Structure-Guided Discovery of Benzoic-Acid-Based TRPC6 Ligands: An Integrated Docking, MD, and MM-GBSA SAR Study: Potential Therapeutic Molecules for Autism Spectrum Disorder
Abstract
1. Introduction
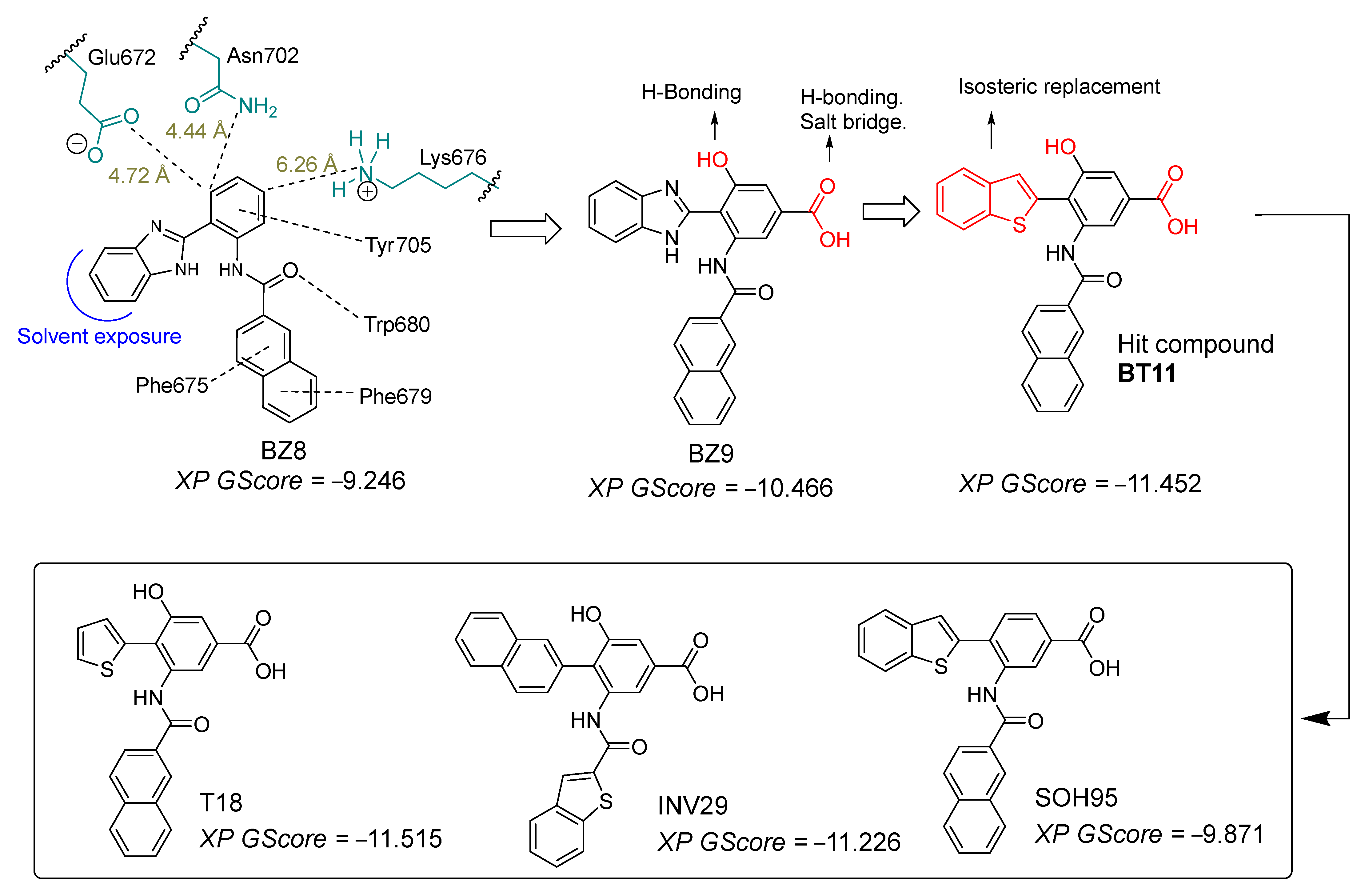
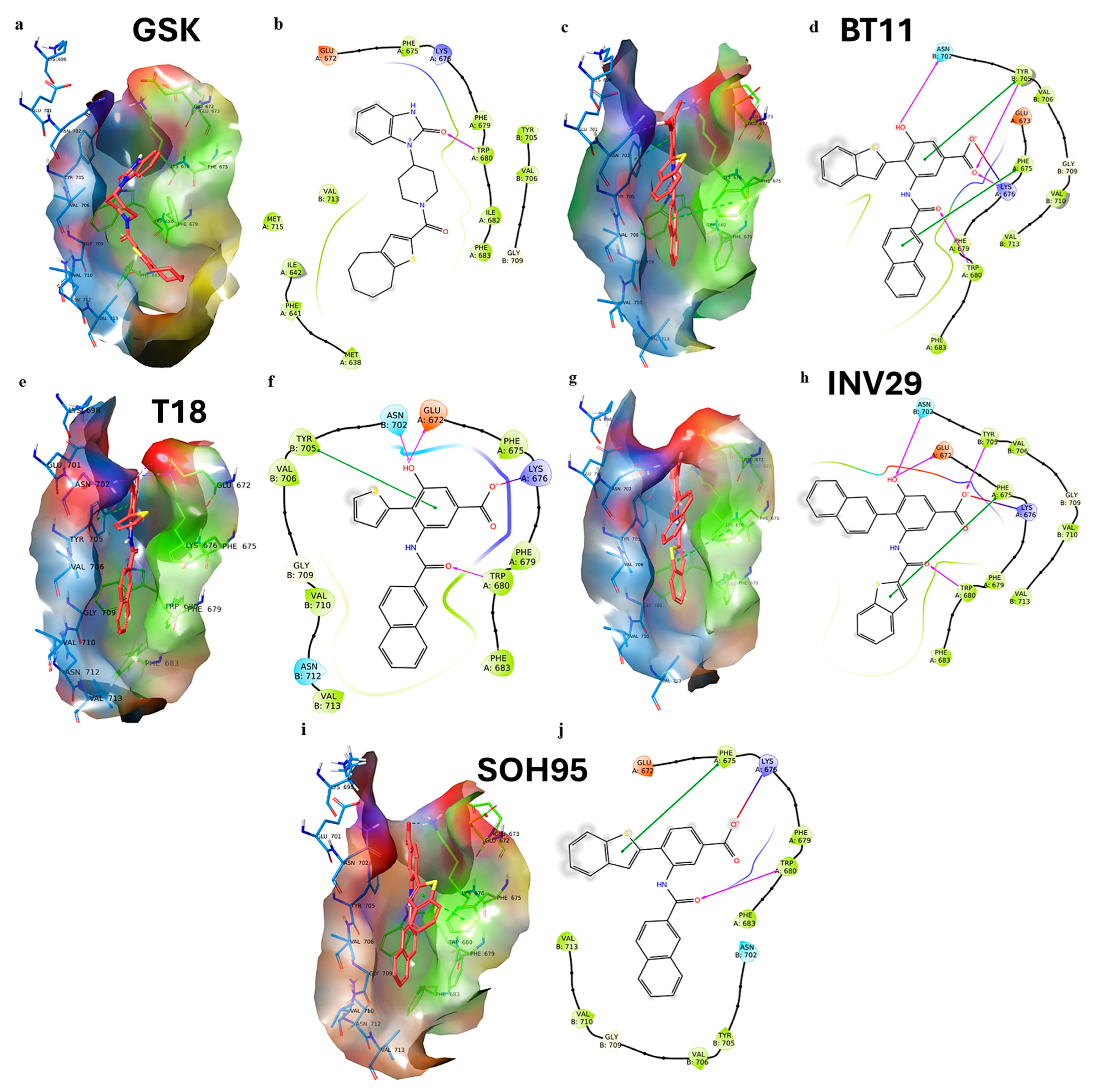
2. Results and Discussion
3. Materials and Methods
3.1. Molecular Docking
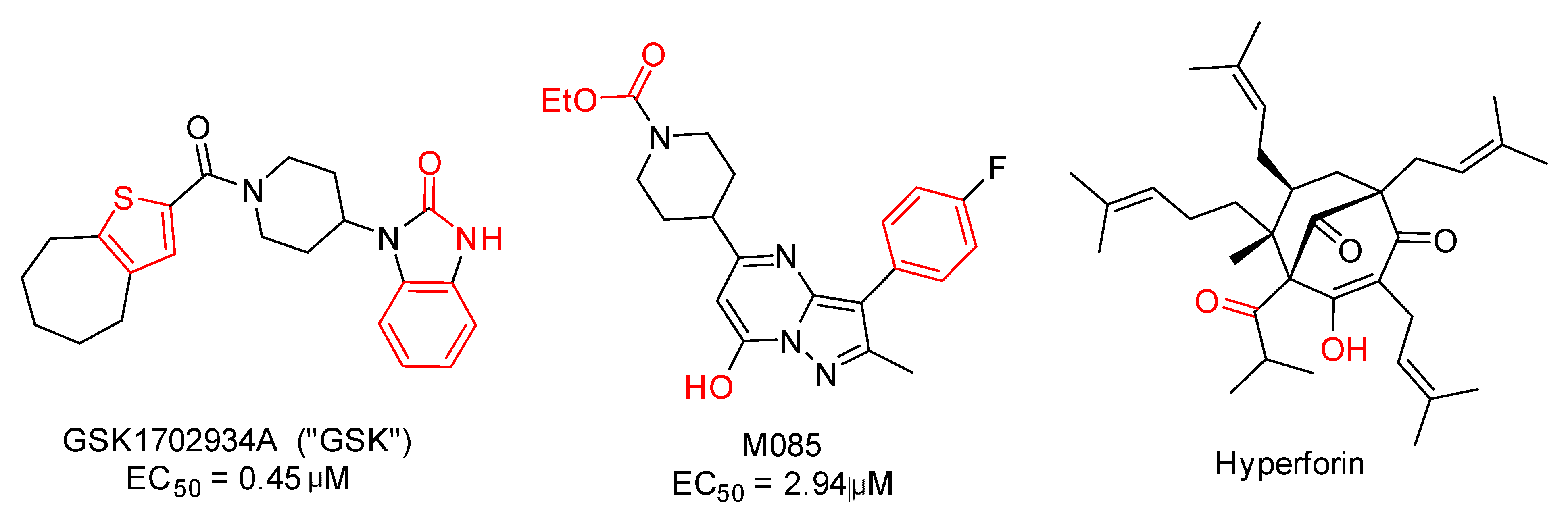
3.2. Initial Virtual Screening
3.3. Molecular Docking Validation
- Molecular Weight (MW), calculated Logarithm of the partition coefficient (cLogP), Topological Polar Surface Area (TPSA): within ±10% of the active’s value (continuous properties).
- Hydrogen Bond Donors (HBD) and Hydrogen Bond Acceptors (HBA): within ±1 of the active’s integer counts.
- Topological dissimilarity. Molecular fingerprints were computed as Morgan Extended-Connectivity Fingerprints (ECFP-like) with radius = 2 and 2048 bits; candidates were required to have Tanimoto similarity ≤ 0.40 to the active (structural dissimilarity constraint).
3.4. Molecular Dynamics Studies
3.4.1. Starting Structures
3.4.2. System Setup
3.4.3. Simulation Protocol
3.4.4. Interaction and Trajectory Analyses
3.4.5. Pore-Diameter Analysis
3.4.6. Molecular Dynamics Validation
3.5. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| Akt | Protein Kinase B |
| apo | Ligand-free |
| ASD | Autism Spectrum Disorder |
| AUC | Area Under Curve |
| BDNF | Brain-derived Neurotrophic Factor |
| CaMKIV | Calcium/Calmodulin-Dependent Protein Kinase IV |
| CI | Confidence Interval |
| cLogP | Calculated Logarithm of the Partition Coefficient |
| CREB | cAMP Response Element–Binding Protein |
| cryo-EM | Cryo-electron Microscopy |
| DAG | Diacylglycerol |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th edition |
| ECFP | Extended-Connectivity Fingerprints |
| EF, EF1%, EF2%, EF5% | Enrichment Factor at the Top 1%, 2%, or 5% of a Ranked List |
| GABA | Gamma-aminobutyric Acid |
| GSK | GSK1702934A |
| HBA | Hydrogen Bond Acceptors |
| HBD | Hydrogen Bond Donors |
| IFD | Induced Fit Docking |
| IGF-1 | Insulin-like Growth Factor 1 |
| iPSC | Induced Pluripotent Stem Cells |
| KS | Kolmogorov–Smirnov |
| MD | Molecular Dynamics |
| mGluR5 | Metabotropic Glutamate Receptor 5 |
| MM-GBSA | Molecular Mechanics/Generalized Born Surface Area |
| MW | Molecular Weight |
| NPT | Isothermal–Isobaric Ensemble with Constant Number of Particles, Pressure and Temperature |
| OPLS3e | Optimized Potentials for Liquid Simulations, Version 3e |
| OPM | Orientations of Proteins in Membranes |
| PDB | Protein Data Bank |
| POPE | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine |
| PR-AUC | Area under the Precision–Recall Curve |
| PROPKA | PROtein pKa Prediction |
| PTPRD | Protein Tyrosine Phosphatase Receptor Delta |
| RMSD | Root-Mean-Square Deviation |
| ROC | Receiver Operating Characteristic |
| ROC-AUC | Area under the Receiver Operating Characteristic Curve |
| SAR | Structure–Activity Relationship |
| SASA | Solvent-Accessible Surface Area |
| SID | Simulation Interaction Diagram |
| TACA | The Autism Community in Action |
| TIP3P | Transferable Intermolecular Potential 3-Point water model |
| TPSA | Topological Polar Surface Area |
| TRPC6 | Transient Receptor Potential Canonical Type 6 |
| XP | Extra-Precision |
| XP GScore | Glide Extra-Precision Docking Score (Reported in kcal/mol) |
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism Spectrum Disorder: Definition, Epidemiology, Causes, and Clinical Evaluation. Transl. Pediatr. 2020, 9, S55. [Google Scholar] [CrossRef]
- Fernandez, B.A.; Scherer, S.W. Syndromic Autism Spectrum Disorders: Moving from a Clinically Defined to a Molecularly Defined Approach. Dialogues Clin. Neurosci. 2022, 19, 353–371. [Google Scholar] [CrossRef]
- Deckers, A.; Roelofs, J.; Muris, P.; Rinck, M. Desire for Social Interaction in Children with Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2014, 8, 449–453. [Google Scholar] [CrossRef]
- Brignell, A.; Chenausky, K.V.; Song, H.; Zhu, J.; Suo, C.; Morgan, A.T. Communication Interventions for Autism Spectrum Disorder in Minimally Verbal Children. CDSR 2018, 11, CD012324. [Google Scholar] [CrossRef]
- Grossi, E.; Caminada, E.; Goffredo, M.; Vescovo, B.; Castrignano, T.; Piscitelli, D.; Valagussa, G.; Franceschini, M.; Vanzulli, F. Patterns of Restricted and Repetitive Behaviors in Autism Spectrum Disorders: A Cross-Sectional Video Recording Study. Preliminary Report. Brain Sci. 2021, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.L.; Kozlowski, A.M. The Increasing Prevalence of Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2011, 5, 418–425. [Google Scholar] [CrossRef]
- AAP News; American Academy of Pediatrics. CDC: Autism Rate Rises to 1 in 36 Children. Available online: https://publications.aap.org/aapnews/news/23904/CDC-Autism-rate-rises-to-1-in-36-children (accessed on 10 October 2025).
- Im, D.S. Treatment of Aggression in Adults with Autism Spectrum Disorder: A Review. Harv. Rev. Psychiatry 2021, 29, 35–80. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, C.; Knudsen, J.; Leutscher, P.D.C.; Lauritsen, M.B.; Nyegaard, M.; Hagstrøm, S.; Sørensen, S. Gut Microbiota Profiles of Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder: A Systematic Literature Review. Gut Microbes 2020, 11, 1172–1187. [Google Scholar] [CrossRef]
- Cortese, S.; Wang, F.; Angriman, M.; Masi, G.; Bruni, O. Sleep Disorders in Children and Adolescents with Autism Spectrum Disorder: Diagnosis, Epidemiology, and Management. CNS Drugs 2020, 34, 415–423. [Google Scholar] [CrossRef]
- Carey, C.; Singh, N.; Dunn, J.T.; Sementa, T.; Mendez, M.A.; Velthuis, H.; Pereira, A.C.; Pretzsch, C.M.; Horder, J.; Hader, S.; et al. From Bench to Bedside: The MGluR5 System in People with and without Autism Spectrum Disorder and Animal Model Systems. Transl. Psychiatry 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. MGluR5-Antagonist Mediated Reversal of Elevated Stereotyped, Repetitive Behaviors in the VPA Model of Autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D.K.; Maloney, B.; Long, J.M.; Ray, B.; Lahiri, D.K. Autism, Alzheimer Disease, and Fragile, X. Neurology 2011, 76, 1344–1352. [Google Scholar] [CrossRef]
- Elia, J.; Gai, X.; Xie, H.M.; Perin, J.C.; Geiger, E.; Glessner, J.T.; D’Arcy, M.; Deberardinis, R.; Frackelton, E.; Kim, C.; et al. Rare Structural Variants Found in Attention-Deficit Hyperactivity Disorder Are Preferentially Associated with Neurodevelopmental Genes. Mol. Psychiatry 2010, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Choucair, N.; Mignon-Ravix, C.; Cacciagli, P.; Abou Ghoch, J.; Fawaz, A.; Mégarbané, A.; Villard, L.; Chouery, E. Evidence That Homozygous PTPRD Gene Microdeletion Causes Trigonocephaly, Hearing Loss, and Intellectual Disability. Mol. Cytogenet. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Choo, H.; Jeon, B. Serotonin Receptors as Therapeutic Targets for Autism Spectrum Disorder Treatment. Int. J. Mol. Sci. 2022, 23, 6515. [Google Scholar] [CrossRef]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The Serotonin System in Autism Spectrum Disorder: From Biomarker to Animal Models. Neuroscience 2016, 321, 24. [Google Scholar] [CrossRef]
- Cellot, G.; Cherubini, E. GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Front. Pediatr. 2014, 2, 70. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Thuras, P.D. GABAA Receptor Downregulation in Brains of Subjects with Autism. J. Autism Dev. Disord. 2009, 39, 223–230. [Google Scholar] [CrossRef]
- Palacios-Muñoz, A.; de Paula Moreira, D.; Silva, V.; García, I.E.; Aboitiz, F.; Zarrei, M.; Campos, G.; Rennie, O.; Howe, J.L.; Anagnostou, E.; et al. Mutations in Trpγ, the Homologue of TRPC6 Autism Candidate Gene, Causes Autism-like Behavioral Deficits in Drosophila. Mol. Psychiatry 2022, 27, 3328–3342. [Google Scholar] [CrossRef]
- Saqib, U.; Munjuluri, S.; Sarkar, S.; Biswas, S.; Mukherjee, O.; Satsangi, H.; Baig, M.S.; Obukhov, A.G.; Hajela, K. Transient Receptor Potential Canonical 6 (TRPC6) Channel in the Pathogenesis of Diseases: A Jack of Many Trades. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Griesi-Oliveira, K.; Acab, A.; Gupta, A.R.; Sunaga, D.Y.; Chailangkarn, T.; Nicol, X.; Nunez, Y.; Walker, M.F.; Murdoch, J.D.; Sanders, S.J.; et al. Modeling Non-Syndromic Autism and the Impact of TRPC6 Disruption in Human Neurons. Mol. Psychiatry 2014, 20, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Beltrão-Braga, P.C.B.; Muotri, A.R. Modeling Autism Spectrum Disorders with Human Neurons. Brain Res. 2017, 1656, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yu, X.; Chen, H.; Horne, D.; White, R.; Wu, X.; Lee, P.; Gu, Y.; Ghimire-Rijal, S.; Lin, D.C.H.; et al. Structural Basis for Pharmacological Modulation of the TRPC6 Channel. Elife 2020, 9, e53311. [Google Scholar] [CrossRef]
- Dietrich, A.; Gudermann, T. TRPC6: Physiological Function and Pathophysiological Relevance. Handb. Exp. Pharmacol. 2014, 222, 157–188. [Google Scholar]
- Wang, H.; Cheng, X.; Tian, J.; Xiao, Y.; Tian, T.; Xu, F.; Hong, X.; Zhu, M.X. TRPC Channels: Structure, Function, Regulation and Recent Advances in Small Molecular Probes. Pharmacol. Ther. 2020, 209, 107497. [Google Scholar] [CrossRef]
- Bellono, N.W.; Kammel, L.G.; Zimmerman, A.L.; Oancea, E. Characterization of Small Molecule TRPC3 and TRPC6 Agonist and Antagonists. Biophys. J. 2013, 104, 454a. [Google Scholar] [CrossRef]
- Yang, P.L.; Li, X.H.; Wang, J.; Ma, X.F.; Zhou, B.Y.; Jiao, Y.F.; Wang, W.H.; Cao, P.; Zhu, M.X.; Li, P.W.; et al. GSK1702934A and M085 Directly Activate TRPC6 via a Mechanism of Stimulating the Extracellular Cavity Formed by the Pore Helix and Transmembrane Helix S6. J. Biol. Chem. 2021, 297, 101125. [Google Scholar] [CrossRef] [PubMed]
- Mella-Raipán, J.A.; Lagos, C.F.; Recabarren-Gajardo, G.; Espinosa-Bustos, C.; Romero-Parra, J.; Pessoa-Mahana, H.H.; Iturriaga-Vásquez, P.; Pessoa-Mahana, C.D.; Mella-Raipan, J.A.; Lagos, C.F.; et al. Design, Synthesis, Binding and Docking-Based 3D-QSAR Studies of 2-Pyridylbenzimidazoles–a New Family of High Affinity CB1 Cannabinoid Ligands. Molecules 2013, 18, 3972–4001. [Google Scholar] [CrossRef] [PubMed]
- Pessoa-Mahana, D.; Espinosa-Bustos, C.; Mella-Raipán, J. Microwave-Assisted Synthesis and Regioisomeric Structural Derivatives. Arkivoc 2009, 2009, 131–140. [Google Scholar] [CrossRef]
- Vasquez, D.; Lagos, C.F.; Mella-Raipan, J.; Gonzalez, L.; Ebensperger, R.; Alvarez-Figueroa, M.J.; Saez, E.; Pessoa-Mahana, H.H.; Araya-Secchp, R.; Gonzalez-Wong, A.; et al. 1-Benzoyl-2-(2-Nitrophenyl)-1H-Benzimidazole Derivatives: A Novel Approach to the Development of New Hiv-1 Reverse Transcriptase Inhibitors. J. Chil. Chem. Soc. 2007, 52, 1281–1287. [Google Scholar] [CrossRef]
- Espinosa-Bustos, C.; Lagos, C.F.; Romero-Parra, J.; Zarate, A.M.; Mella-Raipan, J.; Pessoa-Mahana, H.; Recabarren-Gajardo, G.; Iturriaga-Vasquez, P.; Tapia, R.A.; Pessoa-Mahana, C.D.; et al. Design, Synthesis, Biological Evaluation and Binding Mode Modeling of Benzimidazole Derivatives Targeting the Cannabinoid Receptor Type 1. Arch. Pharm. 2015, 348, 81–88. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and Ligand Preparation: Parameters, Protocols, and Influence on Virtual Screening Enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A Software Program for PKa Prediction and Protonation State Generation for Drug-like Molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of the Crystal Environment in Determining Protein Side-Chain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Orientation of Proteins in Membranes: TRPC6 Channel 6uz8. Available online: https://opm.phar.umich.edu/proteins/5023 (accessed on 10 October 2025).

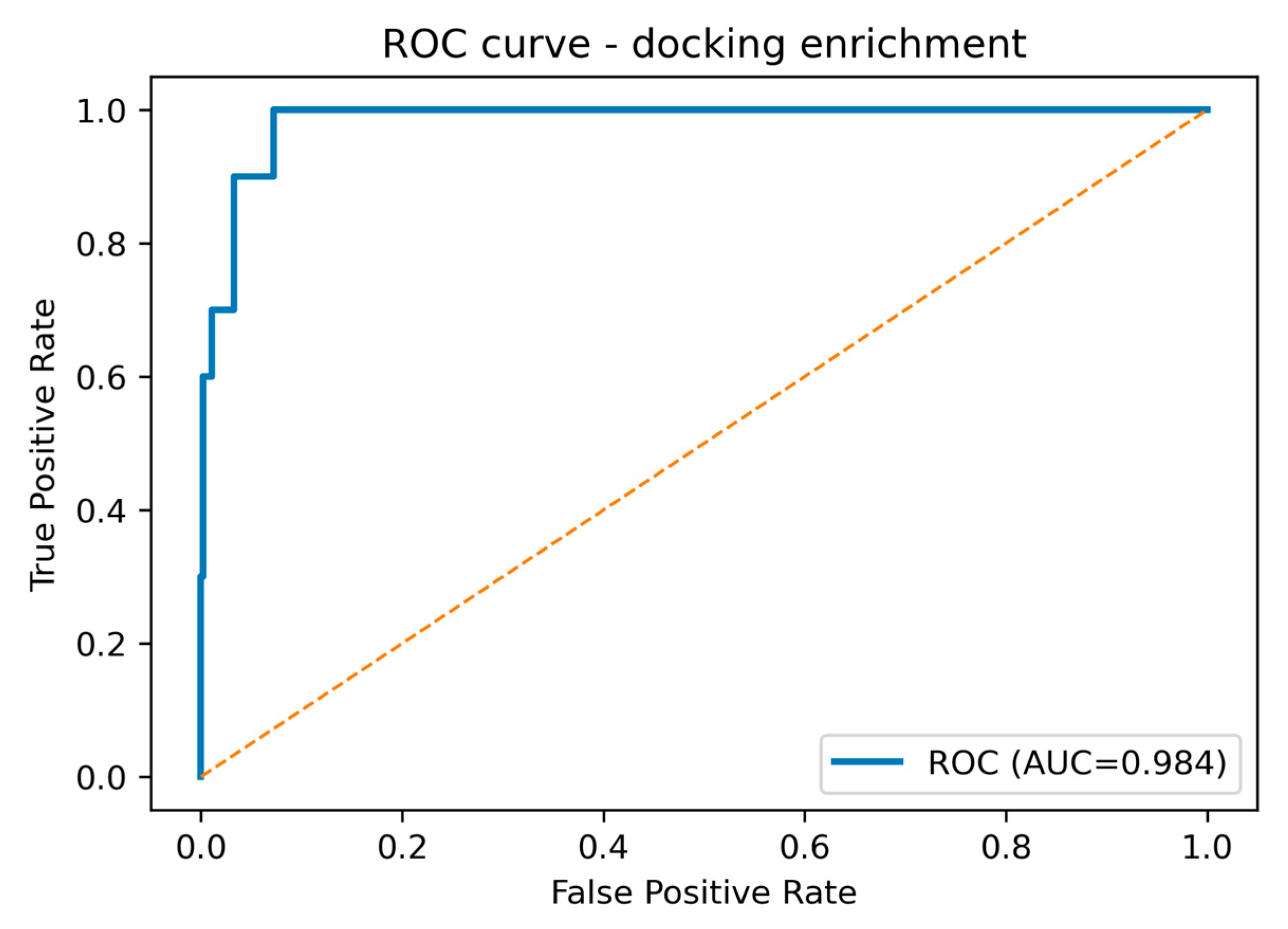
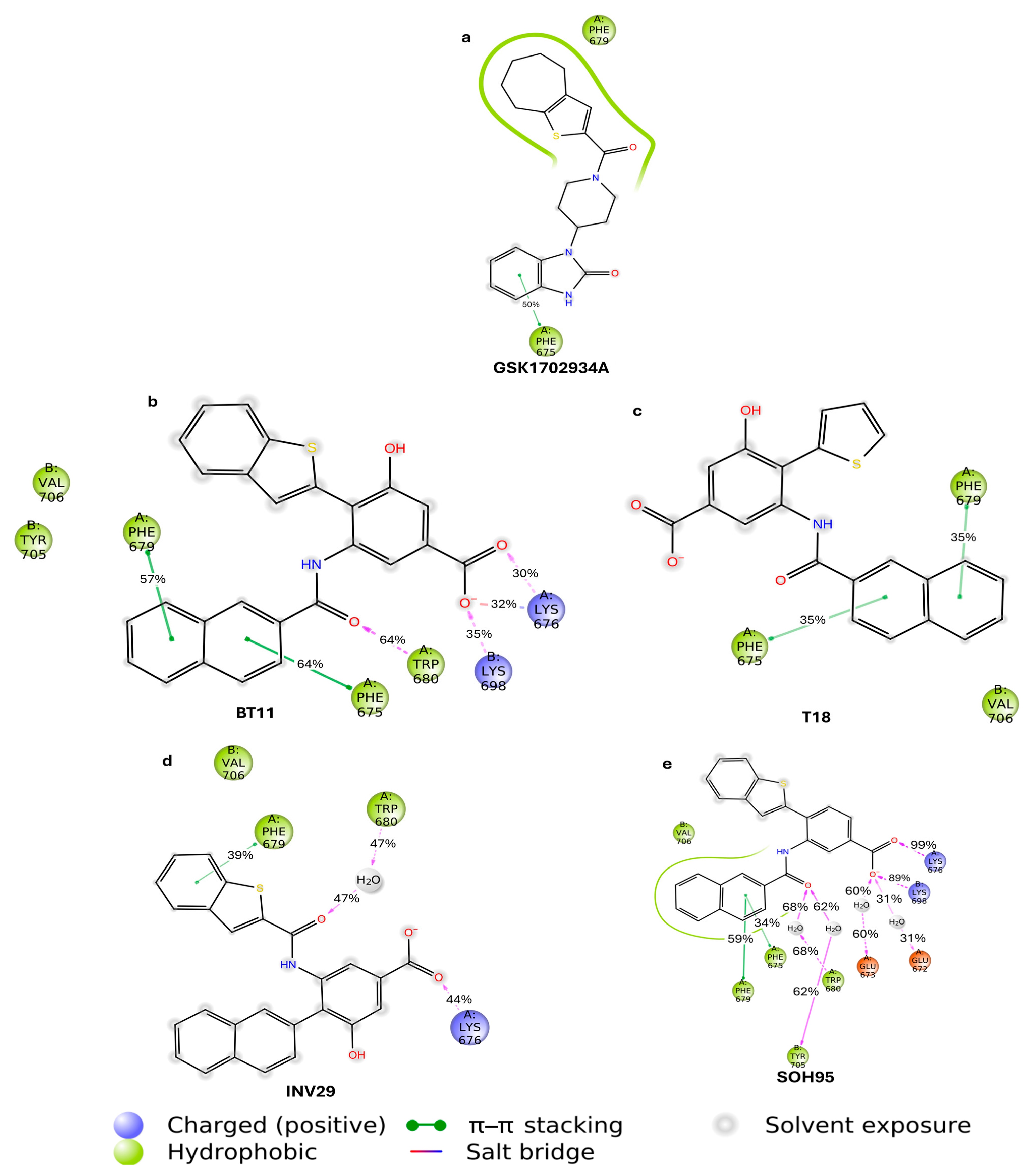
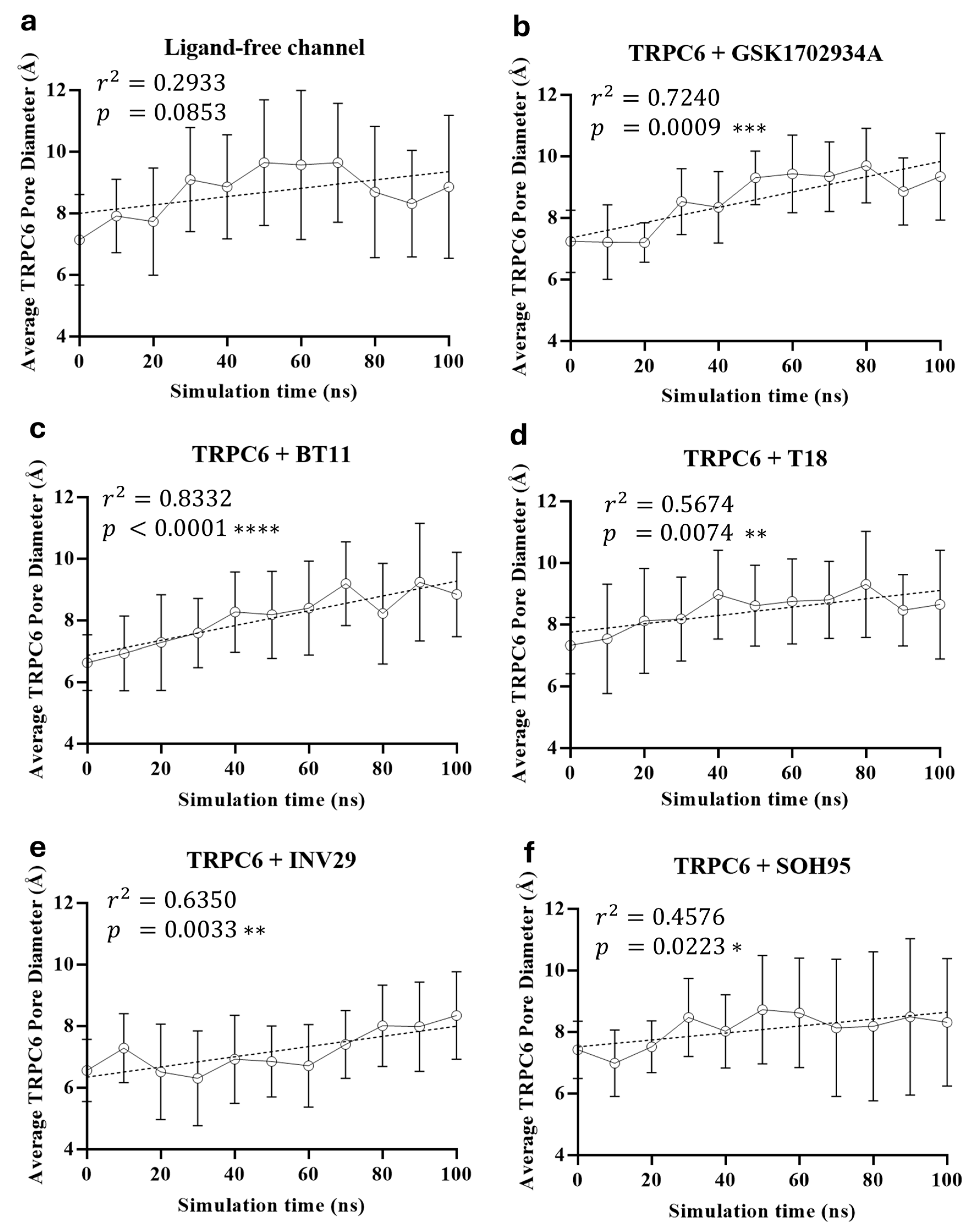
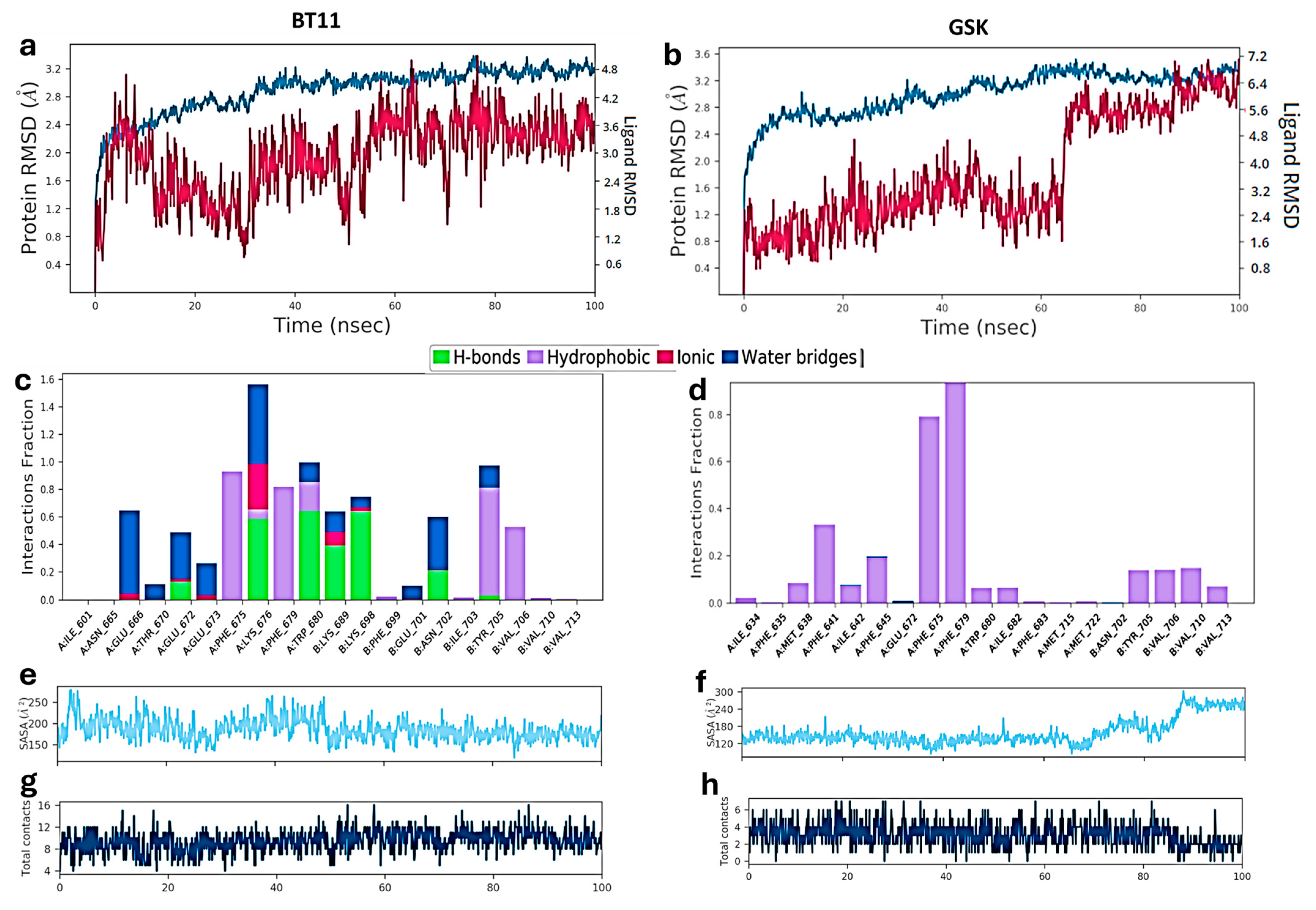

| Metric | Value | CI95_lo | CI95_hi | TopK | Actives in TopK |
|---|---|---|---|---|---|
| ROC-AUC | 0.984 | 0.964 | 0.998 | ||
| EF1% | 37.36 | 18.68 | 70.05 | 5 | 4 |
| EF2% | 28.02 | 15.57 | 42.56 | 10 | 6 |
| EF5% | 17.51 | 9.73 | 19.46 | 24 | 9 |
| PR-AUC | 0.703 |
| GSK | BT11 | T18 | INV29 | SOH95 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frame No. | Time (ns) | ΔG | Frame No. | Time (ns) | ΔG | Frame No. | Time (ns) | ΔG | Frame No. | Time (ns) | ΔG | Frame No. | Time (ns) | ΔG |
| Docking Pose | −70.37 | −66.73 | −71.85 | −67.68 | −60.67 | |||||||||
| 761 | 76 | −52.91 | 861 | 86 | −61.83 | 611 | 61 | −47.99 | 861 | 86 | −52.55 | 811 | 81 | −55.00 |
| 771 | 77 | −54.78 | 871 | 87 | −76.04 | 621 | 62 | −50.23 | 871 | 87 | −48.42 | 821 | 82 | −54.87 |
| 781 | 78 | −58.23 | 881 | 88 | −67.97 | 631 | 63 | −50.98 | 881 | 88 | −52.7 | 831 | 83 | −53.24 |
| 791 | 79 | −52.21 | 891 | 89 | −63.99 | 641 | 64 | −47.87 | 891 | 89 | −54.27 | 841 | 84 | −62.14 |
| 801 | 80 | −55.18 | 901 | 90 | −73.13 | 651 | 65 | −55.82 | 901 | 90 | −56.09 | 851 | 85 | −56.11 |
| 811 | 81 | −64.12 | 911 | 91 | −63.89 | 661 | 66 | −48.86 | 911 | 91 | −57.18 | 861 | 86 | −51.45 |
| 821 | 82 | −56.49 | 921 | 92 | −66.96 | 671 | 67 | −50.4 | 921 | 92 | −59.73 | 871 | 87 | −55.37 |
| 831 | 83 | −71.15 | 931 | 93 | −68.41 | 681 | 68 | −53.06 | 931 | 93 | −55.57 | 881 | 88 | −60.48 |
| 841 | 84 | −59.46 | 941 | 94 | −69.6 | 691 | 69 | −52.78 | 941 | 94 | −58.96 | 891 | 89 | −62.24 |
| 851 | 85 | −54.42 | 951 | 95 | −65.42 | 701 | 70 | −48.48 | 951 | 95 | −55.67 | 901 | 90 | −57.39 |
| Avg. | −57.90 | −67.72 | −50.65 | −55.11 | −56.83 | |||||||||
| ±5.82 | ±4.37 | ±2.58 | ±3.33 | ±3.69 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.I.; Sabadini, G.; Cabezas, D.; González, C.; González, P.; Luo, J.; Salas, C.O.; Mellado, M.; Lorca, M.; Romero-Parra, J.; et al. Structure-Guided Discovery of Benzoic-Acid-Based TRPC6 Ligands: An Integrated Docking, MD, and MM-GBSA SAR Study: Potential Therapeutic Molecules for Autism Spectrum Disorder. Pharmaceuticals 2025, 18, 1577. https://doi.org/10.3390/ph18101577
Silva NI, Sabadini G, Cabezas D, González C, González P, Luo J, Salas CO, Mellado M, Lorca M, Romero-Parra J, et al. Structure-Guided Discovery of Benzoic-Acid-Based TRPC6 Ligands: An Integrated Docking, MD, and MM-GBSA SAR Study: Potential Therapeutic Molecules for Autism Spectrum Disorder. Pharmaceuticals. 2025; 18(10):1577. https://doi.org/10.3390/ph18101577
Chicago/Turabian StyleSilva, Nicolás Ignacio, Gianfranco Sabadini, David Cabezas, Cristofer González, Paulina González, Jiao Luo, Cristian O. Salas, Marco Mellado, Marcos Lorca, Javier Romero-Parra, and et al. 2025. "Structure-Guided Discovery of Benzoic-Acid-Based TRPC6 Ligands: An Integrated Docking, MD, and MM-GBSA SAR Study: Potential Therapeutic Molecules for Autism Spectrum Disorder" Pharmaceuticals 18, no. 10: 1577. https://doi.org/10.3390/ph18101577
APA StyleSilva, N. I., Sabadini, G., Cabezas, D., González, C., González, P., Luo, J., Salas, C. O., Mellado, M., Lorca, M., Romero-Parra, J., & Mella, J. (2025). Structure-Guided Discovery of Benzoic-Acid-Based TRPC6 Ligands: An Integrated Docking, MD, and MM-GBSA SAR Study: Potential Therapeutic Molecules for Autism Spectrum Disorder. Pharmaceuticals, 18(10), 1577. https://doi.org/10.3390/ph18101577









