Valorization of Olive Leaf Extract via Tailored Liposomal Carriers: Comparative Analysis of Physicochemical Features, Antioxidant Capacity, and Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. Polyphenol Profile of Olive Extract and Extract-Loaded Liposomes and Encapsulation Efficiency
2.2. FT-IR Spectra of Olive Leaf Extract-Loaded Liposomes
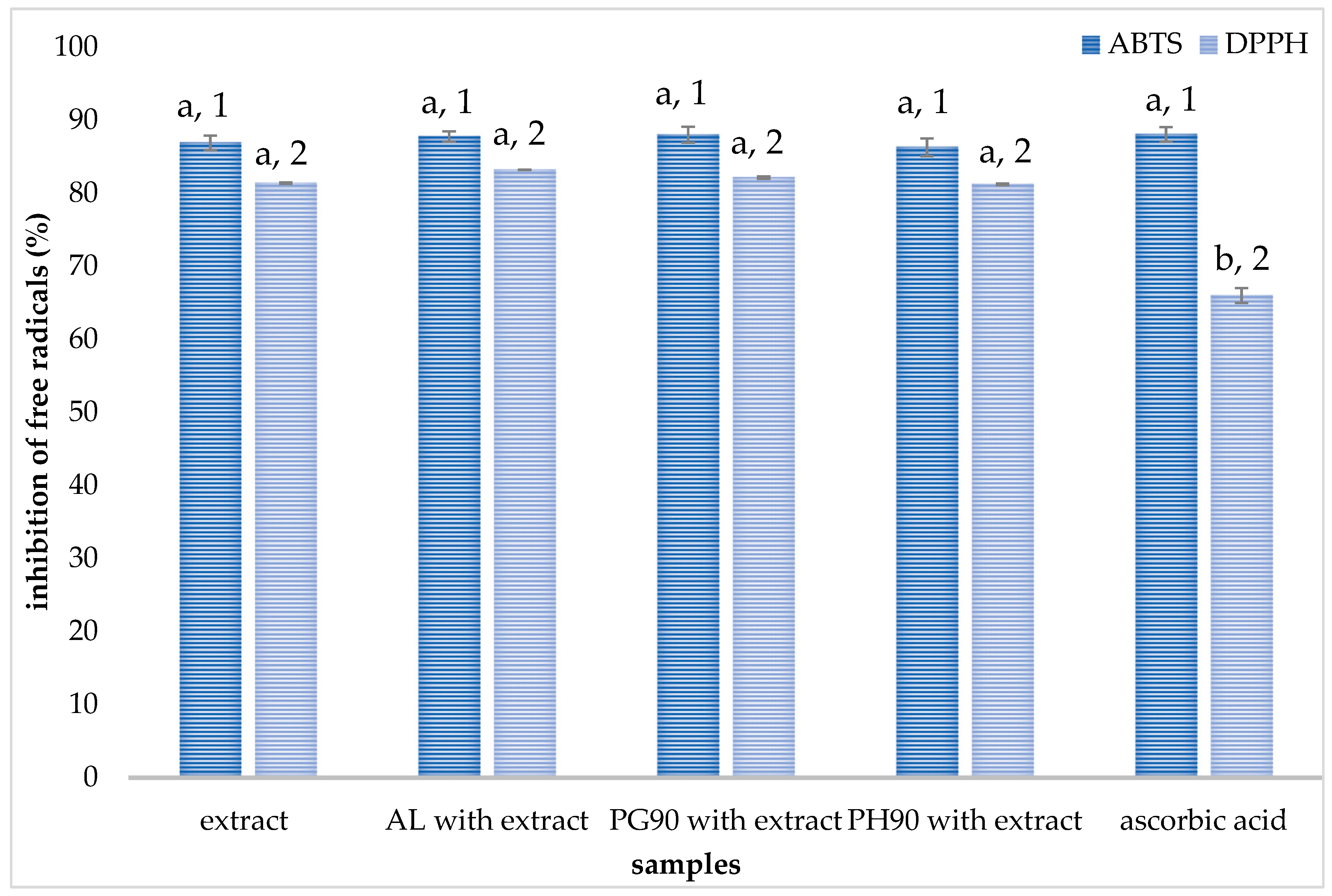
2.3. Antioxidant Capacity of Liposomes
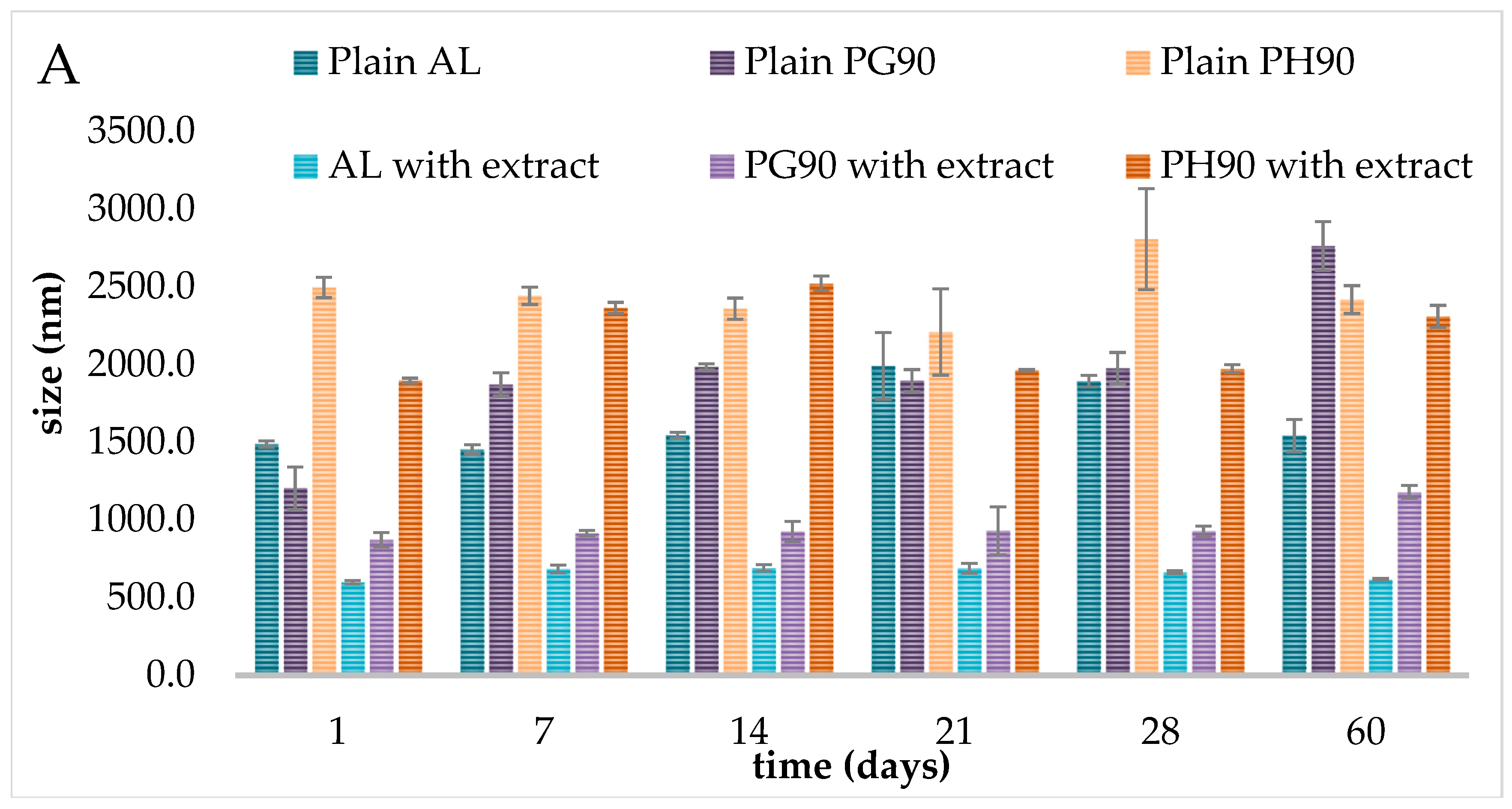
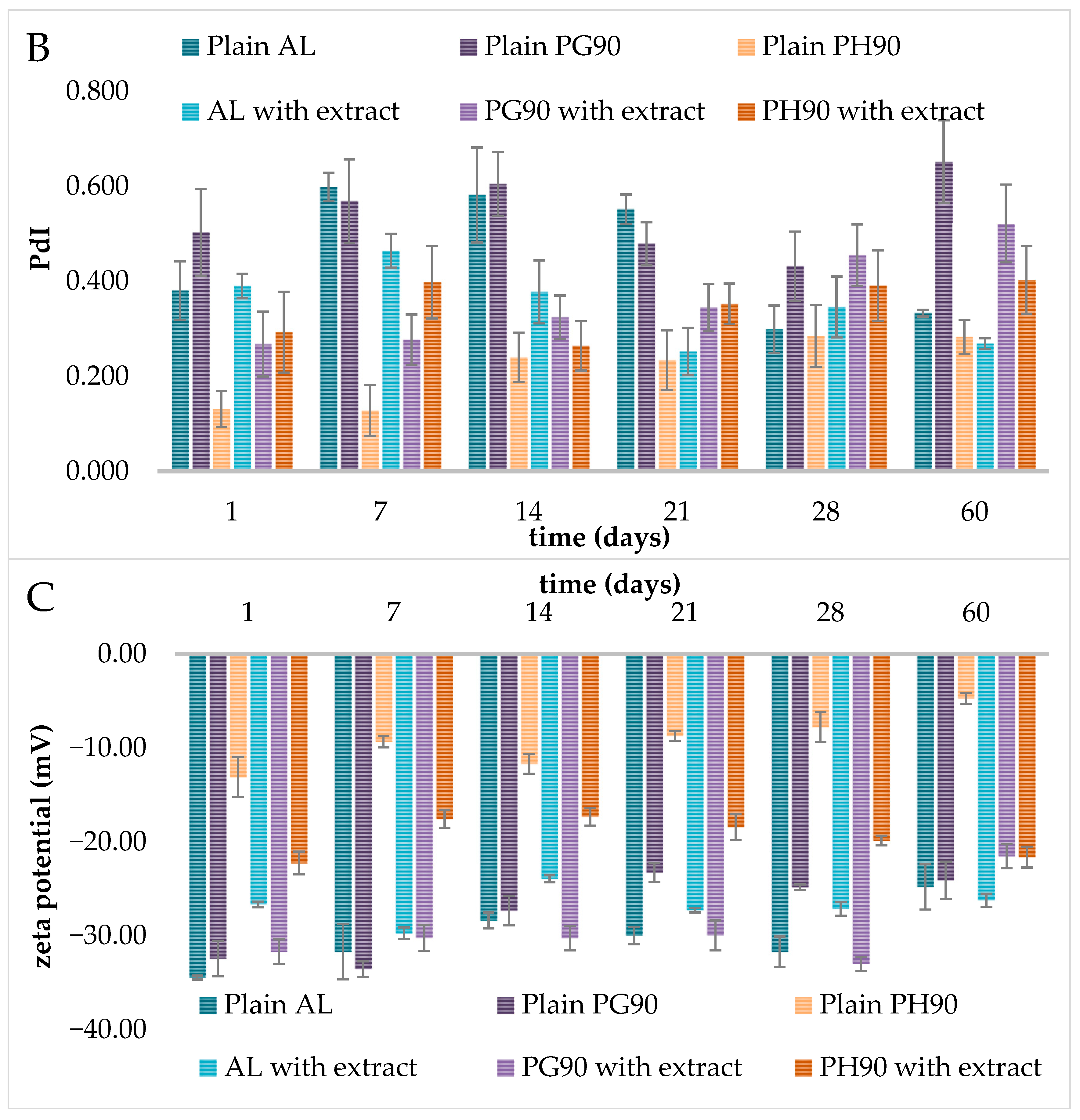
2.4. Stability of Liposomes During Storage
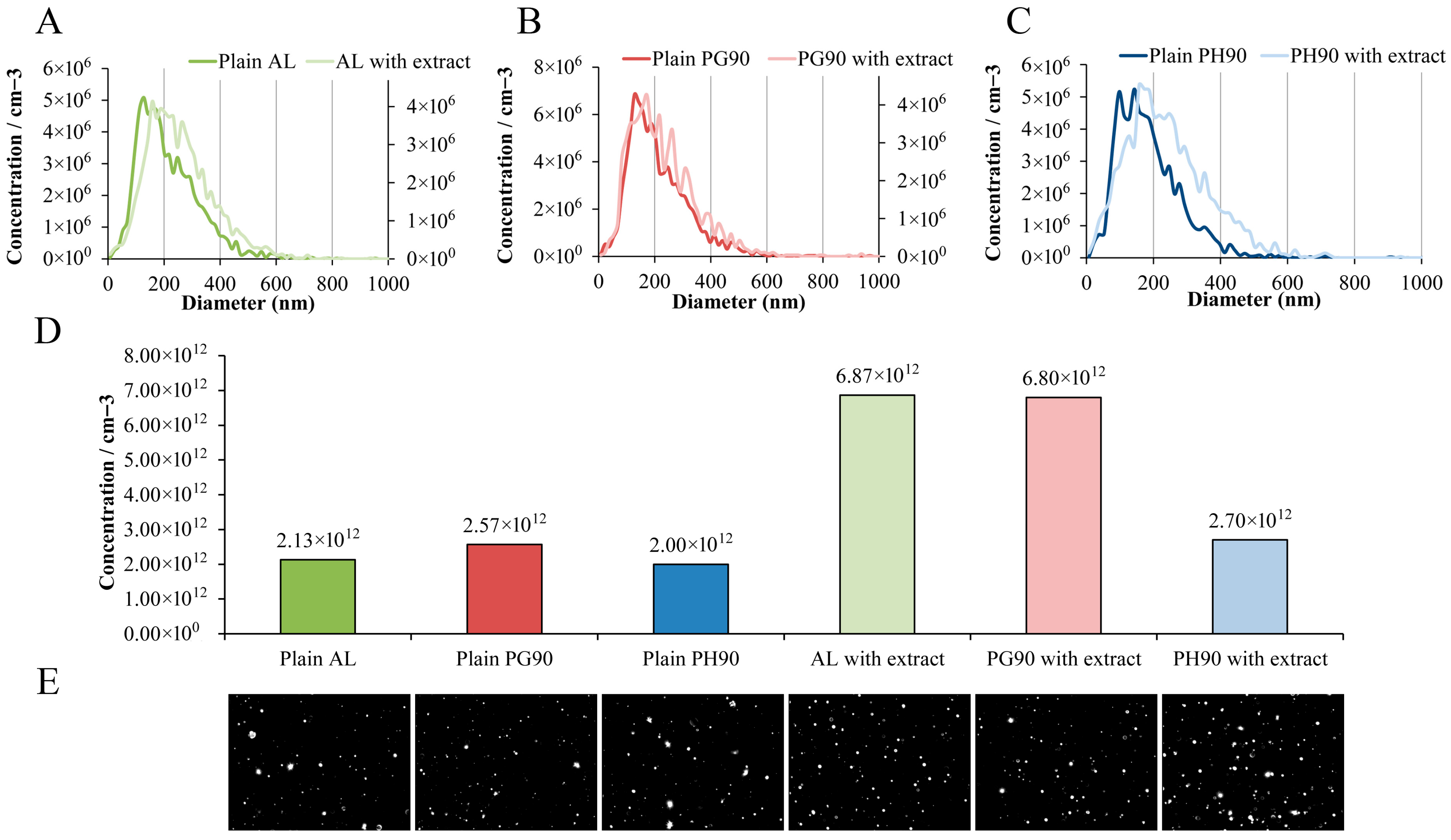
2.5. Nanoparticle Tracking Analysis of Developed Liposomes
2.6. Transmission Electron Microscopy of Olive Leaf Extract-Loaded Liposomes
2.7. Rheological Characteristics of Developed Liposomes
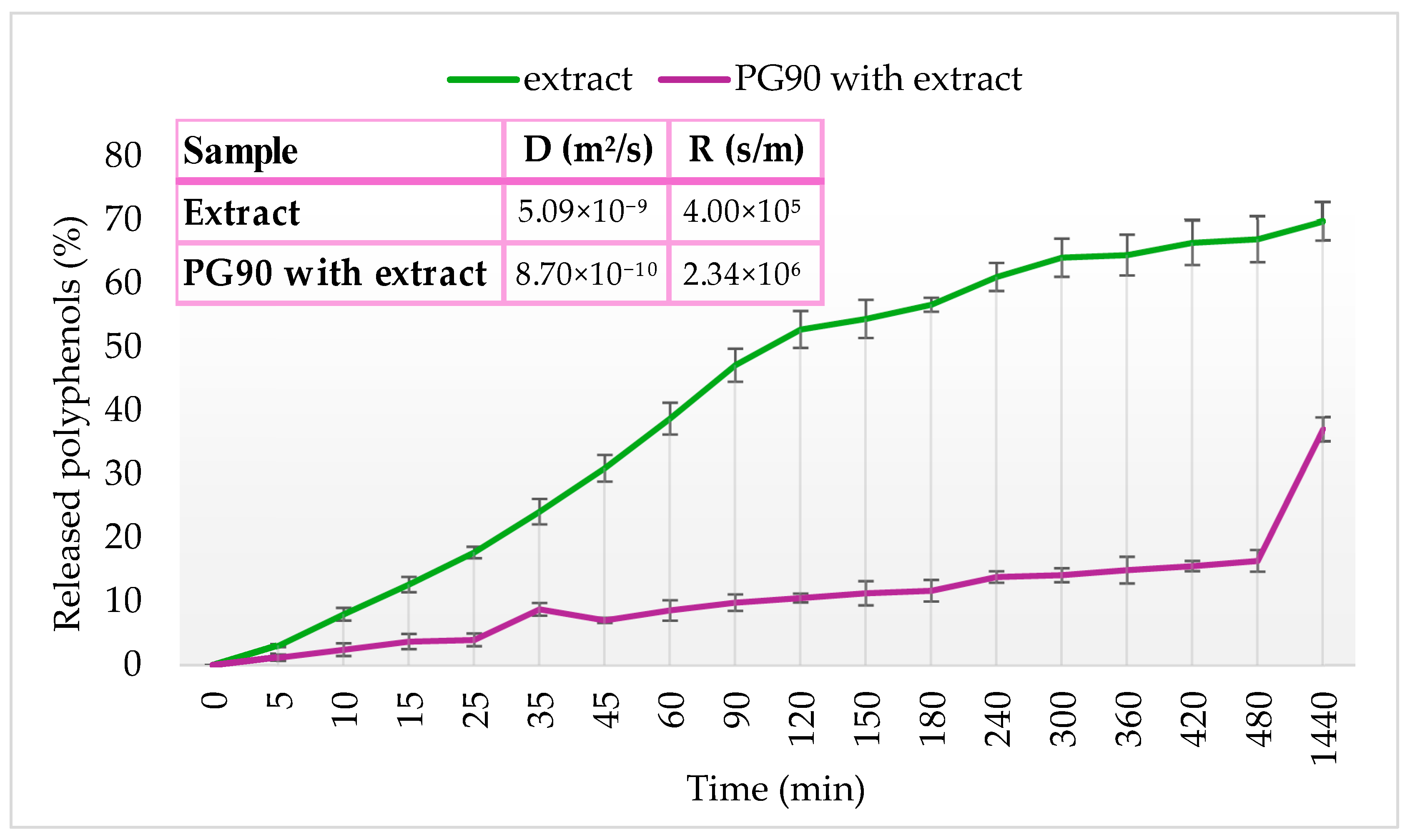
2.8. Release of Olive Leaf Extract Polyphenols Under Simulated Physiological Conditions
3. Materials and Methods
3.1. Chemicals
3.2. Olive Leaf Extract Preparation
3.3. Olive Leaf Extract-Loaded Liposome Preparation
3.4. HPLC and GC-MS Chemical Composition Analysis and Encapsulation Efficiency
3.5. Fourier Transform Infrared Spectroscopy
3.6. Evaluation of the Antioxidant Activity of Liposomes
3.6.1. ABTS Radical Scavenging Assay
3.6.2. DPPH Radical Scavenging Assay
3.7. Monitoring of Liposome Stability
3.8. Nanoparticle Tracking Analysis
3.9. Transmission Electron Microscopy
3.10. Examination of Rheological Characteristics
3.11. In Vitro Release Kinetics Using Franz Diffusion Cell
3.12. Statistical Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rugini, E.; De Pace, C.; Gutiérrez-Pesce, P.; Muleo, R. Olea. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 79–117. [Google Scholar]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. 2015, 2015, 1–29. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, A. Role of Ethnomedicinal Resources to Cure Metabolic Diseases. In Traditional Resources and Tools for Modern Drug Discovery; Anupam, D.T., Ed.; Springer: Singapore, 2024; pp. 129–182. [Google Scholar]
- Alesci, A.; Miller, A.; Tardugno, R.; Pergolizzi, S. Chemical Analysis, Biological and Therapeutic Activities of Olea europaea L. Extracts. Nat. Prod. Res. 2022, 36, 2932–2945. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Papoti, V.T. Bioactive Ingredients in Olive Leaves. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 349–356. [Google Scholar]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.d.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the Usage of Olive Leaves, Bioactive Compounds, Biological Activities, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- Migone, C.; Cerri, L.; Ferreira, B.; Fabiano, A.; Parri, S.; Mezzetta, A.; Guazzelli, L.; Piras, A.M.; Zambito, Y.; Sarmento, B. Olive Leaf Extract-Based Eye Drop Formulations for Corneal Wound Healing. J. Drug Deliv. Sci. Technol. 2025, 110, 107026. [Google Scholar] [CrossRef]
- Panou, A.A.; Karabagias, I.K. Olive Leaf Extracts as a Medicinal Beverage: Origin, Physico-Chemical Properties, and Bio-Functionality. Beverages 2025, 11, 66. [Google Scholar] [CrossRef]
- Aggul, A.G.; Gulaboglu, M.; Cetin, M.; Ozakar, E.; Ozakar, R.S.; Aydin, T. Effects of Emulsion Formulations of Oleuropein Isolated from Ethanol Extract of Olive Leaf in Diabetic Rats. An. Acad. Bras. Cienc. 2020, 92, e20190810. [Google Scholar] [CrossRef]
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors Affecting the Contents of Iridoid Oleuropein in Olive Leaves (Olea europaea L.). J. Agric. Food Chem. 2006, 54, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J. Antioxidant Activity of Phenolics Extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A Natural Antioxidant Molecule in the Treatment of Metabolic Syndrome. Phyther. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Qabaha, K.; AL-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Noori, A.; Dehnoo, M.G.; Bagheri, S.; Cheraghi, R.A. Hypoglycemic, Hypolipidemic and Antiatherogenic Effects of Oleuropein in Alloxan-Induced Type 1 Diabetic Rats. Asian Pacific J. Trop. Dis. 2014, 4, S421–S425. [Google Scholar] [CrossRef]
- Topuz, S.; Bayram, M. Oleuropein Extraction from Leaves of Three Olive Varieties (Olea europaea L.): Antioxidant and Antimicrobial Properties of Purified Oleuropein and Oleuropein Extracts. J. Food Process. Preserv. 2022, 46. [Google Scholar] [CrossRef]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative Metabolic Profiling of Olive Leaf Extracts from Twelve Different Cultivars Collected in Both Fruiting and Flowering Seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological Activities of Natural Triterpenoids and Their Therapeutic Implications. Nat. Prod. Rep. 2006, 23, 394. [Google Scholar] [CrossRef] [PubMed]
- Kuo, R.-Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. Plant-Derived Triterpenoids and Analogues as Antitumor and Anti-HIV Agents. Nat. Prod. Rep. 2009, 26, 1321. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.J.; Režek Jambrak, A.; Lorenzo, J.M.; Barba, F.J. From Extraction of Valuable Compounds to Health Promoting Benefits of Olive Leaves through Bioaccessibility, Bioavailability and Impact on Gut Microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Batarfi, W.A.; Yunus, M.H.M.; Hamid, A.A.; Lee, Y.T.; Maarof, M. Hydroxytyrosol: A Promising Therapeutic Agent for Mitigating Inflammation and Apoptosis. Pharmaceutics 2024, 16, 1504. [Google Scholar] [CrossRef]
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, Distribution, Metabolism, and Excretion of the Main Olive Tree Phenols and Polyphenols: A Literature Review. J. Agric. Food Chem. 2021, 69, 5281–5296. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, M.C.; De Stefani, C.; Vasarri, M.; Ivanova Stojcheva, E.; Ramos-Pineda, A.M.; Baldi, F.; Bilia, A.R.; Degl’Innocenti, D. Encapsulation of Olive Leaf Polyphenol-Rich Extract in Polymeric Micelles to Improve Its Intestinal Permeability. Nanomaterials 2023, 13, 3147. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Qiao, K.; Zhao, M.; Huang, Y.; Liang, L.; Zhang, Y. Bitter Perception and Effects of Foods Rich in Bitter Compounds on Human Health: A Comprehensive Review. Foods 2024, 13, 3747. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.; Petrović, P.M.; Volić, M.; Micić, D.; Živković, J.; Šavikin, K.P. Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior. Plants 2024, 13, 2608. [Google Scholar] [CrossRef]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Jovanović, A.; Grdović, N.; Rajić, J.; Đorđević, M.; Sarić, A.; Bugarski, B.; Vidaković, M.; et al. Liposome Encapsulation Enhances the Antidiabetic Efficacy of Silibinin. Pharmaceutics 2024, 16, 801. [Google Scholar] [CrossRef]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef]
- Paramshetti, S.; Angolkar, M.; Talath, S.; Osmani, R.A.M.; Spandana, A.; Al Fatease, A.; Hani, U.; Ramesh, K.V.R.N.S.; Singh, E. Unravelling the in Vivo Dynamics of Liposomes: Insights into Biodistribution and Cellular Membrane Interactions. Life Sci. 2024, 346, 122616. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.; Volić, M.; Pećinar, I.; Živković, J.; Šavikin, K.P. Rosehip Extract-Loaded Liposomes for Potential Skin Application: Physicochemical Properties of Non- and UV-Irradiated Liposomes. Plants 2023, 12, 3063. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, C.; Yuan, Y.; Zhu, Y.; Wan, J.; Firempong, C.K.; Omari-Siaw, E.; Xu, Y.; Pu, Z.; Yu, J.; et al. Enhanced Oral Bioavailability and in Vivo Antioxidant Activity of Chlorogenic Acid via Liposomal Formulation. Int. J. Pharm. 2016, 501, 342–349. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic Compounds as Antidiabetic, Anti-inflammatory, and Anticancer Agents and Improvement of Their Bioavailability by Liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Camilleri, D.; Attard, K.; Lia, F. Formulation and Evaluation of Liposome-Encapsulated Phenolic Compounds from Olive Mill Waste: Insights into Encapsulation Efficiency, Antioxidant, and Cytotoxic Activities. Molecules 2025, 30, 2351. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical Characterization of Liposomes Containing Nutraceutical Compounds: Tyrosol, Hydroxytyrosol and Oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Prevete, G.; Donati, E.; Ruggiero, A.P.; Fardellotti, S.; Lilla, L.; Ramundi, V.; Nicoletti, I.; Mariani, F.; Mazzonna, M. Encapsulation of Olea europaea Leaf Polyphenols in Liposomes: A Study on Their Antimicrobial Activity to Turn a Byproduct into a Tool to Treat Bacterial Infection. ACS Appl. Mater. Interfaces 2024, 16, 68850–68863. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of Physicochemical and Antioxidant Properties of Yogurt Enriched by Olive Leaf Phenolics within Nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Ghasemi, S.; Koohi, D.E.; Emmamzadehhashemi, M.S.B.; Khamas, S.S.; Moazen, M.; Hashemi, A.K.; Amin, G.; Golfakhrabadi, F.; Yousefi, Z.; Yousefbeyk, F. Investigation of Phenolic Compounds and Antioxidant Activity of Leaves Extracts from Seventeen Cultivars of Iranian Olive (Olea europaea L.). J. Food Sci. Technol. 2018, 55, 4600–4607. [Google Scholar] [CrossRef]
- Aouidi, F.; Dupuy, N.; Artaud, J.; Roussos, S.; Msallem, M.; Perraud Gaime, I.; Hamdi, M. Rapid Quantitative Determination of Oleuropein in Olive Leaves (Olea europaea) Using Mid-Infrared Spectroscopy Combined with Chemometric Analyses. Ind. Crops Prod. 2012, 37, 292–297. [Google Scholar] [CrossRef]
- Tarchi, I.; Olewnik-Kruszkowska, E.; Aït-Kaddour, A.; Bouaziz, M. Innovative Process for the Recovery of Oleuropein-Rich Extract from Olive Leaves and Its Biological Activities: Encapsulation for Activity Preservation with Concentration Assessment Pre and Post Encapsulation. ACS Omega 2025, 10, 6135–6146. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef]
- González-Ortega, R.; Šturm, L.; Skrt, M.; Di Mattia, C.D.; Pittia, P.; Poklar Ulrih, N. Liposomal Encapsulation of Oleuropein and an Olive Leaf Extract: Molecular Interactions, Antioxidant Effects and Applications in Model Food Systems. Food Biophys. 2021, 16, 84–97. [Google Scholar] [CrossRef]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Tanuja; Ain, M.R.; Ahmad, I.; Shahzad, N. Surface Functionalized Folate Targeted Oleuropein Nano-Liposomes for Prostate Tumor Targeting: In Vitro and in Vivo Activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, M.; Faramarzi, B.; Lepore, M. Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy. Biophysica 2023, 3, 524–538. [Google Scholar] [CrossRef]
- Chia, N.C.; Mendelsohn, R. Conformational disorder in unsaturated phospholipids by FTIR spectroscopy. Biochim. Biophys. Acta. 1996, 1283, 141–150. [Google Scholar] [CrossRef]
- Kechagias, A.; Leontiou, A.A.; Vardakas, A.; Stathopoulos, P.; Xenaki, M.; Stathopoulou, P.; Proestos, C.; Giannelis, E.P.; Chalmpes, N.; Salmas, C.E.; et al. Antioxidant Nanohybrid Materials Derived via Olive Leaf Extract Incorporation in Layered Double Hydroxide: Preparation, Characterization, and Evaluation for Applications. Antioxidants 2025, 14, 1010. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Petrovich, D.S.; Ilinichna, K.T.; Morton, D.W. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules 2021, 26, 6892. [Google Scholar] [CrossRef]
- Scarpi-Luttenauer, M.; Boubegtiten-Fezoua, Z.; Hellwig, P.; Chaumont, A.; Vincent, B.; Barloy, L.; Mobian, P. Spectroscopic Evidence of the Interaction of Titanium(IV) Coordination Complexes with a Phosphate Head Group in Phospholipids. Dalton Trans. 2025, 54, 4556–4565. [Google Scholar] [CrossRef]
- Raederstorff, D. Antioxidant activity of olive polyphenols in humans: A review. Int. J. Vitam. Nutr. Res. 2009, 79, 152–165. [Google Scholar] [CrossRef]
- Lins, P.G.; Pugine, S.M.P.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Zhang, Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods. 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Ilgaz, C.; Casula, L.; Sarais, G.; Schlich, M.; Dessì, D.; Cardia, M.C.; Sinico, C.; Kadiroglu, P.; Lai, F. Proniosomal encapsulation of olive leaf extract for improved delivery of oleuropein: Towards the valorization of an agro-industrial byproduct. Food Chem. 2025, 479, 143877. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-J.; Qin, F.G.F.; Tu, J.-L.; Li, B. Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules 2017, 22, 870. [Google Scholar] [CrossRef] [PubMed]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Roberts, S.A.; Lee, C.; Singh, S.; Agrawal, N. Versatile Encapsulation and Synthesis of Potent Liposomes by Thermal Equilibration. Membranes 2022, 12, 319. [Google Scholar] [CrossRef]
- Micheletto, Y.M.S.; Jesus, B.V.d.; Peres, G.L.; Pinto, V.Z. A Systematic Preparation of Liposomes with Yerba Mate (Ilex paraguariensis) Extract. Plants 2025, 14, 1325. [Google Scholar] [CrossRef]
- Yu, X.; Chu, S.; Hagerman, A.E.; Lorigan, G.A. Probing the Interaction of Polyphenols with Lipid Bilayers by Solid-State NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 6783–6789. [Google Scholar] [CrossRef]
- Uekusa, Y.; Kamihira, M.; Nakayama, T. Dynamic Behavior of Tea Catechins Interacting with Lipid Membranes As Determined by NMR Spectroscopy. J. Agric. Food Chem. 2007, 55, 9986–9992. [Google Scholar] [CrossRef]
- Kajiya, K.; Kumazawa, S.; Naito, A.; Nakayama, T. Solid-state NMR Analysis of the Orientation and Dynamics of Epigallocatechin Gallate, a Green Tea Polyphenol, Incorporated into Lipid Bilayers. Magn. Reson. Chem. 2008, 46, 174–177. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-Dependent Interactions of Polyphenols with a Biomimetic Membrane System. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Dag, D.; Oztop, M.H. Formation and Characterization of Green Tea Extract Loaded Liposomes. J. Food Sci. 2017, 82, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.; Majid, A.M.S.A.; Ismail, Z. Preparation and Characterization of Nano Liposomes of Orthosiphon stamineus Ethanolic Extract in Soybean Phospholipids. BMC Biotechnol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.D.; Djordjević, V.B.; Ota, A.; Skrt, M.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Ulrih, N.P. Effect of Gentisic Acid on the Structural-Functional Properties of Liposomes Incorporating β-Sitosterol. Colloid. Surf. B Biointerfaces 2019, 183, 110422. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Yang, G.; Wang, Y.; Tian, S. Effect of Phospholipids on Membrane Characteristics and Storage Stability of Liposomes. Innov. Food Sci. Emerg. Technol. 2022, 81, 103155. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and Release Performance of Curcumin-Loaded Liposomes with Varying Content of Hydrogenated Phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Cho, H.-J.; Wu, Y.; Wright, N.T.; Sum, A.K.; Chan, C. The Role of Fatty Acid Unsaturation in Minimizing Biophysical Changes on the Structure and Local Effects of Bilayer Membranes. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1508–1516. [Google Scholar] [CrossRef]
- Amărandi, R.-M.; Neamṭu, A.; Ştiufiuc, R.-I.; Marin, L.; Drăgoi, B. Impact of Lipid Composition on Vesicle Protein Adsorption: A BSA Case Study. ACS Omega 2024, 9, 17903–17918. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.; Wisniewska-Becker, A. Physical Properties of Lipid Bilayer Membranes: Relevance to Membrane Biological Functions. Acta Biochim. Pol. 2000, 47, 613–625. [Google Scholar] [CrossRef]
- Howard, F.B.; Levin, I.W. Lipid Vesicle Aggregation Induced by Cooling. Int. J. Mol. Sci. 2010, 11, 754–761. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, G.M.; Manohar, B. Nanocharacterization of Liposomes for the Encapsulation of Water Soluble Compounds from Cordyceps sinensis CS1197 by a Supercritical Gas Anti-Solvent Technique. RSC Adv. 2018, 8, 34634–34649. [Google Scholar] [CrossRef]
- Narenji, M.; Talaee, M.R.; Moghimi, H.R. Investigating the Effects of Size, Charge, Viscosity and Bilayer Flexibility on Liposomal Delivery under Convective Flow. Int. J. Pharm. 2016, 513, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative Effects of Cholesterol and Β-Sitosterol on the Liposome Membrane Characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Fathi, M.; Aboutorabzade, S.M.; Sabbagh, F. Development of Curcumin-loaded Prunus armeniaca Gum Nanoparticles: Synthesis, Characterization, Control Release Behavior, and Evaluation of Anticancer and Antimicrobial Properties. Food Sci. Nutr. 2021, 9, 6109–6119. [Google Scholar] [CrossRef]
- Prevete, G.; Carvalho, L.G.; del Carmen Razola-Diaz, M.; Verardo, V.; Mancini, G.; Fiore, A.; Mazzonna, M. Ultrasound Assisted Extraction and Liposome Encapsulation of Olive Leaves and Orange Peels: How to Transform Biomass Waste into Valuable Resources with Antimicrobial Activity. Ultrason. Sonochem. 2024, 102, 106765. [Google Scholar] [CrossRef]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. MiRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef]
- Olfati, A.; Karimi, N.; Arkan, E.; Zhaleh, M.; Mozafari, M.R. Enhancing Bioavailability and Stability of Plant Secondary Metabolites: Formulation and Characterization of Nanophytosomes Encapsulating Red Bryony and Horned Poppy Extracts. J. Funct. Biomater. 2025, 16, 194. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Physical and Chemical Stability of Anthocyanin-Rich Black Carrot Extract-Loaded Liposomes During Storage. Food Res. Int. 2018, 108, 491–497. [Google Scholar] [CrossRef]
- Toopkanloo, S.P.; Tan, T.B.; Abas, F.; Alharthi, F.A.; Nehdi, I.A.; Tan, C.P. Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome. Nanomaterials 2020, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, J.; Duman, G.; Corapcıoğlu, G.; Baranauskas, A.; Taralp, A.; Ivanauskas, L.; Bernatoniene, J. Liposomal Incorporation to Improve Dissolution and Stability of Rosmarinic Acid and Carvacrol Extracted from Oregano (O. onites L.). Biomed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruozi, B.; Tosi, G.; Forni, F.; Fresta, M.; Vandelli, M.A. Atomic Force Microscopy and Photon Correlation Spectroscopy: Two Techniques for Rapid Characterization of Liposomes. Eur. J. Pharm. Sci. 2005, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Knez, Ž.; Primožič, M. Sustainable Technologies for Liposome Preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Bompard, J.; Rosso, A.; Brizuela, L.; Mebarek, S.; Blum, L.J.; Trunfio-Sfarghiu, A.M.; Lollo, G.; Granjon, T.; Girard-Egrot, A.; Maniti, O. Membrane Fluidity as a New Means to Selectively Target Cancer Cells with Fusogenic Lipid Carriers. Langmuir 2020, 36, 5134–5144. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol Modulates the Liposome Membrane Fluidity and Permeability for a Hydrophilic Molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Duffy, C.F.; Gafoor, S.; Richards, D.P.; Admadzadeh, H.; O’Kennedy, R.; Arriaga, E.A. Determination of Properties of Individual Liposomes by Capillary Electrophoresis with Postcolumn Laser-Induced Fluorescence Detection. Anal. Chem. 2001, 73, 1855–1861. [Google Scholar] [CrossRef]
- Yu, M.; Song, W.; Tian, F.; Dai, Z.; Zhu, Q.; Ahmad, E.; Guo, S.; Zhu, C.; Zhong, H.; Yuan, Y.; et al. Temperature- and Rigidity-Mediated Rapid Transport of Lipid Nanovesicles in Hydrogels. Proc. Natl. Acad. Sci. USA 2019, 116, 5362–5369. [Google Scholar] [CrossRef] [PubMed]
- Antohe, F.; Lin, L.; Kao, G.Y.; Poznansky, M.J.; Allen, T.M. Transendothelial Movement of Liposomes In Vitro Mediated by Cancer Cells, Neutrophils or Histamine. J. Liposome Res. 2004, 14, 1–25. [Google Scholar] [CrossRef]
- Qian, S.; Li, C.; Zuo, Z. Pharmacokinetics and Disposition of Various Drug Loaded Liposomes. Curr. Drug Metab. 2012, 13, 372–395. [Google Scholar] [CrossRef]
- Abreu, A.S.; Castanheira, E.M.; Queiroz, M.-J.R.; Ferreira, P.M.; Vale-Silva, L.A.; Pinto, E. Nanoliposomes for Encapsulation and Delivery of the Potential Antitumoral Methyl 6-Methoxy-3-(4-Methoxyphenyl)-1H-Indole-2-Carboxylate. Nanoscale Res. Lett. 2011, 6, 482. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Schlieper, P.; Medda, P.K.; Kaufmann, R. Drug-Induced Zeta Potential Changes in Liposomes Studied by Laser Doppler Spectroscopy. Biochim. Biophys. Acta Biomembr. 1981, 644, 273–283. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.-Y.; Gan, L.-J.; Zhang, H.; Shin, J.-A.; Lee, K.-T.; Hong, S.-T. Effects of Flavonoid Glycosides Obtained from a Ginkgo biloba Extract Fraction on the Physical and Oxidative Stabilities of Oil-in-Water Emulsions Prepared from a Stripped Structured Lipid with a Low Omega-6 to Omega-3 Ratio. Food Chem. 2015, 174, 124–131. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef]
- Chen, Y.; Arriaga, E.A. Individual Electrophoretic Mobilities of Liposomes and Acidic Organelles Displaying PH Gradients Across Their Membranes. Langmuir 2007, 23, 5584–5590. [Google Scholar] [CrossRef]
- Giannopoulos-Dimitriou, A.; Saiti, A.; Petrou, A.; Vizirianakis, I.S.; Fatouros, D.G. Liposome Stability and Integrity. In Liposomes in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 89–121. [Google Scholar]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of Phenolic Compounds with Liposomal Improvement in the Cosmetic Industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef]
- Savaghebi, D.; Barzegar, M.; Mozafari, M.R. Manufacturing of Nanoliposomal Extract from Sargassum boveanum Algae and Investigating Its Release Behavior and Antioxidant Activity. Food Sci. Nutr. 2020, 8, 299–310. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.M.; Machado, A.R.; de Souza da Motta, A.; Costa, J.A.V.; de Souza-Soares, L.A. Development and Characterization of Nanovesicles Containing Phenolic Compounds of Microalgae Spirulina Strain LEB-18 and Chlorella pyrenoidosa. Adv. Mater. Phys. Chem. 2014, 4, 6–12. [Google Scholar] [CrossRef]
- Yarce, C.J.; Alhajj, M.J.; Sanchez, J.D.; Oñate-Garzón, J.; Salamanca, C.H. Development of Antioxidant-Loaded Nanoliposomes Employing Lecithins with Different Purity Grades. Molecules 2020, 25, 5344. [Google Scholar] [CrossRef]
- Yuda, T.; Maruyama, K.; Takizawa, T.; Iwatsuru, M. Long-Circulating Liposomes. Drug Deliv. Syst. 1994, 9, 145–160. [Google Scholar] [CrossRef]
- Ishida, T.; Harashima, H.; Kiwada, H. Interactions of Liposomes with Cells In Vitro and In Vivo: Opsonins and Receptors. Curr. Drug Metab. 2001, 2, 397–409. [Google Scholar] [CrossRef]
- Qi, X.-R.; Zhao, Z. Comparative Study of the in Vitro and in Vivo Characteristics of Cationic and Neutral Liposomes. Int. J. Nanomed. 2011, 3087, 3087–3098. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Sun, X.; Leung, A.W.Y.; Dos Santos, N.; Wretham, N.; Nosrati, Z.; Bally, M.B. Liposomal Formulations of Metal-CX5461 Complexes: Copper-CX5461 Complexation Mediates CX5461 Degradation while Zinc-CX5461 Formulations Are Suitable for Development. J. Drug Deliv. Sci. Technol. 2025, 114, 107586. [Google Scholar] [CrossRef]
- Ramsay, E.; Alnajim, J.; Anantha, M.; Taggar, A.; Thomas, A.; Edwards, K.; Karlsson, G.; Webb, M.; Bally, M. Transition Metal-Mediated Liposomal Encapsulation of Irinotecan (CPT-11) Stabilizes the Drug in the Therapeutically Active Lactone Conformation. Pharm. Res. 2006, 23, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Barlas, F.B.; Guler, E.; Gumus, P.Z.; Can, M.; Yavuz, M.; Coskunol, H.; Timur, S. Gold Nanoparticle Loaded Phytosomal Systems: Synthesis, Characterization and in Vitro Investigations. RSC Adv. 2014, 4, 34687–34695. [Google Scholar] [CrossRef]
- Safta, D.A.; Bogdan, C.; Moldovan, M.L. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics 2022, 14, 991. [Google Scholar] [CrossRef]
- Hammoud, Z.; Kayouka, M.; Trifan, A.; Sieniawska, E.; Jemâa, J.M.B.; Elaissari, A.; Greige-Gerges, H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules 2021, 26, 6840. [Google Scholar] [CrossRef]
- Fernandes, F.; Dias-Teixeira, M.; Delerue-Matos, C.; Grosso, C. Critical Review of Lipid-Based Nanoparticles as Carriers of Neuroprotective Drugs and Extracts. Nanomaterials 2021, 11, 563. [Google Scholar] [CrossRef]
- Wahyudiono; He, J.; Hu, X.; Machmudah, S.; Yasuda, K.; Takami, S.; Kanda, H.; Goto, M. Curcumin-Loaded Liposome Preparation in Ultrasound Environment under Pressurized Carbon Dioxide. Foods 2022, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Hac-Wydro, K.; Wydro, P. The Influence of Fatty Acids on Model Cholesterol/Phospholipid Membranes. Chem. Phys. Lipids 2007, 150, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.E.; Venable, R.M.; Pastor, R.W.; Lyman, E.R. Surface Viscosities of Lipid Bilayers Determined from Equilibrium Molecular Dynamics Simulations. Biophys. J. 2023, 122, 1094–1104. [Google Scholar] [CrossRef]
- Jara-Quijada, E.; Pérez-Won, M.; Tabilo-Munizaga, G.; Lemus-Mondaca, R.; González-Cavieres, L.; Palma-Acevedo, A.; Herrera-Lavados, C. Liposomes Loaded with Green Tea Polyphenols—Optimization, Characterization, and Release Kinetics Under Conventional Heating and Pulsed Electric Fields. Food Bioprocess Technol. 2024, 17, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Karaz, S.; Senses, E. Liposomes Under Shear: Structure, Dynamics, and Drug Delivery Applications. Adv. NanoBiomed Res. 2023, 3, 2200101. [Google Scholar] [CrossRef]
- Santos, S.; Neves, A.R.; Silva, A.; Barbosa, M.; Reis, S.; Barbosa, J. Nanostructured Lipid Carriers Loaded with Resveratrol Modulate Human Dendritic Cells. Int. J. Nanomed. 2016, 11, 3501–3516. [Google Scholar] [CrossRef]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Szalkai, P.; Niczinger, N.A.; Kijima, S.; Sugibayashi, K.; Antal, I.; Kállai-Szabó, N. Viscoelasticity of Liposomal Dispersions. Nanomaterials 2023, 13, 2340. [Google Scholar] [CrossRef]
- Ghazvini, S.; Alonso, R.; Alhakamy, N.; Dhar, P. pH-Induced Changes in the Surface Viscosity of Unsaturated Phospholipids Monitored Using Active Interfacial Microrheology. Langmuir 2018, 34, 1159–1170. [Google Scholar] [CrossRef]
- Zeng, W.; Li, P.; Huang, Y.; Xia, A.; Zhu, X.; Zhu, X.; Liao, Q. How Interfacial Properties Affect Adhesion: An Analysis from the Interactions between Microalgal Cells and Solid Substrates. Langmuir 2022, 38, 3284–3296. [Google Scholar] [CrossRef]
- Zaborowska, M.; Dobrowolski, M.A.; Matyszewska, D. Revealing the Structure and Mechanisms of Action of a Synthetic Opioid with Model Biological Membranes at the Air-Water Interface. Colloid. Surf. B Biointerfaces 2023, 226, 113289. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of the Decrease in Surface Tension by Bulk Nanobubbles (Ultrafine Bubbles). Langmuir 2023, 39, 16574–16583. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, T.; Alakoskela, J.-M.I.; Pakkanen, A.L.; Kinnunen, P.K.J. Comparison of the Effects of Surface Tension and Osmotic Pressure on the Interfacial Hydration of a Fluid Phospholipid Bilayer. Biophys. J. 2003, 85, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Malekar, S.A.; Sarode, A.L.; Bach, A.C.; Worthen, D.R. The Localization of Phenolic Compounds in Liposomal Bilayers and Their Effects on Surface Characteristics and Colloidal Stability. AAPS PharmSciTech. 2016, 17, 1468–1476. [Google Scholar] [CrossRef]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef]
- Fathi Azarbayjani, A.; Jouyban, A.; Chan, S.Y. Impact of Surface Tension in Pharmaceutical Sciences. J. Pharm. Pharm. Sci. 2009, 12, 218. [Google Scholar] [CrossRef]
- Păvăloiu, R.-D.; Sha’at, F.; Bubueanu, C.; Deaconu, M.; Neagu, G.; Sha’at, M.; Anastasescu, M.; Mihailescu, M.; Matei, C.; Nechifor, G.; et al. Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials 2020, 10, 56. [Google Scholar] [CrossRef]
- Iversen, A.; Utterström, J.; Khare, L.P.; Aili, D. Influence of Lipid Vesicle Properties on the Function of Conjugation Dependent Membrane Active Peptides. J. Mater. Chem. B 2024, 12, 10320–10331. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef]
- Pjanović, R.; Bošković-Vragolović, N.; Veljković-Giga, J.; Garić-Grulović, R.; Pejanović, S.; Bugarski, B. Diffusion of Drugs from Hydrogels and Liposomes as Drug Carriers. J. Chem. Technol. Biotechnol. 2010, 85, 693–698. [Google Scholar] [CrossRef]
- Taira, M.C.; Chiaramoni, N.S.; Pecuch, K.M.; Alonso-Romanowski, S. Stability of Liposomal Formulations in Physiological Conditions for Oral Drug Delivery. Drug Deliv. 2004, 11, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Ruedt, C.; Weiss, J. In Vitro Release of Grape-Seed Polyphenols Encapsulated from Uncoated and Chitosan-Coated Liposomes. Food Res. Int. 2016, 88 Pt A, 105–113. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Leyva-Jiménez, F.J.; Quirantes-Piné, R.; López-Bascón, M.A.; Lozano-Sánchez, J.; Borrás-Linares, I. Evaluation of Olive Leaf Phenolic Compounds’ Gastrointestinal Stability Based on Co-Administration and Microencapsulation with Non-Digestible Carbohydrates. Nutrients 2024, 16, 93. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Deriving Valorization of Phenolic Compounds from Olive Oil By-Products for Food Applications through Microencapsulation Approaches: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 920–945. [Google Scholar] [CrossRef] [PubMed]
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring Alginate Beads with Different Biopolymers for the Development of Functional Ingredients Loaded with Olive Leaves Phenolic Extract. Food Hydrocoll. 2020, 108, 105849. [Google Scholar] [CrossRef]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A Simple GC–MS Method for the Screening of Betulinic, Corosolic, Maslinic, Oleanolic and Ursolic Acid Contents in Commercial Botanicals Used as Food Supplement Ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hamishehkar, H.; Ghorbani, M.; Shahvalizadeh, R.; Pateiro, M.; Lorenzo, J.M. Engineering of Liposome Structure to Enhance Physicochemical Properties of Spirulina plantensis Protein Hydrolysate: Stability during Spray-Drying. Antioxidants 2021, 10, 1953. [Google Scholar] [CrossRef]

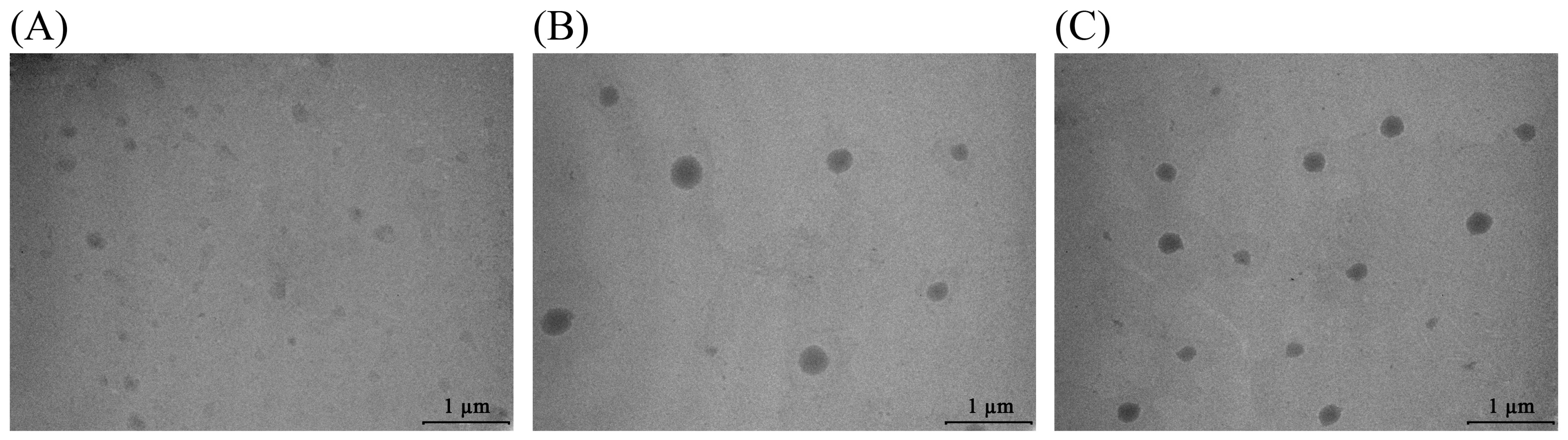
| Sample | Olive Leaf Extract | Extract-Loaded Liposomes | |||
|---|---|---|---|---|---|
| AL | PG90 | PH90 | |||
| Class of Compounds | Compound | µg/mg Dry Extract | |||
| Secoiridoids | Oleuropein | 111.8 ± 10.85 a,* | 98.28 ± 3.50 a | 85.75 ± 4.18 b | 87.18 ± 2.18 d |
| Oleacein | 3.75 ± 0.24 b | 4.23 ± 0.15 a | 4.25 ± 0.15 a | 4.03 ± 0.10 ab | |
| Ligstroside | 1.06 ± 0.14 ab | 0.98 ± 0.06 b | 0.99 ± 0.04 b | 1.28 ± 0.20 a | |
| Oleuropein aglycone | 0.43 ± 0.08 a | 0.24 ± 0.02 b | 0.36 ± 0.05 a | 0.31 ± 0.03 a | |
| Pentacyclic triterpenes | Oleanolic acid | 42.88 ± 3.10 a | 15.61 ± 1.10 c | 20.94 ± 1.6 b | 43.28 ± 2.10 a |
| Maslinic acid | 5.76 ± 0.38 a | 0.18 ± 0.03 d | 0.31 ± 0.05 c | 3.11 ± 0.20 b | |
| Flavonoids and flavonoid glycosides | Luteolin 7-O-glucoside | 9.84 ± 1.21 a | 7.82 ± 0.60 b | 7.04 ± 0.15 c | 7.74 ± 0.31 b |
| Apigenin- 7-O-glucoside | 5.21 ± 0.65 a | 3.94 ± 0.80 ab | 3.75 ± 0.20 b | 4.40 ± 0.18 a | |
| Quercetin | 0.63 ± 0.08 a | 0.50 ± 0.10 ab | 0.45 ± 0.05 b | 0.49 ± 0.07 ab | |
| Quercitrin | 0.18 ± 0.01 ab | 0.20 ± 0.01 a | 0.16 ± 0.03 ab | 0.17 ± 0.01 b | |
| Simple phenols | Hydroxytyrosol | 1.56 ± 0.28 ab | 2.15 ± 0.35 a | 1.90 ± 0.10 a | 1.60 ± 0.05 b |
| Chlorogenic acid | 0.14 ± 0.03 a | 0.08 ± 0.01 b | 0.05 ± 0.01 c | 0.09 ± 0.02 ab | |
| Sample | Encapsulated Extract Fraction in Liposomes | ||||||
|---|---|---|---|---|---|---|---|
| AL | PG90 | PH90 | |||||
| Class of Compounds | Compound | µg/mg d.e. | EE (%) | µg/mg d.e. | EE (%) | µg/mg d.e. | EE (%) |
| Secoiridoids | Oleuropein | 71.65 ± 6.64 a,* | 72.90 ± 2.76 a | 65.18 ± 2.32 a | 76.18 ± 1.29 a | 50.65 ± 1.81 b | 58.09 ± 1.45 b |
| Oleacein | 3.63 ± 0.27 a | 85.76 ± 2.03 a | 3.63 ± 0.14 a | 85.37 ± 2.81 a | 3.17 ± 0.17 b | 78.79 ± 1.51 b | |
| Ligstroside | 0.68 ± 0.05 a | 69.62 ± 4.25 a | 0.75 ± 0.09 a | 75.70 ± 3.06 a | 0.71 ± 0.17 a | 55.40 ± 3.30 b | |
| Oleuropein aglycone | 0.18 ± 0.02 a | 80.62 ± 6.25 a | 0.19 ± 0.05 a | 73.47 ± 3.04 a | 0.16 ± 0.02 a | 79.87 ± 6.21 a | |
| Pentacyclic triterpenes | Oleanolic acid | 13.96 ± 0.05 c | 32.60 ± 6.30 b | 16.66 ± 0.98 b | 38.91 ± 8.22 b | 42.79 ± 1.10 a | 92.93 ± 4.27 a |
| Maslinic acid | 0.06 ± 0.01 b | 32.07 ± 0.18 b | 0.07 ± 0.01 b | 22.88 ± 0.27 c | 3.06 ± 0.10 a | 49.26 ± 0.11 a | |
| Flavonoids and flavonoid glycosides | Luteolin 7-O-glucoside | 6.90 ± 0.62 a | 88.25 ± 6.77 a | 6.32 ± 0.20 a | 89.79 ± 1.62 a | 5.47 ± 0.23 b | 70.65 ± 3.39 b |
| Apigenin 7-O-glucoside | 3.72 ± 0.36 a | 94.38 ± 7.19 a | 3.50 ± 0.14 a | 93.36 ± 4.22 a | 3.55 ± 0.17 a | 80.69 ± 4.44 b | |
| Quercetin | 0.43 ± 0.03 a | 86.40 ± 4.96 a | 0.33 ± 0.02 b | 74.33 ± 2.60 b | 0.42 ± 0.05 a | 86.53 ± 4.15 a | |
| Quercitrin | 0.15 ± 0.01 a | 80.62 ± 4.63 a | 0.12 ± 0.02 a | 73.47 ± 4.69 a | 0.14 ± 0.01 a | 79.87 ± 4.84 a | |
| Simple phenols | Hydroxy tyrosol | 0.97 ± 0.07 a | 45.14 ± 4.08 a | 0.87 ± 0.08 a | 45.68 ± 4.01 a | 0.85 ± 0.06 a | 52.92 ± 3.78 a |
| Chlorogenic acid | 0.02 ± 0.01 | 22.22 ± 2.00 | n.d. | 0.00 | n.d. | 0.00 | |
| Day | Liposomes | Viscosity (mPa·s) | Surface Tension (mN/m) | Density (g/cm3) |
|---|---|---|---|---|
| 1st | Plain AL | 2.63 ± 0.04 h,* | 26.4 ± 1.0 b | 0.999 ± 0.001 c |
| Plain PG90 | 2.68 ± 0.05 h | 15.6 ± 1.3 d | 0.999 ± 0.002 c | |
| Plain PH90 | 3.07 ± 0.08 g | 18.3 ± 0.9 c | 1.002 ± 0.003 bc | |
| AL with extract | 6.04 ± 0.03 c | 29.6 ± 2.0 b | 1.007 ± 0.002 ab | |
| PG90 with extract | 6.78 ± 0.05 b | 27.2 ± 0.7 b | 1.018 ± 0.011 a | |
| PH90 with extract | 4.49 ± 0.12 e | 37.3 ± 1.8 a | 1.005 ± 0.002 ab | |
| 60th | Plain AL | 2.64 ± 0.05 h | 17.9 ± 2.4 cd | 1.001 ± 0.03 bc |
| Plain PG90 | 3.05 ± 0.11 g | 16.0 ± 1.2 d | 1.000 ± 0.02 bc | |
| Plain PH90 | 3.57 ± 0.06 f | 20.3 ± 1.3 c | 1.000 ± 0.03 bc | |
| AL with extract | 7.70 ± 0.07 a | 28.8 ± 1.5 b | 1.008 ± 0.01 a | |
| PG90 with extract | 5.44 ± 0.07 d | 28.1 ± 1.0 b | 1.011 ± 0.02 a | |
| PH90 with extract | 4.36 ± 0.11 e | 39.4 ± 1.9 a | 1.004 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baljak, J.; Dekanski, D.; Pirković, A.; Mitić, N.; Rašković, A.; Kladar, N.; Jovanović, A.A. Valorization of Olive Leaf Extract via Tailored Liposomal Carriers: Comparative Analysis of Physicochemical Features, Antioxidant Capacity, and Stability. Pharmaceuticals 2025, 18, 1639. https://doi.org/10.3390/ph18111639
Baljak J, Dekanski D, Pirković A, Mitić N, Rašković A, Kladar N, Jovanović AA. Valorization of Olive Leaf Extract via Tailored Liposomal Carriers: Comparative Analysis of Physicochemical Features, Antioxidant Capacity, and Stability. Pharmaceuticals. 2025; 18(11):1639. https://doi.org/10.3390/ph18111639
Chicago/Turabian StyleBaljak, Jovan, Dragana Dekanski, Andrea Pirković, Ninoslav Mitić, Aleksandar Rašković, Nebojša Kladar, and Aleksandra A. Jovanović. 2025. "Valorization of Olive Leaf Extract via Tailored Liposomal Carriers: Comparative Analysis of Physicochemical Features, Antioxidant Capacity, and Stability" Pharmaceuticals 18, no. 11: 1639. https://doi.org/10.3390/ph18111639
APA StyleBaljak, J., Dekanski, D., Pirković, A., Mitić, N., Rašković, A., Kladar, N., & Jovanović, A. A. (2025). Valorization of Olive Leaf Extract via Tailored Liposomal Carriers: Comparative Analysis of Physicochemical Features, Antioxidant Capacity, and Stability. Pharmaceuticals, 18(11), 1639. https://doi.org/10.3390/ph18111639









