Phage Encapsulation and Delivery Technology: A Strategy for Treating Drug-Resistant Pathogenic Microorganisms
Abstract
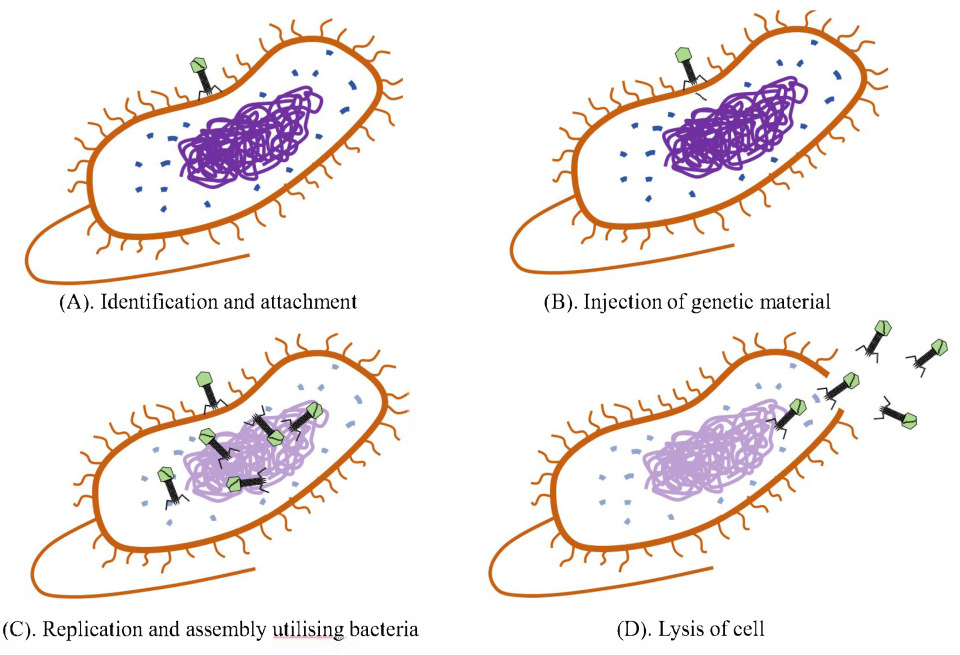
1. Introduction
1.1. Pathogenic Microorganisms
1.2. Antimicrobial Resistance
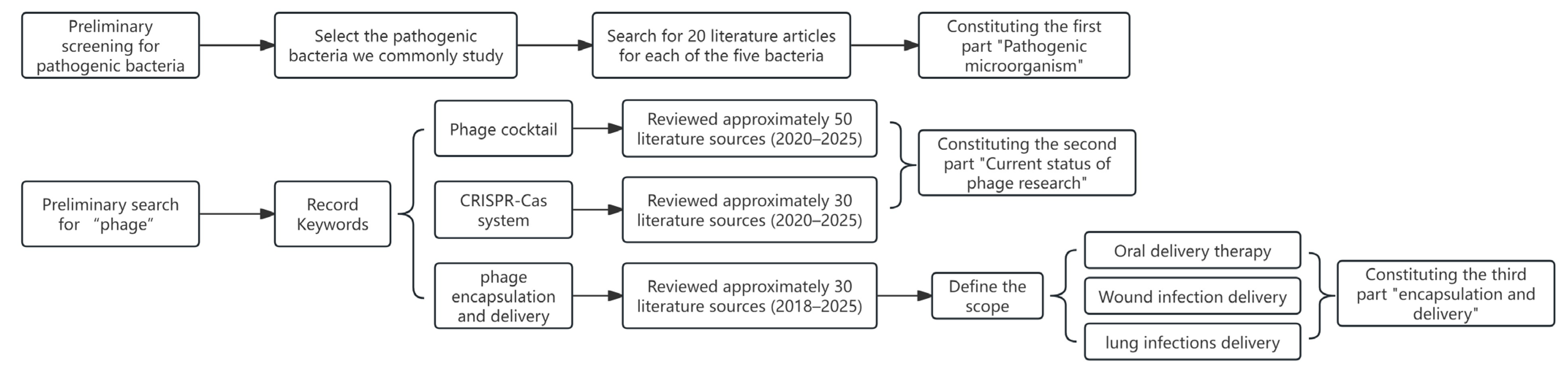
1.3. Materials and Methods
2. Current Status of Phage Research
2.1. Phage Cocktails
2.1.1. Application of Phage Cocktails
2.1.2. Progress in Design Methodology Research
2.2. Engineered Phages Based on the CRISPR-Cas System
2.2.1. Engineered Phages Based on Type I CRISPR-Cas System
2.2.2. Engineered Phages Based on Type II CRISPR-Cas System
3. Recent Research Advances in Phage-Based Encapsulation and Delivery
3.1. Optimization of Traditional Encapsulation Techniques
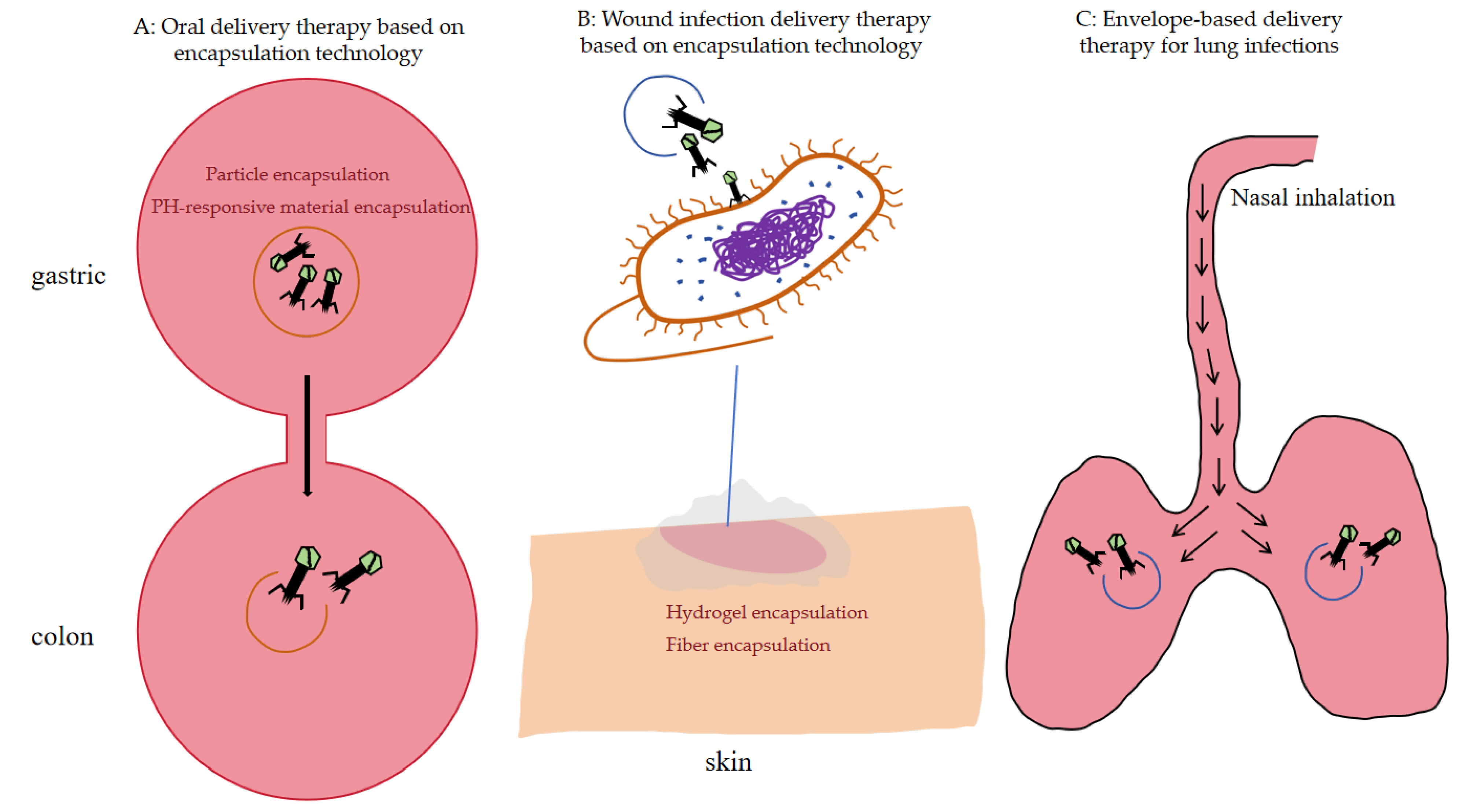
3.2. Oral Delivery
3.2.1. Particle Encapsulation
3.2.2. PH-Responsive Material Encapsulation
3.3. Wound Infection Delivery
3.3.1. Hydrogel Encapsulation
3.3.2. Fiber Encapsulation
3.4. Pulmonary Delivery
3.4.1. Powder Inhalation Therapy
3.4.2. Nebulized Inhalation Therapy
3.5. Novel Encapsulation Material Breakthrough
3.6. Application of Simulation and Prediction Techniques to Phage Encapsulation
4. Limitations
4.1. Research on Encapsulation Technology
4.2. Scale Production
4.3. Security
5. Discussion and Perspective
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Van Overbeek, L.; Burgess, C.M.; Holden, N.; Brennan, F.; Johannessen, G.S.; Allende, A.; Höfte, M.; Cottyn, B.; Pothier, J.F.; et al. Human Pathogenic Microorganisms in Fresh Produce Production: Lessons Learned When Plant Science Meets Food Safety. J. Food Prot. 2025, 88, 100551. [Google Scholar] [CrossRef]
- Chen, B.; Yang, X.; Yang, C.; Chan, E.W.-C.; Wang, X.; Chen, S. Ultrasensitive monoclonal antibody-based Salmonella detection through fluorescence single bacterial cell imaging selectively enhanced by tyramide signal amplification. Innov. Food Sci. Emerg. Technol. 2025, 105, 104229. [Google Scholar] [CrossRef]
- Sripaurya, B.; Pelyuntha, W.; Ngasaman, R.; Ching, C.L.; Guyonnet, V.; Vongkamjan, K. Antibiotic resistance, sequence typing, and virulence gene profiles of Salmonella enterica isolated from the broiler production chain in southern Thailand. Res. Vet. Sci. 2025, 196, 105891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; He, J.; Pei, X.; Lin, Y.; Magnani, M.; Liu, D.; Ding, T.; Feng, J. Milkfat influences thermal tolerance and biofilm formation of Salmonella Typhimurium during pasteurization. Food Res. Int. 2025, 221, 117538. [Google Scholar] [CrossRef] [PubMed]
- Polat, İ.; Güngör, İ.; Şen, B. Prevalence of Salmonella enterica Serotypes Isolated From Broiler Liver and Their Antibiotic Resistance Profiles. J. Food Prot. 2025, 88, 100535. [Google Scholar] [CrossRef]
- Khanal, S.; Luitel, H.; Adhikari, S.; Marasini, A.; Bhattarai, R.K. Molecular characterization, antimicrobial resistance profiling, and biofilm analysis of Salmonella isolates from dead-in-shell embryonated eggs. Poult. Sci. 2025, 104, 105773. [Google Scholar] [CrossRef]
- Woh, P.Y.; Chen, Y.; Nurjadi, D. Bridging the phylodynamic antimicrobial resistance of Salmonella in Southeast Asia and Hong Kong through a One Health Lens. Int. J. Food Microbiol. 2025, 443, 111431. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhou, Z.; Hu, M.; Tai, C.; Shi, X. Characterization of colistin resistance mechanisms development in Salmonella isolates from retail meat in Shanghai, China. LWT 2025, 232, 118424. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Kang, Z.; Xu, H.; Tang, C.; Chen, J.; Hu, H. Mixed-species biofilm with Salmonella in food industry: Persistence, interspecies interaction, and control. Food Res. Int. 2025, 221, 117348. [Google Scholar] [CrossRef]
- Thiers, I.; Lissens, M.; Langie, H.; Lories, B.; Steenackers, H. Salmonella biofilm formation diminishes bacterial proliferation in the C. elegans intestine. Biofilm 2024, 8, 100225. [Google Scholar] [CrossRef]
- Tulin, G.; Méndez, A.A.E.; Figueroa, N.R.; Smith, C.; Folmer, M.P.; Serra, D.; Wade, J.T.; Checa, S.K.; Soncini, F.C. Integration of BrfS into the biofilm-controlling cascade promotes sessile Salmonella growth at low temperatures. Biofilm 2025, 9, 100254. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Liang, Z.; Wu, S.; Ding, Y.; Wu, Q.; Gu, B. Global prevalence of Staphylococcus aureus in food products and its relationship with the occurrence and development of diabetes mellitus. Med. Adv. 2023, 1, 53–78. [Google Scholar] [CrossRef]
- Apiratwarrasakul, S.; Sresuwadjarey, P.; Phumthanakorn, N.; Withatanung, P.; Thongdee, M.; Lerdsittikul, V. The broad-spectrum kayvirus phage disrupts biofilms formed by methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius. Virology 2025, 611, 110657. [Google Scholar] [CrossRef] [PubMed]
- Konatam, S.; Roy, D.N. GC-MS unveils monosaccharide D-Allose in Terminalia arjuna bark extract acting against biofilm regulatory proteins (SrtA and SarA) of Staphylococcus aureus: A drug discovery approach. Int. J. Mass Spectrom. 2025, 518, 117527. [Google Scholar] [CrossRef]
- Rana, E.A.; Nur, S.; Fazal, M.A.; Alam, M.; Ahasan, A.S.M.L.; Rocky, M.J.H.; Rakib, T.M. Genome sequences of three methicillin-resistant Staphylococcus aureus strains isolated from subclinical mastitic cows. Microbiol. Resour. Announc. 2025, 14, e00397-25. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Sivori, F.; Cavallo, I.; Truglio, M.; De Maio, F.; Sanguinetti, M.; Fabrizio, G.; Licursi, V.; Francalancia, M.; Fraticelli, F.; La Greca, I.; et al. Staphylococcus aureus colonizing the skin microbiota of adults with severe atopic dermatitis exhibits genomic diversity and convergence in biofilm traits. Biofilm 2024, 8, 100222. [Google Scholar] [CrossRef]
- Turner, A.B.; Zermeño-Pérez, D.; Mysior, M.M.; Giraldo-Osorno, P.M.; García, B.; O’Gorman, E.; Oubihi, S.; Simpson, J.C.; Lasa, I.; Cróinín, T.Ó; et al. Biofilm morphology and antibiotic susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) on poly-D,L-lactide-co-poly(ethylene glycol) (PDLLA-PEG) coated titanium. Biofilm 2024, 8, 100228. [Google Scholar] [CrossRef]
- Rahal, E.A.; Kazzi, N.; Nassar, F.J.; Matar, G. Escherichia coli O157: H7—Clinical aspects and novel treatment approaches. Front. Cell. Infect. Microbiol. 2012, 2, 138. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D. Epidemiology of Escherichia coli O157: H7 outbreaks, united states, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603. [Google Scholar] [CrossRef]
- Charron, R.; Lemée, P.; Huguet, A.; Minlong, O.; Boulanger, M.; Houée, P.; Soumet, C.; Briandet, R.; Bridier, A. Strain-dependent emergence of aminoglycoside resistance in Escherichia coli biofilms. Biofilm 2025, 9, 100273. [Google Scholar] [CrossRef]
- Zhang, T.; Ray, S.; Melican, K.; Richter-Dahlfors, A. The maturation of native uropathogenic Escherichia coli biofilms seen through a non-interventional lens. Biofilm 2024, 8, 100212. [Google Scholar] [CrossRef]
- Lin, Z.; Duan, X.; Wan, X.; Han, S.; Ji, L.; Zeng, Y.; Shen, J.; Lu, H. Coevolutionary phage training expands phage host range: Driven by tail fiber mutations in Pseudomonas aeruginosa lytic phage? Microbiol. Res. 2025, 301, 128315. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Gales, A.; Isler, B.; Kaye, K.S.; Kwa, A.L.; Landersdorfer, C.B.; Montero, M.M.; Oliver, A.; Pogue, J.M.; Shields, R.K.; et al. How do I manage difficult-to-treat Pseudomonas aeruginosa infections? Key questions for today’s clinicians. Clin. Microbiol. Infect. 2025, 31, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Bello, J.; Vay, C.; Famiglietti, Á.; Nastro, M.; Rodríguez, H. Antimicrobial resistance profiles in multidrug-resistant Pseudomonas aeruginosa isolates in a university hospital in Argentina. Activity of new antibiotic combinations. Rev. Argent. Microbiol. 2025, in press. [CrossRef] [PubMed]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.S.; Furtado, M.M.; Freire, L.; Lee, S.; Jorge, G.P.; Daldosso, G.; Silva, C.E.; Vieira-Junior, L.; Lemos Junior, W.J.F.; Oliveira, C.A.F.; et al. A large sampling study on the occurrence and characteristics of Pseudomonas aeruginosa and heterotrophic bacteria in mineral water over seasons and in different containers. Int. J. Food Microbiol. 2025, 443, 111427. [Google Scholar] [CrossRef]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef]
- Glen, K.A.; Lamont, I.L. β-lactam Resistance in Pseudomonas aeruginosa: Current Status, Future Prospects. Pathogens 2021, 10, 1638. [Google Scholar] [CrossRef]
- Schlichter Kadosh, Y.; Muthuraman, S.; Nisaa, K.; Ben-Zvi, A.; Karsagi Byron, D.L.; Shagan, M.; Brandis, A.; Mehlman, T.; Gopas, J.; Saravana Kumar, R.; et al. Pseudomonas aeruginosa quorum sensing and biofilm attenuation by a di-hydroxy derivative of piperlongumine (PL-18). Biofilm 2024, 8, 100215. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Villeneuve-Faure, C.; Pilloux, L.; Roques, C.; El Garah, F.; Makasheva, K. From adhesion to biofilms formation and resilience: Exploring the impact of silver nanoparticles-based biomaterials on Pseudomonas aeruginosa. Biofilm 2025, 9, 100267. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.S.; Bertolini, A.B.; Pereira, J.G.; Paes, A.C.; da Silva, L.P.; dos Santos Leite, B.; Ribeiro, T.M.; Aires, I.N.; Rangel, M.V.; Curci, V.C.M.; et al. Molecular detection of Listeria monocytogenes in refrigerated raw milk and geospatial distribution in Brazilian small farms. Food Sci. Technol. 2025, 45. [Google Scholar] [CrossRef]
- Fan, C.; Wang, Y.; Zheng, T.; Li, W.; Li, X.; Wu, P. Rapid detection of Listeria monocytogenes in ready-to-eat foods using a one-tube recombinase polymerase amplification and photosensitization colorimetric assay. Sens. Actuators B Chem. 2025, 444, 138489. [Google Scholar] [CrossRef]
- França, L.; Rauecker, U.N.; Santos, D.L.S.d.; Almeida, N.A.; Gebara, C.; Prado, C.S.; Cavicchioli, V.Q.; Lage, M.E.; Duarte, F.O.; Rezende, C.S.M.E.; et al. Occurrence of Listeria spp. and Listeria monocytogenes in half-carcasses, meat cuts, equipment, and the environment of bovine slaughterhouses in Brazil. Res. Vet. Sci. 2025, 196, 105869. [Google Scholar] [CrossRef]
- Li, Z.; Shao, Y.; Liu, X.; Wan, X.; Xiong, P.; Wang, L.; Yuan, J. Steap3 is a key node in regulating the phagosome escape of Listeria monocytogenes. Mol. Immunol. 2025, 182, 96–106. [Google Scholar] [CrossRef]
- Upham, J.P.; Markell, J.A. Challenge Testing to Determine Growth of Listeria monocytogenes on Fresh Enoki Mushrooms at 4 °C and 10 °C Storage Temperatures. J. Food Prot. 2025, 88, 100577. [Google Scholar] [CrossRef]
- Bechtel, T.D.; Hershelman, J.; Ghoshal, M.; McLandsborough, L.; Gibbons, J. Chemical mutagenesis of Listeria monocytogenes for increased tolerance to benzalkonium chloride shows independent genetic underpinnings and off-target antibiotic resistance. PLoS ONE 2024, 19, e0305663. [Google Scholar] [CrossRef]
- Rolon, M.L.; Voloshchuk, O.; Bartlett, K.V.; LaBorde, L.F.; Kovac, J. Multi-species biofilms of environmental microbiota isolated from fruit packing facilities promoted tolerance of Listeria monocytogenes to benzalkonium chloride. Biofilm 2024, 7, 100177. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Wang, Y.; Shi, Y.; Chen, Q. New insights into the antibiofilm activity and mechanism of Mannosylerythritol Lipid-A against Listeria monocytogenes EGD-e. Biofilm 2024, 7, 100201. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Asmare, Z.; Tamrat, E.; Erkihun, M.; Endalamaw, K.; Alelign, D.; Getie, M.; Sisay, A.; Gashaw, Y.; Reta, M.A. Antimicrobial resistance pattern of Acinetobacter baumannii clinical isolate in Ethiopia. A systematic review and meta-analysis. Syst. Rev. Meta-Anal. 2025, 25, 518. [Google Scholar] [CrossRef]
- Antochevis, L.C.; Wilhelm, C.M.; Arns, B.; Sganzerla, D.; Sudbrack, L.O.; Nogueira, T.C.; Guzman, R.D.; Martins, A.S.; Cappa, D.S.; Dos Santos, Â.C.; et al. World Health Organization priority antimicrobial resistance in Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus faecium healthcare-associated bloodstream infections in Brazil (ASCENSION): A prospective, multicentre, observational study. Lancet Reg. Health–Am. 2025, 43, 101004. [Google Scholar] [PubMed]
- Pallett, S.J.; Morkowska, A.; Woolley, S.D.; Potochilova, V.V.; Rudnieva, K.L.; Iungin, O.S.; Sgro, V.; Boyd, S.E.; Reece, N.; Lambert, Z.L.; et al. Evolving antimicrobial resistance of extensively drug-resistant Gram-negative severe infections associated with conflict wounds in Ukraine: An observational study. Lancet Reg. Health–Eur. 2025, 52, 101274. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Alam, M.Z.; Fallon, J.T.; Huang, W. Advances in development of novel therapeutic strategies against multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2024, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Arch. Virol. 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Villalpando-Aguilar, J.L.; Matos-Pech, G.; López-Rosas, I.; Castelán-Sánchez, H.G.; Alatorre-Cobos, F. Phage therapy for crops: Concepts, experimental and bioinformatics approaches to direct Its application. Int. J. Mol. Sci. 2023, 24, 32. [Google Scholar] [CrossRef]
- Ackermann, H.-W. 5500 Phages examined in the electron microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Munteanu, D.I.; Dunn, J.; Apjok, G.; Kintses, B.; Griselain, J.; Steurs, G.; Cochez, C.; Djebara, S.; Merabishvili, M.; Pirnay, J.P.; et al. Phage Therapy for Orthopaedic Infections: The First Three Cases from the United Kingdom. Antibiotics 2025, 14, 114. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Qadri, I.; Alharbi, M.G.; Ramadan, W.S.; Ayubu, J.; Al-Hejin, A.M.; Hakim, R.F.; Hakim, F.F.; Hakim, R.F.; et al. The Battle between Bacteria and Bacteriophages: A Conundrum to Their Immune System. Antibiotics 2023, 12, 381. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Taj, M.I.; Ding, Y.; Bao, Y.; Huang, Y.; Guan, P.; Zhang, Y.; Liu, X.; Ming, Z.; Wang, X. Biocontrol characteristics and application of phage SEP4 against multidrug-resistant Salmonella biofilm on food matrix. Food Biosci. 2024, 60, 104251. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al Hindi, R.; Qadri, I.; Alharbi, M.G.; Hashem, A.M.; Alrefaei, A.A.; Basamad, N.A.; Haque, S.; Alamri, T.; Harakeh, S.; et al. Phage cocktails–an emerging approach for the control of bacterial infection with major emphasis on foodborne pathogens. Biotechnol. Genet. Eng. Rev. 2024, 40, 36–64. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Ngasaman, R.; Yingkajorn, M.; Chukiatsiri, K.; Guyonnet, V.; Vongkamjan, K. Phage cocktail administration to reduce Salmonella load in broilers. Res. Vet. Sci. 2024, 169, 105163. [Google Scholar] [CrossRef]
- Shin, H.; Ahn, E.; Kim, J.; Ryu, S. Development of phage cocktail for controlling colistin-resistant Escherichia coli harboring mcr-1 and its biofilm formation in retail raw chicken. LWT 2024, 200, 116153. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Wu, L.; Xiong, Y.; Xia, L.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Development and mouse model evaluation of a new phage cocktail intended as an alternative to antibiotics for treatment of Staphylococcus aureus-induced bovine mastitis. J. Dairy Sci. 2024, 107, 5974–5987. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Zhu, W. Systemic effects of a phage cocktail on healthy weaned piglets. Biology 2024, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Shiue, S.J.; Wu, M.S.; Chiang, Y.H.; Lin, H.Y. Bacteriophage—Cocktail hydrogel dressing to prevent multiple bacterial infections and heal diabetic ulcers in mice. J. Biomed. Mater. Res. Part A 2024, 112, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gao, Y.; Guo, M.; Zhang, Y.; Zhao, G.; Xia, L.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J. Phage cocktail superimposed disinfection: A ecological strategy for preventing pathogenic bacterial infections in dairy farms. Environ. Res. 2024, 252, 118720. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, D.; Szymańska, M.; Krzyżaniak, M.; Guziński, A.; Matusiak, R.; Kajdanek, A.; Kaczorek-Łukowska, E.; Maszewska, A.; Wójcik, E.A.; Dastych, J. A New Approach for Phage Cocktail Design in the Example of Anti-mastitis Solution. Pathogens 2024, 13, 839. [Google Scholar] [CrossRef]
- Hegarty, B. Making waves: Intelligent phage cocktail design, a pathway to precise microbial control in water systems. Water Res. 2025, 268, 122594. [Google Scholar] [CrossRef]
- Kunisch, F.; Campobasso, C.; Wagemans, J.; Yildirim, S.; Chan, B.K.; Schaudinn, C.; Lavigne, R.; Turner, P.E.; Raschke, M.J.; Trampuz, A. Targeting Pseudomonas aeruginosa biofilm with an evolutionary trained bacteriophage cocktail exploiting phage resistance trade-offs. Nat. Commun. 2024, 15, 8572. [Google Scholar] [CrossRef]
- Li, C.; Shi, T.; Sun, Y.; Zhang, Y. A novel method to create efficient phage cocktails via use of phage-resistant bacteria. Appl. Environ. Microbiol. 2022, 88, e02323-21. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Fan, L.; Jin, H.; Wu, Q.; Ding, Y. Phage engineering using synthetic biology and artificial intelligence to enhance phage applications in food industry. Curr. Opin. Food Sci. 2025, 62, 101274. [Google Scholar] [CrossRef]
- Mahony, J. Biological and bioinformatic tools for the discovery of unknown phage–host combinations. Curr. Opin. Microbiol. 2024, 77, 102426. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; Park de la Torriente, A.; Chalka, A.; Vallejo-Trujillo, A.; McAteer, S.P.; Paterson, G.K.; Low, A.S.; Gally, D.L. Predictive phage therapy for Escherichia coli urinary tract infections: Cocktail selection for therapy based on machine learning models. Proc. Natl. Acad. Sci. USA 2024, 121, e2313574121. [Google Scholar] [CrossRef]
- Lood, C.; Boeckaerts, D.; Stock, M.; De Baets, B.; Lavigne, R.; van Noort, V.; Briers, Y.J.C.O.i.V. Digital phagograms: Predicting phage infectivity through a multilayer machine learning approach. Curr. Opin. Virol. 2022, 52, 174–181. [Google Scholar] [CrossRef]
- Smug, B.J.; Majkowska-Skrobek, G.; Drulis-Kawa, Z. PhREEPred: Phage Resistance Emergence Prediction Web Tool to Foresee Encapsulated Bacterial Escape from Phage Cocktail Treatment. J. Mol. Biol. 2022, 434, 167670. [Google Scholar] [CrossRef]
- Seguritan, V.; Alves Jr, N.; Arnoult, M.; Raymond, A.; Lorimer, D.; Burgin, A.B.; Salamon, P.; Segall, A.M. Artificial neural networks trained to detect viral and phage structural proteins. PLoS Comput. Biol. 2012, 8, e1002657. [Google Scholar] [CrossRef]
- Peng, H.; Chen, I.A.; Qimron, U. Engineering phages to fight multidrug-resistant bacteria. Chem. Rev. 2024, 125, 933–971. [Google Scholar] [CrossRef]
- Nidhi, S.; Anand, U.; Oleksak, P.; Tripathi, P.; Lal, J.A.; Thomas, G.; Kuca, K.; Tripathi, V. Novel CRISPR–Cas systems: An updated review of the current achievements, applications, and future research perspectives. Int. J. Mol. Sci. 2021, 22, 3327. [Google Scholar] [CrossRef]
- Stoltzfus, M.J.; Workman, R.E.; Keith, N.C.; Modell, J.W. A dynamic subpopulation of CRISPR–Cas overexpressers allows Streptococcus pyogenes to rapidly respond to phage. Nat. Microbiol. 2024, 9, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Workman, R.E.; Stoltzfus, M.J.; Keith, N.C.; Euler, C.W.; Bondy-Denomy, J.; Modell, J.W. Anti-CRISPR proteins trigger a burst of CRISPR-Cas9 expression that enhances phage defense. Cell Rep. 2024, 26, 43. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Jia, H.-J.; Jia, P.-P.; Yin, S.; Bu, L.-K.; Yang, G.; Pei, D.-S. Engineering bacteriophages for enhanced host range and efficacy: Insights from bacteriophage-bacteria interactions. Front. Microbiol. 2023, 14, 1172635. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Barrangou, R. Characterization and applications of Type I CRISPR-Cas systems. Biochem. Soc. Trans. 2020, 48, 15–23. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.Ø.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef]
- Chylinski, K.; Makarova, K.S.; Charpentier, E.; Koonin, E.V. Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res. 2014, 42, 6091–6105. [Google Scholar] [CrossRef]
- Agha, A.S.A.; Al-Samydai, A.; Aburjai, T. New frontiers in CRISPR: Addressing antimicrobial resistance with Cas9, Cas12, Cas13, and Cas14. Heliyon 2025, 11, e42013. [Google Scholar] [CrossRef]
- Hosen, A.; Nishat, M.N.H.; Soaib, M.M.H.; Sharkar, O.S.; Sahabuddin, M.; Sharif, I.H.; Bhajan, S.K. A Review: CRISPR Cas System and the Mechanism With an Inhibition of Binding of CRISPR Cas—9. Nano Sel. 2025, 6, e202400009. [Google Scholar] [CrossRef]
- Tsubota, T.; Takasu, Y.; Yonemura, N.; Sezutsu, H. Enhancements of the CRISPR-Cas System in the Silkworm Bombyx mori. Cris. J. 2025, 8, 155–164. [Google Scholar] [CrossRef]
- Park, J.Y.; Moon, B.Y.; Park, J.W.; Thornton, J.A.; Park, Y.H.; Seo, K.S. Genetic engineering of a temperate phage-based delivery system for CRISPR/Cas9 antimicrobials against Staphylococcus aureus. Sci. Rep. 2017, 7, 44929. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P. Positive and negative aspects of bacteriophages and their immense role in the food chain. NPJ Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef]
- Wagh, R.V.; Priyadarshi, R.; Rhim, J.-W. Novel bacteriophage-based food packaging: An innovative food safety approach. Coatings 2023, 13, 609. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, H.; Zou, G.; Song, Z.; Zhou, Y.; Li, H.; Tan, C.; Chen, H.; Fischetti, V.A.; Li, J. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Control. Release 2023, 353, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Koçer Alaşalvar, G.; Yıldırım, Z.J. Encapsulation of SE—P47 phage specific to Salmonella Enteritidis and evaluation of its stability. J. Food Saf. 2024, 44, e13103. [Google Scholar] [CrossRef]
- Selim, H.M.R.M.; Gomaa, F.A.M.; Alshahrani, M.Y.; Morgan, R.N.; Aboshanab, K.M. Phage therapeutic delivery methods and clinical trials for combating clinically relevant pathogens. Ther. Deliv. 2025, 16, 247–269. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.-S.; Han, J. Maltodextrin-trehalose miscible system-based bacteriophage encapsulation: Studies of plasticizing effect on encapsulated phage activity and food application as an antimicrobial agent. Food Control 2023, 146, 109550. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, L.; Ning, M.; Cai, T.; Long, F.; Yuan, Y.; Yue, T. Characterization and release of casein—Sodium alginate embedding phage edible film and the application in controlling of Salmonella contamination in food. Int. J. Food Microbiol. 2025, 434, 111137. [Google Scholar] [CrossRef]
- Simadibrata, D.M.; Syam, A.F.; Lee, Y.Y. A comparison of efficacy and safety of potassium—Competitive acid blocker and proton pump inhibitor in gastric acid—Related diseases: A systematic review and meta—Analysis. J. Gastroenterol. Hepatol. 2022, 37, 2217–2228. [Google Scholar] [CrossRef]
- Javeedvali, S.; Gopalakrishnan, C.; Kannan, R.; Manonmani, S.; Prasanthrajan, M.; Varanavasiappan, S. Strategies for sustainable rice bacterial leaf blight management: A holistic approach through phage biocontrol and nanoparticle encapsulation. J. Plant Pathol. 2024, 1–12. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, T.; Miao, Y.; Zhang, Q.; Yang, M.; Mao, C. Injectable Phage-Loaded Microparticles Effectively Release Phages to Kill Methicillin-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2024, 16, 17232–17241. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M.; Elkenany, R.M.; El-Khateeb, A.Y.; Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Isolation and encapsulation of bacteriophage with chitosan nanoparticles for biocontrol of multidrug-resistant methicillin-resistant Staphylococcus aureus isolated from broiler poultry farms. Sci. Rep. 2024, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Temsaah, H.R.; Abdelkader, K.; Ahmed, A.E.; Elgiddawy, N.; Eldin, Z.E.; Elshebrawy, H.A.; Kasem, N.G.; El-Gohary, F.A.; Azmy, A.F. Chitosan nano-formulation enhances stability and bactericidal activity of the lytic phage HK6. BMC Biotechnol. 2025, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Malligarjunan, N.; Pandian, S.K.; Gowrishankar, S. Chitosan-encapsulated bacteriophage cocktail as promising oral delivery system to surpass gastrointestinal infection caused by Klebsiella aerogenes. Int. J. Biol. Macromol. 2025, 292, 139236. [Google Scholar] [CrossRef]
- Salatin, S.; Yari Khosroushahi, A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Wang, C.; Jiang, P.; Cui, P.; Wang, J. Rationally designed oral DOX gels for colon-specific administration. Gels 2022, 8, 759. [Google Scholar] [CrossRef]
- Guan, Z.; Feng, Q. Chitosan and chitooligosaccharide: The promising non-plant-derived prebiotics with multiple biological activities. Int. J. Mol. Sci. 2022, 23, 6761. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.-M.; Duan, H.-Y.; Yang, K.-D.; Ye, J.-F. Advances and optimization strategies in bacteriophage therapy for treating inflammatory bowel disease. Front. Immunol. 2024, 15, 1398652. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xie, G.; He, J.; Meng, L.; Pang, Y.; Liu, J. Enhancing phage therapy by coating single bacteriophage-infected bacteria with polymer to preserve phage vitality. Nat. Biomed. Eng. 2025, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhi, W.; Bai, B.; Anjum, F.R.; Jia, Z.; Kong, R.; Liu, Q.; Wang, B.; Ma, C.; Ma, D. Impact of sodium alginate hydrogel containing bacteriophage peptides that specifically bind to the EtCab protein on the inhibition of Eimeria tenella infection. Vet. Res. 2025, 56, 18. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, S.B.; Gigante, A.M.; Hooton, S.P.; Atterbury, R.J. Efficacy of different encapsulation techniques on the viability and stability of diverse phage under simulated gastric conditions. Microorganisms 2023, 11, 2389. [Google Scholar] [CrossRef]
- Truong-Le, Q.A.; Lee, S.O.; Ubeyitogullari, A. Encapsulation of Bifidobacterium bifidum into a pH-sensitive alginate-pectin gel system using 3D food printing: Enhanced viability and targeted release. Int. J. Biol. Macromol. 2025, 318, 145134. [Google Scholar] [CrossRef]
- Guadarrama-Escobar, O.R.; Sánchez-Vázquez, I.; Serrano-Castañeda, P.; Chamorro-Cevallos, G.A.; Rodríguez-Cruz, I.M.; Sánchez-Padrón, A.Y.; Anguiano-Almazán, E.; Peña-Juárez, M.C.; Méndez-Albores, A.; Domínguez-Delgado, C.L.; et al. Development, Characterization, Optimization, and In Vivo Evaluation of Methacrylic Acid-Ethyl Acrylate Copolymer Nanoparticles Loaded with Glibenclamide in Diabetic Rats for Oral Administration. Pharmaceutics 2021, 13, 2023. [Google Scholar] [CrossRef]
- Li, N.; Zhang, G.; Zhang, X.; Liu, Y.; Kong, Y.; Wang, M.; Ren, X. A rapid-floating natural polysaccharide gel-raft with double-effect for the treatment of gastroesophageal reflux disease. Int. J. Biol. Macromol. 2024, 261, 129667. [Google Scholar] [CrossRef]
- Brinck, J.E.; Sinha, A.K.; Laursen, M.F.; Dragsted, L.O.; Raes, J.; Uribe, R.V.; Walter, J.; Roager, H.M.; Licht, T.R. Intestinal pH: A major driver of human gut microbiota composition and metabolism. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 639–656. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; To, K.K.; Liu, Y.; Bai, C.; Leung, S.S. A thermosensitive hydrogel formulation of phage and colistin combination for the management of multidrug-resistant Acinetobacter baumannii wound infections. Biomater. Sci. 2024, 12, 151–163. [Google Scholar] [CrossRef]
- Abed, S.; Beig, M.; Barzi, S.M.; Shafiei, M.; Hashemi Shahraki, A.; Sadeghi, S.; Sohrabi, A. Development of phage-containing hydrogel for treating Enterococcus faecalis-infected wounds. PLoS ONE 2024, 19, e0312469. [Google Scholar]
- Khazani Asforooshani, M.; Elikaei, A.; Abed, S.; Shafiei, M.; Barzi, S.M.; Solgi, H.; Badmasti, F.; Sohrabi, A. A novel Enterococcus faecium phage EF-M80: Unveiling the effects of hydrogel-encapsulated phage on wound infection healing. Front. Microbiol. 2024, 15, 1416971. [Google Scholar] [CrossRef]
- Peng, X.; Wang, T.; Dai, B.; Zhu, Y.; Ji, M.; Yang, P.; Zhang, J.; Liu, W.; Miao, Y.; Liu, Y.; et al. Gene Therapy for Inflammatory Cascade in Intrauterine Injury with Engineered Extracellular Vesicles Hybrid Snail Mucus-enhanced Adhesive Hydrogels. Adv. Sci. 2025, 12, e2410769. [Google Scholar] [CrossRef]
- An, D.; Wang, Z.; Ning, Y.; Yue, Y.; Xuan, H.; Hu, Y.; Yang, M.; Zhou, H.; Liu, Q.; Wang, X.; et al. One-Step Physical and Chemical Dual-Reinforcement with Hydrophobic Drug Delivery in Gelatin Hydrogels for Antibacterial Wound Healing. ACS Omega 2024, 9, 34413–34427. [Google Scholar] [CrossRef]
- Shakeri, A.; Najm, L.; Khan, S.; Tian, L.; Ladouceur, L.; Sidhu, H.; Al-Jabouri, N.; Hosseinidoust, Z.; Didar, T.F. Noncontact 3D Bioprinting of Proteinaceous Microarrays for Highly Sensitive Immunofluorescence Detection within Clinical Samples. ACS Nano 2024, 18, 31506–31523. [Google Scholar] [CrossRef]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G.; Tucker, N.; Jafari, S.M. Bioactive-loaded nanovesicles embedded within electrospun plant protein nanofibers; a double encapsulation technique. Food Hydrocoll. 2023, 141, 108683. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Liu, Z.; Wang, W.; Liu, X.; Qian, S.; Chen, L.; Gu, L.; Sun, C.; Hou, J. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023, 402, 134253. [Google Scholar] [CrossRef]
- Ju, T.; Li, J.; Weston, A.; Satta, G.; Bolognini, S.; Di Luca, M.; Gaisford, S.; Williams, G.R. Anti—Pseudomonas Aeruginosa Bacteriophage Loaded Electrospun Fibers for Antibacterial Wound Dressings. Macromol. Rapid Commun. 2025, 13, 2400744. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Chen, Q.; Echterhof, A.; Koff, J.L.; Bollyky, P.L. Phage therapy for respiratory infections: Opportunities and challenges. Lung 2024, 202, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Liang, M. Design of respirable sprayed microparticles of encapsulated bacteriophages. Front. Drug Deliv. 2023, 3, 1209534. [Google Scholar] [CrossRef]
- Sawant, S.S.; Ahmed, M.U.; Gantala, N.-G.; Chiu, C.; Qu, L.; Zhou, Q. Development of Inhalable Bacteriophage Liposomes Against Pseudomonas aeruginosa. Pharmaceutics 2025, 17, 405. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Liu, X.; Wang, C.-Y.; Wan, Q.-Q.; Tian, Y.-F.; Liu, S.-L.; Pang, D.-W.; Wang, Z.-G. Inhalable polymeric microparticles for phage and photothermal synergistic therapy of Methicillin-Resistant Staphylococcus aureus pneumonia. Nano Lett. 2024, 24, 8752–8762. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Leung, S.S.Y. Investigating the effectiveness of liposome-bacteriophage nanocomplex in killing Staphylococcus aureus using epithelial cell coculture models. Int. J. Pharm. 2024, 657, 124146. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Y.; Li, M.; Khanal, D.; Chan, H.K. Can bacteriophage be stabilised by lipid encapsulation when nebulised for inhalation delivery against Pseudomonas aeruginosa? Int. J. Pharm. 2025, 678, 125670. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quek, S.Y.; Huang, K. An Ecofriendly Nature—Inspired Microcarrier for Enhancing Delivery, Stability, and Biocidal Efficacy of Phage—Based Biopesticides. Small 2024, 20, 2403465. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, S.; Jamil, R.Z.R.; Suah, F.B.M. Sporopollenin based materials as a versatile choice for the detoxification of environmental pollutants—A review. Int. J. Biol. Macromol. 2022, 207, 990–1004. [Google Scholar] [CrossRef]
- Singh, L.S. Nano-emulsion encapsulation for the efficient delivery of bacteriophage therapeutics. Biologicals 2024, 85, 101725. [Google Scholar]
- Wdowiak, M.; Raza, S.; Grotek, M.; Zbonikowski, R.; Nowakowska, J.; Doligalska, M.; Cai, N.; Luo, Z.; Paczesny, J. Phage-Nanoparticle Cocktails as a Novel Antibacterial Approach: Synergistic Effects of Bacteriophages and Green-Synthesized Silver Nanoparticles. BioRxiv 2025. [Google Scholar] [CrossRef]
- Easwaran, M.; Raja, N.; Saravanan, M.; Belete, M.A. Liposome-loaded phage cocktail: A promising therapeutic option against postsurgical wound infections–A critical appraisal. Ann. Med. Surg. 2024, 86, 4319–4321. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Wang, F.; Zhang, Y.; Hao, H.; Lv, X.; Hao, L.; Shi, Y. Microencapsulated phage composites with increased gastrointestinal stability for the oral treatment of Salmonella colonization in chicken. Front. Vet. Sci. 2022, 9, 1101872. [Google Scholar] [CrossRef]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Onesto, V.; Manco, R.; Mollo, V.; Vecchione, R.; De Berardinis, P.; Netti, P.A. PLGA microparticle formulations for tunable delivery of a nano-engineered filamentous bacteriophage-based vaccine: In vitro and in silico-supported approach. J. Nanostruct. Chem. 2023, 14, 307–322. [Google Scholar] [CrossRef]
- Tao, S.; Hu, A.; Bavel, E.; Yu, C.H.; Zhang, S.; Kissling, V.M.; Li, Z.; Moriaty, T.F.; Maniura-Weber, K.; Ren, Q. Bio—Responsive Hydrogel for Targeted on—Demand Release of a Phage Cocktail for Treatment of Pseudomonas aeruginosa Infection. Adv. Funct. Mater. 2025, e09360. [Google Scholar] [CrossRef]
- Youssef, R.A.; Sakr, M.M.; Shebl, R.I.; Aboshanab, K.M. Recent insights on challenges encountered with phage therapy against gastrointestinal-associated infections. Gut Pathog. 2025, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Taati Moghadam, M.; Mohebi, S.; Sheikhi, R.; Hasannejad-Bibalan, M.; Shahbazi, S.; Nemati, S. Phage and Endolysin Therapy Against Antibiotics Resistant Bacteria: From Bench to Bedside. MedComm 2025, 6, e70280. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Rahimian, M.; Panahi, B. Bridging the gap: Phage manufacturing processes from laboratory to agri-food industry. Virus Res. 2025, 353, 199537. [Google Scholar] [CrossRef]
- Fatima, R.; Hynes, A.P. Phage-Antibiotic Combinations for Pseudomonas: Successes in the Clinic and In Vitro Tenuously Connected. Microb. Biotechnol. 2025, 18, e70193. [Google Scholar] [CrossRef]
- Banicod, R.J.S.; Javaid, A.; Tabassum, N.; Jo, D.M.; Hassan, M.I.; Kim, Y.M.; Khan, F. Marine Bacteriophages as Next-Generation Therapeutics: Insights into Antimicrobial Potential and Application. Viruses 2025, 17, 971. [Google Scholar] [CrossRef]
- Nang, S.C.; Lin, Y.-W.; Fabijan, A.P.; Chang, R.Y.; Rao, G.G.; Iredell, J.; Chan, H.-K.; Li, J. Pharmacokinetics/pharmacodynamics of phage therapy: A major hurdle to clinical translation. Clin. Microbiol. Infect. 2023, 29, 702–709. [Google Scholar] [CrossRef]
- Akturk, E.; Pinto, G.; Ostyn, L.; Crabbé, A.; Melo, L.D.R.; Azeredo, J.; Coenye, T. Combination of phages and antibiotics with enhanced killing efficacy against dual-species bacterial communities in a three-dimensional lung epithelial model. Biofilm 2025, 9, 100245. [Google Scholar] [CrossRef]
- Akturk, E.; Melo, L.D.R.; Oliveira, H.; Crabbé, A.; Coenye, T.; Azeredo, J. Combining phages and antibiotic to enhance antibiofilm efficacy against an in vitro dual species wound biofilm. Biofilm 2023, 6, 100147. [Google Scholar] [CrossRef]
- Shambharkar, A.; Thompson, T.P.; McClenaghan, L.A.; Bourke, P.; Gilmore, B.F.; Skvortsov, T. Plasma activated water pre-treatment substantially enhances phage activity against Proteus mirabilis biofilms. Biofilm 2024, 8, 100230. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, I. Ultrabright fluorescent particles via physical encapsulation of fluorescent dyes in mesoporous silica: A mini-review. Nanoscale 2024, 16, 10994–11004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Wang, Z.; Zhao, H.; Zhan, L.; Gui, H.; Xu, X.; Ma, X.; Ma, B. pH-responsive and self-adaptive injectable sodium alginate/carboxymethyl chitosan hydrogel accelerates infected wound healing by bacteriostasis and immunomodulation. Carbohydr. Polym. 2025, 354, 123322. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yao, Y.; Wang, M.; Wang, Z.; Xue, Y.; Li, B.; Ma, Y.; Shen, Y.; Wu, H. Recent studies on proteins and polysaccharides-based pH-responsive fluorescent materials. Int. J. Biol. Macromol. 2024, 260, 129534. [Google Scholar] [CrossRef]
- Yadav, P.; Gupta, M.; Singh, S.P.; Parashar, P. Biocompatible Electrospun Hydrogel Fibers for Advanced Wound Healing Therapies. Curr. Pharm. Des. 2024, 30, 3240–3254. [Google Scholar] [CrossRef]
- Gordon, A.; Li, B.; Witten, J.; Nguyen, H.; Anderson, D.G. Inhalable Dry Powders for Lung mRNA Delivery. Adv. Healthc. Mater. 2024, 13, e2400509. [Google Scholar] [CrossRef]
- Naureen, F.; Shah, Y.; Rehman, M.U.; Fazli Nasir, F.N.; Pirzada, A.S.; Al-Otaibi, J.S.; Daglia, M.; Khan, H. Innovative inhalable dry powder: Nanoparticles loaded with Crizotinib for targeted lung cancer therapy. BMC Cancer 2025, 25, 1526. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, R.A.; Leung, C.Y.; Chan, B.K.; Turner, P.E.; Weitz, J.S. Quantitative Models of Phage-Antibiotic Combination Therapy. MSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Parvez, A.; Fahmy, N.A.; Abdel Hady, B.H.; Kumar, S.; Ganguly, A.; Atiya, A.; Elhassan, G.O.; Alfadly, S.O.; Parkkila, S.; et al. Brucellosis: Epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann. Med. 2023, 55, 2295398. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Ruzzi, F.; Semprini, M.S.; Scalambra, L.; Palladini, A.; Angelicola, S.; Cappello, C.; Pittino, O.M.; Nanni, P.; Lollini, P.L. Virus-like Particle (VLP) Vaccines for Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 12963. [Google Scholar] [CrossRef]
- Yue, H.; Li, Y.; Yang, T.; Wang, Y.; Bao, Q.; Xu, Y.; Liu, X.; Miao, Y.; Yang, M.; Mao, C. Filamentous phages as tumour-targeting immunotherapeutic bionanofibres. Nat. Nanotechnol. 2025, 20, 167–176. [Google Scholar] [CrossRef]
- Petrov, G.; Dymova, M.; Richter, V. Bacteriophage-Mediated Cancer Gene Therapy. Int. J. Mol. Sci. 2022, 23, 14245. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Gao, C.; Zheng, Y.M.; Yi, L.; Lu, J.C.; Huang, X.Y.; Cai, J.B.; Zhang, P.F.; Cui, Y.H.; Ke, A.W. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef] [PubMed]
- Dy, R.L.; Rigano, L.A.; Fineran, P.C. Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem. Soc. Trans. 2018, 46, 1605–1613. [Google Scholar] [CrossRef]
- Miller, M.; Deiulio, A.; Holland, C.; Douthitt, C.; McMahon, J.; Wiersma-Koch, H.; Turechek, W.W.; D’Elia, T. Complete genome sequence of Xanthomonas phage RiverRider, a novel N4-like bacteriophage that infects the strawberry pathogen Xanthomonas fragariae. Arch. Virol. 2020, 165, 1481–1484. [Google Scholar] [CrossRef]
- Vique, G.; Mendoza-Barberá, E.; Ramos-Barbero, M.D.; Blanco-Picazo, P.; Sala-Comorera, L.; Quirós, P.; Atares, S.; Salaet, I.; Muniesa, M.; Rodríguez-Rubio, L. Efficacy of Erwinia amylovora and Xanthomonas campestris pv campestris phages to control fire blight and black rot in vivo. Microbiol. Spectr. 2025, 13, e0028025. [Google Scholar] [CrossRef]
| Types | Phages | Targets |
|---|---|---|
| multi-species phage cocktail | S19cd, S143_2, N2, C6, C2 | Salmonella, E. coli |
| Ph(S), Ph(E), Ph(P) | Salmonella, E. coli, P. aeruginosa | |
| VB_ECoM_SYGD1, VB ECoP SYGE1, VB ECOM SYGMH, vB_SauP_SLPW, vB SauM JDYN, vB_SauM JDF86, vB PaeS-PAJD-1 | E. coli, S. aureus, P. aeruginosa | |
| single-strain and single-species phage cocktail | JDYN, JDF86, SLPW | S. aureus |
| vB_SauM_JDYN, vB_SauM_JDF86 and vB_SauP_SLPW | S. aureus | |
| HEMP1, HEMP4, HEMP5 | E. coli |
| Delivery | Encapsulation Methods | Advantages | Disadvantages | Encapsulation Materials and Techniques | Target |
|---|---|---|---|---|---|
| Gastrointestinal | Particle encapsulation | Acid resistance Highly antibacterial Multifunctionality | Complex process Imprecise targeting | Cs-nps | S. aureus |
| PH-responsive material | Acid resistance Precise targeting | Complex process Poor stability | Pectin | ||
| Methacrylic acid-ethyl acrylate copolymer | |||||
| Alginate | |||||
| Alginate-carrageenan | Salmonella | ||||
| Wound | Hydrogel encapsulation | Biocompatibility Sustained effect | High cost Poor stability | Binary temperature-sensitive hydrogel consisting of Pf-127 and HPMC | A. baumannii |
| Hydrogel consisting of sa, cmc and ha | E.faecalis | ||||
| Fiber encapsulation | Sustained effect Good stability | Complex process Material constraints | Electrospun fiber encapsulated materials | P. aeruginosa | |
| Lung | Powder inhalation | Good stability long-term storage | Equipment requirements Complex process | Liposome encapsulation based on the thin-film hydration method | P. aeruginosa |
| Inhalation pmps-pis | S. aureus | ||||
| Nebulized inhalation | Simple process High flexibility | Equipment requirements Poor stability Short shelf life | Liposome atomization encapsulation based on vibrating mesh nebulizer | ||
| Electrospun fiber encapsulated materials | P. aeruginosa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Xu, Z.; Soteyome, T.; Premarathna, M.; Yin, X.; Liu, J. Phage Encapsulation and Delivery Technology: A Strategy for Treating Drug-Resistant Pathogenic Microorganisms. Pharmaceuticals 2025, 18, 1688. https://doi.org/10.3390/ph18111688
Yue Y, Xu Z, Soteyome T, Premarathna M, Yin X, Liu J. Phage Encapsulation and Delivery Technology: A Strategy for Treating Drug-Resistant Pathogenic Microorganisms. Pharmaceuticals. 2025; 18(11):1688. https://doi.org/10.3390/ph18111688
Chicago/Turabian StyleYue, Yang, Zhenbo Xu, Thanapop Soteyome, Mahesh Premarathna, Xiaomao Yin, and Junyan Liu. 2025. "Phage Encapsulation and Delivery Technology: A Strategy for Treating Drug-Resistant Pathogenic Microorganisms" Pharmaceuticals 18, no. 11: 1688. https://doi.org/10.3390/ph18111688
APA StyleYue, Y., Xu, Z., Soteyome, T., Premarathna, M., Yin, X., & Liu, J. (2025). Phage Encapsulation and Delivery Technology: A Strategy for Treating Drug-Resistant Pathogenic Microorganisms. Pharmaceuticals, 18(11), 1688. https://doi.org/10.3390/ph18111688










