze, G.; Ottosson, J.; Marcus, C.; Näslund, I.; Neovius, M. Weight loss and heart failure. Circulation 2017, 135, 1577–1585. [Google Scholar] [CrossRef]
- Barillas-Lara, M.; Faaborg-Andersen, C.; Quintana, R.; Loro-Ferrer, J.; Mandras, S.; daSilva-deAbreu, A. Clinical considerations and pathophysiological associations among obesity, weight loss, heart failure, and hypertension. Curr. Opin. Cardiol. 2023, 38, 447–455. [Google Scholar] [CrossRef]
- Jamaly, S.; Carlsson, L.; Peltonen, M.; Andersson-Assarsson, J.; Karason, K. Heart failure development in obesity: Underlying risk factors and mechanistic pathways. ESC Heart Fail. 2020, 8, 356–367. [Google Scholar] [CrossRef]
- Oga, E.; Eseyin, O. The obesity paradox and heart failure: A systematic review of a decade of evidence. J. Obes. 2016, 2016, 9040248. [Google Scholar] [CrossRef]
- Jung, M.; Shin, M. Obesity-related heart failure with preserved ejection fraction: Diagnostic and therapeutic challenges. Korean J. Intern. Med. 2023, 38, 157–166. [Google Scholar] [CrossRef]
- Voorhees, H.; Sorensen, E.; Pasrija, C.; Kaczorowski, D.; Griffith, B.; Kon, Z. Outcomes of obese patients undergoing less invasive lvad implantation. J. Card. Surg. 2019, 34, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Parto, P.; Lavie, C.; Arena, R.; Bond, S.; Popović, D.; Ventura, H. Body habitus in heart failure: Understanding the mechanisms and clinical significance of the obesity paradox. Future Cardiol. 2016, 12, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.; Pislaru, S.; Melenovský, V.; Borlaug, B. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Lavie, C.; Elagizi, A.; Arena, R.; Ventura, H. The impact of obesity in heart failure. Heart Fail. Clin. 2020, 16, 71–80. [Google Scholar] [CrossRef]
- Niezen, S.; Connelly, M.; Hirsch, C.; Kizer, J.; Benitez, M.; Minchenberg, S.; Perez-Matos, M.C.; Jiang, Z.G.; Mukamal, K.J. Elevated plasma levels of ketone bodies are associated with all-cause mortality and incidence of heart failure in older adults: The chs. J. Am. Heart Assoc. 2023, 12, e029960. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Ebner, N.; Dos Santos, M.R.; Ishida, J.; Hasenfuss, G.; von Haehling, S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur. J. Heart Fail. 2020, 22, 2314–2326. [Google Scholar] [CrossRef]
- Karakousis, N.; Georgakopoulos, P. Loop diuretics and sarcopenia: A potential association. Muscles 2023, 2, 317–326. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Meng, R.; Liu, X. Indirect comparison of sglt2 inhibitors in patients with established heart failure: Evidence based on bayesian methods. ESC Heart Fail. 2023, 10, 1231–1241. [Google Scholar] [CrossRef]
- Jones, N.; Ordóñez-Mena, J.; Roalfe, A.; Taylor, K.; Goyder, C.; Hobbs, R.; Taylor, C.J. Body mass index and survival in people with heart failure. Heart 2023, 109, 1542–1549. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J. Cachexia Sarcopenia Muscle 2020, 11, 698–709. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metabolism 2023, 146, 155639. [Google Scholar] [CrossRef]
- Santos, E.; Moreira, A.; Huguenin, G.; Tibiriçá, E.; Lorenzo, A. Effects of whey protein isolate on body composition, muscle mass, and strength of chronic heart failure patients: A randomized clinical trial. Nutrients 2023, 15, 2320. [Google Scholar] [CrossRef]
- Huerta, L.; Marco-Alacid, C.; Grande, C.; Andrés, C. A narrative review of the diagnosis and treatment of sarcopenia and malnutrition in patients with heart failure. Nutrients 2024, 16, 2717. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Colantuoni, S.; Medicamento, G.; et al. Dysregulated Epicardial Adipose Tissue as a Risk Factor and Potential Therapeutic Target of Heart Failure with Preserved Ejection Fraction in Diabetes. Biomolecules 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639, Erratum in Eur. Heart J. 2024, 45, 53. https://doi.org/10.1093/eurheartj/ehad613. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.A.; Wyne, K.L. Does Renin-Angiotensin-Aldosterone System Inhibition Impact Obesity as a Co–Risk Factor? J. Clin. Hypertens. 2009, 11 (Suppl. 12), S34–S39. [Google Scholar] [CrossRef]
- Armani, A.; Cinti, F.; Marzolla, V.; Morgan, J.; Cranston, G.A.; Antelmi, A.; Carpinelli, G.; Canese, R.; Pagotto, U.; Quarta, C.; et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 2014, 28, 3745–3757. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, P.; Fu, L.; Sun, L.; Shen, W.; Wu, Q. Comparative cardiovascular outcomes of sglt2 inhibitors in type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Front. Endocrinol. 2022, 13, 802992. [Google Scholar] [CrossRef]
- McMurray, J.; Solomon, S.; Inzucchi, S.; Køber, L.; Kosiborod, M.; Martínez, F.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.; McMurray, J.; Claggett, B.; Boer, R.; DeMets, D.; Hernandez, A.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Talha, K.; Anker, S.; Butler, J. SGLT2 inhibitors in heart failure: A review of current evidence. Int. J. Heart Fail. 2023, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Vinjamuri, S.; Salian, R.; Hafeez, N.; Sundaram, D.; Patel, T.; Gudi, T.R.; Vasavada, A.M. Sodium-glucose cotransporter-2 (SGLT2) inhibitors in heart failure: An umbrella review. Cureus 2023, 15, e42113. [Google Scholar] [CrossRef]
- Tsampasian, V.; Baral, R.; Chattopadhyay, R.; Dębski, M.; Joshi, S.; Reinhold, J.; Dweck, M.R.; Garg, P.; Vassiliou, V.S. The role of sglt2 inhibitors in heart failure: A systematic review and meta-analysis. Cardiol. Res. Pract. 2021, 2021, 9927533. [Google Scholar] [CrossRef] [PubMed]
- Dyck, J.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.; Light, P.; Zuurbier, C.J. Cardiac mechanisms of the beneficial effects of sglt2 inhibitors in heart failure: Evidence for potential off-target effects. J. Mol. Cell. Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Yankah, R.; Anku, E.; Eligar, V. Sodium-glucose cotransporter-2 inhibitors and cardiovascular protection among patients with type 2 diabetes mellitus: A systematic review. J. Diabetes Res. 2024, 2024, 9985836. [Google Scholar] [CrossRef]
- Davies, M.J.; Merton, K.; Vijapurkar, U.; Yee, J.; Qiu, R. Efficacy and safety of canagliflozin in patients with type 2 diabetes based on history of cardiovascular disease or cardiovascular risk factors: A post hoc analysis of pooled data. Cardiovasc. Diabetol. 2017, 16, 40. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Cheong, A.; Teo, Y.; Teo, Y.; Syn, N.; Ong, H.; Ting, A.; Chia, A.Z.Q.; Chong, E.Y.; Chan, M.Y.; Lee, C.; et al. Sglt inhibitors on weight and body mass: A meta-analysis of 116 randomized-controlled trials. Obesity 2021, 30, 117–128. [Google Scholar] [CrossRef]
- Gaborit, B.; Ancel, P.; Abdullah, A.; Maurice, F.; Abdesselam, I.; Calen, A.; Soghomonian, A.; Houssays, M.; Varlet, I.; Eisinger, M.; et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The empacef study. Cardiovasc. Diabetol. 2021, 20, 57. [Google Scholar] [CrossRef]
- Jaiswal, A.; Jaiswal, V.; Ang, S.; Hanif, M.; Vadhera, A.; Agrawal, V.; Kumar, T.; Nair, A.M.M.; Borra, V.; Garimella, V.; et al. Sglt2 inhibitors among patients with heart failure with preserved ejection fraction: A meta-analysis of randomised controlled trials. Medicine 2023, 102, e34693. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Laborante, R.; Cappannoli, L.; Morciano, C.; Gugliandolo, S.; Pontecorvi, A.; Burzotta, F.; Donniacuo, M.; Cappetta, D.; Patti, G.; et al. The effects of SGLT2i on cardiac metabolism in patients with HFpEF: Fact or fiction? Cardiovasc. Diabetol. 2025, 24, 208. [Google Scholar] [CrossRef]
- Afsar, B.; Afsar, R.E. Sodium-glucose co-transporter 2 inhibitors and Sarcopenia: A controversy that must be solved. Clin. Nutr. 2023, 42, 2338–2352. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Shiki, K.; Homma, G.; Meinicke, T.; Ogura, Y.; Seino, Y.; EMPA-ELDERLY Investigators. Efficacy and safety of the sodium-glucose co-transporter-2 inhibitor empagliflozin in elderly Japanese adults (≥65 years) with type 2 diabetes: A randomized, double-blind, placebo-controlled, 52-week clinical trial (EMPA-ELDERLY). Diabetes Obes. Metab. 2023, 25, 3538–3548. [Google Scholar] [CrossRef] [PubMed]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef]
- Beltrami, M.; Fumagalli, C.; Milli, M. Frailty, sarcopenia and cachexia in heart failure patients: Different clinical entities of the same painting. World J. Cardiol. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Tschöp, M.; Nogueiras, R.; Åhrén, B. Gut hormone-based pharmacology: Novel formulations and future possibilities for metabolic disease therapy. Diabetologia 2023, 66, 1796–1808. [Google Scholar] [CrossRef]
- Lincoff, A.; Brown-Frandsen, K.; Colhoun, H.; Deanfield, J.; Emerson, S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Piccini, S.; Favacchio, G.; Panico, C.; Morenghi, E.; Folli, F.; Mazziotti, G.; Lania, A.G.; Mirani, M. Time-dependent effect of glp-1 receptor agonists on cardiovascular benefits: A real-world study. Cardiovasc. Diabetol. 2023, 22, 69. [Google Scholar] [CrossRef]
- Ussher, J.; Drucker, D. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Wang, T.; Ding, J.; Cheng, X.; Yang, Q.; Hu, P. Glucagon-like peptide-1 receptor agonists: New strategies and therapeutic targets to treat atherosclerotic cardiovascular disease. Front. Pharmacol. 2024, 15, 1396656. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Y.; Li, J.; Jiang, X.; Chen, J.; Ye, N.; Wu, B.; Sun, Y.; Sun, G. Glp-1: A prospective guardian for comprehensive myocardial perfusion. Diabetes/Metab. Res. Rev. 2024, 40, e70004. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Kuodi, P.; Akunna, C.; McCreedy, N.; Donsmark, M.; Ren, H.; Nnaji, C. Cardiovascular and renal outcomes of glp-1 receptor agonists vs. dpp-4 inhibitors and basal insulin in type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2023, 20, 14791641231221740. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.; Antunes, M.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 esc guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Obata, S.; Miyamoto, Y.; Slipczuk, L.; Takagi, H.; Kuno, T. Effects of glucagon-like peptide-1 receptor agonists on patients with heart failure: A meta-analysis of randomized controlled trials. J. Cardiovasc. Med. 2023, 24, 132–137. [Google Scholar] [CrossRef]
- Neuen, B.; Fletcher, R.; Heath, L.; Perkovic, A.; Vaduganathan, M.; Badve, S.; Tuttle, K.R.; Pratley, R.; Gerstein, H.C.; Perkovic, V.; et al. Cardiovascular, kidney, and safety outcomes with glp-1 receptor agonists alone and in combination with sglt2 inhibitors in type 2 diabetes: A systematic review and meta-analysis. Circulation 2024, 150, 1781–1790. [Google Scholar] [CrossRef]
- Ferreira, J.; Sharma, A.; Butler, J.; Packer, M.; Zannad, F.; Vasques-Nóvoa, F.; Leite-Moreira, A.; Neves, J.S. Glucagon-like peptide-1 receptor agonists across the spectrum of heart failure. J. Clin. Endocrinol. Metab. 2023, 109, 4–9. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Rambaldi, P.; Fumagalli, C.; Marfella, L.; La Grotta, R.; Frigé, C.; Pellegrini, V.; D’andrea, D.; et al. Glp-1 receptor agonists- SGLT2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: An observational study in patients with type 2 diabetes. Cardiovasc. Diabetol. 2024, 23, 10. [Google Scholar] [CrossRef]
- Butler, J.; Abildstrøm, S.; Borlaug, B.; Davies, M.; Kitzman, D.; Petrie, M.; Shah, S.J.; Verma, S.; Abhayaratna, W.P.; Chopra, V.; et al. Semaglutide in patients with obesity and heart failure across mildly reduced or preserved ejection fraction. J. Am. Coll. Cardiol. 2023, 82, 2087–2096. [Google Scholar] [CrossRef]
- Kosiborod, M.; Verma, S.; Borlaug, B.; Butler, J.; Davies, M.; Jensen, T.; Rasmussen, S.; Marstrand, P.E.; Petrie, M.C.; Shah, S.J.; et al. Effects of semaglutide on symptoms, function, and quality of life in patients with heart failure with preserved ejection fraction and obesity: A prespecified analysis of the step-hfpef trial. Circulation 2024, 149, 204–216. [Google Scholar] [CrossRef]
- Papaetis, G.S. GLP-1 receptor agonists, SGLT2 inhibitors, and obstructive sleep apnoea: Can new allies face an old enemy? Arch. Med. Sci. Atheroscler. Dis. 2023, 8, e19–e34. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Deanfield, J.; Pratley, R.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Emerson, S.S.; Kahn, S.E.; Kitzman, D.W.; Lingvay, I.; et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: A pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet 2024, 404, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Bellido, D.; García, C.; Talluri, A.; Lukaski, H.; García-Almeida, J. Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 2023, 24, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Hamaguchi, M.; Fukui, M. Favorable appendicular skeletal muscle mass changes in older patients with type 2 diabetes receiving glp-1 receptor agonist and basal insulin co-therapy. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231161885. [Google Scholar] [CrossRef]
- Nelson, L.W.; Lee, M.H.; Garrett, J.W.; Pickhardt, S.G.; Warner, J.D.; Summers, R.M.; Pickhardt, P.J. Intrapatient Changes in CT-Based Body Composition After Initiation of Semaglutide (Glucagon-Like Peptide-1 Receptor Agonist) Therapy. AJR Am. J. Roentgenol. 2024, 223, e2431805. [Google Scholar] [CrossRef]
- Chun, E.; Siojo, A.; Rivera, D.; Reyna, K.; Legere, H.; Joseph, R.; Pojednic, R. Weight loss and body composition after compounded semaglutide treatment in a real world setting. Diabetes Obes. Metab. 2025, 27, 1536–1543. [Google Scholar] [CrossRef]
- Pandey, A.; Patel, K.V.; Segar, M.W.; Ayers, C.; Linge, J.; Leinhard, O.D.; Anker, S.D.; Butler, J.; Verma, S.; Joshi, P.H.; et al. Effect of liraglutide on thigh muscle fat and muscle composition in adults with overweight or obesity: Results from a randomized clinical trial. J. Cachexia Sarcopenia Muscle 2024, 15, 1072–1083. [Google Scholar] [CrossRef]
- Bikou, A.; Dermiki-Gkana, F.; Penteris, M.; Constantinides, T.K.; Kontogiorgis, C. A systematic review of the effect of semaglutide on lean mass: Insights from clinical trials. Expert Opin. Pharmacother. 2024, 25, 611–619. [Google Scholar] [CrossRef]
- Thomas, M.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual gip and glp-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J. Clin. Endocrinol. Metab. 2020, 106, 388–396. [Google Scholar] [CrossRef]
- Costa, A.; Ai, M.; Nunn, N.; Culotta, I.; Hunter, J.; Boudjadja, M.; Valencia-Torres, L.; Aviello, G.; Hodson, D.J.; Snider, B.M.; et al. Anorectic and aversive effects of glp-1 receptor agonism are mediated by brainstem cholecystokinin neurons, and modulated by gip receptor activation. Mol. Metab. 2022, 55, 101407. [Google Scholar] [CrossRef]
- Zhang, Q.; Challa, T.; Augustin, R.; Bakhti, M.; Colldén, G.; Drucker, D.; Feuchtinger, A.; Caceres, C.G.; Grandl, G.; Harger, A.; et al. The glucose-dependent insulinotropic polypeptide (gip) regulates body weight and food intake via cns-gipr signaling. Cell Metab. 2021, 33, 833–844.e5. [Google Scholar] [CrossRef] [PubMed]
- Bucheit, J.; Ayers, J.; Pamulapati, L.; Browning, A.; Sisson, E. A novel dual incretin agent, tirzepatide (ly3298176), for the treatment of type 2 diabetes mellitus and cardiometabolic health. J. Cardiovasc. Pharmacol. 2022, 80, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.; Davies, M.; Rosenstock, J.; Manghi, F.; Landó, L.; Bergman, B.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Augusto SNJr Kaelber, D.; Tang, W.H.W. Real-world efficacy of tirzepatide in patients with heart failure without diabetes. Curr. Probl. Cardiol. 2025, 50, 102998. [Google Scholar] [CrossRef]
- Lin, Y.M.; Liao, K.M.; Yu, T.; Wu, J.Y.; Lai, C.C. Effectiveness of tirzepatide in patients with HFpEF using a target trial emulation retrospective cohort study. Nat. Commun. 2025, 16, 4471. [Google Scholar] [CrossRef]
- Heise, T.; DeVries, J.; Urva, S.; Li, J.; Pratt, E.; Thomas, M.; Mather, K.J.; Karanikas, C.A.; Dunn, J.; Haupt, A.; et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care 2023, 46, 998–1004. [Google Scholar] [CrossRef]
- Roux, C.; Hankosky, E.; Wang, D.; Malik, R.; Yu, M.; Hickey, A.; Kan, H.; Bunck, M.C.; Stefanski, A.; Garcia-Perez, L.; et al. Tirzepatide 10 and 15 mg compared with semaglutide 2.4 mg for the treatment of obesity: An indirect treatment comparison. Diabetes Obes. Metab. 2023, 25, 2626–2633. [Google Scholar] [CrossRef]
- Wilson, J.; Nikooienejad, A.; Robins, D.; Roell, W.; Riesmeyer, J.; Haupt, A.; Duffin, K.L.; Taskinen, M.; Ruotolo, G. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 2451–2459. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2025, 392, 427–437. [Google Scholar] [CrossRef]
- Kramer, C.M.; Borlaug, B.A.; Zile, M.R.; Ruff, D.; DiMaria, J.M.; Menon, V.; Ou, Y.; Zarante, A.M.; Hurt, K.C.; Murakami, M.; et al. Tirzepatide Reduces LV Mass and Paracardiac Adipose Tissue in Obesity-Related Heart Failure: SUMMIT CMR Substudy. J. Am. Coll. Cardiol. 2025, 85, 699–706. [Google Scholar] [CrossRef]
| Diagnostic Method | Parameter Measured | Advantages | Limitations and Application in the HF Population |
|---|---|---|---|
| DXA (Dual-energy X-ray Absorptiometry) | Muscle quantity (Appendicular Lean Mass, ALM) | Gold standard for body composition assessment; precise. | Availability, cost. Results can be falsely elevated by fluid overload (edema), which is common in HF, masking lean mass loss. |
| BIA (Bioelectrical Impedance Analysis) | Muscle quantity (Skeletal Muscle Mass, SMM) | Portable, inexpensive, fast, non-invasive. | Highly dependent on hydration status. In HF patients, especially during decompensation, results are often unreliable due to edema and fluid shifts. Requires specific equations validated for HF. |
| Handgrip Strength | Muscle strength | Inexpensive, fast, portable. Strong prognostic predictor. Recognized by EWGSOP2 as the primary screening indicator. | Easy to implement. Should be a routine screening test in HF patients to identify sarcopenia risk. |
| Muscle Ultrasound | Muscle quality and quantity (e.g., quadriceps thickness, echogenicity) | Portable (Point-of-Care), non-invasive. Unaffected by edema when assessing muscle thickness. Allows assessment of quality (e.g., fat infiltration). | Requires standardization and trained operators. Increasingly promising for bedside assessment in HF patients. |
| Computed Tomography (CT)/Magnetic Resonance Imaging (MRI) | Muscle quantity and quality (cross-sectional area, fat infiltration) | Highly precise; allows for accurate assessment of muscle fat infiltration (myosteatosis). | High cost, radiation exposure (CT), limited availability. Reserved mainly for research purposes, not routine HF diagnostics. |
| The Causes of Sarcopenia in HFrEF |
|---|
| increase in inflammatory parameters |
| LPS stimulates the production of TNF-α |
| muscle atrophy due to disuse |
| autophagy associated with ROS, damaged proteins, damaged organelles, and hypoxia |
| activation of protein degradation via the ubiquitin-proteasome pathway in skeletal muscles |
| insulin resistance |
| changes in the growth hormone/insulin-like growth factor axis |
| growth hormone insensitivity |
| sympathetic overactivity |
| increased myostatin levels |
| The Causes of Sarcopenia in HFmrEF and HFpEF |
|---|
| inflammation |
| abnormal intramuscular adipose tissue |
| oxidative stress |
| insulin resistance—suppressed growth hormone secretion and decreased insulin-like growth factor I |
| Study (Year) | Number of Patients | Design | Findings |
|---|---|---|---|
| Yankah R. et al. (2024) [37] | 55.501 | Meta-analysis | Reduction in risk of cardiovascular death, reductions in blood pressure and body mass. |
| Davies M. et al. (2022) [38] | 2.313 | Post hoc analysis | Reductions in HBA1c, body mass, and systolic blood pressure in patients with CVD or CVD risk factors. |
| Pan R. et al. (2022) [39] | 1.430 | Meta-analysis | Improving body composition in T2DM, specifically through reductions in key anthropometric measures (body mass, BMI, waist circumference) and adiposity (visceral, subcutaneous, and total fat mass). |
| Cheong A. et al. (2022) [40] | 98.497 | Meta-analysis | A mean weight reduction of −1.79 kg (95% CI: −1.93 to −1.66, p < 0.001) compared with placebo was observed across diabetes status, duration of follow-up, various comorbidities, and all SGLT drug types. |
| Gaborit B. et al. (2021) [41] | 80 | Prospective interventional study | Empagliflozin selectively reduced liver fat content (−27 ± 23 vs. −2 ± 24%, p = 0.0005) and visceral fat (−7.8% [−15.3;−5.6] vs. −0.1% [−1.1;6.5], p = 0.043), with no effect observed on myocardial or epicardial fat. |
| Jaiswal A. et al. (2023) [42] | 15.989 | Meta-analysis | Shifting of fatty acids to ketone bodies as the substrate for myocardial energy generation |
| Yabe et al. (2023) [45] | 129 | Randomized, double-blind, placebo-controlled trial | Empagliflozin improved glucose control and reduced body weight without compromising muscle mass and strength in older adults with T2DM. |
| Study (Year) | Number of Patients | Design | Findings |
|---|---|---|---|
| Obata S. et al. (2023) [56] | 8.965 | Meta-analysis | Reduction MACE in T2DM patients with prior HF compared with the placebo group |
| Neuen B. et al. (2023) [57] | 1.743 | Meta-analysis | Cardiovascular and kidney benefits of GLP-1 receptor agonists in T2DM patients regardless of SGLT2 inhibitor use |
| Ferreira J. et al. (2023) [58] | 11.430 | Meta-analysis | GLP-1 RAs should be considered for improving cardiovascular outcomes in T2DM patients with atherosclerotic risk but no HF. In T2DM patients with HFpEF and high atherosclerotic risk, they may be considered as a second-line agent after an SGLT2 inhibitor. They should, however, be avoided in HFrEF patients until further evidence is available. |
| Marfella R. et al. (2024) [59] | 537 | Observational cohort study | The incidence of MACE was lower in the combination therapy group compared with patients receiving either SGLT2i or GLP-1RA monotherapy |
| Butler J. et al. (2023) [60] | 529 | Randomized, double-blind, placebo-controlled trial | In patients with HFpEF and obesity, semaglutide 2.4 mg improved symptoms, physical limitations, and exercise function, while also reducing inflammation and body mass. These benefits were observed to a similar extent across all LVEF categories. |
| Kosiborod M. et al. (2024) [61] | 529 | Analysis | Benefits of semaglutide extended to all key summary and individual KCCQ domains |
| Kosiborod M. et al. (2024) [63] | 22.282 | Randomized controlled trial | In patients with HFpEF, semaglutide reduced the risk of the combined endpoint of cardiovascular death or worsening HF events, and worsening HF events alone, whereas its effect on cardiovascular death alone was not significant. |
| Nelson L. et al. (2024) [66] | 241 | Retrospective study | Semaglutide may preserve muscle quality during weight loss, as muscle attenuation remained stable despite a slight reduction in muscle mass. Conversely, in patients who gained weight, the decline in attenuation (myosteatosis) and increased intermuscular fat were likely attributable to overall fat accumulation, not a direct drug effect. |
| Chun E. et al. (2025) [67] | 94 | Retrospective study | Semaglutide/ Cyanocobalamin led to significant weight loss, with fat mass reduction and a proportional increase in skeletal muscle mass, suggesting an improvement in body composition despite some lean mass loss. |
| Pandey A. et al. (2024) [68] | 128 | Randomized controlled trial | Liraglutide reduced muscle fat infiltration but did not significantly affect overall muscle volume, suggesting a potential improvement in muscle quality rather than quantity. |
| Study (Year) | Number of Patients | Design | Findings |
|---|---|---|---|
| Frias J. et al. (2021) [74] | 1.879 | Randomized, double-blind, placebo-controlled trial | All tirzepatide doses showed significantly greater HbA1c reductions than semaglutide. Tirzepatide demonstrated superior weight loss across all doses compared to semaglutide. |
| Augusto S. et al. (2025) [75] | 897 | Review | Tirzepatide presents a promising therapeutic option for managing HF, with significant metabolic and cardiovascular benefits. Untreated patients were at higher risk of incident acute HF and MACE, stroke, CKD, and CAD. |
| Lin Y. et al. (2025) [76] | 14.154 | Retrospective cohort study | Tirzepatide use was associated with significantly lower risks of the primary composite outcome of HF exacerbation and all-cause mortality, as well as reductions in major adverse cardiovascular events and major adverse kidney events. |
| Heise T. et al. (2023) [77] | 117 | Randomized, double-blind, placebo-controlled trial | Tirzepatide led to greater reductions in body mass and fat mass than placebo and semaglutide; however, while both active drugs reduced appetite significantly more than placebo, they did not differ from each other on this measure. |
| Roux C. et al. (2023) [78] | 11.430 | Meta-analysis | Tirzepatide (particularly at 15 mg) may offer superior weight loss efficacy compared to semaglutide 2.4 mg, with a similar tolerability profile. |
| Wilson J. et al. (2020) [79] | 316 | Randomized, double-blind, placebo-controlled trial | Tirzepatide treatment dose-dependently decreased levels of apolipoprotein C-III (apoC-III) and apolipoprotein B (apoB) and the number of large triglyceride-rich lipoproteins (TRLP) and small low-density lipoprotein particles (LDLP), suggesting a net improvement in atherogenic lipoprotein profile. |
| Packer M. et al. (2024) [80] | 731 | Randomized, double-blind, placebo-controlled trial | In patients with HFpEF and obesity, tirzepatide led to a lower risk of the composite of death from cardiovascular causes or worsening HF than placebo. Additionally, it improved health status. |
| Kramer CM et al. (2024) [81] | 175 | Randomized, double-blind, placebo-controlled trial | In obesity-related HFpEF, tirzepatide therapy reduced LV mass and paracardiac adipose tissue compared with placebo. Furthermore, the reduction in LV mass paralleled weight loss. |
| Feature | SGLT2 Inhibitors | GLP-1 Receptor Agonists | GIP/GLP-1 Receptor Agonists |
|---|---|---|---|
| Primary mechanism | Inhibits glucose/sodium reabsorption in the proximal tubule. | Activates GLP-1 receptors. | Activates both GIP and GLP-1 receptors. |
| HF indication | HFrEF, HFmrEF and HFpEF | HFpEF with obesity | HFpEF with obesity |
| Body weight loss | Modest (~2–3 kg) | Significant (~15–17%) | Very significant (~20%+) |
| Effect on fat mass | Reduces visceral and epicardial fat. | Strong reduction in total fat mass | Superior reduction in total fat mass. Reduces paracardiac fat. |
| Effect on lean mass | Contentious. Likely reduction in fluid/edema misinterpreted as lean mass loss. Risk of true sarcopenia considered low, but needs study. | Absolute lean mass is lost alongside fat mass. Muscle quality (myosteatosis) may be preserved or improved. | Absolute lean mass is lost, but fat loss is preferential |
| Key cardiovascular mechanism | Hemodynamic (diuresis, preload reduction), metabolic (ketone shift, epicardial adipose tissue reduction) | Anti-inflammatory, hemodynamic (unloading via weight loss), atherosclerotic risk reduction | Superior weight loss (hemodynamic), adipose tissue remodeling (paracardiac fat), improved atherogenic lipids |
| Primary concern | Potential fluid/lean mass ambiguity | Risk of exacerbating sarcopenia due to large absolute lean mass loss, gastrointestinal side effects | Risk of exacerbating sarcopenia due to large absolute lean mass loss, gastrointestinal side effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryglewska-Wawrzak, K.; Kapłon-Cieślicka, A.; Pawlak, A.; Tomaszuk-Kazberuk, A.; Rubiś, P.; Niedziela, J.; Bielecka-Dąbrowa, A. Potential Implications of Body Mass Composition Changes in Heart Failure Patients in the Era of SGLT2i, GLP-1 RA, and GIP/GLP-1 RA. Pharmaceuticals 2025, 18, 1726. https://doi.org/10.3390/ph18111726
Gryglewska-Wawrzak K, Kapłon-Cieślicka A, Pawlak A, Tomaszuk-Kazberuk A, Rubiś P, Niedziela J, Bielecka-Dąbrowa A. Potential Implications of Body Mass Composition Changes in Heart Failure Patients in the Era of SGLT2i, GLP-1 RA, and GIP/GLP-1 RA. Pharmaceuticals. 2025; 18(11):1726. https://doi.org/10.3390/ph18111726
Chicago/Turabian StyleGryglewska-Wawrzak, Katarzyna, Agnieszka Kapłon-Cieślicka, Agnieszka Pawlak, Anna Tomaszuk-Kazberuk, Paweł Rubiś, Jacek Niedziela, and Agata Bielecka-Dąbrowa. 2025. "Potential Implications of Body Mass Composition Changes in Heart Failure Patients in the Era of SGLT2i, GLP-1 RA, and GIP/GLP-1 RA" Pharmaceuticals 18, no. 11: 1726. https://doi.org/10.3390/ph18111726
APA StyleGryglewska-Wawrzak, K., Kapłon-Cieślicka, A., Pawlak, A., Tomaszuk-Kazberuk, A., Rubiś, P., Niedziela, J., & Bielecka-Dąbrowa, A. (2025). Potential Implications of Body Mass Composition Changes in Heart Failure Patients in the Era of SGLT2i, GLP-1 RA, and GIP/GLP-1 RA. Pharmaceuticals, 18(11), 1726. https://doi.org/10.3390/ph18111726









