Phytochemical Profiling and Molecular Insights of Centaurea lycaonica: Apoptosis Induction via the Intrinsic Pathway in Endometrial Cancer Cells
Abstract
1. Introduction
2. Results
2.1. LC-HRMS Results
2.1.1. Qualitative Analyses Results
2.1.2. Quantative Analyses Results
2.2. Effects of the C. lycaonica Extracts on the Cell Viability
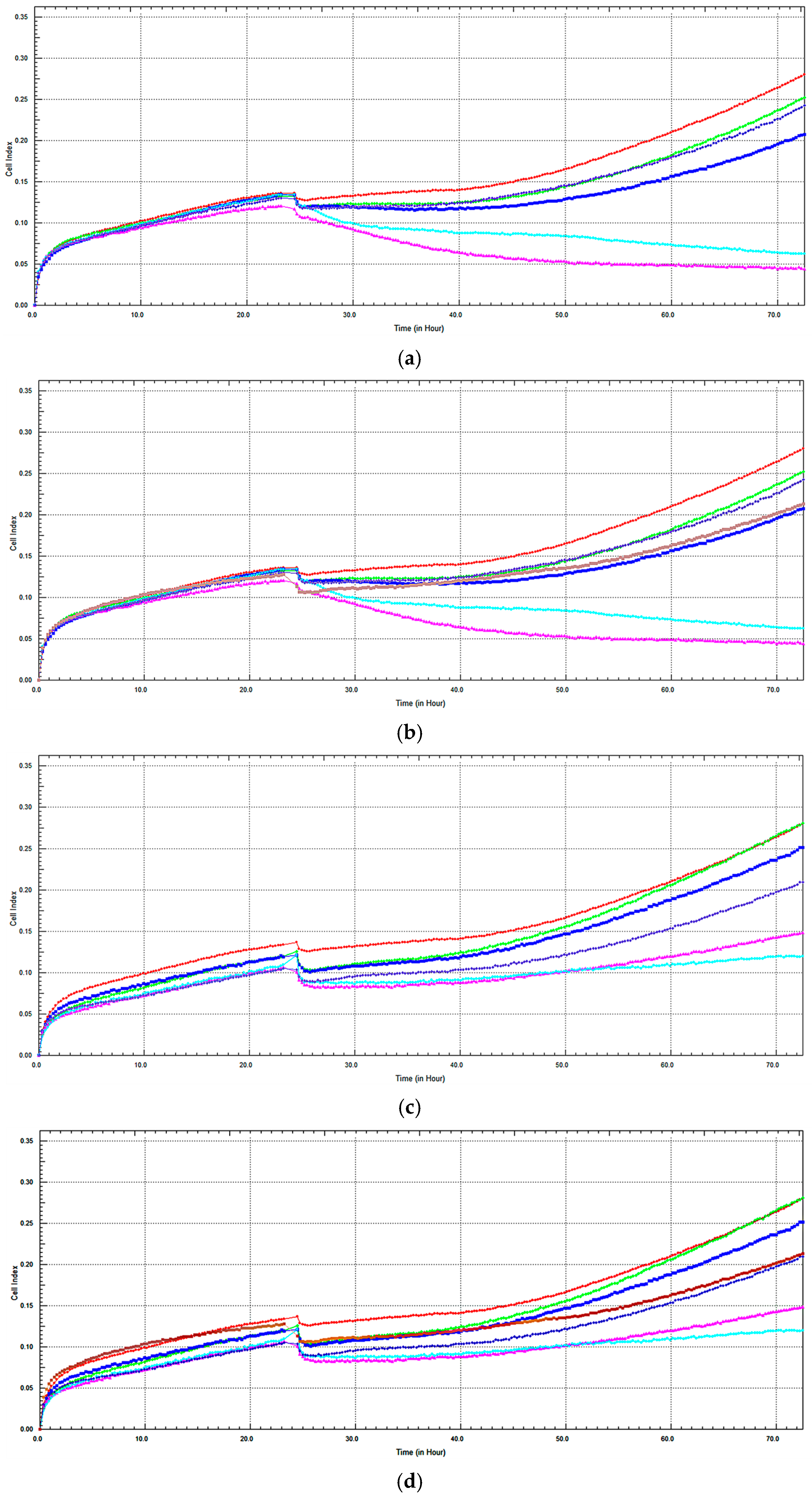
2.3. Monitoring of Cytotoxicity of C. lycaonica Extracts in Real-Time Using xCELLigence System
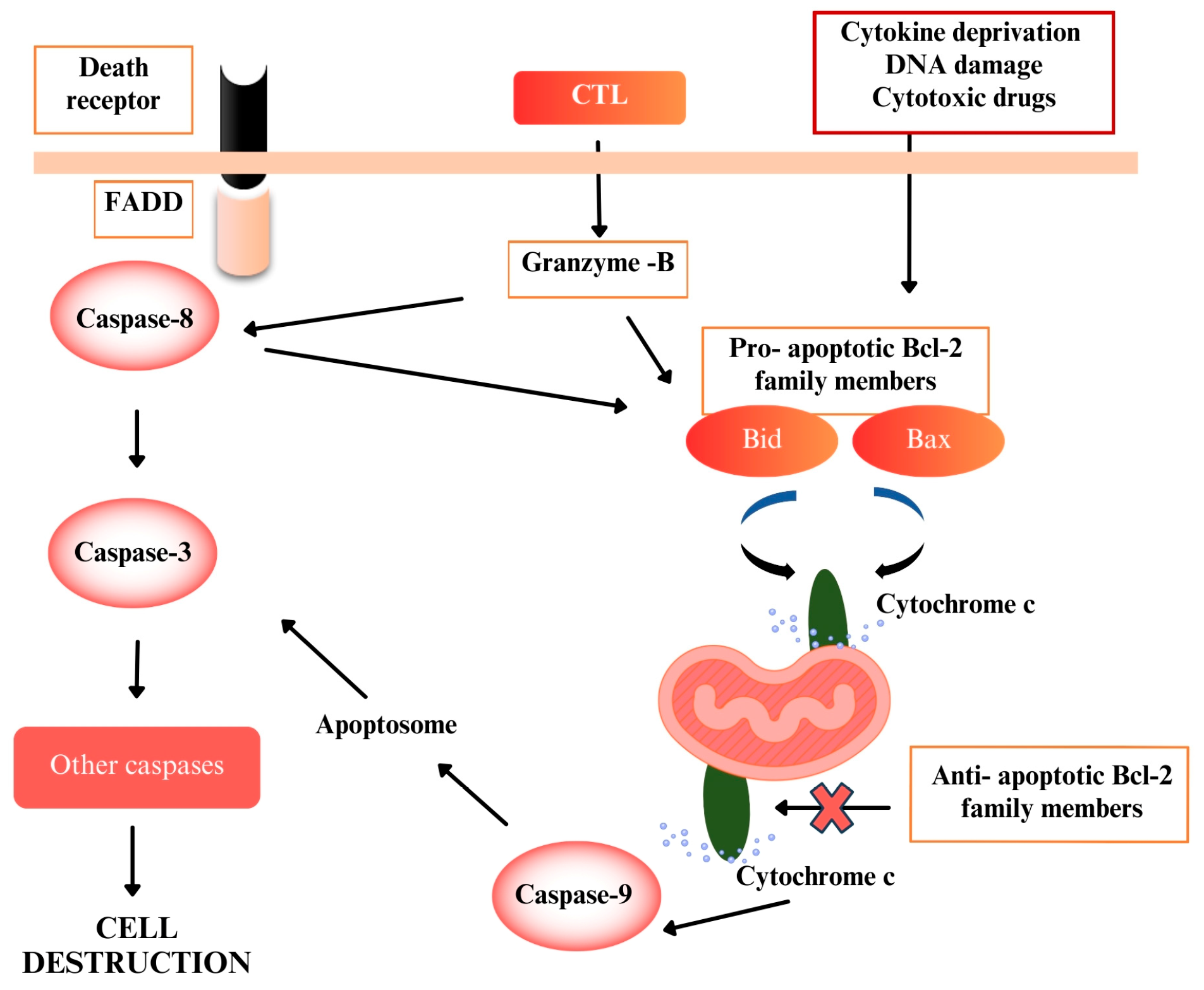
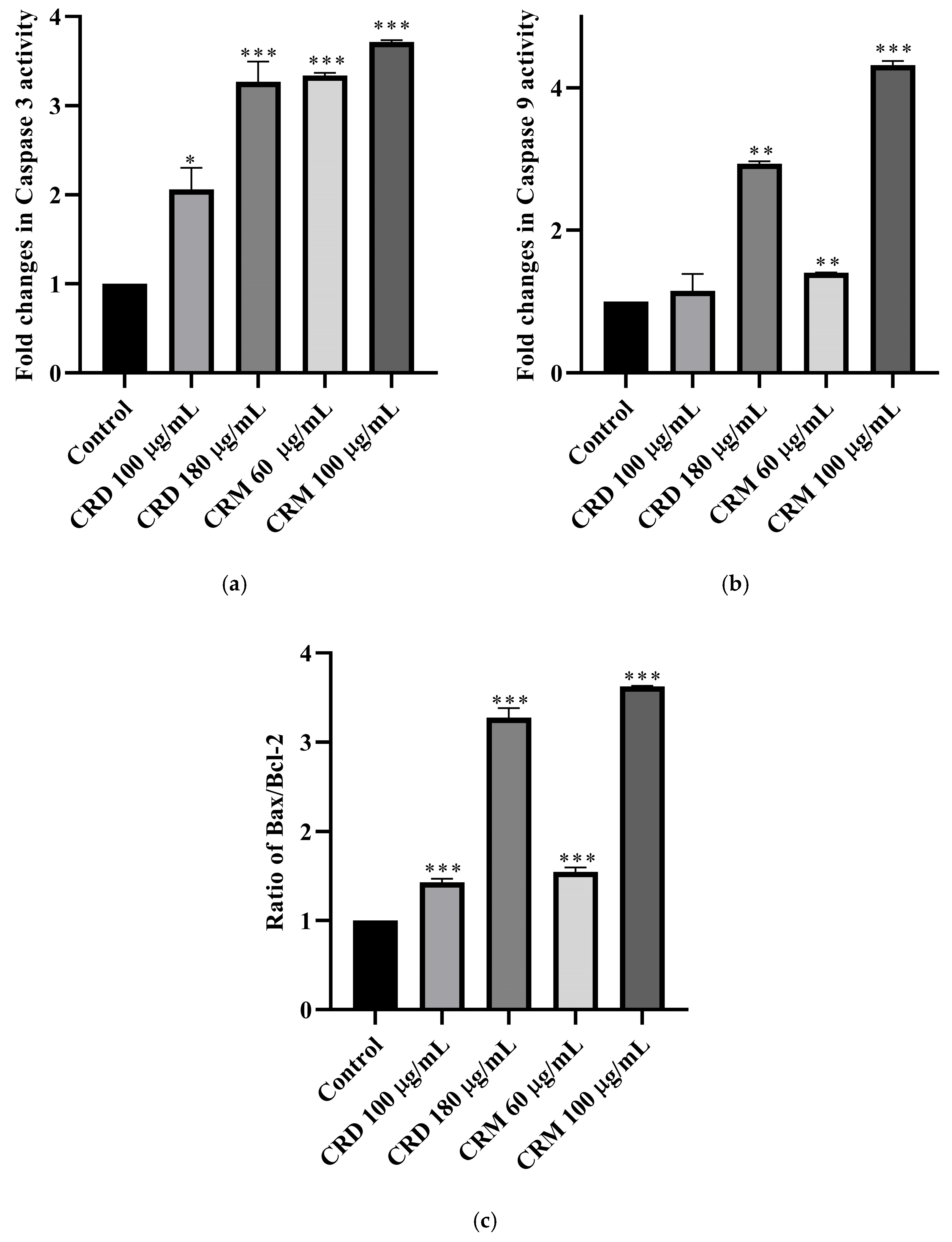
2.4. Apoptotic Analysis of C. lycaonica Extracts
2.4.1. Caspase 3 Assay
2.4.2. Caspase 9 Assay
2.4.3. Bax/Bcl-2 Ratio
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Plant Extract
4.3. RL95-2 Cell Line and Culture
4.4. Cytotoxic Analysis
4.4.1. MTT Viability Assay
4.4.2. xCELLigence RTCA System
4.4.3. Apoptotic Analysis
4.4.4. LC-HRMS Analysis
Qualitative Profile
Quantitative Profile
4.4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A375 | Human melanoma cell line |
| BCA | Bicinchoninic acid |
| CRD | Dichloromethane extract from the root of C. lycaonica |
| CRM | Methanol extract from the root of C. lycaonica |
| CI | Cell Index |
| CTL | Cytotoxic T Lymphocytes |
| DMEM-F12 | Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 |
| Dox | Doxorubicin |
| FADD | Fas-associated death domain protein |
| FBS | Fetal Bovine Serum |
| HeLa | Cervical cancer |
| IC50 | Half-maximal inhibitory concentration |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| MCF-7 | Breast cancer cell line |
| MDA-MB-231 | Human breast cancer cell line |
| RL95-2 | Human endometrial carcinoma |
| RTCA | Real-Time Cell Analyzer |
| Vero | African green monkey kidney cells |
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/en (accessed on 29 February 2024).
- Randall, M.E.; Filiaci, V.L.; Muss, H.; Spirtos, N.M.; Mannel, R.S.; Fowler, J.; Thigpen, J.T.; Benda, J.A.; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Yuan, J. Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell Biol. 2008, 9, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Spierings, D.C.; de Vries, E.G.; Vellenga, E.; van den Heuvel, F.A.; Koornstra, J.J.; Wesseling, J.; Hollema, H.; de Jong, S. Tissue distribution of the death ligand TRAIL and its receptors. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2004, 52, 821–831. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 2001, 26, 61–66. [Google Scholar] [CrossRef]
- Adrain, C.; Martin, S.J. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends Biochem. Sci. 2001, 26, 390–397. [Google Scholar] [CrossRef]
- Alper, M.; Güneş, H. The Anticancer and Anti-inflammatory Effects of Centaurea solstitialis Extract on Human Cancer Cell Lines. Turk. J. Pharm. Sci. 2019, 16, 273–281. [Google Scholar] [CrossRef]
- Kayacan, S.; Sener, L.T.; Melikoglu, G.; Kultur, S.; Albeniz, I.; Ozturk, M. Induction of apoptosis by Centaurea nerimaniae extract in HeLa and MDA-MB-231 cells by a caspase-3 pathway. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2018, 93, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Nasr, F.A.; Shahat, A.A.; Alqahtani, A.S.; Ahmed, M.Z.; Qamar, W.; Al-Mishari, A.A.; Almoqbil, A.N. Centaurea bruguierana inhibits cell proliferation, causes cell cycle arrest, and induces apoptosis in human MCF-7 breast carcinoma cells. Mol. Biol. Rep. 2020, 47, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.; Uysal, I.; Menemen, Y. Morphology, anatomy, ecology and palynology of two Centaurea species from Turkey. Bangladesh J. Bot. 2008, 37, 67–74. [Google Scholar] [CrossRef]
- Khammar, A.; Djeddi, S. Pharmacological and biological properties of some Centaurea species. Eur. J. Sci. Res. 2012, 84, 398–416. [Google Scholar]
- Duman, H.; Uzunhisarcıklı, M.E.; Bahadır, Y.N. A new species of Centaurea (Asteraceae) from northern Turkey. Nord. J. Bot. 2021, 39. [Google Scholar] [CrossRef]
- Erel, S.B.; Demir, S.; Nalbantsoy, A.; Ballar, P.; Khan, S.; Yavasoglu, N.U.; Karaalp, C. Bioactivity screening of five Centaurea species and in vivo anti-inflammatory activity of C. athoa. Pharm. Biol. 2014, 52, 775–781. [Google Scholar] [CrossRef]
- Ozsoy, N.; Kultur, S.; Yilmaz-Ozden, T.; Ozbek Celik, B.; Can, A.; Melikoglu, G. Antioxidant, Anti-Inflammatory, Acetylcholinesterase Inhibitory and Antimicrobial Activities of Turkish Endemic Centaurea antiochia var. Praealta. J. Food Biochem. 2015, 39, 771–776. [Google Scholar] [CrossRef]
- Saroglou, V.; Karioti, A.; Demetzos, C.; Dimas, K.; Skaltsa, H. Sesquiterpene lactones from Centaurea spinosa and their antibacterial and cytotoxic activities. J. Nat. Prod. 2005, 68, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Keser, S.; Keser, F.; Karatepe, M.; Kaygılı, O.; Tekın, S.; Turkoglu, I.; Demır, E.; Yilmaz, O.; Kirbag, S.; Sandal, S.; et al. In vitro biological evaluation and phytochemical contents of three Centaurea L. species growing from Eastern Anatolia in Turkey. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2020, 23, 148–156. [Google Scholar] [CrossRef]
- Aksoy, H. Evaluation of the biological and wound healing activities of Centaurea virgata. Clin. Exp. Health Sci. 2020, 10, 304–308. [Google Scholar] [CrossRef]
- Salachna, P.; Pietrak, A.; Łopusiewicz, Ł. Antioxidant Potential of Flower Extracts from Centaurea spp. Depends on Their Content of Phenolics, Flavonoids and Free Amino Acids. Molecules 2021, 26, 7465. [Google Scholar] [CrossRef] [PubMed]
- Teneva, O.; Petkova, Z.; Dobreva, A.; Dzhurmanski, A.; Stoyanova, L.; Angelova-Romova, M. Centaurea benedicta—A Potential Source of Nutrients and Bioactive Components. Plants 2024, 13, 3579. [Google Scholar] [CrossRef] [PubMed]
- Fatullayev, H.; Paşayeva, L.; Celik, I.; İnce, U.; Tugay, O. Phytochemical Composition, In Vitro Antimicrobial, Antioxidant, and Enzyme Inhibition Activities, and In Silico Molecular Docking and Dynamics Simulations of Centaurea lycaonica: A Computational and Experimental Approach. ACS Omega 2023, 8, 22854–22865. [Google Scholar] [CrossRef]
- Esmaeili, S.; Asadi, M.; Mosaddegh, M.; Motamed, S.M.; Hamzeloo-Moghadam, M. Cytotoxic activity of Centaurea albonitens Turrill aerial parts in colon and breast cancer cell lines. J. Med. Plants 2021, 20, 59–67. [Google Scholar] [CrossRef]
- Yırtıcı, Ü.; Ergene, A.; Adem, Ş.; Atalar, M.N.; Eyüpoğlu, V.; Rawat, R.; Arat, E.; Hamzaoğlu, E. Centaurea mersinensis phytochemical composition and multi-dimensional bioactivity properties supported by molecular modeling. J. Biomol. Struct. Dyn. 2024, 42, 2341–2357. [Google Scholar] [CrossRef]
- Salla, M.; Fakhoury, I.; Saliba, N.; Darwiche, N.; Gali-Muhtasib, H. Synergistic anticancer activities of the plant-derived sesquiterpene lactones salograviolide A and iso-seco-tanapartholide. J. Nat. Med. 2013, 67, 468–479. [Google Scholar] [CrossRef]
- Shoeb, M.; Jaspars, M.; MacManus, S.M.; Celik, S.; Nahar, L.; Kong-Thoo-Lin, P.; Sarker, S.D. Anti-colon cancer potential of phenolic compounds from the aerial parts of Centaurea gigantea (Asteraceae). J. Nat. Med. 2007, 61, 164–169. [Google Scholar] [CrossRef]
- Şekerler, T.; Şen, A.; Bitiş, L.; Şener, A. In vitro antihepatocellular carcinoma activity of secondary metabolites of Centaurea kilaea Boiss. J. Res. Pharm. 2020, 24, 479–486. [Google Scholar] [CrossRef]
- Arslan, A.K.K.; Güngörenler, E.; Paşayeva, L.; Bozkurt, M.; Tugay, O. Centaurea lycaonica Boiss. & Heldr. Bitkisinin İnsan Servikal Kanser Hücre Hattında Sitotoksisitesinin MTT Testi ve xCELLigence Sistemi ile Değerlendirilmesi. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2025, 28, 47–52. [Google Scholar]
- Karaboğa Arslan, A.K.; Eminoğlu, S.; Paşayeva, L.; Bozkurt, N.M.; Tugay, O. Centaurea lycaonica Extracts Induce Apoptosis in HeLa Human Cervical Cancer Cells via Bax/Bcl-2 Modulation and Caspase Activation: An LC-HRMS-Based Study. Food Sci. Nutr. 2025, 13, e70528. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Xu, L.; Jiang, L.; Jiang, Y.; Zhang, J.; Liu, B. Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MSn. Xenobiotica 2020, 50, 1311–1322. [Google Scholar] [CrossRef]
- Şimşek Sezer, E.N. In vitro investigation of cytotoxic, antioxidant, apoptosis-inducing, and wound healing properties of endemic Centaurea fenzlii Reichardt extracts. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.A.M.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M.; et al. Antioxidant, Anti-Proliferative Activity and Chemical Fingerprinting of Centaurea calcitrapa against Breast Cancer Cells and Molecular Docking of Caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef]
- Forgo, P.; Zupkó, I.; Molnár, J.; Vasas, A.; Dombi, G.; Hohmann, J. Bioactivity-guided isolation of antiproliferative compounds from Centaurea jacea L. Fitoterapia 2012, 83, 921–925. Fitoterapia 2012, 83, 921–925. [Google Scholar] [CrossRef]
- Bakr, R.O.; Mohamed, S.A.E.H.; Ayoub, N. Phenolic profile of Centaurea aegyptiaca L. growing in Egypt and its cytotoxic and antiviral activities. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2016, 13, 135–143. [Google Scholar] [CrossRef]
- Sokovic, M.; Ciric, A.; Glamoclija, J.; Skaltsa, H. Biological Activities of Sesquiterpene Lactones Isolated from the Genus Centaurea L. (Asteraceae). Curr. Pharm. Des. 2017, 23, 2767–2786. [Google Scholar] [CrossRef]
- Kubacey, T.M.; Haggag, E.G.; El-Toumy, S.A.; Ahmed, A.A.; El-Ashmawy, I.M.; Youns, M.M. Biological activity and flavonoids from Centaurea alexanderina leaf extract. J. Pharm. Res. 2012, 5, 3352–3361. [Google Scholar]
- Carev, I.; Ruščić, M.; Glumac, M.; Politeo, O.; Siljak-Yakovlev, S. Phytochemical and Cytogenetic Study of Two Centaurea Species from Croatia: The Particular Case of Diploid and Tetraploid C. salonitana. Chem. Biodivers. 2023, 20, e202300092. [Google Scholar] [CrossRef]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative Assessment of the Phytochemical Composition and Biological Activity of Extracts of Flowering Plants of Centaurea cyanus L., Centaurea jacea L. and Centaurea scabiosa L. Plants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Reda, E.H.; Hegazi, N.M.; Marzouk, M.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.A.; Mohamed, T.A.; Ibrahim, M.A.A.; Shams, K.A.; Abdel-Azim, N.S.; et al. Feature-Based Molecular Networking for the Exploration of the Metabolome Diversity of Common Egyptian Centaurea Species in Relation to Their Cytotoxic Activity. Molecules 2023, 28, 674. [Google Scholar] [CrossRef]
- Shakeri, A.; Amini, E.; Asili, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Screening of several biological activities induced by different sesquiterpene lactones isolated from Centaurea behen L. and Rhaponticum repens (L.) Hidalgo. Nat. Prod. Res. 2018, 32, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Seghiri, R.; Boumaza, O.; Mekkiou, R.; Benayache, S.; Mosset, P.; Quintana, J.; Estévez, F.; León, F.; Bermejo, J.; Benayache, F. A flavonoid with cytotoxic activity and other constituents from Centaurea africana. Phytochem. Lett. 2009, 2, 114–118. [Google Scholar] [CrossRef]
- Artun, F.T.; Karagöz, A. Antiproliferative and apoptosis inducing effects of the methanolic extract of Centaurea hermannii in human cervical cancer cell line. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2021, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yaglioglu, A.S.; Demirtas, I.; Goren, N. Bioactivity-guided isolation of antiproliferative compounds from Centaurea carduiformis DC. Phytochem. Lett. 2014, 8, 213–219. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Tuffour, I.; Annor, G.K.; Doris, A.; Blankson-Darku, P.C.; Kissi-Twum, A.A.; Ocloo, A. Antiproliferative, antioxidant activities and apoptosis induction by Morinda lucida and Taraxacum officinale in human HL-60 leukemia cells. J. Glob. Biosci. 2016, 5, 4281–4291. [Google Scholar]
- Bahmani, F.; Esmaeili, S.; Bashash, D.; Dehghan-Nayeri, N.; Mashati, P.; Gharehbaghian, A. Centaurea albonitens extract enhances the therapeutic effects of Vincristine in leukemic cells by inducing apoptosis. Biomed. Pharmacother. 2018, 99, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Cardile, V.; Graziano, A.C.; Rigano, D.; Aktumsek, A.; Zengin, G.; Senatore, F. Effect of Three Centaurea Species Collected from Central Anatolia Region of Turkey on Human Melanoma Cells. Nat. Prod. Commun. 2016, 11, 275–278. [Google Scholar] [CrossRef]
- Bibi, H.; Iqbal, J.; Abbasi, B.A.; Kanwal, S.; Tavafoghi, M.; Ahmed, M.Z.; Mahmood, T. Evaluation and chemical profiling of different Centaurea iberica extracts and investigation of different in vitro biological activities. J. King Saud Univ.-Sci. 2024, 36, 102992. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mahomoodally, M.F.; Picot-Allain, C.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Ak, G.; Polat, R.; Urusan, Z.; et al. A comparative study on biological properties and chemical profiles of different solvent extracts from Centaurea bingoelensis, an endemic plant of Turkey. Process Biochem. 2021, 102, 315–324. [Google Scholar] [CrossRef]
- Cincin, Z.B.; Kiran, B.; Baran, Y.; Cakmakoglu, B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed. Pharmacother. 2018, 103, 336–345. [Google Scholar] [CrossRef]
- Zhao, F.; Hong, X.; Li, D.; Wei, Z.; Ci, X.; Zhang, S. Diosmetin induces apoptosis in ovarian cancer cells by activating reactive oxygen species and inhibiting the Nrf2 pathway. Med. Oncol. 2021, 38, 54. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.S.; Duc, N.M.; Cong, V.T.; Pham, S.Q.T. The syntheses of polymethoxy flavonoid glycosides from apigenin and diosmetin and their cytotoxic activities on two human cancer cell lines. Results Chem. 2022, 4, 100651. [Google Scholar] [CrossRef]
- Zreen, Z.; Hameed, A.; Kiran, S.; Farooq, T.; Zaroog, M.S. A Comparative Study of Diospyros malabarica (Gaub) Extracts in Various Polarity-Dependent Solvents for Evaluation of Phytoconstituents and Biological Activities. BioMed Res. Int. 2022, 2022, 4746223. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Jung, Y.; Kang, J.; Steiner, M.; Qian, P.X.; Banerjee, A.; Mitchell, M.D.; Wu, Z.S.; Zhu, T.; Liu, D.X.; et al. Artemin Reduces Sensitivity to Doxorubicin and Paclitaxel in Endometrial Carcinoma Cells through Specific Regulation of CD24. Transl. Oncol. 2010, 3, 218–229. [Google Scholar] [CrossRef]
- Keogh, R.J. New technology for investigating trophoblast function. Placenta 2010, 31, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Paşayeva, L.; Yetimoğlu, S.; Fatullayev, H.; Ince, U.; Bozkurt, N.M.; Arslan, A.K.K. Optimizing health benefits of walnut (Juglans regia L.) agricultural by-products: Impact of maceration and Soxhlet extraction methods on phytochemical composition, enzyme Inhibition, antioxidant, antimicrobial, and cytotoxic activities. Food Biosci. 2025, 64, 105923. [Google Scholar] [CrossRef]

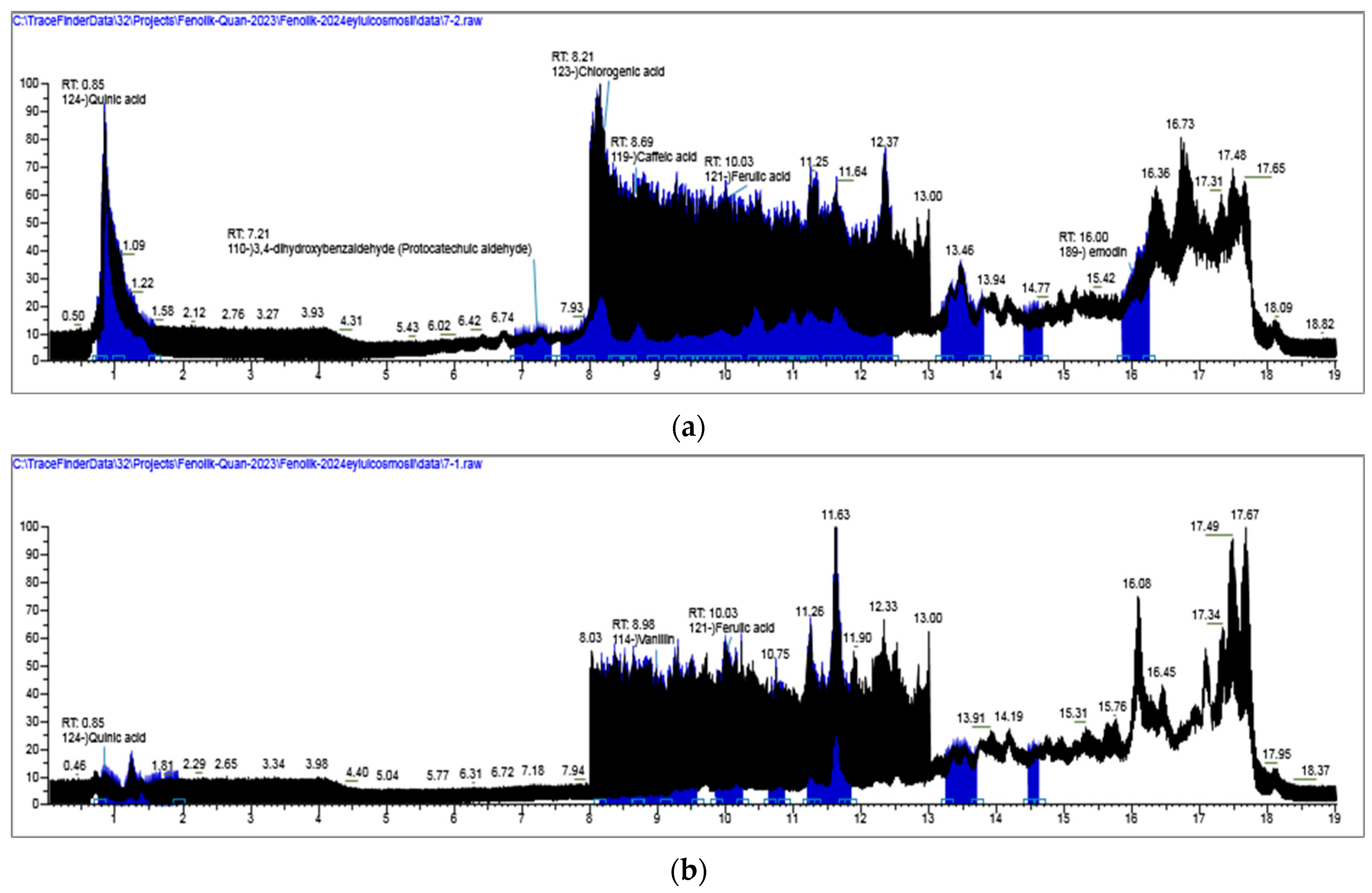

| Compounds | RT (min) | [M − H]− (m/z) | Content (µg/g Extract (dw)) | ||
|---|---|---|---|---|---|
| CRM | CRD | CRM | CRM | ||
| 4-Hydroxybenzoic acid | 7.81 | nd | 137.02442 | 47.670 ± 0.125 | nd 1 |
| Salicylic acid | 10.55 | nd | 137.02442 | 41.194 ± 0.852 | nd |
| Syringic acid | 8.9 | 8.9 | 197.04555 | 49.29 ± 1.243 | 41.598 ± 0.288 |
| 3,4-dihydroxybenzaldehyde | 7.21 | nd | 137.02442 | 12.12 ± 0.218 | nd |
| Vanillic acid | 8.54 | 8.54 | 167.03498 | 219.43 ± 3.874 | 49.1 ± 0.510 |
| Vanilin | 8.97 | 8.98 | 151.04007 | 72.016 ± 1.842 | 177.19 ± 2.557 |
| Coumaric acid | 9.78 | nd | 163.04007 | 38.866 ± 0.973 | nd |
| Caffeic acid | 8.69 | nd | 179.03498 | 91.042 ± 2.184 | nd |
| Ferulic acid | 10.03 | 10.03 | 193.05063 | 147.148 ± 3.236 | 150.306 ± 1.388 |
| Sinapic acid | 10.15 | nd | 223.06120 | 21.426 ± 0.321 | nd |
| Chlorogenic acid | 8.21 | nd | 353.08781 | 2866.98 ± 28.124 | nd |
| Quinic acid | 0.85 | 0.85 | 191.05611 | 361.524 ± 7.231 | 9.52 ± 0.176 |
| 3-(4-Hydroxyphenyl) propionic acid | 9.4 | nd | 165.05572 | 92.384 ± 1.847 | nd |
| Apigenin | 13.31 | nd | 269.04555 | 225.628 ± 4.512 | nd |
| Apigenin 7-glucuronide | 11.41 | nd | 445.07763 | 1317.994 ± 26.359 | nd |
| Rutin | 10.93 | nd | 609.14611 | 3639.834 ± 29.987 | nd |
| Luteolin-7-O-glucuronide | 10.84 | 10.86 | 461.07255 | 74.552 ± 1.491 | 19.492 ± 0.315 |
| Diosmetin | 13.46 | 13.45 | 299.05611 | 1095.524 ± 21.910 | 216.414 ± 2.498 |
| Myricetin | nd | 11.44 | 317.03029 | nd | 8.744 ± 0.227 |
| Orientin | 10.03 | nd | 447.09328 | 5.098 ± 0.051 | nd |
| Afzelin | 12.13 | nd | 431.09837 | 9.436 ± 0.141 | nd |
| Hesperidin | 11.45 | nd | 609.18249 | 48503.884 ± 29.995 | nd |
| Ellagic acid | 11.34 | 11.34 | 300.99899 | 25.312 ± 0.379 | 11.34 ± 0.229 |
| Method | Extract | IC50 (µg/mL) |
|---|---|---|
| MTT | CRD | 98.78 ± 0.88 |
| CRM | 60.02 ± 1.47 | |
| Dox | 3.37 ± 0.74 | |
| XCELLigence | CRD | 139.54 |
| CRM | 94.88 |
| Extract | Fold Changes in Caspase 3 Activity | Fold Changes in Caspase 9 Activity | Bax/Bcl-2 Ratio |
|---|---|---|---|
| CRD 100 µg/mL | +2.06 | +1.15 | +1.43 |
| CRD 180 µg/mL | +3.27 | +2.94 | +3.27 |
| CRM 60 µg/mL | +3.57 | +1.41 | +1.55 |
| CRM 100 µg/mL | +3.62 | +4.32 | +3.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaboğa Arslan, A.K.; Korubaşı, R.; Paşayeva, L.; Bozkurt, N.M.; Tugay, O. Phytochemical Profiling and Molecular Insights of Centaurea lycaonica: Apoptosis Induction via the Intrinsic Pathway in Endometrial Cancer Cells. Pharmaceuticals 2025, 18, 1558. https://doi.org/10.3390/ph18101558
Karaboğa Arslan AK, Korubaşı R, Paşayeva L, Bozkurt NM, Tugay O. Phytochemical Profiling and Molecular Insights of Centaurea lycaonica: Apoptosis Induction via the Intrinsic Pathway in Endometrial Cancer Cells. Pharmaceuticals. 2025; 18(10):1558. https://doi.org/10.3390/ph18101558
Chicago/Turabian StyleKaraboğa Arslan, Ayşe Kübra, Rümeysa Korubaşı, Leyla Paşayeva, Nuh Mehmet Bozkurt, and Osman Tugay. 2025. "Phytochemical Profiling and Molecular Insights of Centaurea lycaonica: Apoptosis Induction via the Intrinsic Pathway in Endometrial Cancer Cells" Pharmaceuticals 18, no. 10: 1558. https://doi.org/10.3390/ph18101558
APA StyleKaraboğa Arslan, A. K., Korubaşı, R., Paşayeva, L., Bozkurt, N. M., & Tugay, O. (2025). Phytochemical Profiling and Molecular Insights of Centaurea lycaonica: Apoptosis Induction via the Intrinsic Pathway in Endometrial Cancer Cells. Pharmaceuticals, 18(10), 1558. https://doi.org/10.3390/ph18101558







