Cuproptosis Contributes to Cisplatin-Induced Nephrotoxicity: Insights into Thymol’s Potential Inhibitory and Protective Effects
Abstract
1. Introduction
2. Results
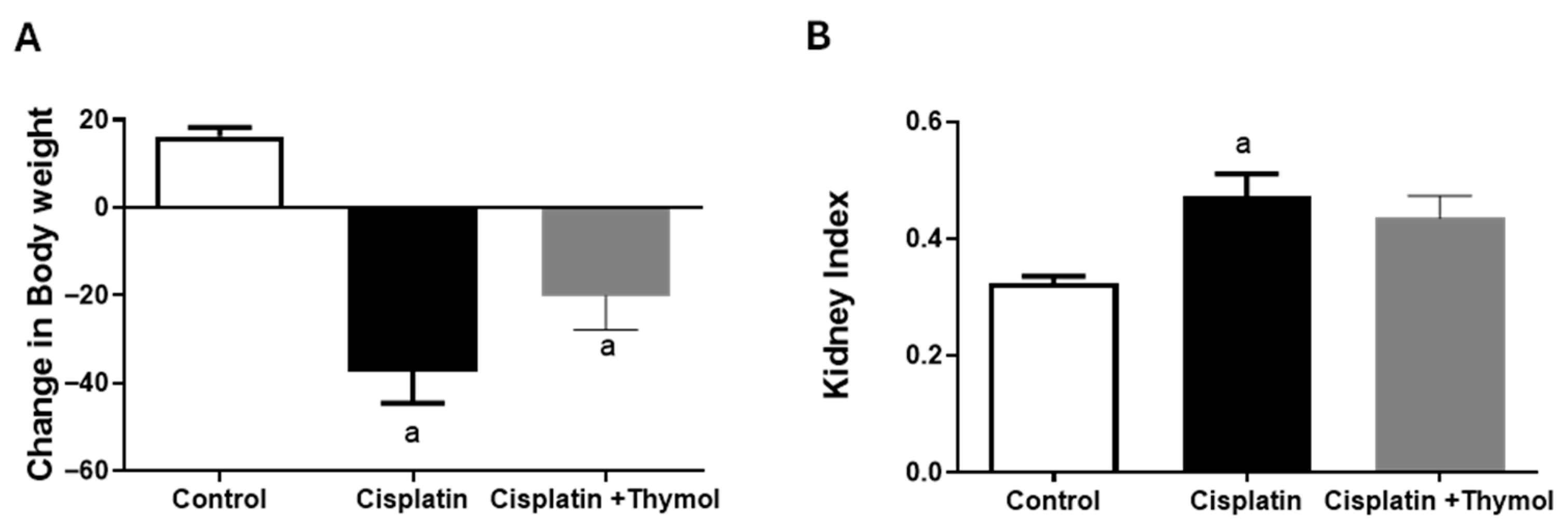
2.1. Effect of Thymol on Changes in Body Weight and Kidney Index
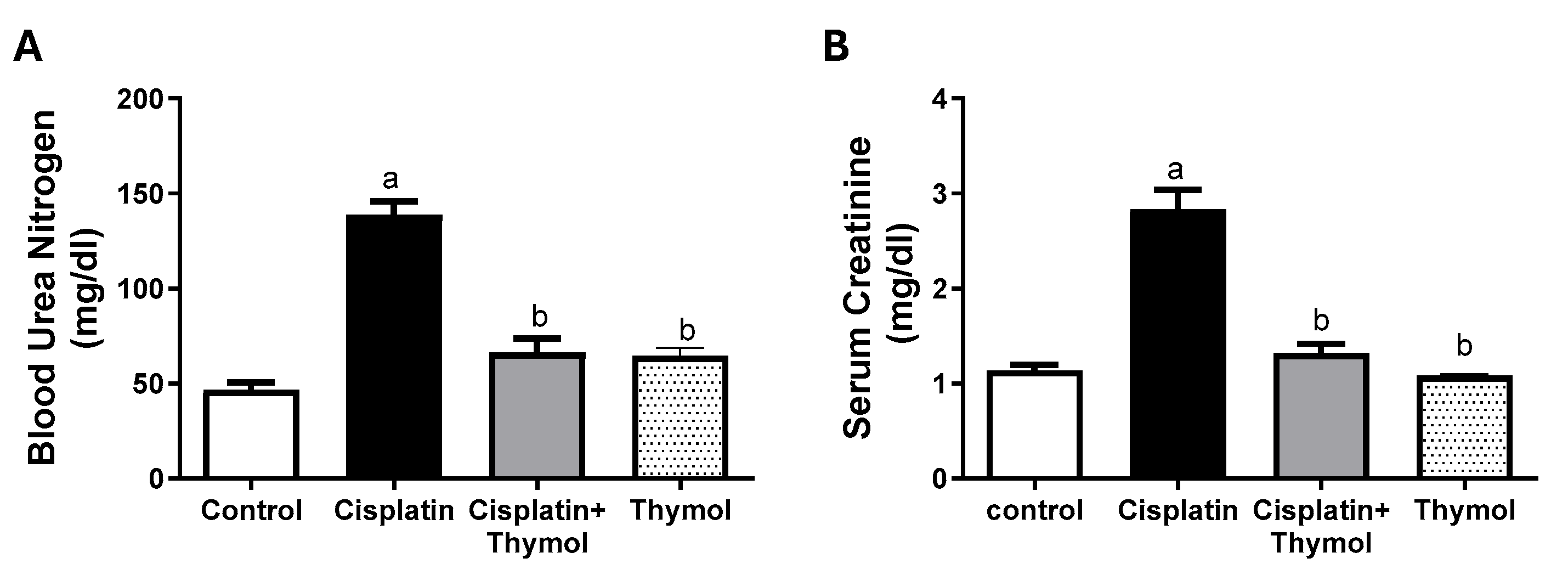
2.2. Effect of Thymol on Blood Urea Nitrogen (BUN) and Serum Creatinine
2.3. Effect of Thymol on Renal Histopathology
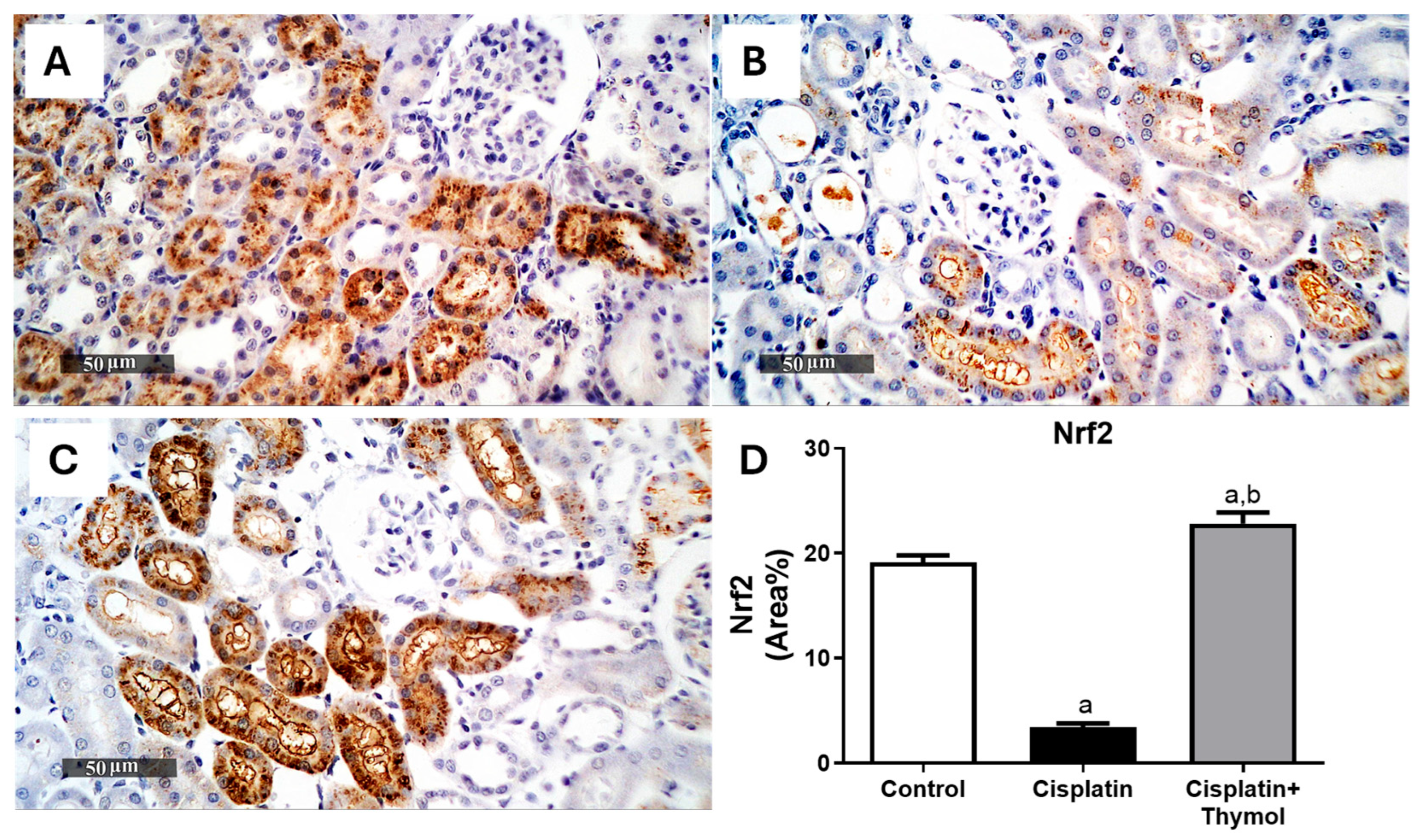
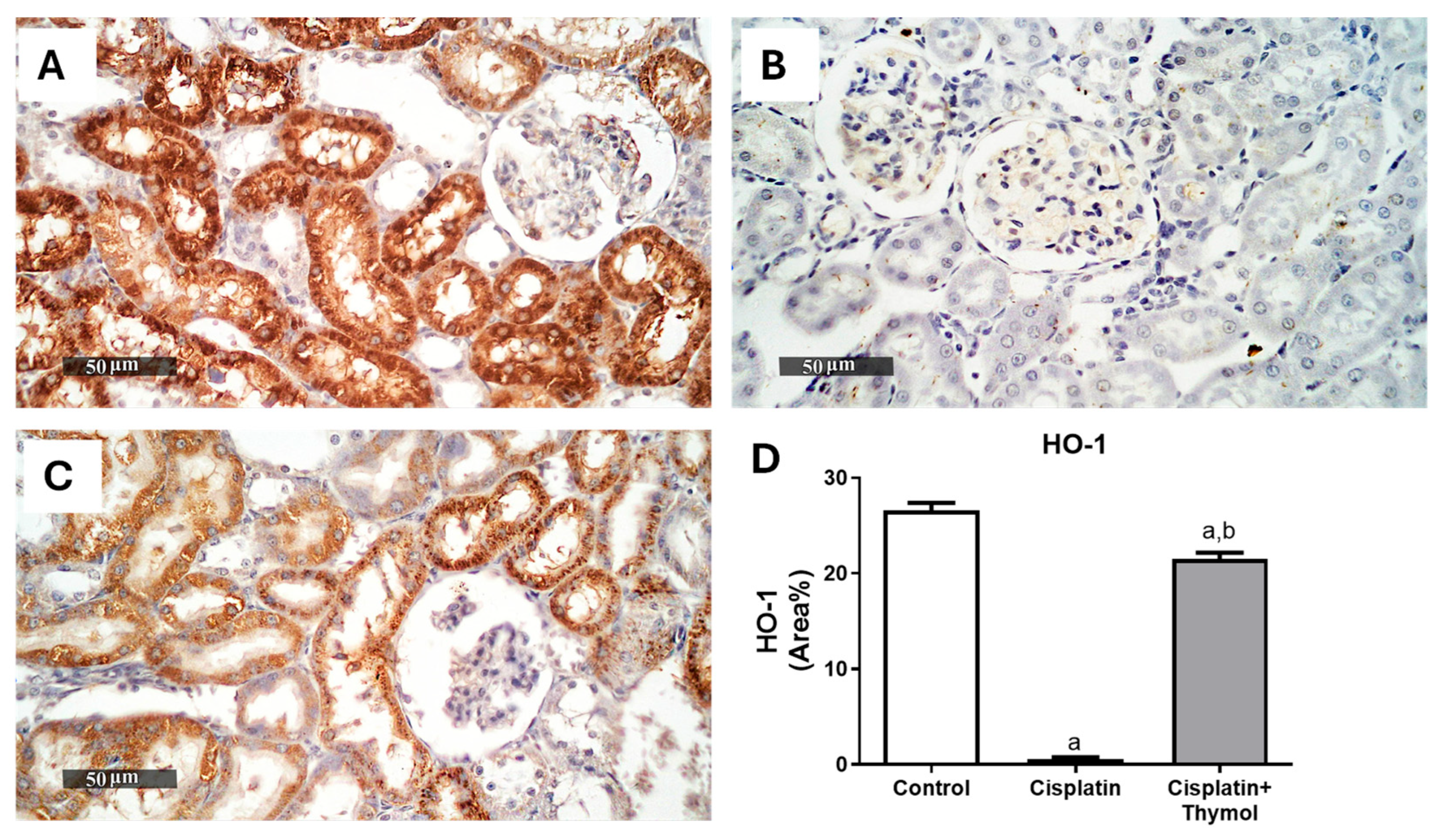
2.4. Effect of Thymol on Kidney Oxidative Stress
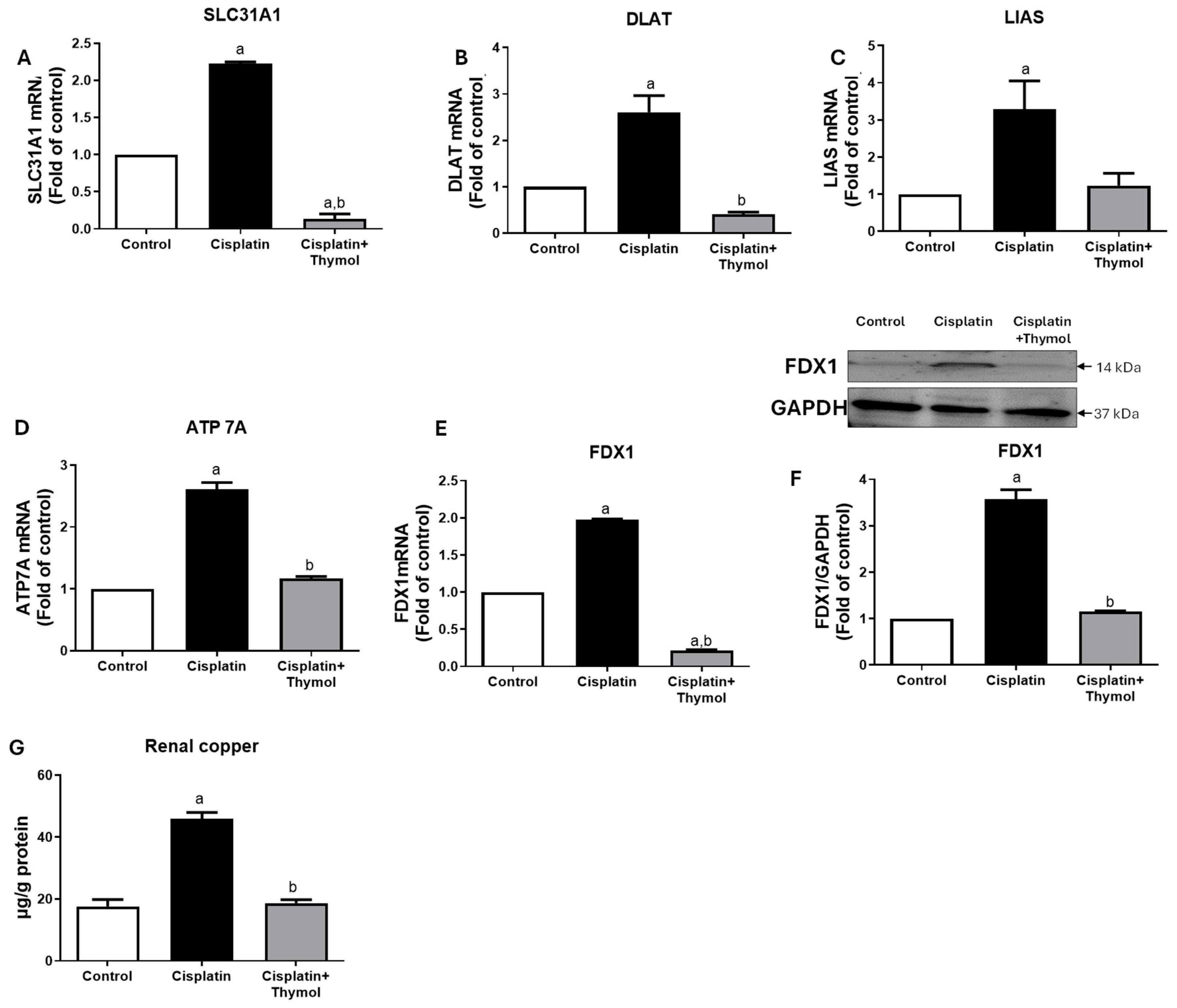
2.5. Effect of Thymol on Markers of Cuproptosis
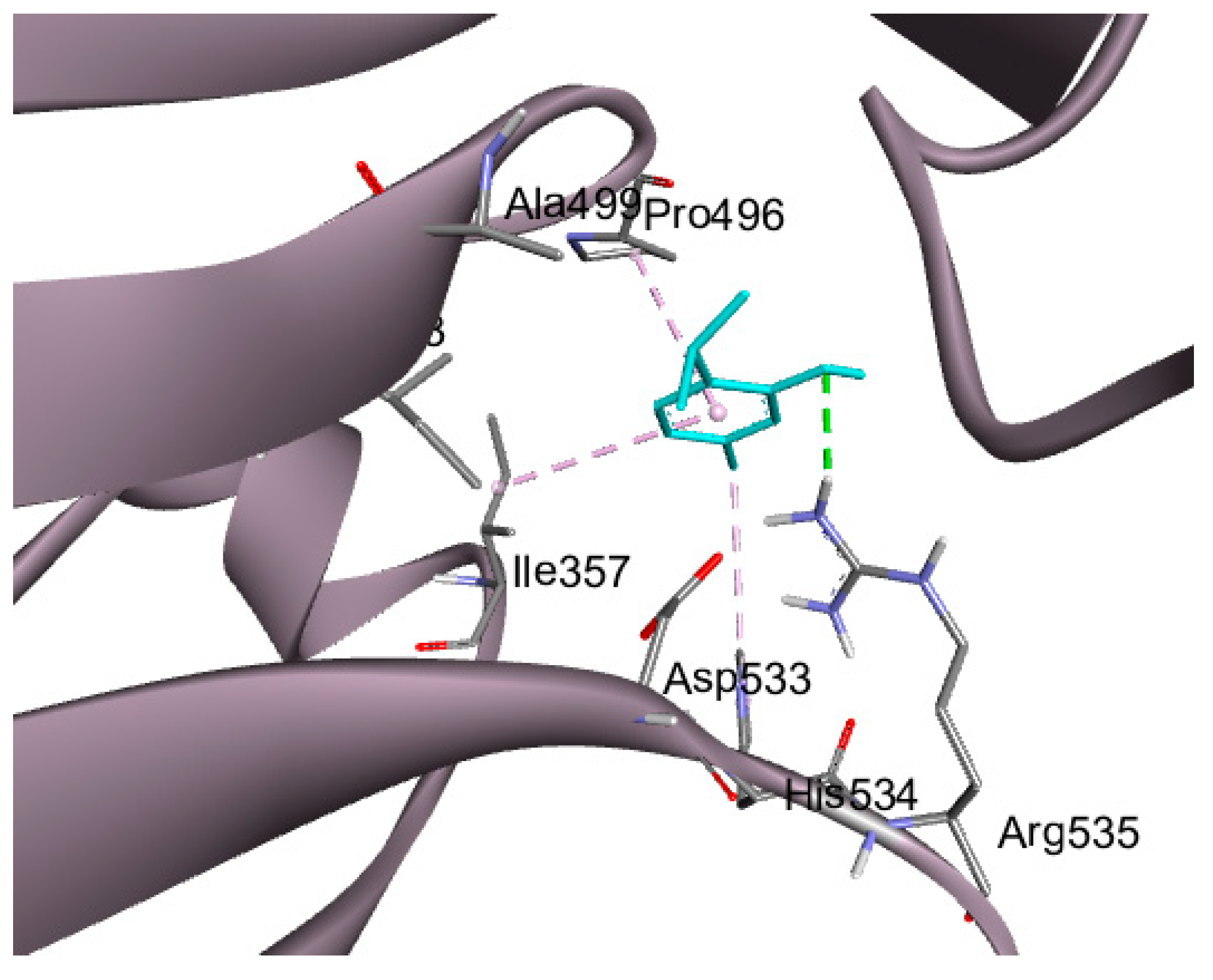
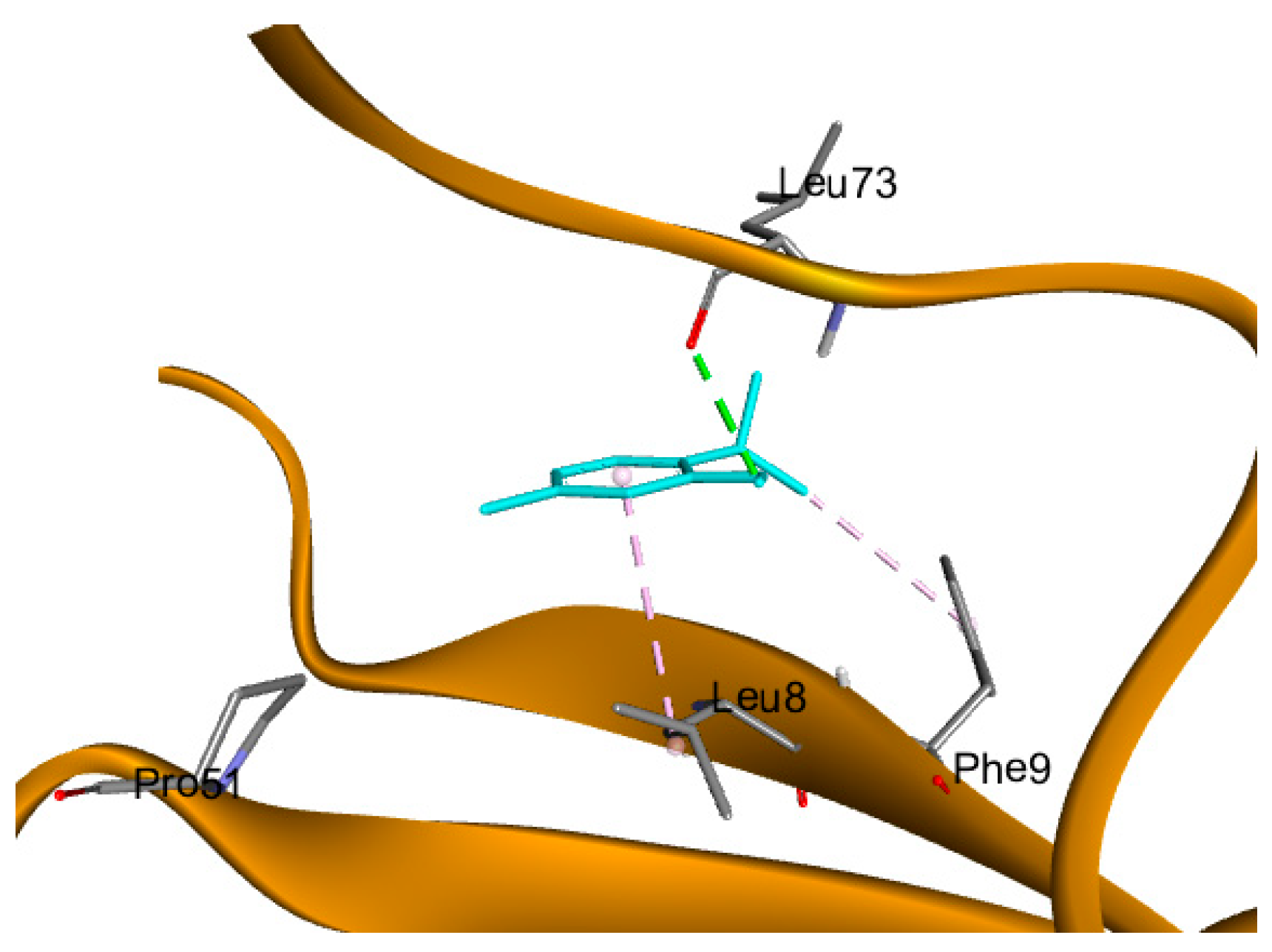
2.6. Molecular Docking Studies
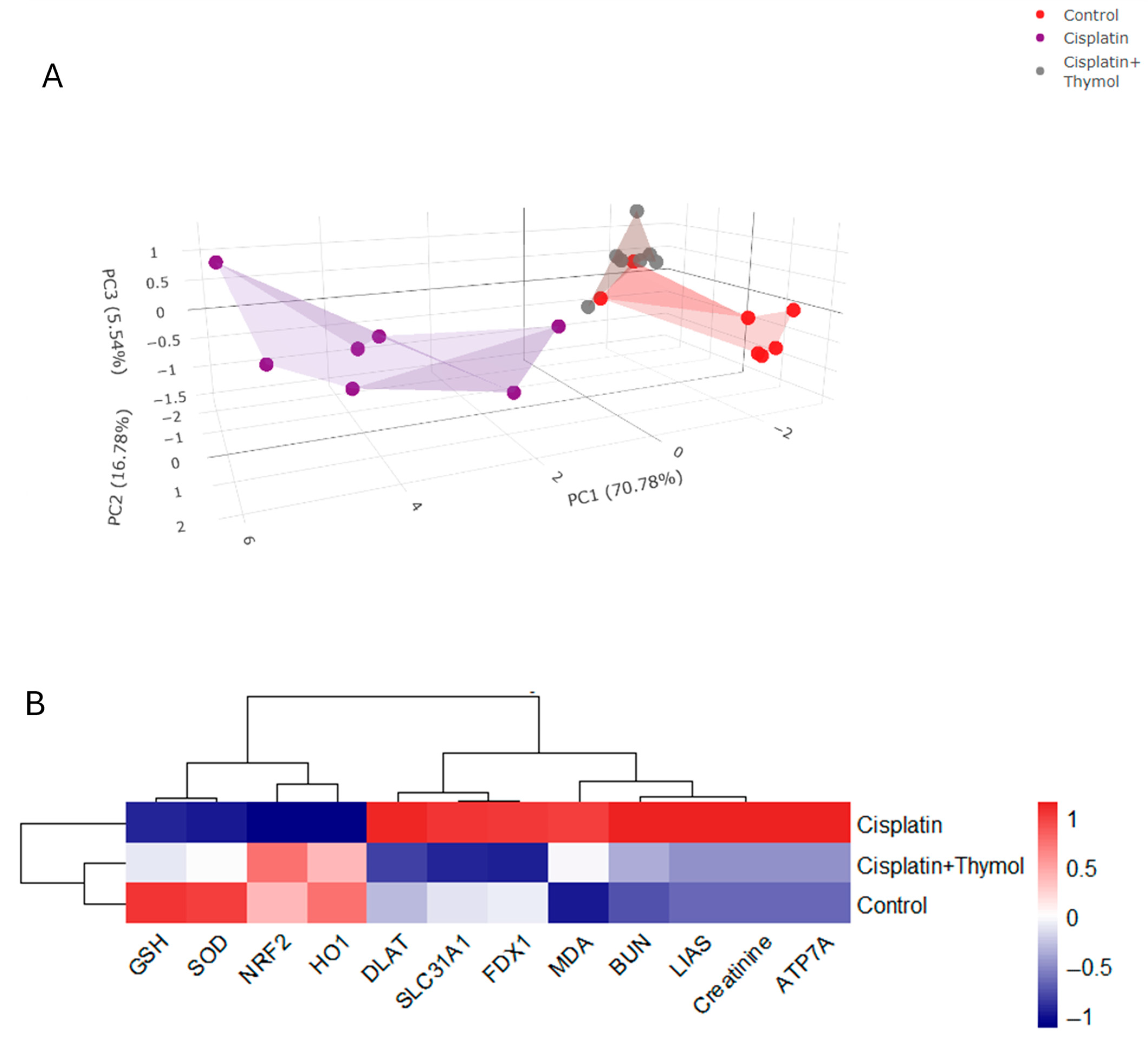
2.7. PCA and Hierarchical Clustering Heatmap
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Experimental Design and Sample Preparation
4.4. Evaluation of Markers of Kidney Function
4.5. Evaluation of Renal Histopathological Architecture
4.6. Evaluation of Renal Nrf2 and HO-1
4.7. Evaluation of Markers of Oxidative Stress and Protein Levels
4.8. Evaluation of Renal mRNA Expression of Cuproptosis Markers (SLC31A1, DLAT, FDX1, LIAS, and ATP 7A)
4.9. Protein Expression of FDX1
4.10. Assessment of Renal Copper Levels
4.11. Docking Studies
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolodziej, M.; Hoverman, J.R.; Garey, J.S.; Espirito, J.; Sheth, S.; Ginsburg, A.; Neubauer, M.A.; Patt, D.; Brooks, B.; White, C. Benchmarks for value in cancer care: An analysis of a large commercial population. J. Oncol. Pract. 2011, 7, 301–306. [Google Scholar] [CrossRef]
- Badary, O.A.; Abdel-Maksoud, S.; Ahmed, W.A.; Owieda, G.H. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci. 2005, 76, 2125–2135. [Google Scholar] [CrossRef]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. Comparative hydrolysis and plasma protein binding of cis-platin and carboplatin in human plasma in vitro. Metallomics 2011, 3, 49–55. [Google Scholar] [CrossRef]
- Beyer, J.; Rick, O.; Weinknecht, S.; Kingreen, D.; Lenz, K.; Siegert, W. Nephrotoxicity after high-dose carboplatin, etoposide and ifosfamide in germ-cell tumors: Incidence and implications for hematologic recovery and clinical outcome. Bone Marrow Transplant. 1997, 20, 813–819. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Lam, A.Q.; Humphreys, B.D. Onco-nephrology: AKI in the cancer patient. Clin. J. Am. Soc. Nephrol. 2012, 7, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zhou, F. Cuproptosis: A new form of programmed cell death. Cell. Mol. Immunol. 2022, 19, 867–868. [Google Scholar] [CrossRef]
- Lutsenko, S. Dynamic and cell-specific transport networks for intracellular copper ions. J. Cell Sci. 2021, 134, jcs240523. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.; Jiang, X.; Wei, Q.; Zhu, L.; Wang, X.; Jin, H.; Feng, L. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J. Exp. Clin. Cancer Res. 2023, 42, 142. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A copper-triggered modality of mitochondrial cell death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Fuss, J.O.; Tsai, C.-L.; Ishida, J.P.; Tainer, J.A. Emerging critical roles of Fe–S clusters in DNA replication and repair. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Springer, C.; Humayun, D.; Skouta, R. Cuproptosis: Unraveling the Mechanisms of Copper-Induced Cell Death and Its Implication in Cancer Therapy. Cancers 2024, 16, 647. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, Q.; Wang, L.; Xiong, Y.; Wu, J.; Wang, M.; Yan, X.; Deng, H. The copper transporter, SLC31A1, transcriptionally activated by ELF3, imbalances copper homeostasis to exacerbate cisplatin-induced acute kidney injury through mitochondrial dysfunction. Chem.-Biol. Interact. 2024, 393, 110943. [Google Scholar] [CrossRef]
- Alamri, M.A.; Abdel-Kader, M.S.; Salkini, M.A.; Alamri, M.A. Thymol and carvacrol derivatives as anticancer agents; synthesis, in vitro activity, and computational analysis of biological targets. RSC Adv. 2024, 14, 30662–30672. [Google Scholar] [CrossRef] [PubMed]
- Mahran, Y.F.; Badr, A.M.; Al-Kharashi, L.A.; Alajami, H.N.; Aldamry, N.T.; Bayoumy, N.M.; Elmongy, E.I.; Soliman, S. Thymol Protects against 5-Fluorouracil-Induced Hepatotoxicity via the Regulation of the Akt/GSK-3β Pathway in In Vivo and In Silico Experimental Models. Pharmaceuticals 2024, 17, 1094. [Google Scholar] [CrossRef]
- Yin, L.; Liang, C.; Wei, W.; Huang, S.; Ren, Y.; Geng, Y.; Huang, X.; Chen, D.; Guo, H.; Fang, J. The antibacterial activity of thymol against drug-resistant Streptococcus iniae and its protective effect on channel catfish (Ictalurus punctatus). Front. Microbiol. 2022, 13, 914868. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Pari, L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem.-Biol. Interact. 2016, 245, 1–11. [Google Scholar] [CrossRef]
- Oskouei, B.G.; Abbaspour-Ravasjani, S.; Musavinejad, S.J.; Salehzadeh, S.A.; Abdolhosseinzadeh, A.; Ghahremanzadeh, K.; Shokouhi, B. In vivo evaluation of anti-hyperglycemic, anti-hyperlipidemic and anti-oxidant status of liver and kidney of thymol in STZ-induced diabetic rats. Drug Res. 2019, 69, 46–52. [Google Scholar] [CrossRef]
- Mahran, Y.F.; Al-Kharashi, L.A.; Atawia, R.T.; Alanazi, R.T.; Dhahi, A.M.B.; Alsubaie, R.; Badr, A.M. Radioprotective effects of Carvacrol and/or Thymol against Gamma irradiation-induced acute nephropathy: In silico and in vivo evidence of the involvement of insulin-like growth factor-1 (IGF-1) and calcitonin gene-related peptide. Biomedicines 2023, 11, 2521. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.M.; Abd-Allah, A.R.; Mansour, A.M.; El-Arabey, A.A. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2015, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef]
- Cobine, P.A.; Brady, D.C. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol. Cell 2022, 82, 1786–1787. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, N.A.G.; Carvalho Rodrigues, M.A.; Martins, N.M.; Dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef]
- Markman, M. Toxicities of the platinum antineoplastic agents. Expert Opin. Drug Saf. 2003, 2, 597–607. [Google Scholar] [CrossRef]
- Kawai, Y.; Taniuchi, S.; Okahara, S.; Nakamura, M.; Gemba, M. Relationship between cisplatin or nedaplatin-induced nephrotoxicity and renal accumulation. Biol. Pharm. Bull. 2005, 28, 1385–1388. [Google Scholar] [CrossRef]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level. Oncol. Rep. 2003, 10, 1663–1682. [Google Scholar] [CrossRef]
- Almanric, K.; Marceau, N.; Cantin, A.; Bertin, É. Risk factors for nephrotoxicity associated with cisplatin. Can. J. Hosp. Pharm. 2017, 70, 99. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, G.; Zhou, H.; Cheng, F.; Yang, X.; Liu, X.; Wang, R. Protective effect of thymol on glycerol-induced acute kidney injury. Ren. Fail. 2023, 45, 2227728. [Google Scholar] [CrossRef]
- Park, J.; Sim, J.; Yi, H.J.; Rhee, S.G.; Woo, H.A. Cisplatin induces kidney damage through the down-regulation of Prx I by autophagic degradation. Free Radic. Biol. Med. 2024, 225, 236–246. [Google Scholar] [CrossRef]
- Baliga, R.; Zhang, Z.; Baliga, M.; Ueda, N.; Shah, S.V. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998, 53, 394–401. [Google Scholar] [CrossRef]
- Kawai, Y.; Nakao, T.; Kunimura, N.; Kohda, Y.; Gemba, M. Relationship of intracellular calcium and oxygen radicals to cisplatin-related renal cell injury. J. Pharmacol. Sci. 2006, 100, 65–72. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, H.; Dong, L.; Chen, L.; Ma, X.; Peng, Y.; Dai, S.; Liu, Q. Activation of the NRF2-ARE signalling pathway by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated cisplatin nephrotoxicity. Int. Immunopharmacol. 2016, 36, 1–8. [Google Scholar] [CrossRef]
- Wu, Q.; Yao, J.; Xiao, M.; Zhang, X.; Zhang, M.; Xi, X. Targeting Nrf2 Signaling Pathway: New Therapeutic Strategy for Cardiovascular Diseases. J. Drug Target. 2024, 32, 874–883. [Google Scholar] [CrossRef]
- Li, N.; Hao, L.; Li, S.; Deng, J.; Yu, F.; Zhang, J.; Nie, A.; Hu, X. The NRF-2/HO-1 signaling pathway: A promising therapeutic target for metabolic dysfunction-associated steatotic liver disease. J. Inflamm. Res. 2024, 17, 8061–8083. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef]
- El-Dessouki, A.M.; Alzokaky, A.A.; Raslan, N.A.; Ibrahim, S.; Salama, L.A.; Yousef, E.H. Piracetam mitigates nephrotoxicity induced by cisplatin via the AMPK-mediated PI3K/Akt and MAPK/JNK/ERK signaling pathways. Int. Immunopharmacol. 2024, 137, 112511. [Google Scholar] [CrossRef] [PubMed]
- Peirovy, Y.; Asle-Rousta, M. Thymol and p-Cymene Protect the Liver by Mitigating Oxidative Stress, Suppressing TNF-α/NF-κ B, and Enhancing Nrf2/HO-1 Expression in Immobilized Rats. Chem. Biol. Drug Des. 2024, 104, e14618. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, H.; Qin, T.; Li, M.; Ma, S. Thymol improves high-fat diet-induced cognitive deficits in mice via ameliorating brain insulin resistance and upregulating NRF2/HO-1 pathway. Metab. Brain Dis. 2017, 32, 385–393. [Google Scholar] [CrossRef]
- Badr, A.M.; Al-Kharashi, L.A.; Attia, H.; Alshehri, S.; Alajami, H.N.; Ali, R.A.; Mahran, Y.F. TLR4/inflammasomes cross-talk and pyroptosis contribute to N-Acetyl cysteine and chlorogenic acid protection against cisplatin-induced nephrotoxicity. Pharmaceuticals 2023, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Chakraborty, K.; Shukla, A. Cellular copper homeostasis: Current concepts on its interplay with glutathione homeostasis and its implication in physiology and human diseases. Metallomics 2017, 9, 1376–1388. [Google Scholar] [CrossRef]
- Guo, B.; Yang, F.; Zhang, L.; Zhao, Q.; Wang, W.; Yin, L.; Chen, D.; Wang, M.; Han, S.; Xiao, H. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with αPD-L1 for enhanced cancer immunotherapy. Adv. Mater. 2023, 35, 2212267. [Google Scholar] [CrossRef]
- Luo, X.; Linghu, M.; Zhou, X.; Ru, Y.; Huang, Q.; Liu, D.; Ji, S.; Ma, Y.; Luo, Y.; Huang, Y. Merestinib inhibits cuproptosis by targeting NRF2 to alleviate acute liver injury. Free Radic. Biol. Med. 2025, 229, 68–81. [Google Scholar] [CrossRef]
- Liu, J.; Tang, H.; Chen, F.; Li, C.; Xie, Y.; Kang, R.; Tang, D. NFE2L2 and SLC25A39 drive cuproptosis resistance through GSH metabolism. Sci. Rep. 2024, 14, 29579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Song, M.; Ren, J.; Liang, L.; Mao, G.; Chen, M. Copper homeostasis and cuproptosis in central nervous system diseases. Cell Death Dis. 2024, 15, 850. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Zhou, L.; Wang, X.; Liu, L.; Wu, M. Copper homeostasis and cuproptosis in atherosclerosis: Metabolism, mechanisms and potential therapeutic strategies. Cell Death Discov. 2024, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, L.; Hao, Y.; Huang, X.; Lv, G.; Zhou, X. Copper-instigated modulatory cell mortality mechanisms and progress in kidney diseases. Ren. Fail. 2025, 47, 2431142. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, H.; Zhu, W.; Ni, S.; Luo, S.; Tang, S.; Chen, Z.; Wang, Q.; Xu, J.; Tu, Q. Cuproptosis Cell Death Molecular Events and Pathways to Liver Disease. J. Inflamm. Res. 2025, 18, 883–894. [Google Scholar] [CrossRef]
- Qi, H.; Shi, H.; Yan, M.; Zhao, L.; Yin, Y.; Tan, X.; Qi, H.; Li, H.; Weng, K.; Tang, Y. Ammonium tetrathiomolybdate relieves oxidative stress in cisplatin-induced acute kidney injury via NRF2 signaling pathway. Cell Death Discov. 2023, 9, 259. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Peng, T.-Y.; Nguyen, T.H.; Bui, T.N.H.; Wang, C.-S.; Lee, W.-J.; Chen, Y.-L.; Wu, Y.-C.; Lee, I.-T. The crosstalk between copper-induced oxidative stress and cuproptosis: A novel potential anticancer paradigm. Cell Commun. Signal. 2024, 22, 353. [Google Scholar] [CrossRef]
- Chen, X.; Li, K.; Xiao, Y.; Wu, W.; Lin, H.; Qing, X.; Tian, S.; Liu, S.; Feng, S.; Wang, B. SP1/CTR1-mediated oxidative stress-induced cuproptosis in intervertebral disc degeneration. Biofactors 2024, 50, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Nevitt, T.; Öhrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Qi, R.-Q.; Song, J.-W.; Wang, S.-Y.; Dong, Z.-J.; Chen, Y.-H.; Liu, Y.; Zhou, X.-Y.; Li, J.; Liu, X.-Y. Sirtuin 7 ameliorates cuproptosis, myocardial remodeling and heart dysfunction in hypertension through the modulation of YAP/ATP7A signaling. Apoptosis 2024, 29, 2161–2182. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Butt, S.S.; Badshah, Y.; Shabbir, M.; Rafiq, M. Molecular docking using chimera and autodock vina software for nonbioinformaticians. JMIR Bioinform. Biotechnol. 2020, 1, e14232. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.; Elmongy, E.I.; Elkhateeb, D.; Moemen, Y.S.; Khalil, A.; Ali, H.; Binsuwaidan, R.; Awadallah, F.; El Sayed, I.E.T. In Silico and In Vitro Investigation of Cytotoxicity and Apoptosis of Acridine/Sulfonamide Hybrids Targeting Topoisomerases I and II. Pharmaceuticals 2024, 17, 1487. [Google Scholar] [CrossRef] [PubMed]


| Group | MDA (nmol/mg Protein) | GSH (µg/mg Protein) | SOD (U/mg Protein) |
|---|---|---|---|
| Control | 0.54 ± 0.05 | 1.83 ± 0.03 | 4.46 ± 0.1 |
| Cisplatin | 2.45 ± 0.07 a | 0.59 ± 0.04 a | 0.83 ± 0.1 a |
| Cisplatin+Thymol | 1.46 ± 0.08 a,b | 1.12 ± 0.03 a,b | 2.61 ± 0.043 a,b |
| Thymol | 0.52 ± 0.06 b,c | 1.7 ± 0.1 b,c | 4.78 ± 0.29 b,c |
| Protein | PDB ID | Binding Energy Kcal/mol | RMSD (Å) | Amino Acid Residues of Interaction | Types of Bonds |
|---|---|---|---|---|---|
| FDX-1 | 3P1M | −3.81 | 1.67 | Ser 177 | H-bond |
| Ser 177 | Pi-donor H-bond | ||||
| Gln 176 | H-bond | ||||
| Tyr 142 | H-bond | ||||
| DLAT | 3B8K | −4.5 | 2.0 | ARG 535 | H-bond |
| ILE 357 | Pi-alkyl hydrophobic | ||||
| HIS 534 | Pi-alkyl hydrophobic | ||||
| Pro 496 | Pi-alkyl hydrophobic | ||||
| ATP7A | 7LU8 | −3.86 | 1.21 | Leu 73 | H-bond |
| Leu 8 | Pi-alkyl hydrophobic | ||||
| Phe 9 | Pi-alkyl hydrophobic |
| Genes | Primer | Sequence (5′-3′) | Accession Number |
|---|---|---|---|
| DLAT | Forward | TGGACCCCGGCTCTTCTCTT | NM_031025.1 |
| Reverse | CATTCCAGGGCTTCTCCACT: | ||
| FDX1 | Forward | CCAGCGTGGAGCGAGTTTG | NM_017126.2 |
| Reverse | TGGCTCCAGGGTTTGTTGTC | ||
| LIAS | Forward | TCCACTCCTGATCTTGGACAC | NM_001012037.1 |
| Reverse | TGGTGCTTCTTGTGGAGTAA | ||
| SLC31A1 | Forward | GGGCTTGGGAGAAGTCCAGA | NM_133600.3 |
| Reverse | TCCTCATGTGGTCCGAAGGA | ||
| ATP7A | Forward | CCAGCGTGGAGCGGACTAC | XM_039099486.2 |
| Reverse | TGGCTCCAGGGTGTCATCTT | ||
| β-actin | Forward | CCTGCTTGCTGATCCACA | NM_031144.3 |
| Reverse | CTGACCGAGCGTGGCTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kharashi, L.A.; Badr, A.M.; Atawia, R.T.; Elmongy, E.I.; Henidi, H.; Ali, R.; Binmughram, A.A.; Al-Abkka, L.; Bayoumy, N.M.K.; Mahran, Y.F. Cuproptosis Contributes to Cisplatin-Induced Nephrotoxicity: Insights into Thymol’s Potential Inhibitory and Protective Effects. Pharmaceuticals 2025, 18, 1686. https://doi.org/10.3390/ph18111686
Al-Kharashi LA, Badr AM, Atawia RT, Elmongy EI, Henidi H, Ali R, Binmughram AA, Al-Abkka L, Bayoumy NMK, Mahran YF. Cuproptosis Contributes to Cisplatin-Induced Nephrotoxicity: Insights into Thymol’s Potential Inhibitory and Protective Effects. Pharmaceuticals. 2025; 18(11):1686. https://doi.org/10.3390/ph18111686
Chicago/Turabian StyleAl-Kharashi, Layla A., Amira M. Badr, Reem T. Atawia, Elshaymaa I. Elmongy, Hanan Henidi, Rehab Ali, Awatif A. Binmughram, Lian Al-Abkka, Nervana Mostafa Kamal Bayoumy, and Yasmen F. Mahran. 2025. "Cuproptosis Contributes to Cisplatin-Induced Nephrotoxicity: Insights into Thymol’s Potential Inhibitory and Protective Effects" Pharmaceuticals 18, no. 11: 1686. https://doi.org/10.3390/ph18111686
APA StyleAl-Kharashi, L. A., Badr, A. M., Atawia, R. T., Elmongy, E. I., Henidi, H., Ali, R., Binmughram, A. A., Al-Abkka, L., Bayoumy, N. M. K., & Mahran, Y. F. (2025). Cuproptosis Contributes to Cisplatin-Induced Nephrotoxicity: Insights into Thymol’s Potential Inhibitory and Protective Effects. Pharmaceuticals, 18(11), 1686. https://doi.org/10.3390/ph18111686








