Enhanced Skin Permeation of Diclofenac Sodium Using Mango Seed Kernel Starch Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Mango Seed Kernel Starch
2.2. Characterization of Mango Seed Kernel Starch Nanoparticles
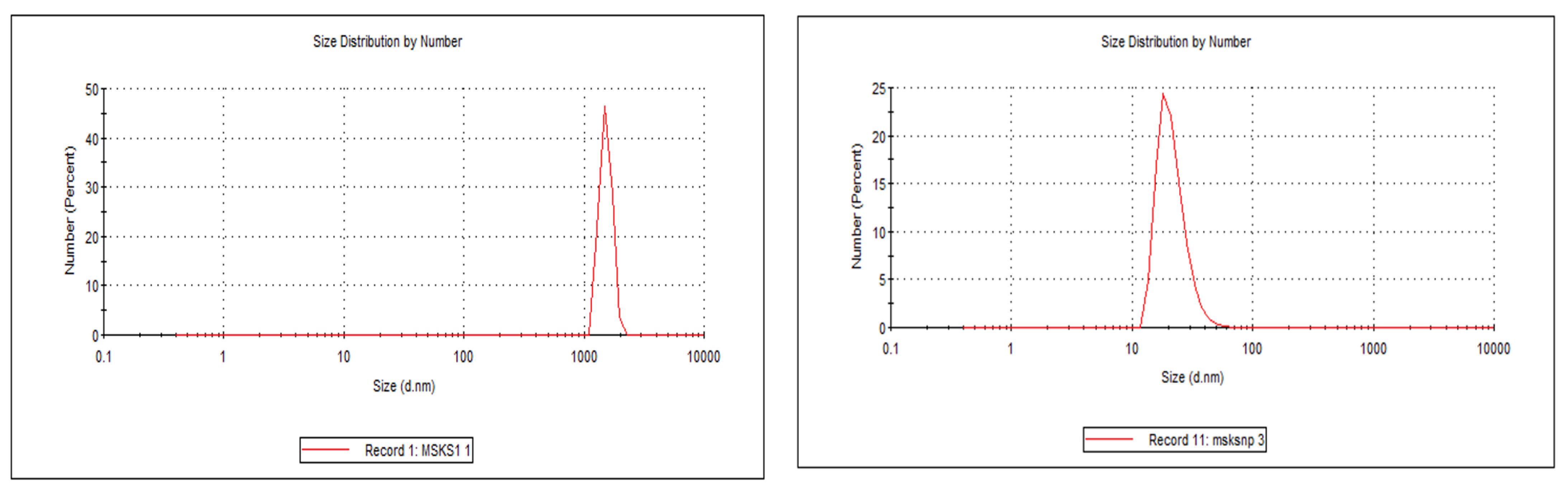
2.2.1. Particle Size Distribution, Zeta Potential, % Encapsulation Efficiency (%EE) and % Drug Loading (%DL)
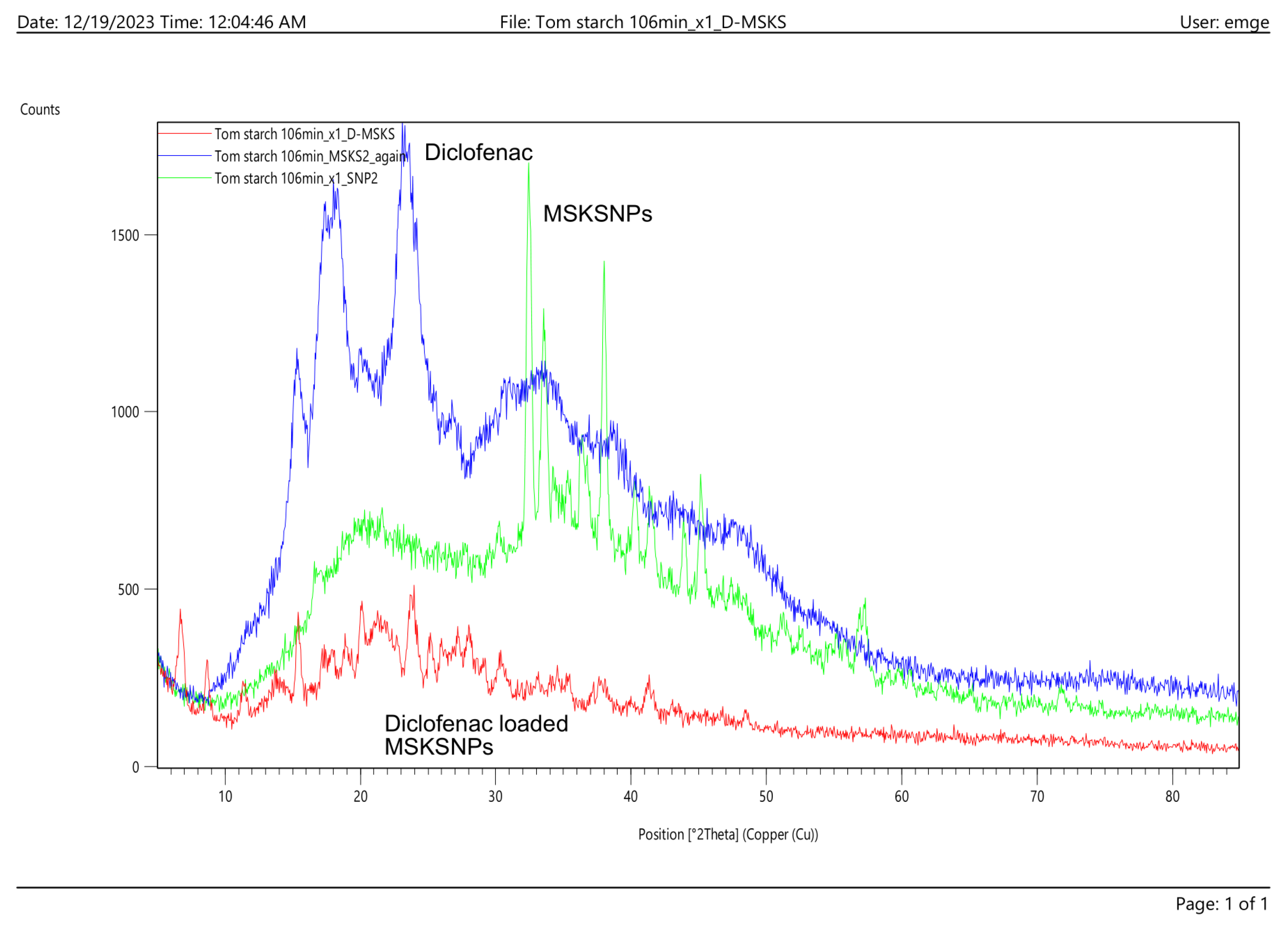
2.2.2. X-ray Diffraction (XRD)
2.2.3. Transmission Electron Microscopy (TEM)
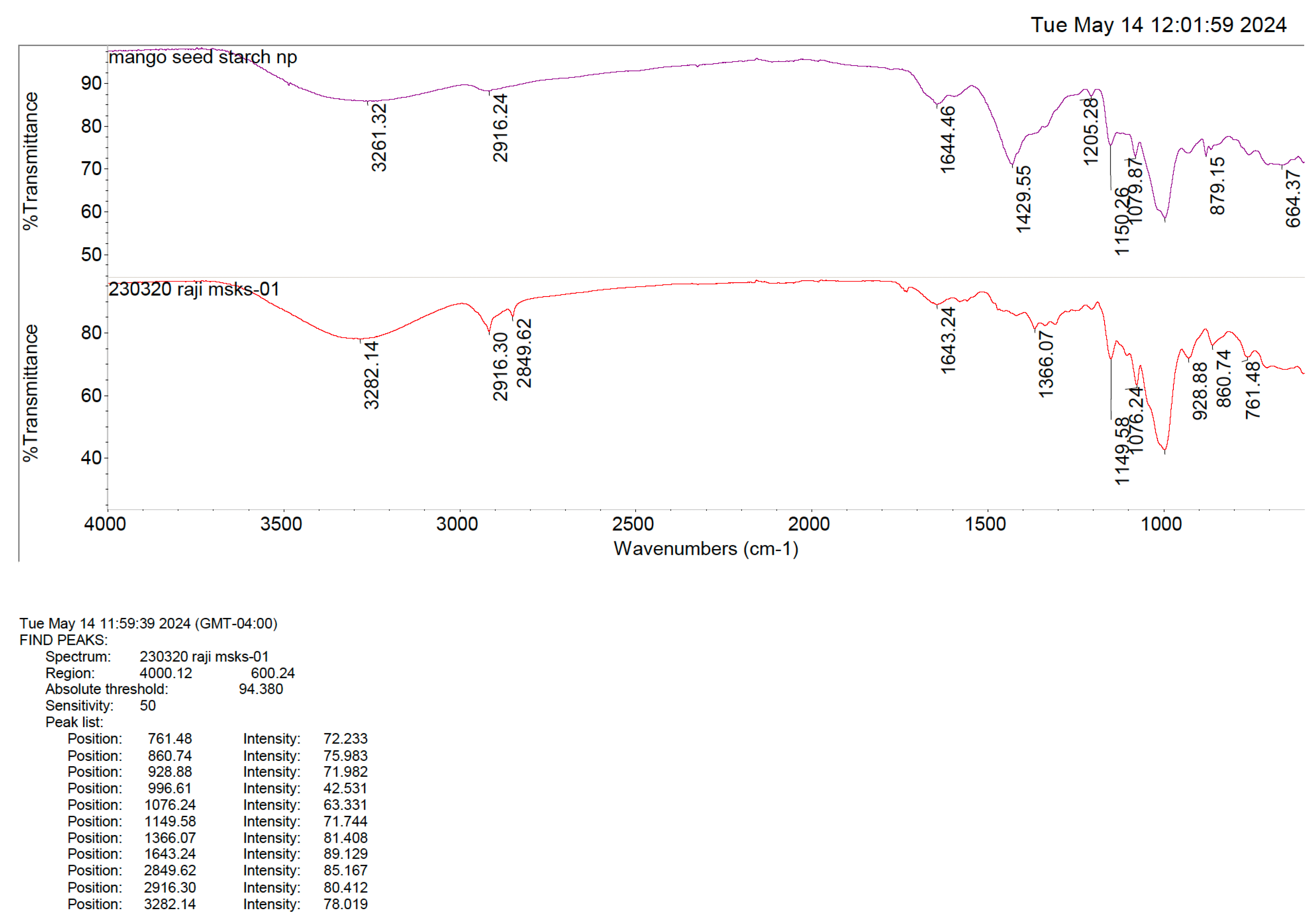
2.2.4. FTIR
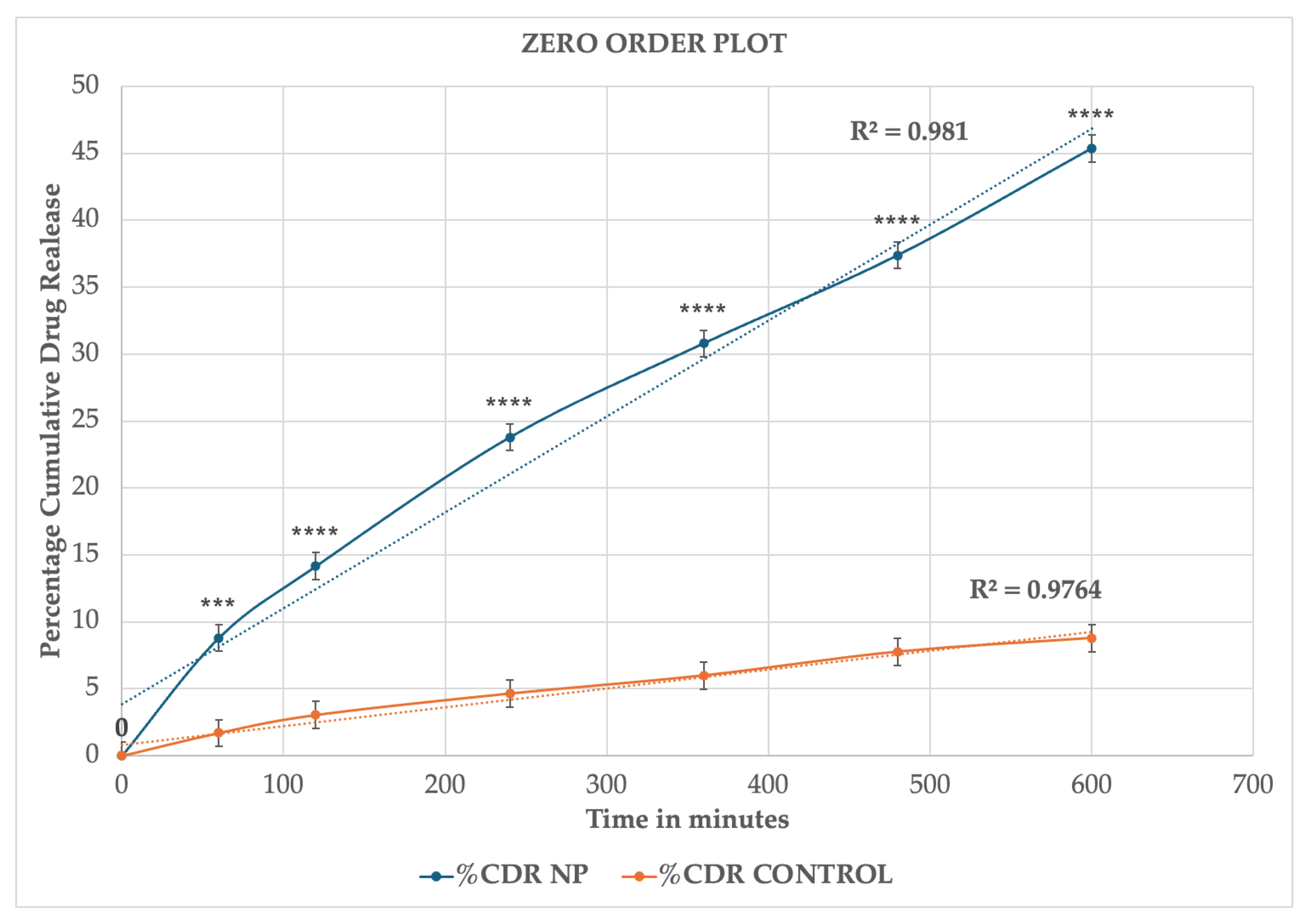
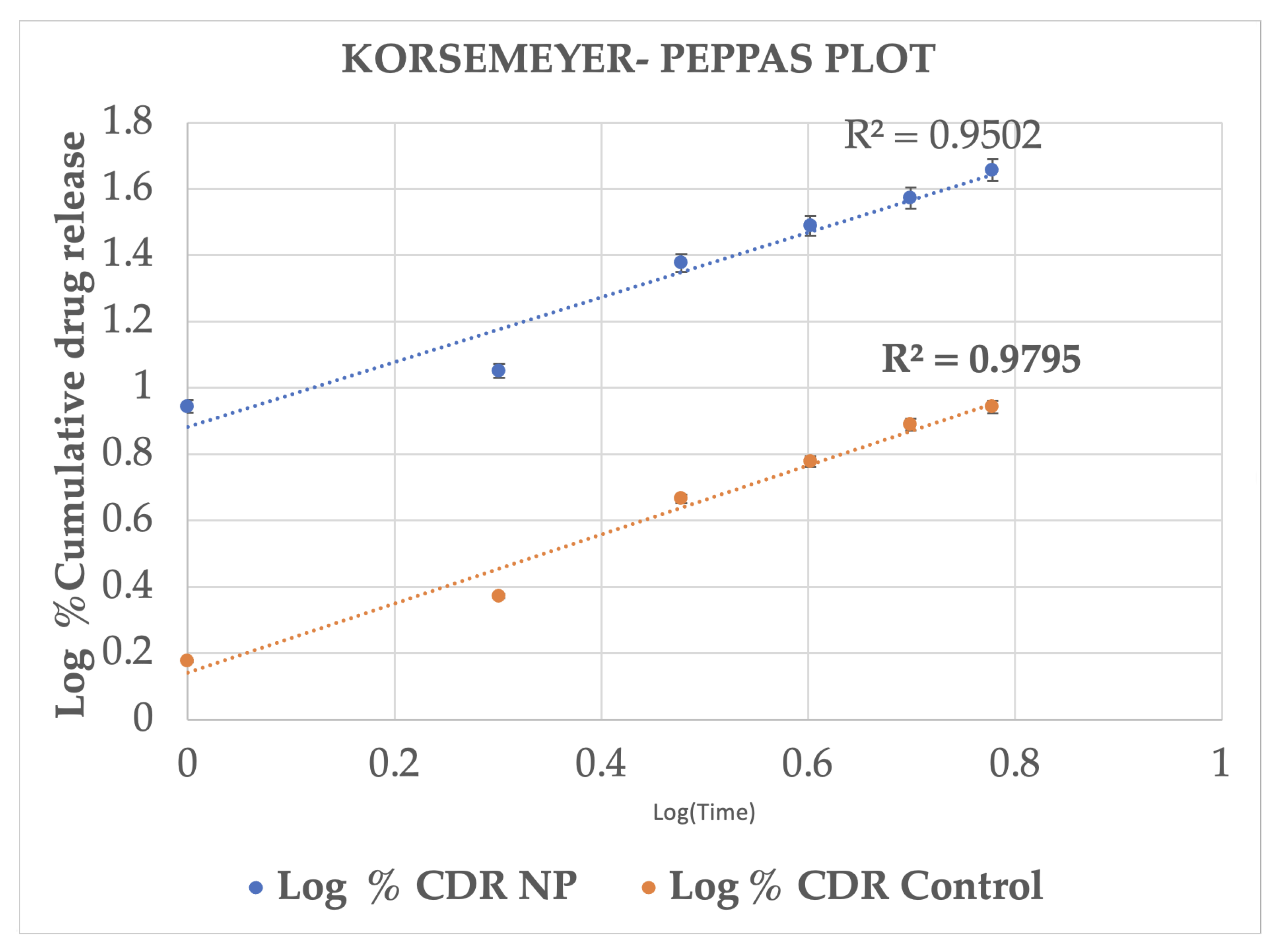
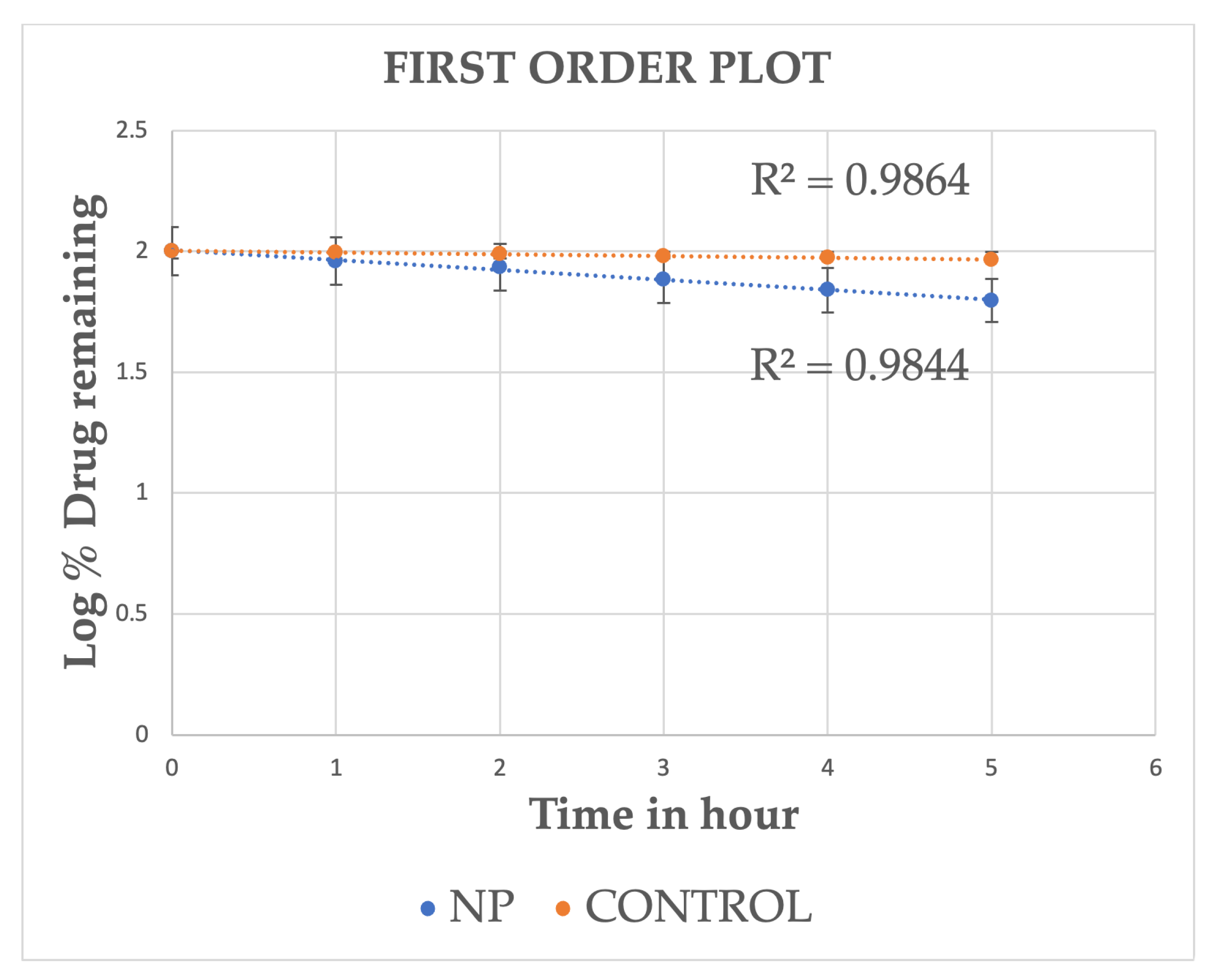
2.3. In Vitro Release Studies
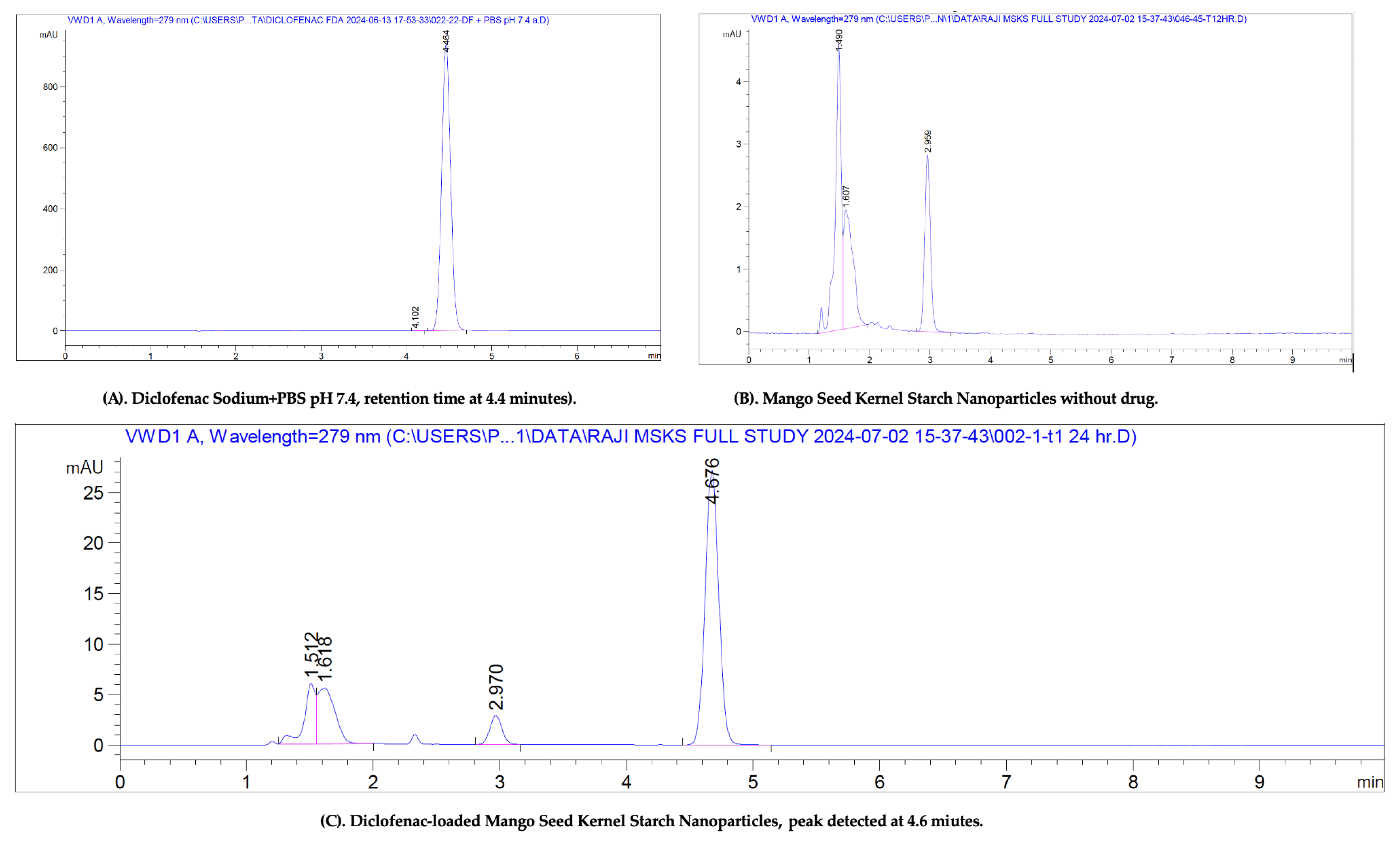
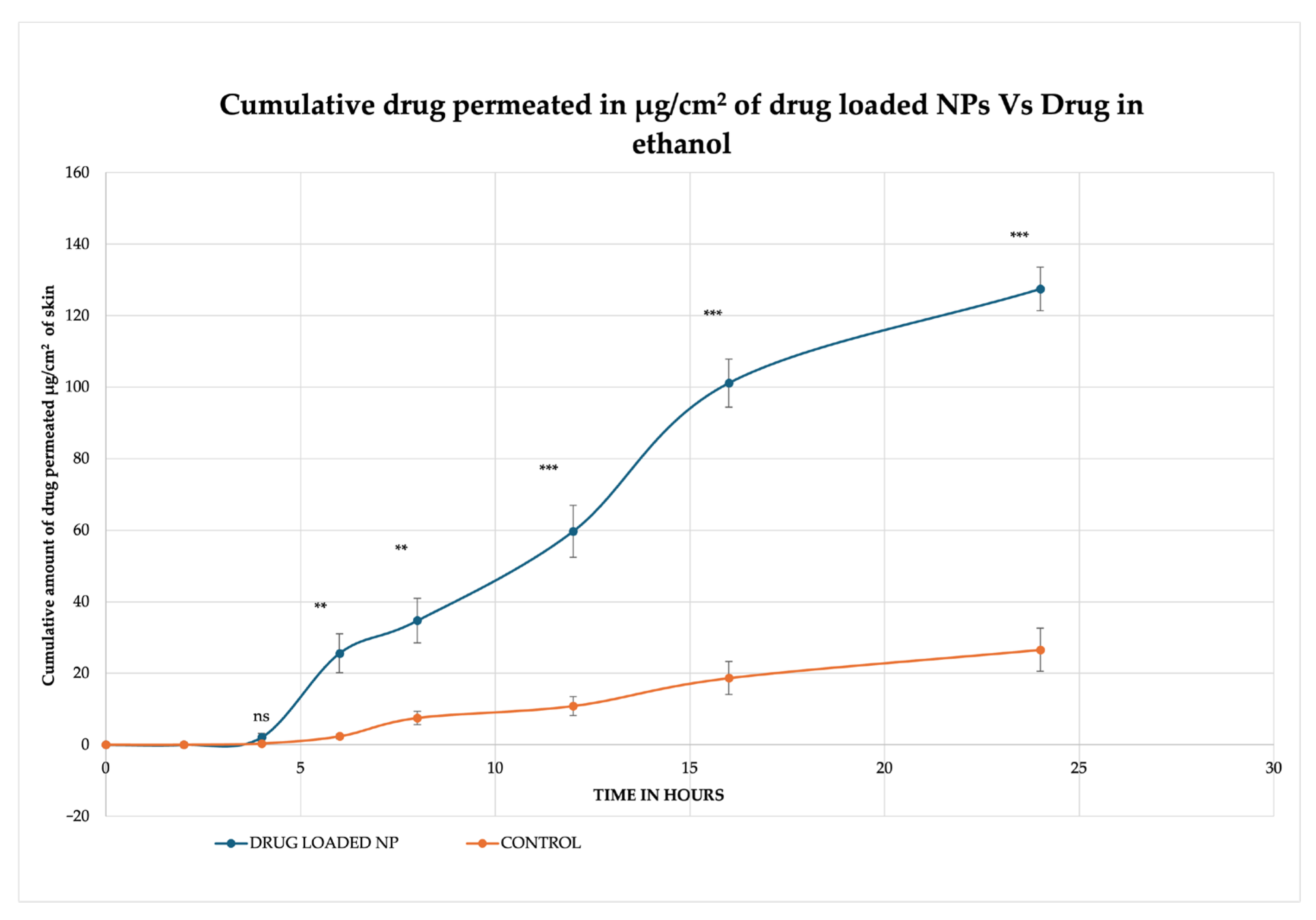
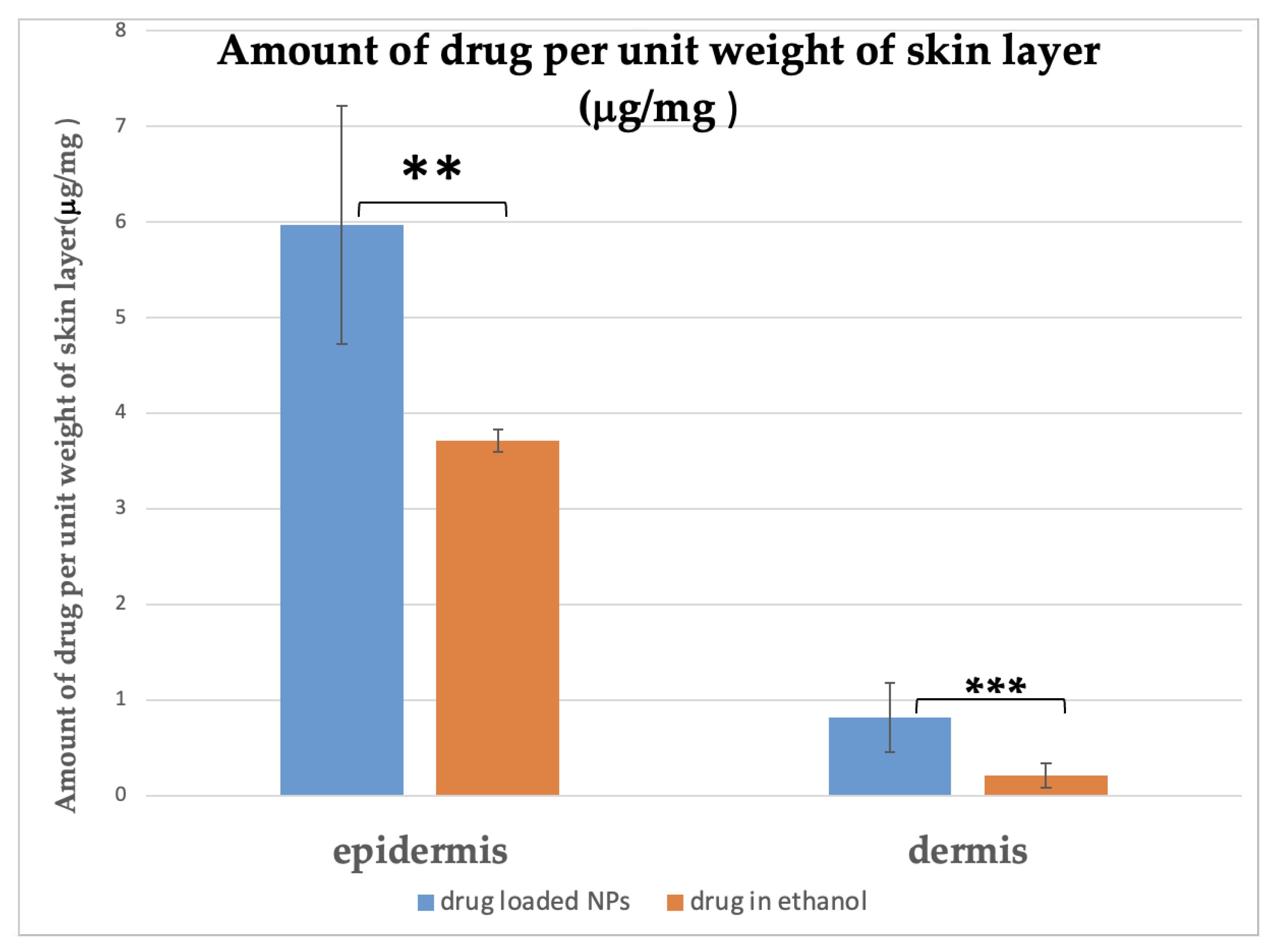
2.4. In Vitro Permeation Studies
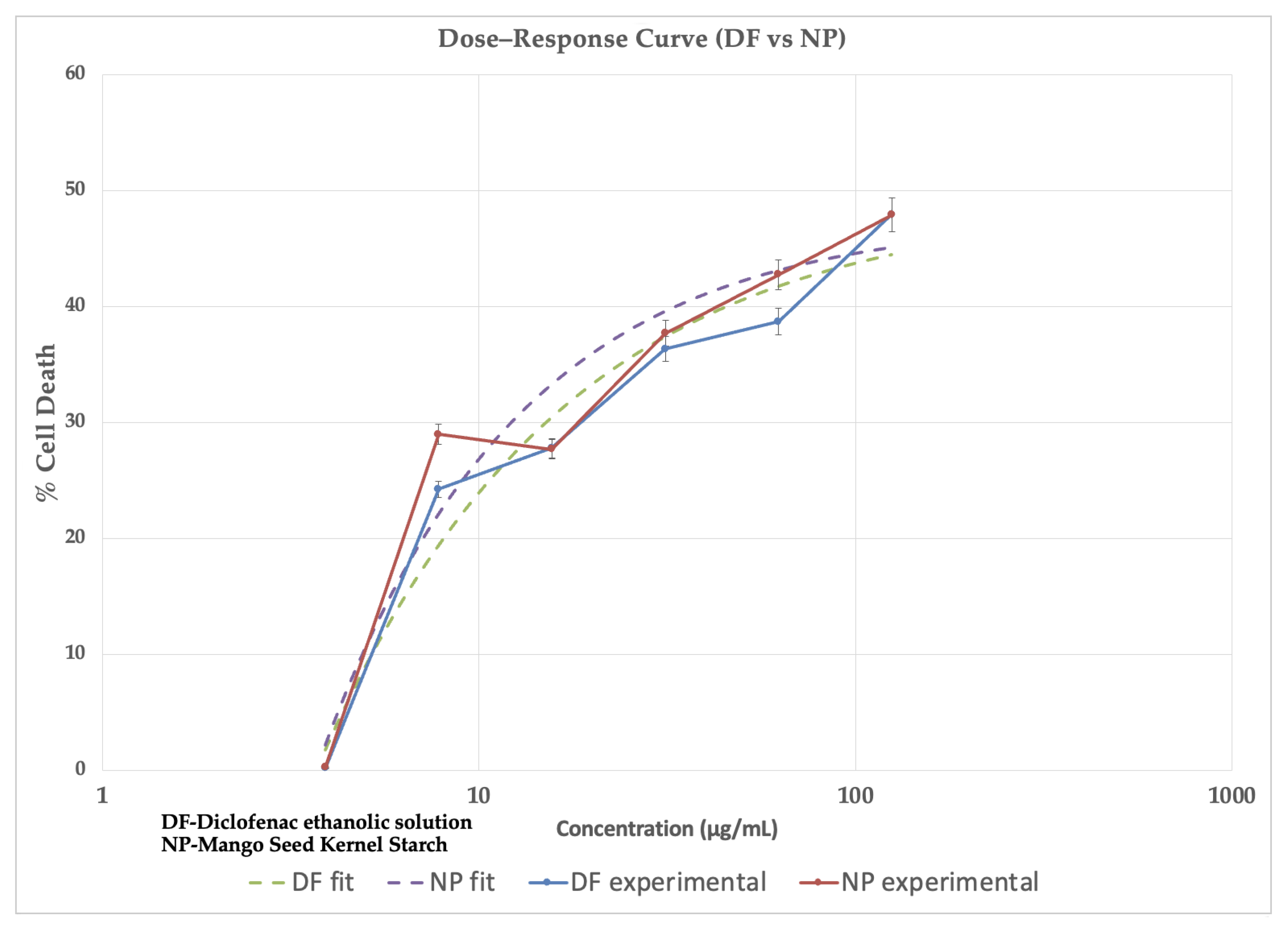
2.5. Cytotoxicity Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Characterization of Mango Seed Kernel Starch (MSKS)
- pH: A 20% w/v dispersion of starch was prepared, and its pH was detected using a pH meter (Mettler-Toledo Seven Compact, Model S220, Columbus, OH, USA), calibrated using standard buffer solutions (pH 4.0, 7.0, and 10.0) before each measurement.
- Moisture content: The moisture content was measured by evaporating 5 g of the starch sample by heating it to 105.0 ± 0.1 °C in an oven until the weight became constant. The percentage moisture content was calculated using the following formula:where Wi = Sample’s initial weight and Wf = final weight of the sample [19].
- Water Holding Capacity: A 5% w/v starch suspension was prepared in a pre-weighed centrifuge tube and centrifuged at 1500 rpm for 5 min. The supernatant was discarded, and the combined weight of the tube and hydrated starch was recorded. Water holding capacity was calculated and expressed as the grams of water retained per 100 g of dry starch. [19].
- Swelling index: A 0.1 g starch sample was placed in a centrifuge tube, and 10 mL of distilled water was added. The mixture was heated in a water bath (Julabo GmbH, Seelbach, Germany) at 50.0 ± 0.1 °C for 30 min with continuous shaking. Following heating, the sample was centrifuged at 1500 rpm for 20 min, the supernatant was carefully removed, and the weight of the starch paste was recorded. Swelling power was then calculated using the following formula [20]:
- Gelatinization temperature: 1 g of MSKS was added to a 20 mL beaker, and 10 mL of deionized water was added. The starch dispersion was subjected to heat at 80 °C on a magnetic heat bench (Benchmark Scientific, Sayreville, NJ, USA). The gelatinization temperature was recorded [21].
- Amylose: Amylopectin content: Amylose content of the isolated starch was determined using the method reported by Williams et al. [22]. A 20 mg starch sample was accurately weighed, and 10 mL of 0.5 N KOH was added to prepare a well-mixed suspension. The mixture was then transferred to a 100 mL volumetric flask, and the volume was adjusted to 100 mL with distilled water. Ten milliliters of the resulting starch solution were pipetted into a 50 mL volumetric flask, followed by the addition of 5 mL of 0.1 N HCl and 0.5 mL of iodine reagent. The volume was diluted to 50 mL, and the absorbance (A) was measured at 625 nm. The amylose and amylopectin were determined using the formula below, and their ratio was determined.
4.3. Preparation of Mango Seed Kernel Starch Nanoparticles (MSKSNPs) Loaded with Diclofenac Sodium
4.4. Characterization of Mango Seed Kernel Starch Nanoparticles
4.4.1. Particle Size Distribution and Zeta Potential
4.4.2. Encapsulation Efficiency and Drug Loading
4.4.3. X-ray Diffraction (XRD)
4.4.4. Transmission Electron Microscopy (TEM)
4.4.5. Fourier Transform Infra-Red Spectrometry (FTIR)
4.5. In Vitro Release Studies (IVRT)
4.6. In Vitro Permeation Studies (IVPT)
Quantification of Drug Content in Skin Samples
4.7. Cytotoxicity Studies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shariatinia, Z. Pharmaceutical applications of natural polysaccharides. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–57. [Google Scholar]
- Joseph, T.M.; Unni, A.B.; Joshy, K.; Kar Mahapatra, D.; Haponiuk, J.; Thomas, S. Emerging Bio-Based Polymers from Lab to Market: Current Strategies, Market Dynamics and Research Trends. C 2023, 9, 30. [Google Scholar] [CrossRef]
- Builders, P.F.; Arhewoh, M.I. Pharmaceutical applications of native starch in conventional drug delivery. Starch-Stärke 2016, 68, 864–873. [Google Scholar] [CrossRef]
- Vilpoux, O.F.; Brito, V.H.; Cereda, M.P. Starch extracted from corms, roots, rhizomes, and tubers for food application. In Starches for Food Application; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–165. [Google Scholar]
- Bangar, S.P.; Dhull, S.B.; Manzoor, M.; Chandak, A.; Esua, O.J. Functionality and Applications of Non-Conventional Starches from Different Sources. Starch-Stärke 2024, 76, 2300073. [Google Scholar] [CrossRef]
- Hassan, L.; Muhammad, A.; Aliyu, R.; Idris, Z.; Izuagie, T.; Umar, K.; Sani, N. Extraction and characterisation of starches from four varieties of Mangifera indica seeds. IOSR J. Appl. Chem. 2013, 3, 16–23. [Google Scholar] [CrossRef]
- Taggart, P.; Mitchell, J. Starch. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 108–141. [Google Scholar]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Caldonazo, A.; Almeida, S.L.; Bonetti, A.F.; Lazo, R.E.L.; Mengarda, M.; Murakami, F.S. Pharmaceutical applications of starch nanoparticles: A scoping review. Int. J. Biol. Macromol. 2021, 181, 697–704. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; El-Rafie, M.; El-Sheikh, M.A.; El-Feky, G.S.; Hebeish, A. Synthesis, characterization, release kinetics and toxicity profile of drug-loaded starch nanoparticles. Int. J. Biol. Macromol. 2015, 81, 718–729. [Google Scholar] [CrossRef]
- Chaksmithanont, P.; Bangsitthideth, K.; Arunprasert, K.; Patrojanasophon, P.; Pornpitchanarong, C. Statistical-Based Optimization of Modified Mangifera indica Fruit Starch as Substituent for Pharmaceutical Tableting Excipient. Polymers 2024, 16, 2653. [Google Scholar] [CrossRef]
- Devi, M.G.; Santosh, K.R.; Kusuma, A. Design, optimization and evaluation of telmisartan fast dissolving film employing mango kernel starch as a new natural super disintegrant. In Advancement in Animal Handling and Generative AI for Pre-clinical Studies; Pattnaik, G., Jal, S., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 130–137. [Google Scholar]
- Zhang, B.; Wang, K.; Hasjim, J.; Li, E.; Flanagan, B.M.; Gidley, M.J.; Dhital, S. Freeze-drying changes the structure and digestibility of B-polymorphic starches. J. Agric. Food Chem. 2014, 62, 1482–1491. [Google Scholar] [CrossRef]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.-S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the skin: A review of current trends and future prospects of transdermal drug delivery systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef]
- Shahrim, N.; Sarifuddin, N.; Ismail, H. Extraction and characterization of starch from mango seeds. J. Phys. Conf. Ser. 2018, 1082, 012019. [Google Scholar] [CrossRef]
- Omojola, M.O.; Akinkunmi, Y.; Olufunsho, K.; Egharevba, H.; Martins, E. Isolation and physico-chemical characterization of cola starch. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 2884–2900. [Google Scholar] [CrossRef]
- Daramola, B.; Osanyinlusi, S.A. Investigation on modification of cassava starch using active components of ginger roots (Zingiber officinale Roscoe). Afr. J. Biotechnol. 2006, 5, 917–920. [Google Scholar]
- Attama, A.; Nnamani, P.; Mbonu, I.; Adiku, M. Effect of hypochlorite oxidation on the physicochemical properties of gladiolus starch. J. Pharm. Allied Sci. 2003, 1, 28–35. [Google Scholar]
- Williams, P. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970, 47, 411–420. [Google Scholar]
- Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H. Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Sci. Rep. 2020, 10, 3533. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Panda, S.S.; Patanaik, D.; Kumar, B.V.R. New stability-indicating RP-HPLC method for determination of diclofenac potassium and metaxalone from their combined dosage form. Sci. Pharm. 2011, 80, 127. [Google Scholar] [CrossRef] [PubMed]
- Virani, A.; Dholaria, N.; Matharoo, N.; Michniak-Kohn, B. A study of microemulsion systems for transdermal delivery of risperidone using penetration enhancers. J. Pharm. Sci. 2023, 112, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Ramanah, A.; Bon, C.; Kanfer, I. Application of a dermatopharmacokinetic (DPK) method for bioequivalence assessment of topical metronidazole creams. J. Pharm. Pharm. Sci. 2020, 23, 437–450. [Google Scholar] [CrossRef] [PubMed]


| Sl. No | Drying Method | Soild–Solvent Ratio | Sedimentation Time (h) | % Yield (Mean ± SD) |
|---|---|---|---|---|
| 1 | Tray Dryer (40 °C) for 6 h | 1:10 | 24 | 17.2 ± 2.0 |
| 2 | Tray Dryer (40 °C) for 6 h | 1:10 | 48 | 20.2 ± 3.2 |
| 3 | Tray Dryer (40 °C) for 6 h | 1:12 | 24 | 15.4 ± 2.4 |
| 4 | Air dry (RT 20 °C) for 24 h | 1:12 | 24 | 18.6 ± 2.3 |
| 5 | Air dry (RT 20 °C) for 24 h | 1:12 | 48 | 36.2 ± 3.2 |
| 6 | Freeze drying for 24 h | 1:10 | 48 | 20.2 ± 2.1 |
| 7 | Freeze drying for 24 h | 1:12 | 48 | 30.4 ± 3.2 |
| 8 | Freeze drying for 24 h | 1:14 | 48 | 67.7 ± 5.2 |
| 9 | Freeze drying for 24 h | 1:15 | 48 | 60.5 ± 3.2 |
| Sl. No. | Parameter | Mango Seed Kernel Starch (Mean ± SD) | Corn Starch (Mean ± SD) |
|---|---|---|---|
| 1 | Solubility (%) | 17.0 ± 2.8 | 14.0 ± 3.2 |
| 2 | pH | 7.0 ± 1.2 | 7.0 ± 0.6 |
| 3 | Moisture Content (%) | 7.4 ± 0.8 | 11.8 ± 1.2 |
| 4 | Water Holding Capacity (%) | 79.35 ± 0.8 | 72.93 ± 0.6 |
| 5 | Swelling Power (g/g) | 3.20 ± 0.16 | 2.30 ± 0.52 |
| 6 | Gelatinization Temperature (°C) | 60.0 ± 2.5 | 66 ± 00 |
| 7 | Amylose/Amylopectin Content | 0.35 | 0.33 |
| Formulation | MSKS:DRUG | Particle Size Distribution (nm) (Mean ± SD) | Polydispersity Index (Mean ± SD) | Zeta Potential (mV) (Mean ± SD) | % Encapsulation Efficiency (EE) (Mean ± SD) | % Drug Loading (DL) (Mean ± SD) |
|---|---|---|---|---|---|---|
| F1 | 1:1 | 167.0 ± 1.3 | 0.34 ± 0.05 | −11.00 ± 0.05 | 83.0 ± 5.0 | 28.4 ± 3.6 |
| F2 | 2:1 | 140.0 ± 3.6 | 0.42 ± 0.03 | −31.20 ± 0.13 | 92.4 ± 3.7 | 31.0 ±2.0 |
| F3 | 1:2 | 123.0 ± 4.2 | 0.47 ± 0.02 | −27.93 ± 0.14 | 87.6 ± 3.0 | 24.0 ±1.4 |
| Drug-Loaded Nanoparticles | ||||||
|---|---|---|---|---|---|---|
| Time (h) | Log Time | SQRT Time | % CDR (M ± SD) | Log % CDR (M ± SD) | % Drug Remaining (M ± SD) | Log % Drug Remaining (M ± SD) |
| 0.00 | 0.00 | 0.00 | 0.00 ± 00 | 00 ± 00 | 100.00 | 2.00 |
| 1.00 | 0.00 | 1.00 | 8.81 ± 0.93 | 0.94 ± 0.04 | 91.19 ± 0.93 | 1.96 ± 0.04 |
| 2.00 | 0.30 | 1.41 | 14.16 ± 0.65 | 1.15 ± 0.02 | 85.84 ± 0.65 | 1.93 ± 0.02 |
| 3.00 | 0.48 | 1.73 | 23.79 ± 0.61 | 1.38 ± 0.01 | 76.21 ± 0.61 | 1.88 ± 0.01 |
| 4.00 | 0.60 | 2.00 | 30.80 ± 1.51 | 1.49 ± 0.02 | 69.20 ± 1.51 | 1.84 ± 0.02 |
| 5.00 | 0.70 | 2.24 | 37.38 ± 0.93 | 1.57 ± 0.01 | 62.62 ± 0.93 | 1.80 ± 0.01 |
| 6.00 | 0.77 | 2.45 | 45.36 ± 1.50 | 1.66 ± 0.01 | 54.63 ± 1.50 | 1.74 ± 0.01 |
| Control | ||||||
| Time (h) | Log Time | SQRT Time | % CDR (M±SD) | Log % CDR (M±SD) | % Drug Remaining (M±SD) | Log % Drug Remaining (M±SD) |
| 0.00 | 0.00 | 0.00 | 0.00 ± 00 | 0.00 ± 00 | 100.00 ± 00 | 2.00 ± 00 |
| 1.00 | 0.00 | 1.00 | 0.84 ± 1.06 | −0.08 ± 0.27 | 99.16 ± 1.06 | 2.00 ± 0.27 |
| 2.00 | 0.30 | 1.41 | 2.53 ± 0.91 | 0.40 ± 0.12 | 97.47 ± 0.91 | 1.99 ± 0.12 |
| 3.00 | 0.48 | 1.73 | 4.72 ± 0.48 | 0.67 ± 0.04 | 95.28 ± 0.48 | 1.98 ± 0.04 |
| 4.00 | 0.60 | 2.00 | 5.61 ± 0.33 | 0.75 ± 0.02 | 94.39 ± 0.33 | 1.97 ± 0.02 |
| 5.00 | 0.70 | 2.24 | 7.51 ± 0.64 | 0.88 ± 0.03 | 92.49 ± 0.64 | 1.97 ± 0.03 |
| 6.00 | 0.78 | 2.45 | 8.42 ± 0.91 | 0.93 ± 0.04 | 91.58 ± 0.91 | 1.96 ± 0.04 |
| Formulation | Zero Order R2 Values | Higuchi R2 Values | First-Order R2 Values | Korsemeyer–Peppas R2 Values |
|---|---|---|---|---|
| Drug-loaded Nanoparticles | 0.981 | 0.995 | 0.98 | 0.95 |
| Control (ethanolic drug solution) | 0.976 | 0.994 | 0.98 | 0.97 |
| Parameter | Control | NP |
|---|---|---|
| Bottom | 0 | 0 |
| Top | 48 | 48 |
| Hill Slope | 1 | 1 |
| logIC50 | 2.2 | 2.2 |
| Conc. (µg/mL) | % Death Control | % Death NP |
|---|---|---|
| 3.9 | 0 | 0 |
| 7.81 | 24.25 ± 3.4 | 28.99 ± 5.2 |
| 15.6 | 27.81 ± 2.8 | 27.72 ± 4.1 |
| 31.25 | 36.35 ± 3.00 | 37.71 ± 3.3 |
| 62.5 | 38.71 ± 3.6 | 42.77 ± 3.4 |
| 125 | 47.92 ± 2.5 | 47.91 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talluri, S.R.; Matharoo, N.S.; Dholaria, N.; Albayati, N.; John, S.; Michniak-Kohn, B. Enhanced Skin Permeation of Diclofenac Sodium Using Mango Seed Kernel Starch Nanoparticles. Pharmaceuticals 2025, 18, 1585. https://doi.org/10.3390/ph18101585
Talluri SR, Matharoo NS, Dholaria N, Albayati N, John S, Michniak-Kohn B. Enhanced Skin Permeation of Diclofenac Sodium Using Mango Seed Kernel Starch Nanoparticles. Pharmaceuticals. 2025; 18(10):1585. https://doi.org/10.3390/ph18101585
Chicago/Turabian StyleTalluri, Sesha Rajeswari, Namrata S. Matharoo, Nirali Dholaria, Nubul Albayati, Shali John, and Bozena Michniak-Kohn. 2025. "Enhanced Skin Permeation of Diclofenac Sodium Using Mango Seed Kernel Starch Nanoparticles" Pharmaceuticals 18, no. 10: 1585. https://doi.org/10.3390/ph18101585
APA StyleTalluri, S. R., Matharoo, N. S., Dholaria, N., Albayati, N., John, S., & Michniak-Kohn, B. (2025). Enhanced Skin Permeation of Diclofenac Sodium Using Mango Seed Kernel Starch Nanoparticles. Pharmaceuticals, 18(10), 1585. https://doi.org/10.3390/ph18101585








