Design of Hybrid Quinoline–Chalcone Compounds Against Leishmania amazonensis Based on Computational Techniques: 2D- and 3D-QSAR with Experimental Validation
Abstract
1. Introduction
2. Results
2.1. Theoretical Models
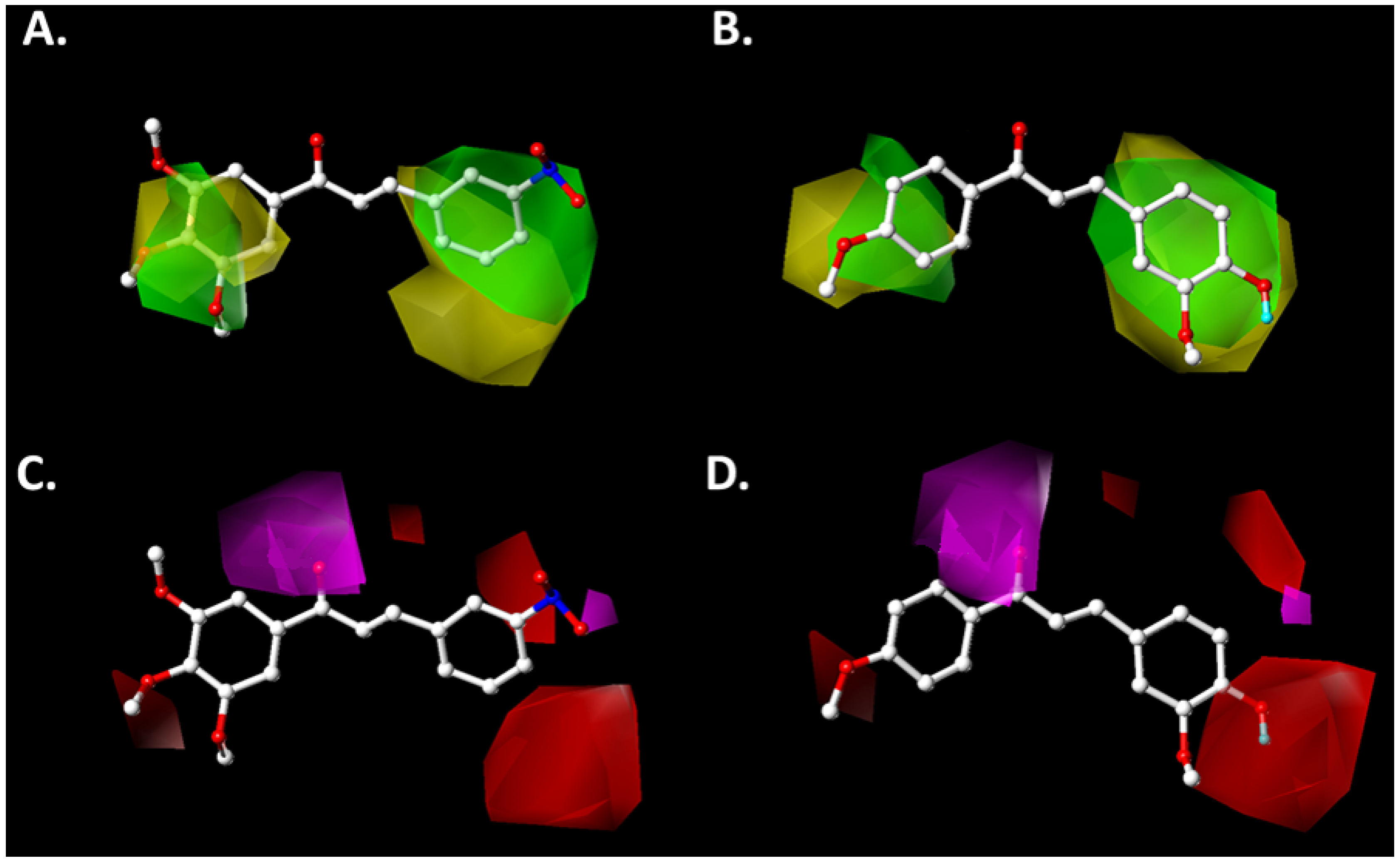
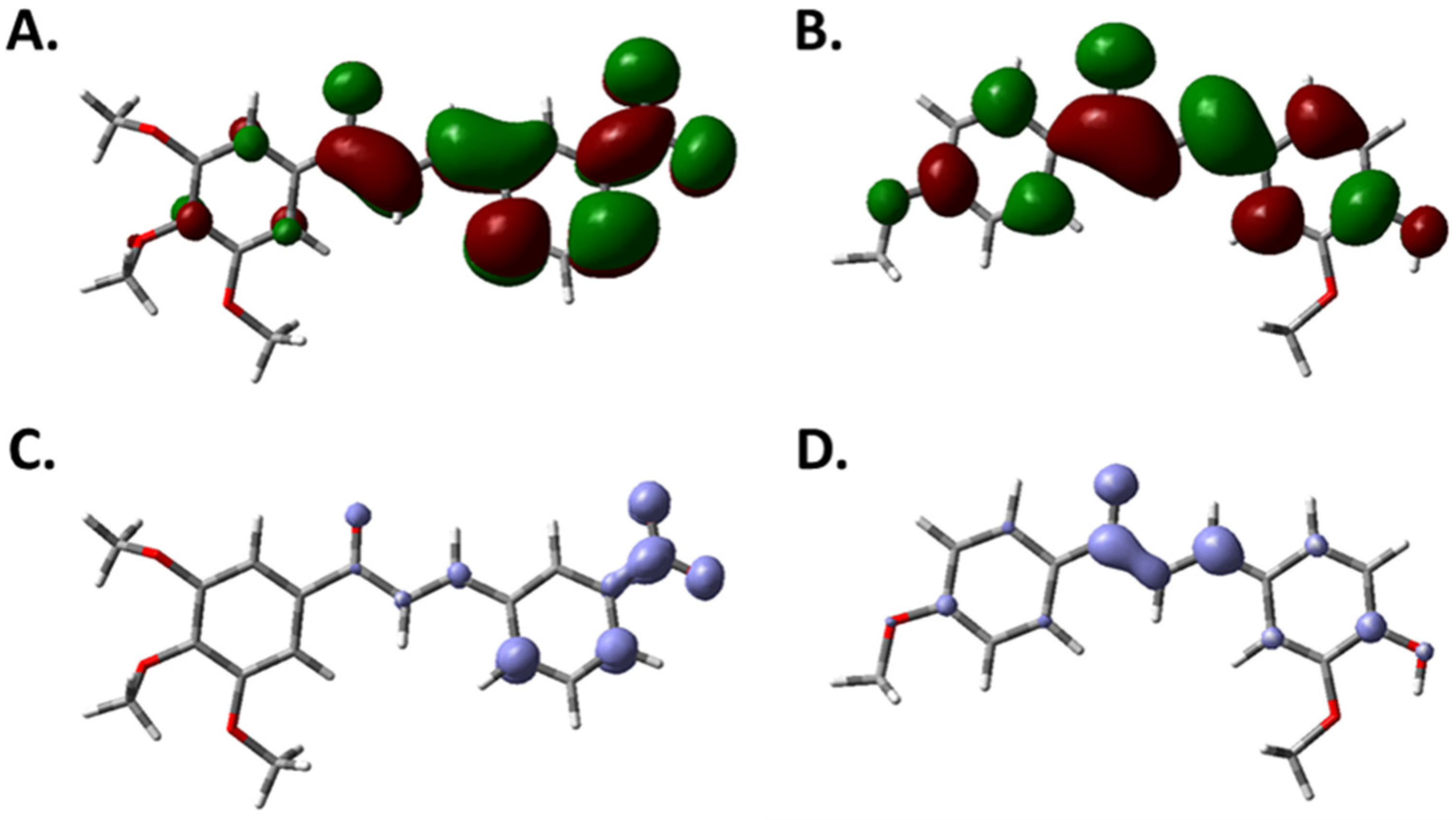
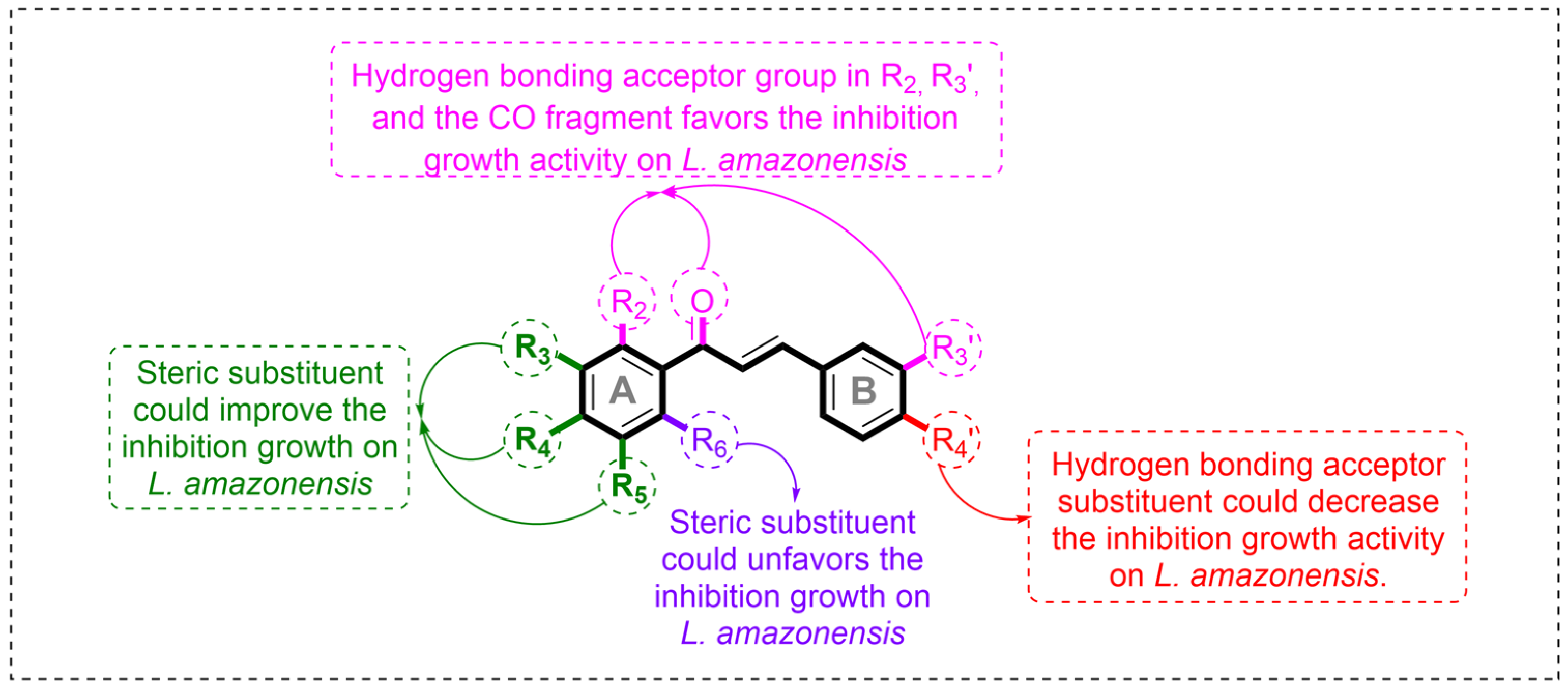
2.2. Contour Map Analysis
2.2.1. Steric Map
2.2.2. Hydrogen-Bonding Acceptor Map
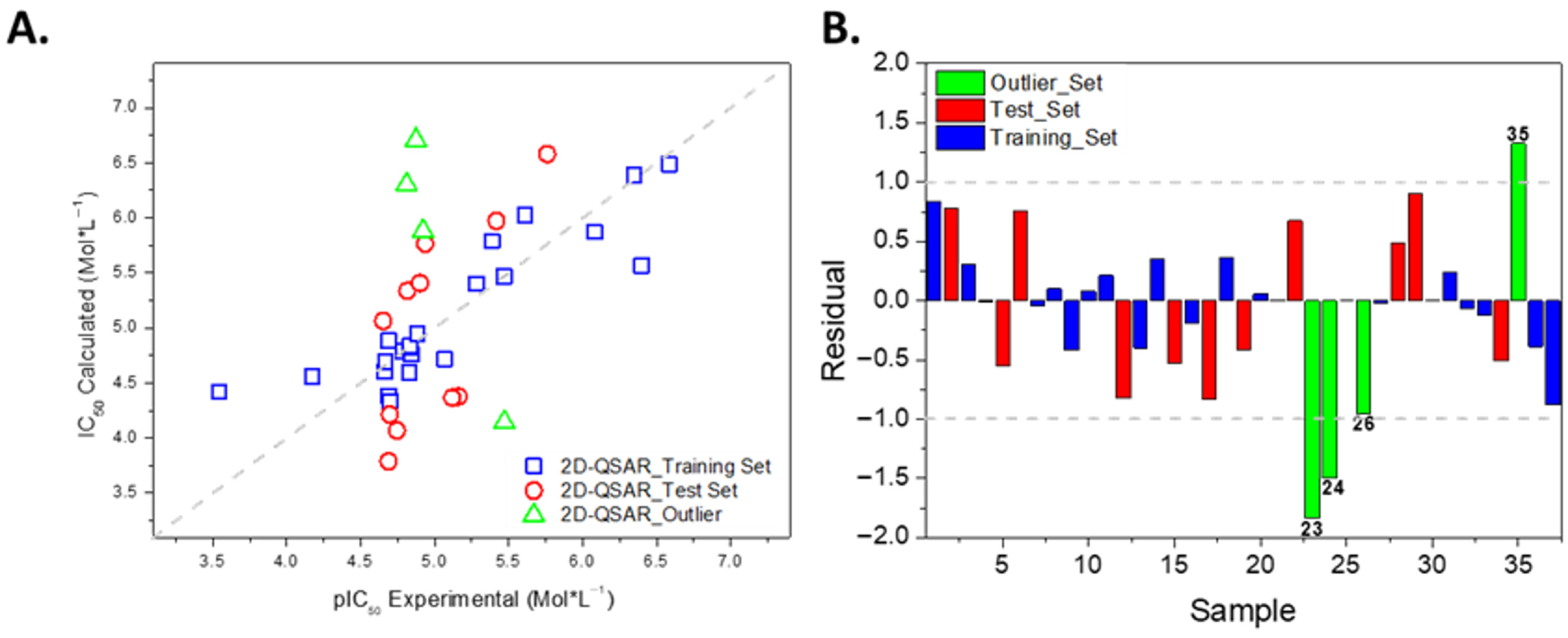
2.3. Development of the 2D-QSAR Model
2.4. Summary of the Principal Results from the Computational Models and Design
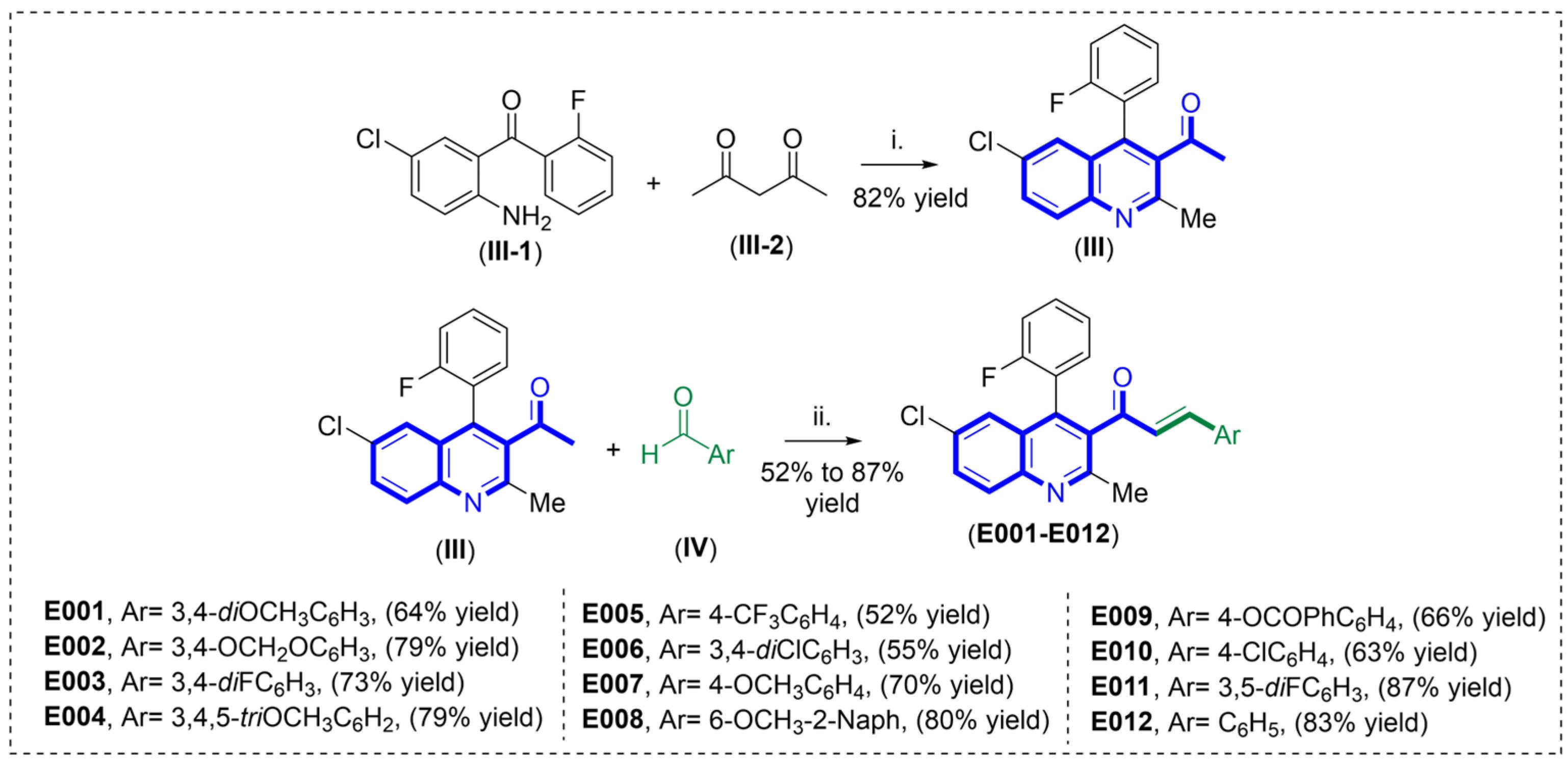
2.5. Synthesis of New Quinoline–Chalcone Hybrids Driven by QSAR Models
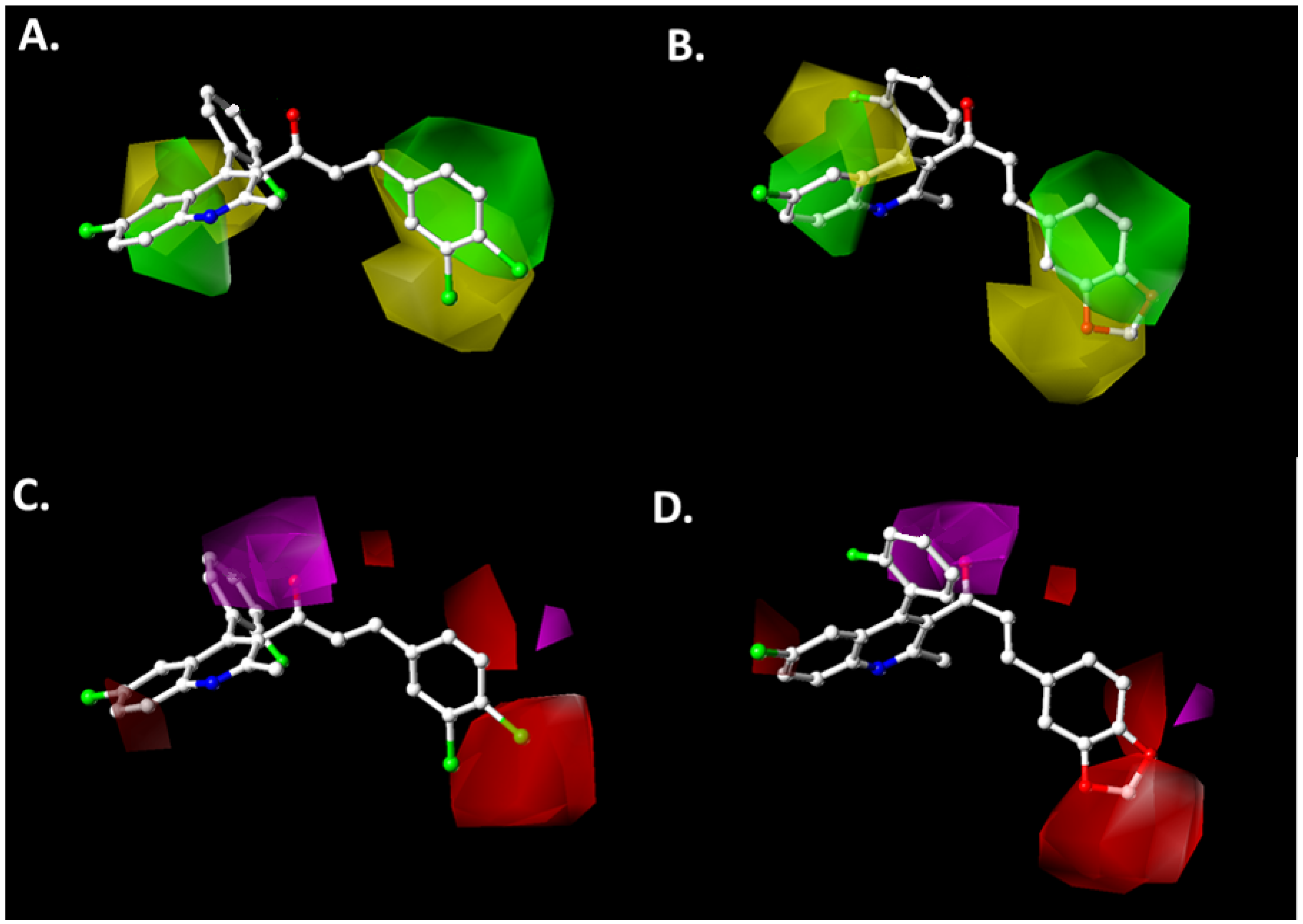
2.6. Assessment of Physicochemical Profiles and ADME Properties
3. Materials and Methods
3.1. Computational Models
3.1.1. Development of 3D-QSAR Model
3.1.2. Comparative Molecular Similarity Index (CoMSIA) Field Generation
3.1.3. Model Construction and Internal Validation
3.1.4. External Validation of the CoMSIA Model
3.1.5. Two-Dimensional Quantitative Structure-Activity Relationship Model
3.1.6. Evaluation of Physicochemical and Pharmacokinetic Properties
3.2. Synthesis of Compounds
3.2.1. Instruments and Chemicals
3.2.2. Chemistry
Synthesis of 1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)ethan-1-one (III)
General Procedure for the Synthesis of Quinoline–Chalcones E001–E012
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(3,4-dimetoxyphenyl)prop-2-en-1-one (E001).
- (E)-3-(benzo[d][1,3]dioxol-5-yl)1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)prop-2-en-1-one (E002)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(3,4-difluorophenyl)prop-2-en-1-one (E003)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(3,4,5-trimetoxyphenyl)prop-2-en-1-one (E004)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (E005)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(3,4-dichlorophenyl)prop-2-en-1-one (E006)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(4-metoxyphenyl)prop-2-en-1-one (E007)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(6-metoxynapthalen-2-yl)prop-2-en-1-one (E008)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-oxoprop-1en-1-yl)phenyl benzoate (E009)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(4-cholrophenyl)prop-2-en-1-one (E010)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-(3,5-difluorophenyl)prop-2-en-1-one (E011)
- (E)-1-(6-chloro-4-(2-fluorophenyl)-2-methylquinolin-3-yl)-3-phenylprop-2-en-1-one (E012)
3.2.3. Biology
Parasites
Viability Assay
Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania Infantum and Dirofilaria Immitis Infections in Italy, 2009–2019: Changing Distribution Patterns. Parasites Vectors 2020, 13, 193. [Google Scholar] [CrossRef]
- Alonso, L.; Menegatti, R.; Gomes, R.S.; Dorta, M.L.; Luzin, R.M.; Liao, L.M.; Alonso, A. Antileishmanial Activity of the Chalcone Derivative LQFM064 Associated with Reduced Fluidity in the Parasite Membrane as Assessed by EPR Spectroscopy. Eur. J. Pharm. Sci. 2020, 151, 105407. [Google Scholar] [CrossRef]
- Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 13 January 2025).
- Sabt, A.; Eldehna, W.M.; Ibrahim, T.M.; Bekhit, A.A.; Batran, R.Z. New Antileishmanial Quinoline Linked Isatin Derivatives Targeting DHFR-TS and PTR1: Design, Synthesis, and Molecular Modeling Studies. Eur. J. Med. Chem. 2023, 246, 114959. [Google Scholar] [CrossRef]
- Glanzmann, N.; Ribeiro Antinarelli, L.M.; da Costa Nunes, I.K.; Gualberto Pereira, H.M.; Ferraz Coelho, E.A.; Coimbra, E.S.; da Silva, A.D. Synthesis and Biological Activity of Novel 4-Aminoquinoline/1,2,3-Triazole Hybrids against Leishmania Amazonensis. Biomed. Pharmacother. 2021, 141, 111857. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Abada, G.; Dammann, M.; Maklad, R.M.; Eldehna, W.M.; Salem, R.; Abdelaziz, M.M.; El-domany, R.A.; Bekhit, A.A.; Beockler, F.M. Tetrahydrobenzo[h]Quinoline Derivatives as a Novel Chemotype for Dual Antileishmanial-Antimalarial Activity Graced with Antitubercular Activity: Design, Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2023, 257, 115534. [Google Scholar] [CrossRef]
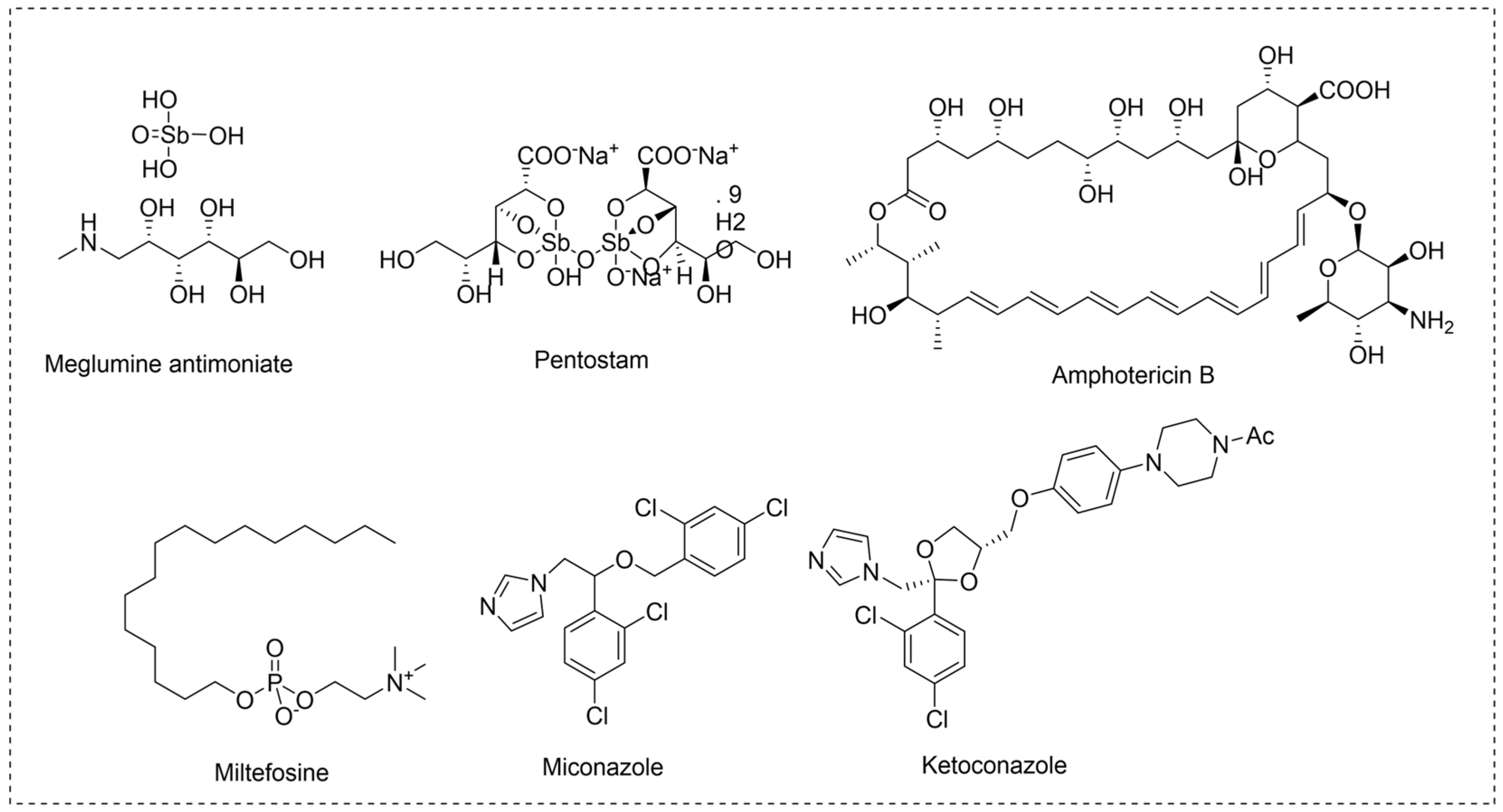
- Sundar, S.; Chakravarty, J. Leishmaniasis: An Update of Current Pharmacotherapy. Expert Opin. Pharmacother. 2013, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Adler-Moore, J.; Berenguer, J.; Boelaert, M.; den Boer, M.; Davidson, R.N.; Figueras, C.; Gradoni, L.; Kafetzis, D.A.; Ritmeijer, K.; et al. Liposomal Amphotericin B for the Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 2006, 43, 917–924. [Google Scholar] [CrossRef]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A Review of Its Pharmacology and Therapeutic Efficacy in the Treatment of Leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Berman, J. Activity of Imidazoles Against Leishmania-Tropica in Human Macrophage Cultures. Am. J. Trop. Med. Hyg. 1981, 30, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, R.; Liu, Y.; Yu, M.; He, Z.; Xiao, J.; Li, K.; Liu, G.; Ning, Q.; Li, Y. Progress in Antileishmanial Drugs: Mechanisms, Challenges, and Prospects. PLoS Neglect. Trop. Dis. 2025, 19, e0012735. [Google Scholar] [CrossRef]
- Moncada-Diaz, M.J.; Rodriguez-Almonacid, C.C.; Quiceno-Giraldo, E.; Khuong, F.T.H.; Muskus, C.; Karamysheva, Z.N. Molecular Mechanisms of Drug Resistance in Leishmania Spp. Pathogens 2024, 13, 835. [Google Scholar] [CrossRef]
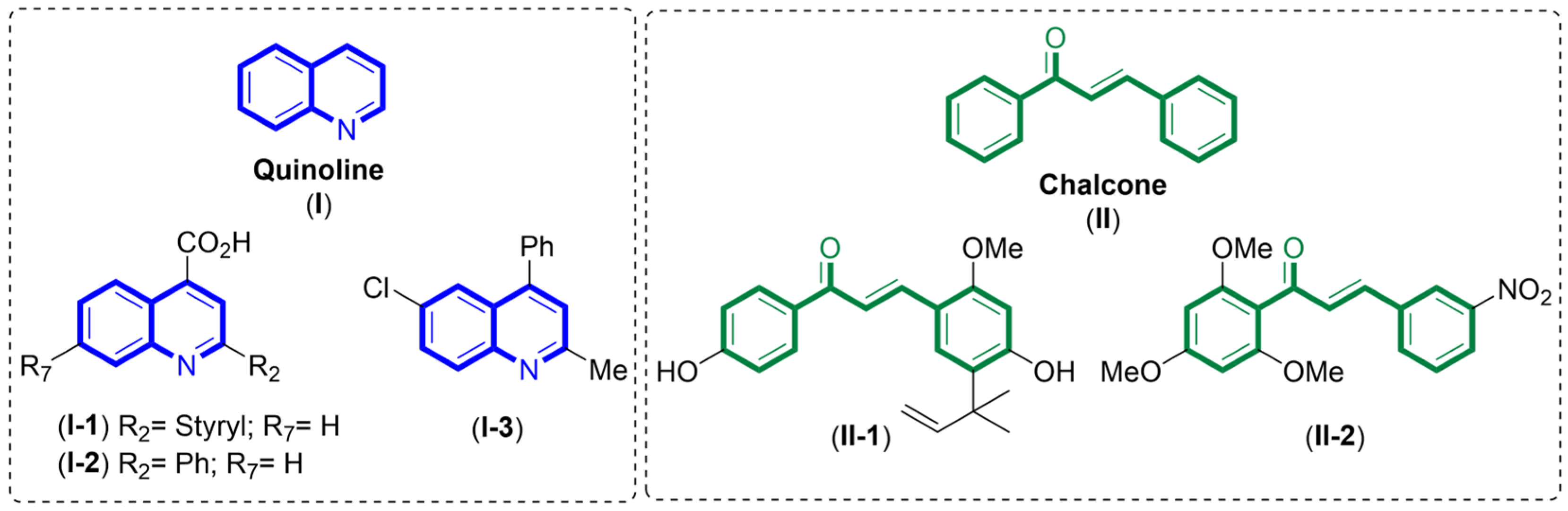
- Guillon, J.; Cohen, A.; Boudot, C.; Valle, A.; Milano, V.; Das, R.N.; Gudin, A.; Moreau, S.; Ronga, L.; Savrimoutou, S.; et al. Design, Synthesis, and Antiprotozoal Evaluation of New 2,4-Bis[(Substituted-Aminomethyl)Phenyl]Quinoline, 1,3-Bis[(Substituted-Aminomethyl)Phenyl]Isoquinoline and 2,4-Bis[(Substituted-Aminomethyl)Phenyl]Quinazoline Derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 432–459. [Google Scholar] [CrossRef]
- Luczywo, A.; Sauter, I.P.; da Silva Ferreira, T.C.; Cortez, M.; Romanelli, G.P.; Sathicq, G.; Asis, S.E. Microwave-Assisted Synthesis of 2-Styrylquinoline-4-Carboxylic Acid Derivatives to Improve the Toxic Effect against Leishmania (Leishmania) amazonensis. J. Heterocycl. Chem. 2021, 58, 822–832. [Google Scholar] [CrossRef]
- Muscia, G.C.; Carnevale, J.P.; Bollini, M.; Asis, S.E. Microwave-Assisted Dobner Synthesis of 2-Phenylquinoline-4-Carboxylic Acids and Their Antiparasitic Activities. J. Heterocycl. Chem. 2008, 45, 611–614. [Google Scholar] [CrossRef]
- Muscia, G.C.; Bollini, M.; Carnevale, J.P.; Bruno, A.M.; Asis, S.E. Microwave-Assisted Friedlander Synthesis of Quinolines Derivatives as Potential Antiparasitic Agents. Tetrahedron Lett. 2006, 47, 8811–8815. [Google Scholar] [CrossRef]
- Boeck, P.; Falcao, C.A.B.; Leal, P.C.; Yunes, R.A.; Cechinel, V.; Torres-Santos, E.C.; Rossi-Bergmann, B. Synthesis of Chalcone Analogues with Increased Antileishmanial Activity. Bioorg. Med. Chem. 2006, 14, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kiderlen, A.F. In Vitro Leishmanicidal Activity of Naturally Occurring Chalcones. Phytother. Res. 2001, 15, 148–152. [Google Scholar] [CrossRef]
- Escrivani, D.O.; Charlton, R.L.; Caruso, M.B.; Burle-Caldas, G.A.; Borsodi, M.P.G.; Zingali, R.B.; Palmeira-Mello, M.; de Jesus, J.B.; Souza, A.M.T.; Abrahim-Vieira, B.; et al. Chalcones Identify cTXNPx as a Potential Antileishmanial Drug Target. PLoS Neglect. Trop. Dis. 2021, 15, e0009951. [Google Scholar] [CrossRef]
- Singh, G.; Arora, A.; Mangat, S.S.; Rani, S.; Kaur, H.; Goya, K.; Sehgal, R.; Maurya, I.K.; Tewari, R.; Choquesillo-Lazarte, D.; et al. Design, Synthesis and Biological Evaluation of Chalconyl Blended Triazole Allied Organosilatranes as Giardicidal and Trichomonacidal Agents. Eur. J. Med. Chem. 2016, 108, 287–300. [Google Scholar] [CrossRef]
- Otero, E.; Vergara, S.; Robledo, S.M.; Cardona, W.; Carda, M.; Velez, I.D.; Rojas, C.; Otalvaro, F. Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids. Molecules 2014, 19, 13251–13266. [Google Scholar] [CrossRef]
- Albratty, M. Quantitative Structure-Activity Relationship Modeling and Docking of Some Synthesized Bioactive Oxopyrolidines against Staphylococcus Aureus. J. Saudi Chem. Soc. 2022, 26, 101509. [Google Scholar] [CrossRef]
- Gianibbi, B.; Visibelli, A.; Spinsanti, G.; Spiga, O. Three-Dimensional Quantitative Structure-Activity Relationship Study of Transient Receptor Potential Vanilloid 1 Channel Antagonists Reveals Potential for Drug Design Purposes. Int. J. Mol. Sci. 2024, 25, 7951. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Espinoza, L.; Madrid, A.; Mella, J.; Chavez-Weisser, E.; Diaz, K.; Cuellar, M. Design, Synthesis, Antifungal Activity, and Structure-Activity Relationship Studies of Chalcones and Hybrid Dihydrochromane-Chalcones. Mol. Divers. 2020, 24, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Lorca, M.; Muscia, G.C.; Perez-Benavente, S.; Bautista, J.M.; Acosta, A.; Gonzalez, C.; Sabadini, G.; Mella, J.; Asis, S.E.; Mellado, M. 2D/3D-QSAR Model Development Based on a Quinoline Pharmacophoric Core for the Inhibition of Plasmodium falciparum: An In Silico Approach with Experimental Validation. Pharmaceuticals 2024, 17, 889. [Google Scholar] [CrossRef]
- Roy, K.; Chakraborty, P.; Mitra, I.; Ojha, P.K.; Kar, S.; Das, R.N. Some Case Studies on Application of “rm2” Metrics for Judging Quality of Quantitative Structure-Activity Relationship Predictions: Emphasis on Scaling of Response Data. J. Comput. Chem. 2013, 34, 1071–1082. [Google Scholar] [CrossRef]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol. Inf. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q. What Is a Randomization Test? J. Am. Stat. Assoc. 2023, 118, 2928–2942. [Google Scholar] [CrossRef]
- Satchell, D.P.N.; Satchell, R.S. Quantitative Aspects of the Lewis Acidity of Covalent Metal Halides and Their Organo Derivatives. Chem. Rev. 1969, 69, 251–278. [Google Scholar] [CrossRef]
- Bakaric, D.; Baranovic, G. The Conformational Equilibrium and Vibrational Properties of Chalcone. J. Mol. Struct. 2019, 1196, 429–438. [Google Scholar] [CrossRef]
- Mellado, M.; Reyna-Jeldes, M.; Weinstein-Oppenheimer, C.; Covarrubias, A.A.; Aguilar, L.F.; Coddou, C.; Mella, J.; Cuellar, M.A. QSAR-Driven Synthesis of Antiproliferative Chalcones against SH-SY5Y Cancer Cells: Design, Biological Evaluation, and Redesign. Arch. Pharm. 2022, 355, e2200042. [Google Scholar] [CrossRef]
- Passalacqua, T.G.; Torres, F.A.E.; Nogueira, C.T.; de Almeida, L.; Del Cistia, M.L.; dos Santos, M.B.; Regasini, L.O.; Graminha, M.A.S.; Marchetto, R.; Zottis, A. The 2′,4′-Dihydroxychalcone Could Be Explored to Develop New Inhibitors against the Glycerol-3-Phosphate Dehydrogenase from Leishmania Species. Bioorg. Med. Chem. Lett. 2015, 25, 3564–3568. [Google Scholar] [CrossRef]
- Borges, E.N.; Alonso, L.; Silveira, M.B.; Balbinot, R.B.; Nakamura, C.V.; da Rocha, A.L.B.; Arruda, E.L.; dos Santos, G.F.; Vaz, B.G.; Gomides, C.D.; et al. Antileishmanial Activities of Three Chalcone Derivatives and Their Association with Plasma Membrane Rigidity as Assessed by EPR Spectroscopy. J. Mol. Struct. 2023, 1292, 136123. [Google Scholar] [CrossRef]
- Batista, A.S.; Oliveira, S.D.S.; Pomel, S.; Commere, P.-H.; Mazan, V.; Lee, M.; Loiseau, P.M.; Rossi-Bergmann, B.; Prina, E.; Duval, R. Targeting Chalcone Binding Sites in Living Leishmania Using a Reversible Fluorogenic Benzochalcone Probe. Biomed. Pharmacother. 2022, 149, 112784. [Google Scholar] [CrossRef] [PubMed]
- Din, Z.U.; Fill, T.P.; de Assis, F.F.; Lazarin-Bidoia, D.; Kaplum, V.; Garcia, F.P.; Nakamura, C.V.; de Oliveira, K.T.; Rodrigues-Filho, E. Unsymmetrical 1,5-Diaryl-3-Oxo-1,4-Pentadienyls and Their Evaluation as Antiparasitic Agents. Bioorg. Med. Chem. 2014, 22, 1121–1127. [Google Scholar] [CrossRef]
- Mendes, E.P.; Goulart, C.M.; Chaves, O.A.; Faioes, V.d.S.; Canto-Carvalho, M.M.; Machado, G.C.; Torres-Santos, E.C.; Echevarria, A. Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies. Biomolecules 2019, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; de Carvalho, E.A.A.; Candido, A.C.B.B.; de Mendonca, R.P.; Fernanda da Silva, M.; Parreira, R.L.T.; Dias, F.G.G.; Ambrosio, S.R.; Arantes, A.T.; da Silva Filho, A.A.; et al. Licochalcone a Exhibits Leishmanicidal Activity in Vitro and in Experimental Model of Leishmania (Leishmania) infantum. Front. Vet. Sci. 2020, 7, 527. [Google Scholar] [CrossRef]
- Klebe, G.; Abraham, U.; Mietzner, T. Molecular Similarity Indexes in a Comparative-Analysis (Comsia) of Drug Molecules to Correlate and Predict Their Biological-Activity. J. Med. Chem. 1994, 37, 4130–4146. [Google Scholar] [CrossRef]
- Clark, M.; Cramer, R.; Vanopdenbosch, N. Validation of the General-Purpose Tripos 5.2 Force-Field. J. Comput. Chem. 1989, 10, 982–1012. [Google Scholar] [CrossRef]
- Mellado, M.; Madrid, A.; Martínez, Ú.; Mella, J.; Salas, C.O.; Cuellar, M. Hansch’s Analysis Application to Chalcone Synthesis by Claisen–Schmidt Reaction Based in DFT Methodology. Chem. Pap. 2018, 72, 703–709. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision A.02 2016; ScienceOpen, Inc.: Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch/index.php (accessed on 8 March 2025).


| Compound | IC50 (µM) | pIC50 | ||||
|---|---|---|---|---|---|---|
| Experimental | CoMSIA-SA | Residual | 2D-QSAR | Residual | ||
| E001 | In | - | - | - | - | - |
| E002 | 13.27 ± 0.43 | 4.876 | 5.212 | −0.336 | 5.562 | −0.686 |
| E003 | 2.38 ± 0.06 | 5.623 | 5.602 | 0.021 | 6.256 | −0.633 |
| E004 | 5.75 ± 0.36 | 5.240 | 5.262 | −0.022 | 5.685 | −0.445 |
| E005 | 2.61 ± 0.32 | 5.583 | 5.552 | 0.031 | 6.522 | −0.939 |
| E006 | 2.23 ± 0.75 | 5.652 | 5.645 | 0.007 | 6.510 | −0.858 |
| E007 | 12.08 ± 0.92 | 4.917 | 5.201 | −0.284 | 5.370 | −0.453 |
| E008 | In | - | - | - | - | - |
| E009 | 4.19 ± 0.31 | 5.378 | 5.474 | −0.096 | 5.843 | −0.465 |
| E010 | In | - | - | - | - | - |
| E011 | 2.67 ± 0.18 | 5.573 | 5.519 | 0.054 | 6.454 | −0.881 |
| E012 | 5.56 ± 0.36 | 5.255 | 5.486 | −0.231 | 5.808 | −0.553 |
| RMSEP | 0.170 | 0.682 | ||||
| MAE | 0.120 | 0.657 | ||||
| Property |  E003 (2.38 µM) | 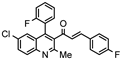 E005 (2.61 µM) | 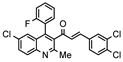 E006 (2.23 µM) | 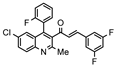 E011 (2.67 µM) |
| H-Bond acceptors | 5 | 6 | 3 | 5 |
| MR | 116.84 | 121.92 | 126.94 | 116.84 |
| iLogP | 4.05 | 4.34 | 4.33 | 4.16 |
| ESOL log S | −7.17 | −7.7 | −8.04 | −7.17 |
| Esol Class | Low | Low | Low | Low |
| GI absorption | Low | Low | Low | Low |
| BBB permeant | No | No | No | No |
| P-gp substract | Yes | Yes | Yes | Yes |
| Inh CYP2C19 | Yes | No | No | Yes |
| Inh CYP2C9 | No | No | No | No |
| Inh CYP2D6 | No | No | No | No |
| Inh CYP3A4 | No | No | No | No |
| Violations Lipinsky | 1 | 1 | 1 | 1 |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorca, M.; Muscia, G.C.; Mella, J.; Thomaz, L.; Yokoyama-Yasunaka, J.K.; Moraga, D.; Rodriguez-Nuñez, Y.A.; Asís, S.E.; Cortez, M.; Mellado, M. Design of Hybrid Quinoline–Chalcone Compounds Against Leishmania amazonensis Based on Computational Techniques: 2D- and 3D-QSAR with Experimental Validation. Pharmaceuticals 2025, 18, 1567. https://doi.org/10.3390/ph18101567
Lorca M, Muscia GC, Mella J, Thomaz L, Yokoyama-Yasunaka JK, Moraga D, Rodriguez-Nuñez YA, Asís SE, Cortez M, Mellado M. Design of Hybrid Quinoline–Chalcone Compounds Against Leishmania amazonensis Based on Computational Techniques: 2D- and 3D-QSAR with Experimental Validation. Pharmaceuticals. 2025; 18(10):1567. https://doi.org/10.3390/ph18101567
Chicago/Turabian StyleLorca, Marcos, Gisela C. Muscia, Jaime Mella, Luciana Thomaz, Jenicer K. Yokoyama-Yasunaka, Daniel Moraga, Yeray A. Rodriguez-Nuñez, Silvia E. Asís, Mauro Cortez, and Marco Mellado. 2025. "Design of Hybrid Quinoline–Chalcone Compounds Against Leishmania amazonensis Based on Computational Techniques: 2D- and 3D-QSAR with Experimental Validation" Pharmaceuticals 18, no. 10: 1567. https://doi.org/10.3390/ph18101567
APA StyleLorca, M., Muscia, G. C., Mella, J., Thomaz, L., Yokoyama-Yasunaka, J. K., Moraga, D., Rodriguez-Nuñez, Y. A., Asís, S. E., Cortez, M., & Mellado, M. (2025). Design of Hybrid Quinoline–Chalcone Compounds Against Leishmania amazonensis Based on Computational Techniques: 2D- and 3D-QSAR with Experimental Validation. Pharmaceuticals, 18(10), 1567. https://doi.org/10.3390/ph18101567








