Cyclodextrin-Based Formulations as a Promising Strategy to Overcome the Blood–Brain Barrier: Historical Overview and Prospects in Glioblastoma Treatment
Abstract
1. Introduction
2. Classification of GB
2.1. World Health Organization Classification
2.2. A Biomarker-Based Classification
2.3. Molecular Subtypes
- -
- Proneural subtype characterized by alterations in Platelet-Derived Growth Factor Receptor Alpha (PDGFRA), IDH1 mutations, and expression of oligodendrocytic lineage markers. This subtype is more common in younger patients and is associated with a relatively better prognosis. Nonetheless, the proneural subtype did not exhibit a markedly different response to chemotherapy and radiotherapy compared to the other molecular subtypes [39].
- -
- Neural subtype exhibiting gene expression profiles that closely resemble those of normal brain tissue and generally showing greater sensitivity to radiation and chemotherapy. GB expressing neural markers such as Synaptotagmin 1, Solute carrier family 12 member 5, Gamma-aminobutyric acid type A receptor alpha1, and Neurofilament light polypeptide are classified within this subtype [39].
- -
- Classical subtype characterized by distinct genomic alterations, including amplification of chromosome 7, loss of chromosome 10, inactivation of the RB (Retinoblastoma-associated protein) pathway, and focal homozygous deletion at 9p21.3. Additionally, this subtype shows high expression of components from the Sonic Hedgehog pathway, the Notch signaling pathway, and the neural precursor/stem cell marker NES. Notably, patients with the classical subtype tend to experience significantly reduced mortality when treated with aggressive radiotherapy and chemotherapy [39].
- -
- Mesenchymal subtype defined by pronounced necrosis and inflammatory activity, along with elevated expression of genes involved in angiogenesis and extracellular matrix remodeling. It frequently presents deletions in key tumor suppressor genes such as TP53, PTEN, and Neurofibromatosis type 1 (NF1), and shows strong activation of the Tumor Necrosis Factor (TNF) superfamily and NF-κB signaling pathways. Despite showing some responsiveness to intensive radiotherapy and chemotherapy, this subtype is associated with the poorest overall prognosis among GB classifications [39].
2.4. Tumor Microenvironment (TME)
3. Etiology of GB
4. Epidemiology of GB
5. Blood–Brain Barrier (BBB) in Brain Cancer
6. State of the Art of Treatment Options
6.1. Surgical Resection
6.2. Radiotherapy
6.3. Pharmacotherapy
- Procarbazine, lomustine, and vincristine regimen (PCV): Procarbazine is a DNA-alkylating agent, lomustine is a nitrosourea compound capable of crossing the BBB, and vincristine is a vinca alkaloid that disrupts microtubule formation, collectively exerting cytotoxic effects on tumor cells [113].
- Repeat surgery: If the location and size of the recurrent tumor allow for safe resection, surgical intervention can be reconsidered [116].
- Enrollment in a clinical trial: Patients may benefit from investigational therapies being tested in ongoing clinical trials [117].
6.4. Targeting Tyrosine Kinases and the Tumor Microenvironment in GB
6.4.1. EGFR-Targeting TKIs
6.4.2. TKIs with Enhanced CNS Bioavailability
6.4.3. Dacomitinib in EGFR-Mutant GB
6.4.4. Regorafenib: A Multi-Kinase Inhibitor
6.4.5. Bevacizumab in GB Management
6.4.6. Bevacizumab for Radionecrosis
6.5. Tumor Treatment Fields (TTFs)
6.6. Supportive Therapy
7. Next-Generation Therapeutics in GB: From Biotechnologies to Smart Delivery Systems
7.1. CAR-T Therapy
7.2. Virotherapy
7.3. RNA Therapy
7.4. Vaccines
7.5. Innovative Drug Delivery System
8. Cyclodextrins (CYDs)
8.1. Discovery: 1891–1911
8.2. CYD Chemistry
8.3. Cyclodextrin Derivatives: HP-β-CYD and SBE-β-CYD
- -
- The primary hydroxyl group at C6 is the most nucleophilic, making it highly reactive under strongly alkaline conditions.
- -
- The secondary hydroxyl group at C2 is the most acidic, and under mildly basic aqueous conditions, it tends to react more readily than C6.
- -
- The secondary hydroxyl group at C3, however, is sterically hindered, making it the least accessible and most difficult to modify.
8.4. CYD and Inclusion Complex Toxicity
8.5. CYDs Regulatory Status
8.6. Mechanisms of Solubility Enhancement by CYDs
8.7. CYDs in GB Treatment
8.7.1. Inclusion Complexes
8.7.2. CYDs in Liposomes
8.7.3. CYD-Based Nanocarriers
8.7.4. CYD-based Systems for RNA Delivery
8.7.5. CYDs as Diagnostic Contrast Agents
9. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDGFR-α | Platelet-derived growth factor receptor-α |
| CA | Contrast agent |
| saRNA | Self-amplifying mRNA |
| 5-ALA | 5-aminolevulinic acid |
| AED | Antiepileptic medication |
| APC | Antigen-presenting cell |
| ASCO | American Society of Clinical Oncology |
| BBB | Blood–Brain Barrier |
| BBTB | Blood–Brain Tumor Barrier |
| BP | Butylidenephthalide |
| CA-4 | Combretastatin A-4 |
| CAR-T | Chimeric Antigen Receptor T-cell |
| CBD | Cannabidiol |
| CBM | Cannabis-based medicine |
| CBT | Cognitive–behavioral therapy |
| CDKN2A/B | Cyclin-dependent kinase inhibitor 2A/B |
| CED | Convection-enhanced delivery |
| CLE | Confocal laser endomicroscopy |
| CMV | Cytomegalovirus |
| CNS | Central nervous system |
| CUR | Curcumin |
| CYD | Cyclodextrin |
| DAMP | Damage-associated molecular pattern |
| DDSs | Drug delivery systems |
| DKD | Diabetic kidney disease |
| DM-β-CYD | 2,6-O-methyl-β-CYD |
| DOAC | Oral anticoagulant |
| DSC | Differential scanning calorimetry |
| DSF | Investigating disulfiram |
| DTX | Docetaxel |
| EGFR | Epidermal growth factor receptor |
| EGFRvIII | Epidermal Growth Factor Receptor Variant III |
| EMA | European Medicines Agency |
| EORTC/NCI | European Organization for Research and Treatment of Cancer/National Cancer Institute |
| Cmax | High concentration peak |
| EPR | Permeability and retention |
| FDA | Food and Drug Administration |
| FGS | Fluorescence-guided surgery |
| FTIR | Fourier-transform infrared spectroscopy |
| GB | Glioblastoma |
| G-CIMP | Hypermethylated phenotype |
| GRAS | Generally Recognized as Safe |
| HER2 | Human Epidermal Growth Factor receptor 2 |
| HPLC | High-performance liquid chromatography |
| HP-β-CD | hydroxypropyl-β-cyclodextrin |
| ICH | Intracranial hemorrhage |
| IDH | Isocitrate dehydrogenase |
| IL-13Ra2 | Interleukin 13Rα2 Receptor |
| iMRI | Intraoperative Magnetic Resonance Imaging |
| IR | Infrared spectroscopy |
| iUS | Intraoperative ultrasound |
| LA | L-arginine |
| LMWH | Low-molecular-weight heparin |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| MTIC | 5-(3-methyl-1-triazen-1-yl)imidazole-4-carboxamide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NC | Nanocapsule |
| NEC | Not Elsewhere Classified |
| Nf1 | Neurofibromatosis type 1 |
| NK | Natural killer ì cell |
| NMR | Nuclear Magnetic Resonance |
| NOS | Not Otherwise Specified |
| NS | Nanospheres |
| NSAID | Non-steroidal anti-inflammatory drug |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| OV | Oncolytic virus |
| PAMPs | Pathogen-associated molecular patterns |
| PCV | Procarbazine, lomustine, and vincristine |
| PDGFRA | Platelet-Derived Growth Factor Receptor Alpha |
| PDT | Photodynamic therapy |
| PES | Progression-free survival |
| PFS | Progression-free survival |
| PTEN | Phosphatase and tensin homolog |
| RADIONECROSIS | Radiation-induced tissue damage |
| RAMEB | Randomly methylated β-CYD |
| RBC | Red blood cell RBC |
| RMS r | Rhabdomyosarcoma |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| RTCT | Radiotherapy and chemotherapy |
| SBE-β-CYD | Sulfobutyl ether β-cyclodextrin |
| SEM | Scanning electron microscopy |
| SF | Sodium fluorescein |
| SFN | Stabilizing sulforaphane |
| SNRI | Serotonin-norepinephrine reuptake inhibitor |
| SSRI | Selective serotonin reuptake inhibitor |
| SuccFerr | N-[4-ferrocenyl,5-5-bis(4-hydroxyphenyl)-pent-4-enyl]-succinimide |
| TAA | Tumor-associated antigen |
| TCA | Tricyclic antidepressant |
| TERT | Telomerase reverse transcriptase |
| TKs | Tyrosine kinases |
| TKIis | Tyrosine kinase inhibitors |
| TMZ | Temozolomide |
| TM-β-CYD | 2,3,6-tri-O-methyl-β-CYD |
| TNF | Tumor Necrosis Factor |
| TP53 | Tumor protein p53 |
| TTFs | Tumor Treatment Fields |
| USPIO | Ultrasmall superparamagnetic iron oxide |
| VEGF | Vascular endothelial growth factor |
| VTE | Venous thromboembolism |
| WHO | World Health Organization |
| XRD | X-ray diffraction |
| β-CYDamine | 6-monodeoxy-6-monoamino-β-CYD hydrochloride |
| βCYDgal | β-CYD derivatives with galactosamine and triazole rings |
References
- Ahmed, M.H.; Canney, M.; Carpentier, A.; Idbaih, A. Overcoming the Blood Brain Barrier in Glioblastoma: Status and Future Perspective. Rev. Neurol. 2023, 179, 430–436. [Google Scholar] [CrossRef]
- Rock, K.; McArdle, O.; Forde, P.; Dunne, M.; Fitzpatrick, D.; O’Neill, B.; Faul, C. A Clinical Review of Treatment Outcomes in Glioblastoma Multiforme--the Validation in a Non-Trial Population of the Results of a Randomised Phase III Clinical Trial: Has a More Radical Approach Improved Survival? Br. J. Radiol. 2012, 85, e729–e733. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Colopi, A.; Fuda, S.; Santi, S.; Onorato, A.; Cesarini, V.; Salvati, M.; Balistreri, C.R.; Dolci, S.; Guida, E. Impact of Age and Gender on Glioblastoma Onset, Progression, and Management. Mech. Ageing Dev. 2023, 211, 111801. [Google Scholar] [CrossRef]
- Rodgers, L.T.; Villano, J.L.; Hartz, A.M.S.; Bauer, B. Glioblastoma Standard of Care: Effects on Tumor Evolution and Reverse Translation in Preclinical Models. Cancers 2024, 16, 2638. [Google Scholar] [CrossRef]
- Rathi, S.; Griffith, J.I.; Zhang, W.; Zhang, W.; Oh, J.-H.; Talele, S.; Sarkaria, J.N.; Elmquist, W.F. The Influence of the Blood-Brain Barrier in the Treatment of Brain Tumours. J. Intern. Med. 2022, 292, 3–30. [Google Scholar] [CrossRef]
- Noorani, I.; de la Rosa, J. Breaking Barriers for Glioblastoma with a Path to Enhanced Drug Delivery. Nat. Commun. 2023, 14, 5909. [Google Scholar] [CrossRef]
- ter Linden, E.; Abels, E.R.; van Solinge, T.S.; Neefjes, J.; Broekman, M.L.D. Overcoming Barriers in Glioblastoma—Advances in Drug Delivery Strategies. Cells 2024, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Digiovanni, S.; Lorenzati, M.; Bianciotto, O.T.; Godel, M.; Fontana, S.; Akman, M.; Costamagna, C.; Couraud, P.-O.; Buffo, A.; Kopecka, J.; et al. Blood-Brain Barrier Permeability Increases with the Differentiation of Glioblastoma Cells in Vitro. Fluids Barriers CNS 2024, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Narsinh, K.H.; Perez, E.; Haddad, A.F.; Young, J.S.; Savastano, L.; Villanueva-Meyer, J.E.; Winkler, E.; de Groot, J. Strategies to Improve Drug Delivery Across the Blood–Brain Barrier for Glioblastoma. Curr. Neurol. Neurosci. Rep. 2024, 24, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Karami, N.; Taheri-Anganeh, M.; Taghvimi, S.; Tondro, G.; Khorsand, M.; Soltani Fard, E.; Sedighimehr, N.; Kazemi, M.; Rahimi Jaberi, K.; et al. Exosomes: Promising Delivery Tools for Overcoming Blood-Brain Barrier and Glioblastoma Therapy. Mol. Neurobiol. 2023, 60, 4659–4678. [Google Scholar] [CrossRef]
- Banks, W.A.; Engelke, K.; Hansen, K.M.; Bullock, K.M.; Calias, P. Modest Blood-Brain Barrier Permeability of the Cyclodextrin Kleptose: Modification by Efflux and Luminal Surface Binding. J. Pharmacol. Exp. Ther. 2019, 371, 121–129. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert. Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-Based Delivery Systems for Cancer Treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Read, 6 Min Biodexa Provides Update on Progression Free and Overall Survival in Phase 1 Study of MTX110 in Recurrent Glioblastoma. Available online: https://www.biospace.com/press-releases/biodexa-provides-update-on-progression-free-and-overall-survival-in-phase-1-study-of-mtx110-in-recurrent-glioblastoma (accessed on 6 October 2025).
- Szalontay, L.; CreveCoeur, T.; Neira, J.; Englander, Z.; Spinazzi, E.; Canoll, P.; Garvin, J.; Fino, J.; Zamoryakhin, D.; Maddocks, A.; et al. A Phase I Study Examining the Feasibility of Intermittent Convection-Enhanced Delivery (CED) of MTX110 for the Treatment of Children with Newly Diagnosed Diffuse Midline Gliomas. Neuro Oncol. 2024, 26 (Suppl. 4), 0. [Google Scholar] [CrossRef]
- Conroy, R. MTX110 May Show Survival Benefits in Recurrent Glioblastoma|CancerNetwork. Available online: https://www.cancernetwork.com/view/mtx110-may-show-survival-benefits-in-recurrent-glioblastoma (accessed on 6 October 2025).
- Mrugala, M.M. Advances and Challenges in the Treatment of Glioblastoma: A Clinician’s Perspective. Discov. Med. 2013, 15, 221–230. [Google Scholar]
- Khaddour, K.; Johanns, T.M.; Ansstas, G. The Landscape of Novel Therapeutics and Challenges in Glioblastoma Multiforme: Contemporary State and Future Directions. Pharmaceuticals 2020, 13, 389. [Google Scholar] [CrossRef]
- Devi, L.S.; Casadidio, C.; Gigliobianco, M.R.; Di Martino, P.; Censi, R. Multifunctionality of Cyclodextrin-Based Polymeric Nanoparticulate Delivery Systems for Chemotherapeutics, Combination Therapy, and Theranostics. Int. J. Pharm. 2024, 654, 123976. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Meng, B.; Chen, Q. Cyclodextrin-Containing Drug Delivery Systems and Their Applications in Neurodegenerative Disorders. Int. J. Mol. Sci. 2024, 25, 10834. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, D.N.; Niculescu, A.-G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. The Definition of Primary and Secondary Glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef]
- Gavrilovic, I.T.; Posner, J.B. Brain Metastases: Epidemiology and Pathophysiology. J. Neurooncol. 2005, 75, 5–14. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW Update 3: Recommended Diagnostic Criteria for “Diffuse Astrocytic Glioma, IDH-Wildtype, with Molecular Features of Glioblastoma, WHO Grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hänggi, D.; Wick, W.; et al. Distribution of EGFR Amplification, Combined Chromosome 7 Gain and Chromosome 10 Loss, and TERT Promoter Mutation in Brain Tumors and Their Potential for the Reclassification of IDHwt Astrocytoma to Glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Dehais, C.; Colin, C.; Alentorn, A.; Carpentier, C.; Ducray, F.; Ibdaih, A.; Kamoun, A.; Mokhtari, K.; Tabouret, E.; et al. PL1.1 CDKN2A Homozygous Deletion Is a Strong Adverse Prognosis Factor in Diffuse Malignant IDHmutant Gliomas. Neuro-Oncology 2019, 21, iii1. [Google Scholar] [CrossRef]
- Gue, R.; Lakhani, D.A. The 2021 World Health Organization Central Nervous System Tumor Classification: The Spectrum of Diffuse Gliomas. Biomedicines 2024, 12, 1349. [Google Scholar] [CrossRef]
- Louis, D.N.; Wesseling, P.; Paulus, W.; Giannini, C.; Batchelor, T.T.; Cairncross, J.G.; Capper, D.; Figarella-Branger, D.; Lopes, M.B.; Wick, W.; et al. cIMPACT-NOW Update 1: Not Otherwise Specified (NOS) and Not Elsewhere Classified (NEC). Acta Neuropathol. 2018, 135, 481–484. [Google Scholar] [CrossRef]
- Sareen, H.; Ma, Y.; Becker, T.M.; Roberts, T.L.; de Souza, P.; Powter, B. Molecular Biomarkers in Glioblastoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 8835. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Genetic Pathways to Primary and Secondary Glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Xu, C.; Hou, P.; Li, X.; Xiao, M.; Zhang, Z.; Li, Z.; Xu, J.; Liu, G.; Tan, Y.; Fang, C. Comprehensive Understanding of Glioblastoma Molecular Phenotypes: Classification, Characteristics, and Transition. Cancer Biol. Med. 2024, 21, 363–381. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef] [PubMed]
- Colman, H.; Zhang, L.; Sulman, E.P.; McDonald, J.M.; Shooshtari, N.L.; Rivera, A.; Popoff, S.; Nutt, C.L.; Louis, D.N.; Cairncross, J.G.; et al. A Multigene Predictor of Outcome in Glioblastoma. Neuro-Oncology 2010, 12, 49–57. [Google Scholar] [CrossRef] [PubMed]
- White, J.; White, M.P.J.; Wickremesekera, A.; Peng, L.; Gray, C. The Tumour Microenvironment, Treatment Resistance and Recurrence in Glioblastoma. J. Transl. Med. 2024, 22, 540. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor Microenvironment in Glioblastoma: Current and Emerging Concepts. Neuro Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, Y. Unraveling the Immunosuppressive Microenvironment of Glioblastoma and Advancements in Treatment. Front. Immunol. 2025, 16, 1590781. [Google Scholar] [CrossRef]
- Tataranu, L.G.; Turliuc, S.; Kamel, A.; Rizea, R.E.; Dricu, A.; Staicu, G.-A.; Baloi, S.C.; Rodriguez, S.M.B.; Manole, A.I.M. Glioblastoma Tumor Microenvironment: An Important Modulator for Tumoral Progression and Therapy Resistance. Curr. Issues Mol. Biol. 2024, 46, 9881–9894. [Google Scholar] [CrossRef] [PubMed]
- Kanderi, T.; Munakomi, S.; Gupta, V. Glioblastoma Multiforme. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hodges, L.C.; Smith, J.L.; Garrett, A.; Tate, S. Prevalence of Glioblastoma Multiforme in Subjects with Prior Therapeutic Radiation. J. Neurosci. Nurs. 1992, 24, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Yamasaki, F.; Amatya, V.J.; Takayasu, T.; Yonezawa, U.; Taguchi, A.; Ohba, S.; Takeshima, Y.; Horie, N.; Sugiyama, K. Characteristics and Therapeutic Strategies of Radiation-Induced Glioma: Case Series and Comprehensive Literature Review. J. Neurooncol. 2022, 159, 531–538. [Google Scholar] [CrossRef]
- Smith, C.J.; Perfetti, T.A.; Chokshi, C.; Venugopal, C.; Ashford, J.W.; Singh, S.K. Risk Factors for Glioblastoma Are Shared by Other Brain Tumor Types. Hum. Exp. Toxicol. 2024, 43, 09603271241241796. [Google Scholar] [CrossRef]
- Yang, T.; Liu, D.; Fang, S.; Ma, W.; Wang, Y. Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. J. Clin. Med. 2022, 11, 5221. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, F.; Toniolo, P.; Cole, P.; Salcman, M. Nonoccupational Risk Indicators of Glioblastoma in Adults. J. Neurooncol. 1990, 8, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Braganza, M.Z.; Rajaraman, P.; Park, Y.; Inskip, P.D.; Freedman, N.D.; Hollenbeck, A.R.; de González, A.B.; Kitahara, C.M. Cigarette Smoking, Alcohol Intake, and Risk of Glioma in the NIH-AARP Diet and Health Study. Br. J. Cancer 2014, 110, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; Codon Publications: Brisbane, Australia, 2017; pp. 143–153. [Google Scholar] [CrossRef]
- Baglietto, L.; Giles, G.G.; English, D.R.; Karahalios, A.; Hopper, J.L.; Severi, G. Alcohol Consumption and Risk of Glioblastoma; Evidence from the Melbourne Collaborative Cohort Study. Int. J. Cancer 2011, 128, 1929–1934. [Google Scholar] [CrossRef]
- Slika, H.; Karimov, Z.; Alimonti, P.; Abou-Mrad, T.; De Fazio, E.; Alomari, S.; Tyler, B. Preclinical Models and Technologies in Glioblastoma Research: Evolution, Current State, and Future Avenues. Int. J. Mol. Sci. 2023, 24, 16316. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical Presentation, Diagnosis, and Management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex Differences in GBM Revealed by Analysis of Patient Imaging, Transcriptome, and Survival Data. Sci. Transl. Med. 2019, 11, eaao5253. [Google Scholar] [CrossRef]
- Bilello, M.; Akbari, H.; Da, X.; Pisapia, J.M.; Mohan, S.; Wolf, R.L.; O’Rourke, D.M.; Martinez-Lage, M.; Davatzikos, C. Population-Based MRI Atlases of Spatial Distribution Are Specific to Patient and Tumor Characteristics in Glioblastoma. NeuroImage Clin. 2016, 12, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Sun, C.-R.; He, M.; Yin, L.-C.; Du, H.-G.; Zhang, J.-M. Correlation Between Tumor Location and Clinical Properties of Glioblastomas in Frontal and Temporal Lobes. World Neurosurg. 2018, 112, e407–e414. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef]
- Malmer, B.; Henriksson, R.; Grönberg, H. Familial Brain Tumours—Genetics or Environment? A Nationwide Cohort Study of Cancer Risk in Spouses and First-Degree Relatives of Brain Tumour Patients. Int. J. Cancer 2003, 106, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Monterroso, P.; Moore, K.J.; Sample, J.M.; Sorajja, N.; Domingues, A.; Williams, L.A. Racial/Ethnic and Sex Differences in Young Adult Malignant Brain Tumor Incidence by Histologic Type. Cancer Epidemiol. 2022, 76, 102078. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood-Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Dréan, A.; Goldwirt, L.; Verreault, M.; Canney, M.; Schmitt, C.; Guehennec, J.; Delattre, J.-Y.; Carpentier, A.; Idbaih, A. Blood-Brain Barrier, Cytotoxic Chemotherapies and Glioblastoma. Expert. Rev. Neurother. 2016, 16, 1285–1300. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to Improve Delivery of Anticancer Drugs across the Blood–Brain Barrier to Treat Glioblastoma. Neuro-Oncology 2016, 18, 27–36. [Google Scholar] [CrossRef]
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue Concentration of Systemically Administered Antineoplastic Agents in Human Brain Tumors. J. Neuro-Oncol. 2011, 104, 629–638. [Google Scholar] [CrossRef]
- Hartz, A.M.S.; Bauer, B. ABC Transporters in the CNS—An Inventory. Curr. Pharm. Biotechnol. 2011, 12, 656–673. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Su, B.; Wang, R.; Xie, Z.; Ruan, H.; Li, J.; Xie, C.; Lu, W.; Wang, J.; Wang, D.; Liu, M. Effect of Retro-Inverso Isomer of Bradykinin on Size-Dependent Penetration of Blood-Brain Tumor Barrier. Small 2018, 14, 1702331. [Google Scholar] [CrossRef]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the Blood-Brain Tumor Barrier for Effective Glioblastoma Treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the Blood–Brain Barrier for the Therapy of Malignant Brain Tumor: Current Status and Prospects of Drug Delivery Approaches. J. Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Feng, Y.; Gao, S.; Mu, Q.; Liu, C. Nanotherapeutics Overcoming the Blood-Brain Barrier for Glioblastoma Treatment. Front. Pharmacol. 2021, 12, 786700. [Google Scholar] [CrossRef]
- Nikfar, B.; Musavi, M.; Chaichian, S.; Guo, G.; Momtazi-Borojeni, A.A. Nanomotor-Mediated Drug Delivery with Efficient Blood–Brain Barrier Crossing for Active Targeting and Therapy of Glioblastomas: A Systematic Review. Nanoscale 2025, 17, 16592–16608. [Google Scholar] [CrossRef]
- Sales, A.H.A.; Beck, J.; Schnell, O.; Fung, C.; Meyer, B.; Gempt, J. Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends. J. Clin. Med. 2022, 11, 5354. [Google Scholar] [CrossRef]
- Barani, I.J.; Larson, D.A. Radiation Therapy of Glioblastoma. Cancer Treat. Res. 2015, 163, 49–73. [Google Scholar] [CrossRef]
- Sevastre, A.-S.; Costachi, A.; Tataranu, L.G.; Brandusa, C.; Artene, S.A.; Stovicek, O.; Alexandru, O.; Danoiu, S.; Sfredel, V.; Dricu, A. Glioblastoma Pharmacotherapy: A Multifaceted Perspective of Conventional and Emerging Treatments (Review). Exp. Ther. Med. 2021, 22, 1408. [Google Scholar] [CrossRef]
- Goel, A. High-Grade Gliomas—Is Radical Resection Needed? Is Radical Resection Possible? Is Surgery Necessary? J. Craniovertebr Junction Spine 2023, 14, 113–115. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Broekman, M.L.D.; De Vleeschouwer, S.; Schucht, P.; Nahed, B.V.; Berger, M.S.; Vincent, A.J.P.E. Safe Surgery for Glioblastoma: Recent Advances and Modern Challenges. Neurooncol. Pract. 2022, 9, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lombardi, G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers 2020, 13, 47. [Google Scholar] [CrossRef]
- Patel, V.; Chavda, V. Intraoperative Glioblastoma Surgery-Current Challenges and Clinical Trials: An Update. Cancer Pathog. Ther. 2024, 2, 256–267. [Google Scholar] [CrossRef]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-Guided Surgery in the Surgical Treatment of Gliomas: Past, Present and Future. Cancers 2021, 13, 3508. [Google Scholar] [CrossRef]
- Chirizzi, C.; Pellegatta, S.; Gori, A.; Falco, J.; Rubiu, E.; Acerbi, F.; Bombelli, F.B. Next-Generation Agents for Fluorescence-Guided Glioblastoma Surgery. Bioeng. Transl. Med. 2024, 9, e10608. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Greco Crasto, S.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci. 2022, 12, 555. [Google Scholar] [CrossRef]
- Choudhri, A.F.; Siddiqui, A.; Klimo, P.; Boop, F.A. Intraoperative MRI in Pediatric Brain Tumors. Pediatr. Radiol. 2015, 45, 397–405. [Google Scholar] [CrossRef]
- Moiyadi, A.V. Intraoperative Ultrasound Technology in Neuro-Oncology Practice—Current Role and Future Applications. World Neurosurg. 2016, 93, 81–93. [Google Scholar] [CrossRef]
- Romanishkin, I.; Savelieva, T.; Kosyrkova, A.; Okhlopkov, V.; Shugai, S.; Orlov, A.; Kravchuk, A.; Goryaynov, S.; Golbin, D.; Pavlova, G.; et al. Differentiation of Glioblastoma Tissues Using Spontaneous Raman Scattering with Dimensionality Reduction and Data Classification. Front. Oncol. 2022, 12, 944210. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Morshed, R.A.; Chunduru, P.; Pandian, B.; Young, J.; Villanueva-Meyer, J.E.; Tihan, T.; Sloan, E.A.; Aghi, M.K.; Molinaro, A.M.; et al. Detection of Glioma Infiltration at the Tumor Margin Using Quantitative Stimulated Raman Scattering Histology. Sci. Rep. 2021, 11, 12162. [Google Scholar] [CrossRef] [PubMed]
- Herta, J.; Cho, A.; Roetzer-Pejrimovsky, T.; Höftberger, R.; Marik, W.; Kronreif, G.; Peilnsteiner, T.; Rössler, K.; Wolfsberger, S. Optimizing Maximum Resection of Glioblastoma: Raman Spectroscopy versus 5-Aminolevulinic Acid. J. Neurosurg. 2023, 139, 334–343. [Google Scholar] [CrossRef]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative Imaging of Brain Tumors with Fluorescein: Confocal Laser Endomicroscopy in Neurosurgery. Clinical and User Experience. Neurosurg. Focus. 2021, 50, E19. [Google Scholar] [CrossRef]
- Abdul-Al, M.; Saeinasab, M.; Zare, A.; Barati, M.; Shakeri, S.; Keykhosravi, E.; Momeni-Moghaddam, M.; Najafzadeh, M.; Keshel, S.H.; Farzi, G.; et al. Application of Biomaterials for Glioblastoma Treatment: Promises, Advances, and Challenges. Mater. Today Commun. 2022, 33, 104562. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Mann, J.; Ramakrishna, R.; Magge, R.; Wernicke, A.G. Advances in Radiotherapy for Glioblastoma. Front. Neurol. 2018, 8, 748. [Google Scholar] [CrossRef]
- Dhermain, F. Radiotherapy of High-Grade Gliomas: Current Standards and New Concepts, Innovations in Imaging and Radiotherapy, and New Therapeutic Approaches. Chin. J. Cancer 2014, 33, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sheline, G.E. Radiation Therapy of Brain Tumors. Cancer 1977, 39, 873–881. [Google Scholar] [CrossRef]
- Skowrońska-Gardas, A. Radiotherapy of Central Nervous System Tumors in Young Children: Benefits and Pitfalls. Med. Pediatr. Oncol. 1999, 33, 572–576. [Google Scholar] [CrossRef]
- Grunert, M.; Kassubek, R.; Danz, B.; Klemenz, B.; Hasslacher, S.; Stroh, S.; Schneele, L.; Langhans, J.; Ströbele, S.; Barry, S.E.; et al. Radiation and Brain Tumors: An Overview. Crit. Rev. Oncog. 2018, 23, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, K.; Abbas, I.; Karandish, A.; Crisman, C.; Eskandar, E.N.; Kobets, A.J. Cognitive Decline in Glioblastoma (GB) Patients with Different Treatment Modalities and Insights on Untreated Cases. Curr. Oncol. 2025, 32, 152. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; T P, A.H.; A J, J.; K M, S.D.; Narayanasamy, A.; Vellingiri, B. Recent Advances in Radiotherapy and Its Associated Side Effects in Cancer—A Review. JoBAZ 2019, 80, 14. [Google Scholar] [CrossRef]
- Fernández, C.; Ciérvide, R.; Díaz, A.; Garrido, I.; Couñago, F. Radiotherapy in Glioblastoma Multiforme: Evolution, Limitations, and Molecularly Guided Future. Biomedicines 2025, 13, 2136. [Google Scholar] [CrossRef]
- Barisano, G.; Bergamaschi, S.; Acharya, J.; Rajamohan, A.; Gibbs, W.; Kim, P.; Zada, G.; Chang, E.; Law, M. Complications of Radiotherapy and Radiosurgery in the Brain and Spine. Neurographics 2018, 8, 167–187. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Liu, H.-L.; Huang, C.-Y.; Chen, J.-Y.; Wang, H.-Y.J.; Chen, P.-Y.; Wei, K.-C. Pharmacodynamic and Therapeutic Investigation of Focused Ultrasound-Induced Blood-Brain Barrier Opening for Enhanced Temozolomide Delivery in Glioma Treatment. PLoS ONE 2014, 9, e114311. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes. Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, J.; Tamangani, J.; Senthil, L.; Cruickshank, G.; Spooner, D.; Jones, B.; Brookes, C.; Sanghera, P. Patterns of Relapse in Glioblastoma Multiforme Following Concomitant Chemoradiotherapy with Temozolomide. Br. J. Radiol. 2013, 86, 20120414. [Google Scholar] [CrossRef]
- Patel, M.; McCully, C.; Godwin, K.; Balis, F.M. Plasma and Cerebrospinal Fluid Pharmacokinetics of Intravenous Temozolomide in Non-Human Primates. J. Neurooncol. 2003, 61, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.; Tsang, L.L.; Slack, J.A. NMR and Molecular Modeling Investigation of the Mechanism of Activation of the Antitumor Drug Temozolomide and Its Interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of Temozolomide Resistance in Glioblastoma—A Comprehensive Review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Garcia, M.; Álvarez-Linera, J.; Carrato, C.; Ley, L.; Luque, R.; Maldonado, X.; Martínez-Aguillo, M.; Navarro, L.M.; Vaz-Salgado, M.A.; Gil-Gil, M. SEOM Clinical Guidelines for Diagnosis and Treatment of Glioblastoma (2017). Clin. Transl. Oncol. 2018, 20, 22–28. [Google Scholar] [CrossRef]
- Solimando, D.A.; Waddell, J.A. Procarbazine, Lomustine, and Vincristine (PCV) Regimen for Central Nervous System Tumors. Hosp. Pharm. 2017, 52, 98–104. [Google Scholar] [CrossRef]
- Wick, A.; Pascher, C.; Wick, W.; Jauch, T.; Weller, M.; Bogdahn, U.; Hau, P. Rechallenge with Temozolomide in Patients with Recurrent Gliomas. J. Neurol. 2009, 256, 734–741. [Google Scholar] [CrossRef]
- Perry, J.R.; Rizek, P.; Cashman, R.; Morrison, M.; Morrison, T. Temozolomide Rechallenge in Recurrent Malignant Glioma by Using a Continuous Temozolomide Schedule: The “Rescue” Approach. Cancer 2008, 113, 2152–2157. [Google Scholar] [CrossRef]
- Kalita, O.; Kazda, T.; Reguli, S.; Jancalek, R.; Fadrus, P.; Slachta, M.; Pospisil, P.; Krska, L.; Vrbkova, J.; Hrabalek, L.; et al. Effects of Reoperation Timing on Survival among Recurrent Glioblastoma Patients: A Retrospective Multicentric Descriptive Study. Cancers 2023, 15, 2530. [Google Scholar] [CrossRef]
- Valerius, A.R.; Webb, L.M.; Thomsen, A.; Lehrer, E.J.; Breen, W.G.; Campian, J.L.; Riviere-Cazaux, C.; Burns, T.C.; Sener, U. Review of Novel Surgical, Radiation, and Systemic Therapies and Clinical Trials in Glioblastoma. Int. J. Mol. Sci. 2024, 25, 10570. [Google Scholar] [CrossRef]
- Roux, A.; Peeters, S.; Zanello, M.; Bou Nassif, R.; Abi Lahoud, G.; Dezamis, E.; Parraga, E.; Lechapt-Zalcmann, E.; Dhermain, F.; Dumont, S.; et al. Extent of Resection and Carmustine Wafer Implantation Safely Improve Survival in Patients with a Newly Diagnosed Glioblastoma: A Single Center Experience of the Current Practice. J. Neurooncol. 2017, 135, 83–92. [Google Scholar] [CrossRef]
- Xiao, Z.-Z.; Wang, Z.-F.; Lan, T.; Huang, W.-H.; Zhao, Y.-H.; Ma, C.; Li, Z.-Q. Carmustine as a Supplementary Therapeutic Option for Glioblastoma: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Mahaley, M.S.; Vick, N.A.; Black, K.L.; Schold, S.C.; Burger, P.C.; Friedman, A.H.; Ciric, I.S.; Eller, T.W.; Cozzens, J.W. Interstitial Chemotherapy with Drug Polymer Implants for the Treatment of Recurrent Gliomas. J. Neurosurg. 1991, 74, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mrugala, M.M.; Crew, L.K.; Fink, J.R.; Spence, A.M. Carboplatin and Bevacizumab for Recurrent Malignant Glioma. Oncol. Lett. 2012, 4, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E.; Dowell, J.M.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Wagner, M.; et al. Phase II Trial of Bevacizumab and Irinotecan in Recurrent Malignant Glioma. Clin. Cancer Res. 2007, 13, 1253–1259. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Sampson, J.H.; Sathornsumetee, S.; McLendon, R.E.; Herndon, J.E.; Marcello, J.E.; Norfleet, J.; et al. Metronomic Chemotherapy with Daily, Oral Etoposide plus Bevacizumab for Recurrent Malignant Glioma: A Phase II Study. Br. J. Cancer 2009, 101, 1986–1994. [Google Scholar] [CrossRef]
- Sathornsumetee, S.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Marcello, J.; Herndon, J.E.; Mathe, A.; Hamilton, M.; Rich, J.N.; Norfleet, J.A.; et al. Phase II Trial of Bevacizumab and Erlotinib in Patients with Recurrent Malignant Glioma. Neuro Oncol. 2010, 12, 1300–1310. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Peters, K.B.; Gururangan, S.; Sampson, J.H.; McLendon, R.E.; Herndon, J.E.; Bulusu, A.; Threatt, S.; Friedman, A.H.; et al. Phase II Study of Carboplatin, Irinotecan, and Bevacizumab for Bevacizumab Naïve, Recurrent Glioblastoma. J. Neurooncol. 2012, 107, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Foroughi-Nia, B.; Barar, J.; Memar, M.Y.; Aghanejad, A.; Davaran, S. Progresses in Polymeric Nanoparticles for Delivery of Tyrosine Kinase Inhibitors. Life Sci. 2021, 278, 119642. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ko, Y.T. Small Molecule Tyrosine Kinase Inhibitors in Glioblastoma. Arch. Pharm. Res. 2020, 43, 385–394. [Google Scholar] [CrossRef]
- Pearson, J.R.D.; Regad, T. Targeting Cellular Pathways in Glioblastoma Multiforme. Signal Transduct. Target. Ther. 2017, 2, 17040. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, E.D.G.; Zhang, L.; Wu, J.; Daly, R.J. The Kinome “at Large” in Cancer. Nat. Rev. Cancer 2016, 16, 83–98. [Google Scholar] [CrossRef]
- Brar, H.K.; Jose, J.; Wu, Z.; Sharma, M. Tyrosine Kinase Inhibitors for Glioblastoma Multiforme: Challenges and Opportunities for Drug Delivery. Pharmaceutics 2022, 15, 59. [Google Scholar] [CrossRef]
- Ezzati, S.; Salib, S.; Balasubramaniam, M.; Aboud, O. Epidermal Growth Factor Receptor Inhibitors in Glioblastoma: Current Status and Future Possibilities. Int. J. Mol. Sci. 2024, 25, 2316. [Google Scholar] [CrossRef]
- D’Incecco, A.; Cappuzzo, F. Gefitinib for Non-Small-Cell Lung Cancer Treatment. Expert. Opin. Drug Saf. 2011, 10, 987–996. [Google Scholar] [CrossRef]
- Shin, J.E.; Jung, H.A.; Park, S.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Ahn, M.-J.; Shim, B.Y. Real-World Data of Dacomitinib as First-Line Treatment for Patients with EGFR-Mutant Non-Small-Cell Lung Cancer. Sci. Rep. 2025, 15, 4593. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-Generation EGFR and ALK Inhibitors: Mechanisms of Resistance and Management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Poels, K.E.; Schoenfeld, A.J.; Makhnin, A.; Tobi, Y.; Wang, Y.; Frisco-Cabanos, H.; Chakrabarti, S.; Shi, M.; Napoli, C.; McDonald, T.O.; et al. Identification of Optimal Dosing Schedules of Dacomitinib and Osimertinib for a Phase I/II Trial in Advanced EGFR-Mutant Non-Small Cell Lung Cancer. Nat. Commun. 2021, 12, 3697. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Tseng, J.-S.; Hsu, K.-H.; Chen, K.-C.; Su, K.-Y.; Yu, S.-L.; Chen, J.J.W.; Yang, T.-Y.; Chang, G.-C. The Impact of Different First-Line EGFR-TKIs on the Clinical Outcome of Sequential Osimertinib Treatment in Advanced NSCLC with Secondary T790M. Sci. Rep. 2021, 11, 12084. [Google Scholar] [CrossRef]
- Wang, Z.; Peet, N.P.; Zhang, P.; Jiang, Y.; Rong, L. Current Development of Glioblastoma Therapeutic Agents. Mol. Cancer Ther. 2021, 20, 1521–1532. [Google Scholar] [CrossRef]
- Sepúlveda, J.M.; Zahonero, C.; Hernandez-Lain, A.; Perez-Nuñez, A.; Bolós, M.V.; Sanhez, P. Targeting EGFR in Glioblastoma: Preclinical Testing of Dacomitinib. JCO 2014, 32, e13015. [Google Scholar] [CrossRef]
- Sepúlveda-Sánchez, J.M.; Vaz, M.Á.; Balañá, C.; Gil-Gil, M.; Reynés, G.; Gallego, Ó.; Martínez-García, M.; Vicente, E.; Quindós, M.; Luque, R.; et al. Phase II Trial of Dacomitinib, a Pan-Human EGFR Tyrosine Kinase Inhibitor, in Recurrent Glioblastoma Patients with EGFR Amplification. Neuro Oncol. 2017, 19, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib Compared with Lomustine in Patients with Relapsed Glioblastoma (REGOMA): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhou, Z.; Huang, X.; Chen, Z.; Zhang, L.; Zhang, J.; Hua, W.; Mao, Y. Use of Bevacizumab in Recurrent Glioblastoma: A Scoping Review and Evidence Map. BMC Cancer 2023, 23, 544. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Pace, A.; Dirven, L.; Koekkoek, J.A.F.; Golla, H.; Fleming, J.; Rudà, R.; Marosi, C.; Le Rhun, E.; Grant, R.; Oliver, K.; et al. European Association for Neuro-Oncology (EANO) Guidelines for Palliative Care in Adults with Glioma. Lancet Oncol. 2017, 18, e330–e340. [Google Scholar] [CrossRef]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-Angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef]
- Mayenga, M.; Falvo, N.; Mahé, I.; Jannot, A.-S.; Gazeau, B.; Meyer, G.; Gendron, N.; Sanchez, O.; Djennaoui, S.; Planquette, B. Cancer-Associated Thrombosis on Bevacizumab: Risk of Recurrence and Bleeding When Bevacizumab Is Stopped or Continued. Cancers 2023, 15, 3893. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Klein, M.; Smits, M.; Reijneveld, J.C.; French, P.J.; Clement, P.; de Vos, F.Y.F.; Wick, A.; Mulholland, P.J.; Taphoorn, M.J.B.; et al. Bevacizumab and Temozolomide in Patients with First Recurrence of WHO Grade II and III Glioma, without 1p/19q Co-Deletion (TAVAREC): A Randomised Controlled Phase 2 EORTC Trial. Lancet Oncol. 2018, 19, 1170–1179. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Reardon, D.A.; de Groot, J.F.; Wick, W.; Weller, M. Antiangiogenic Therapy for Glioblastoma: Current Status and Future Prospects. Clin. Cancer Res. 2014, 20, 5612–5619. [Google Scholar] [CrossRef]
- Pless, M.; Weinberg, U. Tumor Treating Fields: Concept, Evidence and Future. Expert. Opin. Investig. Drugs 2011, 20, 1099–1106. [Google Scholar] [CrossRef]
- Burri, S.H.; Gondi, V.; Brown, P.D.; Mehta, M.P. The Evolving Role of Tumor Treating Fields in Managing Glioblastoma: Guide for Oncologists. Am. J. Clin. Oncol. 2018, 41, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; Pacheco, P.; Stupp, R. Tumor Treating Fields: A Novel Treatment Modality and Its Use in Brain Tumors. Neuro Oncol. 2016, 18, 1338–1349. [Google Scholar] [CrossRef]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Renovanz, M.; Maurer, D.; Lahr, H.; Weimann, E.; Deininger, M.; Wirtz, C.R.; Ringel, F.; Singer, S.; Coburger, J. Supportive Care Needs in Glioma Patients and Their Caregivers in Clinical Practice: Results of a Multicenter Cross-Sectional Study. Front. Neurol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; ISBN 978-0-9944381-2-6. [Google Scholar]
- Forsyth, P.A.; Weaver, S.; Fulton, D.; Brasher, P.M.A.; Sutherland, G.; Stewart, D.; Hagen, N.A.; Barnes, P.; Cairncross, J.G.; DeAngelis, L.M. Prophylactic Anticonvulsants in Patients with Brain Tumour. Can. J. Neurol. Sci. 2003, 30, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.J.; Cole, B.F.; Friedberg, M.H.; Lathi, E.; Choy, H.; Furie, K.; Akerley, W.; Wahlberg, L.; Lekos, A.; Louis, S. A Randomized, Blinded, Placebo-Controlled Trial of Divalproex Sodium Prophylaxis in Adults with Newly Diagnosed Brain Tumors. Neurology 1996, 46, 985–991. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Ohmura, K.; Tomita, H.; Hara, A. Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines 2023, 11, 2731. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Flowers, C.R. Antimicrobial Prophylaxis for Adult Patients with Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018, 14, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, S.; LeBoff, M.S. Osteoporosis: Prevention and Treatment. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Weyant, R.B.; Kabbani, D.; Doucette, K.; Lau, C.; Cervera, C. Pneumocystis Jirovecii: A Review with a Focus on Prevention and Treatment. Expert. Opin. Pharmacother. 2021, 22, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Zoccarato, M.; Nardetto, L.; Basile, A.M.; Giometto, B.; Zagonel, V.; Lombardi, G. Seizures, Edema, Thrombosis, and Hemorrhages: An Update Review on the Medical Management of Gliomas. Front. Oncol. 2021, 11, 617966. [Google Scholar] [CrossRef] [PubMed]
- Edwin, N.C.; Khoury, M.N.; Sohal, D.; McCrae, K.R.; Ahluwalia, M.S.; Khorana, A.A. Recurrent Venous Thromboembolism in Glioblastoma. Thromb. Res. 2016, 137, 184–188. [Google Scholar] [CrossRef]
- Lee, A.Y.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-Molecular-Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef]
- Carney, B.J.; Uhlmann, E.J.; Puligandla, M.; Mantia, C.; Weber, G.M.; Neuberg, D.S.; Zwicker, J.I. Intracranial Hemorrhage with Direct Oral Anticoagulants in Patients with Brain Tumors. J. Thromb. Haemost. 2019, 17, 72–76. [Google Scholar] [CrossRef]
- Beevers, Z.; Hussain, S.; Boele, F.W.; Rooney, A.G. Pharmacological Treatment of Depression in People with a Primary Brain Tumour. Cochrane Database Syst. Rev. 2020, 2020, CD006932. [Google Scholar] [CrossRef]
- Bergo, E.; Lombardi, G.; Pambuku, A.; Della Puppa, A.; Bellu, L.; D’Avella, D.; Zagonel, V. Cognitive Rehabilitation in Patients with Gliomas and Other Brain Tumors: State of the Art. Biomed. Res. Int. 2016, 2016, 3041824. [Google Scholar] [CrossRef]
- Boele, F.W.; Douw, L.; de Groot, M.; van Thuijl, H.F.; Cleijne, W.; Heimans, J.J.; Taphoorn, M.J.B.; Reijneveld, J.C.; Klein, M. The Effect of Modafinil on Fatigue, Cognitive Functioning, and Mood in Primary Brain Tumor Patients: A Multicenter Randomized Controlled Trial. Neuro Oncol. 2013, 15, 1420–1428. [Google Scholar] [CrossRef]
- Butler, J.M.; Case, L.D.; Atkins, J.; Frizzell, B.; Sanders, G.; Griffin, P.; Lesser, G.; McMullen, K.; McQuellon, R.; Naughton, M.; et al. A Phase III, Double-Blind, Placebo-Controlled Prospective Randomized Clinical Trial of d-Threo-Methylphenidate HCl in Brain Tumor Patients Receiving Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Rapp, S.R.; Case, L.D.; Peiffer, A.; Naughton, M.M.; Chan, M.D.; Stieber, V.W.; Moore, D.F.; Falchuk, S.C.; Piephoff, J.V.; Edenfield, W.J.; et al. Donepezil for Irradiated Brain Tumor Survivors: A Phase III Randomized Placebo-Controlled Clinical Trial. J. Clin. Oncol. 2015, 33, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838–6858. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. ESMO Guidelines Committee Management of Cancer Pain in Adult Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; De Conno, F.; Fallon, M.; Hanna, M.; et al. Use of Opioid Analgesics in the Treatment of Cancer Pain: Evidence-Based Recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-155039-0. [Google Scholar]
- Boland, E.G.; Bennett, M.I.; Allgar, V.; Boland, J.W. Cannabinoids for Adult Cancer-Related Pain: Systematic Review and Meta-Analysis. BMJ Support. Palliat. Care 2020, 10, 14–24. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, F.; Ali, H.; Lathia, J.D.; Chen, P. Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives. Cell Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef]
- Sun, B.; Li, R.; Ji, N.; Liu, H.; Wang, H.; Chen, C.; Bai, L.; Su, J.; Chen, J. Brain-Targeting Drug Delivery Systems: The State of the Art in Treatment of Glioblastoma. Mater. Today Bio 2025, 30, 101443. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.; Than, V.T. RNA Therapeutics in Cancer Treatment. Prog. Mol. Biol. Transl. Sci. 2024, 203, 197–223. [Google Scholar] [CrossRef]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Z.; Chen, Y.; Xu, J.; Wang, J.; Wang, Z. Enhancing Cancer Therapy: The Integration of Oncolytic Virus Therapy with Diverse Treatments. Cancer Cell Int. 2024, 24, 242. [Google Scholar] [CrossRef]
- He, W.; Zhang, M.; Zhong, Y.; Gao, Y.; Fan, D.; Lu, X. Diverse Nanoparticles Deliver mRNA to Enhance Tumor Immunotherapy. BMB Rep. 2025, 58, 124–132. [Google Scholar] [CrossRef]
- Fu, R.; Qi, R.; Xiong, H.; Lei, X.; Jiang, Y.; He, J.; Chen, F.; Zhang, L.; Qiu, D.; Chen, Y.; et al. Combination Therapy with Oncolytic Virus and T Cells or mRNA Vaccine Amplifies Antitumor Effects. Signal Transduct. Target. Ther. 2024, 9, 118. [Google Scholar] [CrossRef]
- McGrath, K.; Dotti, G. Combining Oncolytic Viruses with Chimeric Antigen Receptor T Cell Therapy. Hum. Gene Ther. 2021, 32, 150–157. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging Therapies for Glioblastoma: Current State and Future Directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Dotti, G. Chimeric Antigen Receptor (CAR)-Engineered Lymphocytes for Cancer Therapy. Expert. Opin. Biol. Ther. 2011, 11, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Binder, Z.A.; O’Rourke, D.M. Glioblastoma: The Current State of Biology and Therapeutic Strategies. Cancer Res. 2022, 82, 769–772. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A Single Dose of Peripherally Infused EGFRvIII-Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. JCO 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Guzman, G.; Pellot, K.; Reed, M.R.; Rodriguez, A. CAR T-Cells to Treat Brain Tumors. Brain Res. Bull. 2023, 196, 76–98. [Google Scholar] [CrossRef]
- Sharma, P.; Debinski, W. Receptor-Targeted Glial Brain Tumor Therapies. Int. J. Mol. Sci. 2018, 19, 3326. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Starr, R.; Aguilar, B.; Shami, A.F.; Martinez, C.; D’Apuzzo, M.; Barish, M.E.; Forman, S.J.; Jensen, M.C. Stem-like Tumor-Initiating Cells Isolated from IL13Rα2 Expressing Gliomas Are Targeted and Killed by IL13-Zetakine–Redirected T Cells. Clin. Cancer Res. 2012, 18, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Aguilar, B.; Starr, R.; Yang, X.; Chang, W.-C.; Weng, L.; Chang, B.; Sarkissian, A.; Brito, A.; Sanchez, J.F.; et al. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-Tumor Efficacy against Glioblastoma. Mol. Ther. 2018, 26, 31–44. [Google Scholar] [CrossRef]
- Feldman, L.; Brown, C.; Badie, B. Chimeric Antigen Receptor T-Cell Therapy: Updates in Glioblastoma Treatment. Neurosurgery 2021, 88, 1056–1064. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2014, 157, 753. [Google Scholar] [CrossRef]
- Akhavan, D.; Alizadeh, D.; Wang, D.; Weist, M.R.; Shepphird, J.K.; Brown, C.E. CAR T Cells for Brain Tumors: Lessons Learned and Road Ahead. Immunol. Rev. 2019, 290, 60–84. [Google Scholar] [CrossRef]
- Choi, B.D.; Maus, M.V.; June, C.H.; Sampson, J.H. Immunotherapy for Glioblastoma: Adoptive T-Cell Strategies. Clin. Cancer Res. 2019, 25, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Cenciarelli, C. Current Progress in Chimeric Antigen Receptor T Cell Therapy for Glioblastoma Multiforme. Cancer Med. 2021, 10, 5019–5030. [Google Scholar] [CrossRef]
- Ma, K.; Hu, P. Chimeric Antigen Receptor T-Cell Therapy for Glioblastoma. Cancers 2023, 15, 5652. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, X.; Xiao, Y. CAR-T Cell Combination Therapies in Hematologic Malignancies. Exp. Hematol. Oncol. 2024, 13, 69. [Google Scholar] [CrossRef]
- Agosti, E.; Garaba, A.; Antonietti, S.; Ius, T.; Fontanella, M.M.; Zeppieri, M.; Panciani, P.P. CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 7174. [Google Scholar] [CrossRef]
- Johnson, L.A.; Scholler, J.; Ohkuri, T.; Kosaka, A.; Patel, P.R.; McGettigan, S.E.; Nace, A.K.; Dentchev, T.; Thekkat, P.; Loew, A.; et al. Rational Development and Characterization of Humanized Anti–EGFR Variant III Chimeric Antigen Receptor T Cells for Glioblastoma. Science Transl. Med. 2015, 7, 275ra22. [Google Scholar] [CrossRef]
- Ohno, M.; Ohkuri, T.; Kosaka, A.; Tanahashi, K.; June, C.H.; Natsume, A.; Okada, H. Expression of miR-17-92 Enhances Anti-Tumor Activity of T-Cells Transduced with the Anti-EGFRvIII Chimeric Antigen Receptor in Mice Bearing Human GBM Xenografts. J. Immunother. Cancer 2013, 1, 21. [Google Scholar] [CrossRef]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic Virus Immunotherapy: Future Prospects for Oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.L.; Liu, Z.; Sathaiah, M.; Ravindranathan, R.; Guo, Z.; He, Y.; Guo, Z.S. Oncolytic Viruses as Therapeutic Cancer Vaccines. Mol. Cancer 2013, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Gujar, S.; Bell, J.; Diallo, J.-S. SnapShot: Cancer Immunotherapy with Oncolytic Viruses. Cell 2019, 176, 1240–1240.e1. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jiang, H.; Cheng, L.; Ma, B.; Liu, R. Oncolytic Herpes Simplex Virus and Temozolomide Synergistically Inhibit Breast Cancer Cell Tumorigenesis in Vitro and in Vivo. Oncol. Lett. 2021, 21, 99. [Google Scholar] [CrossRef]
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A Phase I/II Study of Triple-Mutated Oncolytic Herpes Virus G47∆ in Patients with Progressive Glioblastoma. Nat. Commun. 2022, 13, 4119. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 Virotherapy plus Pembrolizumab in Recurrent Glioblastoma: A Phase 1/2 Trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Karim, M.E.; Tha, K.K.; Othman, I.; Borhan Uddin, M.; Chowdhury, E.H. Therapeutic Potency of Nanoformulations of siRNAs and shRNAs in Animal Models of Cancers. Pharmaceutics 2018, 10, 65. [Google Scholar] [CrossRef]
- Singh, G.; Rohit; Kumar, P.; Aran, K.R. Targeting EGFR and PI3K/mTOR Pathways in Glioblastoma: Innovative Therapeutic Approaches. Med. Oncol. 2025, 42, 97. [Google Scholar] [CrossRef]
- George, J.; Banik, N.L.; Ray, S.K. Bcl-2 siRNA Augments Taxol Mediated Apoptotic Death in Human Glioblastoma U138MG and U251MG Cells. Neurochem. Res. 2009, 34, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Pu, P.; Jiang, H. Silencing Epidermal Growth Factor Receptor by RNA Interference in Glioma. Methods Mol. Biol. 2009, 542, 335–349. [Google Scholar] [CrossRef]
- Taibi, T.; Cheon, S.; Perna, F.; Vu, L.P. mRNA-Based Therapeutic Strategies for Cancer Treatment. Mol. Ther. 2024, 32, 2819–2834. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Strika, Z.; Petković, K.; Likić, R. Effectiveness and Safety of mRNA Vaccines in the Therapy of Glioblastoma. J. Pers. Med. 2024, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.; Filho, P.N.S.; Moura-Neto, V. Breaking Barriers: A Future Perspective on Glioblastoma Therapy with mRNA-Based Immunotherapies and Oncolytic Viruses. Vaccines 2024, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Song, E. Advancements in Clinical RNA Therapeutics: Present Developments and Prospective Outlooks. Cell Rep. Med. 2024, 5, 101555. [Google Scholar] [CrossRef] [PubMed]
- Naimi, N.; Seyedmirzaei, H.; Hassannejad, Z.; Soltani Khaboushan, A. Advanced Nanoparticle Strategies for Optimizing RNA Therapeutic Delivery in Neurodegenerative Disorders. Biomed. Pharmacother. 2024, 175, 116691. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.-Y. Therapeutic Cancer Vaccines: Past, Present, and Future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef]
- Goforth, R.; Salem, A.K.; Zhu, X.; Miles, S.; Zhang, X.-Q.; Lee, J.H.; Sandler, A.D. Immune Stimulatory Antigen Loaded Particles Combined with Depletion of Regulatory T-Cells Induce Potent Tumor Specific Immunity in a Mouse Model of Melanoma. Cancer Immunol. Immunother. 2009, 58, 517–530. [Google Scholar] [CrossRef]
- Krishnamachari, Y.; Salem, A.K. Innovative Strategies for Co-Delivering Antigens and CpG Oligonucleotides. Adv. Drug Deliv. Rev. 2009, 61, 205–217. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational Heterogeneity in Cancer and the Search for New Cancer Genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of Epidermal Growth Factor Receptor Signalling Networks in Glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef]
- Montano, N.; Cenci, T.; Martini, M.; D’Alessandris, Q.G.; Pelacchi, F.; Ricci-Vitiani, L.; Maira, G.; De Maria, R.; Larocca, L.M.; Pallini, R. Expression of EGFRvIII in Glioblastoma: Prognostic Significance Revisited. Neoplasia 2011, 13, 1113–1121. [Google Scholar] [CrossRef]
- Platten, M. EGFRvIII Vaccine in Glioblastoma—InACT-IVe or Not ReACTive Enough? Neuro Oncol. 2017, 19, 1425–1426. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Barich, D.H.; Munson, E.J.; Zell, M.T. Physicochemical Properties, Formulation, and Drug Delivery. In Drug Delivery; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 57–71. ISBN 978-0-471-47573-6. [Google Scholar]
- Hillery, A.; Park, K. (Eds.) Drug Delivery: Fundamentals and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-38257-9. [Google Scholar]
- Ullyot, G.E.; Ullyot, B.H.; Slater, L.B. The metamorpohsis of smith-kline & french laboratories to smith kline beecham: 1925–1998. Bull. Hist. Chem. 2000, 25, 16–20. [Google Scholar]
- Park, H.; Otte, A.; Park, K. Evolution of Drug Delivery Systems: From 1950 to 2020 and Beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Bawab, A.A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Allen, T.M. The Use of Glycolipids and Hydrophilic Polymers in Avoiding Rapid Uptake of Liposomes by the Mononuclear Phagocyte System. Adv. Drug Deliv. Rev. 1994, 13, 285–309. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Mustafa, G.; Abdel-Wahab, B.A.; Pathak, K.; Das, A.; Sahariah, J.J.; Kalita, P.; Alam, A.; Borthakur, P.P. From Bench to Bedside: Advancing Liposomal Doxorubicin for Targeted Cancer Therapy. Results Surf. Interfaces 2025, 19, 100473. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Pitt, W.G.; Husseini, G.A. Dual-Targeting and Stimuli-Triggered Liposomal Drug Delivery in Cancer Treatment. ACS Pharmacol. Transl. Sci. 2021, 4, 1028–1049. [Google Scholar] [CrossRef]
- Rahman, M.; Beg, S.; Verma, A.; Anwar, F.; Samad, A.; Kumar, V. Chapter 4—Liposomal-Based Therapeutic Carriers for Vaccine and Gene Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 151–166. ISBN 978-0-12-809717-5. [Google Scholar]
- Yasir, M.; Mishra, R.; Tripathi, A.S.; Maurya, R.K.; Shahi, A.; Zaki, M.E.A.; Al Hussain, S.A.; Masand, V.H. Theranostics: A Multifaceted Approach Utilizing Nano-Biomaterials. Discov. Nano 2024, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Ng, V.W.L.; Ono, R.J.; Chan, J.M.W.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.L.; Yang, Y.Y. Role of Non-Covalent and Covalent Interactions in Cargo Loading Capacity and Stability of Polymeric Micelles. J. Control. Release 2014, 193, 9–26. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.; Hariharan, S.; Kumar, M.N.V.R. PLGA Nanoparticles in Drug Delivery: The State of the Art. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef]
- Pacheco, C.; Sousa, F.; Sarmento, B. Chitosan-Based Nanomedicine for Brain Delivery: Where Are We Heading? React. Funct. Polym. 2020, 146, 104430. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef]
- Makharadze, D.; del Valle, L.J.; Katsarava, R.; Puiggalí, J. The Art of PEGylation: From Simple Polymer to Sophisticated Drug Delivery System. Int. J. Mol. Sci. 2025, 26, 3102. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface Functionalization of Polymeric Nanoparticles for Tumor Drug Delivery: Approaches and Challenges. Expert. Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. [Google Scholar] [CrossRef]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic Acid-Coated Chitosan Nanoparticles as Targeted-Carrier of Tamoxifen against MCF7 and TMX-Resistant MCF7 Cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef]
- Kousar, K.; Naseer, F.; Abduh, M.S.; Kakar, S.; Gul, R.; Anjum, S.; Ahmad, T. Green Synthesis of Hyaluronic Acid Coated, Thiolated Chitosan Nanoparticles for CD44 Targeted Delivery and Sustained Release of Cisplatin in Cervical Carcinoma. Front. Pharmacol. 2023, 13, 1073004. [Google Scholar] [CrossRef]
- Mooney, K.L.; Choy, W.; Sidhu, S.; Pelargos, P.; Bui, T.T.; Voth, B.; Barnette, N.; Yang, I. The Role of CD44 in Glioblastoma Multiforme. J. Clin. Neurosci. 2016, 34, 1–5. [Google Scholar] [CrossRef]
- Ahmann, F.R.; Citrin, D.L.; deHaan, H.A.; Guinan, P.; Jordan, V.C.; Kreis, W.; Scott, M.; Trump, D.L. Zoladex: A Sustained-Release, Monthly Luteinizing Hormone-Releasing Hormone Analogue for the Treatment of Advanced Prostate Cancer. JCO 1987, 5, 912–917. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for Oral Delivery: Design, Evaluation and State-of-the-Art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef]
- Ashique, S.; Kumar, P.; Taj, T.; Debnath, B.; Mukherjee, S.; Patel, A.; Sridhar, S.B.; Panigrahy, U.P.; Poonia, P.; Selim, S.; et al. Nanotechnology: A State of the Art for the Management of Ocular Disorders—A Roadmap. BioNanoScience 2025, 15, 285. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and Its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Crini, G. The Contribution of Franz Schardinger to Cyclodextrins: A Tribute on the Occasion of the Centenary of His Death. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 19–28. [Google Scholar] [CrossRef]
- Schardinger, F. Über thermophile Bakterien aus verschiedenen Speisen und Milch. Zeitschr. f. Unters. d. Nahr.-u. Genußmittel 1903, 6, 865–880. [Google Scholar] [CrossRef]
- Biwer, A.; Antranikian, G.; Heinzle, E. Enzymatic Production of Cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–617. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Esteso, M.A.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. ChemInform Abstract: Cyclodextrin-Based Inclusion Complexation Bridging Supramolecular Chemistry and Macromolecular Self-Assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- De Gaetano, F.; Leggio, L.; Celesti, C.; Genovese, F.; Falcone, M.; Giofrè, S.V.; Iraci, N.; Ventura, C.A. Study of Host-Guest Interaction and In Vitro Neuroprotective Potential of Cinnamic Acid/Randomly Methylated β-Cyclodextrin Inclusion Complex. Int. J. Mol. Sci. 2024, 25, 12778. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Scala, A.; Celesti, C.; Lambertsen Larsen, K.; Genovese, F.; Bongiorno, C.; Leggio, L.; Iraci, N.; Mazzaglia, A.; Ventura, C.A. Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study. Molecules 2023, 28, 3023. [Google Scholar] [CrossRef]
- Musumeci, T.; Bonaccorso, A.; De Gaetano, F.; Larsen, K.L.; Pignatello, R.; Mazzaglia, A.; Puglisi, G.; Ventura, C.A. A Physico-Chemical Study on Amphiphilic Cyclodextrin/Liposomes Nanoassemblies with Drug Carrier Potential. J. Liposome Res. 2020, 30, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Zhang, M. General Methods for the Preparation of Cyclodextrin Inclusion Complexes: Preparation and Application in Industry. In Cyclodextrins: Preparation and Application in Industry; World Scientific: Singapore, 2018; pp. 25–50. ISBN 978-981-322-965-5. [Google Scholar]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- De Gaetano, F.; Pastorello, M.; Pistarà, V.; Rescifina, A.; Margani, F.; Barbera, V.; Ventura, C.A.; Marino, A. Rutin/Sulfobutylether-β-Cyclodextrin as a Promising Therapeutic Formulation for Ocular Infection. Pharmaceutics 2024, 16, 233. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Paolino, D.; Celesti, C.; Iannazzo, D.; Pistarà, V.; Iraci, N.; Ventura, C.A. Bicalutamide Anticancer Activity Enhancement by Formulation of Soluble Inclusion Complexes with Cyclodextrins. Biomolecules 2022, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- De Gaetano, F.; Mannino, D.; Celesti, C.; Bulzomí, M.; Iraci, N.; Vincenzo Giofrè, S.; Esposito, E.; Paterniti, I.; Anna Ventura, C. Randomly Methylated β-Cyclodextrin Improves Water—Solubility, Cellular Protection and Mucosa Permeability of Idebenone. Int. J. Pharm. 2024, 665, 124718. [Google Scholar] [CrossRef]
- De Gaetano, F.; Margani, F.; Barbera, V.; D’Angelo, V.; Germanò, M.P.; Pistarà, V.; Ventura, C.A. Characterization and In Vivo Antiangiogenic Activity Evaluation of Morin-Based Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2209. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y. Cooperative Binding and Multiple Recognition by Bridged Bis(Beta-Cyclodextrin)s with Functional Linkers. Acc. Chem. Res. 2006, 39, 681–691. [Google Scholar] [CrossRef]
- De Gaetano, F.; d’Avanzo, N.; Mancuso, A.; De Gaetano, A.; Paladini, G.; Caridi, F.; Venuti, V.; Paolino, D.; Ventura, C.A. Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone. Pharmaceuticals 2022, 15, 1206. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation and Chiral Recognition Thermodynamics of 6-Amino-6-Deoxy-Beta-Cyclodextrin with Anionic, Cationic, and Neutral Chiral Guests: Counterbalance between van Der Waals and Coulombic Interactions. J. Am. Chem. Soc. 2002, 124, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as Food Ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Linde, G.A.; Laverde, A., Jr.; Colauto, N.B. Changes to Taste Perception in the Food Industry: Use of Cyclodextrins. In Handbook of Behavior, Food and Nutrition; Springer: New York, NY, USA, 2011; pp. 99–118. [Google Scholar]
- González Pereira, A.; Rodríguez, M.; García Oliveira, P.; Mejuto, J.; Prieto, M.; Simal-Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Schurig, V. Use of Derivatized Cyclodextrins as Chiral Selectors for the Separation of Enantiomers by Gas Chromatography. Ann. Pharm. Fr. 2010, 68, 82–98. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirino, J.P. Chiral Separation Using Cyclodextrins as Mobile Phase Additives in Open-tubular Liquid Chromatography with a Pseudophase Coating. J. Sep. Sci. 2022, 45, 1195–1201. [Google Scholar] [CrossRef]
- Fejos, I.; Kalydi, E.; Malanga, M.; Benkovics, G.; Béni, S. Single Isomer Cyclodextrins as Chiral Selectors in Capillary Electrophoresis. J. Chromatogr. A 2020, 1627, 461375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Scriba, G. Advances in the Use of Cyclodextrins as Chiral Selectors in Capillary Electrokinetic Chromatography: Fundamentals and Applications. Chromatographia 2016, 79, 1403–1435. [Google Scholar] [CrossRef]
- Wenz, G. Influence of Intramolecular Hydrogen Bonds on the Binding Potential of Methylated β-Cyclodextrin Derivatives. Beilstein J. Org. Chem. 2012, 8, 1890–1895. [Google Scholar] [CrossRef]
- Frijlink, H.W.; Eissens, A.C.; Hefting, N.R.; Poelstra, K.; Lerk, C.F.; Meijer, D.K. The Effect of Parenterally Administered Cyclodextrins on Cholesterol Levels in the Rat. Pharm. Res. 1991, 8, 9–16. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef] [PubMed]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and Derivatives in Drug Delivery: New Developments, Relevant Clinical Trials, and Advanced Products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Harada, A. Supramolecular Polymeric Materials Containing Cyclodextrins. Chem. Pharm. Bull. 2017, 65, 330–335. [Google Scholar] [CrossRef]
- He, Y.; Hou, X.; Liu, Y.; Feng, N. Recent Progress in Synthesis, Structural Diversity and Emerging Applications of Cyclodextrin-Based Carbohydrate Metal-Organic Frameworks. J. Mater. Chem. B 2019, 7, 5602–5619. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Liu, Y.-H.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef]
- Duchêne, D.; Ponchel, G.; Wouessidjewe, D. Cyclodextrins in Targeting. Application to Nanoparticles. Adv. Drug Deliv. Rev. 1999, 36, 29–40. [Google Scholar] [CrossRef]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.-P.; Piel, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.-M.; Cataldo, D. Cyclodextrins as a Potential Carrier in Drug Nebulization. J. Control. Release 2004, 96, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, A.; Delrivo, A.; Aiassa, V.; Longhi, M.R. Binding of Sulfamethazine to β-Cyclodextrin and Methyl-β-Cyclodextrin. AAPS PharmSciTech 2013, 14, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.M.; Alderson, S.R.; Cleven, C.D.; Dixon, D.W.; Ivanyi, R.; Lambert, J.L.; Lowman, D.W.; Offerman, R.J.; Szejtli, J.; Szente, L. Synthesis and Characterization of Water-Soluble Hydroxybutenyl Cyclomaltooligosaccharides (Cyclodextrins). Carbohydr. Res. 2002, 337, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A Review on the Recent Development of Cyclodextrin-Based Materials Used in Oilfield Applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef]
- Ishiguro, T.; Morishita, E.; Iohara, D.; Hirayama, F.; Wada, K.; Motoyama, K.; Arima, H.; Uekama, K. Some Pharmaceutical and Inclusion Properties of 2-Hydroxybutyl-β-Cyclodextrin Derivative. Int. J. Pharm. 2011, 419, 161–169. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Venuti, V.; Crupi, V.; Fazio, B.; Majolino, D.; Acri, G.; Testagrossa, B.; Stancanelli, R.; De Gaetano, F.; Gagliardi, A.; Paolino, D.; et al. Physicochemical Characterization and Antioxidant Activity Evaluation of Idebenone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Biomolecules 2019, 9, 531. [Google Scholar] [CrossRef]
- Cacciola, A.; D’Angelo, V.; De Gaetano, F.; Fais, A.; Germanò, M.P.; Masala, V.; Olla, S.; Pistarà, V.; Stancanelli, R.; Tuberoso, C.I.G.; et al. The Anti-Angiogenic Effect of Cynara Cardunculus L. Subsp. Cardunculus Waste Product. Foods 2025, 14, 2656. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, J.; Mathieu, J.; Alvarez, P. 2-Hydroxypropyl-Beta-Cyclodextrin (HPβCD) Reduces Age-Related Lipofuscin Accumulation through a Cholesterol-Associated Pathway. Sci. Rep. 2017, 7, 2197. [Google Scholar] [CrossRef]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of Cyclodextrins and Drugs after Oral and Parenteral Administration of Drug/Cyclodextrin Complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- ZyVersa Therapeutics, Inc. A Phase 2a Open Label Study to Evaluate Cholesterol Efflux Mediator™ VAR200: 2-Hydroxy-propyl-β-Cyclodextrin (2-HPβCD) in Subjects with Type 2 Diabetic Kidney Disease (DKD); ClinicalTrials.gov Identifier, NCT06489340; ZyVersa Therapeutics, Inc.: Weston, FL, USA, 2025. [Google Scholar]
- Huang, J.; Wang, X.; Huang, T.; Yang, Y.; Tu, J.; Zou, J.; Yang, H.; Yang, R. Application of Sodium Sulfobutylether-β-Cyclodextrin Based on Encapsulation. Carbohydr. Polym. 2024, 333, 121985. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-Cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- Rowe, E.S.; Rowe, V.D.; Biswas, S.; Mosher, G.; Insisienmay, L.; Ozias, M.K.; Gralinski, M.R.; Hunter, J.; Barnett, J.S. Preclinical Studies of a Kidney Safe Iodinated Contrast Agent. J. Neuroimaging 2016, 26, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Rowe, E.S.; Rowe, V.D.; Hunter, J.; Gralinski, M.R.; Neves, L.A. A Nephroprotective Iodinated Contrast Agent with Cardioprotective Properties: A Pilot Study. J. Neuroimaging 2021, 31, 706–713. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Csepregi, R.; Benkovics, G.; Szente, L.; Poór, M. Testing the Protective Effects of Sulfobutylether-Βeta-Cyclodextrin (SBECD) and Sugammadex against Chlorpromazine-Induced Acute Toxicity in SH-SY5Y Cell Line and in NMRI Mice. Pharmaceutics 2022, 14, 1888. [Google Scholar] [CrossRef]
- Trotta, F.; Loftsson, T.; Gaud, R.S.; Trivedi, R.; Shende, P. Integration of Cyclodextrins and Associated Toxicities: A Roadmap for High Quality Biomedical Applications. Carbohydr. Polym. 2022, 295, 119880. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.d.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.d.; Neves de Lima, Á.A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Nasr, G.; Greige-Gerges, H.; Fourmentin, S.; Elaissari, A.; Khreich, N. Cyclodextrins Permeabilize DPPC Liposome Membranes: A Focus on Cholesterol Content, Cyclodextrin Type, and Concentration. Beilstein J. Org. Chem. 2023, 19, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-Beta-Cyclodextrin (HP-Beta-CD): A Toxicology Review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Arima, H.; Toyodome, H.; Irie, T.; Hirayama, F.; Uekama, K. Effect of 2,6-Di-O-Methyl-α-Cyclodextrin on Hemolysis and Morphological Change in Rabbit’s Red Blood Cells. Eur. J. Pharm. Sci. 2006, 29, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mantik, P.; Xie, M.; Wong, H.; La, H.; Steigerwalt, R.W.; Devanaboyina, U.; Ganem, G.; Shih, D.; Flygare, J.A.; Fairbrother, W.J.; et al. Cyclodextrin Reduces Intravenous Toxicity of a Model Compound. J. Pharm. Sci. 2019, 108, 1934–1943. [Google Scholar] [CrossRef]
- Miranda, G.M.; Santos, V.O.R.e.; Bessa, J.R.; Teles, Y.C.F.; Yahouédéhou, S.C.M.A.; Goncalves, M.S.; Ribeiro-Filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef]
- Fenyvesi, É.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Sorrenti, M.; Catenacci, L.; Pavan, B.; Ferraro, L.; Gavini, E.; Bonferoni, M.C.; Giunchedi, P.; Dalpiaz, A. Versatile Nasal Application of Cyclodextrins: Excipients and/or Actives? Pharmaceutics 2021, 13, 1180. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef]
- Cabrera-Quiñones, N.C.; López-Méndez, L.J.; Guadarrama, P. Inclusion and Non-Inclusion Complexes between Curcumin and β-Cyclodextrin with High-Curcumin Loading and Enhanced Aqueous Solubility Obtained by Mechanochemistry. ChemistrySelect 2023, 8, e202303254. [Google Scholar] [CrossRef]
- de Jesus, M.B.; Fraceto, L.F.; Martini, M.F.; Pickholz, M.; Ferreira, C.V.; de Paula, E. Non-Inclusion Complexes between Riboflavin and Cyclodextrins. J. Pharm. Pharmacol. 2012, 64, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic. Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Ganjali Koli, M.; Fogolari, F. Exploring the Role of Cyclodextrins as a Cholesterol Scavenger: A Molecular Dynamics Investigation of Conformational Changes and Thermodynamics. Sci. Rep. 2023, 13, 21765. [Google Scholar] [CrossRef]
- López, C.A.; de Vries, A.H.; Marrink, S.J. Computational Microscopy of Cyclodextrin Mediated Cholesterol Extraction from Lipid Model Membranes. Sci. Rep. 2013, 3, 2071. [Google Scholar] [CrossRef]
- Nazli, A.; Malanga, M.; Sohajda, T.; Béni, S. Cationic Cyclodextrin-Based Carriers for Drug and Nucleic Acid Delivery. Pharmaceutics 2025, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Matencio, A.; Vicente-Herrero, C.; García-Carmona, F.; López-Nicolás, J.M. Study of the Fluorescence and Interaction between Cyclodextrins and Neochlorogenic Acid, in Comparison with Chlorogenic Acid. Sci. Rep. 2021, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. Bioresponsive and Multifunctional Cyclodextrin-Based Non-Viral Nanocomplexes in Cancer Therapy: Building Foundations for Gene and Drug Delivery, Immunotherapy and Bioimaging. Environ. Res. 2023, 234, 116507. [Google Scholar] [CrossRef]
- Wei, X.; Yu, C.-Y.; Wei, H. Application of Cyclodextrin for Cancer Immunotherapy. Molecules 2023, 28, 5610. [Google Scholar] [CrossRef]
- Vecsernyés, M.; Fenyvesi, F.; Bácskay, I.; Deli, M.A.; Szente, L.; Fenyvesi, É. Cyclodextrins, Blood-Brain Barrier, and Treatment of Neurological Diseases. Arch. Med. Res. 2014, 45, 711–729. [Google Scholar] [CrossRef]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-Based Delivery Systems for in Vivo-Tested Anticancer Therapies. Drug Deliv. Transl. Res. 2021, 11, 49–71. [Google Scholar] [CrossRef]
- Pandey, A. Cyclodextrin-Based Nanoparticles for Pharmaceutical Applications: A Review. Env. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Erdoğar, N.; Bilensoy, E. Cyclodextrin-Based Nanosystems in Targeted Cancer Therapy. In Cyclodextrin Applications in Medicine, Food, Environment and Liquid Crystals; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 59–80. ISBN 978-3-319-76162-6. [Google Scholar]
- Najlaoui, F.; Busser, B.; Taïwe, G.S.; Pigeon, P.; Sturm, N.; Giovannini, D.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; Gibaud, S.; et al. Succinimido-Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity. Molecules 2022, 27, 4651. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Gaschard, M.; Othman, M.; Salmain, M.; Jaouen, G. α-Hydroxylactams as Efficient Entries to Diversely Functionalized Ferrociphenols: Synthesis and Antiproliferative Activity Studies. Molecules 2022, 27, 4549. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, C. Application of Ferrocene and Its Derivatives in Cancer Research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Wang, J.; Fan, X.; Xu, X.; Wang, H.; Xiong, Y.; Li, X.; Zhang, X.; Zhang, Q.; et al. How Does Ferrocene Correlate with Ferroptosis? Multiple Approaches to Explore Ferrocene-Appended GPX4 Inhibitors as Anticancer Agents. Chem. Sci. 2024, 15, 10477–10490. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Bethanis, K.; Koniari, E.; Christoforides, E.; Nikola, O.; Andreou, A.; Mantzou, A.; Chrousos, G.P.; Kanaka-Gantenbein, C.; Lambrou, G.I. Biophysical Studies and In Vitro Effects of Tumor Cell Lines of Cannabidiol and Its Cyclodextrin Inclusion Complexes. Pharmaceutics 2022, 14, 706. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, X.; Ma, L.; Li, C.; Xu, Z.; Ma, W.; Zhou, Y.; Zhao, Z.; Ma, D. Therapeutic Effect of Disulfiram Inclusion Complex Embedded in Hydroxypropyl-β-Cyclodextrin on Intracranial Glioma-Bearing Male Rats via Intranasal Route. Eur. J. Pharm. Sci. 2021, 156, 105590. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wu, C.; Wang, L.; Chen, Z.-S.; Cui, W. The Combination of Disulfiram and Copper for Cancer Treatment. Drug Discov. Today 2020, 25, 1099–1108. [Google Scholar] [CrossRef]
- Tawari, P.E.; Wang, Z.; Najlah, M.; Tsang, C.W.; Kannappan, V.; Liu, P.; McConville, C.; He, B.; Armesilla, A.L.; Wang, W. The Cytotoxic Mechanisms of Disulfiram and Copper(Ii) in Cancer Cells. Toxicol. Res. 2015, 4, 1439–1442. [Google Scholar] [CrossRef]
- Deshmukh, V.; Pathan, N.S.; Haldar, N.; Nalawade, S.; Narwade, M.; Gajbhiye, K.R.; Gajbhiye, V. Exploring Intranasal Drug Delivery via Nanocarriers: A Promising Glioblastoma Therapy. Colloids Surf. B: Biointerfaces 2025, 245, 114285. [Google Scholar] [CrossRef]
- Krzak, A.; Bilewicz, R. Voltammetric/UV–Vis Study of Temozolomide Inclusion Complexes with Cyclodextrin Derivatives. Bioelectrochemistry 2020, 136, 107587. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef]
- Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Vanderesse, R.; Acherar, S. Inclusion Complex vs. Conjugation of Hydrophobic Photosensitizers with β-Cyclodextrin: Improved Disaggregation and Photodynamic Therapy Efficacy against Glioblastoma Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110604. [Google Scholar] [CrossRef]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal Delivery of the in Situ Hydrogels of Curcumin and Its Inclusion Complexes of Hydroxypropyl-β-Cyclodextrin for Melanoma Treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Fajardo, A.R.; Nocchi, S.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Starch-Based Microspheres for Sustained-Release of Curcumin: Preparation and Cytotoxic Effect on Tumor Cells. Carbohydr. Polym. 2013, 98, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Gularte, M.S.; Quadrado, R.F.N.; Pedra, N.S.; Soares, M.S.P.; Bona, N.P.; Spanevello, R.M.; Fajardo, A.R. Preparation, Characterization and Antitumor Activity of a Cationic Starch-Derivative Membrane Embedded with a β-Cyclodextrin/Curcumin Inclusion Complex. Int. J. Biol. Macromol. 2020, 148, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Gao, K.; Niu, R.; Wang, J.; Zhang, J.; Gao, C.; Yang, B.; Liao, X. Inclusion Complexes between Chrysin and Amino-Appended β-Cyclodextrins (ACDs): Binding Behavior, Water Solubility, in Vitro Antioxidant Activity and Cytotoxicity. Mater. Sci. Eng. C 2020, 106, 110161. [Google Scholar] [CrossRef]
- Mahdi, W.A.; Alanazi, M.M.; Imam, S.S.; Alshehri, S.; Hussain, A.; Altamimi, M.A.; Alhudaithi, S.S. Formulation of Multicomponent Inclusion Complex of Cyclodextrin-Amino Acid with Chrysin: Physicochemical Characterization, Cell Viability and Apoptosis Assessment in Human Primary Glioblastoma Cell Line. Int. J. Pharm. X 2023, 6, 100211. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Thorat, S.; Nayak, U.; Sherje, A.; Murahari, M. Host-Guest Interaction Study of Efavirenz with Hydroxypropyl-β-cyclodextrin and L-arginine by Computational Simulation Studies: Preparation and Characterization of Supramolecular Complexes. J. Mol. Liq. 2018, 259, 55–64. [Google Scholar] [CrossRef]
- Sherje, A.P.; Patel, F.; Murahari, M.; Suvarna, V.; Patel, K. Study on Effect of L-Arginine on Solubility and Dissolution of Zaltoprofen: Preparation and Characterization of Binary and Ternary Cyclodextrin Inclusion Complexes. Chem. Phys. Lett. 2018, 694, 120–128. [Google Scholar] [CrossRef]
- Mura, P.; Bettinetti, G.P.; Cirri, M.; Maestrelli, F.; Sorrenti, M.; Catenacci, L. Solid-State Characterization and Dissolution Properties of Naproxen–Arginine–Hydroxypropyl-β-Cyclodextrin Ternary System. Eur. J. Pharm. Biopharm. 2005, 59, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Maestrelli, F.; Cirri, M.; Mura, P. Analysis of Physicochemical Properties of Ternary Systems of Oxaprozin with Randomly Methylated-ß-Cyclodextrin and l-Arginine Aimed to Improve the Drug Solubility. J. Pharm. Biomed. Anal. 2016, 129, 350–358. [Google Scholar] [CrossRef]
- Figueiras, A.; Sarraguça, J.M.G.; Pais, A.A.C.C.; Carvalho, R.A.; Veiga, J.F. The Role of L-Arginine in Inclusion Complexes of Omeprazole with Cyclodextrins. AAPS PharmSciTech 2010, 11, 233–240. [Google Scholar] [CrossRef]
- Colapietro, A.; Rossetti, A.; Mancini, A.; Martellucci, S.; Ocone, G.; Pulcini, F.; Biordi, L.; Cristiano, L.; Mattei, V.; Delle Monache, S.; et al. Multiple Antitumor Molecular Mechanisms Are Activated by a Fully Synthetic and Stabilized Pharmaceutical Product Delivering the Active Compound Sulforaphane (SFX-01) in Preclinical Model of Human Glioblastoma. Pharmaceuticals 2021, 14, 1082. [Google Scholar] [CrossRef]
- Sita, G.; Graziosi, A.; Hrelia, P.; Morroni, F. Sulforaphane Causes Cell Cycle Arrest and Apoptosis in Human Glioblastoma U87MG and U373MG Cell Lines under Hypoxic Conditions. Int. J. Mol. Sci. 2021, 22, 11201. [Google Scholar] [CrossRef]
- Lin, E.-Y.; Chen, Y.-S.; Li, Y.-S.; Chen, S.-R.; Lee, C.-H.; Huang, M.-H.; Chuang, H.-M.; Harn, H.-J.; Yang, H.-H.; Lin, S.-Z.; et al. Liposome Consolidated with Cyclodextrin Provides Prolonged Drug Retention Resulting in Increased Drug Bioavailability in Brain. Int. J. Mol. Sci. 2020, 21, 4408. [Google Scholar] [CrossRef]
- Gallego-Yerga, L.; de la Torre, C.; Sansone, F.; Casnati, A.; Mellet, C.O.; García Fernández, J.M.; Ceña, V. Synthesis, Self-Assembly and Anticancer Drug Encapsulation and Delivery Properties of Cyclodextrin-Based Giant Amphiphiles. Carbohydr. Polym. 2021, 252, 117135. [Google Scholar] [CrossRef]
- Turco, V.; Pfleiderer, K.; Hunger, J.; Horvat, N.K.; Karimian-Jazi, K.; Schregel, K.; Fischer, M.; Brugnara, G.; Jähne, K.; Sturm, V.; et al. T Cell-Independent Eradication of Experimental Glioma by Intravenous TLR7/8-Agonist-Loaded Nanoparticles. Nat. Commun. 2023, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Tani, H. Recent Advances and Prospects in RNA Drug Development. Int. J. Mol. Sci. 2024, 25, 12284. [Google Scholar] [CrossRef] [PubMed]
- Traber, G.M.; Yu, A.-M. RNAi-Based Therapeutics and Novel RNA Bioengineering Technologies. J. Pharmacol. Exp. Ther. 2023, 384, 133–154. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, X.; Li, X. Research Progress on siRNA Delivery with Nonviral Carriers. IJN 2011, 2011, 1017–1025. [Google Scholar] [CrossRef]
- de la Torre, C.; Játiva, P.; Posadas, I.; Manzanares, D.; Blanco, J.L.J.; Mellet, C.O.; Fernández, J.M.G.; Ceña, V. A β-Cyclodextrin-Based Nanoparticle with Very High Transfection Efficiency Unveils siRNA-Activated TLR3 Responses in Human Prostate Cancer Cells. Pharmaceutics 2022, 14, 2424. [Google Scholar] [CrossRef]
- Manzanares, D.; Pérez-Carrión, M.D.; Jiménez Blanco, J.L.; Ortiz Mellet, C.; García Fernández, J.M.; Ceña, V. Cyclodextrin-Based Nanostructure Efficiently Delivers siRNA to Glioblastoma Cells Preferentially via Macropinocytosis. Int. J. Mol. Sci. 2020, 21, 9306. [Google Scholar] [CrossRef]
- Franco, L.; Isse, A.A.; Barbon, A.; Altomare, L.; Hyppönen, V.; Rosa, J.; Olsson, V.; Kettunen, M.; Melone, L. Redox Properties and in Vivo Magnetic Resonance Imaging of Cyclodextrin-Polynitroxides Contrast Agents. Chemphyschem 2023, 24, e202300100. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Yang, H.; Ren, J.; Zhao, M.; Chen, C.; Wang, F.; Chen, Z. Novel Electrochemical Immunosensor for O6-Methylguanine-DNA Methyltransferase Gene Methylation Based on Graphene Oxide-Magnetic Nanoparticles-β-Cyclodextrin Nanocomposite. Bioelectrochemistry 2022, 146, 108111. [Google Scholar] [CrossRef] [PubMed]

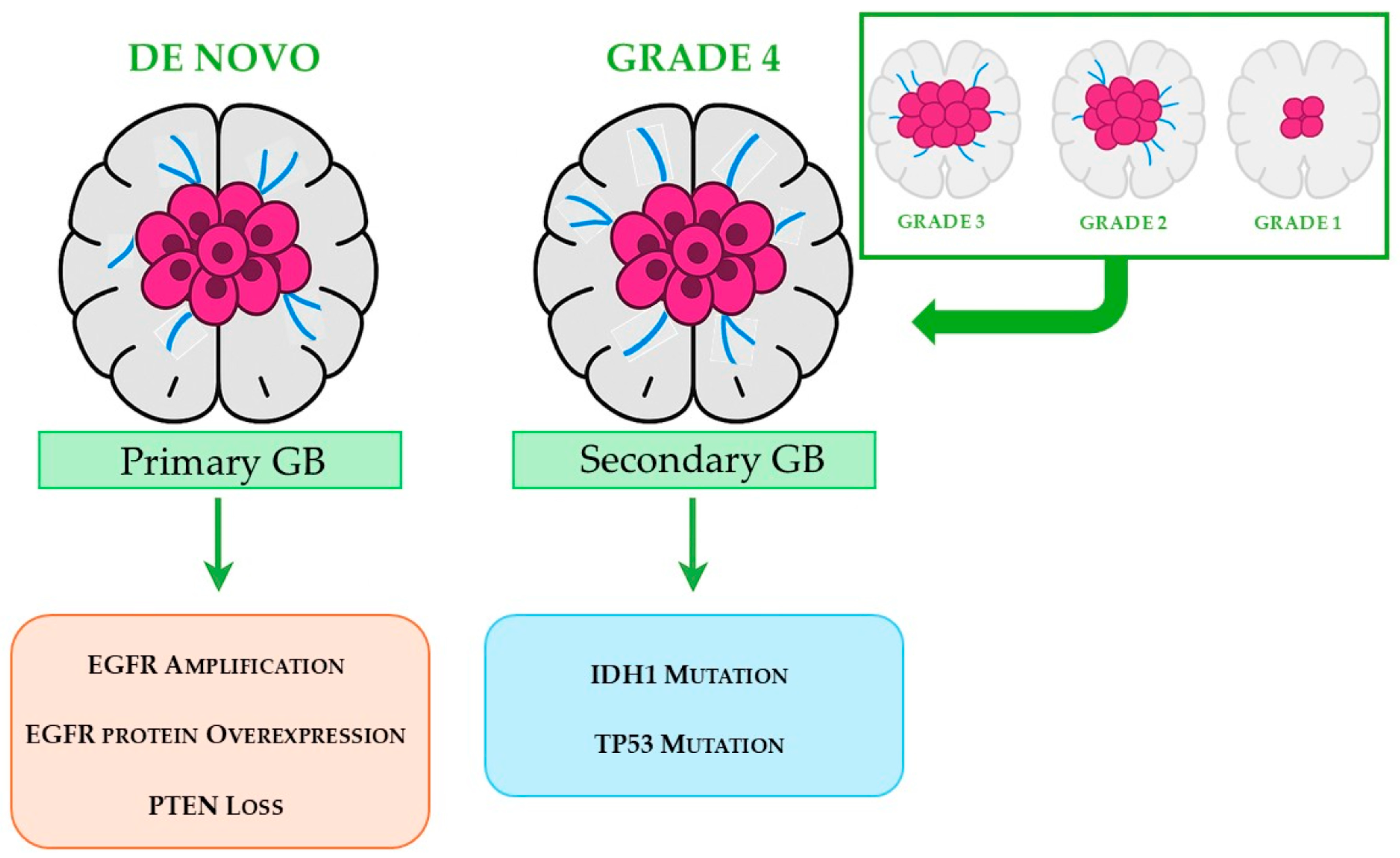
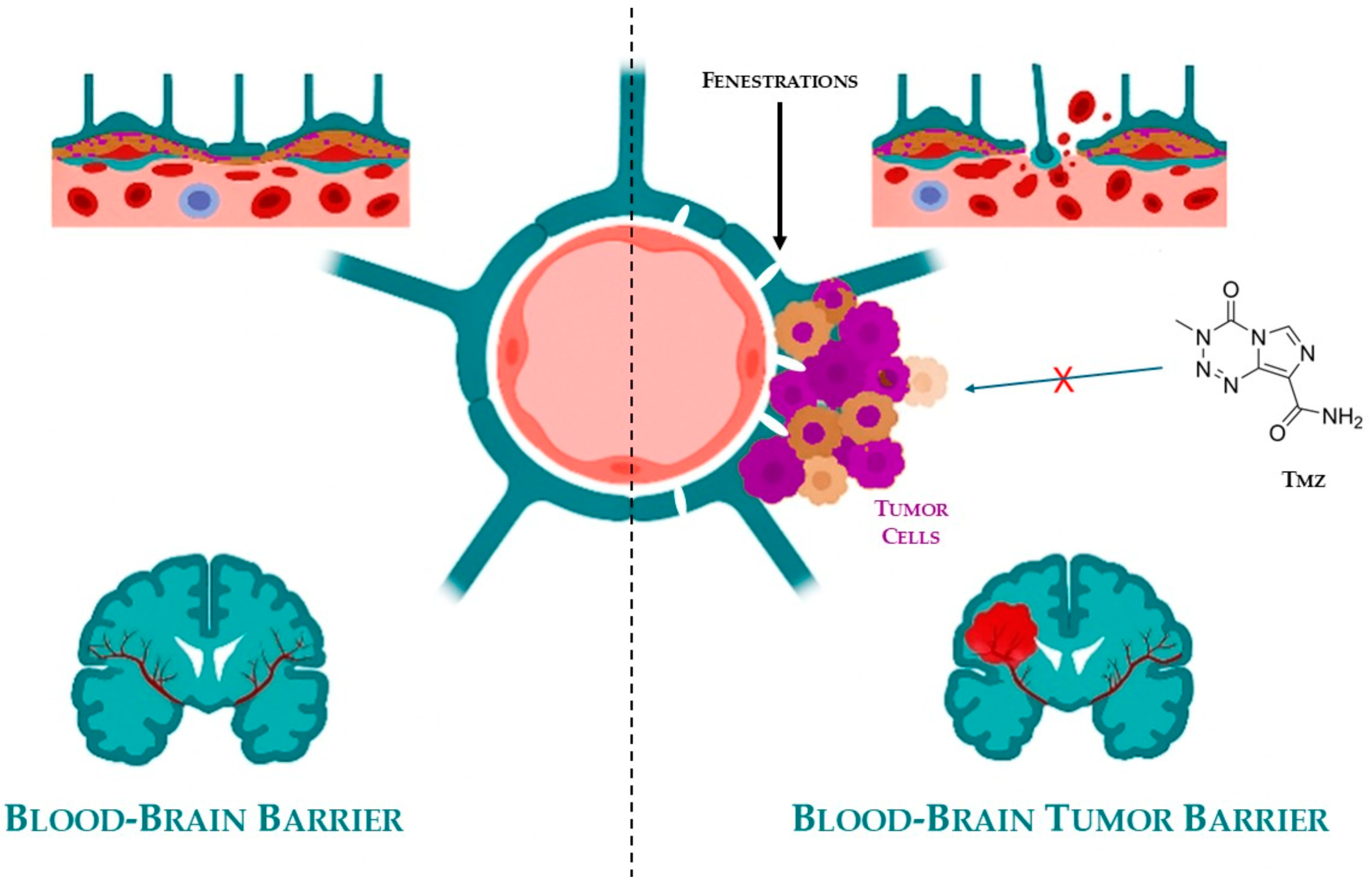
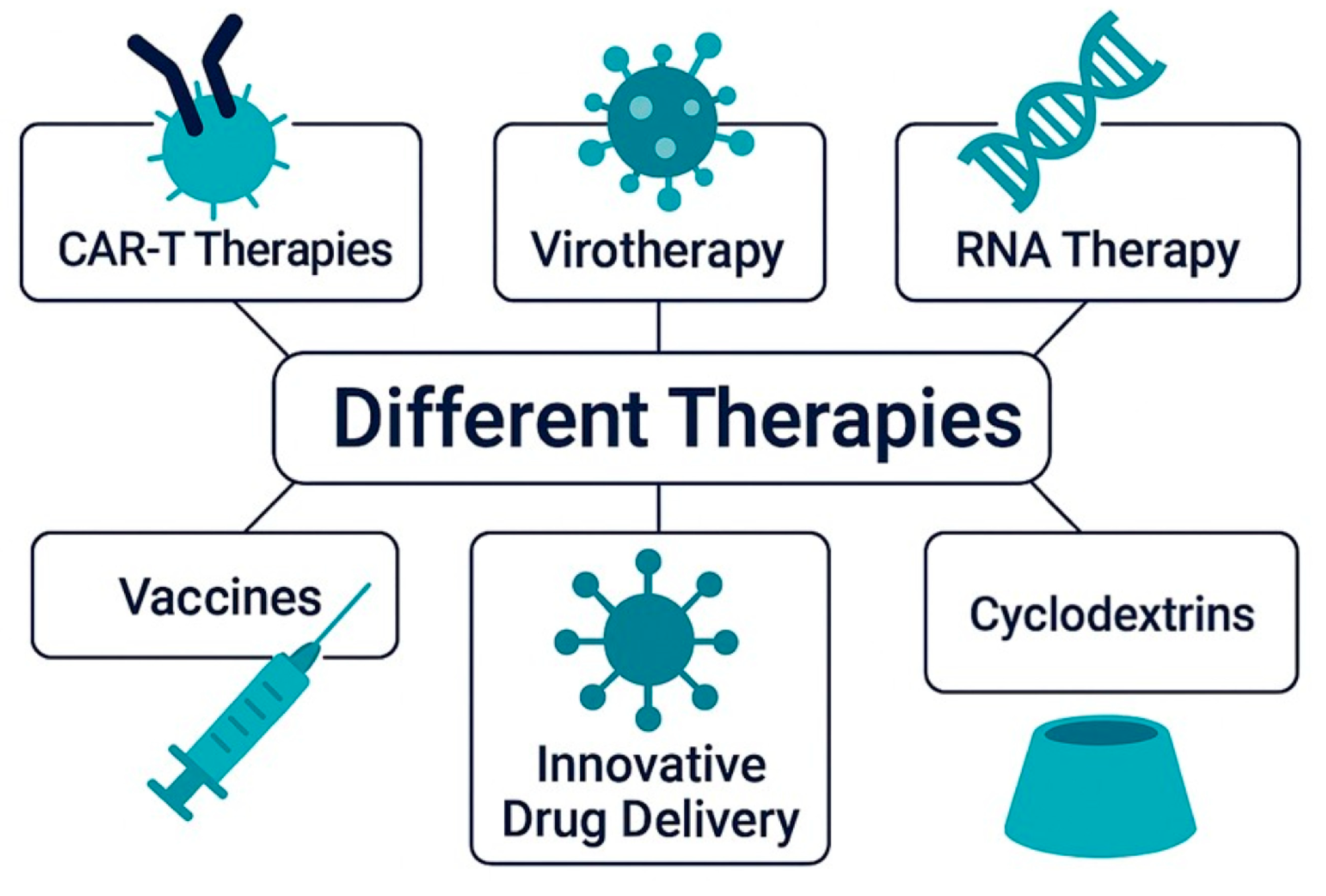

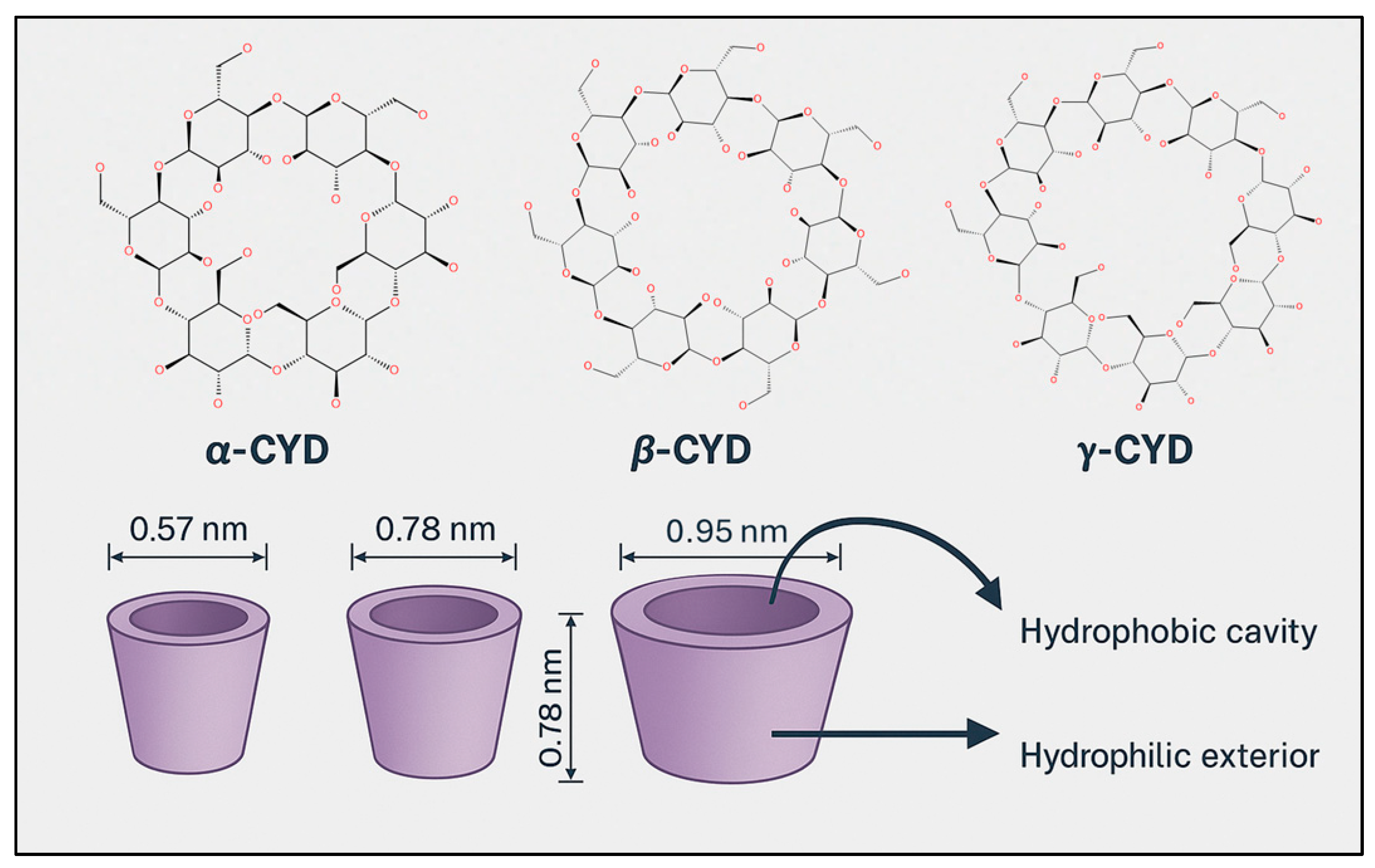


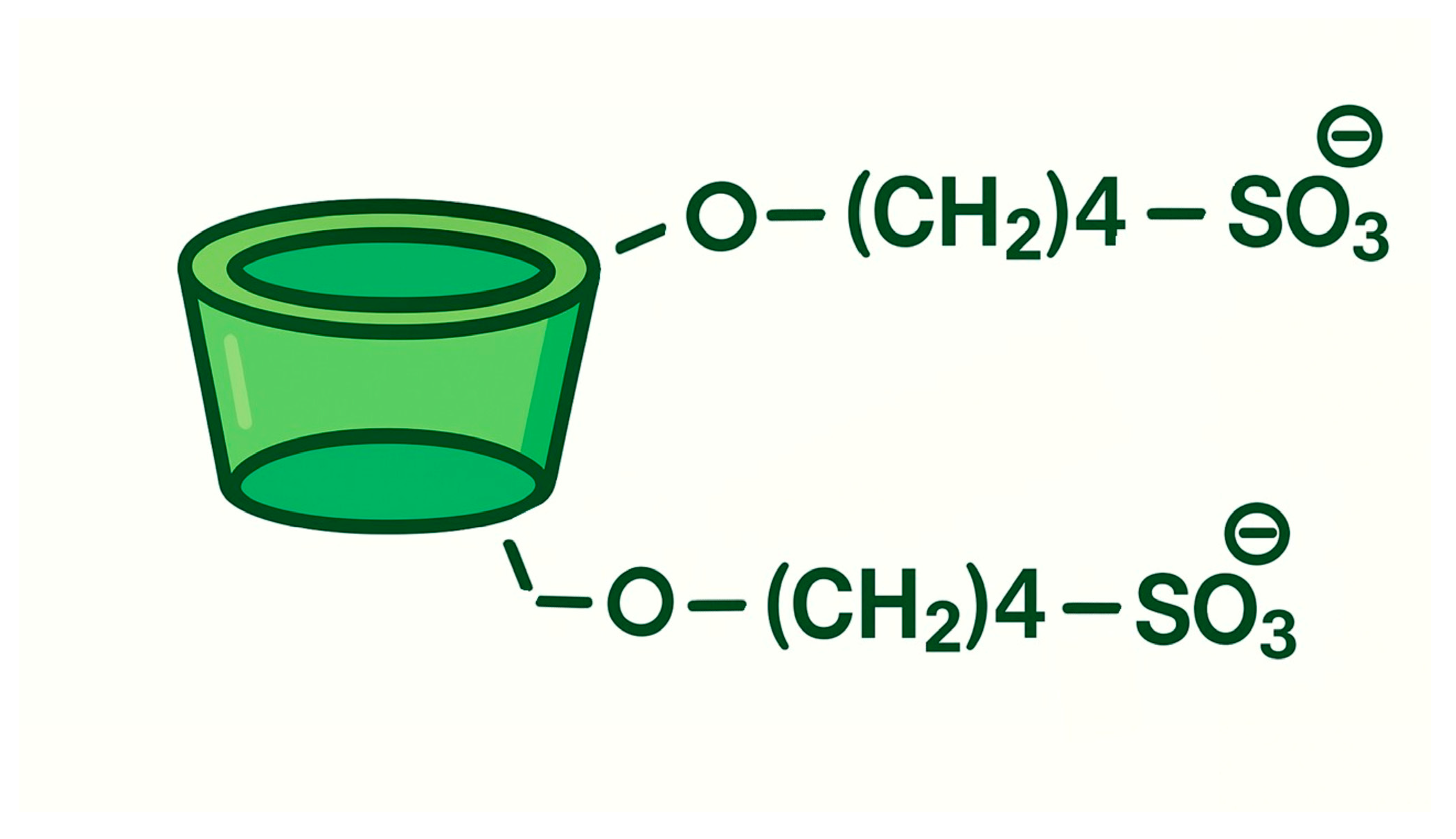


| Treatment Modality | Clinical Benefits | Limitations |
|---|---|---|
| Surgical resection |
- Reduces tumor burden
- Improves clinical symptoms - Enables identifying specific genetic mutations - Enhances efficacy of adjuvant therapies |
- Often incomplete due to an infiltrative nature
- Not feasible in eloquent brain areas - High recurrence rate near the surgical cavity |
| Radiotherapy |
- Targets residual tumor cells
- Extends survival - Palliative approach in inoperable patients to alleviate symptoms - Preserves surrounding healthy tissue |
- Side effects (fatigue, headache, alopecia, cutaneous reactions, edema, cognitive decline)
- Risk of radiation necrosis - Development of radio-resistance - Limited benefit from advanced techniques |
| Pharmacotherapy |
- Prolong survival
- Delay recurrence - Improve overall outcomes |
- Poor infiltration of chemotherapy into tumor cells
- Limited BBB and BBTB penetration - Resistance mechanisms reduce efficacy - Systemic toxicity (especially with IV agents) - Limited options after progression - Combination therapies are still under investigation |
| Tyrosine Kinase Inhibitors (TKIs) |
- Target key molecular pathways
- Potential for personalized therapy - New CNS-penetrant TKIs under development |
- Limited BBB and BBTB penetration
- Resistance mechanisms reduce efficacy - Modest clinical benefit - Significant side effects (e.g., hemorrhage, thrombosis) |
| Tumor Treatment Fields (TTFs) |
- Selective and non-invasive
- Improves survival when combined with TMZ - Minimal systemic toxicity |
- High cost
- Requires prolonged daily use - Hair shaving reduces patients’ compliance |
| Supportive Therapy |
- Improves quality of life
- Manages seizures, edema, pain, mood disorders, and cognitive decline - Reduces complications (e.g., VTE) |
- Risk of side effects from long-term medications (e.g., corticosteroids, anticoagulants)
- Requires individualized management - Limited efficacy |
| Compound | CYDs | Molar Ratio | Key Findings | Applications | Reference |
|---|---|---|---|---|---|
| SuccFerr | RAMEB | 1:2 |
↑ solubility,
↑ cellular uptake, ↑ apoptosis, ↑ drug availability, potent antiproliferative effect |
GB therapy
(in vitro U87/F98; in vivo rat models) | [349] |
| Cannabidiol (CBD) |
HP-β-CYD, RAMEB,
DM-β-CYD, TM-β-CYD |
1:37
1:5 1:1 1:1 |
cytotoxic effect dose- and time-dependent,
↑ solubility, ↑ stability, ↑ bioavailability |
GB and RMS therapy
(in vitro A172 and TE-671) | [353] |
|
Disulfiram
(DSF) | HP-β-CYD | Not specified |
↑ solubility (~2450-fold), combined with Cu → ↑ antitumor activity,
bypass BBB via intranasal delivery |
GB therapy
(in vitro C6, in vivo rat models) | [354] |
| Temozolomide (TMZ) |
β-CYDamine, βCYDgal,
β-CYD | 1:1 |
↑ solubility,
↑ stability, ↑ bioavailability potential to overcome BBB and improve uptake | GB therapy | [358] |
| Photosensitizers | β-CYD | 2:1 |
↑ solubility,
↓ aggregation, ↑ cellular uptake; stable complexes |
GB therapy and photodynamic treatment
(in vitro U251 and U87) | [361] |
|
Curcumin
(CUR) | β-CYD (embedded in cationic starch/PVA membrane) |
1:2
1:4 1:6 |
↑ stability,
controlled release, prolonged cytotoxicity on GB cells, selective toxicity, membrane-based localized delivery |
GB therapy
(in vitro C6 and B16F10) | [364] |
| Chrysin |
HP-β-CYD +
L-arginine | Not specified |
↑ solubility,
↑ dissolution, release profile 99%, ↑ anticancer efficacy (ROS ↑, apoptosis ↑) |
GB therapy
(in vitro U87-MG cells) | [366] |
| Sulforaphane (SFN) |
α-CYD
(SFX-01) | Not specified |
↑ stability,
↓ degradation multifaceted anticancer effects (apoptosis, anti-angiogenic, GB stem cell targeting), improved safety profile |
GB therapy
(preclinical models) | [372] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gaetano, F.; Totaro, N.; Ventura, C.A. Cyclodextrin-Based Formulations as a Promising Strategy to Overcome the Blood–Brain Barrier: Historical Overview and Prospects in Glioblastoma Treatment. Pharmaceuticals 2025, 18, 1626. https://doi.org/10.3390/ph18111626
De Gaetano F, Totaro N, Ventura CA. Cyclodextrin-Based Formulations as a Promising Strategy to Overcome the Blood–Brain Barrier: Historical Overview and Prospects in Glioblastoma Treatment. Pharmaceuticals. 2025; 18(11):1626. https://doi.org/10.3390/ph18111626
Chicago/Turabian StyleDe Gaetano, Federica, Noemi Totaro, and Cinzia Anna Ventura. 2025. "Cyclodextrin-Based Formulations as a Promising Strategy to Overcome the Blood–Brain Barrier: Historical Overview and Prospects in Glioblastoma Treatment" Pharmaceuticals 18, no. 11: 1626. https://doi.org/10.3390/ph18111626
APA StyleDe Gaetano, F., Totaro, N., & Ventura, C. A. (2025). Cyclodextrin-Based Formulations as a Promising Strategy to Overcome the Blood–Brain Barrier: Historical Overview and Prospects in Glioblastoma Treatment. Pharmaceuticals, 18(11), 1626. https://doi.org/10.3390/ph18111626