The Emerging Role of Phosphodiesterase Inhibitors in Fragile X Syndrome and Autism Spectrum Disorder
Abstract
1. Introduction
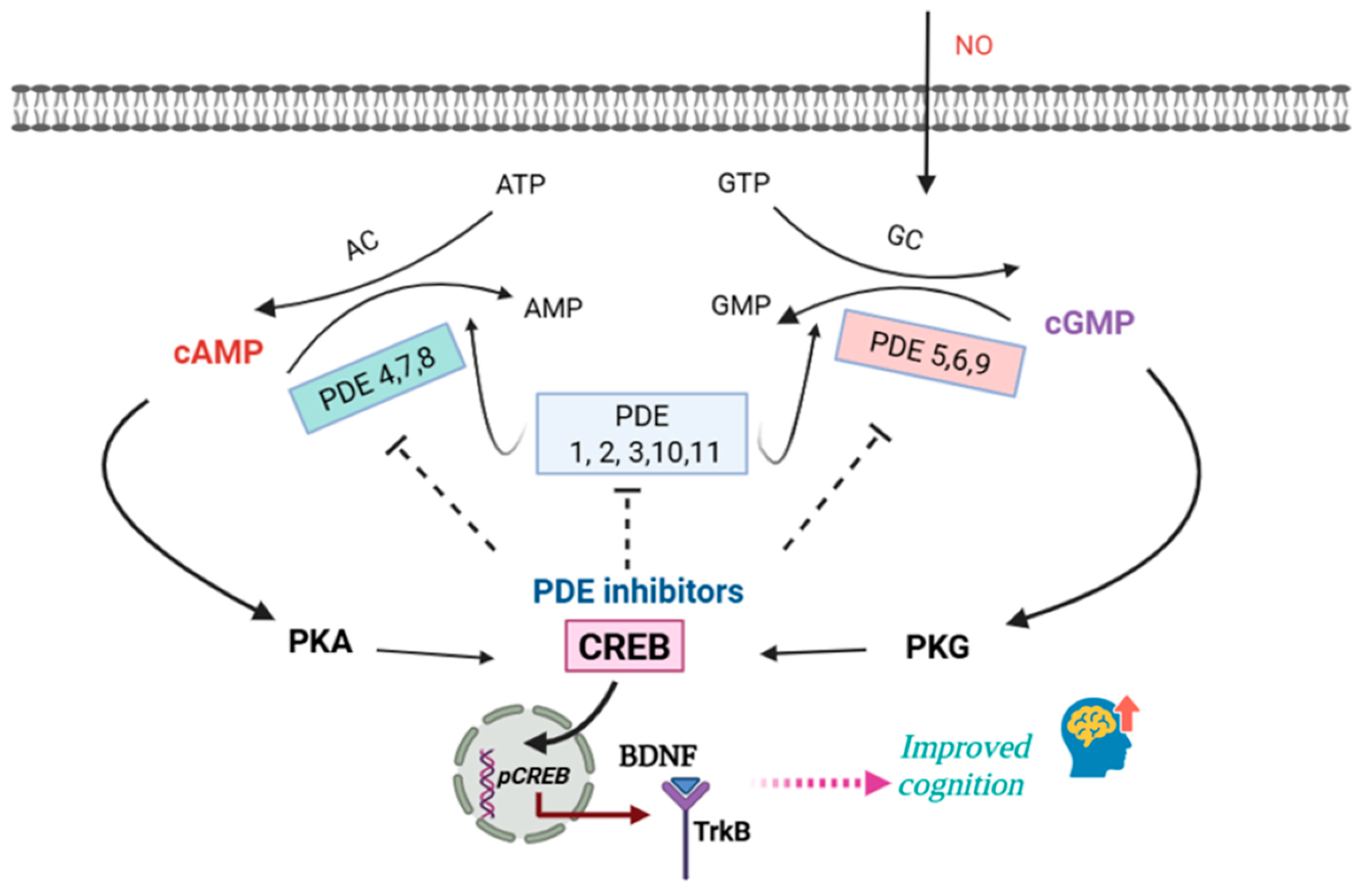
2. Cyclic Nucleotide Signaling and PDE Regulation in the Brain
2.1. Cyclic Nucleotide Signaling and the Role of PDE
2.2. cAMP Signaling Dysregulation in FXS and ASD
3. Abnormalities in PDE Expression and Activity in ASD and FXS
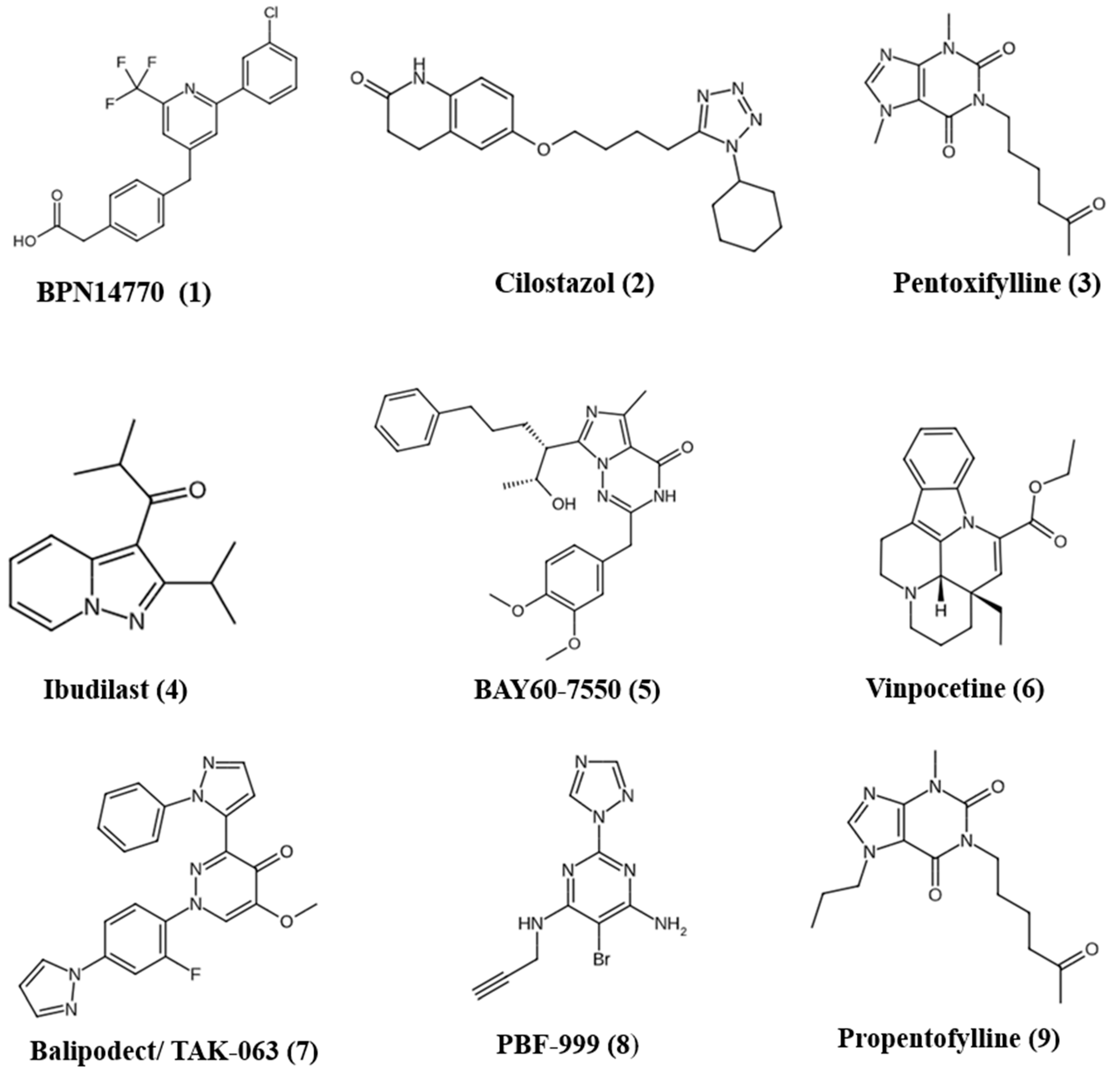
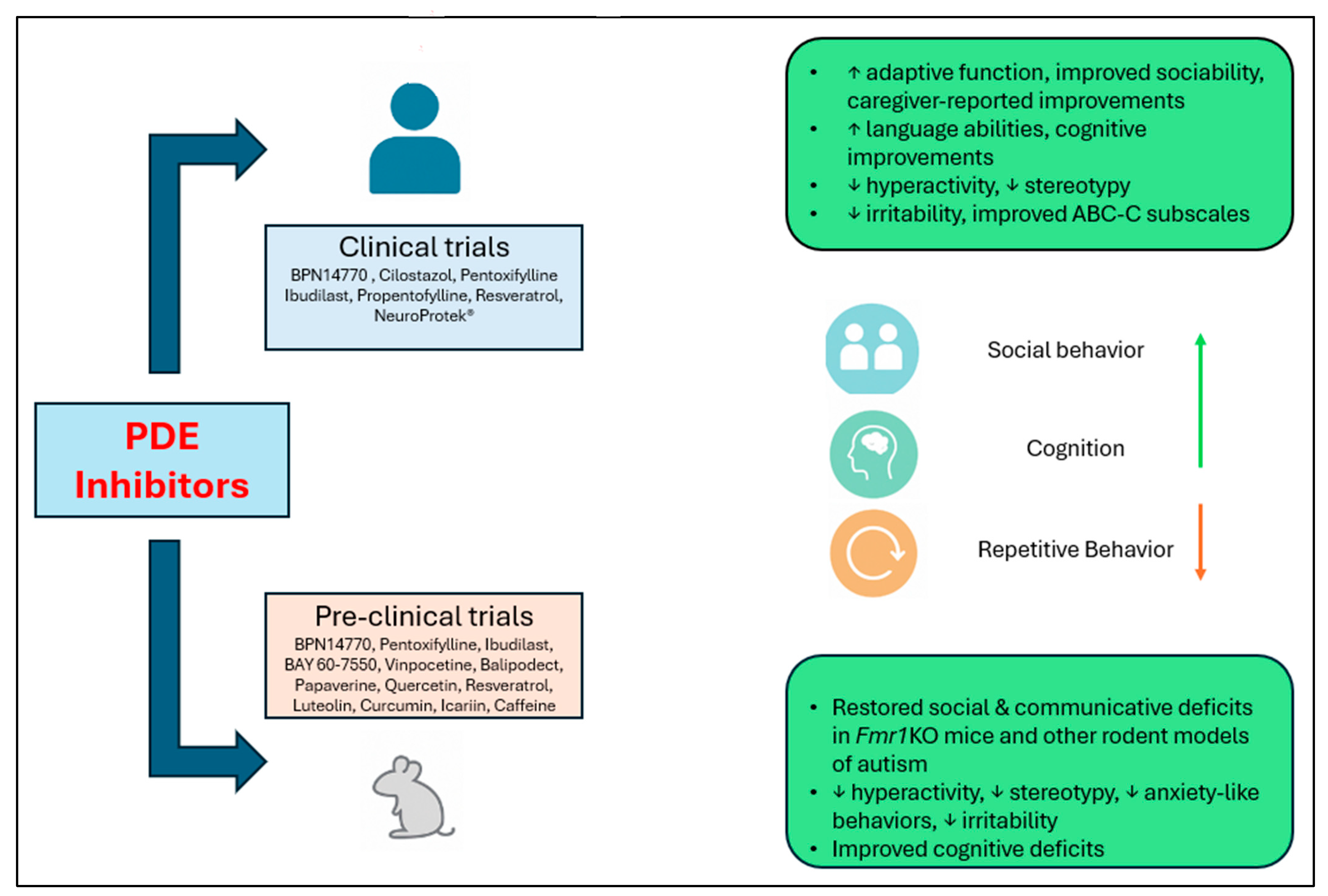
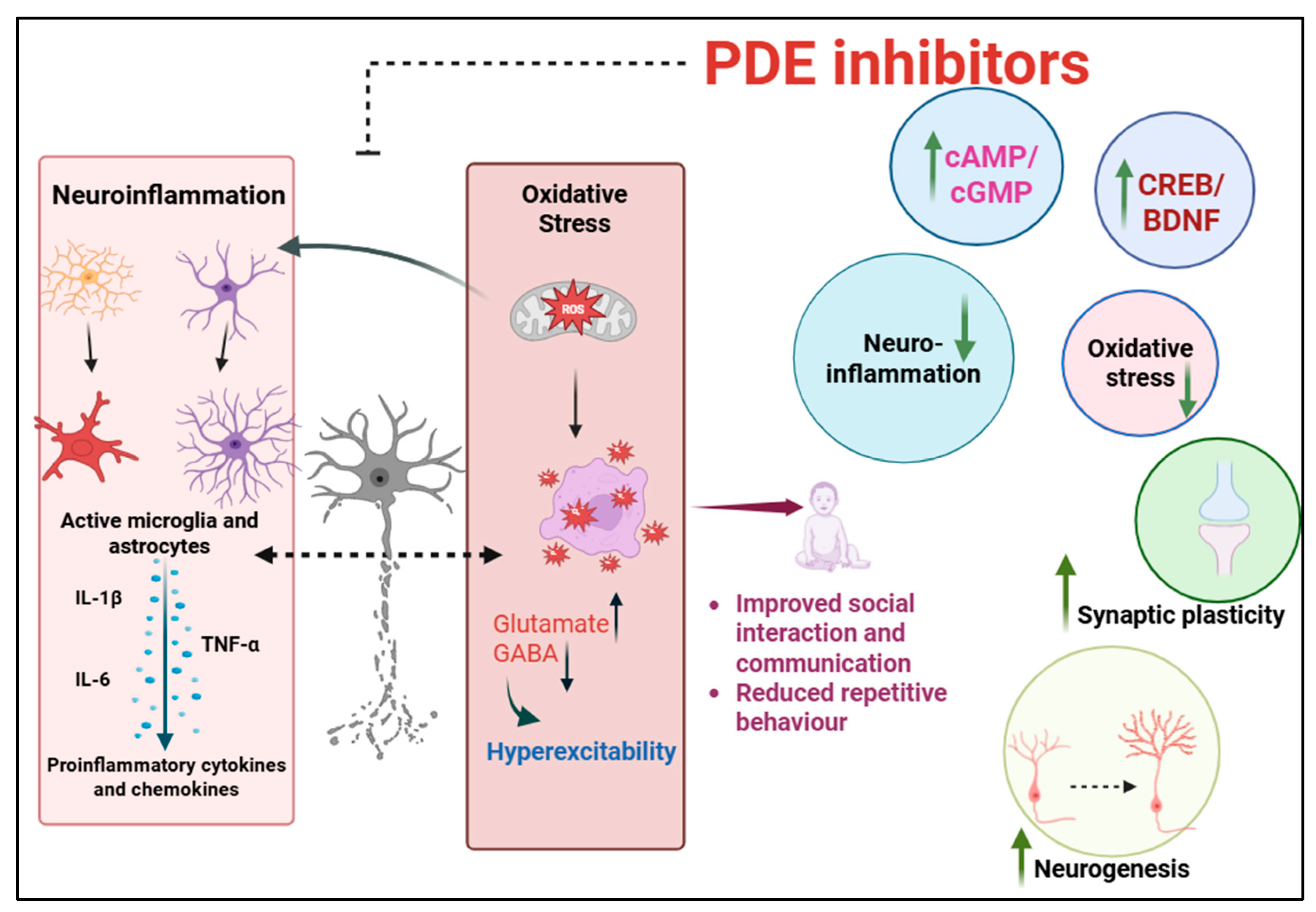
4. PDE Inhibitors for Treatment of FXS and ASD
4.1. BPN14770 (Zatolmilast)
4.2. Cilostazol
4.3. Pentoxifylline
4.4. Ibudilast
4.5. BAY60-7550
4.6. Vinpocetine
4.7. Balipodect/TAK-063
4.8. PBF-999
4.9. Propentofylline
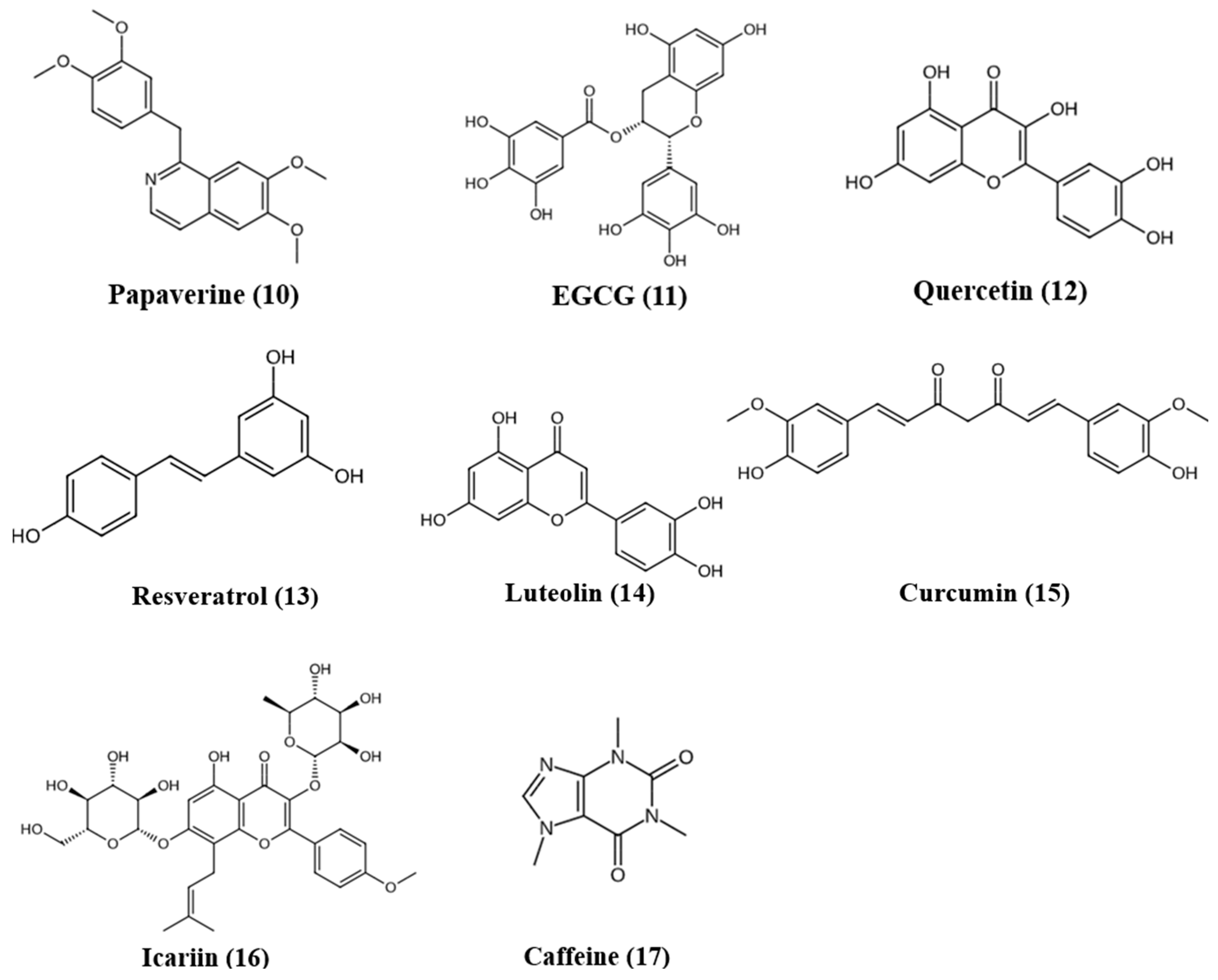
5. Natural Products as PDE Inhibitors
5.1. Papaverine
5.2. (−)-Epigallocatechin-3-gallate (EGCG)
5.3. Quercetin
5.4. Resveratrol
5.5. Luteolin
| Preclinical Investigations of Natural PDE Inhibitors Effective in ASD and FXS | |||
|---|---|---|---|
| Compound | Dose/Model | Outcomes | References |
| Papaverine | 3/10/30 mg/kg; VPA model. | Improved ASD-related behaviors. Increased BDNF, DCX, pCREB, IL-10, and GSH. Decreased TNF-α, IL-6, and TBARS in brain. | [15] |
| 15/30 mg/kg; Developmental hyperserotonemia. | Corrected ASD-related behavioral phenotypes, increased BDNF, IL-10, and GSH; decreased TNF-α, IL-6, and TBARS. | [108] | |
| EGCG (green tea catechin) | 75 and 300 mg/kg orally; mice VPA model. | Improved behavioral deficits at 300 mg/kg. Reduced oxidative stress. | [111] |
| Quercetin | 50 mg/kg orally; rat VPA model. | Prevented behavioral deficits, mitigated oxidative stress in hippocampus and striatum. | [115] |
| 80 mg/kg; rat propionic acid model. | Reduced oxidative stress, neuroinflammation (↓TNF-α), preserved Purkinje cells and neuronal populations. Improved social behavior and learning deficits. | [116] | |
| α-Glycosyl Isoquercitrin (5000 ppm) and α-lipoic acid (1000 ppm in diet); developmental hypothyroidism-induced rat ASD-like model. | Restoration of disrupted hippocampal neurogenesis. AGIQ restored antioxidant enzyme genes. | [117] | |
| Resveratrol | 5, 10, 15 mg/kg orally for 28 days; rat propanoic acid model. | Suppressed oxidative/nitrosative stress, mitochondrial dysfunction, TNF-α, and MMP-9. Improved behavioral deficits. | [120] |
| Co-ultramicronized Palmitoylethanolamide + Luteolin (co-ultraPEA-LUT) | 1 mg/kg; mice VPA model. | Improved social and nonsocial behaviors in mice. Neuroprotective and anti-inflammatory effects attributed to mast cell and microglial modulation. | [125] |
| Curcumin | 50, 100, 200 mg/kg, orally for 28 days; rat propanoic acid model. | Suppressed oxidative/nitrosative stress, mitochondrial dysfunction, TNF-α, and MMP-9. Improved social interaction and reduced stereotypy. | [128] |
| 25, 50, 100 mg/kg; BTBR mice. | Ameliorated social deficits without affecting locomotion or anxiety and restored oxidative stress markers (SOD, CAT) in hippocampus and cerebellum. | [129] | |
| Icariin | 80 mg/kg; BTBR mice. | Ameliorated social deficits, repetitive stereotypical behaviors, and short-term memory deficits; reduced hippocampal neuroinflammation. Rescued excitatory-inhibitory synaptic imbalance by reducing vGlut1 without affecting vGAT. | [130] |
| Caffeine | 1 mg/mL; male rat pups prenatally exposed to VPA. | Improved learning and memory, reduced anxiety-like behaviors, and enhanced social interaction deficits. | [131] |
5.6. Curcumin
5.7. Icariin
5.8. Caffeine
6. Possible Mechanisms Underlying the Therapeutic Potential of PDE Inhibition
7. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef]
- Lai, M.-C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of Co-Occurring Mental Health Diagnoses in the Autism Population: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Lin, L.-S.; Long, S.; Ke, X.-Y.; Fukunaga, K.; Lu, Y.-M.; Han, F. Signalling Pathways in Autism Spectrum Disorder: Mechanisms and Therapeutic Implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Parkhill, M.; Salmaso, N.; D’Angiulli, A.; Lee, V.; Aguilar-Valles, A. Emerging Autism and Fragile X Syndrome Treatments. Trends Pharmacol. Sci. 2025, 46, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef] [PubMed]
- Rosenheck, M.; Sheeler, C.; Saré, R.M.; Gurney, M.E.; Smith, C.B. Effects of Chronic Inhibition of Phosphodiesterase-4D on Behavior and Regional Rates of Cerebral Protein Synthesis in a Mouse Model of Fragile X Syndrome. Neurobiol. Dis. 2021, 159, 105485. [Google Scholar] [CrossRef]
- Barbagallo, F.; Assenza, M.R.; Messina, A. In the Brain of Phosphodiesterases: Potential Therapeutic Targets for Schizophrenia. Clin. Psychopharmacol. Neurosci. 2025, 23, 15–31. [Google Scholar] [CrossRef]
- Kelley, D.; Bhattacharyya, A.; Lahvis, G.; Yin, J.; Malter, J.; Davidson, R. The Cyclic AMP Phenotype of Fragile X and Autism. Neurosci. Biobehav. Rev. 2008, 32, 1533–1543. [Google Scholar] [CrossRef]
- Bollen, E.; Prickaerts, J. Phosphodiesterases in Neurodegenerative Disorders. IUBMB Life 2012, 64, 965–970. [Google Scholar] [CrossRef]
- Aishworiya, R.; Valica, T.; Hagerman, R.; Restrepo, B. An Update on Psychopharmacological Treatment of Autism Spectrum Disorder. Neurotherapeutics 2022, 19, 248–262. [Google Scholar] [CrossRef]
- Padovan-Neto, F.E.; Cerveira, A.J.d.O.; da Silva, A.; Ribeiro, D.L. Beyond Traditional Pharmacology: Evaluating Phosphodiesterase Inhibitors in Autism Spectrum Disorder. Neuropsychopharmacology 2024, 49, 1359–1360. [Google Scholar] [CrossRef]
- Gurney, M.E.; Cogram, P.; Deacon, R.M.; Rex, C.; Tranfaglia, M. Multiple Behavior Phenotypes of the Fragile-X Syndrome Mouse Model Respond to Chronic Inhibition of Phosphodiesterase-4D (PDE4D). Sci. Rep. 2017, 7, 14653. [Google Scholar] [CrossRef]
- Maurin, T.; Lebrigand, K.; Castagnola, S.; Paquet, A.; Jarjat, M.; Popa, A.; Grossi, M.; Rage, F.; Bardoni, B. HITS-CLIP in Various Brain Areas Reveals New Targets and New Modalities of RNA Binding by Fragile X Mental Retardation Protein. Nucleic Acids Res. 2018, 46, 6344–6355. [Google Scholar] [CrossRef] [PubMed]
- Zamarbide, M.; Mossa, A.; Muñoz-Llancao, P.; Wilkinson, M.K.; Pond, H.L.; Oaks, A.W.; Manzini, M.C. Male-Specific cAMP Signaling in the Hippocampus Controls Spatial Memory Deficits in a Mouse Model of Autism and Intellectual Disability. Biol. Psychiatry 2019, 85, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Luhach, K.; Kulkarni, G.T.; Singh, V.P.; Sharma, B. Attenuation of Neurobehavioural Abnormalities by Papaverine in Prenatal Valproic Acid Rat Model of ASD. Eur. J. Pharmacol. 2021, 890, 173663. [Google Scholar] [CrossRef] [PubMed]
- Argyrousi, E.K.; Heckman, P.R.A.; Prickaerts, J. Role of Cyclic Nucleotides and Their Downstream Signaling Cascades in Memory Function: Being at the Right Time at the Right Spot. Neurosci. Biobehav. Rev. 2020, 113, 12–38. [Google Scholar] [CrossRef]
- Kleppisch, T. Phosphodiesterases in the Central Nervous System. In cGMP: Generators, Effectors and Therapeutic Implications; Schmidt, H.H.H.W., Hofmann, F., Stasch, J.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 71–92. ISBN 978-3-540-68964-5. [Google Scholar]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular cAMP Signaling by Soluble Adenylyl Cyclase. Kidney Int. 2011, 79, 1277–1288. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kim, J.-E.; Lee, R.; Malberg, J.E.; Chen, J.; Steffen, C.; Zhang, Y.-J.; Nestler, E.J.; Duman, R.S. Regulation of Neurogenesis in Adult Mouse Hippocampus by cAMP and the cAMP Response Element-Binding Protein. J. Neurosci. 2002, 22, 3673–3682. [Google Scholar] [CrossRef]
- Tojima, T.; Kobayashi, S.; Ito, E. Dual Role of Cyclic AMP-Dependent Protein Kinase in Neuritogenesis and Synaptogenesis during Neuronal Differentiation. J. Neurosci. Res. 2003, 74, 829–837. [Google Scholar] [CrossRef]
- Ji, Y.; Pang, P.T.; Feng, L.; Lu, B. Cyclic AMP Controls BDNF-Induced TrkB Phosphorylation and Dendritic Spine Formation in Mature Hippocampal Neurons. Nat. Neurosci. 2005, 8, 164–172. [Google Scholar] [CrossRef]
- Kelly, M.P. Cyclic Nucleotide Signaling Changes Associated with Normal Aging and Age-Related Diseases of the Brain. Cell Signal 2018, 42, 281–291. [Google Scholar] [CrossRef]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [PubMed]
- Knott, E.P.; Assi, M.; Rao, S.N.R.; Ghosh, M.; Pearse, D.D. Phosphodiesterase Inhibitors as a Therapeutic Approach to Neuroprotection and Repair. Int. J. Mol. Sci. 2017, 18, 696. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic Targeting of 3’,5’-Cyclic Nucleotide Phosphodiesterases: Inhibition and Beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- West, A.R.; Tseng, K.Y. Nitric Oxide-Soluble Guanylyl Cyclase-Cyclic GMP Signaling in the Striatum: New Targets for the Treatment of Parkinson’s Disease? Front. Syst. Neurosci. 2011, 5, 55. [Google Scholar] [CrossRef]
- Rodríguez-Moreno, A.; Sihra, T.S. Presynaptic Kainate Receptor-Mediated Facilitation of Glutamate Release Involves Ca2+-Calmodulin and PKA in Cerebrocortical Synaptosomes. FEBS Lett. 2013, 587, 788–792. [Google Scholar] [CrossRef]
- Scott Bitner, R. Cyclic AMP Response Element-Binding Protein (CREB) Phosphorylation: A Mechanistic Marker in the Development of Memory Enhancing Alzheimer’s Disease Therapeutics. Biochem. Pharmacol. 2012, 83, 705–714. [Google Scholar] [CrossRef]
- Nishi, A.; Shuto, T. Potential for Targeting Dopamine/DARPP-32 Signaling in Neuropsychiatric and Neurodegenerative Disorders. Expert. Opin. Ther. Targets 2017, 21, 259–272. [Google Scholar] [CrossRef]
- Xiang, Y.; Naik, S.; Zhao, L.; Shi, J.; Ke, H. Emerging Phosphodiesterase Inhibitors for Treatment of Neurodegenerative Diseases. Med. Res. Rev. 2024, 44, 1404–1445. [Google Scholar] [CrossRef]
- Ortega-Martínez, S. A New Perspective on the Role of the CREB Family of Transcription Factors in Memory Consolidation via Adult Hippocampal Neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef]
- Kanellopoulos, A.K.; Semelidou, O.; Kotini, A.G.; Anezaki, M.; Skoulakis, E.M.C. Learning and Memory Deficits Consequent to Reduction of the Fragile X Mental Retardation Protein Result from Metabotropic Glutamate Receptor-Mediated Inhibition of cAMP Signaling in Drosophila. J. Neurosci. 2012, 32, 13111–13124. [Google Scholar] [CrossRef]
- Choi, C.H.; Schoenfeld, B.P.; Weisz, E.D.; Bell, A.J.; Chambers, D.B.; Hinchey, J.; Choi, R.J.; Hinchey, P.; Kollaros, M.; Gertner, M.J.; et al. PDE-4 Inhibition Rescues Aberrant Synaptic Plasticity in Drosophila and Mouse Models of Fragile X Syndrome. J. Neurosci. 2015, 35, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Hicar, M.; Ciurlionis, R. Reduced Cyclic AMP Production in Fragile X Syndrome: Cytogenetic and Molecular Correlations. Pediatr. Res. 1995, 38, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Ciurlionis, R. Overexpression of Fragile X Gene (FMR-1) Transcripts Increases cAMP Production in Neural Cells. J. Neurosci. Res. 1998, 51, 41–48. [Google Scholar] [CrossRef]
- Hwu, W.L.; Wang, T.R.; Lee, Y.M. FMR1 Enhancer Is Regulated by cAMP through a cAMP-Responsive Element. DNA Cell Biol. 1997, 16, 449–453. [Google Scholar] [CrossRef]
- Choi, C.H.; Schoenfeld, B.P.; Bell, A.J.; Hinchey, J.; Rosenfelt, C.; Gertner, M.J.; Campbell, S.R.; Emerson, D.; Hinchey, P.; Kollaros, M.; et al. Multiple Drug Treatments That Increase cAMP Signaling Restore Long-Term Memory and Aberrant Signaling in Fragile X Syndrome Models. Front. Behav. Neurosci. 2016, 10, 136. [Google Scholar] [CrossRef]
- Delhaye, S.; Bardoni, B. Role of Phosphodiesterases in the Pathophysiology of Neurodevelopmental Disorders. Mol. Psychiatry 2021, 26, 4570–4582. [Google Scholar] [CrossRef]
- Nickl-Jockschat, T.; Habel, U.; Michel, T.M.; Manning, J.; Laird, A.R.; Fox, P.T.; Schneider, F.; Eickhoff, S.B. Brain Structure Anomalies in Autism Spectrum Disorder--a Meta-Analysis of VBM Studies Using Anatomic Likelihood Estimation. Hum. Brain Mapp. 2012, 33, 1470–1489. [Google Scholar] [CrossRef]
- Fuccillo, M.V. Striatal Circuits as a Common Node for Autism Pathophysiology. Front. Neurosci. 2016, 10, 27. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Ercument Cicek, A.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, Transcriptional and Chromatin Genes Disrupted in Autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Reed, T.M.; Repaske, D.R.; Snyder, G.L.; Greengard, P.; Vorhees, C.V. Phosphodiesterase 1B Knock-out Mice Exhibit Exaggerated Locomotor Hyperactivity and DARPP-32 Phosphorylation in Response to Dopamine Agonists and Display Impaired Spatial Learning. J. Neurosci. 2002, 22, 5188–5197. [Google Scholar] [CrossRef]
- Siuciak, J.A.; McCarthy, S.A.; Chapin, D.S.; Reed, T.M.; Vorhees, C.V.; Repaske, D.R. Behavioral and Neurochemical Characterization of Mice Deficient in the Phosphodiesterase-1B (PDE1B) Enzyme. Neuropharmacology 2007, 53, 113–124. [Google Scholar] [CrossRef]
- Luhach, K.; Kulkarni, G.T.; Singh, V.P.; Sharma, B. Vinpocetine Amended Prenatal Valproic Acid Induced Features of ASD Possibly by Altering Markers of Neuronal Function, Inflammation, and Oxidative Stress. Autism Res. 2021, 14, 2270–2286. [Google Scholar] [CrossRef]
- Stephenson, D.T.; Coskran, T.M.; Kelly, M.P.; Kleiman, R.J.; Morton, D.; O’Neill, S.M.; Schmidt, C.J.; Weinberg, R.J.; Menniti, F.S. The Distribution of Phosphodiesterase 2A in the Rat Brain. Neuroscience 2012, 226, 145–155. [Google Scholar] [CrossRef]
- Gaigg, S.B. The Interplay between Emotion and Cognition in Autism Spectrum Disorder: Implications for Developmental Theory. Front. Integr. Neurosci. 2012, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Imada, H.; Shiraishi, E.; Ito, Y.; Suzuki, N.; Miyamoto, M.; Taniguchi, T.; Iwashita, H. Phosphodiesterase 2A Inhibitor TAK-915 Ameliorates Cognitive Impairments and Social Withdrawal in N-Methyl-d-Aspartate Receptor Antagonist–Induced Rat Models of Schizophrenia. J. Pharmacol. Exp. Ther. 2018, 365, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.; Jalkh, N.; Corbani, S.; Abou-Ghoch, J.; Fawaz, A.; Mehawej, C.; Chouery, E. A Homozygous Splicing Mutation in PDE2A in a Family With Atypical Rett Syndrome. Mov. Disord. 2020, 35, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Maurin, T.; Melancia, F.; Jarjat, M.; Castro, L.; Costa, L.; Delhaye, S.; Khayachi, A.; Castagnola, S.; Mota, E.; Di Giorgio, A.; et al. Involvement of Phosphodiesterase 2A Activity in the Pathophysiology of Fragile X Syndrome. Cereb. Cortex 2019, 29, 3241–3252. [Google Scholar] [CrossRef]
- Schiavi, S.; Carbone, E.; Melancia, F.; di Masi, A.; Jarjat, M.; Brau, F.; Cardarelli, S.; Giorgi, M.; Bardoni, B.; Trezza, V. Phosphodiesterase 2A Inhibition Corrects the Aberrant Behavioral Traits Observed in Genetic and Environmental Preclinical Models of Autism Spectrum Disorder. Transl. Psychiatry 2022, 12, 119. [Google Scholar] [CrossRef]
- Delhaye, S.; Jarjat, M.; Boulksibat, A.; Sanchez, C.; Tempio, A.; Turtoi, A.; Giorgi, M.; Lacas-Gervais, S.; Baj, G.; Rovere, C.; et al. Defects in AMPAR Trafficking and Microglia Activation Underlie Socio-Cognitive Deficits Associated to Decreased Expression of Phosphodiesterase 2a. Neurobiol. Dis. 2024, 191, 106393. [Google Scholar] [CrossRef]
- Bardoni, B.; Gwizdek, C.; Maurin, T. How Close Are We to a cAMP- and cGMP-Theory-Based Pharmacological Therapy for Fragile X Syndrome? CR Med. 2025, 6, 101972. [Google Scholar] [CrossRef]
- Richter, W.; Menniti, F.S.; Zhang, H.-T.; Conti, M. PDE4 as a Target for Cognition Enhancement. Expert. Opin. Ther. Targets 2013, 17, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Sklena, P. Demonstration of Abnormal Cyclic AMP Production in Platelets from Patients with Fragile X Syndrome. Am. J. Med. Genet. 1993, 45, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Bailey, A.M.; Matthies, H.J.G.; Renden, R.B.; Smith, M.A.; Speese, S.D.; Rubin, G.M.; Broadie, K. Drosophila Fragile X-Related Gene Regulates the MAP1B Homolog Futsch to Control Synaptic Structure and Function. Cell 2001, 107, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Dockendorff, T.C.; Su, H.S.; McBride, S.M.J.; Yang, Z.; Choi, C.H.; Siwicki, K.K.; Sehgal, A.; Jongens, T.A. Drosophila Lacking Dfmr1 Activity Show Defects in Circadian Output and Fail to Maintain Courtship Interest. Neuron 2002, 34, 973–984. [Google Scholar] [CrossRef]
- Lee, H.; Graham, J.M.; Rimoin, D.L.; Lachman, R.S.; Krejci, P.; Tompson, S.W.; Nelson, S.F.; Krakow, D.; Cohn, D.H. Exome Sequencing Identifies PDE4D Mutations in Acrodysostosis. Am. J. Hum. Genet. 2012, 90, 746–751. [Google Scholar] [CrossRef]
- Michot, C.; Le Goff, C.; Blair, E.; Blanchet, P.; Capri, Y.; Gilbert-Dussardier, B.; Goldenberg, A.; Henderson, A.; Isidor, B.; Kayserili, H.; et al. Expanding the Phenotypic Spectrum of Variants in PDE4D/PRKAR1A: From Acrodysostosis to Acroscyphodysplasia. Eur. J. Hum. Genet. 2018, 26, 1611–1622. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Huang, Y.; Masood, A.; Stolinski, L.R.; Li, Y.; Zhang, L.; Dlaboga, D.; Jin, S.-L.C.; Conti, M.; O’Donnell, J.M. Anxiogenic-like Behavioral Phenotype of Mice Deficient in Phosphodiesterase 4B (PDE4B). Neuropsychopharmacology 2008, 33, 1611–1623. [Google Scholar] [CrossRef]
- Rutten, K.; Wallace, T.L.; Works, M.; Prickaerts, J.; Blokland, A.; Novak, T.J.; Santarelli, L.; Misner, D.L. Enhanced Long-Term Depression and Impaired Reversal Learning in Phosphodiesterase 4B-Knockout (PDE4B-/-) Mice. Neuropharmacology 2011, 61, 138–147. [Google Scholar] [CrossRef]
- Manzini, M.C.; Xiong, L.; Shaheen, R.; Tambunan, D.E.; Di Costanzo, S.; Mitisalis, V.; Tischfield, D.J.; Cinquino, A.; Ghaziuddin, M.; Christian, M.; et al. CC2D1A Regulates Human Intellectual and Social Function as Well as NF-κB Signaling Homeostasis. Cell Rep. 2014, 8, 647–655. [Google Scholar] [CrossRef]
- Oaks, A.W.; Zamarbide, M.; Tambunan, D.E.; Santini, E.; Di Costanzo, S.; Pond, H.L.; Johnson, M.W.; Lin, J.; Gonzalez, D.M.; Boehler, J.F.; et al. Cc2d1a Loss of Function Disrupts Functional and Morphological Development in Forebrain Neurons Leading to Cognitive and Social Deficits. Cereb. Cortex 2017, 27, 1670–1685. [Google Scholar] [CrossRef] [PubMed]
- Mironov, S.L.; Skorova, E.Y.; Kügler, S. Epac-Mediated cAMP-Signalling in the Mouse Model of Rett Syndrome. Neuropharmacology 2011, 60, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Wang, A.; Hamzah, H.; Waldman, A.; Jiang, K.; Dong, Q.; Li, R.; Kim, J.; Turner, D.; Chang, Q. CREB Signaling Is Involved in Rett Syndrome Pathogenesis. J. Neurosci. 2017, 37, 3671–3685. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Z.-M.; Tan, W.; Wang, X.; Li, Y.; Bai, B.; Li, Y.; Zhang, S.-F.; Yan, H.-L.; Chen, Z.-L.; et al. Partial Loss of Psychiatric Risk Gene Mir137 in Mice Causes Repetitive Behavior and Impairs Sociability and Learning via Increased Pde10a. Nat. Neurosci. 2018, 21, 1689–1703. [Google Scholar] [CrossRef]
- Siuciak, J.A.; McCarthy, S.A.; Chapin, D.S.; Martin, A.N.; Harms, J.F.; Schmidt, C.J. Behavioral Characterization of Mice Deficient in the Phosphodiesterase-10A (PDE10A) Enzyme on a C57/Bl6N Congenic Background. Neuropharmacology 2008, 54, 417–427. [Google Scholar] [CrossRef]
- Kelly, M.P.; Logue, S.F.; Brennan, J.; Day, J.P.; Lakkaraju, S.; Jiang, L.; Zhong, X.; Tam, M.; Sukoff Rizzo, S.J.; Platt, B.J.; et al. Phosphodiesterase 11A in Brain Is Enriched in Ventral Hippocampus and Deletion Causes Psychiatric Disease-Related Phenotypes. Proc. Natl. Acad. Sci. USA 2010, 107, 8457–8462. [Google Scholar] [CrossRef]
- Hegde, S.; Capell, W.R.; Ibrahim, B.A.; Klett, J.; Patel, N.S.; Sougiannis, A.T.; Kelly, M.P. Phosphodiesterase 11A (PDE11A), Enriched in Ventral Hippocampus Neurons, Is Required for Consolidation of Social but Not Nonsocial Memories in Mice. Neuropsychopharmacology 2016, 41, 2920–2931. [Google Scholar] [CrossRef]
- Hegde, S.; Ji, H.; Oliver, D.; Patel, N.S.; Poupore, N.; Shtutman, M.; Kelly, M.P. PDE11A regulates social behaviors and is a key mechanism by which social experience sculpts the brain. Neuroscience 2016, 335, 151–169. [Google Scholar] [CrossRef]
- Gurney, M.E.; Nugent, R.A.; Mo, X.; Sindac, J.A.; Hagen, T.J.; Fox, D.I.; O’Donnell, J.M.; Zhang, C.; Xu, Y.; Zhang, H.-T.; et al. Design and Synthesis of Selective Phosphodiesterase 4D (PDE4D) Allosteric Inhibitors for the Treatment of Fragile X Syndrome and Other Brain Disorders. J. Med. Chem. 2019, 62, 4884–4901. [Google Scholar] [CrossRef]
- Berry-Kravis, E.M.; Harnett, M.D.; Reines, S.A.; Reese, M.A.; Ethridge, L.E.; Outterson, A.H.; Michalak, C.; Furman, J.; Gurney, M.E. Inhibition of Phosphodiesterase-4D in Adults with Fragile X Syndrome: A Randomized, Placebo-Controlled, Phase 2 Clinical Trial. Nat. Med. 2021, 27, 862–870. [Google Scholar] [CrossRef]
- Norris, J.E.; Berry-Kravis, E.M.; Harnett, M.D.; Reines, S.A.; Reese, M.A.; Outterson, A.H.; Michalak, C.; Furman, J.; Gurney, M.E.; Ethridge, L.E. Auditory N1 Event-Related Potential Amplitude Is Predictive of Serum Concentration of BPN14770 in Fragile X Syndrome. Mol. Autism 2024, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.D.; Chung, W.K. Clinical Features of PPP2 Syndrome Type R5D (Jordan’s Syndrome) to Support Standardization of Care. Cold Spring Harb. Mol. Case Stud. 2023, 9, a006285. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Hara, H. Protective Effects of Cilostazol against Hemorrhagic Stroke: Current and Future Perspectives. J. Pharmacol. Sci. 2016, 131, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Chen, M.-J.; Hsiao, C.-D.; Hu, R.-Z.; Huang, Y.-S.; Chen, Y.-F.; Yang, T.-H.; Tsai, G.-Y.; Chou, C.-W.; Chen, R.-S.; et al. The Anti-Platelet Drug Cilostazol Enhances Heart Rate and Interrenal Steroidogenesis and Exerts a Scant Effect on Innate Immune Responses in Zebrafish. PLoS ONE 2023, 18, e0292858. [Google Scholar] [CrossRef]
- Zhao, J.; Harada, N.; Kurihara, H.; Nakagata, N.; Okajima, K. Cilostazol Improves Cognitive Function in Mice by Increasing the Production of Insulin-like Growth Factor-I in the Hippocampus. Neuropharmacology 2010, 58, 774–783. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Seyedmirzaei, H.; Moradi, K.; Bagheri, S.; Moeini, M.; Mohammadi, M.-R.; Akhondzadeh, S. Cilostazol as Adjunctive Therapy in Treatment of Children with Autism Spectrum Disorders: A Double-Blind and Placebo-Controlled Randomized Trial. Int. Clin. Psychopharmacol. 2023, 38, 89. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; Hegazy, S.K.; Abd-Elsalam, S.M.; Bahaa, M.M. Pentoxifylline, a Nonselective Phosphodiesterase Inhibitor, in Adjunctive Therapy in Patients with Irritable Bowel Syndrome Treated with Mebeverine. Biomed. Pharmacother. 2022, 145, 112399. [Google Scholar] [CrossRef]
- Shamabadi, A.; Karimi, H.; Arabzadeh Bahri, R.; Motavaselian, M.; Akhondzadeh, S. Emerging Drugs for the Treatment of Irritability Associated with Autism Spectrum Disorder. Expert Opin. Emerg. Drugs 2024, 29, 45–56. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Fallah, J.; Mohammadi, M.-R.; Imani, R.; Mohammadi, M.; Salehi, B.; Ghanizadeh, A.; Raznahan, M.; Mohebbi-Rasa, S.; Rezazadeh, S.-A.; et al. Double-Blind Placebo-Controlled Trial of Pentoxifylline Added to Risperidone: Effects on Aberrant Behavior in Children with Autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 32–36. [Google Scholar] [CrossRef]
- Erdoğan, M.A.; Tunç, K.C.; Daştan, A.İ.; Tomruk, C.; Uyanıkgil, Y.; Erbaş, O. Therapeutic Effects of Pentoxifylline in Propionic Acid-Induced Autism Symptoms in Rat Models: A Behavioral, Biochemical, and Histopathological Study. Int. J. Dev. Neurosci. 2024, 84, 991–1005. [Google Scholar] [CrossRef]
- Mizuno, T.; Kurotani, T.; Komatsu, Y.; Kawanokuchi, J.; Kato, H.; Mitsuma, N.; Suzumura, A. Neuroprotective Role of Phosphodiesterase Inhibitor Ibudilast on Neuronal Cell Death Induced by Activated Microglia. Neuropharmacology 2004, 46, 404–411. [Google Scholar] [CrossRef]
- Gibson, L.C.D.; Hastings, S.F.; McPhee, I.; Clayton, R.A.; Darroch, C.E.; Mackenzie, A.; MacKenzie, F.L.; Nagasawa, M.; Stevens, P.A.; MacKenzie, S.J. The Inhibitory Profile of Ibudilast against the Human Phosphodiesterase Enzyme Family. Eur. J. Pharmacol. 2006, 538, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Rawat, K.; Gautam, V.; Sharma, A.; Kumar, A.; Saha, L. Phosphodiesterase Inhibitor, Ibudilast Alleviates Core Behavioral and Biochemical Deficits in the Prenatal Valproic Acid Exposure Model of Autism Spectrum Disorder. Brain Res. 2023, 1815, 148443. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, W.; Angulo-Herrera, I.; Cogram, P.; Deacon, R.J.M.; Mason, D.J.; Brown, D.; Roberts, I.; O’Donovan, D.J.; Tranfaglia, M.R.; Guilliams, T.; et al. A Novel Combination Treatment for Fragile X Syndrome Predicted Using Computational Methods. Brain Commun. 2024, 6, fcad353. [Google Scholar] [CrossRef] [PubMed]
- Deidda, G.; Bozarth, I.F.; Cancedda, L. Modulation of GABAergic Transmission in Development and Neurodevelopmental Disorders: Investigating Physiology and Pathology to Gain Therapeutic Perspectives. Front. Cell Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Zürcher, N.R.; Rogier, O.; Ruest, T.; Hippolyte, L.; Ben-Ari, Y.; Lemonnier, E. Improving Emotional Face Perception in Autism with Diuretic Bumetanide: A Proof-of-Concept Behavioral and Functional Brain Imaging Pilot Study. Autism 2015, 19, 149–157. [Google Scholar] [CrossRef]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Cellot, G.; Cherubini, E. GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Front. Pediatr. 2014, 2, 70. [Google Scholar] [CrossRef]
- Contestabile, A.; Magara, S.; Cancedda, L. The GABAergic Hypothesis for Cognitive Disabilities in Down Syndrome. Front. Cell Neurosci. 2017, 11, 54. [Google Scholar] [CrossRef]
- Lemonnier, E.; Villeneuve, N.; Sonie, S.; Serret, S.; Rosier, A.; Roue, M.; Brosset, P.; Viellard, M.; Bernoux, D.; Rondeau, S.; et al. Effects of Bumetanide on Neurobehavioral Function in Children and Adolescents with Autism Spectrum Disorders. Transl. Psychiatry 2017, 7, e1056. [Google Scholar] [CrossRef]
- Kharod, S.C.; Kang, S.K.; Kadam, S.D. Off-Label Use of Bumetanide for Brain Disorders: An Overview. Front. Neurosci. 2019, 13, 310. [Google Scholar] [CrossRef]
- Erickson, C.A.; Perez-Cano, L.; Pedapati, E.V.; Painbeni, E.; Bonfils, G.; Schmitt, L.M.; Sachs, H.; Nelson, M.; De Stefano, L.; Westerkamp, G.; et al. Safety, Tolerability, and EEG-Based Target Engagement of STP1 (PDE3,4 Inhibitor and NKCC1 Antagonist) in a Randomized Clinical Trial in a Subgroup of Patients with ASD. Biomedicines 2024, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.M.; Meyer, E.; Milani, H.; Steinbusch, H.W.M.; Prickaerts, J.; de Oliveira, R.M.W. The Phosphodiesterase Type 2 Inhibitor BAY 60-7550 Reverses Functional Impairments Induced by Brain Ischemia by Decreasing Hippocampal Neurodegeneration and Enhancing Hippocampal Neuronal Plasticity. Eur. J. Neurosci. 2017, 45, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Du, K.; Tao, M.; Shan, C.; Ye, R.; Tang, Y.; Pan, H.; Lv, J.; Zhang, M.; Pan, J. Phosphodiesterase-2 Inhibitor Bay 60-7550 Ameliorates Aβ-Induced Cognitive and Memory Impairment via Regulation of the HPA Axis. Front. Cell Neurosci. 2019, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Yan, C. An Update on Vinpocetine: New Discoveries and Clinical Implications. Eur. J. Pharmacol. 2018, 819, 30–34. [Google Scholar] [CrossRef]
- Sharma, N.; Luhach, K.; Golani, L.K.; Singh, B.; Sharma, B. Vinpocetine, a PDE1 Modulator, Regulates Markers of Cerebral Health, Inflammation, and Oxidative Stress in a Rat Model of Prenatal Alcohol-Induced Experimental Attention Deficit Hyperactivity Disorder. Alcohol. 2022, 105, 25–34. [Google Scholar] [CrossRef]
- Paine, T.A.; Neve, R.L.; Carlezon, W.A. Attention Deficits and Hyperactivity Following Inhibition of cAMP-Dependent Protein Kinase within the Medial Prefrontal Cortex of Rats. Neuropsychopharmacology 2009, 34, 2143–2155. [Google Scholar] [CrossRef]
- Nunes, F.; Ferreira-Rosa, K.; Pereira, M.d.S.; Kubrusly, R.C.; Manhães, A.C.; Abreu-Villaça, Y.; Filgueiras, C.C. Acute Administration of Vinpocetine, a Phosphodiesterase Type 1 Inhibitor, Ameliorates Hyperactivity in a Mice Model of Fetal Alcohol Spectrum Disorder. Drug Alcohol. Depend. 2011, 119, 81–87. [Google Scholar] [CrossRef]
- Araujo, U.C.; Nunes, F.; Gonçalves, B.S.; Gomes, R.A.A.; Moreira, M.d.F.R.; Nunes-Freitas, A.; Krahe, T.E.; de Abreu-Villaça, Y.; Manhães, A.C.; Filgueiras, C.C. Vinpocetine, a Phosphodiesterase Type 1 Inhibitor, Mitigates Locomotor Hyperactivity in Female Mice Exposed to Lead During Development. Brain Sci. 2025, 15, 150. [Google Scholar] [CrossRef]
- Macek, T.A.; Suzuki, K.; Asin, K.; Kimura, H. Translational Development Strategies for TAK-063, a Phosphodiesterase 10A Inhibitor. Int. J. Neuropsychopharmacol. 2020, 23, 524–532. [Google Scholar] [CrossRef]
- Jonak, C.R.; Sandhu, M.S.; Assad, S.A.; Barbosa, J.A.; Makhija, M.; Binder, D.K. The PDE10A Inhibitor TAK-063 Reverses Sound-Evoked EEG Abnormalities in a Mouse Model of Fragile X Syndrome. Neurotherapeutics 2021, 18, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Behmanesh, H.; Moghaddam, H.S.; Mohammadi, M.-R.; Akhondzadeh, S. Risperidone Combination Therapy With Propentofylline for Treatment of Irritability in Autism Spectrum Disorders: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin. Neuropharmacol. 2019, 42, 189. [Google Scholar] [CrossRef] [PubMed]
- Hendouei, F.; Sanjari Moghaddam, H.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as Adjunctive Therapy in Treatment of Irritability in Children with Autism: A Double-Blind and Placebo-Controlled Randomized Trial. J. Clin. Pharm. Ther. 2020, 45, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S. A Case Series of a Luteolin Formulation (NeuroProtek®) in Children with Autism Spectrum Disorders. Int. J. Immunopathol. Pharmacol. 2012, 25, 317–323. [Google Scholar] [CrossRef]
- Saglam, E.; Zırh, S.; Aktas, C.C.; Muftuoglu, S.F.; Bilginer, B. Papaverine Provides Neuroprotection by Suppressing Neuroinflammation and Apoptosis in the Traumatic Brain Injury via RAGE- NF-B Pathway. J. Neuroimmunol. 2021, 352, 577476. [Google Scholar] [CrossRef]
- Bhat, A.; Tan, V.; Heng, B.; Chow, S.; Basappa, S.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J. Papaverine, a Phosphodiesterase 10A Inhibitor, Ameliorates Quinolinic Acid-Induced Synaptotoxicity in Human Cortical Neurons. Neurotox. Res. 2021, 39, 1238–1250. [Google Scholar] [CrossRef]
- Luhach, K.; Kulkarni, G.T.; Singh, V.P.; Sharma, B. Effect of Papaverine on Developmental Hyperserotonemia Induced Autism Spectrum Disorder Related Behavioural Phenotypes by Altering Markers of Neuronal Function, Inflammation, and Oxidative Stress in Rats. Clin. Exp. Pharmacol. Physiol. 2021, 48, 614–625. [Google Scholar] [CrossRef]
- Sharma, N.; Dhiman, N.; Golani, L.K.; Sharma, B. Papaverine Ameliorates Prenatal Alcohol-Induced Experimental Attention Deficit Hyperactivity Disorder by Regulating Neuronal Function, Inflammation, and Oxidative Stress. Int. J. Dev. Neurosci. 2021, 81, 71–81. [Google Scholar] [CrossRef]
- Álvarez, E.; Campos-Toimil, M.; Justiniano-Basaran, H.; Lugnier, C.; Orallo, F. Study of the Mechanisms Involved in the Vasorelaxation Induced by (−)-Epigallocatechin-3-Gallate in Rat Aorta. Br. J. Pharmacol. 2006, 147, 269–280. [Google Scholar] [CrossRef]
- Banji, D.; Banji, O.J.F.; Abbagoni, S.; Hayath, M.S.; Kambam, S.; Chiluka, V.L. Amelioration of Behavioral Aberrations and Oxidative Markers by Green Tea Extract in Valproate Induced Autism in Animals. Brain Res. 2011, 1410, 141–151. [Google Scholar] [CrossRef]
- Kumaravel, P.; Melchias, G.; Vasanth, N.; Manivasagam, T. Epigallocatechin Gallate Attenuates Behavioral Defects in Sodium Valproate Induced Autism Rat Model. Res. J. Pharm. Technol. 2017, 10, 1477–1480. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; Moneti, C.; Serrano-Ballesteros, P.; Castellano, G.; Bayona-Babiloni, R.; Carriquí-Suárez, A.B.; Motos-Muñoz, M.; Proaño, B.; Benlloch, M. Liposomal Epigallocatechin-3-Gallate for the Treatment of Intestinal Dysbiosis in Children with Autism Spectrum Disorder: A Comprehensive Review. Nutrients 2023, 15, 3265. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; de Bari, L.; de Rasmo, D.; Signorile, A.; Henrion-Caude, A.; Contestabile, A.; Vacca, R.A. The Polyphenols Resveratrol and Epigallocatechin-3-Gallate Restore the Severe Impairment of Mitochondria in Hippocampal Progenitor Cells from a Down Syndrome Mouse Model. Biochim. Biophys. Acta 2016, 1862, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, B.d.S.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Teixeira, F.C.; de Souza, A.A.; Stefanello, F.M.; Baldissarelli, J.; Gamaro, G.D.; Spanevello, R.M. Quercetin Prevents Alterations of Behavioral Parameters, Delta-Aminolevulinic Dehydratase Activity, and Oxidative Damage in Brain of Rats in a Prenatal Model of Autism. Int. J. Dev. Neurosci. 2020, 80, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, K.D.; Garipoğlu, G.; Çakar, B.; Uyanıkgil, Y.; Erbaş, O. Antioxidant-Effective Quercetin Through Modulation of Brain Interleukin-13 Mitigates Autistic-Like Behaviors in the Propionic Acid-Induced Autism Model in Rats. J. Neuroimmune Pharmacol. 2025, 20, 36. [Google Scholar] [CrossRef]
- Tanaka, T.; Masubuchi, Y.; Okada, R.; Nakajima, K.; Nakamura, K.; Masuda, S.; Nakahara, J.; Maronpot, R.R.; Yoshida, T.; Koyanagi, M.; et al. Ameliorating Effect of Postweaning Exposure to Antioxidant on Disruption of Hippocampal Neurogenesis Induced by Developmental Hypothyroidism in Rats. J. Toxicol. Sci. 2019, 44, 357–372. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Li, Y.-J.; Zhang, X.; Li, Y.-M. Antineuroinflammatory Therapy: Potential Treatment for Autism Spectrum Disorder by Inhibiting Glial Activation and Restoring Synaptic Function. CNS Spectr. 2020, 25, 493–501. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Resveratrol Suppresses Neuroinflammation in the Experimental Paradigm of Autism Spectrum Disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Zanatta, G.; Della Flora Nunes, G.; Mueller de Melo, G.; Michels, M.; Fontes-Dutra, M.; Nogueira Freire, V.; Riesgo, R.; Gottfried, C. Resveratrol Prevents Social Deficits in Animal Model of Autism Induced by Valproic Acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Khan, H.; Cauli, O. Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects. Antioxidants 2020, 9, 188. [Google Scholar] [CrossRef]
- Yu, M.-C.; Chen, J.-H.; Lai, C.-Y.; Han, C.-Y.; Ko, W.-C. Luteolin, a Non-Selective Competitive Inhibitor of Phosphodiesterases 1–5, Displaced [3H]-Rolipram from High-Affinity Rolipram Binding Sites and Reversed Xylazine/Ketamine-Induced Anesthesia. Eur. J. Pharmacol. 2010, 627, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An Open-Label Pilot Study of a Formulation Containing the Anti-Inflammatory Flavonoid Luteolin and Its Effects on Behavior in Children With Autism Spectrum Disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2016, 23, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, M.; Hu, J.; Du, Z.; Su, Q.; Xiang, Z. Luteolin Suppresses Microglia Neuroinflammatory Responses and Relieves Inflammation-Induced Cognitive Impairments. Neurotox. Res. 2021, 39, 1800–1811. [Google Scholar] [CrossRef]
- Singh, N.K.; Bhushan, B.; Singh, P.; Sahu, K.K. Therapeutic Expedition of Luteolin against Brain-Related Disorders: An Updated Review. Comb. Chem. High. Throughput Screen. 2025, 28, 371–391. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Neuropsychopharmacotherapeutic Efficacy of Curcumin in Experimental Paradigm of Autism Spectrum Disorders. Life Sci. 2015, 141, 156–169. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Isaev, D.; Shabbir, W.; Lorke, D.E.; Sadek, B.; Oz, M. Curcumin Potentiates A7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice. Int. J. Mol. Sci. 2021, 22, 7251. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, L.; Du, Z.; Zhao, L.; Tang, Y.; Fei, X.; Wang, L.; Li, D.; Li, S.; Yang, H.; et al. Icariin Alleviates Autistic-like Behavior, Hippocampal Inflammation and vGlut1 Expression in Adult BTBR Mice. Behav. Brain Res. 2023, 445, 114384. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Afarinesh, M.R.; Zarif, M.; Mirasadi, A.; Esmaeilpour, K. Does Caffeine Therapy Improve Cognitive Impairments in Valproic Acid Rat Model of Autism? Toxin Rev. 2021, 40, 654–664. [Google Scholar] [CrossRef]
- Kuo, C.-J.; Huang, C.-C.; Chou, S.-Y.; Lo, Y.-C.; Kao, T.-J.; Huang, N.-K.; Lin, C.; Lin, H.-C.; Lin, H.-C.; Lee, Y.-C. Potential Therapeutic Effect of Curcumin, a Natural mTOR Inhibitor, in Tuberous Sclerosis Complex. Phytomedicine 2019, 54, 132–139. [Google Scholar] [CrossRef]
- Xin, Z.C.; Kim, E.K.; Lin, C.S.; Liu, W.J.; Tian, L.; Yuan, Y.M.; Fu, J. Effects of Icariin on cGMP-Specific PDE5 and cAMP-Specific PDE4 Activities. Asian J. Androl. 2003, 5, 15–18. [Google Scholar] [PubMed]
- Jin, J.; Wang, H.; Hua, X.; Chen, D.; Huang, C.; Chen, Z. An Outline for the Pharmacological Effect of Icariin in the Nervous System. Eur. J. Pharmacol. 2019, 842, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-Inflammatory and Immunoregulatory Effects of Icariin and Icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Man, H.-Y. Fundamental Elements in Autism: From Neurogenesis and Neurite Growth to Synaptic Plasticity. Front. Cell. Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Gong, H.; Liu, T.; Li, X.; Fan, X. Implication of Hippocampal Neurogenesis in Autism Spectrum Disorder: Pathogenesis and Therapeutic Implications. Curr. Neuropharmacol. 2023, 21, 2266. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Depino, A.M. Peripheral and Central Inflammation in Autism Spectrum Disorders. Mol. Cell. Neurosci. 2013, 53, 69–76. [Google Scholar] [CrossRef]
- Lucchina, L.; Depino, A.M. Altered Peripheral and Central Inflammatory Responses in a Mouse Model of Autism. Autism Res. 2014, 7, 273–289. [Google Scholar] [CrossRef]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front. Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jayaprakash, P.; Marwan, N.Z.H.J.; Aziz, E.A.B.A.; Kuder, K.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice. Pharmaceuticals 2024, 17, 1293. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.K.; Onore, C.E.; Careaga, M.; Rogers, S.J.; Ashwood, P. Increased Monocyte Production of IL-6 after Toll-like Receptor Activation in Children with Autism Spectrum Disorder (ASD) Is Associated with Repetitive and Restricted Behaviors. Brain Sci. 2022, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P. Preliminary Findings of Elevated Inflammatory Plasma Cytokines in Children with Autism Who Have Co-Morbid Gastrointestinal Symptoms. Biomedicines 2023, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Bujnakova, I.; Kovacova, V.; Macejova, A.; Tonhajzerova, I. Peripheral Inflammatory Markers in Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder at Adolescent Age. Int. J. Mol. Sci. 2023, 24, 11710. [Google Scholar] [CrossRef]
- Bordt, E.A.; Polster, B.M. NADPH Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Proinflammatory Microglial Activation: A Bipartisan Affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- Di, J.; Li, J.; O’Hara, B.; Alberts, I.; Xiong, L.; Li, J.; Li, X. The Role of GABAergic Neural Circuits in the Pathogenesis of Autism Spectrum Disorder. Int. J. Dev. Neurosci. 2020, 80, 73–85. [Google Scholar] [CrossRef]
- Sanderson, T.M.; Sher, E. The Role of Phosphodiesterases in Hippocampal Synaptic Plasticity. Neuropharmacology 2013, 74, 86–95. [Google Scholar] [CrossRef]
- Rombaut, B.; Kessels, S.; Schepers, M.; Tiane, A.; Paes, D.; Solomina, Y.; Piccart, E.; van den Hove, D.; Brône, B.; Prickaerts, J.; et al. PDE Inhibition in Distinct Cell Types to Reclaim the Balance of Synaptic Plasticity. Theranostics 2021, 11, 2080–2097. [Google Scholar] [CrossRef]
- Huang, P.; Wei, S.; Luo, M.; Tang, Z.; Lin, Q.; Wang, X.; Luo, M.; He, Y.; Wang, C.; Wei, D.; et al. MiR-139-5p Has an Antidepressant-like Effect by Targeting Phosphodiesterase 4D to Activate the cAMP/PKA/CREB Signaling Pathway. Ann. Transl. Med. 2021, 9, 1594. [Google Scholar] [CrossRef]
- Jino, K.; Miyamoto, K.; Kanbara, T.; Unemura, C.; Horiguchi, N.; Ago, Y. Allosteric Inhibition of Phosphodiesterase 4D Induces Biphasic Memory-Enhancing Effects Associated with Learning-Activated Signaling Pathways. Psychopharmacology 2024, 241, 805–816. [Google Scholar] [CrossRef]
- Li, Y.-F.; Cheng, Y.-F.; Huang, Y.; Conti, M.; Wilson, S.P.; O’Donnell, J.M.; Zhang, H.-T. Phosphodiesterase-4D Knock-Out and RNA Interference-Mediated Knock-Down Enhance Memory and Increase Hippocampal Neurogenesis via Increased cAMP Signaling. J. Neurosci. 2011, 31, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Reneerkens, O.A.H.; Rutten, K.; Steinbusch, H.W.M.; Blokland, A.; Prickaerts, J. Selective Phosphodiesterase Inhibitors: A Promising Target for Cognition Enhancement. Psychopharmacology 2009, 202, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Castrén, M.L.; Castrén, E. BDNF in Fragile X Syndrome. Neuropharmacology 2014, 76 Pt C, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Taylor, C.; Williamson, M.; Newton, S.S.; Qin, L. Diminished Activity-Dependent BDNF Signaling Differentially Causes Autism-like Behavioral Deficits in Male and Female Mice. Front. Psychiatry 2023, 14, 1182472. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Glass, L.; Snape, M.; Hurley, M.J.; Altimiras, F.J.; Biekofsky, R.R.; Cogram, P. NNZ-2566, a Novel Analog of (1-3) IGF-1, as a Potential Therapeutic Agent for Fragile X Syndrome. Neuromolecular Med. 2015, 17, 71–82. [Google Scholar] [CrossRef]
- Heckman, P.R.A.; van Duinen, M.A.; Bollen, E.P.P.; Nishi, A.; Wennogle, L.P.; Blokland, A.; Prickaerts, J. Phosphodiesterase Inhibition and Regulation of Dopaminergic Frontal and Striatal Functioning: Clinical Implications. Int. J. Neuropsychopharmacol. 2016, 19, pyw030. [Google Scholar] [CrossRef]
- Pieretti, S.; Dominici, L.; Di Giannuario, A.; Cesari, N.; Dal Piaz, V. Local Anti-Inflammatory Effect and Behavioral Studies on New PDE4 Inhibitors. Life Sci. 2006, 79, 791–800. [Google Scholar] [CrossRef]
- Ribaudo, G.; Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A. Therapeutic Potential of Phosphodiesterase Inhibitors against Neurodegeneration: The Perspective of the Medicinal Chemist. ACS Chem. Neurosci. 2020, 11, 1726–1739. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Park, J.-S.; Leem, Y.-H.; Park, J.-E.; Kim, H.-S. The Potent PDE10A Inhibitor MP-10 (PF-2545920) Suppresses Microglial Activation in LPS-Induced Neuroinflammation and MPTP-Induced Parkinson’s Disease Mouse Models. J. Neuroimmune Pharmacol. 2021, 16, 470–482. [Google Scholar] [CrossRef]
- Mosenden, R.; Taskén, K. Cyclic AMP-Mediated Immune Regulation--Overview of Mechanisms of Action in T Cells. Cell Signal 2011, 23, 1009–1016. [Google Scholar] [CrossRef]
- Zhou, Q.; Le, M.; Yang, Y.; Wang, W.; Huang, Y.; Wang, Q.; Tian, Y.; Jiang, M.; Rao, Y.; Luo, H.-B.; et al. Discovery of Novel Phosphodiesterase-1 Inhibitors for Curing Vascular Dementia: Suppression of Neuroinflammation by Blocking NF-κB Transcription Regulation and Activating cAMP/CREB Axis. Acta Pharm. Sin. B 2023, 13, 1180–1191. [Google Scholar] [CrossRef]
- You, T.; Cheng, Y.; Zhong, J.; Bi, B.; Zeng, B.; Zheng, W.; Wang, H.; Xu, J. Roflupram, a Phosphodiesterase 4 Inhibitior, Suppresses Inflammasome Activation through Autophagy in Microglial Cells. ACS Chem. Neurosci. 2017, 8, 2381–2392. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Park, J.-S.; Leem, Y.-H.; Park, J.-E.; Kim, D.-Y.; Choi, Y.-H.; Park, E.-M.; Kang, J.L.; Kim, H.-S. The Phosphodiesterase 10 Inhibitor Papaverine Exerts Anti-Inflammatory and Neuroprotective Effects via the PKA Signaling Pathway in Neuroinflammation and Parkinson’s Disease Mouse Models. J. Neuroinflammation 2019, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Lionnard, L.; Singh, I.; Karim, M.A.; Chajra, H.; Frechet, M.; Kissa, K.; Racine, V.; Ammanamanchi, A.; McCarty, P.J.; et al. Mitochondrial Morphology Is Associated with Respiratory Chain Uncoupling in Autism Spectrum Disorder. Transl. Psychiatry 2021, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rincon, N.; McCarty, P.J.; Brister, D.; Scheck, A.C.; Rossignol, D.A. Biomarkers of Mitochondrial Dysfunction in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Neurobiol. Dis. 2024, 197, 106520. [Google Scholar] [CrossRef]
- Jung, N.H.; Janzarik, W.G.; Delvendahl, I.; Münchau, A.; Biscaldi, M.; Mainberger, F.; Bäumer, T.; Rauh, R.; Mall, V. Impaired Induction of Long-Term Potentiation-like Plasticity in Patients with High-Functioning Autism and Asperger Syndrome. Dev. Med. Child. Neurol. 2013, 55, 83–89. [Google Scholar] [CrossRef]
- El-Ansary, A. Data of Multiple Regressions Analysis between Selected Biomarkers Related to Glutamate Excitotoxicity and Oxidative Stress in Saudi Autistic Patients. Data Brief. 2016, 7, 111–116. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Siracusano, M.; Savino, R.; Saso, L.; Scapagnini, G.; Mazzone, L. Oxidative Stress Response and NRF2 Signaling Pathway in Autism Spectrum Disorder. Redox Biol. 2025, 83, 103661. [Google Scholar] [CrossRef]
- Alabdali, A.; Ben Bacha, A.; Alonazi, M.; Al-Ayadhi, L.Y.; Alanazi, A.S.J.; El-Ansary, A. Comparative Evaluation of Certain Biomarkers Emphasizing Abnormal GABA Inhibitory Effect and Glutamate Excitotoxicity in Autism Spectrum Disorders. Front. Psychiatry 2025, 16, 1562631. [Google Scholar] [CrossRef]
- Eleftheriou, C.; Cesca, F.; Maragliano, L.; Benfenati, F.; Maya-Vetencourt, J.F. Optogenetic Modulation of Intracellular Signalling and Transcription: Focus on Neuronal Plasticity. J. Exp. Neurosci. 2017, 11, 1179069517703354. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The Development and Application of Optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef]
- Hufgard, J.R.; Williams, M.T.; Skelton, M.R.; Grubisha, O.; Ferreira, F.M.; Sanger, H.; Wright, M.E.; Reed-Kessler, T.M.; Rasmussen, K.; Duman, R.S.; et al. Phosphodiesterase-1b (Pde1b) Knockout Mice Are Resistant to Forced Swim and Tail Suspension Induced Immobility and Show Upregulation of Pde10a. Psychopharmacology 2017, 234, 1803–1813. [Google Scholar] [CrossRef]
| PDE Family | Isoform | Substrate | Expression in Brain |
|---|---|---|---|
| PDE 1 | IA, IB, IC | cAMP and cGMP | 1A—striatum, hippocampus |
| IB—striatum | |||
| 1C—spinal cord, cerebellum | |||
| PDE 2 | 2A | cAMP and cGMP | frontal lobe, hypothalamus, hippocampus, striatum, substantia nigra, amygdala |
| PDE 3 | 3A, 3B | cAMP and cGMP | 3A—frontal lobe, cingulate cortex, corpus callosum, striatum, cerebellum |
| 3B—frontal lobe, striatum, cerebellum | |||
| PDE 4 | 4A, 4B, 4C, 4D | cAMP | 4A—frontal lobe, parietal lobe, striatum, thalamus, hypothalamus, hippocampus, cerebellum |
| 4B—frontal lobe, parietal lobe, striatum, thalamus, hippocampus, cerebellum | |||
| 4D—frontal lobe, parietal lobe, striatum, thalamus, hippocampus, cerebellum | |||
| PDE 5 | 5A | cGMP | hippocampus, cerebellum |
| PDE 6 | 6A, 6B, 6C, 6D, 6H, 6G | cGMP | rod and cone |
| PDE 7 | 7A, 7B | cAMP | 7A—frontal lobe, temporal lobe, parietal lobe, cingulate cortex, striatum, thalamus, hypothalamus, hippocampus, spinal cord, cerebellum |
| 7B—striatum | |||
| PDE 8 | 8A, 8B | cAMP | 8A—cingulate cortex, corpus callosum, striatum, hypothalamus, spinal cord |
| 8B—frontal lobe, occipital lobe, striatum, thalamus, hippocampus, pons, medulla oblongata, spinal cord | |||
| PDE 9 | 9A | cGMP | Striatum, hypothalamus, hippocampus, cerebellum |
| PDE 10 | 10A | cAMP and cGMP | Striatum, hippocampus, cerebellum |
| PDE 11 | 11A | cAMP and cGMP | hippocampus, cerebellum |
| Isoform | Findings from ASD/FXS Models | Therapeutic Potential of PDE Inhibitor |
|---|---|---|
| PDE1B | Inherited missense PDE1B variants reported in individuals with ASD; Pde1b KO mice show hyperactivity, learning/cognitive deficits | Vinpocetine improves behavioral deficits in rodent models. |
| PDE2A | Elevated PDE2A in Fmr1KO mice reduces cAMP/cGMP, associated with exaggerated mGluR-LTD, immature dendritic spines, and social/cognitive impairments. Heterozygous Pde2a KO led to sex-dependent socio-cognitive deficits. | BAY60-7550 normalizes hippocampal mGluR-LTD, restores axonal/spine morphology, and improves social/cognitive behaviors in FXS models; early postnatal treatment produced long-lasting developmental benefits. Timing (developmental window) is critical for efficacy. |
| PDE4 (B, D) | PDE4 dysregulation contributes to learning/memory deficits. Drosophila and mouse FXS models show PDE4 inhibition rescues mGluR-LTD, cognitive deficits, and brain structural defects. Pde4b KO mice show hyperactivity, impaired associative learning, anxiety-like behavior; Pde4d KO mice show reduced immobility in behavioral despair models. Cc2d1a KO mice reveal male-specific PDE4D hyperactivity, reduced CREB signaling, and spatial memory deficits. | Rolipram (PDE4 inhibitor) rescued learning, memory, and synaptic deficits in FXS and Rett syndrome models. BPN14770 (PDE4D negative allosteric modulator) improves behavior and dendritic spine morphology with persistent effects post-treatment. Isoform and sex-specific effects are critical for efficacy. |
| PDE10A | target of miR-137- critical for neurogenesis and neuronal development. Heterozygous mice show repetitive behaviors, deficits in learning and social behavior, disrupted synaptic plasticity; Pde10a KO mice show decreased exploratory activity, delayed conditioned avoidance, altered dopamine turnover. Elevated PDE10A observed in Fmr1KO mice. | Papaverine (PDE10A inhibitor) or genetic knockdown partially reverses behavioral and cognitive impairments in rodent models of ASD. |
| PDE11A | Dual-specific PDE with splice variant PDE11A4 highly expressed in ventral hippocampus. Essential for normal social interactions; RNA-seq analyses show involvement in oxytocin signaling and membrane signaling, highlighting a role in social behavior regulation. | No specific pharmacological inhibitors tested in ASD/FXS preclinical models. |
| Clinical Investigations of PDE Inhibitors in ASD and FXS | |||
|---|---|---|---|
| Compound | Population | Outcomes | References/Trial |
| BPN14770 | A 2-period crossover study of BPN14770 in adult males with fragile X syndrome (phase 2). | Cognitive gains in language abilities, along with improvements in caregiver assessments of language and daily functioning. | NCT03569631 |
| A randomized study of BPN14770 in male adolescents (aged 9 to <18 years) with fragile X syndrome (phase 2/3). | Active, not recruiting. | NCT05163808 | |
| A study of BPN14770 in male adults (aged 18 to 45) with Fragile X syndrome (phase 3). | Completed, no study results posted. | NCT05358886 | |
| An open-label extension study of BPN14770 in subjects with fragile X syndrome (phase 3). | Active, not recruiting. | NCT0536790 | |
| Cilostazol | Cilostazol (50–100 mg/day) adjunct to risperidone—61 children (aged 5–11 years) with ASD (double-blind, randomized clinical trial). | Safe and well-tolerated; significant improvement in hyperactivity subscale in children with higher baseline hyperactivity. | [77] |
| Pentoxifylline | Forty children with ASD (4–12 years); double-blind, placebo-controlled 10-week trial; adjunct to risperidone. | Significant improvement across multiple ABC-C subscales: irritability, social withdrawal, stereotypic behavior and hyperactivity. | [80] |
| STP1 (Ibudilast + bumetanide) | 12 ASD patients (phase 1b, randomized, double-blind, placebo-controlled). | Safe and well-tolerated. EEG markers showed dose-related reduction in gamma power (linked to executive function/memory). Numerical but not statistically significant improvements in clinical scales. | NCT04644003 |
| Propentofylline | Forty-eight children with ASD; randomized, double-blind, placebo-controlled (10 weeks); adjunctive treatment with risperidone. | Adjunctive propentofylline + risperidone showed significant improvement in irritability subscale and CARS scores vs. placebo. | [103] |
| Resveratrol | Sixty-two patients with ASD; randomized, placebo-controlled (10 weeks); adjunct to risperidone. | Resveratrol significantly improved hyperactivity/non-compliance scores. No significant effect on irritability. | [104] |
| NeuroProtek® (Luteolin +Quercetin + Rutin) | Thirty-seven children with ASD (4–14 years). | Improved sociability/adaptive functioning; safe and well-tolerated. | [105] |
| Fifty children with ASD (4–10 yrs); 26-week open-label trial. | Significant improvement in adaptive functioning; effective in reducing ASD symptoms, with no major adverse effects. | NCT01847521 | |
| Preclinical Investigations of Classical PDE Inhibitors Effective in FXS and ASD | |||
|---|---|---|---|
| Compound | Dose/Model | Outcomes | References |
| BPN14770 | 0.3, 1, and 3.0 mg/kg dietary administration; Fmr1 KO mice | Ameliorated hyperactivity in open field. Improved social behavior. | [6] |
| 0.3 mg/kg, Fmr1 KO mice | Improved social interaction, improved dendritic spine morphology. Behavioral improvements persisted 2 weeks after washout. | [12] | |
| Pentoxifylline | 300 mg/kg/day, rat PPA model | ↓ TNF-α, oxidative stress; NGF; improved autism-like behaviors. | [81] |
| Ibudilast | 5 and 10 mg/kg, VPA rat model | Improved social interaction, learning, memory; ↓ anxiety and hyperactivity, ↓oxidative stress, ↓pro-inflammatory cytokines. | [84] |
| Ibudilast (6mg/kg) + Gaboxadol (1.5 mg/kg), Fmr1 KO mice | Combination treatment rescued both cognitive and behavioral deficits. No adverse effects observed. | [85] | |
| BAY60-7550 | 0.05 mg/kg at infancy; 0.1 mg/kg adolescence/adulthood Fmr1-Δexon 8 rat, VPA rat model | Improved communicative, social, and cognitive impairments. | [50] |
| Fmr1KO mouse Fmr1KO rat | PDE2A inhibition rescued dendritic spine maturity and exaggerated mGluR-LTD. Restored social and communicative deficits in Fmr1KO mice and rats. | [49] | |
| Vinpocetine | 10/20 mg/kg, VPA rat model | Improved ASD-like behaviors, increased BDNF, synapsin-IIa, DCX, pCREB/CREB, IL-10, GSH; decreased TNF-α, IL-6, TBARS. | [44] |
| Balipodect (TAK-063) | 0.5 and 5 mg/kg, Fmr1KO mice | Improved EEG biomarkers. Normalized cortical auditory processing without depressing baseline EEG power or causing any noticeable sedation or behavioral side effects. | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.D.; Mohammed, H.A.; Hamad, M.I.K.; Oz, M.; Statsenko, Y.; Sadek, B. The Emerging Role of Phosphodiesterase Inhibitors in Fragile X Syndrome and Autism Spectrum Disorder. Pharmaceuticals 2025, 18, 1507. https://doi.org/10.3390/ph18101507
Thomas SD, Mohammed HA, Hamad MIK, Oz M, Statsenko Y, Sadek B. The Emerging Role of Phosphodiesterase Inhibitors in Fragile X Syndrome and Autism Spectrum Disorder. Pharmaceuticals. 2025; 18(10):1507. https://doi.org/10.3390/ph18101507
Chicago/Turabian StyleThomas, Shilu Deepa, Hend Abdulaziz Mohammed, Mohammad I. K. Hamad, Murat Oz, Yauhen Statsenko, and Bassem Sadek. 2025. "The Emerging Role of Phosphodiesterase Inhibitors in Fragile X Syndrome and Autism Spectrum Disorder" Pharmaceuticals 18, no. 10: 1507. https://doi.org/10.3390/ph18101507
APA StyleThomas, S. D., Mohammed, H. A., Hamad, M. I. K., Oz, M., Statsenko, Y., & Sadek, B. (2025). The Emerging Role of Phosphodiesterase Inhibitors in Fragile X Syndrome and Autism Spectrum Disorder. Pharmaceuticals, 18(10), 1507. https://doi.org/10.3390/ph18101507









