In Vitro Characterization of the Published Glypican-3-Targeting Peptide TJ12P2 Reveals a Lack of Specificity and Potency
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization
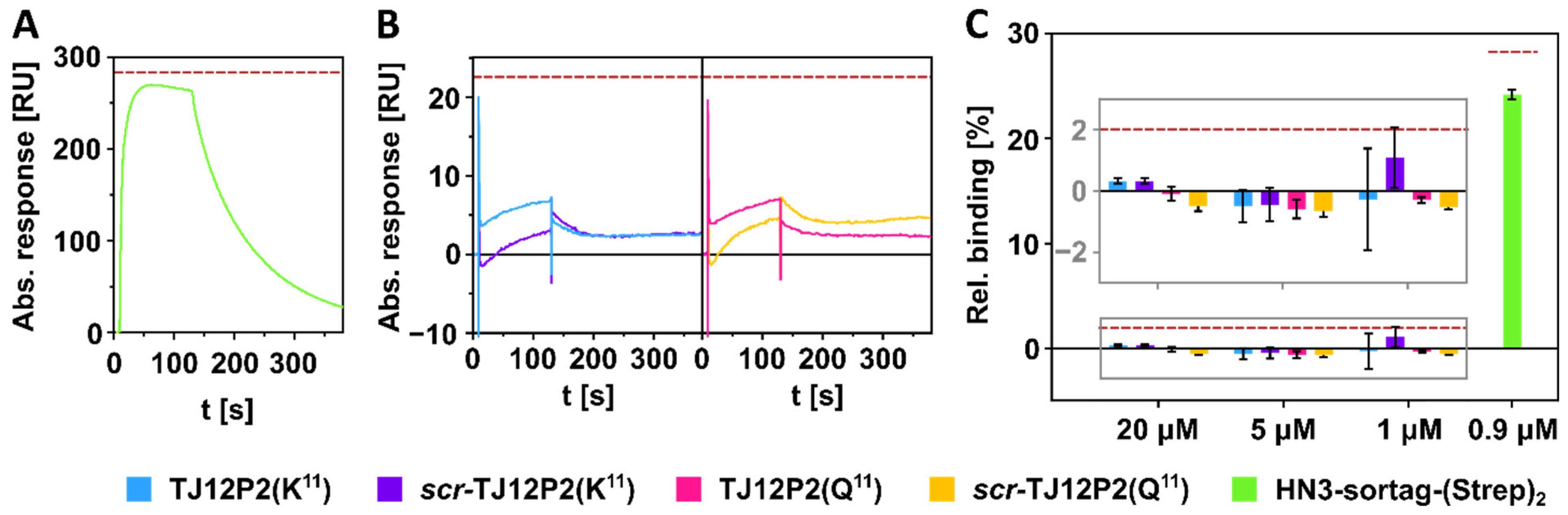
2.2. SPR Interaction Analysis Experiments
2.3. Fluorescence Microscopy
2.4. Radiolabeling and Characterization
2.5. Real-Time Radioligand Binding
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis and Characterization
4.2. Nanobody Synthesis and Isolation
4.3. SPR Interaction Analysis
4.4. Cell Culture
4.5. Western Blotting
4.6. Fluorescence Microscopy
4.7. Radiochemistry
4.8. LogD7.4 Determination
4.9. Stability Studies
4.10. Real-Time Radioligand Binding Using the Ligand Tracer System
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alloc | Allyloxycarbonyl |
| DBCO | Dibenzocyclooctin |
| BCN | Bicyclo[6.1.0]non-4-yne |
| Df | Deferoxamine |
| DTT | Dithiothreitol |
| DMSO | Dimethyl sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| FC | Flow cells |
| FDG | Fluordesoxyglucose |
| Fmoc | Fluorenylmethoxycarbonyl |
| GPC3 | Glypican-3 |
| HCC | Hepatocellular carcinoma |
| HPLC | High-performance liquid chromatography |
| HR | High resolution |
| MALDI | Matrix-assisted laser desorption/ionization |
| MS | Mass spectrometry |
| NODA-GA | 1,4,7-triazacyclononane-1-glutaric acid-4,7-diacetic acid |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| PAGE | Polyacrylamide gel electrophoresis |
| PBS | Phosphate-buffered saline |
| PET | Positron emission tomography |
| PMSF | Phenylmethylsulfonyl fluoride |
| POD | Peroxidase |
| PVDF | Polyvinylidene fluoride |
| RIPA | Radioimmunoprecipitation assay buffer |
| RP | Reverse phase |
| SDS | Sodium dodecyl sulfate |
| SPR | Surface plasmon resonance |
| TLC | Thin layer chromatography |
| TOF | Time of flight |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.E.; Soerjomataram, I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef]
- Ding, J.; Wen, Z. Survival improvement and prognosis for hepatocellular carcinoma: Analysis of the SEER database. BMC Cancer 2021, 21, 1157. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kokudo, N.; Makuuchi, M.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J. Hepatol. 2013, 58, 724–729. [Google Scholar] [CrossRef]
- Kim, K.M.; Sinn, D.H.; Jung, S.; Gwak, G.; Paik, Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. The recommended treatment algorithms of the BCLC and HKLC staging systems: Does following these always improve survival rates for HCC patients? Liver Int. 2016, 36, 1490–1497. [Google Scholar] [CrossRef]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the Use of 18F-FDG PET in Oncology. J. Nucl. Med. 2008, 49, 480–508. [Google Scholar] [CrossRef]
- Singh, P.; Singhal, T.; Parida, G.K.; Rahman, A.; Agrawal, K. Diagnostic Performance of FAPI PET/CT vs. 18F-FDG PET/CT in Evaluation of Liver Tumors: A Systematic Review and Meta-analysis. Mol. Imaging Radionucl. Ther. 2024, 33, 77–89. [Google Scholar] [CrossRef]
- Haug, A.R. Imaging of primary liver tumors with positron-emission tomography. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 292–300. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Lu, W.; Zeng, Y. Prognostic significance of glypican-3 expression in hepatocellular carcinoma: A meta-analysis. Medicine 2018, 97, e9702. [Google Scholar] [CrossRef]
- Shirakawa, H.; Suzuki, H.; Shimomura, M.; Kojima, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T.; Konishi, M.; Kobayashi, N.; Kinoshita, T.; et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009, 100, 1403–1407. [Google Scholar] [CrossRef]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; O’dOnoghue, J.A.; Beylergil, V.; Ruan, S.; Pandit-Taskar, N.; Larson, S.M.; Smith-Jones, P.M.; Lyashchenko, S.K.; Ohishi, N.; Ohtomo, T.; et al. I-124 codrituzumab imaging and biodistribution in patients with hepatocellular carcinoma. EJNMMI Res. 2018, 8, 20. [Google Scholar] [CrossRef]
- Sham, J.G.; Kievit, F.M.; Grierson, J.R.; Miyaoka, R.S.; Yeh, M.M.; Zhang, M.; Yeung, R.S.; Minoshima, S.; Park, J.O. Glypican-3–Targeted 89Zr PET Imaging of Hepatocellular Carcinoma. J. Nucl. Med. 2014, 55, 799–804. [Google Scholar] [CrossRef]
- Natarajan, A.; Zhang, H.; Ye, W.; Huttad, L.; Tan, M.; Chua, M.-S.; Gambhir, S.S.; So, S.K. A Humanized Anti-GPC3 Antibody for Immuno-Positron Emission Tomography Imaging of Orthotopic Mouse Model of Patient-Derived Hepatocellular Carcinoma Xenografts. Cancers 2021, 13, 3977. [Google Scholar] [CrossRef]
- Karlsson, J.; Siegel, F.; Moen, I.; Scholz, A.; Mobergslien, A.; Suurs, F.; Oteiza, A.; Pelekanou, V.; Glaus, C.; Zimmermann, S. Abstract ND09: BAY 3547926: Novel targeted radionuclide therapy for hepatocellular carcinoma. Cancer Res. 2025, 85, ND09. [Google Scholar] [CrossRef]
- Sham, J.G.; Kievit, F.M.; Grierson, J.R.; Chiarelli, P.A.; Miyaoka, R.S.; Zhang, M.; Yeung, R.S.; Minoshima, S.; Park, J.O. Glypican-3–Targeting F(ab′)2 for 89Zr PET of Hepatocellular Carcinoma. J. Nucl. Med. 2014, 55, 2032–2037. [Google Scholar] [CrossRef]
- Fayn, S.; King, A.P.; Gutsche, N.T.; Duan, Z.; Buffington, J.; Olkowski, C.P.; Fu, Y.; Hong, J.; Sail, D.; Baidoo, K.E.; et al. Site-Specifically Conjugated Single-Domain Antibody Successfully Identifies Glypican-3–Expressing Liver Cancer by Immuno-PET. J. Nucl. Med. 2023, 64, 1017–1023. [Google Scholar] [CrossRef]
- An, S.; Zhang, D.; Zhang, Y.; Wang, C.; Shi, L.; Wei, W.; Huang, G.; Liu, J. GPC3-targeted immunoPET imaging of hepatocellular carcinomas. Eur. J. Nucl. Med. Mol. Imag. 2022, 49, 2682–2692. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.-J.; Huang, S.; Wang, M.; Zhou, W.-L.; Li, H.-S.; Wang, Q.-S.; Wu, H.-B. Imaging the expression of glypican-3 in hepatocellular carcinoma by PET. Amino Acids 2018, 50, 309–320. [Google Scholar] [CrossRef]
- Zhu, D.; Qin, Y.; Wang, J.; Zhang, L.; Zou, S.; Zhu, X.; Zhu, L. Novel Glypican-3-Binding Peptide for in Vivo Hepatocellular Carcinoma Fluorescent Imaging. Bioconjug. Chem. 2016, 27, 831–839. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Z.; Tao, J.; Zhao, M.; Zhang, W.; Li, P.; Tang, L.; Gu, Y. An innovative peptide with high affinity to GPC3 for hepatocellular carcinoma diagnosis. Biomater. Sci. 2018, 7, 159–167. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, S.; Li, Y.; Zou, S.; Chen, M.; Zhu, D.; Gao, S.; Wu, H.; Zhu, L.; Zhu, X. The development of a Glypican-3-specific binding peptide using in vivo and in vitro two-step phage display screening for the PET imaging of hepatocellular carcinoma. Biomater. Sci. 2020, 8, 5656–5665. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, J.; Wang, Y.; Wang, G.; Wang, X.; Zhou, Z.; Liu, G.; Gao, S.; Zhu, L. Identification of a Glypican-3-Binding Peptide for In Vivo Non-Invasive Human Hepatocellular Carcinoma Detection. Macromol. Biosci. 2017, 17, 1600335. [Google Scholar] [CrossRef]
- Berman, R.M.; Kelada, O.J.; Gutsche, N.T.; Natarajan, R.; Swenson, R.E.; Fu, Y.; Hong, J.; Ho, M.; Choyke, P.L.; Escorcia, F.E. In Vitro Performance of Published Glypican 3-Targeting Peptides TJ12P1 and L5 Indicates Lack of Specificity and Potency. Cancer Biother. Radiopharm. 2019, 34, 498–503. [Google Scholar] [CrossRef]
- Pan, S.; Sha, L.; Wu, F.; Wang, N.; Yang, H.; Zhang, X.; Zhou, S.; Hu, C.; Ding, G.; Xu, J.; et al. MMAE-Based Peptide–Drug Conjugates Targeting GPC3 for Precision Chemoradiotherapy in Hepatocellular Carcinoma. J. Med. Chem. 2025, 68, 12950–12968. [Google Scholar] [CrossRef]
- Feng, M.; Gao, W.; Wang, R.; Chen, W.; Man, Y.-G.; Figg, W.D.; Wang, X.W.; Dimitrov, D.S.; Ho, M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, E1083–E1091. [Google Scholar] [CrossRef]
- Singh, G.; Zarschler, K.; Hunoldt, S.; Martínez, I.I.S.; Ruehl, C.L.; Matterna, M.; Bergmann, R.; Máthé, D.; Hegedüs, N.; Bachmann, M.; et al. Versatile Bispidine-Based Bifunctional Chelators for 64CuII-Labelling of Biomolecules. Chem. Eur. J. 2020, 26, 1989–2001. [Google Scholar] [CrossRef]
- Powell, M.F.; Stewart, T.; Otvos, L., Jr.; Urge, L.; Gaeta, F.C.A.; Sette, A.; Arrhenius, T.; Thomson, D.; Soda, K.; Colon, S.M. Peptide Stability in Drug Development. II. Effect of Single Amino Acid Substitution and Glycosylation on Peptide Reactivity in Human Serum. Pharm. Res. 1993, 10, 1268–1273. [Google Scholar] [CrossRef]
- Isaac, R.E.; Bland, N.D.; Shirras, A.D. Neuropeptidases and the metabolic inactivation of insect neuropeptides. Gen. Comp. Endocrinol. 2009, 162, 8–17. [Google Scholar] [CrossRef]
- Lin, F.; Clift, R.; Ehara, T.; Yanagida, H.; Horton, S.; Noncovich, A.; Guest, M.; Kim, D.; Salvador, K.; Richardson, S.; et al. Peptide Binder to Glypican-3 as a Theranostic Agent for Hepatocellular Carcinoma. J. Nucl. Med. 2024, 65, 586–592. [Google Scholar] [CrossRef]
- Poot, A.J.; Lapa, C.; Weber, W.A.; Lam, M.G.; Eiber, M.; Dierks, A.; Bundschuh, R.A.; Braat, A.J. [68Ga]Ga-RAYZ-8009: A Glypican-3–Targeted Diagnostic Radiopharmaceutical for Hepatocellular Carcinoma Molecular Imaging—A First-in-Human Case Series. J. Nucl. Med. 2024, 65, 1597–1603. [Google Scholar] [CrossRef]
- Scholtissek, H.; Reitsam, N.G.; Dierks, A.; Kröncke, T.; Märkl, B.; Trepel, M.; Bundschuh, R.A.; Lapa, C. Noninvasive Characterization of Hepatic Lesions by Means of Glypican-3–Directed PET/CT. J. Nucl. Med. 2025, 66, 1314. [Google Scholar] [CrossRef]
- Kreller, M.; Brühlmann, S.A.; Knieß, T.; Kopka, K.; Walther, M. Production of Medical Radionuclides in the Center for Radiopharmaceutical Tumor Research—A Status Report. Instruments 2024, 8, 10. [Google Scholar] [CrossRef]
- Önell, A.; Andersson, K. Kinetic determinations of molecular interactions using Biacore—Minimum data requirements for efficient experimental design. J. Mol. Recognit. 2005, 18, 307–317. [Google Scholar] [CrossRef]
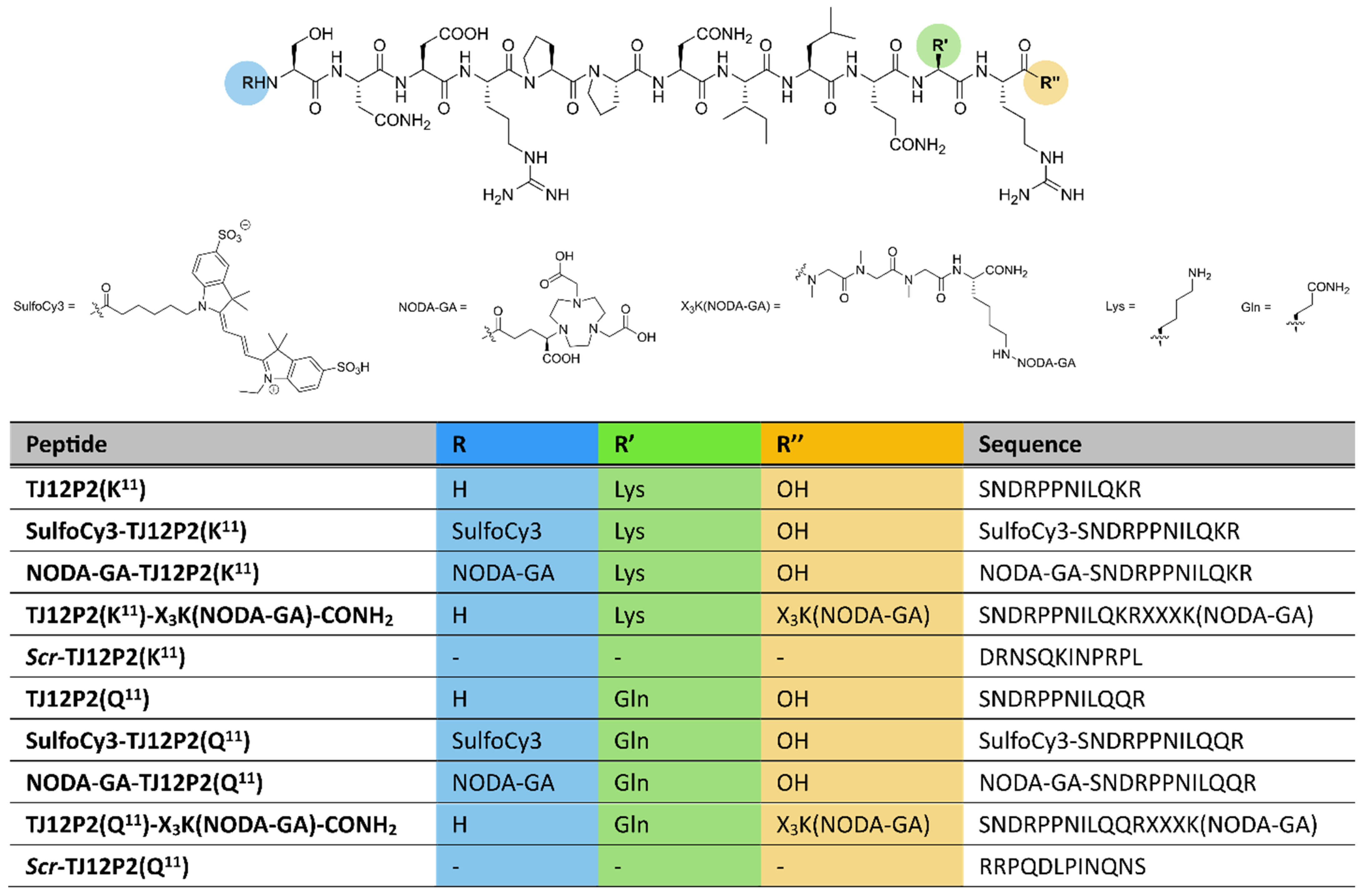


| Peptide | Chem. Formula | m/z calcd. | m/z found | Purity [%] |
|---|---|---|---|---|
| TJ12P2(K11) | C60H104N22O19 | 719.3997 [M+2H]2+ | 719.3994 | ≥99.8 |
| SulfoCy3-TJ12P2(K11) | C91H140N24O26S2 | 684.3355 [M+3H]3+ | 684.3349 | ≥98.2 |
| NODA-GA-TJ12P2(K11) | C75H127N25O26 | 598.9868 [M+3H]3+ | 598.9865 | ≥98.5 |
| TJ12P2(K11)-X3K(NODA-GA)-CONH2 | C90H155N31O29 | 712.3942 [M+3H]3+ | 712.3936 | ≥98.6 |
| Scr-TJ12P2(K11) | C60H104N22O19 | 479.9356 [M+3H]3+ | 479.9354 | ≥99.0 |
| TJ12P2(Q11) | C59H100N22O20 | 719.3815 [M+2H]2+ | 719.3813 | ≥99.1 |
| SulfoCy3-TJ12P2(Q11) | C90H136N24O27S2 | 684.3233 [M+3H]3+ | 684.3229 | ≥99.8 |
| NODA-GA-TJ12P2(Q11) | C74H123N25O27 | 598.9746 [M+3H]3+ | 598.9744 | ≥96.4 |
| TJ12P2(Q11)-X3K(NODA-GA)-CONH2 | C89H151N31O30 | 712.7165 [M+3H]3+ | 712.7159 | ≥98.5 |
| Scr-TJ12P2(Q11) | C59H100N22O20 | 719.3815 [M+2H]2+ | 719.3812 | ≥99.0 |
| Peptide | RCC [%] | AM [MBq/nmol] | LogD7.4 |
|---|---|---|---|
| [67Ga]Ga-NODA-GA-TJ12P2(K11) | 98.8 ± 0.6 (n = 3) | 5.5 ± 0.5 (n = 3) | −4.78 ± 0.05 (n = 4) |
| TJ12P2(K11)-X3K-[67Ga]Ga-NODA-GA-CONH2 | 99.3 ± 0.6 (n = 3) | 5.9 ± 0.8 (n = 3) | −4.79 ± 0.03 (n = 4) |
| [67Ga]Ga-NODA-GA-TJ12P2(Q11) | 99.7 | 5.13 | −4.79 ± 0.03 (n = 4) |
| TJ12P2(Q11)-X3K-[67Ga]Ga-NODA-GA-CONH2 | 99.7 | 3.30 | −4.61 ± 0.05 (n = 4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, E.-M.; Hill, C.; Wodtke, R.; Zarschler, K.; Laube, M.; Donat, C.K.; Hauser, S.; Kopka, K.; Pietzsch, J.; Stadlbauer, S. In Vitro Characterization of the Published Glypican-3-Targeting Peptide TJ12P2 Reveals a Lack of Specificity and Potency. Pharmaceuticals 2025, 18, 1656. https://doi.org/10.3390/ph18111656
Burger E-M, Hill C, Wodtke R, Zarschler K, Laube M, Donat CK, Hauser S, Kopka K, Pietzsch J, Stadlbauer S. In Vitro Characterization of the Published Glypican-3-Targeting Peptide TJ12P2 Reveals a Lack of Specificity and Potency. Pharmaceuticals. 2025; 18(11):1656. https://doi.org/10.3390/ph18111656
Chicago/Turabian StyleBurger, Eva-Maria, Charlice Hill, Robert Wodtke, Kristof Zarschler, Markus Laube, Cornelius K. Donat, Sandra Hauser, Klaus Kopka, Jens Pietzsch, and Sven Stadlbauer. 2025. "In Vitro Characterization of the Published Glypican-3-Targeting Peptide TJ12P2 Reveals a Lack of Specificity and Potency" Pharmaceuticals 18, no. 11: 1656. https://doi.org/10.3390/ph18111656
APA StyleBurger, E.-M., Hill, C., Wodtke, R., Zarschler, K., Laube, M., Donat, C. K., Hauser, S., Kopka, K., Pietzsch, J., & Stadlbauer, S. (2025). In Vitro Characterization of the Published Glypican-3-Targeting Peptide TJ12P2 Reveals a Lack of Specificity and Potency. Pharmaceuticals, 18(11), 1656. https://doi.org/10.3390/ph18111656








