A Review of the Main Biologically Active Compounds of the Genus Echium L., Naturally Distributed in Bulgaria, and Their Pharmacological Potential
Abstract
1. Introduction
2. Materials and Methods
- The publication is a peer-reviewed scientific article, review, dissertation, or academic report;
- Written in English or Bulgarian;
- Contains relevant keywords in the title, abstract, keywords section, or full text;
- Provides information on phytochemical composition, biological activity, or medical/pharmaceutical application of any of the target Echium species.
- Preliminary screening of titles and abstracts;
- Full-text review of eligible articles. Extraction and tabulation of relevant data, including: identified bioactive compounds, reported pharmacological activities, and toxicological profiles, where available;
- Comparative analysis of the phytochemical profiles and documented applications of the Bulgarian Echium species.
3. Botanical Description of Echium Spp. Distributed in Bulgaria
3.1. Echium italicum
3.2. Echium russicum
3.3. Echium plantagineum
3.4. Echium vulgare
4. Ethnopharmacological Use of Echium Species in Folk Traditional Medicine
4.1. Echium italicum
4.2. Echium russicum
4.3. Echium plantagineum
4.4. Echium vulgare
5. Phytochemical Profile of Studied Echium Species
5.1. Phenolic Compounds
5.2. Flavonoids
5.3. Fatty Acids
5.4. Pyrrolizidine Alkaloids
5.5. Quinones
5.6. Terpenes
5.7. Phytosterols
6. Pharmacological Activity and Medical Applications
6.1. Antioxidant and Anti-Inflammatory Activity
6.2. Anxiolytic and Neuroprotective Effects
6.3. Antimicrobial Activity
6.4. Cytotoxicity and Antitumor Activity
6.5. Application in Dermatology and Cosmetics
7. Toxicological Profile and Safety of Echium L. Representatives
8. Relevance of the Topic and Current Research Trends
- Phase 1 (2000–2007)—characterized by low intensity, dominated by studies on E. vulgare and E. plantagineum.
- Phase 2 (2008–2016)—a period of diversification, marked by the emergence of research on E. italicum and sporadic publications on E. russicum.
- Phase 3 (2017–2023)—a peak activity phase, with simultaneous maxima for several species, particularly in 2017.
9. Future Perspectives and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Retief, E.; Van Wyk, A.E. The genus Echium (Boraginaceae) in southern Africa. Bothalia 1998, 28, 167–177. [Google Scholar] [CrossRef]
- Hu, A.; Meng, Q.; Borris, R.P.; Kim, H. Herbal Extract-Induced DNA Damage, Apoptosis, and Antioxidant Effects of C. elegans: A Comparative Study of Mentha longifolia, Scrophularia orientalis, and Echium biebersteinii. Pharmaceuticals 2025, 18, 1030. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.N. Plant naturalizations and invasions in the eastern United States: 1634–1860. Ann. Mo. Bot. Gard. 2003, 90, 77–90. [Google Scholar] [CrossRef]
- Zhu, X.C.; Gopurenko, D.; Skoneczny, D.; Lepschi, B.J.; Spencer, M.A.; Gurr, G.M.; Callaway, R.M.; Serrano, M.; Weston, L.A. Introduction of Paterson’s curse (Echium plantagineum) to Australia-unravelling the story by DNA sequence analysis. In Proceedings of the 20th Australasian Weeds Conference (2016), Perth, Australia, 11–15 September 2016; Weed Society of Western Australia: East Victoria Park, Australia, 2016; pp. 157–161. Available online: https://researchoutput.csu.edu.au/en/publications/introduction-of-patersons-curse-echium-plantagineum-to-australia- (accessed on 15 July 2025).
- Petreska Stanoeva, J.; Stefova, M.; Matevski, V. Extraction, distribution and diversity of phenolic compounds in most widespread boraginaceae species from Macedonia. Chem. Biodivers. 2023, 20, e202201149. [Google Scholar] [CrossRef]
- Sheydaei, P.; Amaral, M.E.; Duarte, A.P. Genus Echium L.: Phytochemical Characterization and Bioactivity Evaluation for Drug Discovery. Plants 2025, 14, 2548. [Google Scholar] [CrossRef]
- Shafaghi, B.; Naderi, N.; Tahmasb, L.; Kamalinezhad, M. Anxiolytic effect of Echium amoenum in mice. Iran. J. Pharmacol. Ther. 2002, 1, 37–41. [Google Scholar]
- Rabbani, M.; Sajjadi, S.E.; Vaseghi, G.; Jafarian, A. Anxiolytic effect of Echium amoenum on the elevated plus-maze model of anxiety in mice. Fitoterapia 2004, 75, 457–464. [Google Scholar] [CrossRef]
- Azizi, H.; Ghafari, S.; Ghods, R.; Shojaii, A.; Salmanian, M.; Ghafarzadeh, J. A review study on pharmacological activities, chemical constituents, and traditional uses of Echium amoenum. Pharmacogn. Rev. 2018, 12, 208–213. [Google Scholar] [CrossRef]
- Nouri, M.; Farajdokht, F.; Torbati, M.; Ranjbar, F.; Hamedyazdan, S.; Sadigh-Eteghad, S.; Araj-Khodaei, M. Antidepressant and anxiolytic effect of Echium amoenum in restraint stress model: The role of neuroinflammation in the prefrontal cortex and hippocampus. Iran. Red Crescent Med. J. 2019, 21, 1–14. [Google Scholar] [CrossRef]
- Gohari, A.R.; Saeidnia, S.; Gohari, M.R.; Moradi-Afrapoli, F.; Malmir, M.; Hadjiakhoondi, A.; Yazdanpanah, M. Shrimp Cytotoxicity of Some Medicinal Plants Belongs to Lamiaceae, Asteracea, Rosaceae and Boraginaceae Families. 2009. Available online: http://jmp.ir/article-1-388-en.html (accessed on 17 July 2025).
- Chiocchio, I.; Marincich, L.; Mandrone, M.; Trincia, S.; Tarozzi, C.; Poli, F. Saving the local tradition: Ethnobotanical survey on the use of plants in Bologna district (Italy). J. Ethnobiol. Ethnomed. 2024, 20, 33. [Google Scholar] [CrossRef]
- Wang, W.; Jin, J.; Xu, H.; Shi, Y.; Boersch, M.; Yin, Y. Comparative analysis of the main medicinal substances and applications of Echium vulgare L. and Echium plantagineum L.: A review. J. Ethnopharmacol. 2022, 285, 114894. [Google Scholar] [CrossRef]
- Nadaf, M.; Joharchi, M.; Amiri, M.S. Ethnomedicinal uses of plants for the treatment of nervous disorders at the herbal markets of Bojnord, North Khorasan Province, Iran. Avicenna J. Phytomed. 2019, 9, 153. [Google Scholar] [PubMed] [PubMed Central]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Garcıa-Maroto, F.; Campra-Madrid, P. Occurrence and characterization of oils rich in γ-linolenic acid: Part I: Echium seeds from Macaronesia. Phytochemistry 2000, 53, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; García-Maroto, F.; Campra-Madrid, P.; Gómez-Mercado, F. Occurrence and characterization of oils rich in γ-linolenic acid Part II: Fatty acids and squalene from Macaronesian Echium leaves. Phytochemistry 2000, 54, 525–529. [Google Scholar] [CrossRef]
- Berti, M.; Johnson, B.L.; Dash, S.; Fischer, S.; Wilckens, R.; Hevia, F. Echium: A source of stearidonic acid adapted to the northern Great Plains in the US. In Issues in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2007; pp. 120–125. Available online: http://www.cytothesis.us/3.0/n-3_Echium_SDA_18.4.pdf (accessed on 17 July 2025).
- Roeder, E. Medicinal plants in China containing pyrrolizidine alkaloids. Pharmazie 2000, 55, 711–726. Available online: https://www.researchgate.net/profile/Erhard-Roeder-Roeder/publication/12244620_Medicinal_plants_in_China_containing_pyrrolizidine_alkaloids/links/0fcfd508950457deb5000000/Medicinal-plants-in-China-containing-pyrrolizidine-alkaloids.pdf (accessed on 8 July 2025). [PubMed]
- EFSA (European Food Safety Authority). Scientific Opinion on Pyrrolizidine Alkaloids in Food and Feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Betteridge, K.; Cao, Y.; Colegate, S.M. Improved method for extraction and LC-MS analysis of pyrrolizidine alkaloids and their N-oxides in honey: Application to Echium vulgare honeys. J. Agric. Food Chem. 2005, 53, 1894–1902. [Google Scholar] [CrossRef]
- Leiss, K.A.; Vrieling, K.; Klinkhamer, P.G.L. Heritability of nectar production in Echium vulgare. Heredity 2004, 92, 446–451. [Google Scholar] [CrossRef]
- Corbet, S.A.; Delfosse, E.S. Honeybees and the nectar of Echium plantagineum L. in southeastern Australia. Aust. J. Ecol. 1984, 9, 125–139. [Google Scholar] [CrossRef]
- Medicinal Plants Act in Bulgaria (State Gazette No. 29/2000), No. 29, 7 April 2000; pp. 9–29; Last Amended in State Gazette, No. 82, 26 October 2012. Available online: https://eea.government.bg/bg/legislation/biodiversity/ZLR_en.pdf (accessed on 10 July 2025).
- Nikolov, S. Specialized Encyclopedia of Medicinal Plants in Bulgaria; Trud Publishing House: Sofia, Bulgaria, 2006; p. 566. [Google Scholar]
- Kaniskov, V. Medicinal Plants of Bulgaria; Iztok-Zapad: Sofia, Bulgaria, 2011; pp. 176–177. Available online: https://iztok-zapad.eu/image/catalog/materials/Bilkite_v_Bulgaria.pdf (accessed on 28 July 2025).
- Kozhuharov, S. (Ed.) Flora Reipublicae Popularis Bulgaricae; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1989; Volume IX. [Google Scholar]
- Delipavlov, D.; Cheshmedzhiev, I.; Popova, M.; Terziiski, D.; Kovachev, I. Opredelitel na Rasteniyata v Balgariya [Flora of Bulgaria]; Pensoft: Sofia, Bulgaria, 2003; Available online: https://botanica.gallery/wp/archives/2102 (accessed on 13 July 2025).
- Assyov, B.; Petrova, A. Conspectus of the Bulgarian Vascular Flora; Bulgarian Biodiversity Foundation: Sofia, Bulgaria, 2012; Available online: https://archive.org/details/ConspectusOfTheBulgarianVascularFlora.DistributionMapsAndFloristic/page/n15/mode/2up (accessed on 10 July 2025).
- Biodiversity Act in Bulgaria Ministry of Environment and Water in Bulgaria. Available online: https://www.moew.government.bg/static/media/ups/tiny/filebase/Nature/Legislation/Zakoni/English_versions/Biodiversity_Act2002-2018-EN.pdf (accessed on 10 July 2025).
- Peev, D.; Vassilev, R.; Petrova, A.; Meshinev, T.; Denchev, C.M. (Eds.) Red Data Book of the Republic of Bulgaria. Volume 1. Plants and Fungi; Bulgarian Academy of Sciences & Ministry of Environment and Water: Sofia, Bulgaria, 2011.
- Kelley, R.B. Echium plantagineum. In Jepson Flora; Jepson eFlora. 2012. Copyright © 2025 Regents of the University of California. Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=23837 (accessed on 18 August 2025).
- Klemow, K.M.; Clements, D.R.; Threadgill, P.F.; Cavers, P.B. The biology of Canadian weeds. 116 Echium vulgare L. Can. J. Plant Sci. 2002, 82, 235–248. [Google Scholar] [CrossRef]
- Jin, J.; Boersch, M.; Nagarajan, A.; Davey, A.K.; Zunk, M. Antioxidant properties and reported ethnomedicinal use of the genus Echium (Boraginaceae). Antioxidants 2020, 9, 722. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Rajabian, T.; Taghizadeh, M. Antioxidant activity, phenolic and flavonoid contents of Echium species from different geographical locations of Iran. J. Med. Plants By-Prod. 2013, 2, 23–31. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Gonzales-Tejero, M.R.; Sanchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia-Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Bošković, I.; Đukić, D.; Mašković, P.; Mandić, L. Phytochemical Composition and Biological Activity of Echium italicum L. Plant Extracts. Bulg. Chem. Commun. 2017, 49, 836–845. Available online: https://scidar.kg.ac.rs/handle/123456789/20254 (accessed on 16 July 2025).
- Demirci Kayiran, S.; Parlak, M.; Yilmaz Oral, D. Ethnobotany of medicinal plants used in dermatology in Türkiye: A review. Turk. J. Bot. 2023, 47, 408–463. [Google Scholar] [CrossRef]
- De Natale, A.; Pollio, A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 2007, 109, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Cakilcioglu, U.; Turkoglu, I. An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Yilmaz, G.; Üstün, O. Analgesic and antioxidant activity of some Echium species wild growing in Turkey. FABAD J. Pharm. Sci. 2012, 37, 151. Available online: https://dergi.fabad.org.tr/pdf/volum37/issue3/151-159.pdf (accessed on 10 August 2025).
- Gürbüz, İ.; Özatkan, G.; Akaydin, G.; Günbatan, T. Ethnopharmacobotanical findings of medicinal plants in the Kızılcahamam District of Ankara, Turkey. Turk. J. Pharm. Sci. 2021, 18, 667. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8744430/ (accessed on 1 August 2025). [CrossRef]
- Sârbu, I.; Ştefan, N.; Oprea, A. Vascular Plants of Romania: Illustrated Field Guide; Victor B. Victor: Bucureşti, Romania, 2011. (In Romanian) [Google Scholar]
- Batsatsashvili, K.; Mehdiyeva, N.; Fayvush, G.; Kikvidze, Z.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D.; Aleksanyan, A.; Alizade, V.M.; et al. Echium maculatum L. In Ethnobotany of the Caucasus; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Tardío, J.; Pascual, H.; Morales, R. Wild food plants traditionally used in the province of Madrid, Central Spain. Econ. Bot. 2005, 59, 122–136. [Google Scholar] [CrossRef]
- Eruygur, N.; Yılmaz, G.; Kutsal, O.; Yücel, G.; Üstün, O. Bioassay-guided isolation of wound healing active compounds from Echium species growing in Turkey. J. Ethnopharmacol. 2016, 185, 370–376. [Google Scholar] [CrossRef]
- Jarić, S.; Popović, Z.; Mačukanović-Jocić, M.; Djurdjević, L.; Mijatović, M.; Karadžić, B.; Mitrović, M.; Pavlović, P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia). J. Ethnopharmacol. 2007, 111, 160–175. [Google Scholar] [CrossRef]
- Rigat, M.; Valles, J.; Gras, A.; Iglésias, J.; Garnatje, T. Plants with topical uses in the Ripollès district (Pyrenees, Catalonia, Iberian Peninsula): Ethnobotanical survey and pharmacological validation in the literature. J. Ethnopharmacol. 2015, 164, 162–179. [Google Scholar] [CrossRef]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.D.F. Medicinal plants for the treatment of local tissue damage induced by snake venoms: An overview from traditional use to pharmacological evidence. Evid.-Based Complement. Altern. Med. 2017, 2017, 5748256. [Google Scholar] [CrossRef] [PubMed]
- Lyoussi, B.; Bakour, M.; Cherkaoui-Tangi, K.; El-Hilaly, J.; Hano, C. Ethnobotanical survey and pharmacological screening of medicinal plants used as antihypertensive in Sefrou province (middle-north of Morocco): Benefits and challenges. Front. Biosci.-Sch. 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.E.E. Folk medicinal plants used for treatment of gynecological disorders by rural population of Zorlu village (in Turkey). Ethnobot. Res. Appl. 2021, 22, 1–17. Available online: https://ethnobotanyjournal.org/index.php/era/article/view/2695 (accessed on 12 August 2025).
- El-Shazly, A.; Wink, M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Miquel, M.I.R. Echium oil: A valuable source of n-3 and n-6 fatty acids. Oléagineux Corps Gras Lipides 2008, 15, 252–256. [Google Scholar] [CrossRef]
- Taravati, G.; Masoudian, N.; Gholamian, A. Evaluation of medical metabolites in Boraginaceae family. J. Chem. Health Risks 2014, 4, 53–61. [Google Scholar]
- Bošković, I.; Đukić, D.; Mašković, P.; Mandić, L. HPLC analysis and antimicrobial potential of plant extracts. In Book of Proceedings XI International Scientific Agriculture Symposium “AGROSYM 2020”; Jahorina, October 8–9, 2020; [editor in chief Dusan Kov acevic]. -Onlajn izd. -El. zbornik. -East Sarajevo: Faculty of Agriculture, 2020.- Ilustr; Available online: https://scidar.kg.ac.rs/bitstream/123456789/19707/1/HPLC%20ANALYSIS%20AND%20ANTIMICROBIAL%20POTENTIAL%20OF%20PLANT%20EXTRACTS.pdf (accessed on 12 August 2025).
- Alamholo, M. Antiradical and antibacterial activity of Echium altissimum extracts on human infective bacteria and chemical composition analysis. Microbiol. Metab. Biotechnol. 2020, 3, 19–27. [Google Scholar] [CrossRef]
- Gheisary, B.; Fattahi, M.; Ashrafi-Saeidlou, S. Selenium Nanoparticles Enhance Secondary Metabolite Accumulation, Antioxidant Defense, and Antimicrobial Activity in Echium italicum: A Study Supported by Molecular Docking Analysis. 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5363354 (accessed on 19 July 2025).
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, S.; Radjabian, T.; Taghizadeh, M.; Fazeli, F.; Salmaki, Y. Characterization of fatty acids in different organs of some Iranian Echium plants. J. Med. Plants Res. 2011, 5, 4814–4821. Available online: http://www.academicjournals.org/JMPR (accessed on 17 July 2025).
- Özcan, T. Molecular (RAPDs and Fatty acid) and micromorphological variations of Echium italicum L. populations from Turkey. Plant Syst. Evol. 2013, 299, 631–641. [Google Scholar] [CrossRef]
- Özcan, T.; Süzerer, V. Variation of some seed oil components at altitidunal range in a widely distributed species, Echium italicum L.(Boraginaceae) from Turkey. J. Mater. Environ. Sci. 2020, 11, 540–550. Available online: https://jmaterenvironsci.com/Document/vol11/vol11_N4/JMES-2020-1148-Ozcan.pdf (accessed on 16 July 2025).
- Al-Snafi, A.E. Oils and fats contents of medicinal plants, as natural ingredients for many therapeutic purposes—A review. IOSR J. Pharm 2020, 10, 1–41. Available online: https://www.researchgate.net/profile/Ali-Al-Snafi/publication/343111268_Oils_and_fats_contents_of_medicinal_plants_as_natural_ingredients_for_many_therapeutic_purposes-_A_review/links/5f17314592851cd5fa39fc42/Oils-and-fats-contents-of-medicinal-plants-as-natural-ingredients-for-many-therapeutic-purposes-A-review.pdf (accessed on 26 July 2025).
- Stefova, E.; Cvetanoska, M.; Bogdanov, J.; Matevski, V.; Stanoeva, J.P. Assessment of distribution and diversity of pyrrolizidine alkaloids in the most prevalent Boraginaceae species in Macedonia. Chem. Biodivers. 2022, 19, e202200066. [Google Scholar] [CrossRef]
- Tepe, M. Antihistaminic Activity of Shikonin from Biotechnologically Grown Echium italicum L. In Biotechnology of Medicinal Plants with Antiallergy Properties: Research Trends and Prospects; Springer: Singapore, 2024; pp. 219–234. [Google Scholar] [CrossRef]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J. Chromatogr. A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef]
- Tepe, M.; Atilla, D.; Özden, Y.Ç. Phytochemical analysis of shikonin derıvatıve which is produced from Echium italicum L. haıry roots. Kongre Kitabi 2023, 1, 369–376. [Google Scholar]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef]
- Zare, K.; Khosrowshahli, M.; Nazemiyeh, H.; Movafeghi, A.; Azar, A.M.; Omidi, Y. Callus culture of Echium italicum L. towards production of a shikonin derivative. Nat. Prod. Res. 2011, 25, 1480–1487. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. Chemical composition and antimicrobial activity of essential oil of Echium italicum L. J. Essent. Oil Bear. Plants 2009, 12, 557–561. [Google Scholar] [CrossRef]
- Chari, S.A.; Radjabian, T.; Taghizadeh, M. Extraction and identification of phytosterols in different organs of some Iranian Echium plants. J. Appl. Plant Biol. 2023, 1, 12–19. [Google Scholar] [CrossRef]
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of shikonin and rosmarinic acid in Echium vulgare L. and Echium russicum JF Gmel. by capillary electrophoresis. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 698–701. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Daironas, Z.V.; Zilfikarov, I.N. Shikonin and rosmarinic-acid derivatives from Echium russicum roots. Chem. Nat. Compd. 2017, 53, 953–955. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Garcıa-Maroto, F.; Vilches-Ferrón, M.A.; López-Alonso, D. Gamma-linolenic acid from fourteen Boraginaceae species. Ind. Crop. Prod. 2003, 18, 85–89. [Google Scholar] [CrossRef]
- Jin, J.; Holland, D.C.; Carroll, A.R.; Zunk, M. Echiumin E, an aryl dihydronaphthalene lignan from the Australian invasive plant Paterson’s Curse (Echium plantagineum). J. Nat. Prod. 2022, 85, 2474–2479. [Google Scholar] [CrossRef]
- Carlini, G.C.G.; Roschel, G.G.; Ferrari, R.A.; Alencar, S.M.; Ota, H.C.; da Silveira, T.F.F.; Castro, I.A. Chemical characterization of Echium plantagineum seed oil obtained by three methods of extraction. J. Food Sci. 2021, 86, 5307–5317. [Google Scholar] [CrossRef]
- Bastürk, F.N.; Özcan, T.; Gercek, Y.C. Populational segregation of Echium plantagineum L. based on seed oil fatty acid ratios as chemotaxonomical marker sets. J. Food Compos. Anal. 2024, 136, 106752. [Google Scholar] [CrossRef]
- García-Maroto, F.; Manas-Fernández, A.; Garrido-Cárdenas, J.A.; Alonso, D.L. Substrate specificity of acyl-Δ6-desaturases from Continental versus Macaronesian Echium species. Phytochemistry 2006, 67, 540–544. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Replacing dietary fish oil with Echium oil enriched barramundi with C18 PUFA rather than long-chain PUFA. Aquaculture 2011, 312, 162–171. [Google Scholar] [CrossRef]
- Eberle, C.A.; Forcella, F.; Gesch, R.; Weyers, S.; Peterson, D.; Eklund, J. Flowering dynamics and pollinator visitation of oilseed Echium (Echium plantagineum). PLoS ONE 2014, 9, e113556. [Google Scholar] [CrossRef] [PubMed]
- Castejón, N.; Luna, P.; Señoráns, F.J. Alternative oil extraction methods from Echium plantagineum L. seeds using advanced techniques and green solvents. Food Chem. 2018, 244, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, N.; Kusmenoglu, S.; Vural, M. γ-Linolenic acid content and fatty acid composition of Boraginaceae seed oils. Eur. J. Lipid Sci. Technol. 2004, 106, 160–164. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; García Maroto, F.F.; Gimenez Gimenez, A. Fatty acid profiles from forty-nine plant species that are potential new sources of γ-linolenic acid. J. Am. Oil Chem. Soc. 2001, 78, 677–684. [Google Scholar] [CrossRef]
- Gray, D.A.; Payne, G.; McClements, D.J.; Decker, E.A.; Lad, M. Oxidative stability of Echium plantagineum seed oil bodies. Eur. J. Lipid Sci. Technol. 2010, 112, 741–749. [Google Scholar] [CrossRef]
- Kavanagh, K.; Flynn, D.M.; Jenkins, K.A.; Wilson, M.D.; Chilton, F.H. Stearidonic and γ-linolenic acids in Echium oil improves glucose disposal in insulin resistant monkeys. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 39–45. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Guil-Guerrero, J.L. Preparation of stearidonic acid-enriched triacylglycerols from Echium plantagineum seed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 227–232. [Google Scholar] [CrossRef]
- Quinn, J.C.; Kessell, A.; Weston, L.A. Secondary plant products causing photosensitization in grazing herbivores: Their structure, activity and regulation. Int. J. Mol. Sci. 2014, 15, 1441–1465. [Google Scholar] [CrossRef]
- Skoneczny, D.; Weston, P.A.; Zhu, X.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Metabolic profiling of pyrrolizidine alkaloids in foliage of two Echium spp. invaders in Australia—A case of novel weapons? Int. J. Mol. Sci. 2015, 16, 26721–26737. [Google Scholar] [CrossRef]
- Sixto, A.; Pérez-Parada, A.; Niell, S.; Heinzen, H. GC–MS and LC–MS/MS workflows for the identification and quantitation of pyrrolizidine alkaloids in plant extracts, a case study: Echium plantagineum. Rev. Bras. Farmacogn. 2019, 29, 500–503. [Google Scholar] [CrossRef]
- Weston, P.A.; Weston, L.A.; Hildebrand, S. Metabolic profiling in Echium plantagineum: Presence of bioactive pyrrolizidine alkaloids and napthoquinones from accessions across southeastern Australia. Phytochem. Rev. 2013, 12, 831–837. [Google Scholar] [CrossRef]
- Skoneczny, D.; Weston, P.A.; Zhu, X.; Gurr, G.M.; Callaway, R.M.; Barrow, R.A.; Weston, L.A. Metabolic profiling and identification of shikonins in root periderm of two invasive Echium spp. weeds in Australia. Molecules 2017, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ren, R.; Jin, S.; Fang, R.; Wen, Z.; Yang, M.; Wang, X.; Liu, B.; Yin, T.; Lu, G.; et al. Overexpression of a putative 12-oxophytodienoate reductase gene, EpOPR1, enhances acetylshikonin production in Echium plantagineum. In Vitr. Cell. Dev. Biol.-Plant 2022, 58, 311–320. [Google Scholar] [CrossRef]
- Durán, A.G.; Gutiérrez, M.T.; Rial, C.; Torres, A.; Varela, R.M.; Valdivia, M.M.; Molinillo, J.M.G.; Skoneczny, D.; Weston, L.A.; Macías, F.A. Bioactivity and quantitative analysis of isohexenylnaphthazarins in root periderm of two Echium spp.: E. plantagineum and E. gaditanum. Phytochemistry 2017, 141, 162–170. [Google Scholar] [CrossRef]
- Weston, L.; Weston, P.; McCully, M. Production of bioactive napthoquinones by roots of Paterson’s Curse (Echium plantagineum L.): Implications for invasion success? In Proceedings of the 23rd Asian-Pacific Weed Science Society Conference (APWSS): Weed Management in a Changing World, Cairns, Australia, 26–29 September 2011; APWSS: Perth, Australia, 2011; pp. 576–581. [Google Scholar]
- Zhu, X.; Skoneczny, D.; Weidenhamer, J.D.; Mwendwa, J.M.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Identification and localization of bioactive naphthoquinones in the roots and rhizosphere of Paterson’s curse (Echium plantagineum), a noxious invader. J. Exp. Bot. 2016, 67, 3777–3788. [Google Scholar] [CrossRef]
- Fu, J.Y.; Zhao, H.; Bao, J.X.; Wen, Z.L.; Fang, R.J.; Fazal, A.; Yang, M.K.; Liu, B.; Yin, T.M.; Pang, Y.J.; et al. Establishment of the hairy root culture of Echium plantagineum L. and its shikonin production. 3 Biotech 2020, 10, 429. [Google Scholar] [CrossRef]
- Mitkov, S.; Obreshkova, D.; Ilieva, I.; Pangarova, T.; Pencheva, I. Phenolcarboxylic acids in Echium vulgare L. Acta Pharm. Turc. 2002, 44, 43–48. [Google Scholar]
- Czaplicki, S.; RZadernowski, H. Nowak-Polakowska Phenolic compounds of blueweed (Echium vulgare L.) seeds. Bromat. Chem. Toksykol. 2011, 44, 815–882. [Google Scholar] [CrossRef]
- Alsanie, W.F.; El-Hallous, E.I.; Dessoky, E.S.; Ismail, I.A. Viper’s Bugloss (Echium vulgare L) Extract as A Natural Antioxidant and Its Effect on Hyperlipidemia. Int. J. Pharm. Phytopharm. Res. (eIJPPR) 2018, 8, 81–89. Available online: https://www.researchgate.net/profile/Eldessoky-Aboelhana/publication/332111625_Viper’s_Bugloss_Echium_Vulgare_L_Extract_as_A_Natural_Antioxidant_and_Its_Effect_on_Hyperlipidemia/links/628581e9247e622c2efb4370/Vipers-Bugloss-Echium-Vulgare-L-Extract-as-A-Natural-Antioxidant-and-Its-Effect-on-Hyperlipidemia.pdf (accessed on 2 August 2025).
- Boskovic, I.; Đukić, D.; Mašković, P.; Mandić, L. Influence of solvent type on the phenolic content and antimicrobial and antioxidant properties of Echium vulgare L. extracts. FARMACIA 2022, 70, 4. Available online: https://scidar.kg.ac.rs/handle/123456789/14898 (accessed on 25 July 2025). [CrossRef]
- Kuruüzüm-Uz, A.; Güvenalp, Z.; Ströch, K.; Demirezer, L.Ö.; Zeeck, A. Phytochemical and antimicrobial investigation of Echium vulgare growing in Turkey. Biochem. Syst. Ecol. 2004, 32, 833–836. [Google Scholar] [CrossRef]
- Nićiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Muratspahić, D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem. 2009, 112, 587–594. [Google Scholar] [CrossRef]
- Kapusterynska, A.R.; Hamada, V.R.; Krvavych, A.S.; Konechna, R.T.; Kurka, M.S.; Novikov, V.P. Investigation of the extract’s composition of Viper’s bugloss (Echium vulgare). Ukr. Bioorganica Acta 2020, 15, 42–46. [Google Scholar] [CrossRef]
- Bilgiç-Keleş, S.; Şahin-Yeşilçubuk, N.; Barla-Demirkoz, A.; Karakaş, M. Response surface optimization and modelling for supercritical carbon dioxide extraction of Echium vulgare seed oil. J. Supercrit. Fluids 2019, 143, 365–369. [Google Scholar] [CrossRef]
- Czaplicki, S.; Zadernowski, R.; Ogrodowska, D. Triacylglycerols from viper bugloss (Echium vulgare L.) seed bio-oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1266–1269. [Google Scholar] [CrossRef]
- Cisowski, W.; Zielińska-Stasiek, M.; Stołyhwo, A.; Migas, P.; Kamieniec, A. Gas-liquid chromatographic analysis of fatty acids obtained from the seeds of some Boraginaceae plants. Acta Chromatogr. 2001, 215–223. Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BAT3-0025-0097 (accessed on 17 August 2025).
- Stolyhwo, A.; Mol, J. Changes in the content of γ-linolenic C18: 3 (n-6) and stearidonic C18: 4 (n-3) acids in developing seeds of viper’s bugloss Echium vulgare L. Acta Biochim. Pol. 2007, 54, 741–746. Available online: https://www.frontierspartnerships.org/articles/10.18388/abp.2007_3148/pdf (accessed on 4 August 2025). [CrossRef]
- Ogrodowska, D.; Tańska, M.; Brandt, W.; Czaplicki, S. The influence of emulsion drying on the fatty acid composition, bioactive compounds content and oxidative stability of encapsulated bio-oils. CyTA-J. Food 2019, 17, 949–959. [Google Scholar] [CrossRef]
- Kempf, M.; Heil, S.; Haßlauer, I.; Schmidt, L.; von der Ohe, K.; Theuring, C.; Reinhard, A.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in pollen and pollen products. Mol. Nutr. Food Res. 2010, 54, 292–300. [Google Scholar] [CrossRef]
- Boppré, M.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloids of Echium vulgare honey found in pure pollen. J. Agric. Food Chem. 2005, 53, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Kast, C.; Kilchenmann, V.; Reinhard, H.; Droz, B.; Lucchetti, M.A.; Dübecke, A.; Beckh, G.; Zoller, O. Chemical fingerprinting identifies Echium vulgare, Eupatorium cannabinum and Senecio spp. as plant species mainly responsible for pyrrolizidine alkaloids in bee-collected pollen. Food Addit. Contam. Part A 2018, 35, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Kast, C.; Kilchenmann, V.; Reinhard, H.; Bieri, K.; Zoller, O. Pyrrolizidine alkaloids: The botanical origin of pollen collected during the flowering period of Echium vulgare and the stability of pyrrolizidine alkaloids in bee bread. Molecules 2019, 24, 2214. [Google Scholar] [CrossRef] [PubMed]
- Mädge, I.; Gehling, M.; Schöne, C.; Winterhalter, P.; These, A. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit. Contam. Part A 2020, 37, 1339–1358. [Google Scholar] [CrossRef]
- Erenler, R. ON NATURAL & MEDICAL SCIENCES-V. 2022. Available online: https://www.researchgate.net/profile/ramazan-erenler/publication/381155334_chemical_constituents_of_the_essential_oil_of_echium_vulgare/links/665f54bb2f32b240a563b8b9/chemical-constituents-of-the-essential-oil-of-echium-vulgare.pdf (accessed on 20 July 2025).
- Pardo, F.; Perich, F.; Torres, R.; Delle Monache, F. Stigmast-4-ene-3, 6-dione an unusual phytotoxic sterone from the roots of Echium vulgare L. Biochem. Syst. Ecol. 2000, 28, 911–913. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Galleguillos-Fernández, R.; González-Barriga, V.; Valenzuela, R.; Speisky, H.; Fuentes, J.; Valenzuela, A. Fatty acid profile and bioactive compound extraction in purple viper’s bugloss seed oil extracted with green solvents. J. Am. Oil Chem. Soc. 2020, 97, 319–327. [Google Scholar] [CrossRef]
- Benbrinis, S. In Vitro and In Vivo Anti-Inflammatory and Hepatoprotector Effects of Algerian Echium plantagineum and Brassica rapa Extracts. Doctoral Dissertation, Ferhat Abbas University, Setif 1 Faculty of Natural and Life Sciences, Setif, Algeria, 2024. Available online: http://dspace.univ-setif.dz:8888/jspui/handle/123456789/4292 (accessed on 10 July 2025).
- Bağcı, E.; Bruehl, L.; Aitzetmuller, K.; Altan, Y. Fatty acid and tocochromanol patterns of some Turkish Boraginaceae. Nord. J. Bot. 2004, 22, 719–726. [Google Scholar] [CrossRef]
- García-Maroto, F.; Garrido-Cárdénas, J.A.; Rodríguez-Ruiz, J.; Vilches-Ferrón, M.; Adam, A.C.; Polaina, J.; López Alonso, D. Cloning and molecular characterization of the Δ6-desaturase from two Echium plant species: Production of GLA by heterologous expression in yeast and tobacco. Lipids 2002, 37, 417–426. [Google Scholar] [CrossRef]
- Özcan, T. Analysis of the total oil and fatty acid composition of seeds of some Boraginaceae taxa from Turkey. Plant Syst. Evol. 2008, 274, 143–153. [Google Scholar] [CrossRef]
- Kuhnt, K.; Degen, C.; Jaudszus, A.; Jahreis, G. Searching for health beneficial n-3 and n-6 fatty acids in plant seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Nogueira, M.; Scolaro, B.; Thomazini, M.; Ferro-Furtado, R.; de Castro, I.A.; Favaro-Trindade, C.S. Enhancing stability of echium seed oil and beta-sitosterol by their coencapsulation by complex coacervation using different combinations of wall materials and crosslinkers. Food Chem. 2018, 252, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Robert, S.; Singh, S.; Green, A. Heterologous production of GLA and SDA by expression of an Echium plantagineum Δ6-desaturase gene. Plant Sci. 2006, 170, 665–673. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L. Stearidonic acid (18: 4n-3): Metabolism, nutritional importance, medical uses and natural sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1226–1236. [Google Scholar] [CrossRef]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Maes, A.; Goemaere, S.; Rottey, S.; Foubert, I.; et al. Echium oil is not protective against weight loss in head and neck cancer patients undergoing curative radio (chemo) therapy: A randomised-controlled trial. BMC Complement. Altern. Med. 2014, 14, 382. [Google Scholar] [CrossRef]
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit. Rev. Food Sci. Nutr. 2021, 61, 1725–1737. [Google Scholar] [CrossRef]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; van Egmond, H.P.; Omar, M.F.; Mofleh, J. An outbreak of hepatic veno-occlusive disease in western Afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J. Toxicol. 2010, 2010, 313280. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef]
- Dübecke, A.; Beckh, G.; Lüllmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. Part A 2011, 28, 348–358. [Google Scholar] [CrossRef]
- Martinello, M.; Manzinello, C.; Gallina, A.; Mutinelli, F. In-house validation and application of UHPLC-MS/MS method for the quantification of pyrrolizidine and tropane alkaloids in commercial honey bee-collected pollen, teas and herbal infusions purchased on Italian market in 2019–2020 referring to recent European Union regulations. Int. J. Food Sci. Technol. 2022, 57, 7505–7516. [Google Scholar] [CrossRef]
- Skoneczny, D.; Zhu, X.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Production of pyrrolizidine alkaloids and shikonins in Echium plantagineum L. in response to various plant stressors. Pest Manag. Sci. 2019, 75, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Gurusinghe, S.; Weston, L.A. The potential role of allelopathy in the persistence of invasive weeds. In Persistence Strategies of Weeds; Wiley: Hoboken, NJ, USA, 2022; pp. 271–301. [Google Scholar] [CrossRef]
- Tandon, V.K.; Kumar, S. Recent development on naphthoquinone derivatives and their therapeutic applications as anticancer agents. Expert Opin. Ther. Pat. 2013, 23, 1087–1108. [Google Scholar] [CrossRef] [PubMed]
- El-Najjar, N.; Gali-Muhtasib, H.; Vuorela, P.; Urtti, A.; Vuorela, H. Naphthoquinones and anthraquinones: Chemical, analytical, and biological overview. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley: Hoboken, NJ, USA, 2006; pp. 1–16. [Google Scholar] [CrossRef]
- El-Najjar, N.; Gali-Muhtasib, H.; Ketola, R.A.; Vuorela, P.; Urtti, A.; Vuorela, H. The chemical and biological activities of quinones: Overview and implications in analytical detection. Phytochem. Rev. 2011, 10, 353–370. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Assimopoulou, A.N.; Papageorgiou, V.P.; Gavalas, A.; Kourounakis, P.N. Alkannin and Shikonin: Effect on free radical processes and on inflammation—A preliminary pharmacochemical investigation. Arch. Pharm. Int. J. Pharm. Med. Chem. 2002, 335, 262–266. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of shikonin and derivatives: Isolation, chemistry, biosynthesis, pharmacology and toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef]
- Akduman, D.; Akduman, R.; Geyik, M.Ş.; Akyüz, M. Investigated the effects of Echium italicum root extracts on human dermal fibroblast cells, demonstrating potential wound healing activity through increased cell viability and migration. Eurasian Mol. Biochem. Sci. 2024, 3, 14–21. [Google Scholar] [CrossRef]
- Karakaş, F.P.; Yildirim, A.; Türker, A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turk. J. Biol. 2012, 36, 641–652. [Google Scholar] [CrossRef]
- Ateşşahin, D.A.; Dalkılıç, L.K.; Özeren, Y.; Dalkılıç, S.; Çakmak, K.; Çiçek, T.A. Investigation of cytotoxic, antimicrobial and antioxidant activity of Echium vulgare L. seed. Int. J. Nat. Life Sci. 2023, 7, 129–135. [Google Scholar] [CrossRef]
- Atessahin, D.A.; Dalkilic, S.; Dalkilic, L.K.; Bayindir, D.; Cetinkaya, E. Evaluation of cytotoxic, antimicrobial, and antioxidant activi-ties of Echium italicum L. in MCF-7 and HepG2 cell lines. Int. J. Plant Based Pharm. 2025, 5, 25–32. [Google Scholar] [CrossRef]
- Fazilati, M.; Dousti, B. Evaluation of the Antifungal effects of various Extracts of the Aerial part and Root of Echium italicum on Candida albicans compared with two common antibiotics. Yafteh 2019, 21, 122–134. Available online: http://eprints.lums.ac.ir/id/eprint/1736 (accessed on 16 August 2025).
- Karadağ, A.E.; Çaşkurlu, A.; Tosun, F. In vitro anti-helicobacter pylori and antimycobacterial activity evaluation of selected plants from Turkey. J. Anatol. Environ. Anim. Sci. 2020, 5, 231–235. [Google Scholar] [CrossRef]
- Figat, R.; Zgadzaj, A.; Geschke, S.; Sieczka, P.; Pietrosiuk, A.; Sommer, S.; Skrzypczak, A. Cytotoxicity and antigenotoxicity evaluation of acetylshikonin and shikonin. Drug Chem. Toxicol. 2021, 44, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Shahandeh, S.; Shahsavand, S. Anxiolytic and hypnotic effects of aqueous and ethanolic extracts of aerial parts of Echium italicum L. in mice. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Medicinal plants possess sedative and anxiolytic effect with emphasis on their mechanisms of action. GSC Biol. Pharm. Sci. 2021, 17, 61–77. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Ballis, A.C. Alkannins and shikonins: A new class of wound healing agents. Curr. Med. Chem. 2008, 15, 3248–3267. [Google Scholar] [CrossRef]
- Malik, S.; Bhushan, S.; Sharma, M.; Ahuja, P.S. Biotechnological approaches to the production of shikonins: A critical review with recent updates. Crit. Rev. Biotechnol. 2016, 36, 327–340. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentao, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Moita, E.; Sousa, C.; Andrade, P.B.; Fernandes, F.; Pinho, B.R.; Silva, L.R.; Valentão, P. Effects of Echium plantagineum L. bee pollen on basophil degranulation: Relationship with metabolic profile. Molecules 2014, 19, 10635–10649. [Google Scholar] [CrossRef]
- Sousa, C.; Moita, E.; Valentao, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of colored and noncolored phenolics of Echium plantagineum L. bee pollen in Caco-2 cells under oxidative stress induced by tert-butyl hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef]
- Bautista-Sopelana, L.M.; Bolívar, P.; Gómez-Muñoz, M.T.; Martínez-Díaz, R.A.; Andrés, M.F.; Alonso, J.C.; Bravo, C.; González-Coloma, A. Bioactivity of plants eaten by wild birds against laboratory models of parasites and pathogens. Front. Ecol. Evol. 2022, 10, 1027201. [Google Scholar] [CrossRef]
- Moreira, R.; Fernandes, F.; Valentão, P.; Pereira, D.M.; Andrade, P.B. Echium plantagineum L. honey: Search of pyrrolizidine alkaloids and polyphenols, anti-inflammatory potential and cytotoxicity. Food Chem. 2020, 328, 127169. [Google Scholar] [CrossRef]
- Nagai, T.; Tanoue, Y.; Kai, N.; Suzuki, N. Functional property of honey from Echium vulgare. Food Nutr. Sci. 2012, 3, 614–620. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef]
- Conforti, P.A.; Patrignani, M. Antioxidant activity from non-conventional beverage plant sources in Argentina. Beverage Plant Res. 2025, 5, e006. [Google Scholar] [CrossRef]
- Gecer, E.N.; Erenler, R. Biogenic synthesis of silver nanoparticles using Echium vulgare: Characterisation, quantitative analysis of bioactive compounds, antioxidant activity and catalytic degradation. J. Indian Chem. Soc. 2023, 100, 101003. [Google Scholar] [CrossRef]
- Tahmouzi, S. Extraction, antioxidant and antilisterial activities of polysaccharides from the flower of viper’s bugloss. Int. J. Biol. Macromol. 2014, 69, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Uysal, İ.; Mohammed, F.S.; Şabik, A.E.; Kına, E.; Sevindik, M. Antioxidant and Oxidant status of medicinal plant Echium italicum collected from different regions. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 1902–1904. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological and therapeutic importance of Echium italicum—A review. Indo Am. J. Pharm. Sci. 2017, 4, 394–398. [Google Scholar] [CrossRef]
- Sousa, C.; Andrade, P.B.; Valentão, P. Relationships of Echium plantagineum L. bee pollen, dietary flavonoids and their colonic metabolites with cytochrome P450 enzymes and oxidative stress. RSC Adv. 2016, 6, 6084–6092. [Google Scholar] [CrossRef]
- Dias Moita, M.E.D.R.P. Effects of Echium plantagineum L. Bee Pollen on Macrophages and Basophils: Metabolic Profile vs Inflammatory Mediators Degranulation and Oxidative Stress. Doctoral Dissertation, Universidade do Porto, Porto, Portugal, 2015. Available online: https://www.proquest.com/openview/abe1ad20251c104ddae9ffe2d37a06bf/1?pq-origsite=gscholar&cbl=2026366&diss=y (accessed on 17 August 2025).
- Moalem, S.A.; Hosseinzadeh, H.; Ghoncheh, F. Evaluation of antidepressant effects of aerial parts of Echium vulgare on mice. Iran. J. Basic Med. Sci. 2007, 10, 189–196. Available online: https://www.sid.ir/paper/551783/en (accessed on 27 August 2025).
- Thuwaini, M.M. Antibacterial spectrum of medicinal plants: A review. GSC Biol. Pharm. Sci. 2023, 24, 239–284. [Google Scholar] [CrossRef]
- Gautam, S.; Lapčík, L.; Lapčíková, B. Pharmacological significance of boraginaceae with special insights into shikonin and its potential in the food industry. Foods 2024, 13, 1350. [Google Scholar] [CrossRef]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, O.W. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implications for commercial processing of bee pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Peloso, M.; Minkoumba Sonfack, G.; Paduano, S.; De Martino, M.; De Santis, B.; Caprai, E. Pyrrolizidine alkaloids in food on the Italian market. Molecules 2023, 28, 5346. [Google Scholar] [CrossRef]
- Field, R.A.; Stegelmeier, B.L.; Colegate, S.M.; Brown, A.W.; Green, B.T. An in vitro comparison of the cytotoxic potential of selected dehydropyrrolizidine alkaloids and some N-oxides. Toxicon 2015, 97, 36–45. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, B.L. Pyrrolizidine Alkaloid–Containing Toxic Plants (Senecio, Crotalaria, Cynoglossum, Amsinckia, Heliotropium, and Echium spp.). Vet. Clin. Food Anim. Pract. 2011, 27, 419–428. Available online: https://www.vetfood.theclinics.com/article/S0749-0720(11)00015-6/abstract (accessed on 22 August 2025). [CrossRef]
- Beales, K.A.; Betteridge, K.; Colegate, S.M.; Edgar, J.A. Solid-phase extraction and LC− MS analysis of pyrrolizidine alkaloids in honeys. J. Agric. Food Chem. 2004, 52, 6664–6672. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.P.; Mulder, P.P.; Zeilmaker, M.J.; Van den Top, H.J.; Remmelink, G.J.; Brandon, E.F.; Klijnstra, M.; Meijer, G.A.L.; Schothorst, R.; Van Egmond, H.P. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit. Contam. Part A 2011, 28, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.A.; Smith, L.W. Transfer of pyrrolizidine alkaloids into eggs: Food safety implications. In Natural and Selected Synthetic Toxins; ACS Publication: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Edgar, J.A.; Roeder, E.; Molyneux, R.J. Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. J. Agric. Food Chem. 2002, 50, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal-and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and analyzing pyrrolizidine alkaloids in medicinal plants: A review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef]
- Colegate, S.M.; Edgar, J.A.; Knill, A.M.; Lee, S.T. Solid-phase extraction and HPLC-MS profiling of pyrrolizidine alkaloids and their N-oxides: A case study of Echium plantagineum. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 108–119. [Google Scholar] [CrossRef]
- GGleńsk, M.; Dudek, M.K.; Kinkade, P.; Santos, E.C.S.; Glinski, V.B.; Ferreira, D.; Seweryn, E.; Kaźmierski, S.; Calixto, J.B.; Glinski, J.A. Isolation of echimidine and its C-7 isomers from Echium plantagineum L. and their hepatotoxic effect on rat hepatocytes. Molecules 2022, 27, 2869. [Google Scholar] [CrossRef]
- Chojkier, M. Hepatic sinusoidal-obstruction syndrome: Toxicity of pyrrolizidine alkaloids. J. Hepatol. 2003, 39, 437–446. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yan, J.; Fu, P.P.; Chou, M.W. Human liver microsomal reduction of pyrrolizidine alkaloid N-oxides to form the corresponding carcinogenic parent alkaloid. Toxicol. Lett. 2005, 155, 411–420. [Google Scholar] [CrossRef]
- Cao, Y.; Colegate, S.M.; Edgar, J.A. Persistence of echimidine, a hepatotoxic pyrrolizidine alkaloid, from honey into mead. J. Food Compos. Anal. 2013, 29, 106–109. [Google Scholar] [CrossRef]
- Egebjerg, M.M.; Olesen, P.T.; Eriksen, F.D.; Ravn-Haren, G.; Bredsdorff, L.; Pilegaard, K. Are wild and cultivated flowers served in restaurants or sold by local producers in Denmark safe for the consumer? Food Chem. Toxicol. 2018, 120, 129–142. [Google Scholar] [CrossRef]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. Occurrence of pyrrolizidines and other alkaloids of plant origin in foods. In Encyclopedia of Food Safety, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72. [Google Scholar] [CrossRef]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. Int. J. 2010, 30, 183–196. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine alkaloids: Chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Griffin, C.T.; Danaher, M.; Elliott, C.T.; Kennedy, D.G.; Furey, A. Detection of pyrrolizidine alkaloids in commercial honey using liquid chromatography–ion trap mass spectrometry. Food Chem. 2013, 136, 1577–1583. [Google Scholar] [CrossRef]







| Characteristic | E. italicum | E. russicum | E. plantagineum | E. vulgare |
|---|---|---|---|---|
| life form | biennial | biennial | annual–biennial | biennial–short-lived perennial |
| stem height (cm) | 40–150 | 25–80 | 20–60 | 20–100 |
| stem indumentum | appressed simple hairs + bristly hairs on tubercles | stiff hairs on small tubercles | bristly hairs with bulbous bases | stout spreading bristles + fine appressed hairs |
| basal leaves | lanceolate, 20–35 × 1.5–4.0 cm | linear-lanceolate, 2–11 × 0.7–1.2 cm | ovate, 3–12 × 1.2–1.5 cm | oblanceolate, 2–25 × 0.5–3 cm |
| cauline leaves | narrowly elliptic | narrow, linear-lanceolate | narrow-lanceolate, cordate base | linear-lanceolate, sessile |
| inflorescence | branched, pyramidal | narrow, spike-like | broad, paniculate or racemose | panicle of helicoid cymes |
| corolla size and color | 10–12 mm, whitish to blue-violet | 8–12 mm, dark red | 15–20 mm, blue to deep blue (rarely white) | 10–20 mm, pink in bud, bright blue at anthesis |
| stamens | 4–5 exserted | exserted, longer than corolla | 2 shortly exserted, 3 included | exserted |
| nutlet size (mm) | 2.8–3.8 × 2.3–2.5 | 1.8–3.2 × 1.5–1.8 | 2.0–3.0 × 2.0–2.3 | 1.8–3.2 × 1.5–1.8 |
| Species | Traditional Uses | Plant Parts Used | Mode of Application | Geographic Region | References |
|---|---|---|---|---|---|
| Echium italicum | anti-inflammatory, antiseptic, analgesic, depurative, diuretic, emollient for respiratory infections, sudorific, treatment of burns, wounds, abscesses | leaves, roots | decoctions, infusions, ointments with oil, poultices | Anatolia, Southern Europe (Italy, Croatia, Montenegro), Turkey | [35,36,37,38,39,40,41,42] |
| Echium russicum | anemia, fatigue, gynecology, hemorrhoids, osteomyelitis, snake bites, wound-healing | leaves, flowers | tea, macerations, poultices | Eastern Europe, Georgia, Turkey | [36,43,44] |
| Echium plantagineum | chest pain, cough, fever, inflammation, insect bites, muscle strain, skin conditions, urinary tract infections | leaves, flowers, seeds | pastes, tea, syrup, topical oil | Africa, America, Asia, Australia (introduced use), Eastern Europe, Europe, and Oceania, Iberian Peninsula, Mediterranean, North Africa | [13,33,45] |
| Echium vulgare | anti-inflammatory, balsamic agents, blood purifier, cough suppressant, depuratives, diuretic, epilepsy, expectorant, fever, gynecological disorders, lactogenic remedies, laxative effects, muscle strain, snake bites, ulcers, urinary tract infections, wound-healing | bark, flowering tops, flowers, leaves, root, seeds, stem | compresses, decoction, infusion (tea), ointments, poultices, syrups | Africa, America, Asia, Balkans, Bulgaria, Central and Eastern Europe, Europe and Oceania, Mediterranean, Morocco, Serbia, Turkey | [13,24,36,41,45,46,47,48,49,50,51] |
| Class of Compound | Reported Compounds | Analytical/Isolation Methods | Pharmacological Properties | References |
|---|---|---|---|---|
| Echium italicum | ||||
| Phenolic compounds | caffeic acid, chlorogenic acid, ferulic acid, p-coumaric acid, hydrocaffeic acid, p-hydroxybenzoic acid, rosmarinic acid, sinapic acid, tannins | Folin–Ciocalteu colorimetric assay; UV–Vis spectrophotometry (λ = 765 nm); HPLC-DAD (C18 column, multiwavelength detection); PVP-Folin–Ciocalteu method | antibacterial, antimicrobial, antioxidant, radiation protection | [37,41,54,55,56,57] |
| Flavonoids | anthocyanins, apigenin, apigenin glycoside, kaempferol, luteolin glycoside, myricitrin, naringenin, quercetin, rutin | Aluminum chloride (AlCl3) spectrophotometric method (λ = 415 nm); HPLC-DAD; HPCE-UV–Vis DAD (190–600 nm) | anxiolytic, sedative | [37,41,54,55,56,57,58] |
| Fatty acids | arachidonic acid, caproic acid, erucic acid, heptadecanoic, lauric acid, linoleic acid, myristic acid, oleic acid, stearidonic acid, α-linolenic acid, γ-linolenic acid, palmitic acid, palmitoleic acid, pentadecanoic, stearic acid | Gas Chromatography (GC) with Flame Ionization Detector (FID); GC-MS; FAME (Fatty Acid Methyl Ester) derivatization | anti-inflammatory | [54,59,60,61,62] |
| Pyrrolizidine alkaloids | echimin, echinin, leptanthine, lycopsamine, uplandicine | LC–MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) | potential toxicity | [63] |
| Quinones | 2-methyl-n-butyryl shikonin, 3,3-dimethyl acrylyshikonin, acetyl shikonin, alkanin, angeloylshikonin, deoxyshikonin, isobutyryl shikonin, isovalerylshikonin, propionyl shikonin, shikonin | HPCE (High-Performance Capillary Electrophoresis) with UV–Vis DAD; HPLC-VIS/MS (APCI-mode); TLC and preparative HPLC; 1H/13C NMR | antibacterial, anti-allergic, antimicrobial, antioxidant, antitumor, antithrombotic, wound-healing | [58,64,65,66,67,68] |
| Terpenes | limonene, pulegone | GC, GC-MS | antimicrobial, aromatherapeutic potential | [69] |
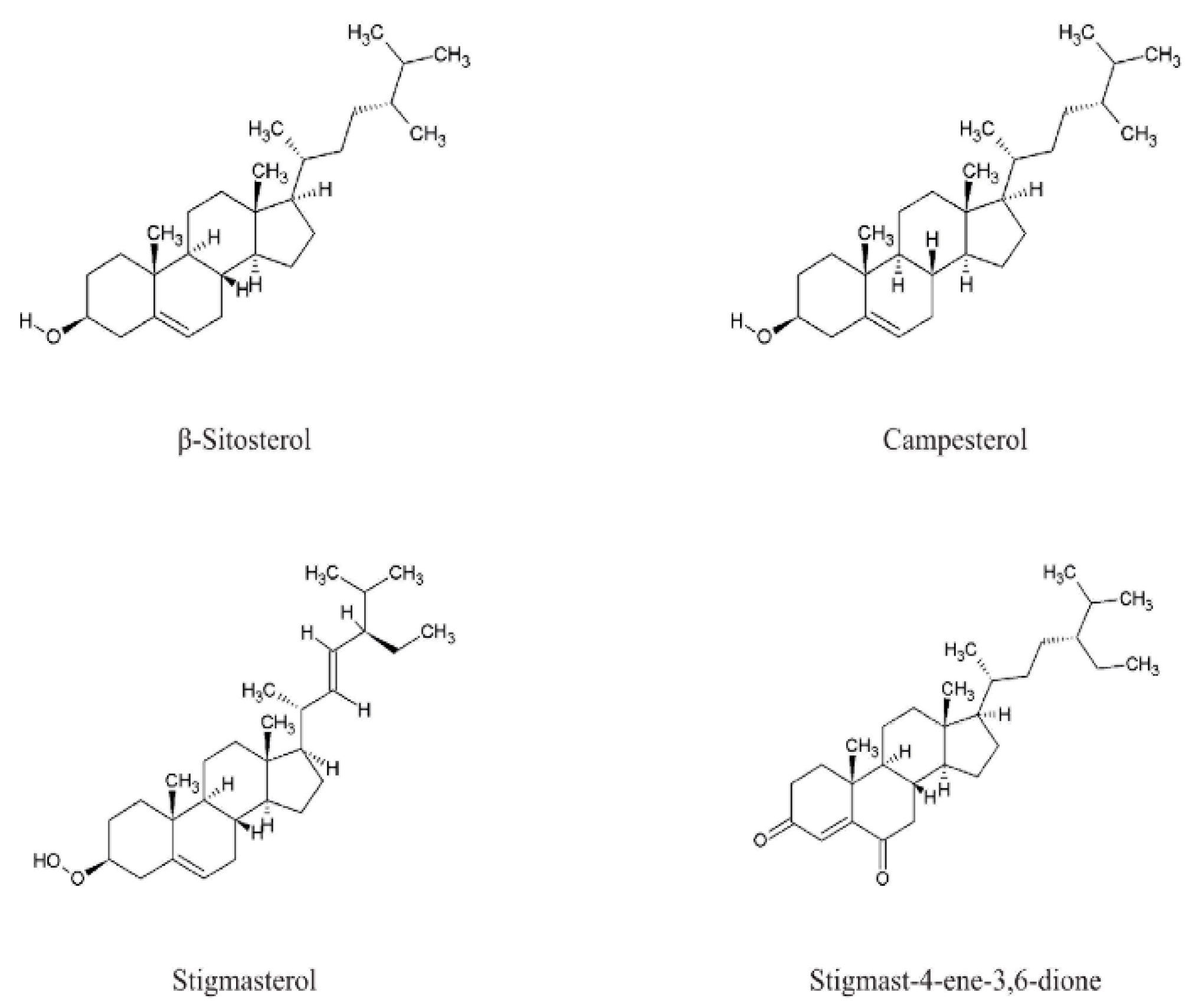
| Phytosterols | 24-methylenecholesterol, β-sitosterol, δ5,23-stigmastadienone, δ5-avenasterol, campesterol, clerosterol, stigmast-4-ene-3,6-dione, sitostanol, stigmasterol | GC-FID (after silylation); HPLC; Preparative TLC + 1H/13C NMR | cardiovascular protection | [6,61,70] |
| Echium russicum | ||||
| Phenolic compounds | caffeoylquinic acid, chlorogenic acid, globoidnan a, rabdossin (disodium salt), rosmarinic acid, tannins | Folin–Ciocalteu colorimetric assay; PVP-Folin–Ciocalteu method; Capillary Zone Electrophoresis (CZE), UV detection | antioxidant, hepatoprotective, radiation protection | [54,58,71,72] |
| Flavonoids | anthocyanins, apigenin-7-o-glucoside, naringin, rutin, anthocyanins | HPCE (High-Performance Capillary Electrophoresis) with UV–Vis DAD (190–600 nm) | anti-inflammatory, antiviral | [54,58] |
| Fatty acids | arachidonic acid, linoleic acid, oleic acid, stearidonic acid, α-linolenic acid, γ-linolenic acid, palmitic acid, stearic acid | Gas Chromatography-Mass Spectrometry (GC-MS); Gas-Liquid Chromatography (GLC) | anti-inflammatory | [54,73] |
| Pyrrolizidine alkaloids | No specific data were found for the species. | - | - | - |
| Quinones | shikonin | Capillary Zone Electrophoresis (CZE), UV detection | anti-inflammatory, antimicrobial, antitumor, wound-healing | [44,58,71,72] |
| Terpenes | No specific data were found for the species. | - | - | - |
| Phytosterols | No specific data were found for the species. | - | - | - |
| Echium plantagineum | ||||
| Phenolic compounds | caffeic acid, globoidnan a, ferulic acid, rosmarinic acid, sinapic acid, γ-tocopherol, rabdosiin | 1D/2D NMR spectroscopy; HRMS; GC-FID; HPLC-DAD; UV–Vis spectrophotometry (Folin–Ciocalteu assay) | antioxidant, anticancer | [74,75] |
| Flavonoids | petunidin-3-o-rutinoside; delphinidin; cyanidin; peonidin; malvidin-3-o-rutinoside and cyanidin-3-(6″-malonylglucoside), kaempferol, quercetin, rutin | HPLC-DAD; HPLC-MS (ESI mode); LC–MS/MS | anti-allergic, photoprotective | [67] |
| Fatty acids | α-linolenic acid, γ-linolenic acid, caproic acid, caprylic acid, capric acid, stearidonic acid, undecanoic acid | GC-FID; GC-MS; GLC-FID; GC-MS (FAME derivatization); NMR; Silver-ion HPLC; TLC-FID | anti-inflammatory | [15,16,17,53,75,76,77,78,79,80,81,82,83,84,85] |
| Pyrrolizidine alkaloids | 3′-o-acetylechiumine-n-oxide, 3′-o-acetylintermedine [sol] lycopsamine, 7,9-ditigloylretronecine n-oxide, 9-o-angelyl retronecine-n-oxide, acetyl lycopsamine, echimin, echimidine, echimidine n-oxide, echimiplatine-n-oxide, echinin, echi-uplatine-n-oxide, echiumine, echiumine n-oxide, intermedine, leptanthine-n-oxide, lycopsamine, lycopsamine n-oxide, retrorsine | UHPLC-QTOF-MS; LC-ESI/MS; GC-MS; SPE (solid-phase extraction, cation-exchange); LC–MS/MS (QTRAP) | potentially hepatotoxic, hepatotoxic photosensitization | [86,87,88,89] |
| Quinones | 1,8-dihydroxy-3-methylanthraquinone, acetylshikonin, angelicshikonin, dimethylacrylshikonin, isovalerylshikonin, shikonin | UHPLC–QTOF-MS; LC-ESI/MS; GC-MS; Spectrophotometry (Nanodrop 2000c, λ = 493–562 nm); Ethanolic extraction | antibacterial, wound-healing | [15,89,90,91,92,93,94,95] |
| Terpenes | β-carotene | HPLC-DAD; HRMS; Spectrophotometric β-carotene quantification; Oil extraction (hydraulic, solvent, cold-press) | antioxidant | [75] |
| Phytosterols | β-sitosterol, campesterol | HPLC; HRMS; Spectrophotometric quantification of sterols/tocopherols; Oil extraction and oxidative stability testing | cardiovascular protection | [75] |
| Echium vulgare | ||||
| Phenolic compounds | 3-(3′,4′-dihydroxyphenyl)-(2R)-lactic acid, caffeic acid, catechol, chlorogenic acid, cis-cinnamic acid, ellagic acid, ferulic acid, gallic acid, hydrocaffeic acid, isoferulic acid, p-coumaric acid, protocatechuic acid, rosmarinic acid, salicylic acid, tannins, vanillic acid | Ethanolic maceration (70–96% MeOH/EtOH); Folin–Ciocalteu spectrophotometric assay; UV–Vis (DPPH, Fe2+-Ferrozine, β-carotene test); Capillary Zone Electrophoresis (CZE); Column chromatography (SiO2, Sephadex LH-20); 1H/13C NMR, COSY, HMQC, HMBC; ESI-MS | anti-inflammatory, antioxidant, antimicrobial | [41,71,96,97,98,99,100,101,102] |
| Flavonoids | apigenin, hesperetin, hesperidin, kaempferol, kaempferol 3-o-neohesperidoside, naringen, naringin, quercetin, quercetrin, rutin | MeOH maceration; UV–Vis spectrophotometry (AlCl3 colorimetric assay at 415 nm); HPLC-DAD; HPCE/CZE (UV–VIS DAD); 1H/13C NMR, COSY, HMQC, HMBC; ESI-MS | anticonvulsant, neuroprotective | [41,58,98,99,100,101,102,103] |
| Fatty acids | α-linolenic acid, γ-linolenic acid, stearidonic acid | GC-FID; GC-MS; GLC-MS; HPLC; Silver-ion TLC (Ag+-TLC); Supercritical CO2 (SC-CO2) oil extraction; NMR | anti-inflammatory | [16,17,73,104,105,106,107,108] |
| Pyrrolizidine alkaloids | 7-o-acetylvulgarin, echiimin, echimidine, echinin, echinatine, echivulgarine, intermedin, leptanthine, lycopsamine, uplandicine, vulgarin | MeOH extraction; Aqueous acid extraction; Strong cation-exchange solid-phase extraction (SCX-SPE); LC–MS/MS; LC-HR-MS (Orbitrap, QTOF); UHPLC-MS/MS (TSQ Quantiva); 1H/13C NMR | potentially toxic | [63,87,100,109,110,111,112,113] |
| Quinones | acetylshikonin, dimethylacrylshikonin, isovalerylshikonin, shikonin | Ethanolic extraction; Capillary Zone Electrophoresis (CZE, UV–VIS DAD); UHPLC-QTOF-MS; Spectrophotometry (Nanodrop 2000c, λ = 493–562 nm) | anti-inflammatory, antimicrobial, antitumor, wound-healing | [32,58,71,90,98] |
| Terpenes | α-bisabolol, camphor, caryophyllene oxide isomers, cis-geranyl acetate, endo-borneol, lavandulyl acetate, linalool, trans-geraniol, trans-geraniol acetate, α-terpineol | GC; GC-MS; HPCE with chemometric analysis; CZE | anti-inflammatory, antimicrobial, antitumor, aromatherapeutic potential, emollient, soothing | [114] |
| Phytosterols | β-sitosterol, campesterol, sterone stigmast-4-ene-3,6-dione, stigmasterol, sitostanol | Column chromatography (silica gel; CHCl3, Et2O, EtOAc, Me2CO); Preparative TLC; GC-MS; HPLC; 1H/13C NMR, INEPT | cardiovascular protection | [6,70,108,115] |
| Species | Type of Extract/Active | Experimental Model | Observed Effects | References |
|---|---|---|---|---|
| E. italicum | Acetone extract | In vitro antibacterial | Strong activity vs. S. enteritidis, P. vulgaris | [55] |
| E. italicum | Methanolic/hexane seed extracts; crude extracts | Cancer cell lines (MCF-7, HepG2, RD, Hep2c) | Cytotoxic/antitumor effects | [37,140,141,142] |
| E. italicum | Essential oil | Disk diffusion; MIC assays | Concentration-dependent activity vs. B. subtilis, S. aureus, E. coli, S. typhi, P. aeruginosa, A. niger, C. albicans | [69,143] |
| E. italicum | Various extracts | In vitro antibacterial | Weak inhibition vs. H. pylori, M. smegmatis, M. avium | [144] |
| E. italicum | Shikonin/derivatives (incl. acetylshikonin) | In vitro (multiple cancer models); pharmacology reviews | Anticancer/antibacterial/wound-healing; acetylshikonin with antitumor potential | [57,65,138,145] |
| E. italicum | Aqueous and ethanolic (aerial parts) | Mice: elevated plus-maze; pentobarbital sleep | Anxiolytic and sedative without motor impairment | [146,147] |
| E. italicum (roots) | Allantoin; shikonin pigments | Dermatology/cosmetics context; phytochemical reports | Soothing/regenerative (allantoin); antioxidant/antimicrobial pigments | [58,148,149] |
| E. plantagineum | Bee-pollen extracts | RAW 264.7 macrophages; basophils; Caco-2 cells | Reduction of NO, iNOS, and COX-2 mediators; effects on degranulation; antioxidant protection | [150,151,152] |
| E. plantagineum | Leaves/flowers extracts | In vitro antiparasitic/antifungal (nematodes, Trichomonas gallinae, A. niger) | Antinematodal/antitrichomonad/antifungal activity | [153] |
| E. plantagineum, E. vulgare (seed oil) | Seed oils rich in GLA/SDA | Cosmetic/dermatological applications (reported); formulation/processing studies | Anti-inflammatory/skin-barrier support (reported); high ω-3 content relevant for cosmetics/nutraceuticals | [80,83,127] |
| E. vulgare | Methanolic extract (aerial parts) | In vitro antioxidant assays | Strong OH scavenging; iron-chelating capacity | [101] |
| E. vulgare | Ethanolic extracts (various parts) | In vitro antioxidant assays; phenolics/flavonoids profiling | Good antioxidant activity consistent with high phenolic/flavonoid content | [33] |
| E. vulgare | Crude extracts (unspecified) | Animal model (hyperlipidemia) | Improved blood parameters, lipid profile, liver function; histopathology improvement | [98] |
| E. vulgare | Extracts (unspecified) | In vitro/ex vivo inflammatory readouts | Inhibition of IL-1β, TNF-α, COX-2 | [154] |
| E. vulgare | Aqueous/ethanol/methanol extracts | Disk diffusion vs. 10 bacteria (Gram±) | Broad antibacterial inhibition | [99,140] |
| E. vulgare (seeds) | Seed extracts | Antimicrobial screens (incl. E. coli) | Antimicrobial activity; notable activity against E. coli | [55,100,141] |
| Echium spp. honey | Phenolic-rich honey | In vitro antioxidant assays; dermal uses (review) | Antioxidant, radical scavenging; traditional wound/skin care uses | [155,156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzieva, S.; Grozeva, N.; Tzanova, M. A Review of the Main Biologically Active Compounds of the Genus Echium L., Naturally Distributed in Bulgaria, and Their Pharmacological Potential. Pharmaceuticals 2025, 18, 1618. https://doi.org/10.3390/ph18111618
Terzieva S, Grozeva N, Tzanova M. A Review of the Main Biologically Active Compounds of the Genus Echium L., Naturally Distributed in Bulgaria, and Their Pharmacological Potential. Pharmaceuticals. 2025; 18(11):1618. https://doi.org/10.3390/ph18111618
Chicago/Turabian StyleTerzieva, Svetoslava, Neli Grozeva, and Milena Tzanova. 2025. "A Review of the Main Biologically Active Compounds of the Genus Echium L., Naturally Distributed in Bulgaria, and Their Pharmacological Potential" Pharmaceuticals 18, no. 11: 1618. https://doi.org/10.3390/ph18111618
APA StyleTerzieva, S., Grozeva, N., & Tzanova, M. (2025). A Review of the Main Biologically Active Compounds of the Genus Echium L., Naturally Distributed in Bulgaria, and Their Pharmacological Potential. Pharmaceuticals, 18(11), 1618. https://doi.org/10.3390/ph18111618









