Systemic Metabolic Alterations Induced by Etodolac in Healthy Individuals
Abstract
1. Introduction
2. Results
2.1. Demographics and Clinical Characteristics of Participants
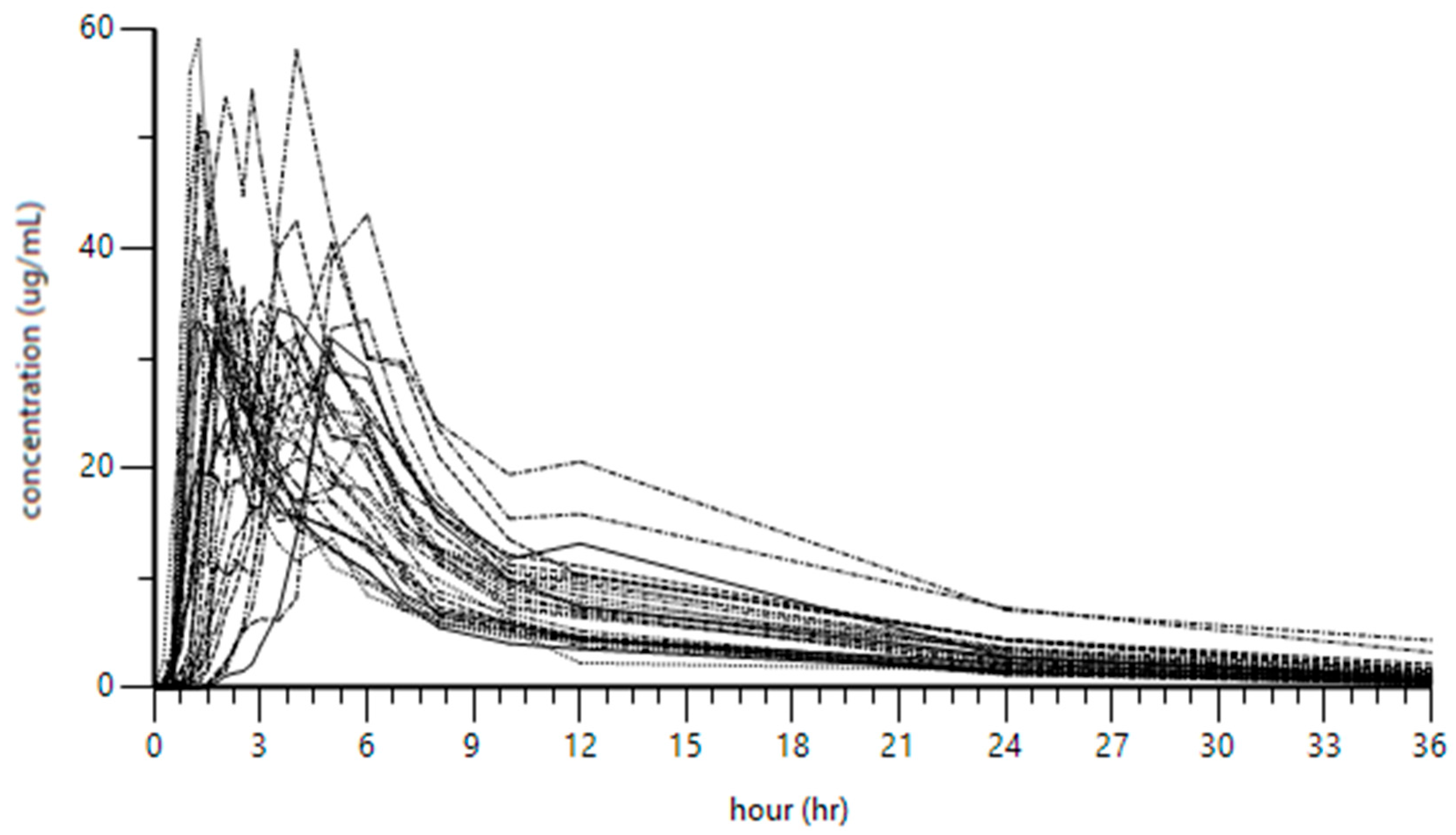
2.2. Pharmacokinetics of Etodolac in All Participants
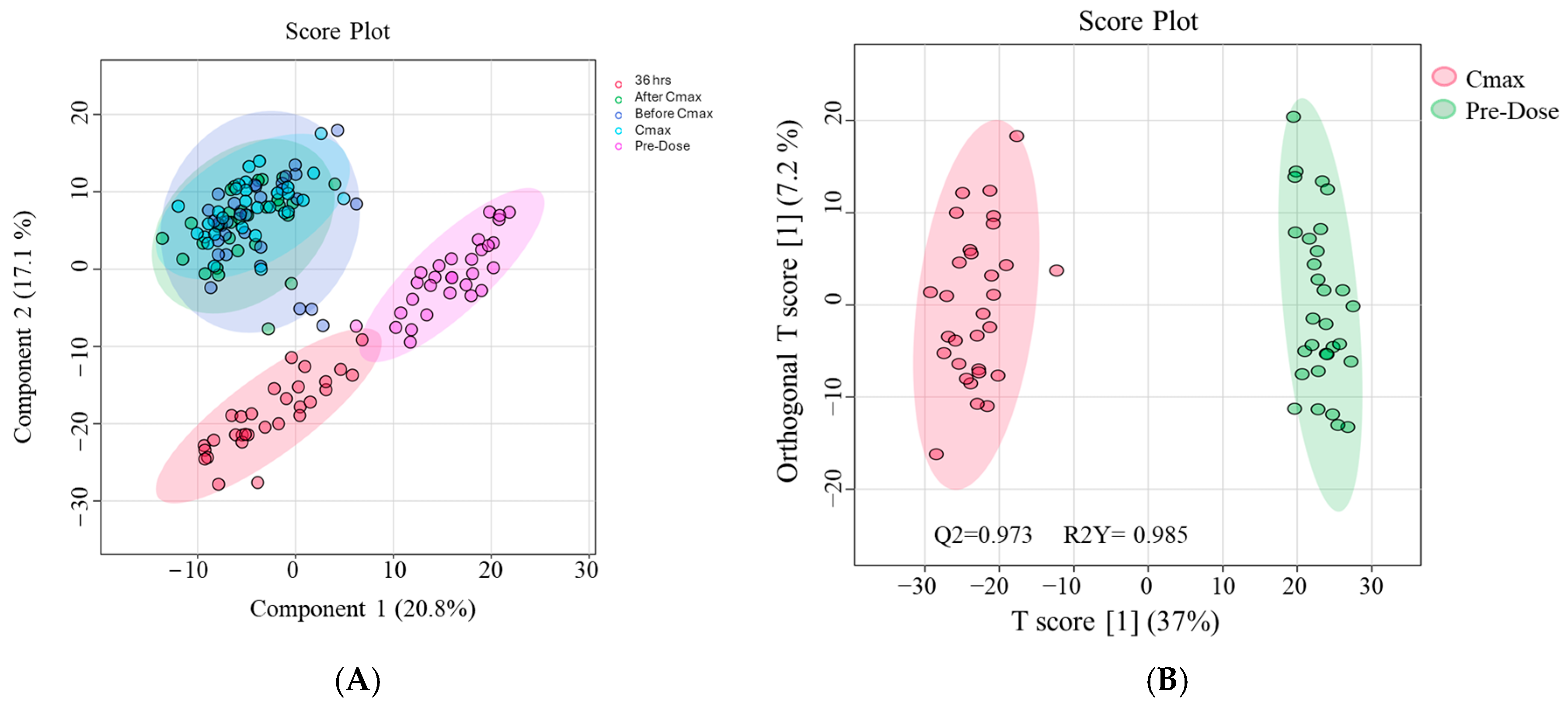
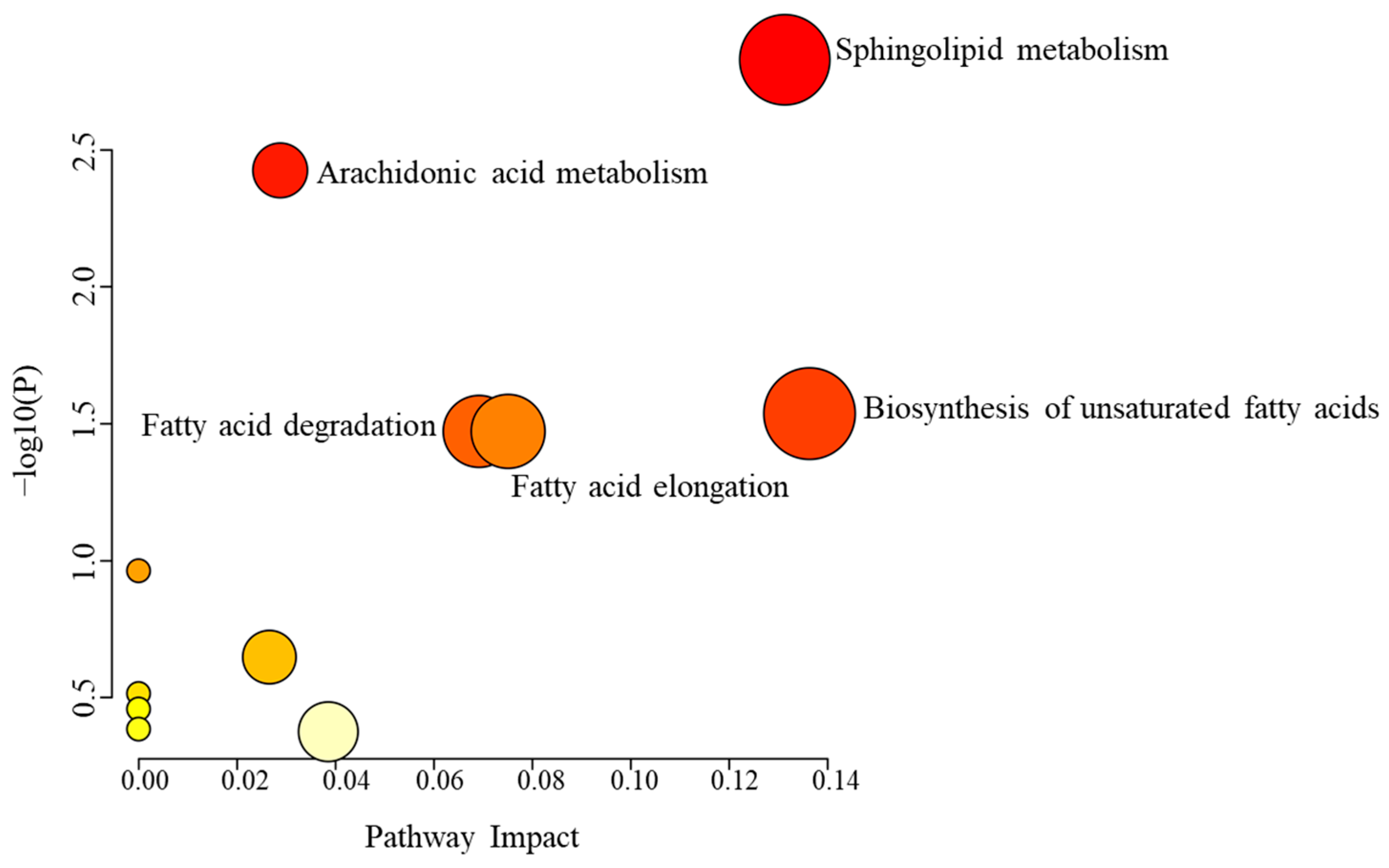
2.3. Metabolic Alterations Associated with Etodolac Administration
3. Discussion
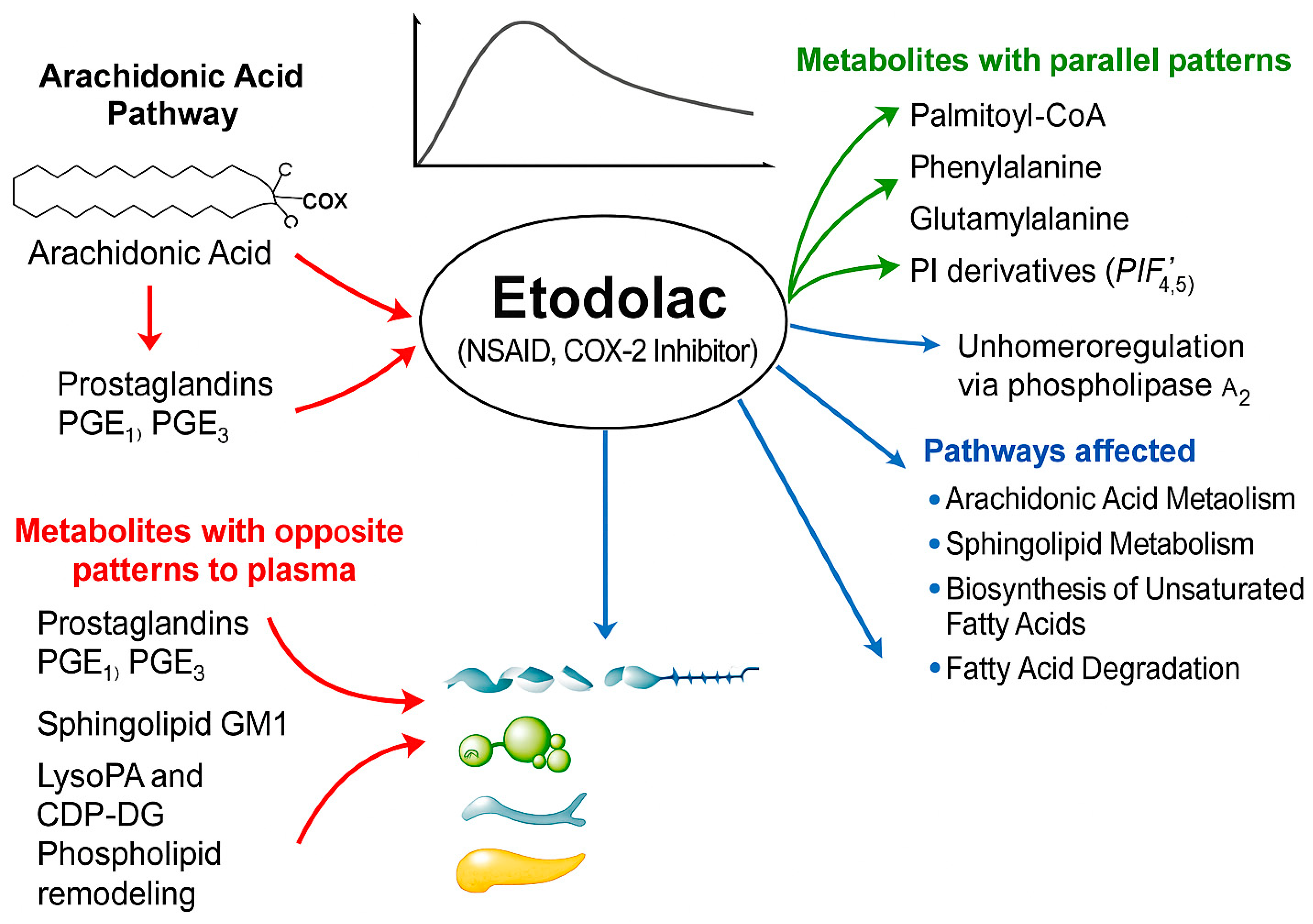
3.1. Characterizing Systemic Metabolomic Changes Induced by Etodolac in Healthy Individuals
3.1.1. Metabolites Exhibiting Parallel or Divergent Kinetics Relative to Etodolac Pharmacokinetics
3.1.2. Metabolic Alterations Specifically at the Cmax Time Point of Etodolac Administration
4. Materials and Methods
4.1. Ethical Approval
4.2. Participants’ Demographic and Clinical Data
4.3. Blood Sample Collection Pre- and Post-Etodolac Administration
4.4. Assessment of Etodolac Pharmacokinetics
4.5. Sample Preparation and Metabolite Extraction
4.6. LC-MS-Based Untargeted Metabolomics Analysis
4.7. Statistical Analysis
4.8. Metabolite Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellamy, N. Etodolac in the management of pain: A clinical review of a multipurpose analgesic. Inflammopharmacology 1997, 5, 139–152. [Google Scholar] [CrossRef]
- Chadha, N.; Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef]
- Moore, N. Ibuprofen: A Journey from Prescription to Over-the-Counter Use. J. R. Soc. Med. 2007, 100, 2–6. [Google Scholar] [CrossRef]
- Weideman, R.A.; Kelly, K.C.; Kazi, S.; Cung, A.; Roberts, K.W.; Smith, H.J.; Sarosi, G.A.; Little, B.B.; Cryer, B. Risks of clinically significant upper gastrointestinal events with etodolac and naproxen: A historical cohort analysis. Gastroenterology 2004, 127, 1322–1328. [Google Scholar] [CrossRef]
- Warner, T.D.; Giuliano, F.; Vojnovic, I.; Bukasa, A.; Mitchell, J.A.; Vane, J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Maccagno, A.; Di Giorgio, E.; Romanowicz, A. Effectiveness of etodolac (‘Lodine’) compared with naproxen in patients with acute gout. Curr. Med. Res. Opin. 1991, 12, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655–683. [Google Scholar] [PubMed]
- Glaser, K.B. Cyclooxygenase selectivity and NSAIDs: Cyclooxygenase-2 selectivity of etodolac (LODINE). Inflammopharmacology 1995, 3, 335–345. [Google Scholar] [CrossRef]
- Tirunagari, S.K.; Derry, S.; Moore, R.A.; McQuay, H.J. Single dose oral etodolac for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2009, 2009, CD007357. [Google Scholar] [CrossRef]
- 1Brocks, D.R.; Jamali, F. Etodolac Clinical Pharmacokinetics. Clin. Pharmacokinet. 1994, 26, 259–274. [Google Scholar] [CrossRef]
- Daly, A.; Rettie, A.; Fowler, D.; Miners, J. Pharmacogenomics of CYP2C9: Functional and Clinical Considerations. J. Pers. Med. 2017, 8, 1. [Google Scholar] [CrossRef]
- Zeng, W.; Han, C.; Mohammed, S.; Li, S.; Song, Y.; Sun, F.; Du, Y. Indole-containing pharmaceuticals: Targets, pharmacological activities, and SAR studies. RSC Med. Chem. 2024, 15, 788–808. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Xu, Z.; Dou, J. Mass spectrometry-based metabolomics in health and medical science: A systematic review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef]
- Backshall, A.; Sharma, R.; Clarke, S.J.; Keun, H.C. Pharmacometabonomic Profiling as a Predictor of Toxicity in Patients with Inoperable Colorectal Cancer Treated with Capecitabine. Clin. Cancer Res. 2011, 17, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Aa, J.; Jia, H.; Xin, X.; Tao, C.; Liu, L.; Zou, B.; Song, Q.; Shi, J.; Cao, B.; et al. A Pharmacometabonomic Approach To Predicting Metabolic Phenotypes and Pharmacokinetic Parameters of Atorvastatin in Healthy Volunteers. J. Proteome Res. 2015, 14, 3970–3981. [Google Scholar] [CrossRef]
- Du Preez, I.; Loots, D.T. Novel insights into the pharmacometabonomics of first-line tuberculosis drugs relating to metabolism, mechanism of action and drug-resistance. Drug Metab. Rev. 2018, 50, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Luquez, K.; Reis Silveira, A.M.; Sánchez-Vinces, S.; Rosini Silva, A.A.; Barreto, J.; Lemos De Brito, R.B.S.; Garcia, C.D.M.; Vieira, A.L.; Antonio, M.A.; De Oliveira Carvalho, P. Etodolac Single Dose Metabolic Profile Elucidation: Pharmacokinetics and Adverse Events in Healthy Volunteers. Pharmaceuticals 2025, 18, 82. [Google Scholar] [CrossRef]
- Davies, N.M.; Skjodt, N.M. Choosing the Right Nonsteroidal Anti-Inflammatory Drug for the Right Patient: A Pharmacokinetic Approach. Clin. Pharmacokinet. 2000, 38, 377–392. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef][Green Version]
- Burt, T.; Yoshida, K.; Lappin, G.; Vuong, L.; John, C.; De Wildt, S.; Sugiyama, Y.; Rowland, M. Microdosing and Other Phase 0 Clinical Trials: Facilitating Translation in Drug Development. Clin. Transl. Sci. 2016, 9, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Laine, L.; Sloane, R.; Ferretti, M.; Cominelli, F. A randomized, double-blind comparison of placebo, etodolac, and naproxen on gastrointestinal injury and prostaglandin production. Gastrointest. Endosc. 1995, 42, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.J.; Constantine, G. Etodolac (Lodine) in the treatment of osteoarthritis: Recent studies. J. Rheumatol. Suppl. 1997, 47, 23–31. [Google Scholar]
- Hatori, M.; Kokubun, S. Clinical Use of Etodolac for the Treatment of Lumbar Disc Herniation. Curr. Med. Res. Opin. 1999, 15, 193–201. [Google Scholar] [CrossRef]
- Balfour, J.A.; Buckley, M.M.-T. Etodolac: A Reappraisal of its Pharmacology and Therapeutic Use in Rheumatic Diseases and Pain States. Drugs 1991, 42, 274–299. [Google Scholar] [CrossRef]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and Pharmacodynamics: A Comprehensive Analysis of the Absorption, Distribution, Metabolism, and Excretion of Psychiatric Drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny Lipids With Giant Impact on Cell Regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2018, 506, 307–314. [Google Scholar] [CrossRef]
- Fooksman, D.R.; Shaikh, S.R.; Boyle, S.; Edidin, M. Cutting Edge: Phosphatidylinositol 4,5-Bisphosphate Concentration at the APC Side of the Immunological Synapse Is Required for Effector T Cell Function. J. Immunol. 2009, 182, 5179–5182. [Google Scholar] [CrossRef]
- Boyle, D.L.; Kim, H.-R.; Topolewski, K.; Bartok, B.; Firestein, G.S. Novel Phosphoinositide 3-Kinase δ,γ Inhibitor: Potent Anti-Inflammatory Effects and Joint Protection in Models of Rheumatoid Arthritis. J. Pharmacol. Exp. Ther. 2014, 348, 271–280. [Google Scholar] [CrossRef]
- Raghu, P.; Joseph, A.; Krishnan, H.; Singh, P.; Saha, S. Phosphoinositides: Regulators of Nervous System Function in Health and Disease. Front. Mol. Neurosci. 2019, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.; Wright, B.D.; Zylka, M.J. Lipid kinases as therapeutic targets for chronic pain. Pain 2015, 156, S2–S10. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Kazberuk, A.; Chalecka, M.; Palka, J.; Surazynski, A. Nonsteroidal Anti-Inflammatory Drugs as PPARγ Agonists Can Induce PRODH/POX-Dependent Apoptosis in Breast Cancer Cells: New Alternative Pathway in NSAID-Induced Apoptosis. Int. J. Mol. Sci. 2022, 23, 1510. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, H.-J.; Jeong, J.-A.; Jung, J.-W. Palmitic acid inhibits inflammatory responses in lipopolysaccharide-stimulated mouse peritoneal macrophages. Orient. Pharm. Exp. Med. 2010, 10, 37–43. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Yang, M.; Cai, J.; Fu, Q.; Ma, J.; Zhu, L. The mitochondrial unfolded protein response (UPRmt) protects against osteoarthritis. Exp. Mol. Med. 2022, 54, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Rogero, M.M.; Tirapegui, J. Effects of glutamine and alanine supplementation on muscle fatigue parameters of rats submitted to resistance training. Nutrition 2019, 65, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mehta, O.; Vijay, A.; Gohir, S.A.; Kelly, T.; Zhang, W.; Doherty, M.; Walsh, D.A.; Aithal, G.; Valdes, A.M. Serum Metabolome Analysis Identified Amino-Acid Metabolism Associated With Pain in People With Symptomatic Knee Osteoarthritis—A Cross-Sectional Study. J. Pain 2023, 24, 1251–1261. [Google Scholar] [CrossRef]
- Zhai, G.; Sun, X.; Randell, E.W.; Liu, M.; Wang, N.; Tolstykh, I.; Rahman, P.; Torner, J.; Lewis, C.E.; Nevitt, M.C.; et al. Phenylalanine Is a Novel Marker for Radiographic Knee Osteoarthritis Progression: The MOST Study. J. Rheumatol. 2021, 48, 123–128. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Gong, L. Biomarker identification and pathway analysis of rheumatoid arthritis based on metabolomics in combination with ingenuity pathway analysis. Proteomics 2021, 21, 2100037. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Mechanism of Action of Nonsteroidal Anti-inflammatory Drugs. Am. J. Med. 1998, 104, 2S–8S. [Google Scholar] [CrossRef]
- Guo, Z. Ganglioside GM1 and the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 9558. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.; Williams, L.; Arnold, N.; Larsen, J. Therapeutic developments for neurodegenerative GM1 gangliosidosis. Front. Neurosci. 2024, 18, 1392683. [Google Scholar] [CrossRef] [PubMed]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Pereira-Leite, C.; Nunes, C.; Reis, S. Interaction of nonsteroidal anti-inflammatory drugs with membranes: In vitro assessment and relevance for their biological actions. Prog. Lipid Res. 2013, 52, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A. Etodolac: An overview of a selective COX-2 inhibitor. InflammoPharmacology 1999, 7, 269–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef]
- Nakamura, H.; Murayama, T. The Role of Sphingolipids in Arachidonic Acid Metabolism. J. Pharmacol. Sci. 2014, 124, 307–312. [Google Scholar] [CrossRef]
- Xiao, C.-Y.; Yuhki, K.; Hara, A.; Fujino, T.; Kuriyama, S.; Yamada, T.; Takayama, K.; Takahata, O.; Karibe, H.; Taniguchi, T.; et al. Prostaglandin E2 Protects the Heart From Ischemia-Reperfusion Injury via Its Receptor Subtype EP4. Circulation 2004, 109, 2462–2468. [Google Scholar] [CrossRef]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Motonaga, A.; Dainaka, J.; Nishimura, T.; Hashii, H.; Yamate, K.; Ueda, F.; Kimura, K. Effect of etodolac on prostaglandin E2 biosynthesis, active oxygen generation and bradykinin formation. Prostaglandins Leukot. Essent. Fatty Acids 1994, 51, 457–462. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Author Correction: Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef]
- Nixon, G.F. Sphingolipids in inflammation: Pathological implications and potential therapeutic targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef]
- Benabdelkamel, H.; Sebaa, R.; AlMalki, R.H.; Masood, A.; Alfadda, A.A.; Abdel Rahman, A.M. Untargeted metabolomics reveals the impact of Liraglutide treatment on metabolome profiling and metabolic pathways in type-2 diabetes mellitus. Saudi Pharm. J. 2024, 32, 102172. [Google Scholar] [CrossRef]
- Alshahrani, A.; Aleidi, S.M.; Al Dubayee, M.; AlMalki, R.; Sebaa, R.; Zhra, M.; Abdel Rahman, A.M.; Aljada, A. Postprandial Metabolomic Profiling: Insights into Macronutrient-Specific Metabolic Responses in Healthy Individuals. Nutrients 2024, 16, 3783. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]

| Demographic Data | Mean ± STD (n = 30) | Normal Range * |
| Age (y) | 25 ± 6.8 | NA |
| Height (m) | 1.73 ± 0.049 | NA |
| Weight (Kg) | 71 ± 9.7 | NA |
| * Body mass index (BMI) (Weight/“Height^2”) | 23.8 ± 3.29 | NA |
| Smoker (%) | 80% | NA |
| Biochemical Data | Mean ± STD (n = 30) | Normal Range * |
| Fasting blood sugar (mg/dL) | 95.50 ± 8.577 | 70.00–115.00 mg/dL |
| Urea (mg/dL) | 28.6 ± 7.72 | 10.0–50.0 mg/dL |
| Creatinine (mg/dL) | 0.85 ± 0.146 | 0.60–1.30 mg/dL |
| Sodium (mmol/L) | 143 ± 4.8 | 135–153 mmol/L |
| Potassium (mmol/L) | 4.2 ± 0.39 | 3.50–5.30 mmol/L |
| Aspartate transaminase (SGOT) (U/L) | 23 ± 3.4 | Up to 42 U/L |
| Serum glutamate pyruvate transaminase (SGPT) (U/L) | 18 ± 5.7 | Up to 50 U/L |
| Alkaline phosphatase (ALP) (IU/L) | 99 ± 22.2 | Up to 40–150 U/L |
| Total bilirubin (mg/dL) | 0.5 ± 0.25 | Up to 1.40 mg/dL |
| Hematological Data | Mean ± STD (n = 30) | Normal Range * |
| Red blood cells (RBCs) | 5.30 ± 0.383 | 4.20–6.10 1012/L |
| Hemoglobin | 15.82 ± 0.846 | 14.00–18.00 G/DL |
| Hematocrit | 46.62 ± 2.343 | 40.00–54.00% |
| Mean corpuscular volume (MCV) | 88.20 ± 3.763 | 76.00–94.00 FL |
| Mean corpuscular hemoglobin (MCH) | 29.90 ± 1.432 | 26.00–31.00 PG |
| Mean corpuscular hemoglobin concentration (MCHC) | 33.90 ± 0.583 | 31.00–36.00 G/DL |
| White blood cells (WBCs) | 7.75 ± 2.233 | 4.50–11.00 109/L |
| Neutrophils | 55.87 ± 6.056 | 45.00–75.00% |
| Lymphocytes | 36.20 ± 5.610 | 25.00–40.00% |
| Monocytes | 5.07 ± 1.617 | 0.00–7.00% |
| Eosinophils | 2.33 ± 1.768 | 0.00–4.00% |
| Basophils | 0.53 ± 0.507 | 0.00–1.00% |
| Platelets | 229.43 ± 50.665 | 150.00–450.00 109/L |
| Immunological Data | Mean ± STD (n = 30) | Normal Range * |
| Hepatitis B surface antigen (HBs Ag) | Negative | Negative < 1.00 S/CO |
| Hepatitis C virus antibody (HCV Ab) | Negative | Negative < 1.00 S/CO |
| Human immunodeficiency virus types I and II (HIV I and II) | Negative | Negative < 1.00 S/CO |
| Pattern | Annotated Metabolites | Reference |
|---|---|---|
| Similar pattern | CDP-DG(18:1)-O(12,13)/20:4), lysoPI(20:4/0:0), PIP(TXB2/22:4), PIP(22:5)/PGE2), palmitoyl-CoA, D-phenylalanine, 3-oxotetradecanoyl-CoA, 6-hydroxytetradecanedioyl-CoA, glutamylalanine, 10-nitrooctadec-9-enoyl-CoA, O-acetyl-ADP-ribose, 3-oxooctadecanoyl-CoA, PGP(a-13:0/i-12:0), 4-hydroxy-5-phenyltetrahydro-1,3-oxazin-2-one, CDP-glycerol | Figure 3A and Table S8 |
| Opposite pattern | Glycineamideribotide, MG(0:0/TXB2/0:0), prostaglandin E2, Glc-Cer(d18:1/25:0), ganglioside GM1(18:1/12:0), prostaglandin E1, 15d PGD2, lysoPA(19:0/0:0), 8,9-DiHETrE, deoxycholic acid glycine conjugate, prostaglandin B-1, chenodeoxycholylproline, PGP(i-12:0/i-12:0), cGAMP(2′-5′), 14,15-DiHETrE, MG(18:4/0:0/0:0), prostaglandin B1, lysoPA(i-14:0/0:0), CL(8:0/8:0/11:0/18:2), CDP-DG(18:1/18:1-2OH(9,10)), PC(PGE1/P-18:1), PS(22:4/6 keto-PGF1alpha), MG(0:0/16:0/0:0), PC(22:5/20:5), Isodocosanoyl-CoA, PE(22:6-OH(4)/22:4), docosanoyl-CoA | Figure 3B and Table S9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebaa, R.; AlMalki, R.H.; Sukkarieh, H.; Dahabiyeh, L.A.; Mogren, M.A.; Arafat, T.; Mujamammi, A.H.; Sabi, E.M.; Abdel Rahman, A.M. Systemic Metabolic Alterations Induced by Etodolac in Healthy Individuals. Pharmaceuticals 2025, 18, 1155. https://doi.org/10.3390/ph18081155
Sebaa R, AlMalki RH, Sukkarieh H, Dahabiyeh LA, Mogren MA, Arafat T, Mujamammi AH, Sabi EM, Abdel Rahman AM. Systemic Metabolic Alterations Induced by Etodolac in Healthy Individuals. Pharmaceuticals. 2025; 18(8):1155. https://doi.org/10.3390/ph18081155
Chicago/Turabian StyleSebaa, Rajaa, Reem H. AlMalki, Hatouf Sukkarieh, Lina A. Dahabiyeh, Maha Al Mogren, Tawfiq Arafat, Ahmed H. Mujamammi, Essa M. Sabi, and Anas M. Abdel Rahman. 2025. "Systemic Metabolic Alterations Induced by Etodolac in Healthy Individuals" Pharmaceuticals 18, no. 8: 1155. https://doi.org/10.3390/ph18081155
APA StyleSebaa, R., AlMalki, R. H., Sukkarieh, H., Dahabiyeh, L. A., Mogren, M. A., Arafat, T., Mujamammi, A. H., Sabi, E. M., & Abdel Rahman, A. M. (2025). Systemic Metabolic Alterations Induced by Etodolac in Healthy Individuals. Pharmaceuticals, 18(8), 1155. https://doi.org/10.3390/ph18081155






