Repurposing Auranofin for Oncology and Beyond: A Brief Overview of Clinical Trials as Mono- and Combination Therapy
Abstract
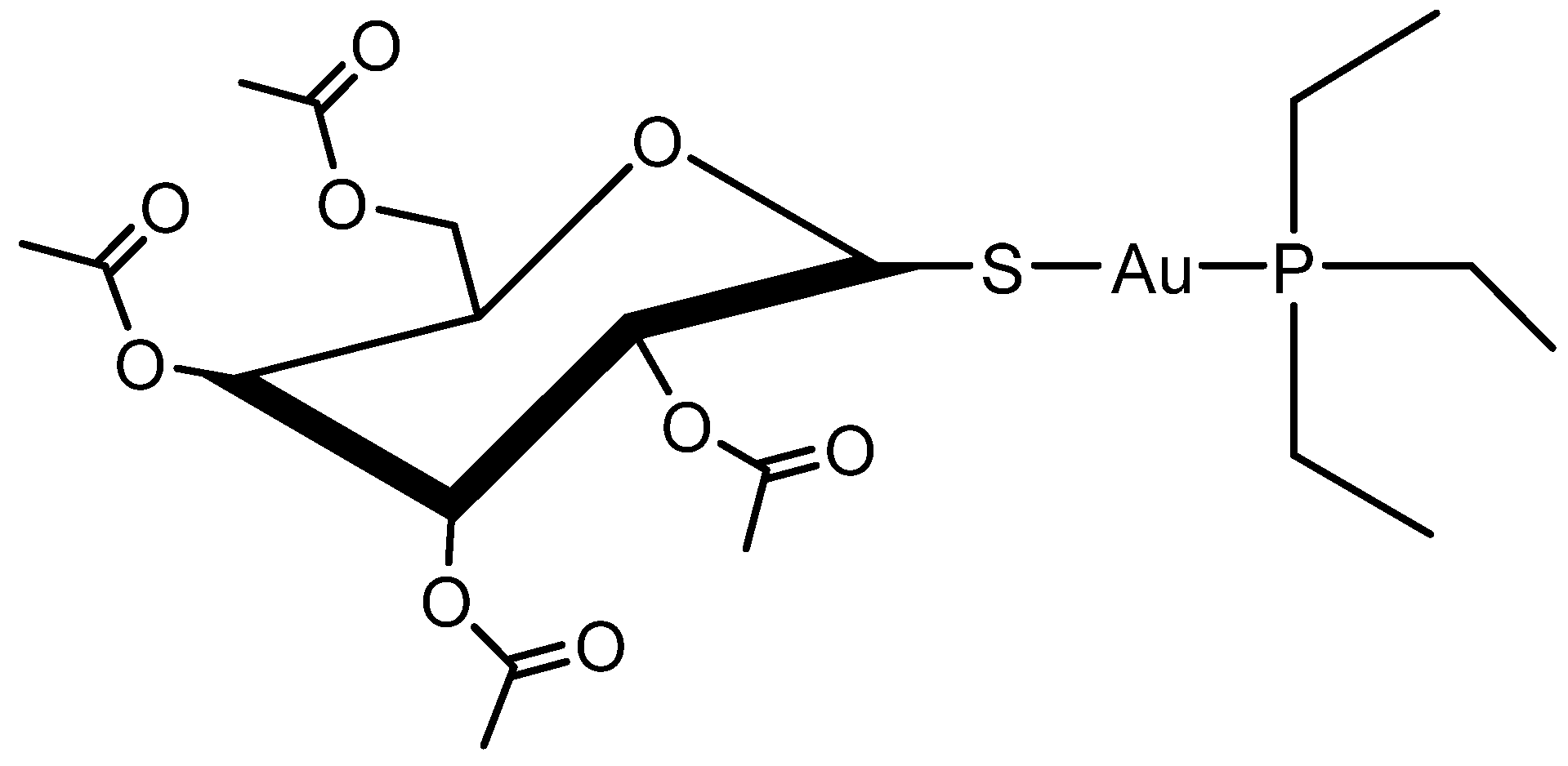
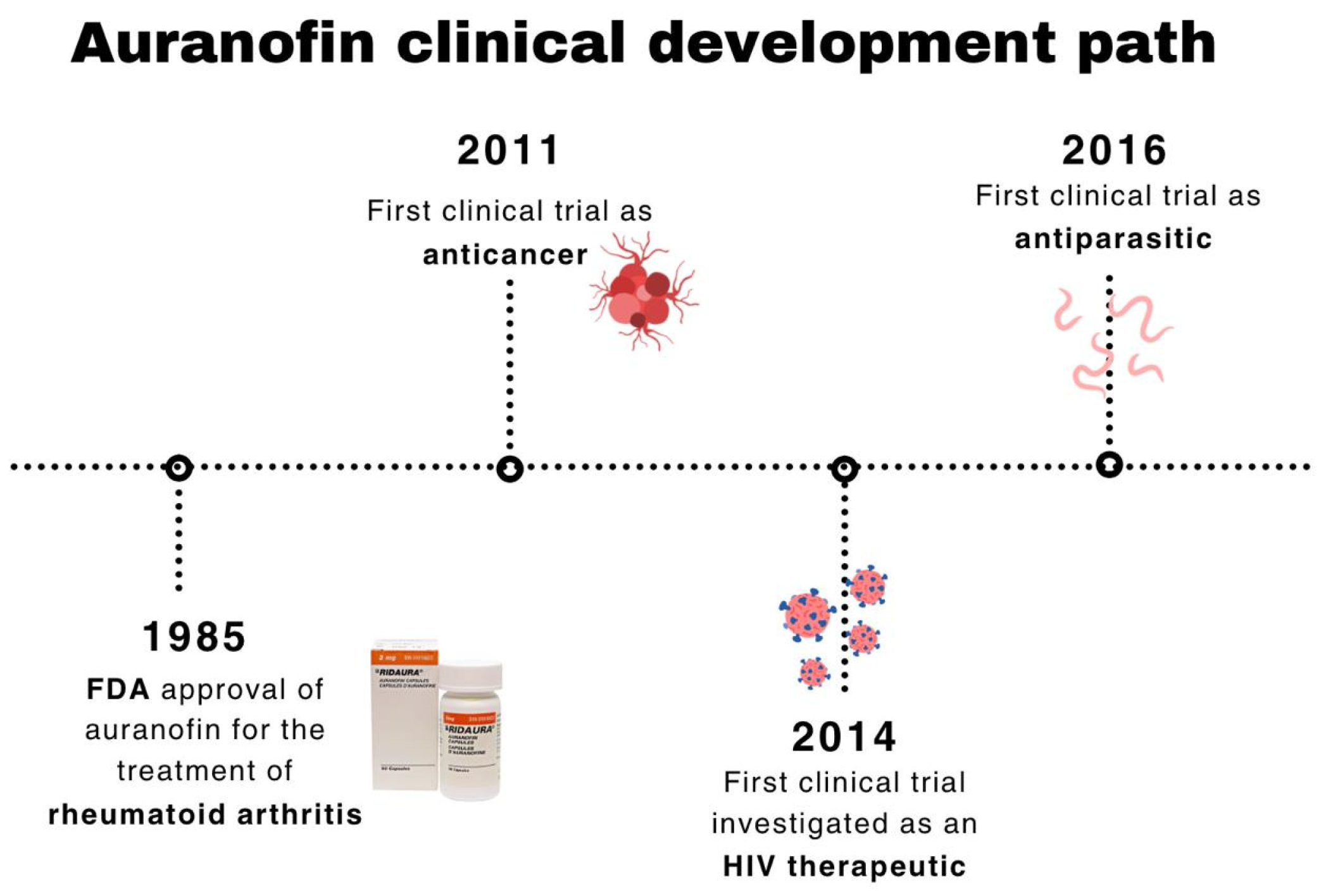
1. Introduction
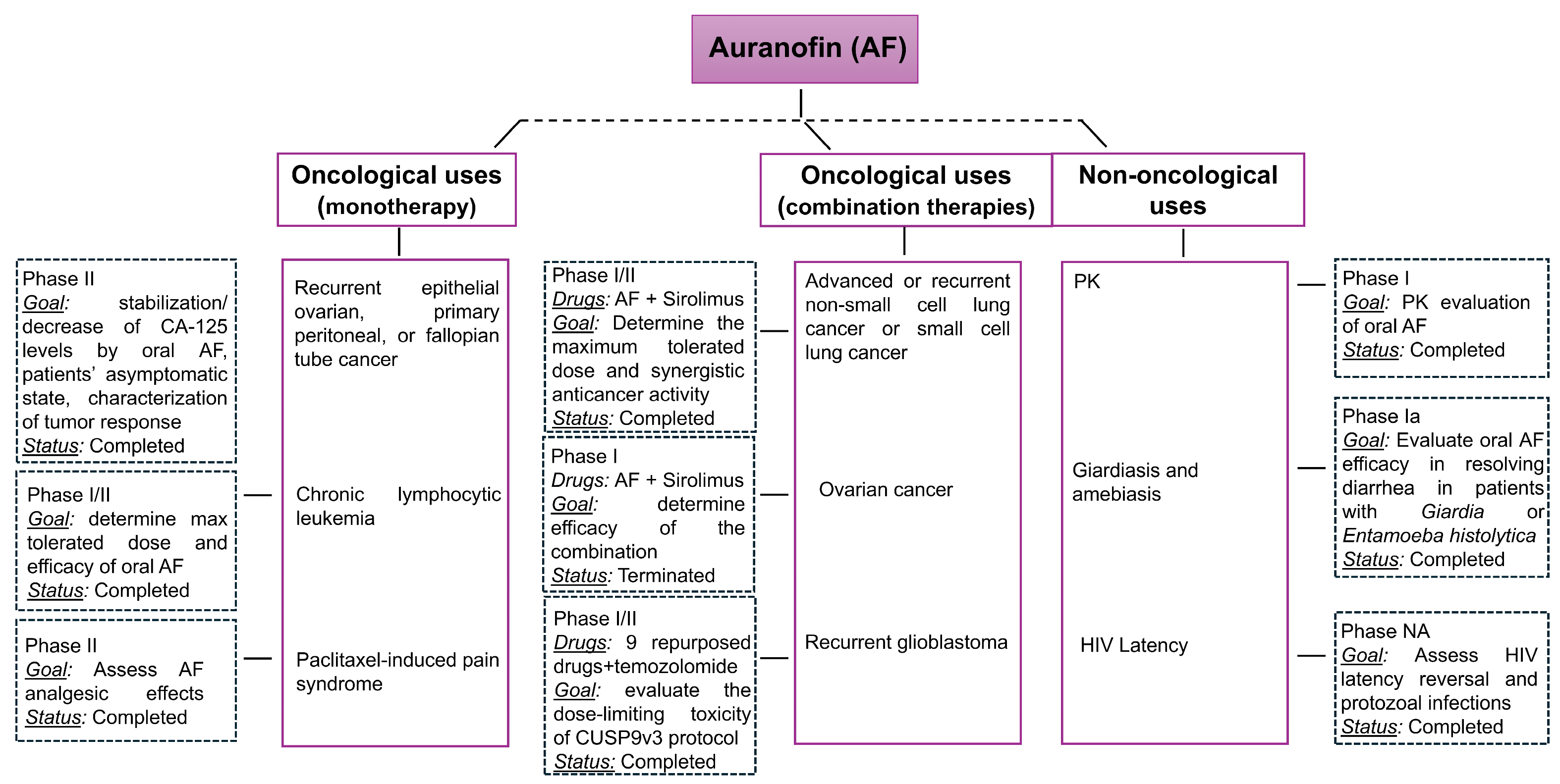
2. Auranofin-Based Monotherapy for Cancer Management
2.1. NCT01747798—Auranofin in Treating Patients with Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer https://clinicaltrials.gov/study/NCT01747798 (Accessed on 20 July 2025)
2.2. NCT01419691—Phase I and II Study of AF in Chronic Lymphocytic Leukemia (CLL) https://clinicaltrials.gov/study/NCT01419691 (Accessed on 20 July 2025)
2.3. NCT02063698—Auranofin in Decreasing Pain in Patients with Paclitaxel-Induced Pain Syndrome https://clinicaltrials.gov/study/NCT02063698 (Accessed on 20 July 2025)
3. Combinatory Regimens for Cancer Management Involving Auranofin
3.1. NCT01737502—Sirolimus and Auranofin in Treating Patients with Advanced or Recurrent Non-Small Cell Lung Cancer or Small Cell Lung Cancer https://clinicaltrials.gov/study/NCT01737502 (Accessed on 20 July 2025)
3.2. NCT03456700—Auranofin and Sirolimus in Treating Participants with Ovarian Cancer https://clinicaltrials.gov/study/NCT03456700 (Accessed on 20 July 2025)
3.3. NCT02770378—A Proof-of-Concept Clinical Trial Assessing the Safety of the Coordinated Undermining of Survival Paths by 9 Repurposed Drugs Combined with Metronomic Temozolomide (CUSP9v3 Treatment Protocol) for Recurrent Glioblastoma https://clinicaltrials.gov/study/NCT02770378 (Accessed on 20 July 2025)
4. Auranofin in Non-Oncological Diseases
4.1. NCT02089048—Auranofin Pharmacokinetic (PK) Following Oral Dose Administration https://clinicaltrials.gov/study/NCT02089048 (Accessed on 20 July 2025)
4.2. NCT02736968—Auranofin for Giardia Protozoa and Entamoeba histolytica http://clinicaltrials.gov/study/NCT02736968 (Accessed on 20 July 2025)
4.3. NCT02961829—Multi Interventional Study Exploring HIV-1 Residual Replication: A Step Towards HIV-1 Eradication and Sterilizing Cure https://clinicaltrials.gov/study/NCT02961829 (Accessed on 20 July 2025)
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamashita, M. Auranofin: Past to Present, and Repurposing. Int. Immunopharmacol. 2021, 101, 108272. [Google Scholar] [CrossRef]
- Betts, K.A.; Griffith, J.; Ganguli, A.; Li, N.; Douglas, K.; Wu, E.Q. Economic Burden and Treatment Patterns of Cycling between Conventional Synthetic Disease-Modifying Antirheumatic Drugs among Biologic-Treated Patients with Rheumatoid Arthritis. Clin. Ther. 2016, 38, 1205–1216. [Google Scholar] [CrossRef]
- Marzo, T.; Messori, L. A Role for Metal-Based Drugs in Fighting COVID-19 Infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Xu, Z.; Ma, X.; Chen, X.; Liu, W. Repurposing of the Gold Drug Auranofin and a Review of Its Derivatives as Antibacterial Therapeutics. Drug Discov. Today 2022, 27, 1961–1973. [Google Scholar] [CrossRef]
- Gamberi, T.; Chiappetta, G.; Fiaschi, T.; Modesti, A.; Sorbi, F.; Magherini, F. Upgrade of an Old Drug: Auranofin in Innovative Cancer Therapies to Overcome Drug Resistance and to Increase Drug Effectiveness. Med. Res. Rev. 2022, 42, 1111–1146. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, E.V.; Bricker-Ford, R.; Rogers, M.J.; McKerrow, J.H.; Reed, S.L. Phase I Clinical Trial Results of Auranofin, a Novel Antiparasitic Agent. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Selvaraju, K.; Saei, A.A.; D’Arcy, P.; Zubarev, R.A.; Arnér, E.S.; Linder, S. Repurposing of Auranofin: Thioredoxin Reductase Remains a Primary Target of the Drug. Biochimie 2019, 162, 46–54. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin Reductase as a Pharmacological Target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef] [PubMed]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin Reductase: A Target for Gold Compounds Acting as Potential Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Marzano, C.; Gandin, V.; Folda, A.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of Thioredoxin Reductase by Auranofin Induces Apoptosis in Cisplatin-Resistant Human Ovarian Cancer Cells. Free Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef]
- Jackson-Rosario, S.; Cowart, D.; Myers, A.; Tarrien, R.; Levine, R.L.; Scott, R.A.; Self, W.T. Auranofin Disrupts Selenium Metabolism in Clostridium Difficile by Forming a Stable Au-Se Adduct. J. Biol. Inorg. Chem. 2009, 14, 507–519. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Holmgren, A. The Thioredoxin System in Cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef]
- Jia, J.J.; Geng, W.S.; Wang, Z.Q.; Chen, L.; Zeng, X.S. The Role of Thioredoxin System in Cancer: Strategy for Cancer Therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Gencheva, R.; Arner, E.S.J. Thioredoxin Reductase Inhibition for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2021, 62, 177–196. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.N.; Xie, L.Z.; Shen, Y.; Li, J.; Guo, Y.; Fu, Y.; Liu, F.Y.; Han, F.J. Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 8388258. [Google Scholar] [CrossRef]
- Abdalbari, F.H.; Telleria, C.M. The Gold Complex Auranofin: New Perspectives for Cancer Therapy. Discov. Oncol. 2021, 12, 42. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Mazidimoradi, A.; Allahqoli, L.; Salehiniya, H. The Role of CA-125 in the Management of Ovarian Cancer: A Systematic Review. Cancer Rep. 2025, 8, e70142. [Google Scholar] [CrossRef]
- Ghia, P.; Ferreri, A.M.; Galigaris-Cappio, F. Chronic Lymphocytic Leukemia. Crit. Rev. Oncol. Hematol. 2007, 64, 234–246. [Google Scholar] [CrossRef]
- Fiskus, W.; Saba, N.; Shen, M.; Ghias, M.; Liu, J.; Das Gupta, S.; Chauhan, L.; Rao, R.; Gunewardena, S.; Schorno, K.; et al. Auranofin Induces Lethal Oxidative and Endoplasmic Reticulum Stress and Exerts Potent Preclinical Activity against Chronic Lymphocytic Leukemia. Cancer Res. 2014, 74, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Shen, M.; Ghias, M.; Farooqui, M.; Austin, C.; Schorno, K.; Weir, S.; Bhalla, K.; Wiestner, A. The Gold Compound Auranofin Induces Oxidative Stress and Apoptosis in Primary CLL Cells Independent of Classic Prognostic Markers and the Protective Effect of the Tissue Microenvironment. Blood 2012, 120, 865. [Google Scholar] [CrossRef]
- Blodgett, R.C.; Pietrusko, R.G. Long-Term Efficacy and Safety of Auranofin: A Review of Clinical Experience. Scand. J. Rheumatol. 1987, 16, 67–78. [Google Scholar] [CrossRef]
- Hleuhel, M.H.; Ben-Dali, Y.; Da Cunha-Bang, C.; Brieghel, C.; Clasen-Linde, E.; Niemann, C.U.; Andersen, M.A. Risk Factors Associated with Richter’s Transformation in Patients with Chronic Lymphocytic Leukaemia: Protocol for a Retrospective Population-Based Cohort Study. BMJ Open 2019, 9, e023566. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxid. Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Paclitaxel. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 44, pp. 205–238. ISBN 9780128171653. [Google Scholar]
- Yan, X.; Maixner, D.W.; Yadav, R.; Gao, M.; Li, P.; Bartlett, M.G.; Weng, H.R. Paclitaxel Induces Acute Pain via Directly Activating Toll like Receptor 4. Mol. Pain 2015, 11, 10. [Google Scholar] [CrossRef]
- Da̧bek, J.; Kułach, A.; Ga̧sior, Z. Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-ΚB): A New Potential Therapeutic Target in Atherosclerosis? Pharmacol. Rep. 2010, 62, 778–783. [Google Scholar] [CrossRef]
- Chen, W.H.; Chan, K.S.; Gan, T.J.; Chen, C.; Lakshminarayanan, M.; Revicki, D.A. Validation of the Modified Brief Pain Inventory-Exploratory Form in Surgery Patients. Health Outcomes Res. Med. 2010, 1, e17–e28. [Google Scholar] [CrossRef]
- Rehan, M. An Anti-Cancer Drug Candidate OSI-027 and Its Analog as Inhibitors of MTOR: Computational Insights Into the Inhibitory Mechanisms. J. Cell. Biochem. 2017, 118, 4558–4567. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, J.; Yu, Y.; Wu, F.; Shen, X.; Qiu, C.; Zhang, T.; Hong, L.; Zheng, P.; Shao, R.; et al. Compensatory Combination of MTOR and TrxR Inhibitors to Cause Oxidative Stress and Regression of Tumors. Theranostics 2021, 11, 4335–4350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hill, K.S.; Fields, A.P. PKCi Maintains a Tumor-Initiating Cell Phenotype That Is Required for Ovarian Tumorigenesis. Mol. Cancer Res. 2013, 11, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Roy, A.; Mandal, S. Protein Kinase C Iota Promotes Glycolysis via PI3K/AKT/MTOR Signalling in High Grade Serous Ovarian Cancer. Mol. Biol. Rep. 2024, 51, 983. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro. Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Jezierzański, M.; Nafalska, N.; Stopyra, M.; Furgoł, T.; Miciak, M.; Kabut, J.; Gisterek-Grocholska, I. Temozolomide (TMZ) in the Treatment of Glioblastoma Multiforme—A Literature Review and Clinical Outcomes. Curr. Oncol. 2024, 31, 3994–4002. [Google Scholar] [CrossRef]
- Pendergrass, K.; Hargreaves, R.; Petty, K.J.; Carides, A.D.; Evans, J.K.; Horgan, K.J. Aprepitant: An Oral NK1 Antagonist for the Prevention of Nausea and Vomiting Induced by Highly Emetogenic Chemotherapy. Drugs Today 2004, 40, 853–863. [Google Scholar] [CrossRef]
- Elewa, H.F.; Hilali, R.; Hess, D.C.; Machado, L.S.; Fagan, S.C. Minocycline for Short-Term Neuroprotection. Pharmacotherapy 2006, 26, 515–521. [Google Scholar] [CrossRef]
- Caminear, M.W.; Harrington, B.S.; Kamdar, R.D.; Kruhlak, M.J.; Annunziata, C.M. Disulfiram Transcends ALDH Inhibitory Activity When Targeting Ovarian Cancer Tumor-Initiating Cells. Front. Oncol. 2022, 12, 762820. [Google Scholar] [CrossRef]
- Rosas, C.; Sinning, M.; Ferreira, A.; Fuenzalida, M.; Lemus, D. Celecoxib Decreases Growth and Angiogenesis and Promotes Apoptosis in a Tumor Cell Line Resistant to Chemotherapy. Biol. Res. 2014, 47, 27. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, S.; Atia-Tul-Wahab; Irshad, R.; Siddiqui, R.A.; Choudhary, M.I. Antidepressant Sertraline Hydrochloride Inhibits the Growth of HER2+ AU565 Breast Cancer Cell Line through Induction of Apoptosis and Cell Cycle Arrest. Anticancer. Agents Med. Chem. 2024, 24, 1038–1046. [Google Scholar] [CrossRef]
- Wysocki, P.J.; Kwiatkowska, E.P.; Kazimierczak, U.; Suchorska, W.; Kowalczyk, D.W.; Mackiewicz, A. Captopril, an Angiotensin-Converting Enzyme Inhibitor, Promotes Growth of Immunogenic Tumors in Mice. Clin. Cancer Res. 2006, 12, 4095–4102. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tsai, T.F. Itraconazole in the Treatment of Nonfungal Cutaneous Diseases: A Review. Dermatol. Ther. 2019, 9, 271–280. [Google Scholar] [CrossRef]
- Laurence, J.; Elhadad, S.; Gostynska, S.; Yu, Z.; Terry, H.; Varshney, R.; Fung, K.M.; Choi, M.E.; Ahamed, J. HIV Protease Inhibitor Ritonavir Induces Renal Fibrosis and Dysfunction: Role of Platelet-Derived TGF-SS1 and Intervention via Antioxidant Pathways. AIDS 2020, 34, 989–1000. [Google Scholar] [CrossRef]
- Halatsch, M.E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A Phase Ib/IIa Trial of 9 Repurposed Drugs Combined with Temozolomide for the Treatment of Recurrent Glioblastoma: CUSP9v3. Neuro-Oncol. Adv. 2021, 3, vdab075. [Google Scholar] [CrossRef]
- Diaz, R.S.; Shytaj, I.L.; Giron, L.B.; Obermaier, B.; della Libera, E.; Galinskas, J.; Dias, D.; Hunter, J.; Janini, M.; Gosuen, G.; et al. Potential Impact of the Antirheumatic Agent Auranofin on Proviral HIV-1 DNA in Individuals under Intensified Antiretroviral Therapy: Results from a Randomised Clinical Trial. Int. J. Antimicrob. Agents 2019, 54, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Coscione, F.; Zineddu, S.; Vitali, V.; Fondi, M.; Messori, L.; Perrin, E. The Many Lives of Auranofin: How an Old Anti-Rheumatic Agent May Become a Promising Antimicrobial Drug. Antibiotics 2024, 13, 652. [Google Scholar] [CrossRef]
- Wassmann, C.; Hellberg, A.; Tannich, E.; Bruchhaus, I. Metronidazole Resistance in the Protozoan Parasite Entamoeba Histolytica Is Associated with Increased Expression of Iron-Containing Superoxide Dismutase and Peroxiredoxin and Decreased Expression of Ferredoxin 1 and Flavin Reductase. J. Biol. Chem. 1999, 274, 26051–26056. [Google Scholar] [CrossRef]
- Upcroft, P.; Upcroft, J.A. Drug Targets and Mechanisms of Resistance in the Anaerobic Protozoa. Clin. Microbiol. Rev. 2001, 14, 150–164. [Google Scholar] [CrossRef]
- Debnath, A.; Parsonage, D.; Andrade, R.M.; He, C.; Cobo, E.R.; Hirata, K.; Chen, S.; García-Rivera, G.; Orozco, E.; Martínez, M.B.; et al. A High-Throughput Drug Screen for Entamoeba Histolytica Identifies a New Lead and Target. Nat. Med. 2012, 18, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Tejman-Yarden, N.; Miyamoto, Y.; Leitsch, D.; Santini, J.; Debnath, A.; Gut, J.; McKerrow, J.H.; Reed, S.L.; Eckmann, L. A Reprofiled Drug, Auranofin, Is Effective against Metronidazole-Resistant Giardia Lamblia. Antimicrob. Agents Chemother. 2013, 57, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Ocholaid, E.A.; Karanja, D.M.S.; Elliott, S.J. The Impact of Neglected Tropical Diseases (Ntds) on Health and Wellbeing in Sub-Saharan Africa (Ssa): A Case Study of Kenya. PLoS Negl. Trop. Dis. 2021, 15, e0009131. [Google Scholar] [CrossRef]
- Ilari, A.; Baiocco, P.; Messori, L.; Fiorillo, A.; Boffi, A.; Gramiccia, M.; Di Muccio, T.; Colotti, G. A Gold-Containing Drug against Parasitic Polyamine Metabolism: The X-Ray Structure of Trypanothione Reductase from Leishmania Infantum in Complex with Auranofin Reveals a Dual Mechanism of Enzyme Inhibition. Amino Acids 2012, 42, 803–811. [Google Scholar] [CrossRef]
- Auclair, M.; Guénantin, A.C.; Fellahi, S.; Garcia, M.; Capeau, J. HIV Antiretroviral Drugs, Dolutegravir, Maraviroc and Ritonavir-Boosted Atazanavir Use Different Pathways to Affect Inflammation, Senescence and Insulin Sensitivity in Human Coronary Endothelial Cells. PLoS ONE 2020, 15, e0226924. [Google Scholar] [CrossRef]
- Shirakawa, K.; Chavez, L.; Hakre, S.; Calvanese, V.; Verdin, E. Reactivation of Latent HIV by Histone Deacetylase Inhibitors. Trends Microbiol. 2013, 21, 277–285. [Google Scholar] [CrossRef]
- de Almeida Baptista, M.V.; da Silva, L.T.; Samer, S.; Oshiro, T.M.; Shytaj, I.L.; Giron, L.B.; Pena, N.M.; Cruz, N.; Gosuen, G.C.; Ferreira, P.R.A.; et al. Immunogenicity of Personalized Dendritic-Cell Therapy in HIV-1 Infected Individuals under Suppressive Antiretroviral Treatment: Interim Analysis from a Phase II Clinical Trial. AIDS Res. Ther. 2022, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Cirri, D.; Chiaverini, L.; Pratesi, A.; Marzo, T. Is the Next Cisplatin Already in Our Laboratory? Comments Inorg. Chem. 2022, 43, 465–478. [Google Scholar] [CrossRef]
- Chiaverini, L.; Di Leo, R.; Famlonga, L.; Pacini, M.; Baglini, E.; Barresi, E.; Peana, M.F.; Tolbatov, I.; Marrone, A.; La Mendola, D.; et al. The Metal(Loid)s’ Dilemma. What’s the next Step for a New Era of Inorganic Molecules in Medicine? Metallomics 2025, 17, 13. [Google Scholar] [CrossRef]
- Cirri, D.; Bartoli, F.; Pratesi, A.; Baglini, E.; Barresi, E.; Marzo, T. Strategies for the Improvement of Metal-Based Chemotherapeutic Treatments. Biomedicines 2021, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R&D 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Ma, J.; van de Sande, W.; Biersack, B. Exploring a Therapeutic Gold Mine: The Antifungal Potential of the Gold-Based Antirheumatic Drug Auranofin. Int. J. Mol. Sci. 2025, 26, 7909. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Relevance of Half-Life in Drug Design. J. Med. Chem. 2018, 61, 4273–4282. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Wu, S.; Wang, L.; Cao, X.; Zhang, X.; Dai, B.; Cao, M.; Shao, R.; Zhang, R.; et al. Auranofin-Mediated Inhibition of PI3K/AKT/MTOR Axis and Anticancer Activity in Non-Small Cell Lung Cancer Cells. Oncotarget 2016, 7, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- Deben, C.; Boullosa, L.F.; Fortes, F.R.; De La Hoz, E.C.; Le Compte, M.; Seghers, S.; Peeters, M.; Vanlanduit, S.; Lin, A.; Dijkstra, K.K.; et al. Auranofin Repurposing for Lung and Pancreatic Cancer: Low CA12 Expression as a Marker of Sensitivity in Patient-Derived Organoids, with Potentiated Efficacy by AKT Inhibition. J. Exp. Clin. Cancer Res. 2024, 43, 88. [Google Scholar] [CrossRef] [PubMed]
- Kupersmith, M.J.; Jette, N. Specific Recommendations to Improve the Design and Conduct of Clinical Trials. Trials 2023, 24, 263. [Google Scholar] [CrossRef]
- Wang, J.; Yu, B.; Dou, Y.N.; Mascaro, J. Biomarker-Driven Oncology Trial Design and Subgroup Characterization: Challenges and Potential Solutions. JCO Precis. Oncol. 2024, 8, e2400116. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Okuyama, R. Advancements in Drug Repurposing: Examples in Psychiatric Medications. Int. J. Mol. Sci. 2023, 24, 11000. [Google Scholar] [CrossRef] [PubMed]

| ClinicalTrials.gov Identifier | Indication | Sponsor | Status a | Phase b | Results c |
|---|---|---|---|---|---|
| NCT01747798 | Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | Mayo Clinic | C (2019) | Early 1 (0) | N/A |
| NCT01419691 | Chronic Lymphocytic Leukemia (CLL) | University of Kansas Medical Center | C (2016) | 2 | N/A |
| NCT02063698 | Paclitaxel-Induced Pain Syndrome | Mayo Clinic | C (2019) | 2 | Y |
| NCT01737502 | Lung cancer | Mayo Clinic | C (2024) | 1/2 | N/A |
| NCT03456700 | Ovarian Cancer | Mayo Clinic | T (2025) | 2 | Y |
| NCT02770378 | Glioblastoma | University of Ulm | C (2021) | 1/2 | N/A |
| NCT02089048 | PK | National Institute of Allergy and Infectious Diseases (NIAID) | C (2017) | 1 | N/A |
| NCT02736968 | Amoebiasis or giardiasis | National Institute of Allergy and Infectious Diseases (NIAID) | C (2023) | 2 | Y |
| NCT02961829 | HIV | Federal University of São Paulo | C (2020) | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgini, D.; Chiaverini, L.; Viviano, M.; Belvedere, R.; Salerno, S.; Baglini, E.; Da Settimo, F.; Marzo, T.; Taliani, S.; Barresi, E. Repurposing Auranofin for Oncology and Beyond: A Brief Overview of Clinical Trials as Mono- and Combination Therapy. Pharmaceuticals 2025, 18, 1628. https://doi.org/10.3390/ph18111628
Giorgini D, Chiaverini L, Viviano M, Belvedere R, Salerno S, Baglini E, Da Settimo F, Marzo T, Taliani S, Barresi E. Repurposing Auranofin for Oncology and Beyond: A Brief Overview of Clinical Trials as Mono- and Combination Therapy. Pharmaceuticals. 2025; 18(11):1628. https://doi.org/10.3390/ph18111628
Chicago/Turabian StyleGiorgini, Doralice, Lorenzo Chiaverini, Monica Viviano, Raffaella Belvedere, Silvia Salerno, Emma Baglini, Federico Da Settimo, Tiziano Marzo, Sabrina Taliani, and Elisabetta Barresi. 2025. "Repurposing Auranofin for Oncology and Beyond: A Brief Overview of Clinical Trials as Mono- and Combination Therapy" Pharmaceuticals 18, no. 11: 1628. https://doi.org/10.3390/ph18111628
APA StyleGiorgini, D., Chiaverini, L., Viviano, M., Belvedere, R., Salerno, S., Baglini, E., Da Settimo, F., Marzo, T., Taliani, S., & Barresi, E. (2025). Repurposing Auranofin for Oncology and Beyond: A Brief Overview of Clinical Trials as Mono- and Combination Therapy. Pharmaceuticals, 18(11), 1628. https://doi.org/10.3390/ph18111628









