In Vitro and in Vivo Efficacy of Different Ointment Formulations Containing Centaurium erythraea Rafn. Aerial Extract
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profile
2.2. Determination of Particle Size, PDI and Zeta Potential of Pre-Dissolved C. erythraea Lyophilized Product
2.3. Results of Texture Analysis
2.4. Results of pH Measurement
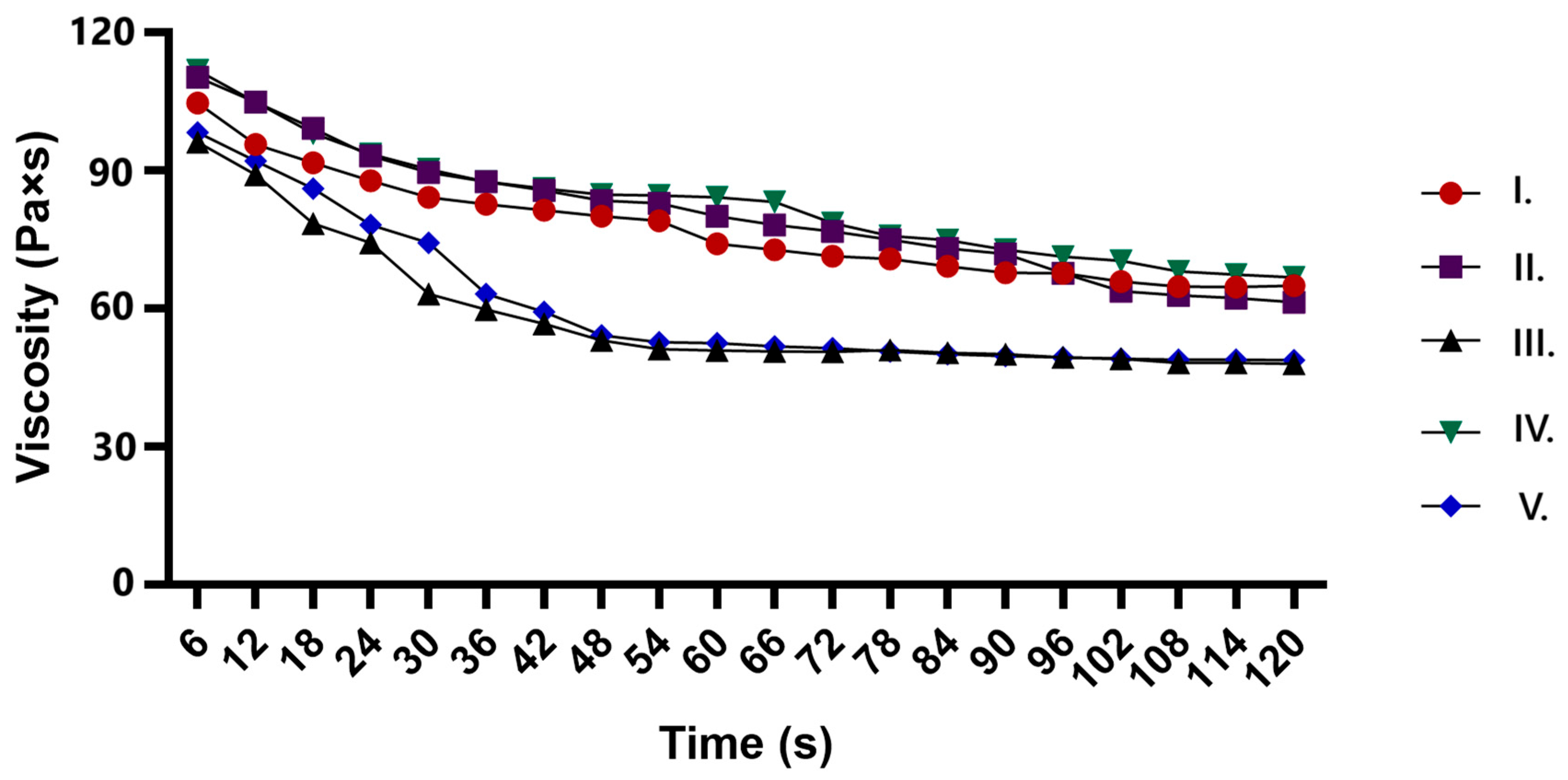
2.5. Results of Rheological Properties of Ointments
2.6. Results of Synthetic Membrane Diffusion Model
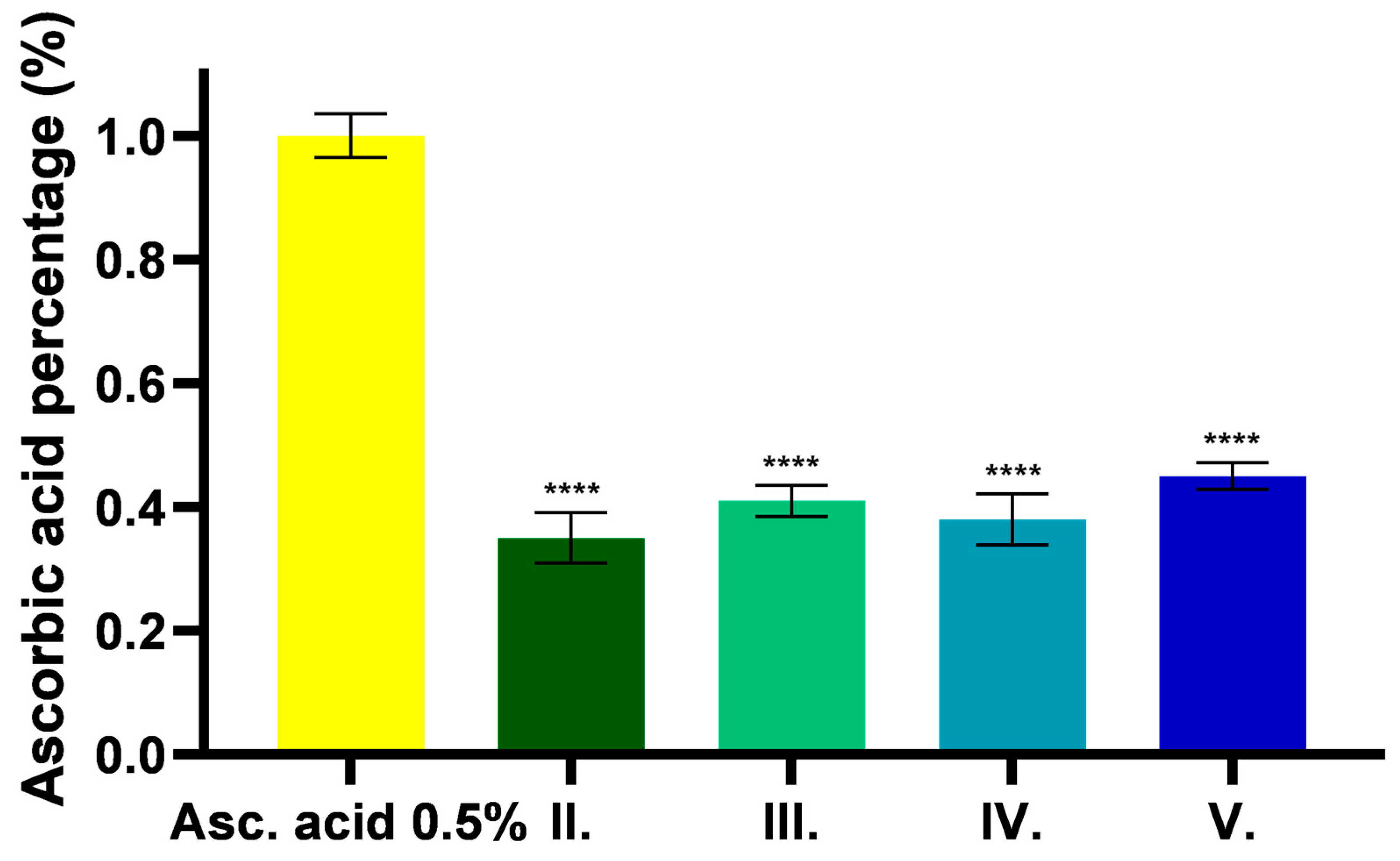
2.7. Antioxidant Effect of C. erythraea-Containing Ointments
2.7.1. DPPH
2.7.2. FRAP Assay
2.7.3. Determination of TPC (Total Phenolic Content)
2.8. MTT Cytotoxicity Assay
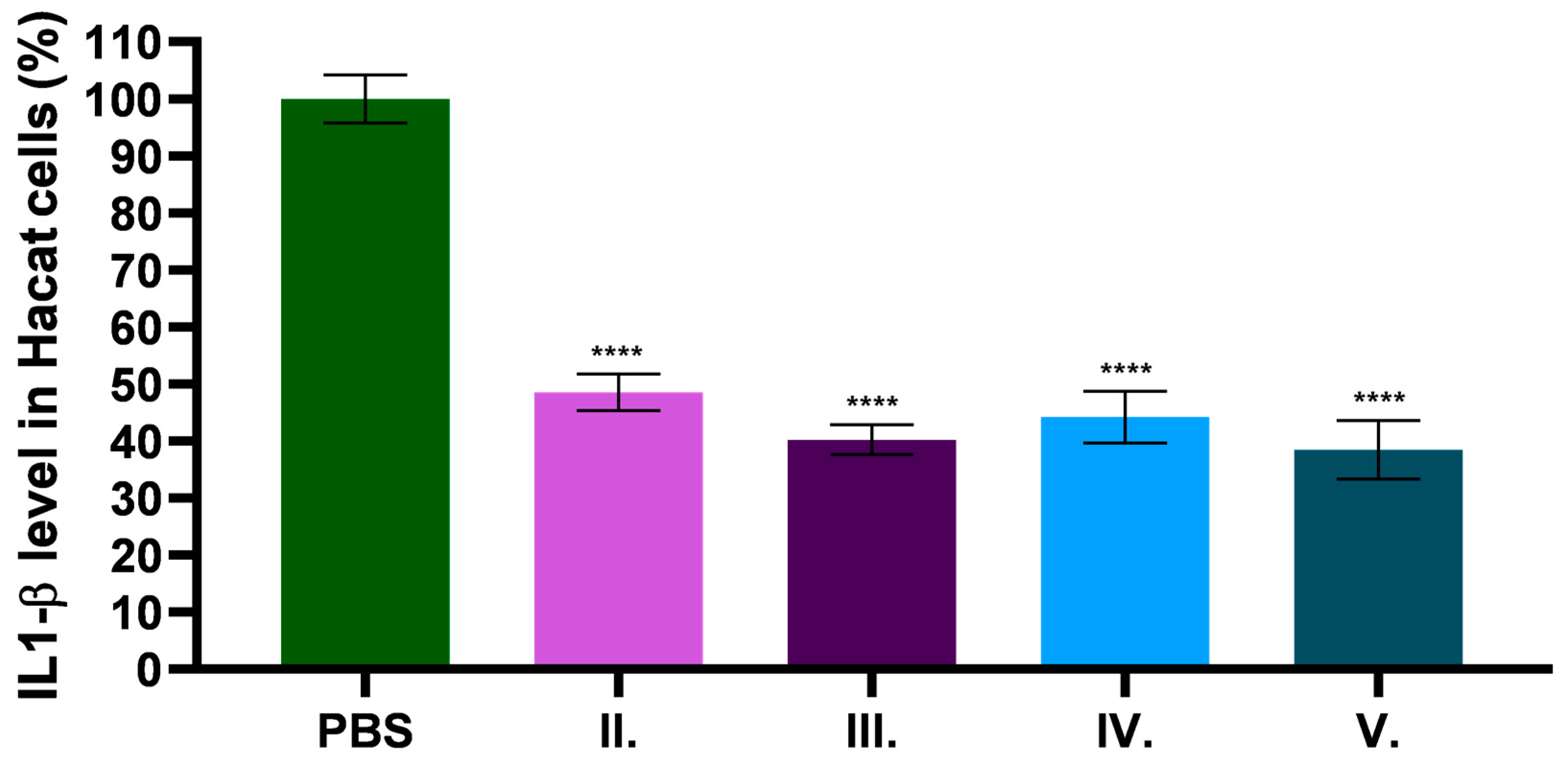
2.9. In Vitro Anti-Inflammatory Effect of C. erythraea Extract
2.10. Results of In Vivo Carrageenan-Induced Rat Paw Edema Model
3. Discussion
4. Materials and Methods
4.1. Preparation of C. erythraea Lyophilized Product
4.2. Phytochemical Analysis Using LC-MS/MS
4.3. Measurement of Particle Size, PDI and Zeta Size of Pre-Dissolved C. erythraea Lyophilized Product
4.4. Formulation of Ointments Containing C. erythraea Lyophilized Product
4.5. pH Measurement
4.6. Texture Analysis
4.7. Viscosity Measurement of Different Formulations
4.8. Determination of Diffused API Through Synthetic Membrane for Release Testing
4.9. In Vitro Antioxidant Activity Tests
4.9.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
4.9.2. FRAP Assay (Ferric Reducing Antioxidant Potential)
4.9.3. Total Polyphenol Content (TPC)
4.10. Cell Culturing
4.11. MTT Cytotoxicity Assay
4.12. Investigation of IL-1β, Enzyme-Linked Immunosorbent Assay (ELISA) on HaCaT Cells
4.13. Experimental Animals
4.14. In Vivo Carrageenan-Induced Inflammation Assay
4.15. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing Herbal Medicine: Enhancing Product Quality and Safety through Robust Quality Control Practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Buhse, L.; Kolinski, R.; Westenberger, B.; Wokovich, A.; Spencer, J.; Chen, C.W.; Turujman, S.; Gautam-Basak, M.; Kang, G.J.; Kibbe, A.; et al. Topical Drug Classification. Int. J. Pharm. 2005, 295, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Guedes, L.; Reis, P.B.P.S.; Machuqueiro, M.; Ressaissi, A.; Pacheco, R.; Serralheiro, M.L. Bioactivities of Centaurium Erythraea (Gentianaceae) Decoctions: Antioxidant Activity, Enzyme Inhibition and Docking Studies. Molecules 2019, 24, 3795. [Google Scholar] [CrossRef]
- Šiler, B.; Mišić, D. Biologically Active Compounds from the Genus Centaurium s.l. (Gentianaceae). Stud. Nat. Prod. Chem. 2016, 49, 363–397. [Google Scholar]
- Kültür, Ş. Medicinal Plants Used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Tahraoui, A.; Israili, Z.H.; Lyoussi, B. Acute and Sub-Chronic Toxicity of a Lyophilised Aqueous Extract of Centaurium Erythraea in Rodents. J. Ethnopharmacol. 2010, 132, 48–55. [Google Scholar] [CrossRef]
- Đorđević, M.; Grdović, N.; Mihailović, M.; Arambašić Jovanović, J.; Uskoković, A.; Rajić, J.; Sinadinović, M.; Tolić, A.; Mišić, D.; Šiler, B.; et al. Centaurium Erythraea Extract Improves Survival and Functionality of Pancreatic Beta-Cells in Diabetes through Multiple Routes of Action. J. Ethnopharmacol. 2019, 242, 112043. [Google Scholar] [CrossRef]
- Petrović, A.; Madić, V.; Stojanović, G.; Zlatanović, I.; Zlatković, B.; Vasiljević, P.; Đorđević, L. Antidiabetic Effects of Polyherbal Mixture Made of Centaurium Erythraea, Cichorium Intybus and Potentilla Erecta. J. Ethnopharmacol. 2024, 319, 117032. [Google Scholar] [CrossRef]
- El Menyiy, N.; Guaouguaou, F.-E.; El Baaboua, A.; El Omari, N.; Taha, D.; Salhi, N.; Shariati, M.A.; Aanniz, T.; Benali, T.; Zengin, G.; et al. Phytochemical Properties, Biological Activities and Medicinal Use of Centaurium Erythraea Rafn. J. Ethnopharmacol. 2021, 276, 114171. [Google Scholar] [CrossRef] [PubMed]
- Berkan, T.; Üstünes, L.; Lermioglu, F.; Özer, A. Antiinflammatory, Analgesic, and Antipyretic Effects of an Aqueous Extract of Erythraea Centaurium. Planta Med. 1991, 57, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of Iridoids, Secoiridoids and Xanthones in Centaurium Erythraea, Frasera Caroliniensis and Gentiana Lutea Using LC-MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, Y.; Nahar, L.; Cox, P.J.; Jaspars, M.; Sarker, S.D. Bioactivity of Secoiridoid Glycosides from Centaurium Erythraea. Phytomedicine 2003, 10, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the Skin: Understanding Formulation and Efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidant Activity of Centaurium Erythraea Infusion Evidenced by Its Superoxide Radical Scavenging and Xanthine Oxidase Inhibitory Activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating Inflammation and Infection in the 21st Century: New Hints from Decoding Resolution Mediators and Mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic Diseases, Inflammation, and Spices: How Are They Linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation – Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Pelaia, G.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Calabrese, C.; Terracciano, R.; Maselli, R. Cellular Mechanisms Underlying Eosinophilic and Neutrophilic Airway Inflammation in Asthma. Mediators Inflamm. 2015, 2015, 879783. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; van de Kerkhof, P.; Czarnecka-Operacz, M. Psoriasis and Atopic Dermatitis. Dermatol. Ther. 2017, 7, 31–41. [Google Scholar] [CrossRef]
- Barnes, P.J. Glucocorticoids. Chem. Immunol. Allergy 2014, 100, 311–316. [Google Scholar]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Razzaq Chaudhry, W.; Akbar, A. Effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural Terpenoids with Anti-Inflammatory Activities: Potential Leads for Anti-Inflammatory Drug Discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Savjani, J.K. Systematic Review of Plant Steroids as Potential Antiinflammatory Agents: Current Status and Future Perspectives. J. Phytopharm. 2015, 4, 121–125. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Nasser Singab, A.; Chaubey, K.K.; Bachheti, R.K. Anti-Inflammatory Activity of Phytochemicals from Medicinal Plants and Their Nanoparticles: A Review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Censi, R.; Martena, V.; Hoti, E.; Malaj, L.; Di Martino, P. Permeation and Skin Retention of Quercetin from Microemulsions Containing Transcutol ® P. Drug Dev. Ind. Pharm. 2012, 38, 1128–1133. [Google Scholar] [CrossRef]
- Hernandez, C.; Jain, P.; Sharma, H.; Lam, S.; Sonti, S. Investigating the Effect of Transcutol on the Physical Properties of an O/W Cream. J. Dispers. Sci. Technol. 2020, 41, 600–606. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Impact of Various Nonionic Surfactants on Self-Nanoemulsification Efficiency of Two Grades of Capryol (Capryol-90 and Capryol-PGMC). J. Mol. Liq. 2013, 182, 57–63. [Google Scholar] [CrossRef]
- Musakhanian, J.; Osborne, D.W.; Rodier, J.-D. Skin Penetration and Permeation Properties of Transcutol® in Complex Formulations. AAPS PharmSciTech 2024, 25, 201. [Google Scholar] [CrossRef] [PubMed]
- Birngruber, T.; Vought, K.; Schwingenschuh, S.; Reisenegger, P.; Maibach, H.; Lissin, D. Topical Delivery Systems Effectively Transport Analgesics to Areas of Localized Pain via Direct Diffusion. Pharmaceutics 2023, 15, 2563. [Google Scholar] [CrossRef]
- INAKI, T. Ointments for Antiinflammatory Drugs. In Gels Handbook; Elsevier: Amsterdam, The Netherlands, 2001; pp. 211–220. [Google Scholar]
- Sandru, D.; Niculescu, V.; Lengyel, E.; Tița, O. Identification and Quantification of Total Polyphenols in Plants with Bioactive Potentially. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 4, 47–51. [Google Scholar] [CrossRef]
- Brudzińska-Kosior, A.; Kosior, G.; Sporek, M.; Ziembik, Z.; Zinicovscaia, I.; Frontasyeva, M.; Dołhańczuk-Śródka, A. Nuclear Analytical Techniques Used to Study the Trace Element Content of Centaurium Erythraea Rafn, a Medicinal Plant Species from Sites with Different Pollution Loads in Lower Silesia (SW Poland). PLoS ONE 2023, 18, e0285306. [Google Scholar] [CrossRef]
- Chda, A.; El Kabbaoui, M.; Fresco, P.; Silva, D.; Gonçalves, J.; Oliveira, A.P.; Andrade, P.B.; Valentão, P.; Tazi, A.; El Abida, K.; et al. Centaurium Erythraea Extracts Exert Vascular Effects through Endothelium- and Fibroblast-Dependent Pathways. Planta Med. 2020, 86, 121–131. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Brudzińska-Kosior, A.; Kosior, G.; Samecka-Cymerman, A.; Kolon, K.; Mróz, L.; Kempers, A.J. Metal Contents in Centaurium Erythraea and Its Biometry at Various Levels of Environmental Pollution. Ecotoxicol. Environ. Saf. 2012, 80, 349–354. [Google Scholar] [CrossRef]
- Mijajlovic, N.; Grubisic, D.; Giba, Z.; Konjevic, R. The Effect of Plant Growth Regulators on Centaury (Centaurium Erythraea Rafn) Seeds Germination. Arch. Biol. Sci. 2005, 57, 25–28. [Google Scholar] [CrossRef]
- Reiné, R.; Chocarro, C.; Fillat, F. Spatial Patterns in Seed Bank and Vegetation of Semi-Natural Mountain Meadows. Plant Ecol. 2006, 186, 151–160. [Google Scholar] [CrossRef]
- Mihaylova, D.; Vrancheva, R.; Popova, A. Phytochemical Profile and in Vitro Antioxidant Activity of Centaurium Erythraea Rafn. Bulg. Chem. Commun. 2019, 51, 95–100. [Google Scholar]
- ElNaker, N.A.; Daou, M.; Ochsenkühn, M.A.; Amin, S.A.; Yousef, A.F.; Yousef, L.F. A Metabolomics Approach to Evaluate the Effect of Lyophilization versus Oven Drying on the Chemical Composition of Plant Extracts. Sci. Rep. 2021, 11, 22679. [Google Scholar] [CrossRef] [PubMed]
- Nwadibia, J.A.; Fasogbon, I.V.; Musyoka, A.M.; Ekpono, E.U.; Ibiam, U.A.; Orji, O.U.; Eze, E.D.; Onaadepo, O.; Agu, P.C.; Aja, P.M. Protective Effect of Ficus Capensis Lyophilized Extract against Carboplatin-Induced Liver Injury via Inhibition of Oxidative Stress and Inflammation in Rats. Toxicol. Rep. 2024, 13, 101734. [Google Scholar] [CrossRef]
- Sibri, J.F.; Akakpo-Akue, J.; Okou, O.C.; Kple, T.K.M. Evaluation of the Antioxidant Activity of Aqueous and Hydro-Ethanolic Extracts of Ficus Capensis, Newbouldia Laevis and Carpolobia Lutea. Asian J. Res. Biochem. 2023, 13, 9–15. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Di Filippo, L.D.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid in Dermatological Formulations: In Vitro Release Profile and Skin Absorption. Comb. Chem. High Throughput Screen. 2017, 20, 675–681. [Google Scholar] [CrossRef]
- Song, H.S.; Park, T.W.; Sohn, U.D.; Shin, Y.K.; Choi, B.C.; Kim, C.J.; Sim, S.S. The Effect of Caffeic Acid on Wound Healing in Skin-Incised Mice. Korean J. Physiol. Pharmacol. 2008, 12, 343. [Google Scholar] [CrossRef]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin Aging Properties of Protocatechuic Acid in Vitro and in Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Yang, D.; Moh, S.; Son, D.; You, S.; Kinyua, A.; Ko, C.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Okselni, T.; Septama, A.W.; Juliadmi, D.; Dewi, R.T.; Angelina, M.; Yuliani, T.; Saragih, G.S.; Saputri, A. Quercetin as a Therapeutic Agent for Skin Problems: A Systematic Review and Meta-Analysis on Antioxidant Effects, Oxidative Stress, Inflammation, Wound Healing, Hyperpigmentation, Aging, and Skin Cancer. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025, 398, 5011–5055. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy. Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia Cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef]
- Moukova, A.; Malina, L.; Kolarova, H.; Bajgar, R. Hyperoside as a UV Photoprotective or Photostimulating Compound—Evaluation of the Effect of UV Radiation with Selected UV-Absorbing Organic Compounds on Skin Cells. Int. J. Mol. Sci. 2023, 24, 9910. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Yao, Y.; Ru, G.; Lan, C.; Li, L.; Huang, T. Protective Effect of Isoquercitrin on UVB-induced injury in HaCaT cells and mice skin through anti-inflammatory, antioxidant, and regulation of MAPK and JAK2-STAT3 Pathways. Photochem. Photobiol. 2024, 100, 1507–1518. [Google Scholar] [CrossRef]
- Lee, E.; Park, H.; Kim, H.; Jung, H.; Kang, I.; Cho, Y. Isolated Isoquercitrin from Green Ball Apple Peel Inhibits Photoaging in CCD-986Sk Fibroblasts Cells via Modulation of the MMPs Signaling. J. Cosmet. Dermatol. 2021, 20, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Matsuda, S. The Chemopreventive Effects of Chlorogenic Acids, Phenolic Compounds in Coffee, against Inflammation, Cancer, and Neurological Diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef]
- LI, L.; ZHANG, X.-H.; LIU, G.-R.; LIU, C.; DONG, Y.-M. Isoquercitrin Suppresses the Expression of Histamine and Pro-Inflammatory Cytokines by Inhibiting the Activation of MAP Kinases and NF-ΚB in Human KU812 Cells. Chin. J. Nat. Med. 2016, 14, 407–412. [Google Scholar] [CrossRef]
- Kuppan, G.; Balasubramanyam, J.; Monickaraj, F.; Srinivasan, G.; Mohan, V.; Balasubramanyam, M. Transcriptional Regulation of Cytokines and Oxidative Stress by Gallic Acid in Human THP-1 Monocytes. Cytokine 2010, 49, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Stefkov, G.; Miova, B.; Dinevska-Kjovkarovska, S.; Stanoeva, J.P.; Stefova, M.; Petrusevska, G.; Kulevanova, S. Chemical Characterization of Centaurium Erythrea L. and Its Effects on Carbohydrate and Lipid Metabolism in Experimental Diabetes. J. Ethnopharmacol. 2014, 152, 71–77. [Google Scholar] [CrossRef]
- Mitsutake, H.; Ribeiro, L.N.M.; Rodrigues da Silva, G.H.; Castro, S.R.; de Paula, E.; Poppi, R.J.; Breitkreitz, M.C. Evaluation of Miscibility and Polymorphism of Synthetic and Natural Lipids for Nanostructured Lipid Carrier (NLC) Formulations by Raman Mapping and Multivariate Curve Resolution (MCR). Eur. J. Pharm. Sci. 2019, 135, 51–59. [Google Scholar] [CrossRef]
- Braun-Falco, O.; Korting, H.C. Normal PH Value of Human Skin. Hautarzt 1986, 37, 126–129. [Google Scholar]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The Mechanism of Skin Lipids Influencing Skin Status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef]
- Danso, M.; Boiten, W.; van Drongelen, V.; Gmelig Meijling, K.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered Expression of Epidermal Lipid Bio-Synthesis Enzymes in Atopic Dermatitis Skin Is Accompanied by Changes in Stratum Corneum Lipid Composition. J. Dermatol. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between Surfactants and the Skin – Theory and Practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Wagner, H. PH Profiles in Human Skin: Influence of Two in Vitro Test Systems for Drug Delivery Testing. Eur. J. Pharm. Biopharm. 2003, 55, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, Y.G.; Patravale, V.B. SMEDDS of Glyburide: Formulation, In Vitro Evaluation, and Stability Studies. AAPS PharmSciTech 2009, 10, 482–487. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the Stratum Corneum: The Modulation of Skin Penetration. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Godwin, D.A.; Kim, N.-H.; Felton, L.A. Influence of Transcutol® CG on the Skin Accumulation and Transdermal Permeation of Ultraviolet Absorbers. Eur. J. Pharm. Biopharm. 2002, 53, 23–27. [Google Scholar] [CrossRef]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.Ø.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The Effects of Polar Excipients Transcutol and Dexpanthenol on Molecular Mobility, Permeability, and Electrical Impedance of the Skin Barrier. J. Colloid Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Korhonen, M.; Hellen, L.; Hirvonen, J.; Yliruusi, J. Rheological Properties of Creams with Four Different Surfactant Combinations—Effect of Storage Time and Conditions. Int. J. Pharm. 2001, 221, 187–196. [Google Scholar] [CrossRef]
- Sarkar, R.; Mandal, N. Hydroalcoholic Extracts of Indian Medicinal Plants Can Help in Amelioration from Oxidative Stress through Antioxidant Properties. J. Complement. Integr. Med. 2012, 9. [Google Scholar] [CrossRef]
- Šiler, B.; Živković, S.; Banjanac, T.; Cvetković, J.; Nestorović Živković, J.; Ćirić, A.; Soković, M.; Mišić, D. Centauries as Underestimated Food Additives: Antioxidant and Antimicrobial Potential. Food Chem. 2014, 147, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea Europaea L.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Valicherla, G.R.; Dave, K.M.; Syed, A.A.; Riyazuddin, M.; Gupta, A.P.; Singh, A.; Wahajuddin; Mitra, K.; Datta, D.; Gayen, J.R. Formulation Optimization of Docetaxel Loaded Self-Emulsifying Drug Delivery System to Enhance Bioavailability and Anti-Tumor Activity. Sci. Rep. 2016, 6, 26895. [Google Scholar] [CrossRef]
- Mony, R.S. Studies on Anti-Inflammatory Activity of Herbal Extract Mixture Using Inflammation Induced Human Keratinocytes (HaCaT) Cells. J. Pharmacogn. Phytochem. 2023, 12, 8–10. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Deyrieux, A.F.; Wilson, V.G. In Vitro Culture Conditions to Study Keratinocyte Differentiation Using the HaCaT Cell Line. Cytotechnology 2007, 54, 77–83. [Google Scholar] [CrossRef]
- Saah, S.; Wiwattanapatapee, R. Cytotoxic Effect of Surfactants Used in Self-Microemulsifying Drug Delivery Systems (SMEDDS) on Normal and Cancer Gastrointestinal Cell Lines. Lat. Am. J. Pharm. 2018, 37, 2244–2253. [Google Scholar]
- Schwartz, C.; Moran, T.; Saunders, S.P.; Kaszlikowska, A.; Floudas, A.; Bom, J.; Nunez, G.; Iwakura, Y.; O’Neill, L.; Irvine, A.D.; et al. Spontaneous Atopic Dermatitis in Mice with a Defective Skin Barrier Is Independent of ILC2 and Mediated by IL-1β. Allergy 2019, 74, 1920–1933. [Google Scholar] [CrossRef]
- Vazquez, E.; Navarro, M.; Salazar, Y.; Crespo, G.; Bruges, G.; Osorio, C.; Tortorici, V.; Vanegas, H.; López, M. Systemic Changes Following Carrageenan-Induced Paw Inflammation in Rats. Inflamm. Res. 2015, 64, 333–342. [Google Scholar] [CrossRef]
- Luger, T. Balancing Efficacy and Safety in the Management of Atopic Dermatitis: The Role of Methylprednisolone Aceponate. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 251–258. [Google Scholar] [CrossRef]
- Torrelo, A. Methylprednisolone Aceponate for Atopic Dermatitis. Int. J. Dermatol. 2017, 56, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2022, 12, 91. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef]
- Solcan, M.-B.; Fizeșan, I.; Vlase, L.; Vlase, A.-M.; Rusu, M.E.; Mateș, L.; Petru, A.-E.; Creștin, I.-V.; Tomuțǎ, I.; Popa, D.-S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes Nigrum L.), European Blueberry (Vaccinium Myrtillus L.), and Mountain Cranberry (Vaccinium Vitis-Idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Solcan, M.-B.; Vlase, A.-M.; Marc, G.; Muntean, D.; Casian, T.; Nadăș, G.C.; Novac, C.; Ștefania; Popa, D.-S.; Vlase, L. Antimicrobial Effectiveness of Ribes Nigrum L. Leaf Extracts Prepared in Natural Deep Eutectic Solvents (NaDESs). Antibiotics 2024, 13, 1118. [Google Scholar] [CrossRef]
- Karbab, A.; Charef, N.; Jaber, A.M.; Siedat, A.M.; Kharchi, M.; Nini, R.; Sanabrah, A. Antioxidant, Anti-Inflammatory Effects of Centaurium Erythraea Rafn. Aerial Part Extracts and Identification of Its Bioactive Constituents by LC-MS/MS Analysis. Int. J. Comput. Exp. Sci. Eng. 2025, 11, 4237–4245. [Google Scholar] [CrossRef]
- Gaber, D.A.; Alsubaiyel, A.M.; Alabdulrahim, A.K.; Alharbi, H.Z.; Aldubaikhy, R.M.; Alharbi, R.S.; Albishr, W.K.; Mohamed, H.A. Nano-Emulsion Based Gel for Topical Delivery of an Anti-Inflammatory Drug: In Vitro and in Vivo Evaluation. Drug Des. Devel. Ther. 2023, Volume 17, 1435–1451. [Google Scholar] [CrossRef] [PubMed]
- Bhagurkar, A.M.; Angamuthu, M.; Patil, H.; Tiwari, R.V.; Maurya, A.; Hashemnejad, S.M.; Kundu, S.; Murthy, S.N.; Repka, M.A. Development of an Ointment Formulation Using Hot-Melt Extrusion Technology. AAPS PharmSciTech 2016, 17, 158–166. [Google Scholar] [CrossRef]
- Jurca, T.; Józsa, L.; Suciu, R.; Pallag, A.; Marian, E.; Bácskay, I.; Mureșan, M.; Stan, R.L.; Cevei, M.; Cioară, F.; et al. Formulation of Topical Dosage Forms Containing Synthetic and Natural Anti-Inflammatory Agents for the Treatment of Rheumatoid Arthritis. Molecules 2020, 26, 24. [Google Scholar] [CrossRef]
- Ivko, T.; Hrytsenko, V.; Kienko, L.; Bobrytska, L.; Kukhtenko, H.; Germanyuk, T. Investigation of the Rheological Properties of Ointment Bases as a Justification of the Ointment Composition for Herpes Treatment. Turkish J. Pharm. Sci. 2021, 18, 628–636. [Google Scholar] [CrossRef]
- Kocabaş, N.Ö.; Kahraman, E.; Güngör, S. Assessment of Membrane Type Effects on in Vitro Performance of Topical Semi-Solid Products. J. Drug Deliv. Sci. Technol. 2021, 64, 102646. [Google Scholar] [CrossRef]
- Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef]
- Gavra, D.I.; Endres, L.; Pető, Á.; Józsa, L.; Fehér, P.; Ujhelyi, Z.; Pallag, A.; Marian, E.; Vicas, L.G.; Ghitea, T.C.; et al. In Vitro and Human Pilot Studies of Different Topical Formulations Containing Rosa Species for the Treatment of Psoriasis. Molecules 2022, 27, 5499. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Morar, I.I.; Pop, R.M.; Peitzner, E.; Ranga, F.; Orăsan, M.S.; Cecan, A.D.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; Țicolea, M.; et al. Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey. Nutrients 2025, 17, 276. [Google Scholar] [CrossRef]
- Michiu, D.; Socaciu, M.-I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Mureşan, V.; Semeniuc, C.A. Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef]
- Papp, B.; Le Borgne, M.; Perret, F.; Marminon, C.; Józsa, L.; Pető, Á.; Kósa, D.; Nagy, L.; Kéki, S.; Ujhelyi, Z.; et al. Formulation and Investigation of CK2 Inhibitor-Loaded Alginate Microbeads with Different Excipients. Pharmaceutics 2023, 15, 2701. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-Induced Paw Edema in the Rat and Mouse. In Inflammation Protocols; Humana Press: Totowa, NJ, USA; pp. 115–122.





| No. | Compounds | Concentration (µg/mL) |
|---|---|---|
| 1 | Gentisic acid | <LOQ |
| 2 | Caffeic acid | 0.479 ± 0.052 |
| 3 | Chlorogenic acid | 1.069 ± 0.064 |
| 4 | 4-O-caffeoylquinic acid | 0.966 ± 0.057 |
| 5 | Ferulic acid | 0.481 ± 0.043 |
| 6 | Hyperoside | 3.778 ± 0.075 |
| 7 | Isoquercitrin | 0.561 ± 0.067 |
| 8 | Rutin | <LOQ |
| 9 | Quercitrin | 2.613 ± 0.156 |
| 10 | Quercetin | 0.527 ± 0.026 |
| 11 | Luteolin | <LOQ |
| 12 | Kaempferol | <LOQ |
| 13 | Apigenin | <LOQ |
| 14 | Epicatechin | 0.026 ± 0.002 |
| 15 | Syringic acid | 0.04 ± 0.001 |
| 16 | Gallic acid | 0.181 ± 0.009 |
| 17 | Protocatechuic acid | 1.126 ± 0.101 |
| 18 | Epigallocatechin gallate | 0.179 ± 0.023 |
| Entry | Particle Size0 (nm) | PDI0 | Particle Size30 (nm) | PDI30 | Zeta Potential (mV) | Zeta Potential30 (mV) |
|---|---|---|---|---|---|---|
| 1. | 161.42 ± 2.48 | 0.241 ± 0.014 | 185.96 ± 7.96 | 0.417 ± 0.008 | −5.570 ± 0.49 | −5.324 ± 0.35 |
| 2. | 188.90 ± 0.70 | 0.303 ± 0.015 | 240.90 ± 3.70 | 0.506 ± 0.032 | −4.596 ± 0.26 | −3.984 ± 0.28 |
| 3. | 113.56 ± 2.04 | 0.306 ± 0.033 | 196.67 ± 3.56 | 0.421 ± 0.006 | −6.497 ± 0.56 | −6.287 ± 0.42 |
| Entry | pH0 | pH30 |
|---|---|---|
| I. | 5.92 ± 0.05 | 5.87 ± 0.04 |
| II. | 5.78 ± 0.04 | 5.72 ± 0.03 |
| III. | 5.47 ± 0.04 | 5.46 ± 0.06 |
| IV. | 5.12 ± 0.03 | 5.13 ± 0.03 |
| V. | 5.39 ± 0.04 | 5.41 ± 0.04 |
| Entry | Release Rate k·102 (µg/cm2·min1/2) ± SD | Diffusion Coefficient D·105 (cm2/min) ± SD |
|---|---|---|
| II. | 0.41 ± 0.04 | 7.99 ± 2.13 |
| III. | 0.78 ± 0.04 | 27.05 ± 3.27 |
| IV. | 0.81 ± 0.07 | 23.93 ± 2.19 |
| V. | 0.86 ± 0.06 | 35.12 ± 3.39 |
| Entry | Zero-Order R2 | First-Order R2 | Higuchi R2 | Korsmeyer–Peppas R2 | Weibull R2 |
|---|---|---|---|---|---|
| II. | 0.782 | — | 0.945 | 0.973 | — |
| III. | 0.679 | — | 0.975 | 0.986 | — |
| IV. | 0.797 | — | 0.929 | 0.965 | — |
| V. | 0.536 | — | 0.978 | 0.980 | — |
| Entry | TPC (mg GAE/mL) |
|---|---|
| II. | 59.07 ± 3.43 |
| III. | 68.43 ± 1.84 |
| IV. | 66.84 ± 0.57 |
| V. | 69.71 ± 0.83 |
| Entry | Lyophilized C. erythraea (g) | Transcutol® P (g) | Capryol® 90 (g) |
|---|---|---|---|
| 1. | 1.00 | 5.00 | - |
| 2. | 1.00 | - | 2.00 |
| 3. | 1.00 | 5.00 | 2.00 |
| Composition | I.(g) | II. (g) | III. (g) | IV. (g) | V. (g) |
|---|---|---|---|---|---|
| White Vaseline | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 |
| Cetylstearyl alcohol | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Beeswax | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Polysorbate 60 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Methylparahydroxybenzoate solution (1 w/V) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Distilled water | up to 100.00 | up to 100.00 | up to 100.00 | up to 100.00 | up to 100.00 |
| Transcutol® P | - | - | 5.00 | - | 5.00 |
| Capryol® 90 | - | - | - | 2.00 | 2.00 |
| Lyophilized C. erythraea | - | 1.00 | 1.00 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karetka, A.J.; Papp, B.; Lekli, I.; Vlase, A.-M.; Pallag, A.; Vicaș, L.G.; Lestyán, A.-M.; Józsa, L.; Kósa, D.; Pető, Á.; et al. In Vitro and in Vivo Efficacy of Different Ointment Formulations Containing Centaurium erythraea Rafn. Aerial Extract. Pharmaceuticals 2025, 18, 1681. https://doi.org/10.3390/ph18111681
Karetka AJ, Papp B, Lekli I, Vlase A-M, Pallag A, Vicaș LG, Lestyán A-M, Józsa L, Kósa D, Pető Á, et al. In Vitro and in Vivo Efficacy of Different Ointment Formulations Containing Centaurium erythraea Rafn. Aerial Extract. Pharmaceuticals. 2025; 18(11):1681. https://doi.org/10.3390/ph18111681
Chicago/Turabian StyleKaretka, Anett Jolán, Boglárka Papp, István Lekli, Ana-Maria Vlase, Annamária Pallag, Laura Grațiela Vicaș, Antonia-Maria Lestyán, Liza Józsa, Dóra Kósa, Ágota Pető, and et al. 2025. "In Vitro and in Vivo Efficacy of Different Ointment Formulations Containing Centaurium erythraea Rafn. Aerial Extract" Pharmaceuticals 18, no. 11: 1681. https://doi.org/10.3390/ph18111681
APA StyleKaretka, A. J., Papp, B., Lekli, I., Vlase, A.-M., Pallag, A., Vicaș, L. G., Lestyán, A.-M., Józsa, L., Kósa, D., Pető, Á., Ujhelyi, Z., Nacsa, F., Bácskay, I., Fehér, P., & Jurca, T. (2025). In Vitro and in Vivo Efficacy of Different Ointment Formulations Containing Centaurium erythraea Rafn. Aerial Extract. Pharmaceuticals, 18(11), 1681. https://doi.org/10.3390/ph18111681














