Fluid Therapy in Acute Pancreatitis—Current Knowledge and Future Perspectives
Abstract
1. Introduction
2. Search Strategy
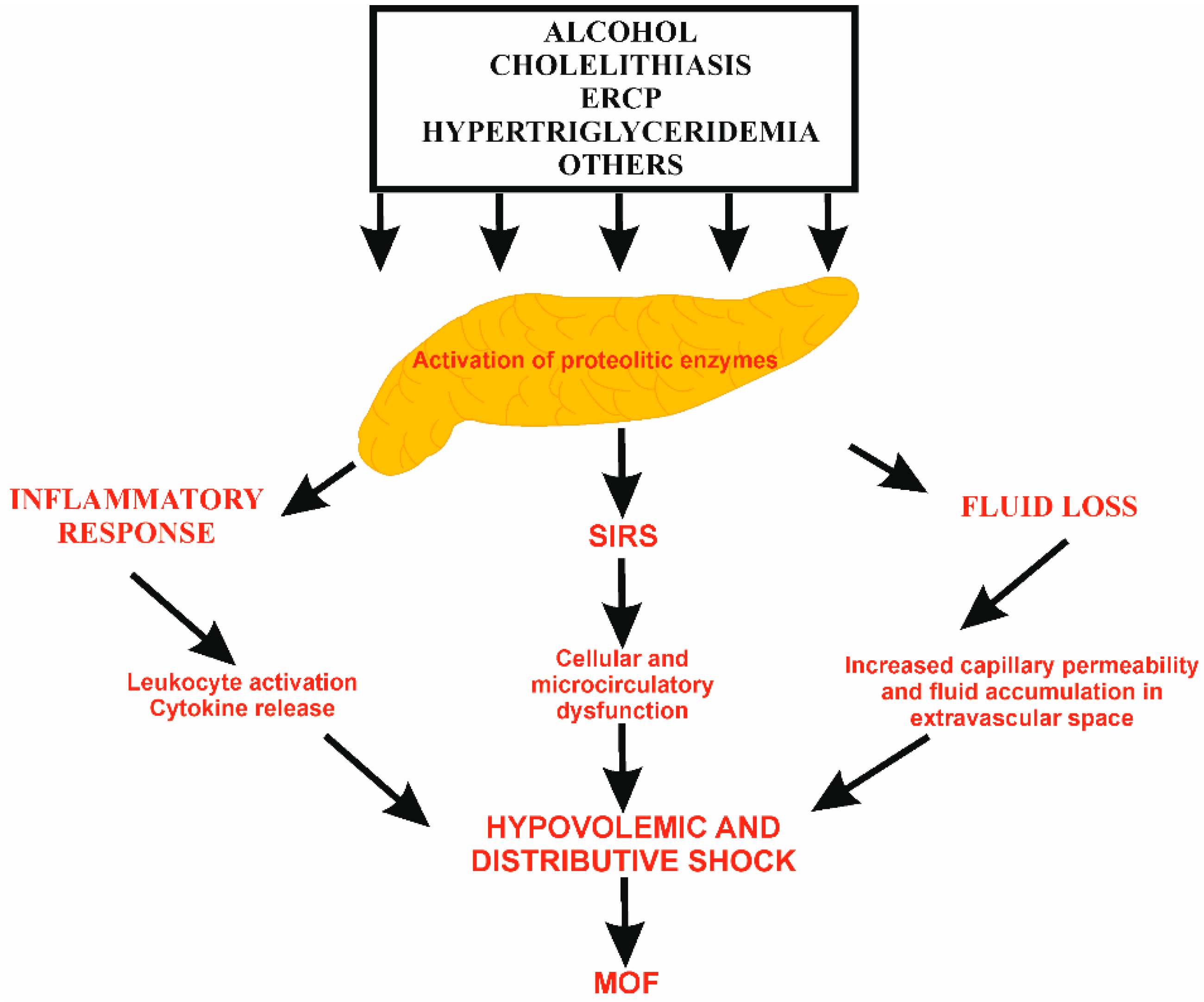
3. The Rationale for Fluid Therapy in AP
4. Severity of AP
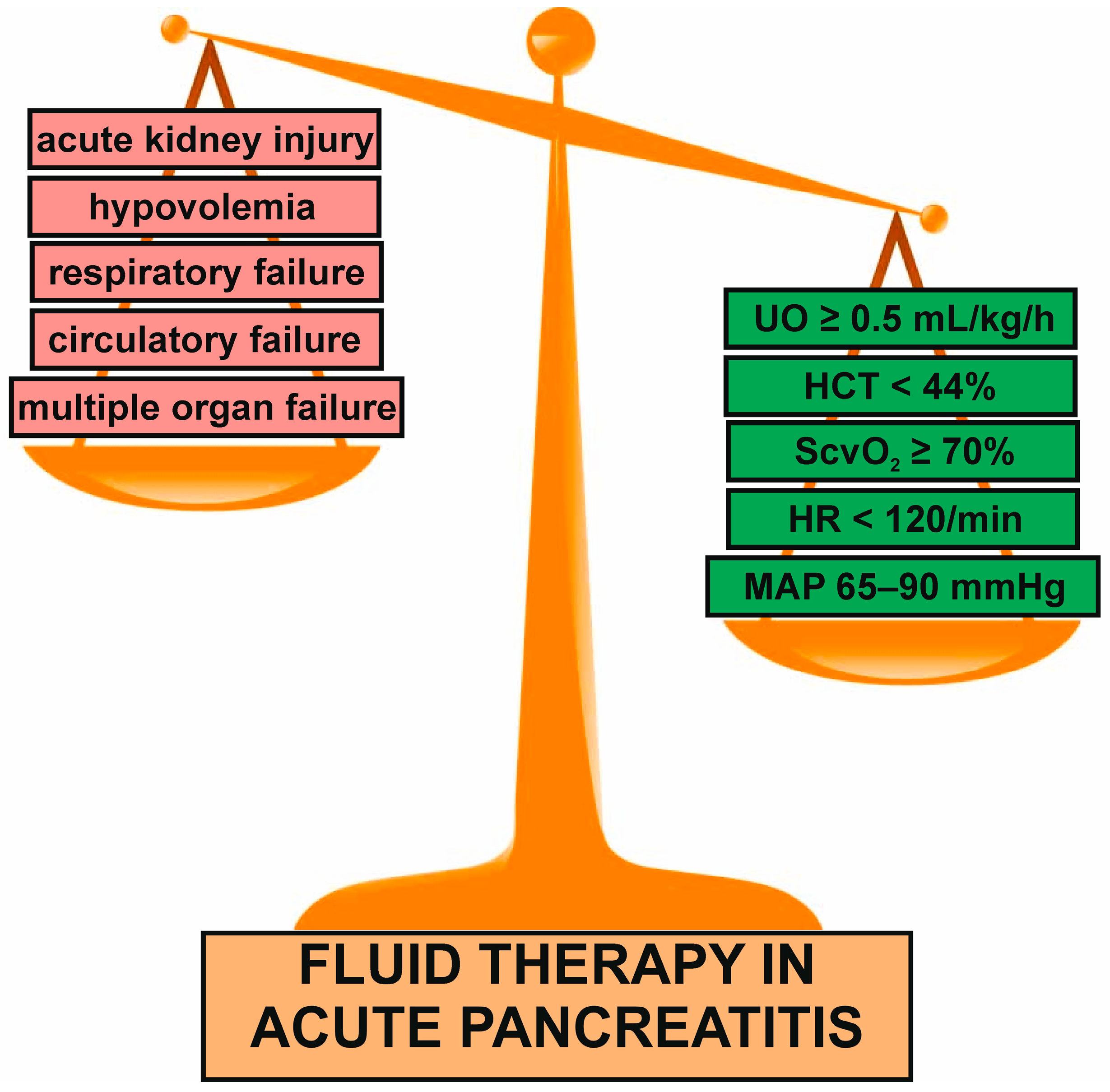
5. General Characteristics of Fluid Management in AP
6. Fluid Rate and Volume
7. Fluid Type
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wos-Wroniewicz, E.; Caban, M.; Malecka-Panas, E. Role of adipokines in the assessment of severity and predicting the clinical course of acute pancreatitis. J. Physiol. Pharmacol. 2020, 71, 605–614. [Google Scholar]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute pancreatitis: A review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Jabłońska, B.; Mrowiec, S. Nutritional support in patients with severe acute pancreatitis-current standards. Nutrients 2021, 13, 1498. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Yadav, D. Epidemiology of recurrent acute and chronic pancreatitis: Similarities and differences. Dig. Dis. Sci. 2017, 62, 1683–1691. [Google Scholar] [CrossRef]
- Guda, N.M.; Muddana, V.; Whitcomb, D.C.; Levy, P.; Garg, P.; Cote, G.; Uc, A.; Varadarajulu, S.; Vege, S.S.; Chari, S.T.; et al. Recurrent acute pancreatitis: International state-of-the-science conference with recommendations. Pancreas 2018, 47, 653–666. [Google Scholar] [CrossRef]
- Fonseca Sepúlveda, E.V.; Guerrero-Lozano, R. Acute pancreatitis and recurrent acute pancreatitis: An exploration of clinical and etiologic factors and outcomes. J. Pediatr. 2019, 95, 713–719. [Google Scholar] [CrossRef]
- Párniczky, A.; Lantos, T.; Tóth, E.M.; Szakács, Z.; Gódi, S.; Hágendorn, R.; Illés, D.; Koncz, B.; Márta, K.; Mikó, A.; et al. Antibiotic therapy in acute pancreatitis: From global overuse to evidence based recommendations. Pancreatology 2019, 19, 488–499. [Google Scholar] [CrossRef]
- Demcsák, A.; Soós, A.; Kincses, L.; Capunge, I.; Minkov, G.; Kovacheva-Slavova, M.; Nakov, R.; Wu, D.; Huang, W.; Xia, Q.; et al. Acid suppression therapy, gastrointestinal bleeding and infection in acute pancreatitis—An international cohort study. Pancreatology 2020, 20, 1323–1331. [Google Scholar] [CrossRef]
- Zerem, E.; Kurtcehajic, A.; Kunosić, S.; Zerem Malkočević, D.; Zerem, O. Current trends in acute pancreatitis: Diagnostic and therapeutic challenges. World J. Gastroenterol. 2023, 29, 2747–2763. [Google Scholar] [CrossRef]
- Windsor, J.A.; Escott, A.; Brown, L.; Phillips, A.R. Novel strategies for the treatment of acute pancreatitis based on the determinants of severity. J. Gastroenterol. Hepatol. 2017, 32, 1796–1803. [Google Scholar] [CrossRef]
- Szatmary, P.; Grammatikopoulos, T.; Cai, W.; Huang, W.; Mukherjee, R.; Halloran, C.; Beyer, G.; Sutton, R. Acute pancreatitis: Diagnosis and treatment. Drugs 2022, 82, 1251–1276. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-H. Recent treatment strategies for acute pancreatitis. J. Clin. Med. 2024, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Papachristou, G.I. Intravenous fuid resuscitation in the management of acute pancreatitis. Curr. Opin. Gastroenterol. 2020, 36, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Burdett, E.; Roche, A.M.; Mythen, M.G. Hyperchloremic acidosis: Pathophysiology and clinical impact. Transfus. Altern. Transfus. Med. 2003, 5, 424–430. [Google Scholar] [CrossRef]
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segovia-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.; et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef]
- Rosołowski, M.; Lipiński, M.; Dobosz, M.; Durlik, M.; Głuszek, S.; Kuśnierz, K.; Lampe, P.; Małecka-Panas, E.; Nowakowska-Duława, E.; Nowak-Niezgoda, M.; et al. Management of acute pancreatitis (AP)—Polish Pancreatic Club recommendations. Prz. Gastroenterol. 2016, 11, 65–72. [Google Scholar] [CrossRef]
- Singh, P.; Garg, P.K. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J. Gastroenterol. 2016, 35, 153–166. [Google Scholar] [CrossRef]
- Voronina, S.G.; Barrow, S.L.; Simpson, A.W.; Gerasimenko, O.V.; Xavier, G.S.; Rutter, G.A.; Petersen, O.H.; Tepikin, A.V. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology 2010, 138, 1976–1987. [Google Scholar] [CrossRef]
- Telek, G.; Ducroc, R.; Scoazec, J.Y.; Pasquier, C.; Feldmann, G.; Roze, C. Differential up-regulation of cellular adhesion molecules at the sites of oxidative stress in experimental acute pancreatitis. J. Surg. Res. 2001, 1, 56–67. [Google Scholar] [CrossRef]
- Mentula, P.; Kylänpää, M.L.; Kemppainen, E.; Jansson, S.E.; Sarna, S.; Puolakkainen, P.; Haapiainen, R.; Repo, H. Plasma anti-inflammatory cytokines and monocyte human leucocyte antigen DR expression in patients with acute pancreatitis. Scand. J. Gastroenterol. 2004, 39, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Pitkaranta, P.; Kivisaari, L.; Nordling, S.; Nuutinen, P.; Schroder, T. Vascular changes of pancreatic ducts and vessels in acute necrotizing, and in chronic pancreatitis in humans. Int. J. Pancreatol. 1991, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, T.; Eibl, G.; Hotz, H.G.; Faulhaber, J.; Kirchengast, M.; Buhr, H.J. Endothelin receptor blockade in severe acute pan creatitis leads to systemic enhancement of microcirculation, stabilization of capillary permeability, and improved survival rates. Surgery 2000, 128, 399–407. [Google Scholar] [CrossRef]
- Cuthbertson, C.M.; Christophi, C. Disturbances of the microcirculation in acute pancreatitis. Br. J. Surg. 2006, 93, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B.; Vege, S.S.; Pearson, R.K.; Chari, S.T. Fluid resuscitation in acute pancreatitis. Clin. Gastroenterol. Hepatol. 2008, 6, 1070–1076. [Google Scholar] [CrossRef]
- Komara, N.L.; Paragomi, P.; Greer, P.J.; Wilson, A.S.; Breze, C.; Papachristou, G.I.; Whitcomb, D.C. Severe acute pancreatitis: Capillary permeability model linking systemic inflammation to multiorgan failure. Am. J. Physiol. Liver Physiol. 2020, 319, G573–G583. [Google Scholar] [CrossRef]
- Juvonen, P.O.; Tenhunen, J.J.; Heino, A.A.; Merasto, M.; Paajanen, H.E.; Alhava, E.M.; Takala, J.A. Splanchnic tissue perfusion in acute experimental pancreatitis. Scand. J. Gastroenterol. 1999, 34, 308–314. [Google Scholar] [CrossRef]
- Tyberg, A.; Karia, K.; Gabr, M.; Desai, A.; Doshi, R.; Gaidhane, M.; Sharaiha, R.Z.; Kahaleh, M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J. Gastroenterol. 2016, 22, 2256–2270. [Google Scholar] [CrossRef]
- De Laet, I.E.; Malbrain, M.L.N.G.; De Waele, J.J. A Clinician’s guide to manage ment of intra-abdominal hypertension and abdominal compartment syndrome in critically Ill patients. Crit. Care 2020, 24, 97. [Google Scholar] [CrossRef]
- Hu, J.X.; Zhao, C.F.; Wang, S.L.; Tu, X.Y.; Huang, W.B.; Chen, J.N.; Xie, Y.; Chen, C.R. Acute pancreatitis: A review of diagnosis, severity prediction and prognosis assessment from imaging technology, scoring system and artificial intelligence. World J. Gastroenterol. 2023, 29, 5268–5291. [Google Scholar] [CrossRef]
- Hamesch, K.; Hollenbach, M.; Guilabert, L.; Lahmer, T.; Koch, A. Practical management of severe acute pancreatitis. Eur. J. Intern. Med. 2025, 133, 1–13. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, T.N.; Chung, H.H.; Kim, K.H. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J. Gastroenterol. 2015, 21, 2387–2394. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Xing, Y.; Du, L.; Chen, J.; Liu, X.; Hao, J. Comparison of BISAP, Ranson, MCTSI, and APACHE II in Predicting Severity and Prognoses of Hyperlipidemic Acute Pancreatitis in Chinese Patients. Gastroenterol. Res. Pract. 2016, 2016, 1834256. [Google Scholar] [CrossRef]
- Harshit Kumar, A.; Singh Griwan, M. A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol. Rep. 2018, 6, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zou, J. Evaluation of four scoring systems in prognostication of acute pancreatitis for elderly patients. BMC Gastroenterol. 2020, 20, 165. [Google Scholar] [CrossRef]
- Teng, T.Z.J.; Tan, J.K.T.; Baey, S.; Gunasekaran, S.K.; Junnarkar, S.P.; Low, J.K.; Huey, C.W.T.; Shelat, V.G. Sequential organ failure assessment score is superior to other prognostic indices in acute pancreatitis. World J. Crit. Care Med. 2021, 10, 355–368. [Google Scholar] [CrossRef]
- Strand, K.; Møller, J.H. Fluid treatment in acute pancreatitis: A careful balancing act. Br. J. Surg. 2023, 110, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Bassett, P. Crystalloids vs. colloids for fluid resuscitation in the Intensive Care Unit: A systematic review and meta-analysis. J. Crit. Care 2019, 50, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Costea, C.-N.; Pojoga, C.; Seicean, A. Advances in the Management of Fluid Resuscitation in Acute Pancreatitis: A Systematic Review. Diagnostics 2025, 15, 810. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Baillie, J. AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007, 132, 2022–2044. [Google Scholar] [CrossRef]
- Aggarwal, A.; Manrai, M.; Kocchar, R. Fluid resuscitation in acute pancreatitis. World J. Gastroenterol. 2014, 20, 18092–18103. [Google Scholar] [CrossRef]
- Wilms, H.; Mittal, A.; Haydock, M.D.; van den Heever, M.; Devaud, M.; Windsor, J.A. A systematic review of goal directed fluid therapy: Rating of evidence for goals and monitoring methods. J. Crit. Care 2014, 29, 204–209. [Google Scholar] [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N. American Gastroenterological Association Institute Clinical Guidelines Committee. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef]
- Wenkui, Y.; Ning, L.; Jianfeng, G.; Weiqin, L.; Shaoqiu, T.; Zhihui, T.; Tao, G.; Juanjuan, Z.; Fengchan, X.; Hui, S.; et al. Restricted peri-operative fluid administration adjusted by serum lactate level improved outcome after major elective surgery for gastrointestinal malignancy. Surgery 2010, 147, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Froghi, F.; Soggiu, F.; Ricciardi, F.; Gurusamy, K.; Martin, D.S.; Singh, J.; Siddique, S.; Eastgate, C.; Ciaponi, M.; McNeil, M.; et al. Ward-based Goal-Directed Fluid Therapy (GDFT) in acute pancreatitis (GAP) trial: Study protocol for a feasibility randomised controlled trial. BMJ Open 2019, 9, e028783. [Google Scholar] [CrossRef] [PubMed]
- Froghi, F.; Soggiu, F.; Ricciardi, F.; Vindrola-Padros, C.; Floros, L.; Martin, D.; Filipe, H.; Varcada, M.; Gurusamy, K.; Bhattacharya, S.; et al. Ward based goal directed fluid therapy (GDFT) in acute pancreatitis (GAP) trial: A feasibility randomised controlled trial. Int. J. Surg. 2022, 104, 106737. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, A.; Spina, S.; Marrazzo, F.; Cimbanassi, S.; Malbrain, M.; Van Regenmortel, N.; Fumagalli, R.; Langer, T. Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: A narrative review. Ann. Intensive Care 2022, 12, 98. [Google Scholar] [CrossRef]
- de-Madaria, E.; Soler-Sala, G.; Sánchez-Payá, J.; Lopez-Font, I.; Martínez, J.; Gómez-Escolar, L.; Sempere, L.; Sánchez-Fortún, C.; Pérez-Mateo, M. Influence of fluid therapy on the prognosis of acute pancreatitis: A prospective cohort study. Am. J. Gastroenterol. 2011, 106, 1843–1850. [Google Scholar] [CrossRef]
- Yaowmaneerat, T.; Sirinawasatien, A. Update on the strategy for intravenous fluid treatment in acute pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2023, 14, 22–32. [Google Scholar] [CrossRef]
- Beagle, A.J.; Prasad, P.A.; Hubbard, C.C.; Walderich, S.; Oreper, S.; Abe-Jones, Y.; Fang, M.C.; Kangelaris, K.N. Associations between volume of early intravenous fluid and hospital outcomes in septic patients with and without heart failure: A retrospective cohort study. Crit. Care Explor. 2024, 6, e1082. [Google Scholar] [CrossRef] [PubMed]
- Nassar, T.I.; Qunibi, W.Y. AKI Associated with acute pancreatitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1106–1115. [Google Scholar] [CrossRef]
- Padula, D.; Mauro, A.; Maggioni, P.; Kurihara, H.; Di Sabatino, A.; Anderloni, A. Practical approach to acute pancreatitis: From diagnosis to the management of complications. Intern. Emerg. Med. 2024, 19, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.L.; Quezada, M.; Da, B.; Jani, N.; Lane, C.; Mwengela, D.; Kelly, T.; Jhun, P.; Dhanireddy, K.; Laine, L. Early aggressive hydration hastens clinical improvement in mild acute pancreatitis. Am. J. Gastroenterol. 2017, 112, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.; Liang, H.; Toklu, H.Z.; Fritze, S.; Luu, S.W. Early aggressive hydration is associated with decreased opioid use and readmission in mild acute pancreatitis. HCA Healthc. J. Med. 2020, 1, 223–229. [Google Scholar] [CrossRef]
- Wu, F.; She, D.; Ao, Q.; Zhang, S.; Li, J. Aggressive intravenous hydration protocol of Lactated Ringer’s solution benefits patients with mild acute pancreatitis: A meta-analysis of 5 randomized controlled trials. Front. Med. 2022, 9, 966824. [Google Scholar] [CrossRef]
- Cuéllar-Monterrubio, J.E.; Monreal-Robles, R.; González-Moreno, E.I.; Borjas-Almaguer, O.D.; Herrera-Elizondo, J.L.; García-Compean, D.; Maldonado-Garza, H.J.; González-González, J.A. Nonaggressive versus aggressive intravenous fluid therapy in acute pancreatitis with more than 24 hours from disease onset: A randomized controlled trial. Pancreas 2020, 49, 579–583. [Google Scholar] [CrossRef]
- Malbrain, M.; Langer, T.; Annane, D.; Gattinoni, L.; Elbers, P.; Hahn, R.G.; De Laet, I.; Minini, A.; Wong, A.; Ince, C.; et al. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann. Intensive Care 2020, 10, 64. [Google Scholar] [CrossRef]
- de-Madaria, E.; Buxbaum, J.L.; Maisonneuve, P.; García García de Paredes, A.; Zapater, P.; Guilabert, L.; Vaillo-Rocamora, A.; Rodríguez-Gandía, M.A.; Donate-Ortega, J.; Lozada-Hernández, E.E.; et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N. Engl. J. Med. 2022, 387, 989–1000. [Google Scholar] [CrossRef]
- Li, X.W.; Wang, C.H.; Dai, J.W.; Tsao, S.H.; Wang, P.H.; Tai, C.C.; Chien, R.N.; Shao, S.C.; Lai, E.C. Comparison of clinical outcomes between aggressive and non-aggressive intravenous hydration for acute pancreatitis: A systematic review and meta-analysis. Crit. Care 2023, 27, 122. [Google Scholar] [CrossRef]
- Gad, M.M.; Simons-Linares, C.R. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? A meta-analysis of randomized control trials and cohort studies. World J. Gastroenterol. 2020, 26, 1098–1106. [Google Scholar] [CrossRef]
- Liao, J.; Zhan, Y.; Wu, H.; Yao, Z.; Peng, X.; Lai, J. Effect of aggressive versus conservative hydration for early phase of acute pancreatitis in adult patients: A meta-analysis of 3,127 cases. Pancreatology 2022, 22, 226–234. [Google Scholar] [CrossRef]
- He, K.; Gao, L.; Yang, Z.; Zhang, Y.; Hua, T.; Hu, W.; Wu, D.; Ke, L. Aggressive versus controlled fluid resuscitation in acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Chin. Med. J. 2023, 136, 1166–1173. [Google Scholar] [CrossRef]
- Guo, J.; Hong, J.; He, Y.; Li, Q.; Huang, T.; Lou, D.; Zhang, J. Comparison of early aggressive versus nonaggressive fluid resuscitation in acute pancreatitis: A meta-analysis. Therap. Adv. Gastroenterol. 2023, 16, 17562848231192144. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, B. Effect of aggressive intravenous fluid resuscitation versus nonaggressive fluid resuscitation in the treatment of acute pancreatitis: A systematic review and meta-analysis. Pancreas 2023, 52, e89–e100. [Google Scholar] [CrossRef]
- Kumari, R.; Sadarat, F.; Luhana, S.; Parkash, O.; Lohana, A.C.; Rahaman, Z.; Wang, H.Y.; Mohammed, Y.N.; Kumar, S.K.; Chander, S. Evaluating the efficacy of different volume resuscitation strategies in acute pancreatitis patients: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 119. [Google Scholar] [CrossRef]
- Angsubhakorn, A.; Tipchaichatta, K.; Chirapongsathorn, S. Comparison of aggressive versus standard intravenous hydration for clinical improvement among patients with mild acute pancreatitis: A randomized controlled trial. Pancreatology 2021, 21, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Chen, Y.; Zhao, B.; Ma, L.; Yao, Y.; Chen, E.; Zhou, W.; Mao, E. The impact of fluid resuscitation via colon on patients with severe acute pancreatitis. Sci. Rep. 2021, 11, 12488. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, H.; Yuan, Y.; Liu, C.; Mo, S.; He, F.; Fu, X. Efficacy and safety of early enteral and intravenous fluid resuscitation in severe acute pancreatitis: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2023, 38, 36. [Google Scholar] [CrossRef]
- Tomanguillo, J.; Searls, L.; Annie, F.H.; Kemper, S.; Drabish, K.; Naravadi, V. A Nationwide Analysis of Fluid Resuscitation Outcomes in Patients with Acute Pancreatitis. Cureus 2023, 15, e50182. [Google Scholar] [CrossRef]
- Messallam, A.A.; Body, C.B.; Berger, S.; Sakaria, S.S.; Chawla, S. Impact of early aggressive fluid resuscitation in acute pancreatitis. Pancreatology 2021, 21, 69–73. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Finfer, S.; Bellomo, R.; Billot, L.; Cass, A.; Gattas, D.; Glass, P.; Lipman, J.; Liu, B.; McArthur, C.; et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N. Engl. J. Med. 2012, 367, 1901–1911. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, J.G.; Wu, H.S.; Tao, J.; Qin, Q.; Deng, S.C.; Liu, Y.; Liu, L.; Wang, B.; Tian, K.; et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J. Gastroenterol. 2013, 19, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Calderón, E.; Diaz-Arocutipa, C.; Monge, E. Lactate Ringer’s versus normal saline in the management of acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Dig. Dis. Sci. 2022, 67, 4131–4139. [Google Scholar] [CrossRef]
- Chen, H.; Lu, X.; Xu, B.; Meng, C.; Xie, D. Lactated Ringer solution is superior to normal saline solution in managing acute pancreatitis: An updated meta-analysis of randomized controlled trials. J. Clin. Gastroenterol. 2022, 56, e114–e120. [Google Scholar] [CrossRef]
- Kow, C.S.; Burud, I.A.S.; Hasan, S.S. Fluid resuscitation with Lactated Ringer’s solution versus normal saline in acute pancreatitis: A systematic review and meta-analysis of randomized trials. Pancreas 2022, 51, 752–755. [Google Scholar] [CrossRef]
- Dawson, A.; Karunakaran, M.; Sharma, Z.D.; Ullah, S.; Barreto, S.G. Fluid resuscitation in the early management of acute pancreatitis—Evidence from a systematic review and meta-analysis. HPB 2023, 25, 1451–1465. [Google Scholar] [CrossRef]
- Ocskay, K.; Mátrai, P.; Hegyi, P.; Párniczky, A. Lactated Ringer’s solution reduces severity, mortality, systemic and local complications in acute pancreatitis: A systematic review and meta-analysis. Biomedicines 2023, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, K.; Mo, S.; Liu, Z.; Yao, J. A meta-analysis of Lactate Ringer’s solution versus Normal Saline in the treatment of acute pancreatitis. Gastroenterol. Hepatol. 2024, 47, 876–887. [Google Scholar] [CrossRef]
- Hong, J.; Li, Q.; Wang, Y.; Xiang, L.; Zhou, Y.; Fan, M.; Lin, R. Comparison of fluid resuscitation with Lactate Ringer’s versus normal saline in acute pancreatitis: An updated meta-analysis. Dig. Dis. Sci. 2024, 69, 262–274. [Google Scholar] [CrossRef]
- Antoniak, D.; Twohig, P.; Olson, K.; Samson, K.; Mitchell, C.; Eichelle, D. Lactated Ringer’s or normal saline for initial resuscitation in patients hospitalized with acute pancreatitis: A retrospective database analysis. Pancreas 2023, 52, e203–e209. [Google Scholar] [CrossRef]
- Ichai, C.; Orban, J.-C.; Fontaine, E. Sodium lactate for fluid resuscitation: The preferred solution for the coming decades? Crit. Care 2014, 18, 163. [Google Scholar] [CrossRef]
- Singh, S.; Kerndt, C.C.; Davis, D. Ringer’s Lactate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Khatua, B.; Yaron, J.R.; El-Kurdi, B.; Kostenko, S.; Papachristou, G.I.; Singh, V.P. Ringer’s Lactate prevents early organ failure by providing extracellular calcium. J. Clin. Med. 2020, 9, 263. [Google Scholar] [CrossRef]
- Blumberg, N.; Cholette, J.M.; Pietropaoli, A.P.; Phipps, R.; Spinelli, S.L.; Eaton, M.P.; Noronha, S.A.; Seghatchian, J.; Heal, J.M.; Refaai, M.A. 0.9% NaCl (Normal Saline)—Perhaps not so normal after all? Transfus. Apher. Sci. 2018, 57, 127–131. [Google Scholar] [CrossRef]
- Bihari, S.; Wiersema, U.F.; Schembri, D.; De Pasquale, C.G.; Dixon, D.L.; Prakash, S.; Lawrence, M.D.; Bowden, J.J.; Bersten, A.D. Bolus intravenous 0.9% saline, but not 4% albumin or 5% glucose, causes interstitial pulmonary edema in healthy subjects. J. Appl. Physiol. 2015, 119, 783–792. [Google Scholar] [CrossRef]
- Li, H.; Bersten, A.; Wiersema, U.; Schembri, D.; Cavallaro, E.; Dixon, D.L.; Bihari, S. Bolus intravenous 0.9% saline leads to interstitial permeability pulmonary edema in healthy volunteers. Eur. J. Appl. Physiol. 2021, 121, 3409–3419. [Google Scholar] [CrossRef]
- Wu, B.U.; Hwang, J.Q.; Gardner, T.H.; Repas, K.; Delee, R.; Yu, S.; Smith, B.; Banks, P.A.; Conwell, D.L. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 710–717.e1. [Google Scholar] [CrossRef]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in Toll-Like Receptor– and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef]
- Lee, A.; Ko, C.; Buitrago, C.; Hiramoto, B.; Hilson, L.; Buxbaum, J.; NS-LR Study Group. Lactated Ringers vs normal saline resuscitation for mild acute pancreatitis: A randomized trial. Gastroenterology 2021, 160, 955–957.e4. [Google Scholar] [CrossRef]
- Iqbal, U.; Anwar, H.; Scribani, M. Ringer’s lactate versus normal saline in acute pancreatitis: A systematic review and meta-analysis. J. Dig. Dis. 2018, 19, 335–341. [Google Scholar] [CrossRef]
- Aziz, M.; Ahmed, Z.; Weissman, S.; Ghazaleh, S.; Beran, A.; Kamal, F.; Lee-Smith, W.; Assaly, R.; Nawras, A.; Pandol, S.J. Lactated Ringer’s vs normal saline for acute pancreatitis: An updated systematic review and meta-analysis. Pancreatology 2021, 21, 1217–1223. [Google Scholar] [CrossRef]
- Zhou, S.; Buitrago, C.; Foong, A.; Lee, V.; Dawit, L.; Hiramoto, B.; Chang, P.; Schilperoort, H.; Lee, A.; de-Madaria, E.; et al. Comprehensive meta-analysis of randomized controlled trials of Lactated Ringer’s versus Normal Saline for acute pancreatitis. Pancreatology 2021, 21, 1405–1410. [Google Scholar] [CrossRef]
- Vedantam, S.; Tehami, N.; de-Madaria, E.; Barkin, J.A.; Amin, S. Lactated Ringers does not reduce SIRS in acute pancreatitis compared to normal saline: An updated meta-analysis. Dig. Dis. Sci. 2022, 67, 3265–3274. [Google Scholar] [CrossRef]
- Ke, L.; Ye, B.; Huang, M.; Chen, T.; Doig, G.; Li, C.; Chen, Y.; Zhang, H.; Zhao, L.; Chen, G.; et al. Balanced solution versus normal saline in predicted severe acute pancreatitis: A stepped wedge cluster randomized trial. Ann. Surg. 2025, 281, 86–94. [Google Scholar] [CrossRef]
- Guilabert, L.; Cárdenas-Jaén, K.; Vaillo-Rocamora, A.; de Paredes, A.G.G.; Chhoda, A.; Sheth, S.G.; López-Valero, C.; Zapater, P.; Navarrete-Muñoz, E.M.; Maisonneuve, P.; et al. Normal saline versus lactated Ringer’s solution for acute pancreatitis resuscitation, an open-label multicenter randomized controlled trial: The WATERLAND trial study protocol. Trials 2024, 25, 699. [Google Scholar] [CrossRef]
- Kayhan, S.; Selcan Akyol, B.; Ergul, M.; Baysan, C. The effect of type of fluid on disease severity in acute pancreatitis treatment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7460–7467. [Google Scholar]
- Karki, B.; Thapa, S.; Khadka, D.; Karki, S.; Shrestha, R.; Khanal, A.; Shrestha, R.; Paudel, B.N. Intravenous Ringers lactate versus normal saline for predominantly mild acute pancreatitis in a Nepalese Tertiary Hospital. PLoS ONE 2022, 17, e0263221. [Google Scholar] [CrossRef]
| Clinical Features and Parameters of Adverse Prognosis in AP | |
|---|---|
| Characteristic of patient | Age > 55 years |
| BMI ≥ 25 kg/m2 | |
| Alcohol abuse | |
| Altered mental status | |
| Comorbid diseases | |
| SIRS (≥2 criteria) | Body temperature < 36 °C or > 38 °C |
| HR > 90/min | |
| RR > 20/min (PaCO2 < 32 mmHg) | |
| WBC > 12 G/L or < 4 G/L (or > 10% immature leukocytes) | |
| Laboratory tests | HCT ≥ 40% (women)/≥ 44% (men) |
| Glucose > 200 mg/dL | |
| Calcium < 1.97 mmol/L | |
| CRP > 15 mg/dL | |
| BUN ≥ 20 mg/dL | |
| LDH > 350 U/L | |
| Increased level of creatinine | |
| Radiological imaging | Pleural effusions |
| Pulmonary infiltrates | |
| Multiple or extensive peripancreatic fluid collections | |
| Scoring scales/systems | BISAP ≥ 3 points |
| APACHE-II ≥ 8 points on admission or within first 72 h | |
| SOFA elevation ≥ 2 points | |
| Ranson criteria ≥ 3; assessment on admission and after first 48 h | |
| ClinicalTrials.gov Identifier/Phase (If Specified) | Participants/Enrollment | Study Endpoint | Rate and Volume of Administration | Clinical Effects/Findings Results | Author, Year Reference |
|---|---|---|---|---|---|
| Retrospective cohort study | 500 patients with MAP; Hydration group A (n = 252); hydration group B (n = 161); hydration group C (n = 87) | The first 12 h of vitals check | Hydration group A (0–1.5 mL/kg/h); Hydration group B (>1.5–3 mL/kg/h); Hydration group C (>3 mL/kg/h) | ↓ opioid use ↓ readmission to the hospital | Doshi et al., 2020 [55] |
| Randomized controlled clinical trial | 88 patients with AP; aggressive fluid arm (n = 43) or standard fluid arm (n = 45) | Development of SIRS, MODS; Hospital length of stay | Aggressive arm: 20 mL/kg bolus + 3 mL/kg/h for the first 24 h and then 30 mL/kg for the next 24 h Standard arm: 1.5 mL/kg/h for the first 24 h and 30 mL/kg during the next 24 h; 6400 mL vs. 2795 mL | ↔ persistent SIRS ↔ pancreatic necrosis ↔ respiratory complications, AKI ↔ hospital stay length | Cuéllar-Monterrubio et al., 2020 [57] |
| Multicentre, open-label, parallel-group, randomized, controlled trial | 249 patients with AP; aggressive fluid arm (n = 122) or moderate fluid arm (n = 127) | Development of MSAP/SAP during hospitalization; Fluid overload | Aggressive arm: 20 mL/kg bolus + 3 mL/kg/h Moderate arm: 10 mL/kg bolus + 1.5 mL/kg/h 5400 mL vs. 3310 mL | ↑ fluid overload ↑ hospital stay | de-Madaria et al., 2022 [59] |
| Meta-analysis | Patients with AP; aggressive fluid arm (n = 1229) or moderate fluid arm (n = 1397) | NA | Aggressive: 3–5 mL/kg/h in first 24 h | ↔ SIRS, MODS, pancreatic necrosis, mortality risk ↑ AKI, ARDS risk | Gad et al., 2020 [61] |
| Meta-analysis | 3127 patients with SAP | NA | Aggressive: 250–500 mL/h or 5–10 mL/kg/h | ↑ AKI, ARDS, persistent SIRS, pancreatic necrosis risk ↑ mortality risk ↑ hospital stay | Liao et al., 2022 [62] |
| Meta-analysis | 4072 patients with AP; aggressive fluid resuscitation (n = 1653) or controlled fluid resuscitation (n = 2419) | NA | Aggressive: >250 mL/h or >5 mL/kg/h | ↑ mortality ↑ MODS and infection risk ↑ hospital stay | Guo et al., 2023 [64] |
| Systematic review and meta-analysis | 3423 patients with AP | NA | ↑ AKI, ARDS risk ↔ mortality | Ding et al., 2023 [65] | |
| Systematic review and meta-analysis | 4667 patients with MAP, MSAP and SAP | NA | High: (≥20 mL/kg/h) Moderate: (≥10 to <20 mL/kg/h) Low: (5 to <10 mL/kg/h) | ↑ clinical outcomes ↓ mortality | Kumari et al., 2024 [66] |
| Randomized controlled trial | 44 patientd with AP; aggressive fluid arm (n = 22) or moderate fluid arm (n = 22) | Clinical improvement within 36 h; Decrease in HCT, BUN, creatinine and reduced epigastric pain level tolerance of oral intake, development of SIRS | Aggressive arm: 20 mL/kg bolus + 3 mL/kg/h Standard arm: 10 mL/kg bolus + 1.5 mL/kg/h 4886 mL vs. 3985 mL | ↔ clinical improvement (expect of obese patients) | Angsubhakorn et al., 2021 [67] |
| Retrospective cohort study | 10,400 patients with AP | 30-day mortality; mechanical ventilation rates; severe sepsis | Aggressive: >3 mL/kg/h Standard: ≤1.5 mL/kg/h | ↔ mortality ↔ hospital stay length | Tomanguillo et al., 2023 [70] |
| Retrospective cohort study | 310 patients with AP | MODS; In-hospital mortality | Aggressive: ≥4.475 L/24 h Moderate: 2.8–4.475 L/24 h Conservative: <2.8 L/24 h | Aggressive fluid therapy increased MODS occurrence and hospital length stay | Messallam et al., 2021 [71] |
| ClinicalTrials.gov Identifier/Phase (If Specified) | Participants/Enrollment | Type of Fluid | Rate of Administration | Clinical Effects/Findings | References |
|---|---|---|---|---|---|
| Double-blinded andomized controlled trial | 331 patients with AP | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | 10 mL/kg bolus followed by continuous infusion at 3 mL/kg/h | RL: ↔ SIRS development ↓ hospital stay ↓ ICU admission ↓ local complications development | Lee et al., 2021 [90] |
| Prospective, single-center study, ranodmized controlled trial | 142 patients with AP (77 with LR and 65 with NS) | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | 1000 mL within the first hour after randomization, and then 3 mL/kg/h until oral feeding | RL: ↓ AP severity ↓ CRP level | Kayhan et al., 2021 [97] |
| Open-label randomized controlled trial | 51 patients with AP (26 with LR and 25 with NS) | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | 10 mL/kg within the first 60 min followed by infusion at the rate of 1.5 mL/kg/h until oral feeding | RL: ↓ SIRS development ↓ CRP level after 72 h | Karki et al., 2022 [98] |
| Systematic review and meta-analysis | 549 patients with AP (230 with LR and 319 with NS) | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↔ mortality ↔ SIRS development ↓ hospital stay | Aziz et al., 2021 [92] |
| Multicenter, stepped-wedge, cluster-randomized trial | 259 patients with predicted SAP (112 with BMESs and 147 with NS) | BMESs, NS; Comparison of intravenous hydration with BMESs and NS | No detailed data | BMES: ↓ SIRS development ↓ organ failure | Ke et al., 2025 [95] |
| Meta-analysis | 546 patients with AP | RL, NS; Comparison of intravenous hydration with RL and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↔ mortality ↔ SIRS development ↓ ICU admission | Vedantam et al., 2022 [94] |
| Meta-analysis | 248 patients with AP | RL, NS; Comparison of intravenous hydration with RL and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↔ mortality ↔ SIRS, MODS, pancreatic necrosis development ↔ hospital stay ↓ ICU admission | Chen et al., 2022 [75] |
| Systematic review and meta-analysis | 3752 patients with AP; aggressive fluid resuscitation (n = 1386) or controlled fluid resuscitation (n = 2366) for comparison intensify of fluid therapy; 631 patients with AP (251 with RL, 359 with NS and 21 with HES) for comparison type of fluid therapy | RL, NS, HES; Comparison of intravenous hydration with RL and NS, as well as aggressive and moderate fluid therapy | The regimen of fluid resuscitation differed across the included trials | RL: ↔ mortality ↓ hospital stay ↑ MODS risk | Dawson et al., 2023 [77] |
| Systematic review and meta-analysis | 557 patients with AP (278 with RL and 279 with NS) | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↓ mortality ↓ MODS, local complications risk ↓ hospital stay ↓ MSAP risk | Ocskay et al., 2023 [78] |
| Meta-analysis | 431 patients with AP | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↓ SIRS risk ↓ hospital stay ↓ local complications risk ↓ ICU admission | Wang et al., 2024 [79] |
| Meta-analysis | 1424 patients with AP (651 with RL and 773 with NS) | RL solution, NS; Comparison of intravenous hydration with RL solution and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↓ MSAP, SAP risk ↓ ICU admission ↓ local complications risk ↓ hospital stay | Hong et al., 2024 [80] |
| Retrospective analysis | 20,049 admissions with AP | RL, NS; Comparison of intravenous hydration with RL and NS | The regimen of fluid resuscitation differed across the included trials | RL: ↓ 1-year mortality | Antoniak et al., 2023 [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caban, M.; Zatorski, H.; Małecka-Wojciesko, E. Fluid Therapy in Acute Pancreatitis—Current Knowledge and Future Perspectives. Pharmaceuticals 2025, 18, 1601. https://doi.org/10.3390/ph18111601
Caban M, Zatorski H, Małecka-Wojciesko E. Fluid Therapy in Acute Pancreatitis—Current Knowledge and Future Perspectives. Pharmaceuticals. 2025; 18(11):1601. https://doi.org/10.3390/ph18111601
Chicago/Turabian StyleCaban, Miłosz, Hubert Zatorski, and Ewa Małecka-Wojciesko. 2025. "Fluid Therapy in Acute Pancreatitis—Current Knowledge and Future Perspectives" Pharmaceuticals 18, no. 11: 1601. https://doi.org/10.3390/ph18111601
APA StyleCaban, M., Zatorski, H., & Małecka-Wojciesko, E. (2025). Fluid Therapy in Acute Pancreatitis—Current Knowledge and Future Perspectives. Pharmaceuticals, 18(11), 1601. https://doi.org/10.3390/ph18111601







