Photothermal Therapy Combined with Chemotherapy and Anti-Inflammation Therapy Weakens the Immunosuppression of Cervical Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of DDP@ZIF-8@PDA and DDP-ADL@ZIF-8@PDA
2.2. Photothermal Effect of DDP-ADL@ZIF-8@PDA
2.3. In Vitro Dual-Stimulation Responsive Release of DDP-ADL@ZIF-8@PDA
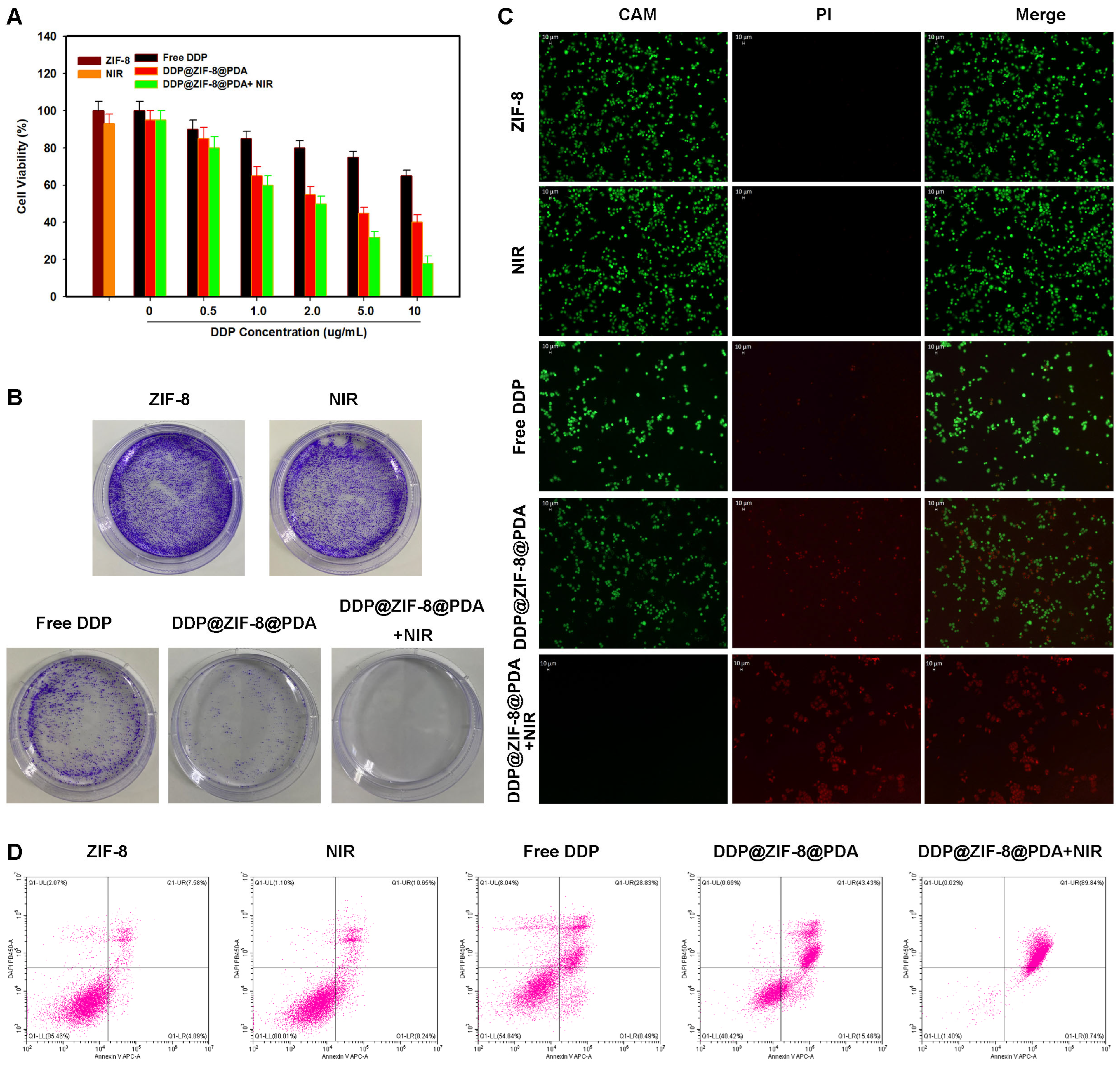
2.4. In Vitro Combination of Chemo-PTT
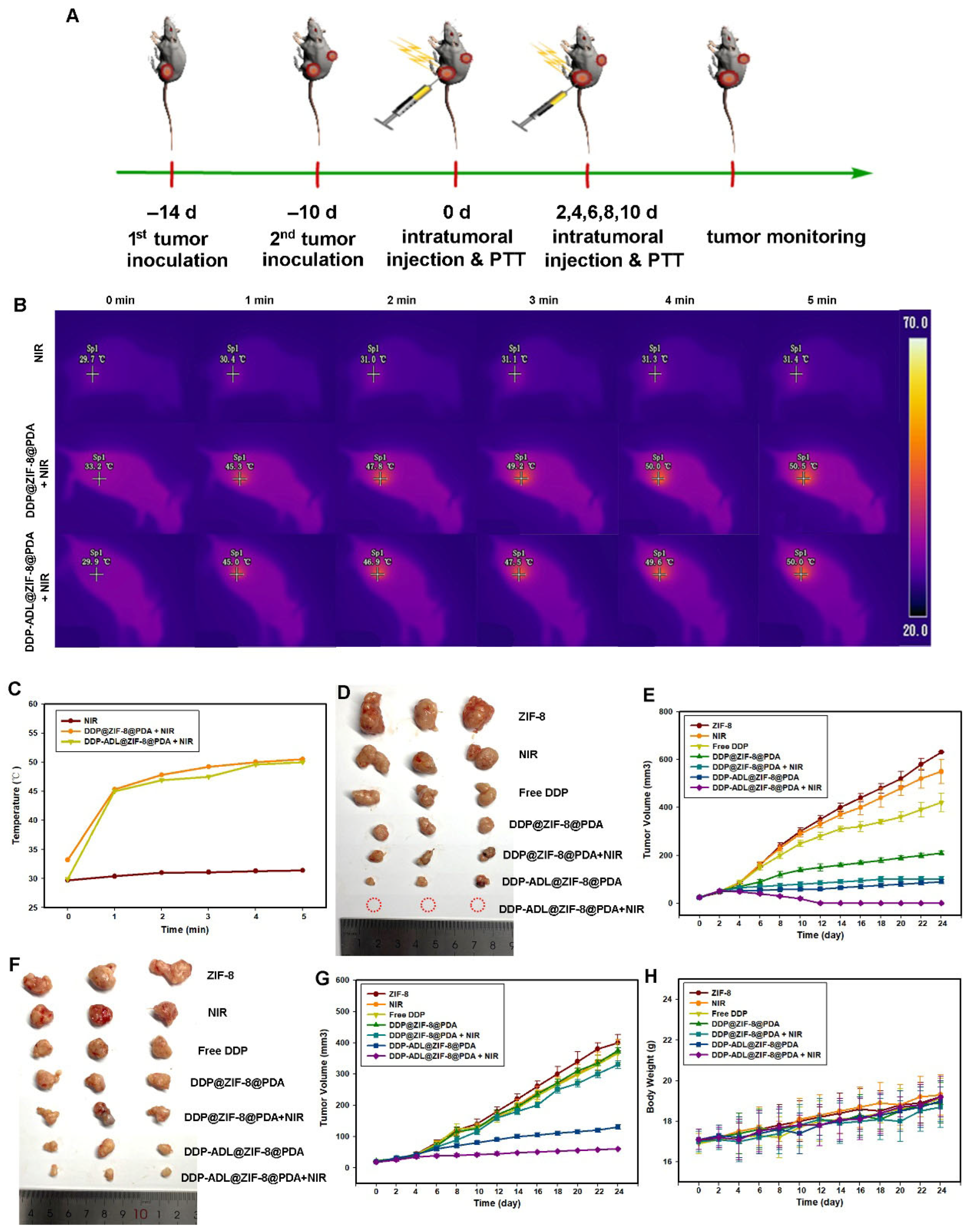
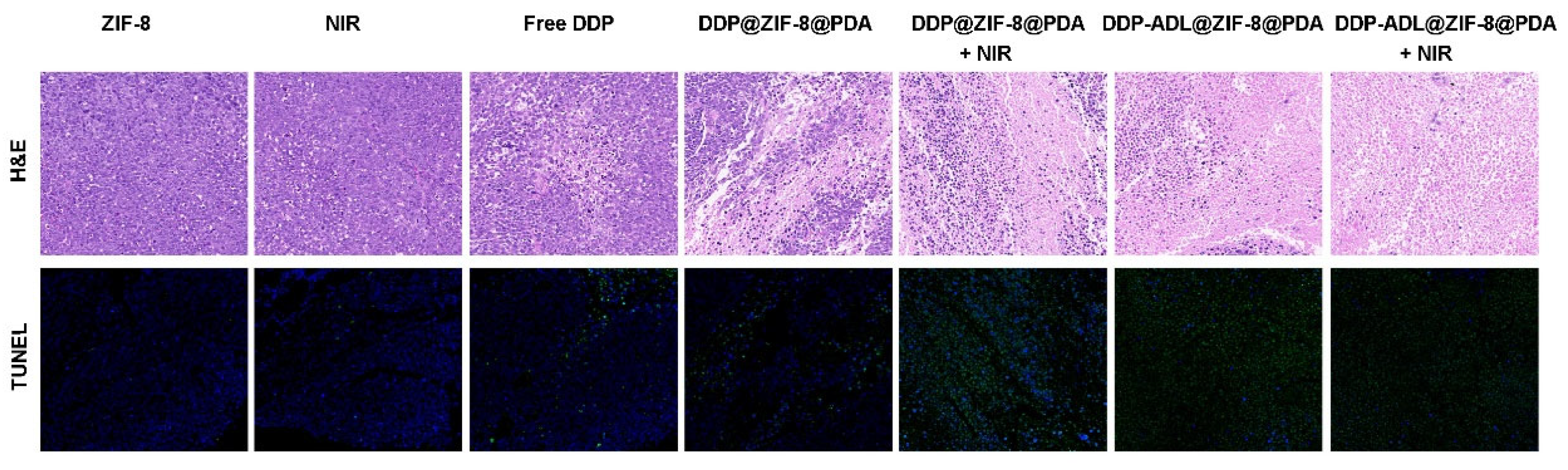
2.5. In Vivo Combination of Chemo–Anti-Inflammatory–PTT
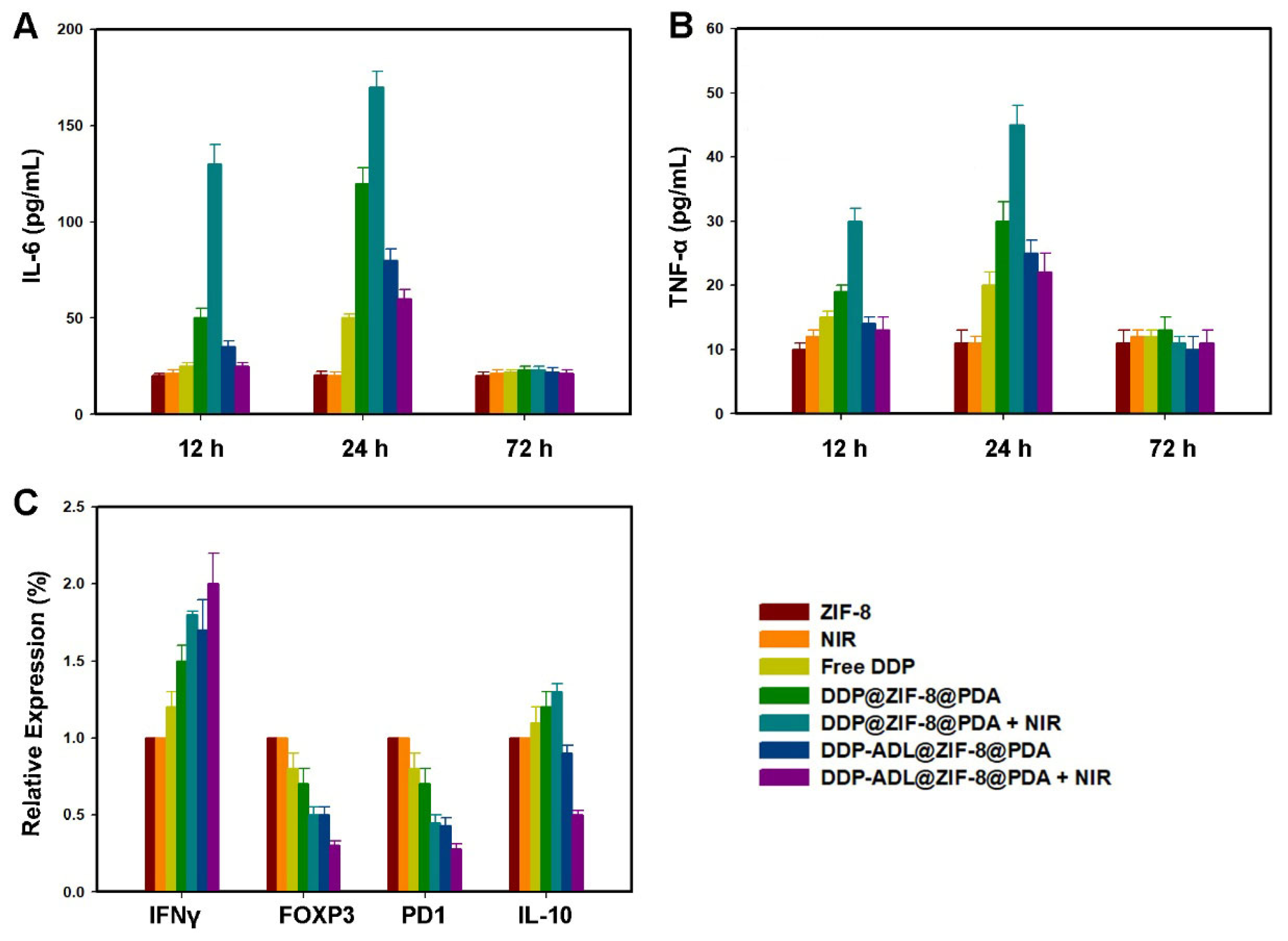
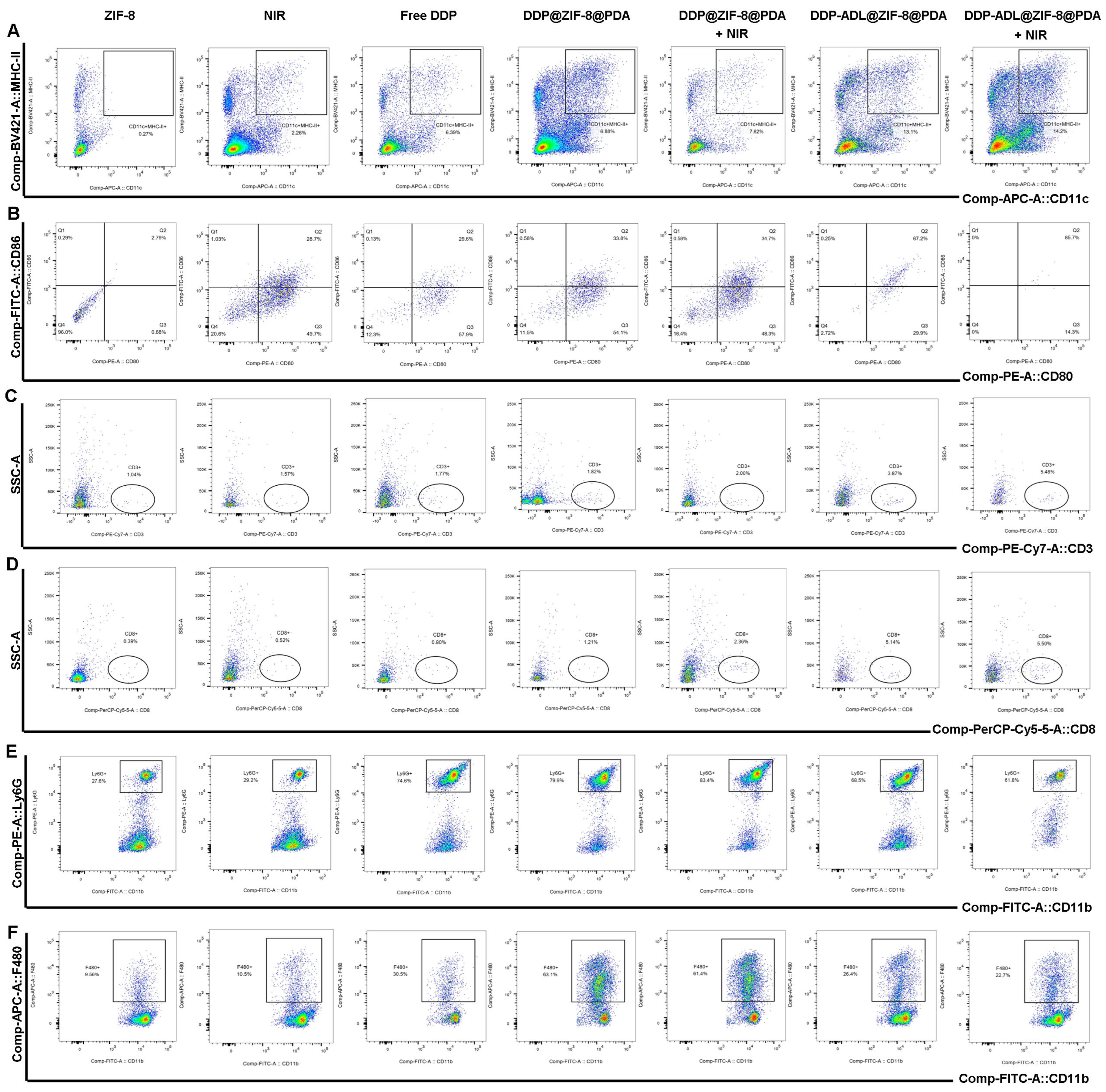
2.6. The Enhanced Antitumor Mechanism of Chemo–Anti-Inflammatory–PTT
2.7. Biosafety Assessment
3. Materials and Methods
3.1. Materials
3.2. Synthesis of DDP@ZIF-8@PDA and DDP-ADL@ZIF-8@PDA Nanoparticles
3.3. Characterization
3.4. Measurement of Photothermal Performance
3.5. In Vitro Drug Release
3.6. In Vitro Biodegradation Evaluation of DDP-ADL@ZIF-8@PDA
3.7. In Vitro PA Imaging
3.8. In Vitro Cytotoxicity Assay of Nanoparticles
3.9. In Vitro Cellular Uptake of DDP-ADL@ZIF-8@PDA Nanoparticles
3.10. In Vitro Chemo–Anti-Inflammatory–PTT Synergistic Effect
3.11. Live/Dead Assay
3.12. Animal and Tumor Models
3.13. Detection of Cytokines
3.14. Detection of Antibody Expression in Distal Tumors
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahasrabuddhe, V.V. Cervical Cancer: Precursors and Prevention. Hematol. Oncol. Clin. N. Am. 2024, 38, 771–781. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines(R) Insights: Cervical Cancer, Version 1. 2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1224–1233. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Vlajkovic, S.M. Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions. Int. J. Mol. Sci. 2023, 24, 16545. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020, 10, 37600–37620. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, C.; Zhu, L.; Yu, C.; Lu, B.; Wang, Y.; Ding, Y.; Dong, C.M.; Yao, Y. Polydopamine-drug conjugate nanocomposites based on ZIF-8 for targeted cancer photothermal-chemotherapy. J. Biomed. Mater. Res. A 2022, 110, 954–963. [Google Scholar] [CrossRef]
- Shang, T.; Yu, X.; Han, S.; Yang, B. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater. Sci. 2020, 8, 5241–5259. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Chen, S.; Ahmadi, S.; Veedu, R.N. Aptamer-engineered (nano)materials for theranostic applications. Theranostics 2023, 13, 5183–5206. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Zou, W.; Miao, Y.; Zhu, Y.; Cao, M.; Yu, B.; Cong, H.; Shen, Y. Donor-acceptor-donor small molecules for fluorescence/photoacoustic imaging and integrated photothermal therapy. Acta Biomater. 2023, 164, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hong, C.; Shen, C.; Xie, D.; Chen, T.; Wu, A.; Li, Q. Polydopamine nanomaterials and their potential applications in the treatment of autoimmune diseases. Drug Deliv. 2023, 30, 2289846. [Google Scholar] [CrossRef]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-Based Nanoparticles for Photothermal Therapy/Chemotherapy and their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, K.; Wu, Y.; Yue, Y.; Cheng, K.; Feng, Q.; Ma, X.; Liang, J.; Ma, N.; Liu, G.; et al. Antigen Capture and Immune Modulation by Bacterial Outer Membrane Vesicles as In Situ Vaccine for Cancer Immunotherapy Post-Photothermal Therapy. Small 2022, 18, e2107461. [Google Scholar] [CrossRef] [PubMed]
- Heras-Murillo, I.; Adán-Barrientos, I.; Galán, M.; Wculek, S.K.; Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 257–277. [Google Scholar] [CrossRef]
- Mitlehner, W. Non-Steroidal Anti-Inflammatory Drugs. Dtsch. Arztebl. Int. 2022, 119, 566. [Google Scholar]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Garcia-Quiroz, J.; Vázquez-Almazán, B.; García-Becerra, R.; Díaz, L.; Avila, E. The Interaction of Human Papillomavirus Infection and Prostaglandin E(2) Signaling in Carcinogenesis: A Focus on Cervical Cancer Therapeutics. Cells 2022, 11, 2528. [Google Scholar] [CrossRef]
- Lu, W.; Liu, W.; Hu, A.; Shen, J.; Yi, H.; Cheng, Z. Combinatorial Polydopamine-Liposome Nanoformulation as an Effective Anti-Breast Cancer Therapy. Int. J. Nanomed. 2023, 18, 861–879. [Google Scholar] [CrossRef]
- Ma, X.; Cui, Y.; Zhang, M.; Lyu, Q.; Zhao, J. A Multifunctional Nanodrug Co-Delivering VEGF-siRNA and Dexamethasone for Synergistic Therapy in Ocular Neovascular Diseases. Int. J. Nanomed. 2024, 19, 12369–12387. [Google Scholar] [CrossRef]
- Zaman, M.S.; Chauhan, N.; Yallapu, M.M.; Gara, R.K.; Maher, D.M.; Kumari, S.; Sikander, M.; Khan, S.; Zafar, N.; Jaggi, M.; et al. Curcumin Nanoformulation for Cervical Cancer Treatment. Sci Rep. 2016, 6, 20051. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Hu, M.; Dong, M.; Yang, Z.; Zhan, X.; Chang, X.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-gamma: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Shiri, A.M.; Zhang, T.; Bedke, T.; Zazara, D.E.; Zhao, L.; Lücke, J.; Sabihi, M.; Fazio, A.; Zhang, S.; Tauriello, D.V.; et al. IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction. J. Hepatol. 2024, 80, 634–644. [Google Scholar] [CrossRef]
- Natalini, A.; Simonetti, S.; Favaretto, G.; Peruzzi, G.; Antonangeli, F.; Santoni, A.; Muñoz-Ruiz, M.; Hayday, A.; Di Rosa, F. OMIP-079: Cell cycle of CD4(+) and CD8(+) naive/memory T cell subsets, and of Treg cells from mouse spleen. Cytometry A 2021, 99, 1171–1175. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Qin, Q.; Chao, N.; Huang, T.; Chen, C.; Lu, X.; Huang, R.; Pan, J. Dendritic cells modified by tumor associated antigen SMP30 have enhanced antitumor effect against mouse hepatocarcinoma cells in vitro and in vivo. Am. J. Transl. Res. 2022, 14, 5785–5799. [Google Scholar]
- Kawakubo, A.; Uchida, K.; Miyagi, M.; Nakawaki, M.; Satoh, M.; Sekiguchi, H.; Yokozeki, Y.; Inoue, G.; Takaso, M. Investigation of resident and recruited macrophages following disc injury in mice. J. Orthop. Res. 2020, 38, 1703–1709. [Google Scholar] [CrossRef]
- Vafadarnejad, E.; Rizzo, G.; Krampert, L.; Arampatzi, P.; Arias-Loza, A.-P.; Nazzal, Y.; Rizakou, A.; Knochenhauer, T.; Bandi, S.R.; Nugroho, V.A.; et al. Dynamics of Cardiac Neutrophil Diversity in Murine Myocardial Infarction. Circ. Res. 2020, 127, e232–e249. [Google Scholar] [CrossRef] [PubMed]
- Carai, P.; González, L.F.; Van Bruggen, S.; Spalart, V.; De Giorgio, D.; Geuens, N.; Martinod, K.; Jones, E.A.V.; Heymans, S. Neutrophil inhibition improves acute inflammation in a murine model of viral myocarditis. Cardiovasc. Res. 2023, 118, 3331–3345. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Fu, J.; Xu, Y.; Li, D.; Ren, H. Photothermal Therapy Combined with Chemotherapy and Anti-Inflammation Therapy Weakens the Immunosuppression of Cervical Cancer. Pharmaceuticals 2025, 18, 1657. https://doi.org/10.3390/ph18111657
Yang X, Fu J, Xu Y, Li D, Ren H. Photothermal Therapy Combined with Chemotherapy and Anti-Inflammation Therapy Weakens the Immunosuppression of Cervical Cancer. Pharmaceuticals. 2025; 18(11):1657. https://doi.org/10.3390/ph18111657
Chicago/Turabian StyleYang, Xiaojing, Jie Fu, Yi Xu, Dejian Li, and Hanru Ren. 2025. "Photothermal Therapy Combined with Chemotherapy and Anti-Inflammation Therapy Weakens the Immunosuppression of Cervical Cancer" Pharmaceuticals 18, no. 11: 1657. https://doi.org/10.3390/ph18111657
APA StyleYang, X., Fu, J., Xu, Y., Li, D., & Ren, H. (2025). Photothermal Therapy Combined with Chemotherapy and Anti-Inflammation Therapy Weakens the Immunosuppression of Cervical Cancer. Pharmaceuticals, 18(11), 1657. https://doi.org/10.3390/ph18111657






