Abstract
Background: Qishen granule (QSG) is a widely prescribed herbal formula for the treatment of chronic heart failure. The mechanisms of action of QSG have been clarified; however, the effective substances remain unclear. This lack of clarity hinders quality control and the consistency of the clinical efficacy of QSG. Methods: In the present study, an integrated strategy for an efficacy- and in vivo exposure-oriented study involving metabolite profiling, molecular docking, in vitro bioassays, and in vivo pharmacokinetics was proposed for investigating the potentially effective components of QSG. Results: In total, 101 prototypes/metabolites were preliminarily identified and characterized by UHPLC-Q TOF-MS/MS. Molecular docking of the absorbed constituents with targeted proteins suggested that 49 potential components were highly related to chronic heart failure (CHF). Then, the effectiveness of these potential compounds was verified by the oxygen glucose deprivation/re-oxygenation (OGD/R)-induced H9c2 cell model. As a result, 14 active components were screened, and their median effective concentration (EC50) was calculated and utilized to generate the weight coefficient for the bioeffect of each constituent. By exploring the kinetic parameters of the active compounds in a pharmacokinetic study, the exposure levels of these pharmacologically active compounds were determined by area under the curve (AUC0→∞) calculations. Finally, by calculating the effect–constituent index (ECI) for each compound, five key active components (cryptochlorogenic acid, chlorogenic acid, isochlorogenic acid C, salvianolic acid B, and neochlorogenic acid), which possess both pharmacological activities and higher exposure levels, were revealed to be the key effective substances of QSG. Conclusions: This study is the first to combine pharmacological activities with in vivo exposure for investigating the effective components of QSG. The identification of key active components provides a foundation for improving the quality control of QSG in clinics. The efficacy- and in vivo exposure-oriented integrated method could provide reliable references for other traditional Chinese medicines (TCMs).
1. Introduction
Qishen granule (QSG), a widely prescribed herbal formula for the treatment of chronic heart failure (CHF), is derived from two popular formulated traditional Chinese medicines (TCMs), which are Simiaoyongan and Zhenwu decoctions, and it is composed of six herbal medicines: Astragali Radix Preparata cum Melle (zhihuangqi, ZHQ), Salvia Miltiorrhiza Radix et Rhizoma (danshen, DS), Aconiti Laterlis Radix Preparata (heishunpian, HSP), Lonicerae Japonicae Flos (jinyinhua, JYH), Scrophulariae Radix (xuanshen, XS), and Glycyrrhizae Radix et Rhizoma Preparata cum Melle (zhigancao, ZGC). The efficacy and safety of QSG in CHF patients have been verified in clinics [1]. The mechanisms of action have also been thoroughly investigated and documented [2,3,4,5,6,7,8]. Quality control based on the content of some vital active compounds is essential to ensure both the safety and efficacy of QSG in clinical applications. However, little research has been carried out to investigate the material’s basis and reveal the effective substances of QSG with respect to CHF. Our previous study identified 213 constituents in QSG, among which the main types were flavonoids, chlorogenic acids, salvianolic acids, tanshinones, iridoid glucoside, alkaloids, phenolic acids, triterpenoid saponins, and phenylpropanoids [9]. While compounds absorbed into the blood are most likely to be responsible for the effects of drugs, investigating the effective substances by exploring the in vivo processes of QSG is an efficient method. There are some reports that focused on pharmacokinetic studies of QSG. The kinetic parameters of the main components were characterized; several compounds, such as glycyrrhizic acid and glycyrrhetic acid, were recognized as the effective components of different kinetic parameters in sham and model rats [10,11,12]. However, these studies focused exclusively on a limited number of compounds selected by the researchers, without comprehensive metabolite profiling of QSG. In addition, the biological activities of the selected compounds were not experimentally validated. Consequently, several vital compounds, including salvianolic acid B from DS, were overlooked despite their high exposure levels in rat plasma, and they exhibited pharmacological activity against CHF.
It is known that TCMs contain numerous ingredients, among which the active compounds exert their effects mostly by being absorbed into the blood and accumulating to an effective concentration [13]. Thus, the investigation of the absorbed components, as well as their exposure levels and efficacies, is of great importance and could provide scientific data for clarifying the effective material basis of TCMs. Pharmacokinetic studies characterize the in vivo exposures of absorbed compounds [13], while in vitro bioassays provide preliminary efficacy evaluations [14]. Usually, exposure is not directly correlated with efficacy. High-exposure compounds may exhibit low activity, while low-exposure compounds can demonstrate high activity. Selecting compounds that possess both substantial exposure and significant pharmacological activity is therefore critical. Nevertheless, an integrated approach combining efficacy and in vivo exposure to identify active constituents of a specific TCM has not yet been reported.
In recent years, the quality marker of the effect–constituent index (ECI) has been proposed for the advancement of the quality control of TCMs [15,16]. This method determines ECI values by measuring the contents of active constituents, with each assigned an effect weight based on its relative pharmacological activities, thereby correlating the results with clinical efficacy and accounting for differential contributions among constituents. Given that TCM quality can be assessed by quantifying both its quality markers and relevant bioeffect indicators, identifying effective constituents may be accomplished by determining the in vivo exposure weighted by the bioactivity coefficient.
In this study, an efficacy- and in vivo exposure-oriented integrated method was first developed and utilized for the investigation of the effective components of QSG. Figure 1 shows the experimental flow chart of this study. The ECI for each component was calculated by introducing the bioeffect weight coefficient of median effective concentration (EC50) with in vivo exposure; thus, those with both effectiveness and higher exposure levels could be recognized as the effective components of QSG. The results of this study could be helpful for the clarification of the effective substances of QSG, and the identified key active components could also establish a basis for enhancing the quality control of QSG in clinical practice. The efficacy- and in vivo exposure-oriented integrated method for the investigation of effective components could provide reliable references for other TCMs.
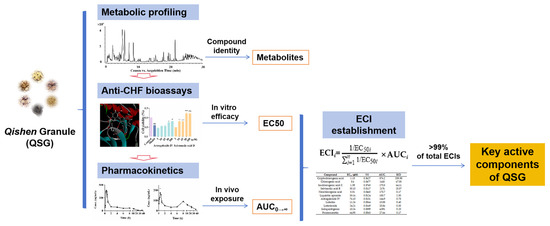
Figure 1.
The experimental flow chart of this study.
2. Results
2.1. Exploring the Absorbed Components of QSG in Rat Plasma
After oral administration of QSG, a total of 101 components were identified in rat plasma, which included 49 prototypes and 51 metabolites. The detailed identification information is listed in Table 1 and Table 2.

Table 1.
Identification of absorbed components in rat plasma after oral administration of QSG using UHPLC-Q TOF-MS in negative ion mode.

Table 2.
Identification of absorbed components in rat plasma after oral administration of QSG using UHPLC-Q TOF-MS in positive ion mode.
2.1.1. Identification of Prototype Components
A total of 49 prototype components in administered plasma were matched with the established chemical component database of QSG in Metabolite ID software (version B.04.00) [9], which included the chromatographic behaviors and fragmentation patterns of all components identified in QSG. The data of all components, including 16 alkaloids, 11 flavonoids, 6 iridoid glucosides, 5 chlorogenic acids, 5 triterpenoid saponins, 3 tanshinones, 1 salvianolic acid, 1 phenolic acid, and 1 phenylpropanoid, are displayed in Table 1 and Table 2. A pair of isomers, compounds 38 and 50, were selected as the examples for the stepwise elucidations of the molecular structures. Liquiritin and isoliquiritin are two important compounds previously identified in QSG [9]; thus their MS information such as molecular formulas and retention times as well as their MS2 spectrum was imported into Metabolite ID for their metabolite identification. There were two isomers screened with precursor ions of m/z 417.1191 in QSG-administered rat plasma, and diagnostic fragment ions at m/z 135.008 and m/z 119.0502 generated from Retro-Diels–Alder (RDA) cleavage of Ring C confirmed that they were flavanones and/or chalcones [17]; they were further identified as liquiritin and isoliquiritin by comparing the MS information with that of the standards imported in Metabolite ID (Figure S1).
2.1.2. Identification of Metabolites
Based on the metabolic prediction and screening function of Metabolite ID, a total of 52 metabolites were identified, which included 21 flavonoid metabolites, 16 phenolic acid metabolites, 8 tanshinone metabolites, 5 alkaloid metabolites, and 2 salvianolic acid metabolites. The metabolic phase II reactions were mainly glucuronide and sulfate conjugation, phase I reactions mainly consisted of hydrogenation and hydrolysis, and phase I+phase II conjugations such as glucuronidation+hydrogenation were also detected in this study.
A total of 21 flavonoid metabolites including 17 phase II and 4 phase I+II conjugation metabolites were identified. Fifteen compounds (18, 20, 24, 29, 31, 33, 36, 41, 48, 52, 55–58, 60) were characterized as glucuronide conjugates due to the neutral loss of 176.0321 Da. Most of these compounds were deduced as isomers because of their identical molecular weights as well as similar fragment pathways; thus glucuronide should be bonded to different substituent positions of one compound or the same substituent position of different isomers. In our study, the structures of most compounds were characterized based on MS2 fragmentation patterns and comparisons with literature data, enabling their use in subsequent molecular docking experiments. For example, 18 (tR = 5.86 min) and 29 (tR = 8.70 min) displayed [M−H]− ions at m/z 593.1512 and 593.1501, with fragment ions such as m/z 417.1189 and 255.0671 indicating that they are glucuronidation metabolites of liquiritin and isoliquiritin, and conjugated positions should be 7-OH. By comparing the ClogP values of 18 and 29, they were deduced as liquiritin-7-O-GluA (ClogP, −1.22) and isoliquiritin-7-O-GluA (ClogP, −0.69) [18,19,20]. Similarly, 31, 33, and 55 were confirmed as glucuronidation metabolites of liquiritigenin and isoliquiritigenin, and 31 and 33 were deduced as liquiritigenin-7-O-glucuronide (ClogP, 0.27) and liquiritigenin-4′-O-glucuronide (ClogP, 0.56) [18,19,20]. However, the substituted position of 55 could not be accurately determined due to the presence of two hydroxyl substituents in the isoliquiritigenin structure. In addition, 27 was identified as a glucuronidation+sulfation metabolite of liquiritigenin, and 43 was a sulfation metabolite of calycosin. Four compounds (34, 42, 46, 54) were identified as phase I+II conjugation metabolites; among them, 54 was identified as a hydrogenation+glucuronidation metabolite of liquiritigenin or isoliquiritigenin [18,19,20]. The fragment ion was at m/z 257.0818, which was 2 Da more than liquiritigenin or isoliquiritigenin, indicating that it is metabolized by a hydrogenation reaction. To determine whether metabolite 54 originated from liquiritigenin or isoliquiritigenin, we compared the behaviors of compounds 54 and 55. Compound 55 (tR = 13.31 min) was identified as the glucuronide conjugate of isoliquiritigenin. A hydrogenated and glucuronidated derivative of isoliquiritigenin would be expected to display greater hydrophobicity. The shorter retention time of compound 54 (tR = 12.93 min), however, indicated that it more likely arises from liquiritigenin through hydrogenation and subsequent glucuronidation. Precursor ions of m/z 513.071 [M−H]− were found for 34, 42, and 46, which were 80 Da higher than 54, indicating that they undergo another sulfation reaction. Based on the ClogP values of the three metabolites, 34 was identified as a hydrogenation+glucuronidation+sulfation metabolite of liquiritigenin, and 42 and 46 were isoliquiritigenin+H2-7-O-GluA-4′-SO3 and isoliquiritigenin+H2-4′-O-GluA-7-SO3. Moreover, 41, 48, and 56 were preliminarily identified as glucuronidation metabolites of naringenin by Metabolite ID. According to the identification results of the chemical components of QSG [9], the three metabolites were position isomers distinguished by different conjugating sites on the three hydroxyl groups of naringenin; according to their ClogP values, they were identified as naringenin-5-O-GluA (ClogP, −0.05), naringenin-7-O-GluA (ClogP, 0.41) and naringenin-4′-O-GluA (ClogP, 0.47). Similarly, 20, 24, 36, 52, and 57 were identified as glucuronidation metabolites of liquiritin apioside, calycosin-7-O-D-glucoside, calycosin, formononetin, and 3′-methoxy-luteolin. 43 was a sulfation metabolite of calycosin.
A total of 16 phenolic acid metabolites including 11 phase II and 5 phase I metabolites were identified. 2–5, 7, 15, 21, and 28 were identified as sulfonic acid conjugates due to the neutral loss of SO3 residues (79.9568 Da). With their identical precursor ions at m/z 232.9771 and similar fragment ions of 2 and 4 indicating that they are isomers, they were further confirmed as protocatechuic acid-3-O-SO3 and protocatechuic acid-4-O-SO3 with substitution positions at the two hydroxyl sites. 5 and 7 were confirmed to be sulfation metabolites of vanillic acid. 3 and 15 were recognized as sulfation metabolites of danshensu and caffeic acid. 11, 17, and 19 were metabolites of glucuronidation, confirmed by the neutral loss of 176.0321 Da. 17 and 19, two isomers, were identified as (E)-ferulic acid-4-O-GluA and (Z)-ferulic acid-4-O-GluA based on their ClogP values. Similarly, 21 and 28 were characterized as (E)-ferulic acid-4-O-SO3 and (Z)-ferulic acid-4-O-SO3. The fragment ions of 11, such as m/z 179.0357 and 135.0450, confirmed that it is the glucuronidation metabolite of caffeic acid.
91, 93, 94, 96, and 98–101 were characterized as metabolites of tanshinone IIA, and the metabolized reactions mainly involve hydration, hydrogenation, and demethylation. For example, 94 and 101 were screened out as the hydration+hydrogenation metabolites of tanshinone IIA, and the fragment ion at m/z 297.1494, which is 2 Da higher than tanshinone IIA, was generated by the neutral loss of H2O; 94 and 101 were thus confirmed as the hydration+hydrogenation product of tanshinone IIA. 96, which was observed at m/z 301.1439 [M+H]+, is 15 Da lower than 94 and 101, with similar fragmentation patterns indicating that they are isomers of the hydration+hydrogenation+demethylation products of tanshinone IIA. These three metabolites (94, 96 and 101) were first reported in this study, and they were identified as potential new ones; however, detailed structure information still needs more research. Moreover, 93 and 100 were screened as hydrogenation metabolites of tanshinone IIA, 98 and 99 as hydroxylation+demethylation and hydroxylation metabolites of tanshinone IIA, and 91 as a hydration metabolite of tanshinone IIA.
Five alkaloid-related metabolites (71, 72, 77, 79) were identified by Metabolite ID, all of which were isomers of the prototype components in QSG, though their specific structures could not be determined yet. Two metabolite isomers related to rosmarinic acid (45 and 51) were identified, which were the methylation metabolites of rosmarinic acid with different reaction positions.
2.2. Anti-CHF Screening and Experimental Verification
2.2.1. Molecular Docking Assessments
Among the 101 identified components, the structures of 22 could not be accurately determined. The remaining 79 small molecules (Table S1) were therefore eventually utilized for subsequent molecular docking assessment with 36 target proteins (Table S2). As a result, a total of 49 components, including 24 prototypes and 25 metabolites, with a total score ≥ 7 for at least one target protein were screened. Tables S3 and S4 show the molecular docking results of 49 components with 24 target proteins. Twenty-five metabolites primarily originated from these 24 prototypes; these prototype components were therefore identified as potential active constituents closely associated with CHF. Figure 2 displays the associations of the 24 prototypes with the top 10 target proteins. Five components, namely calycosin, calycosin-7-O-glucoside, formononetin, formononetin-7-O-glucoside, and astragaloside IV, are derived from ZHQ. Salvianolic acid B was derived from DS, and hypaconitine was derived from HSP. Nine components including luteolin, luteoloside, neochlorogenic acid, cryptochlorogenic acid, chlorogenic acid, isochlorogenic acid C, 7-epi-loganin, secoxyloganin, and secologanoside were derived from JYH; 10-methoxyl catalpol, morroniside, and angoroside C were three components derived from XS. The five components of isoliquiritigenin, liquiritigenin, liquiritin, isoliquiritin, and liquiritin apioside were derived from ZGC.
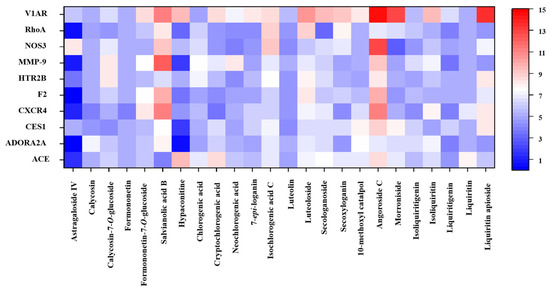
Figure 2.
Heatmap of molecular docking results for 24 components with 10 core targets.
Here, the bindings between salvianolic acid B and the core proteins related to myocardial fibrosis are introduced as an example. ROCK1 and ROCK2 were the key molecules in the RhoA/ROCK signaling pathway; as shown in Figure 3, salvianolic acid B could tightly bind to ROCK1 and ROCK2 (Total score > 10), and electrostatic, van der Waals, and covalent bond interactions were mainly formed between the amino acid residues of the target proteins and the nucleus of salvianolic acid B. The complexes of salvianolic acid B with ROCK1 and ROCK2 were stabilized by interactions with amino acid residues LYS D:200, THR D:219, ASP D:216, GLY D:218, PHE D:87, GLU D:154, MET D:156 and ASN D:219, LYS A:216, GLU A:170 MET A:172, LYS A:121, ASP A: 218, and ILE A:98, as well as by hydrogen bonds. Salvianolic acid B formed one pi–sigma interaction with ASP D: 216 in ROCK 1. Docking results of salvianolic acid B and other components with target proteins are listed in Tables S3 and S4. It is worth mentioning that salvianolic acid B is present in the highest concentrations of all components in QSG as well as in the QSG-administered plasma among all target compounds (Table S5); thus it is considered the key active compound in QSG, which is worthy of in-depth research.
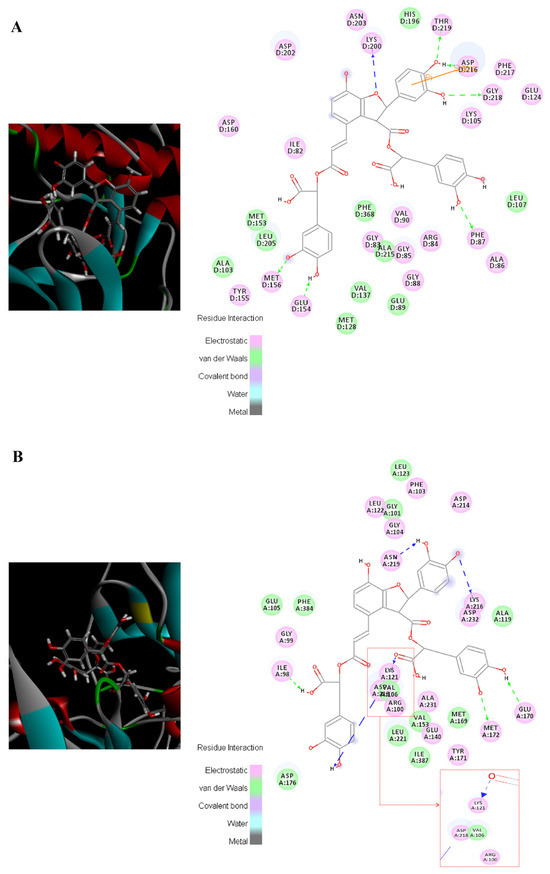
Figure 3.
Visualization of the binding of salvianolic acid B with ROCK1 and ROCK2. (A) ROCK1; (B) ROCK 2.
2.2.2. Experimental Verification by Oxygen Glucose Deprivation/Re-Oxygenation (OGD/R)-Induced H9c2 Cells
Firstly, CCK-8 results demonstrated that most components exhibited no cytotoxicity toward H9c2 cells (Table S6). Notably, treatment with cryptochlorogenic acid, isochlorogenic acid C, and salvianolic acid B significantly enhanced cell viabilities compared to the control group, indicating their potent pro-proliferative effects. In the OGD/R model, H9c2 cell viability showed a significant decline (p < 0.001), which confirmed the successful induction of cell injury and apoptosis.
Afterward, the 24 screened potentially effective components toward CHF were verified by assessing the protective effects in alleviating OGD/R injury on rat myocardial H9c2 cells. Results suggested that 14 analytes, which were cryptochlorogenic acid, isochlorogenic acid C, salvianolic acid B, luteolin, loganin, morroniside, isoliquiritigenin, chlorogenic acid, neochlorogenic acid, angoroside C, luteoloside, liquiritin apioside, formononetin, and astragaloside IV, could significantly improve H9c2 cell survival rates at different concentrations, as shown in Figure 4 (the pharmacological activities for the remaining 10 compounds are shown in Figure S2). Among them, astragaloside IV and formononetin, which originated from the monarch drug ZHQ, exhibited significant protective effects at 50 and 100 μM. Salvianolic acid B in ministerial drug DS, which showed the highest content in vitro and in vivo, exerted strong cardioprotective activity, thus highlighting its crucial role in the therapeutic effects of QSG. The “jiedu” principle embodied by adjunct drugs JYH and XS distinguishes QSG from other cardiovascular formulas [21]. As could be seen in the results, chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, isochlorogenic acid C, luteolin, luteoloside, and 7-epi-loganin in JYH, as well as angoroside C and morroniside in XS, significantly enhanced cardiomyocyte survival, which validated their efficacy-enhancing roles in CHF treatment. Moreover, isoliquiritigenin and liquiritin apioside, two active constituents, originated from the courier drug ZGC.
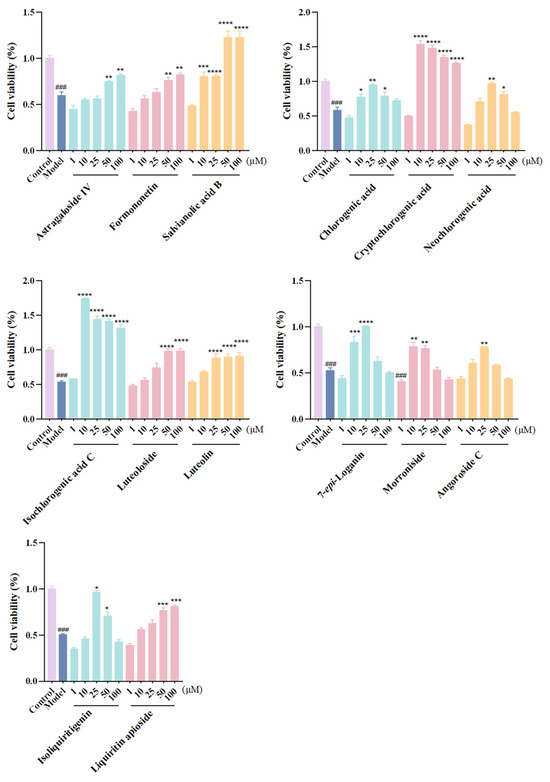
Figure 4.
Anti-CHF effects of active ingredients on OGD/R-induced H9c2 cells. Data are expressed as the mean ± SD. n = 6; ###, p < 0.001; compared with the control group. ****, p < 0.0001; ***, p < 0.001; **, p < 0.01; *, p < 0.05; compared with the model group.
It is reported that astragaloside IV could activate PPARα, shifting energy metabolism from glycolysis toward fatty acid β-oxidation and promoting angiogenesis [22,23]. Formononetin reduces myocardial ischemia/reperfusion injury by alleviating thrombosis and inflammation [24]. Salvianolic acid B markedly preserves left ventricular structure and cardiac function through its action on matrix metalloproteinase-9 [25]. Chlorogenic acid protects cardiomyocytes from TNF-α-induced injury by targeting NF-κB and JNK signaling pathways [26,27]. Cryptochlorogenic acid enhances hemodynamic function and ameliorates cellular morphology in myocardial tissues subjected to ischemia/reperfusion [28]. Both neochlorogenic acid and isochlorogenic acid C exhibit protective effects on H9c2 cells against ISO-induced injury [29]. Luteolin safeguards myocardial cells by suppressing apoptosis and oxidative stress [30]. Luteoloside mitigates damage to myocardial cells caused by hypoxia/reoxygenation [31]. 7-epi-loganin inhibits angiotensin II-induced cardiac hypertrophy [32]. Angoroside C improves ventricular remodeling in rats under pressure overload [33]. Morroniside promotes cardiac repair and exerts cardioprotective effects following myocardial infarction in adult rats [34]. Isoliquiritigenin protects against heart failure in mice via anti-inflammatory and anti-remodeling mechanisms [35].
All 14 active components, representing either quality control marker compounds or high-content constituents in their respective herbs, demonstrated protective effects against OGD/R injury and should be the material basis for the anti-CHF efficacy of QSG.
2.3. In Vivo Exposure Determination of Screened Active Components by Pharmacokinetic Study
Previous studies suggested that of the 14 active compounds, 7-epi-loganin, morroniside, and angoroside C exhibit relatively lower exposure levels in vivo (Table S5); thus the other 11 components were applied for the pharmacokinetic study to explore their exposures as well as other kinetic parameters.
2.3.1. Method Validation
Figure 5 displays the representative chromatograms of the blank plasma sample, quality control (QC) plasma sample, and plasma sample obtained 30 min after QSG treatment, with no apparent interference from the endogenous matrix interfering with the quantitation of both the analytes and internal standard (IS). Compound parameters, such as ion transitions, fragmentors, and collision energies (CEs), are summarized in Table S7. Calibration curves are presented in Table S8, and the correlation coefficients (R2) were all above 0.9973. Limits of detection (LODs) for 11 analytes ranged from 0.01 to 1.0 ng/mL. As shown in Table S9, the relative standard deviation (RSD) of intra-day and inter-day precision varied within the ranges of 1.02–13.68% and 2.59–12.02%, respectively. The accuracy ranged from 85.10 to 114.70% and 85.32 to 114.74%. Results of stability tests showed that the RSDs of all analytes were within 15% and the accuracy data ranged from 85.10% to 114.83% (Table S10), indicating good stabilities. As illustrated in Table S11, matrix effects ranged from 85.12% to 108.33%, and the recoveries of these 11 analytes ranged from 85.81% to 114.73%.
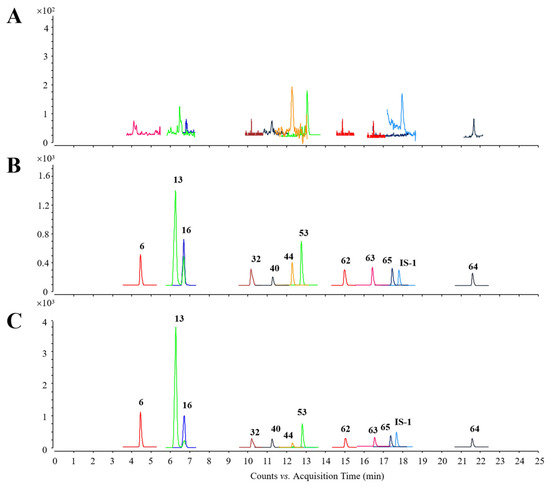
Figure 5.
Representative chromatograms. (A) Blank plasma sample. (B) QC plasma sample. (C) Plasma sample obtained 30 min after QSG treatment.
2.3.2. Pharmacokinetic Analysis
The developed method was applied for pharmacokinetic investigations of 11 analytes in rats after oral administration of 7 g/kg QSG. Figure 6 shows the mean plasma concentration–time curves of all analytes, with the key parameters such as area under the curve (AUC0→∞) summarized in Table 3.
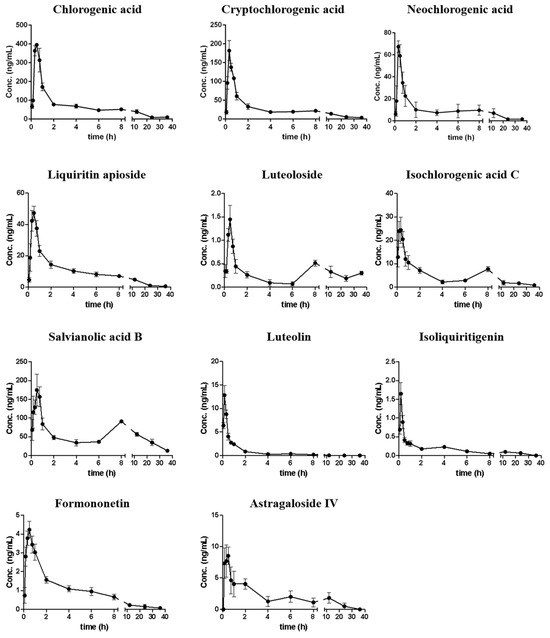
Figure 6.
Mean (SD, n = 6) plasma concentration–time curves of 11 analytes after oral administration of QSG in rats.

Table 3.
Pharmacokinetic parameters of analytes after oral administration of QSG in rats (n = 6).
It was observed that 11 compounds could be detected in plasma within 5 min, and most compounds reach their Cmax within 40 min, which indicated that these compounds could be rapidly absorbed into the circulation system. In addition, the mean residence time (MRT) of each analyte ranged from 1.839 to 16.01 h, with most compounds retained for more than 5 h. Among them, the MRTs of luteoloside and salvianolic acid B were more than 10 h, indicating that they were retained in the circulation system for a relatively long time; however, most compounds could be eliminated within 36 h. Double peaks were observed for luteoloside, isoliquiritigenin, isochlorogenic acid C, salvianolic acid B, and astragaloside IV. They reached the first peak at 10–30 min, and then reached the second peak at 4–12 h, with the concentrations of their second peak lower than those of the first peak, which might be attributed to the hepatic and intestinal circulations. In addition, the second peak for compounds like isoliquiritigenin might also be due to the glycolysis of liquiritin apioside, liquiritin, and isoliquiritin existing in QSG [36].
AUC0→∞ results suggested that salvianolic acid B exhibited the highest in vivo exposure level of 2176 ± 842.3 ng h/mL, accounting for 43.48% of the total exposures of all analytes. The exposure levels of four chlorogenic acid compounds (chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, and isochlorogenic acid C) accounted for another 46.76%. By comparing the exposure level (AUC0→∞) with the dosage of each compound, the oral relative bioavailability was obtained (Table S12). Among them, astragaloside IV had relatively higher absorption efficiency. It is worth noting that although salvianolic acid B had the highest content in QSG and the highest exposure level in vivo, the bioavailability was much lower than most compounds, which might be related to the physicochemical properties and/or the oversaturation condition for salvianolic acid B in vivo. Most flavonoid aglycones had a higher absorption rate than flavonoid glycosides, which might have resulted from the hydrolysis of glycosides into their aglycones in vivo. The four chlorogenic acid analogs exhibited markedly different absorption efficiencies. Notably, neochlorogenic acid and cryptochlorogenic acid demonstrated relatively higher absorption rates, whereas isochlorogenic acid C and chlorogenic acid were less readily absorbed. These results suggested that neochlorogenic acid and cryptochlorogenic acid might represent the dominant conformers in vivo. The other two compounds may undergo hydrolysis or isomerization to be converted into these two analogs. Overall, the results are helpful in understanding the kinetic parameters of active compounds of QSG and provide scientific data for ECI calculation.
2.4. Exploration of the Effective Components by the Efficacy- and In Vivo Exposure-Oriented Integrated Method
According to the in vitro bioeffect assay, the EC50 values for 11 constituents were calculated and are presented in Table 4; the ranking of the 11 compounds is clearly different from the AUC0→∞ ranking. It is difficult to identify the key effective components in the anti-CHF activities of QSG according to the distinct bioeffects and in vivo exposures.

Table 4.
ECIs for 11 active compounds.
To address this challenge, we developed an efficacy- and in vivo exposure-oriented integrated strategy to identify the key active constituents of QSG, using a comparison of their ECIs. The ECI for each constituent was calculated as the product of its in vivo exposure (AUC0→∞) and a weighting factor (Wi) derived from its bioactivity (EC50). Since EC50 values are inversely related to compound activity, each constituent was assigned a normalized weight using the reciprocal of its EC50. We then applied Equation (1) to compute Wi from the EC50 values of the 11 compounds, and subsequently used Equation (2) to determine their respective ECIs by incorporating both Wi and AUC0→∞.
According to the results shown in Table 4, the key effective components could be readily recognized by their individual indicator of the ECI, which ranked active constituents by correlating bioeffects and dealing with the in vivo exposure differences. For example, salvianolic acid B exhibited the highest exposure level with a moderate bioeffect, while isocholorgenic acid C possessed lower exposure but the strongest bioeffect, and their contributions to the effective material basis were ranked as isocholorgenic acid C > salvianolic acid B by their ECIs. Consequently, cryptochlorogenic acid, chlorogenic acid, isochlorogenic acid C, neochlorogenic acid, and salvianolic acid B were preliminarily identified as the key active components of QSG, with each exhibiting an ECI > 5, collectively accounting for approximately 99% of the total ECIs.
3. Discussion
In this study, we developed and applied an integrated strategy oriented toward efficacy and in vivo exposure to identify effective constituents of QSG. Identifying effective compounds requires consideration of both high pharmacological activities and adequate in vivo exposures. Our results indicated, however, that exposure levels are not directly correlated with efficacies: high-exposure compounds such as salvianolic acid B showed relatively low activity, whereas isochlorogenic acid C showed a strong effect with lower exposure. To pinpoint key compounds combining substantial exposures with high pharmacological activities, we introduced the multi-criteria evaluation metric ECI to integrate both exposure and efficacy contributions. By incorporating bioactivities as weighting factors for in vivo exposure levels, five kinetic active components demonstrating both anti-CHF effects and elevated in vivo exposure levels were identified as key effective constituents of QSG. Consequently, the content of these active ingredients can be controlled to ensure the efficacy and safety of QSG, thereby enhancing the quality control in clinical applications.
To the best of our knowledge, this is the first study to combine the in vivo exposures of the constituents (considering the in vivo kinetic parameters) with their bioeffects for the exploration of the effective components in a TCM. However, there are also some limitations in our study; for instance, the bioeffects of the absorbed components were only judged by one single-cell model, and the ECI threshold for key effective components needs in-depth studies.
4. Materials and Methods
4.1. Chemicals and Materials
Chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, isochlorogenic acid C, calycosin-7-O-D-glucoside, formononetin-7-O-D-glucoside, salvianolic acid B, astragaloside II, astragaloside IV, morroniside, sweroside, angoroside C, luteolin, glycyrrhetic acid, 7-epi-loganin, secologanoside, secoxyloganin, and 10-methoxyl catalpol were obtained from Chengdu Herb purify Co., Ltd. (Chengdu, China). Formononetin, luteoloside, calycosin, liquiritin apioside, liquiritin, liquiritigenin, isoliquiritin, isoliquiritigenin, benzoylmesaconine, benzoylhypacoitine, hypaconitine, dihydrotanshinone I, and glycyrrhizic acid were purchased from Shanghai Standard Biotech Co. Ltd. (Shanghai, China). Benzoylaconine and tanshinone IIA were obtained from National Institutes for Food and Drug Control (Beijing, China). The purities of these standards were all above 98%.
LC-MS-grade acetonitrile, methanol, and formic acid were purchased from Fisher-Scientific (Fair Lawn, NJ, USA). Ultrapure water was prepared by a Milli-Q system (Millipore, Bedford, MA, USA).
QSG was prepared in our group following a previous protocol [9].
4.2. Instrument and Conditions
4.2.1. UHPLC-Q TOF-MS Conditions
An Agilent Q-TOF 6550 iFunnel mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) was operated using the following parameters according to our previous study [9]: the fragmentor and nebulizer were set at 380 V and 35 psig, temperatures of drying gas and sheath gas were 150 °C and 275 °C, flow rates of drying gas and sheath gas were 16 L/min and 11 L/min, capillary voltages were set as 4000 V (+) and 3500 V (−), the full scan mass range was m/z 100–1200, and PIS-MS/MS, following the previous protocols, was utilized for MS2 data acquisition. UHPLC was performed on an Agilent 1290 Infinity UHPLC system (Agilent Technologies, CA, USA). The separation of metabolites was achieved on an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm, Waters, Wexford, Ireland); 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) were used as the mobile phases, the flow rate was set as 0.4 mL/min, the column oven was maintained at 35 °C, and the gradient procedure was as follows: 0–10 min, 5–23% B; 10–15 min, 23–30% B; 15–20 min, 30–60% B; 20–25 min, 60–95% B; and 25–30 min, 95% B. The injection volume was set at 5 μL.
4.2.2. HPLC-QQQ-MS Conditions
An Agilent 6470 QQQ mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) via an electrospray ionization (ESI) interface was employed for analyte determination, with dynamic MRM mode in negative mode utilized. Ion source parameters were set as follows: temperatures of drying gas and sheath gas were 300 °C and 300 °C, flow rates of drying gas and sheath gas were 7 L/min and 11 L/min, the nebulizer was set at 35 psig, capillary voltage was 3500 V (−), and nozzle voltage was 2000 V (−). As for the HPLC domain, an Agilent 1260 Infinity HPLC system was utilized. Chromatographic separations were conducted on a ZORBAX Eclipse Plus C18 column (3.0 × 50 mm, 1.8 μm, Agilent Technologies, California, USA). The mobile phase was composed of 0.1% aqueous FA (A, (v/v)) and 0.1% formic acid methanol (B), the flow rate was set at 0.5 mL/min, and the column oven was maintained at 40 °C. The gradient elution was programmed as follows: 0–5 min, 5–25% B; 5–15 min, 25–55% B; 15–20 min, 55–85% B; 20–22 min, 85–95% B; and 22–25 min, 95% B. The injection volume was set at 10 μL.
4.3. Experimental Animals
Male Sprague Dawley rats (200 ± 20 g) were bought from Vital River Laboratory Animal Technology Co., Ltd. {(SCXK (Jing) 2021~0006, Beijing, China}. The rats were fed under a humidity of 50–70% and temperature of 22–25 °C with a light–dark cycle of 12 h. All animals were fasted overnight with free access to water prior to drug administration.
4.4. Identification of the Absorbed Components of QSG in Rat Plasma
4.4.1. Preparations of Standard Solutions and Plasma Samples
Stock standard solutions of 25 compounds were mixed and diluted to 500 ng/mL for compound confirmation. For metabolic profiling, 10 rats were randomly divided into the QSG group (n = 5) and control group (n = 5), and after a single oral administration of 7 g/kg of QSG or water, blood was collected from each rat at 0.5, 1, 4, 8, and 12 h. Afterward, blood samples of each time point were mixed in equal amounts, and 9 mL of acetonitrile was immediately added to 3 mL of the pooled plasma for protein precipitation. After 5 min of centrifugation (13,201× g, 4 °C), the supernatant was moved to a new vial for concentration, and the dried residue was reconstituted with 150 μL 50% aqueous acetonitrile (v/v) and centrifuged for another 5 min (13,201× g, 4 °C).
4.4.2. Metabolite Identification
A database of 213 chemical components including their MS and MS2 information was established for QSG based on results previously reported [9]; the database was further imported into Metabolite ID so as to predict possible metabolites of QSG in vivo. Then, the acquired data files of administered plasma were introduced into Metabolite ID, and parameters such as error tolerance (±10 ppm) and peak abundance threshold (>103) were set. Meanwhile, the data files of blank plasma were also imported into Metabolite ID; thus components detected in blank plasma samples could be automatically excluded, making the analysis results more reliable. As a result, compounds that met the requirements were extracted and highlighted, and MS/MS data of all targeted metabolites were further compared with their prototypes for structure identities.
4.5. Screening of the Anti-CHF Active Components
4.5.1. Molecular Docking Screening
To evaluate the binding affinity of the absorbed components to CHF-related target proteins, molecular docking studies were performed by using SYBYL-X software (version 2.0). The three-dimensional structures of the absorbed components were constructed and energy-minimized. The target proteins were acquired by combining the reported anti-CHF targets of QSG [3,4,5,6,7,37,38,39] and targets in the Therapeutic Target Database (https://db.idrblab.net/ttd/, accessed on 9 September 2023) of marketed drugs, and three-dimensional structures of these targets were acquired from the Protein Data Bank (https://www.rcsb.org/, accessed on 9 September 2023) database. Docking was performed using the Surflex-Dock Geom (SFXC) mode. The target protein was prepared by adding hydrogen atoms, followed by the removal of ligands, water molecules, and heteroatoms. The therapeutic effects were assessed by the docking scores; the higher the total score, the more stable the binding conformation. Typically, a total score ≥ 7 often denotes great docking [40]; thus molecules with docking scores above the threshold were considered to be potential effective components.
4.5.2. Bioassay Screening
Rat myocardial H9c2 cells (purchased from China Infrastructure of Cell Line Resources, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), Penicillin (100 U/mL), and Streptomycin (100 μg/mL) at 37 °C under a humidified atmosphere of 5% CO2 and 95% air. For OGD/R treatment, H9c2 cells were cultured in glucose-free DMEM under hypoxic conditions (95%N2 + 5%CO2) for 4 h at 37 °C followed by complete medium under normal conditions with air (95%) and CO2 (5%) for 2 h. A control group was maintained under normal culture conditions (with glucose-containing DMEM and 5% CO2) without undergoing OGD/R treatment. CCK8 was used to assess cell viability. After treatment, H9c2 cells were seeded in 96-well plates, with each well supplemented with 10 μL of CCK-8 solution and then incubated for 1 h. Finally, the absorbance at 450 nm was detected by a microplate reader (Model INFINITE 200 PRO, TECAN, Grodig, Austria).
When evaluating the cytotoxicities of the absorbed compounds, the H9c2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well; when cells reached 80% confluence, they were treated with 25 and 50 μM of the 24 analytes to screen the non-toxic concentration ranges. Afterward, the protective effects were verified by assessing the cell viability when treating with different concentrations (1, 10, 25, 50, and 100 μM) of the analytes prior to OGD/R treatment.
4.6. In Vivo Exposure Determination of Screened Active Components by Pharmacokinetic Study
4.6.1. Preparations of Standard Solutions and Plasma Samples
Working mixed standard solutions were made by diluting the stock solutions to a series of concentrations. QC samples were three concentration levels of calibration samples. Afterward, 10 μL of each working solution or QC solution was spiked into 100 μL blank plasma to generate calibration samples or QC samples with desired concentrations.
4.6.2. Method Development
Several columns and mobile phases were assayed to achieve satisfactory separations. Different biosample pretreatment methods, such as protein precipitation and liquid–liquid extraction, were also compared to enhance the recoveries of these analytes.
The developed method was fully carried out according to the FDA guidelines [41]. The calibration curve was plotted by the peak area ratio of analyte/IS against the theoretical concentration. Linearity for each analyte was assessed with over seven concentrations. Concentrations at a signal-to-noise ratio (S/N) of about 3 were determined as the LODs. The selectivity of the method was validated by comparing the blank plasma, QC plasma, and QSG-administered plasma samples. Precision and accuracy were examined by measuring six replicate QC samples on one day and two replicate QC samples on three consecutive days. The RSD should be within 15%. Stabilities such as in the short term, in the long term, and under three cycles of freeze–thaw assays were assessed by storing the QC samples at room temperature (25 °C) for 24 h, −80 °C for 30 days, −80 °C for 24 h and thawing at 25 °C for three cycles. The stability was acceptable when that of all analytes ranged from 85% to 115%, with the RSD not exceeding 15%. The matrix effects were evaluated by comparing the post-extracted samples with those of standard solutions. The recoveries were evaluated by calculating the peak areas between QC samples and spiked-after-extraction samples.
4.6.3. Pharmacokinetic Study
For the pharmacokinetic study, each rat (n = 6) received a single oral administration of 7 g/kg of QSG, and the collection time points were 0 (pre-dose), 0.03, 0.08, 0.17, 0.33, 0.5, 0.75, 1, 2, 4, 6, 8, 12, 24, and 36 h. The blood samples were immediately centrifugated at 1467× g (4 °C) for 20 min, and the homogenate supernatants were collected for the following analysis. Protein precipitation was carried out for each time point for plasma samples, calibration samples, or QC samples by introducing four volumes of acetonitrile containing 400 ng/mL of icariin. Then, 10 μL 10% aqueous ascorbic acid (v/v) was spiked into each sample to prevent the oxidation of phenolic compounds. The precipitates were removed by 10 min of centrifugation (13,201× g, 4 °C). An aliquot (450 μL) of the supernatant was transferred and dried under nitrogen vacuum. The residues were reconstituted with 100 μL of 50% aqueous methanol (v/v) and centrifuged for another 5 min at 13,201× g (4 °C).
The pharmacokinetic parameters were calculated by DAS software (version 2.0) for a non-compartmental analysis.
4.7. ECI Establishment
It is known that the greater the EC50 value, the lower the pharmacological activity. Thus, we calculated the protective effect coefficient for each constituent using the normalization reciprocal value of EC50 in Equation (1):
Wi refers to the weight coefficient for the bioeffect of each constituent in Table 4. EC50i is the EC50 of constituent i. n is the number of constituents. By inputting EC50 values of each constituent to the above equation, the ECI models of anti-CHF activity for each active compound can be derived, as shown in Equation (2):
ECIi = Wi × AUCi
In the equations shown above, ECIi represents the predicted protective effect in alleviating OGD/R injury on H9c2 cells. AUCi is the exposure of each active component in QSG.
5. Conclusions
In the present study, an efficacy- and in vivo exposure-oriented integrated strategy of metabolite profiling, molecular docking, in vitro bioassays, and in vivo pharmacokinetics was established to systematically characterize the absorbed components as well as reveal the key effective components of QSG. Five kinetic active components (cryptochlorogenic acid, chlorogenic acid, isochlorogenic acid C, salvianolic acid B, and neochlorogenic acid) that possess both pharmacological activities and higher exposure levels were regarded as the key effective substances of QSG. Overall, this study provides a comprehensive understanding of the effective components of QSG and could offer a novel strategy for systematically identifying the active components of other TCMs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18101584/s1. Figure S1: MS2 spectrums and proposed fragmentation pathways of liquiritin (A) and isoliquiritin (B); Figure S2: Anti-CHF effects of 10 ingredients on OGD/R-induced H9c2 cells; Table S1: Small molecules for molecular docking; Table S2: Target proteins for molecular docking; Table S3: Molecular docking results of the 49 components with the first 12 target proteins; Table S4: Molecular docking results of the 49 components with the last 12 target proteins; Table S5: Concentrations of 24 analytes in QSG-treated plasma (n = 5); Table S6: The effect of compounds on the cytotoxicities of H9c2 cells; Table S7: The retention times (tR), fragmentors, and collision energies (CEs) of the analytes; Table S8: Regression equations, linear ranges, and LODs of each analyte; Table S9: Precision and accuracy data for each analyte (n = 6); Table S10: Stability data for each analyte (n = 6); Table S11: Matrix effect and recovery data for three analytes (n = 6); Table S12: Oral relative bioavailabilities of components in QSG.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L.; software, T.W.; validation, C.C.; formal analysis, Y.H.; investigation, Y.X.; data curation, Y.T.; writing—original draft preparation, Y.L.; writing—review and editing, J.L.; visualization, J.G.; project administration, H.X.; funding acquisition, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant numbers 82204599, 82173957).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Laboratory Animals of Beijing University of Chinese Medicine (approval code: BUCM-2024090504-3185; approval date: 29 September 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | Area under the curve |
| CE | Collision energy |
| CHF | Chronic heart failure |
| DS | Danshen |
| ECI | Effect–constituent index |
| EC50 | Median effective concentration |
| HSP | Heishunpian |
| IS | Internal standard |
| JYH | Jinyinhua |
| LOD | Limit of detection |
| MRT | Mean residence time |
| OGD/R | Oxygen glucose deprivation/re-oxygenation |
| QC | Quality control |
| QSG | Qishen granule |
| RSD | Relative standard deviation |
| S/N | Signal-to-noise ratio |
| TCMs | Traditional Chinese medicines |
| XS | Xuanshen |
| ZGC | Zhigancao |
| ZHQ | Zhihuangqi |
References
- Wang, J.P.; Shi, J.; Wei, J.W.; Wang, J.; Gao, K.; Li, X.L.; Chen, J.X.; Li, S.J.; Zhao, H.H.; Wang, W. Safety and efficacy of Qishen granules in patients with chronic heart failure: Study protocol for a randomized controlled trial. Trials 2017, 18, 468. [Google Scholar] [CrossRef]
- Guo, S.Z.; Li, P.; Fu, B.Z.; Chuo, W.J.; Gao, K.; Zhang, W.X.; Wang, J.Y.; Chen, J.X.; Wang, W. Systems-biology dissection of mechanisms and chemical basis of herbal formula in treating chronic myocardial ischemia. Pharmacol. Res. 2016, 114, 196–208. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Q.Y.; Zhang, Y.; Zhang, N.; Lu, L.H.; Wu, Y.; Zhang, Q.; Wang, W.; Wang, Y.; et al. Qishen granules inhibit myocardial inflammation injury through regulating arachidonic acid metabolism. Sci. Rep. 2016, 6, 36949. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Ouyang, Y.L.; Yu, J.D.; Guo, S.Z.; Liu, Z.Y.; Li, D.; Han, J.; Wang, W. Cardioprotective effects of Qishenyiqi mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin-converting enzyme 2. Evid.-Based Complement. Altern. Med. 2012, 2012, 978127. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.Y.; Li, C.; Li, D.; Ouyang, Y.L.; Yu, J.D.; Guo, S.Z.; He, F.C.; Wang, W. Drug Target prediction based on the herbs components: The study on the multitargets pharmacological mechanism of Qishenkeli acting on the coronary heart disease. Evid.-Based Complement Altern. Med. 2012, 2012, 698531. [Google Scholar] [CrossRef]
- Xia, K.; Wang, Q.Y.; Li, C.; Zeng, Z.F.; Wang, Y.; Wang, W. Effect of QSKL on MAPK and RhoA pathways in a rat model of heart failure. Evid.-Based Complement Altern. Med. 2017, 2017, 3903898. [Google Scholar] [CrossRef]
- Lu, W.J.; Wang, Q.Y.; Sun, X.Q.; He, H.; Wang, Q.X.; Wu, Y.; Liu, Y.; Wang, Y.; Li, C. Qishen Granule improved cardiac remodeling via balancing M1 and M2 macrophages. Front. Pharmacol. 2019, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wang, Q.; Yang, X.; Ren, Y.; Jiao, S.; Zhu, Q.; Guo, D.; Xia, K.; Wang, Y.; Li, C.; et al. Qishen granule attenuates cardiac fibrosis by regulating TGF-β/Smad3 and GSK-3β pathway. Phytomedicine 2019, 62, 152949. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Liu, J.; Su, R.B.; Li, Q.; Chen, Y.J.; Yang, J.; Zhao, S.J.; Jia, Z.X.; Xiao, H.B. Pseudotargeted screening and determination of constituents in Qishen granule based on compound biosynthetic correlation using UHPLC coupled with high-resolution MS. J. Sep. Sci. 2020, 43, 1032–1042. [Google Scholar] [CrossRef]
- Zhou, H.; He, Y.; Zheng, Z.; Liu, Z.; Song, F.; Liu, S. Quantitative analysis and pharmacokinetic comparison of multiple bioactive components in rat plasma after oral administration of Qi-Shen-Ke-Li formula and its single-herb extracts using ultra-high-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2020, 34, e4959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, Y.; Zheng, Z.; Xing, J.; Liu, Z.; Pi, Z.; Liu, S. Pharmacokinetics and tissue distribution study of 18 bioactive components in healthy and chronic heart failure rats after oral administration of Qi-Shen-Ke-Li formula using ultra-high-performance liquid chromatography/triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e9060. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Sun, J.; Li, C.; Song, Y.; Li, J.; Tu, P.; Zhao, Y. Simultaneous determination of twenty-five compounds in rat plasma using ultra-high performance liquid chromatography-polarity switching tandem mass spectrometry and its application to a pharmacokinetic study. Molecules 2017, 22, 1853. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, L.; Fang, C.; Pan, M.; Cong, S.; Guo, Z.; Yang, X.; Liu, J.; Li, Y.; Xiao, H. A network-pharmacology-combined integrated pharmacokinetic strategy to investigate the mechanism of potential liver injury due to polygonum multiflorum. Molecules 2022, 27, 8592. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Qi, J. Identification of active markers of Chinese formula yupingfeng san by network pharmacology and HPLC-Q-TOF-MS/MS analysis in experimental allergic rhinitis models of mice and isolated basophilic leukemia cell line RBL-2H3. Pharmaceuticals 2025, 18, 540. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Y.; Li, F.; Chen, L.; Dong, Q.; Wang, J.; Gullen, E.A.; Cheng, Y.C.; Xiao, X. Promotion of quality standard of Chinese herbal medicine by the integrated and efficacy-oriented quality marker of Effect-constituent Index. Phytomedicine 2018, 45, 26–35. [Google Scholar] [CrossRef]
- Zhang, D.K.; Li, R.S.; Han, X.; Li, C.Y.; Zhao, Z.H.; Zhang, H.Z.; Yang, M.; Wang, J.B.; Xiao, X.H. Toxic constituents index: A toxicity-calibrated quantitative evaluation approach for the precise toxicity prediction of the hypertoxic phytomedicine-aconite. Front. Pharmacol. 2016, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Song, W.; Ji, S.; Wang, Q.; Guo, D.A.; Ye, M. Separation and characterization of phenolic compounds and triterpenoid saponins in licorice (Glycyrrhiza uralensis) using mobile phase-dependent reversed-phase×reversed-phase comprehensive two-dimensional liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2015, 1402, 36–45. [Google Scholar]
- Hu, L.F.; Yao, Z.H.; Qin, Z.F.; Liu, L.Y.; Song, X.J.; Dai, Y.; Kiyohara, H.; Yamada, H.; Yao, X.S. In vivo metabolic profiles of Bu-Zhong-Yi-Qi-Tang, a famous traditional Chinese medicine prescription, in rats by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 171, 81–98. [Google Scholar] [CrossRef]
- Miao, W.J.; Wang, Q.; Bo, T.; Ye, M.; Qiao, X.; Yang, W.Z.; Xiang, C.; Guan, X.Y.; Guo, D.A. Rapid characterization of chemical constituents and rats metabolites of the traditional Chinese patent medicine Gegen-Qinlian-Wan by UHPLC/DAD/qTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, Q.; Wang, S.; Miao, W.J.; Li, Y.J.; Xiang, C.; Guo, D.A.; Ye, M. Compound to extract to formulation: A knowledge-transmitting approach for metabolites identification of Gegen-Qinlian Decoction, a traditional Chinese medicine formula. Sci. Rep. 2016, 6, 39534. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Q.; Tian, C.; Li, C.; Lin, Y.Z.; Gao, W.X.; Wu, D.F.; Jiao, N.; Zhu, L.X.; Li, W.Z.; et al. The roles of Qishen granules recipes, Qingre Jiedu, Wenyang Yiqi and Huo Xue, in the treatment of heart failure. J. Ethnopharmacol. 2020, 249, 112372. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhao, P.; Xu, M.; Zhang, C.; Guo, W.; Chen, H.; Tian, J.; Wei, H.; Lu, R.; Cao, T. Astragaloside IV alleviates heart failure via activating PPARα to switch glycolysis to fatty acid β-oxidation. Sci. Rep. 2017, 7, 2691. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.B.; Wang, Y.; Liu, L.; Liu, F.; Zhang, Y.Q. Astragaloside IV alleviates heart failure by promoting angiogenesis through the JAK-STAT3 pathway. Pharm. Biol. 2019, 57, 48–54. [Google Scholar] [CrossRef]
- Tang, S.; Ye, J.X.; Li, R.Y.; Wang, J.L.; Xie, H.C.; Zhang, Y.Q.; Wang, M.; Sun, G.B. Formononetin attenuates myocardial ischemia/reperfusion injury by regulating neutrophil extracellular traps formation and platelet activation via platelet CD36. Phytomedicine 2025, 141, 156736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Chen, J.; Xu, L.; Gao, Z.; Deng, Y.; Wang, Y.; Xu, F.; Shen, X.; Guo, D.A. Salvianolic acid B functioned as a competitive inhibitor of matrix metalloproteinase-9 and efficiently prevented cardiac remodeling. BMC Pharmacol. 2010, 10, 10. [Google Scholar] [CrossRef]
- Li, Y.; Shen, D.; Tang, X.; Li, X.; Wo, D.; Yan, H.; Song, R.; Feng, J.; Li, P.; Zhang, J.; et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014, 226, 257–263. [Google Scholar] [CrossRef]
- Tian, L.; Su, C.P.; Wang, Q.; Wu, F.J.; Bai, R.; Zhang, H.M.; Liu, J.Y.; Lu, W.J.; Wang, W.; Lan, F.; et al. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. J. Cell. Mol. Med. 2019, 23, 4666–4678. [Google Scholar] [CrossRef]
- Tong, Y.; Li, G.; Shi, X.; Wang, L.; Zhou, J.; Chu, M.; Wang, Z.; Abd El-Aty, A.M.; Dang, J. Protection against myocardial ischemia/reperfusion injury in mice by 3-caffeoylquinic acid isomers isolated from Saxifraga tangutica. RSC Adv. 2024, 14, 6642–6655. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, X.H.; Li, L.; Su, C.P.; Zhang, Y.L.; Jiang, Y.Y.; Guo, S.Z.; Liu, B. Deciphering the effective combinatorial components from Si-Miao-Yong-An decoction regarding the intervention on myocardial hypertrophy. J. Ethnopharmacol. 2021, 271, 113833. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L.; Huang, J.; Xu, P.; Zhang, Z.; Yin, D.; Liu, J.; He, H.; He, M. Luteoloside attenuates anoxia/reoxygenation-induced cardiomyocytes injury via mitochondrial pathway mediated by 14-3-3η protein. Phytother. Res. 2018, 32, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Li, R.J.; Zhang, Z.H.; Yang, C.; Liu, S.X.; Li, Y.L.; Chen, M.W.; Wang, W.W.; Zhang, G.Y.; Song, G.; et al. Loganin inhibits angiotensin II-induced cardiac hypertrophy through the JAK2/STAT3 and NF-κB signaling pathways. Front. Pharmacol. 2021, 12, 678886. [Google Scholar] [CrossRef]
- Gu, W.L.; Chen, C.X.; Huang, X.Y.; Gao, J.P. The effect of angoroside C on pressure overload-induced ventricular remodeling in rats. Phytomedicine 2015, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Liu, T.; Chen, M.; Sun, F.; Fei, Y.; Chen, Y.; Tian, X.; Wu, Z.; Zhu, Z.; Zheng, W.; et al. Morroniside induces cardiomyocyte cell cycle activity and promotes cardiac repair after myocardial infarction in adult rats. Front. Pharmacol. 2023, 14, 1260674. [Google Scholar] [CrossRef]
- Zhang, L.; Luan, Y.; Ding, X.; Yang, C.; Xing, L.; Zhang, H.; Liu, Z. Integration of network pharmacology and transcriptomics to explore the mechanism of isoliquiritigenin in treating heart failure induced by myocardial infarction. Toxicol. Appl. Pharmacol. 2024, 492, 117114. [Google Scholar] [CrossRef]
- Qiao, X.; Ye, M.; Xiang, C.; Wang, Q.; Liu, C.F.; Miao, W.J.; Guo, D.A. Analytical strategy to reveal the in vivo process of multi-component herbal medicine: A pharmacokinetic study of licorice using liquid chromatography coupled with triple quadrupole mass spectrometry. J. Chromatogr. A 2012, 1258, 84–93. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Ouyang, Y.; Shi, T.; Yang, X.; Yu, J.; Qiu, Q.; Han, J.; Wu, Y.; Tang, B.; et al. QSYQ attenuates oxidative stress and apoptosis induced heart remodeling rats through different subtypes of NADPH-Oxidase. Evid.-Based Complement Altern. Med. 2013, 2013, 824960. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Qiu, Q.; Shi, T.; Wu, Y.; Han, J.; Chai, X.; Wang, W. Qishenyiqi protects ligation-induced left ventricular remodeling by attenuating inflammation and fibrosis via STAT3 and NF-κB signaling pathway. PLoS ONE 2014, 9, e104255. [Google Scholar] [CrossRef]
- Chang, H.; Li, C.; Wang, Q.; Lu, L.; Zhang, Q.; Zhang, Y.; Zhang, N.; Wang, Y.; Wang, W. QSKL protects against myocardial apoptosis on heart failure via PI3K/Akt-p53 signaling pathway. Sci. Rep. 2017, 7, 16986. [Google Scholar] [CrossRef]
- Guan, H.R.; Li, B.; Zhang, Z.H.; Wu, H.S.; He, X.L.; Dong, Y.J.; Su, J.; Lv, G.Y.; Chen, S.H. Integrated bioinformatics and network pharmacology to explore the therapeutic target and molecular mechanisms of Bailing capsule on polycystic ovary syndrome. BMC Complement. Med. Ther. 2023, 23, 458. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Guidance for Industry: Bioanalytical Method; Validation US Department of Health and Human Services: Washington, DC, USA; Food and Drug Administration: Silver Spring, MD, USA; Center for Drug Evaluation and Research: Silver Spring, MD, USA; Center for Veterinary Medicine: Rockville, MD, USA, 2001.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).