Rubus occidentalis Ethanol Extract Attenuates Neuroinflammation and Cognitive Impairment in Lipopolysaccharide-Stimulated Microglia and Scopolamine-Induced Amnesic Mice
Abstract
1. Introduction
2. Results
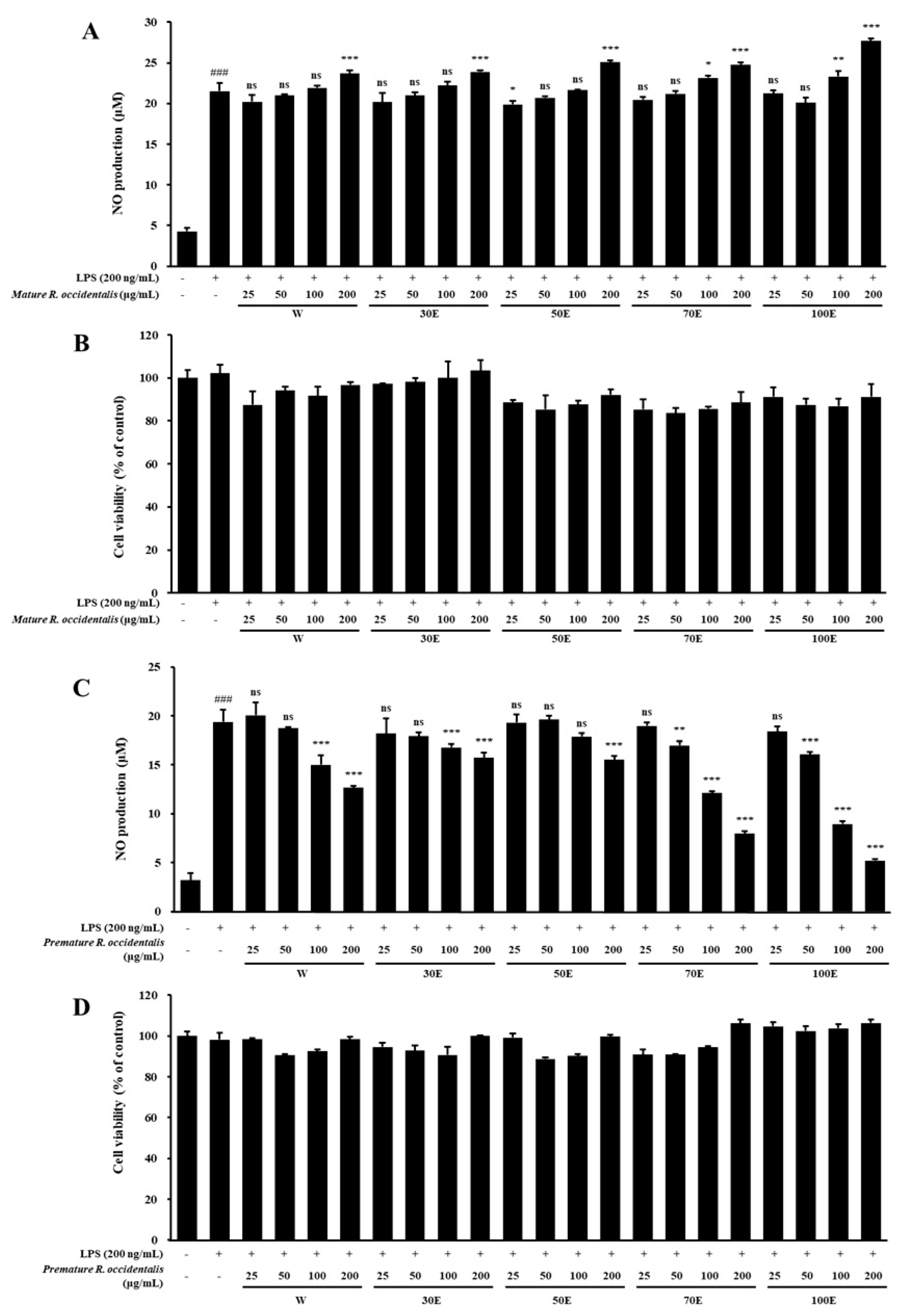
2.1. Inhibitory Effect of NO Production and Cell Viability of Mature and Premature R. occidentalis Extracts in LPS-Stimulated BV-2 Microglia Cells
2.2. Total Polyphenol and Flavonoid Content of R. occidentalis Extracts
2.3. ROE Inhibits mRNA and Protein Expression of iNOS, COX-2, and Pro-Inflammatory Cytokines in LPS-Stimulated BV-2 Microglia Cells
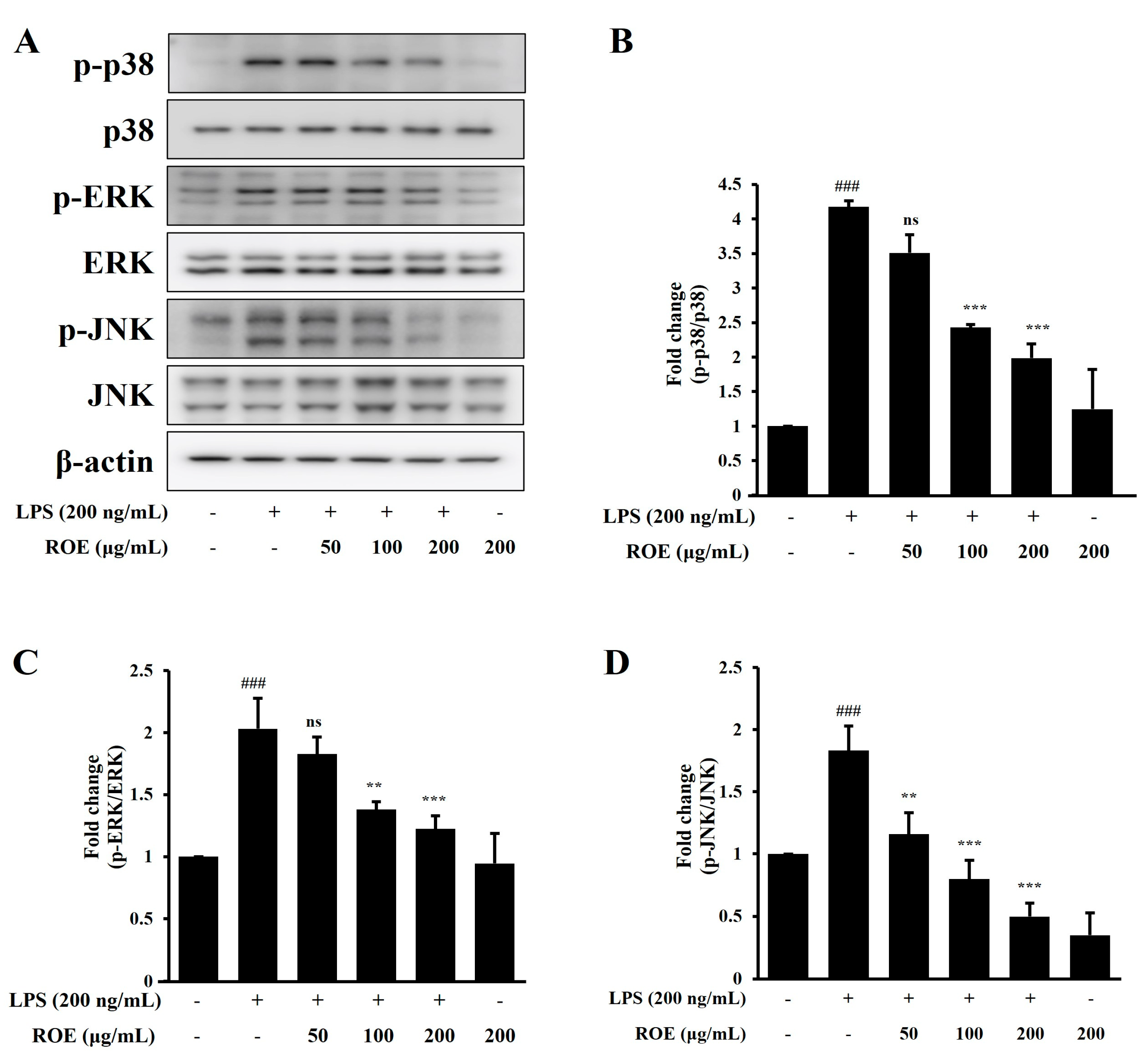
2.4. ROE Inhibited the Phosphorylation of MAPK Signaling in LPS-Stimulated BV-2 Microglia Cells
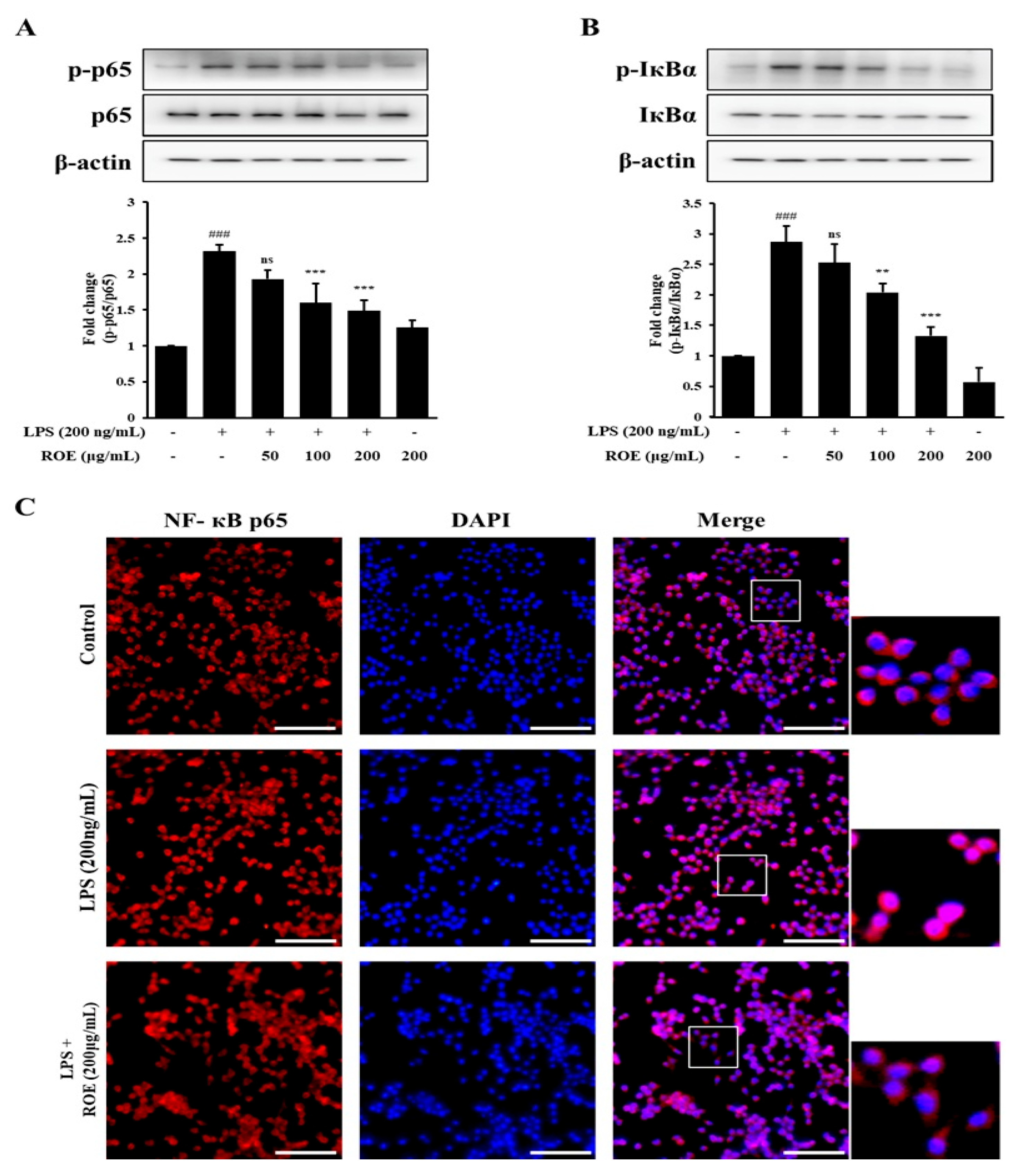
2.5. ROE Inhibited IκB-α Degradation and Nuclear Translocation of NF-κB in LPS-Stimulated BV-2 Microglia Cells
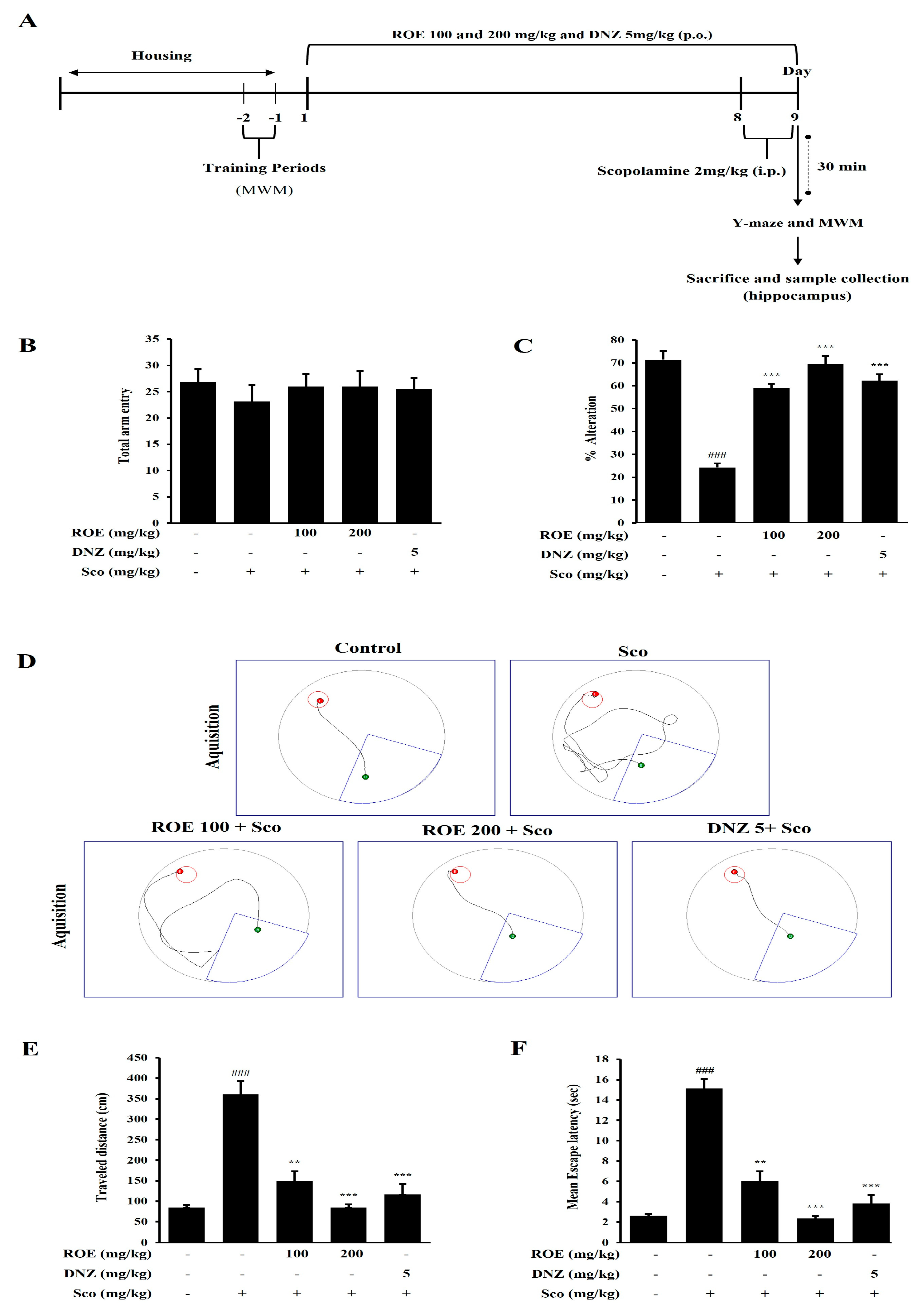
2.6. Effects of ROE on Spatial Learning and Cognitive Impairment in a Scopolamine-Treated Amnesic Mouse Model
2.7. ROE Modulated BDNF/CREB Signaling and Inflammatory Mediator Expression in the Hippocampus of Scopolamine-Treated Amnesic Mice
2.8. Analysis of Miquelianin Using High-Performance Liquid Chromatography
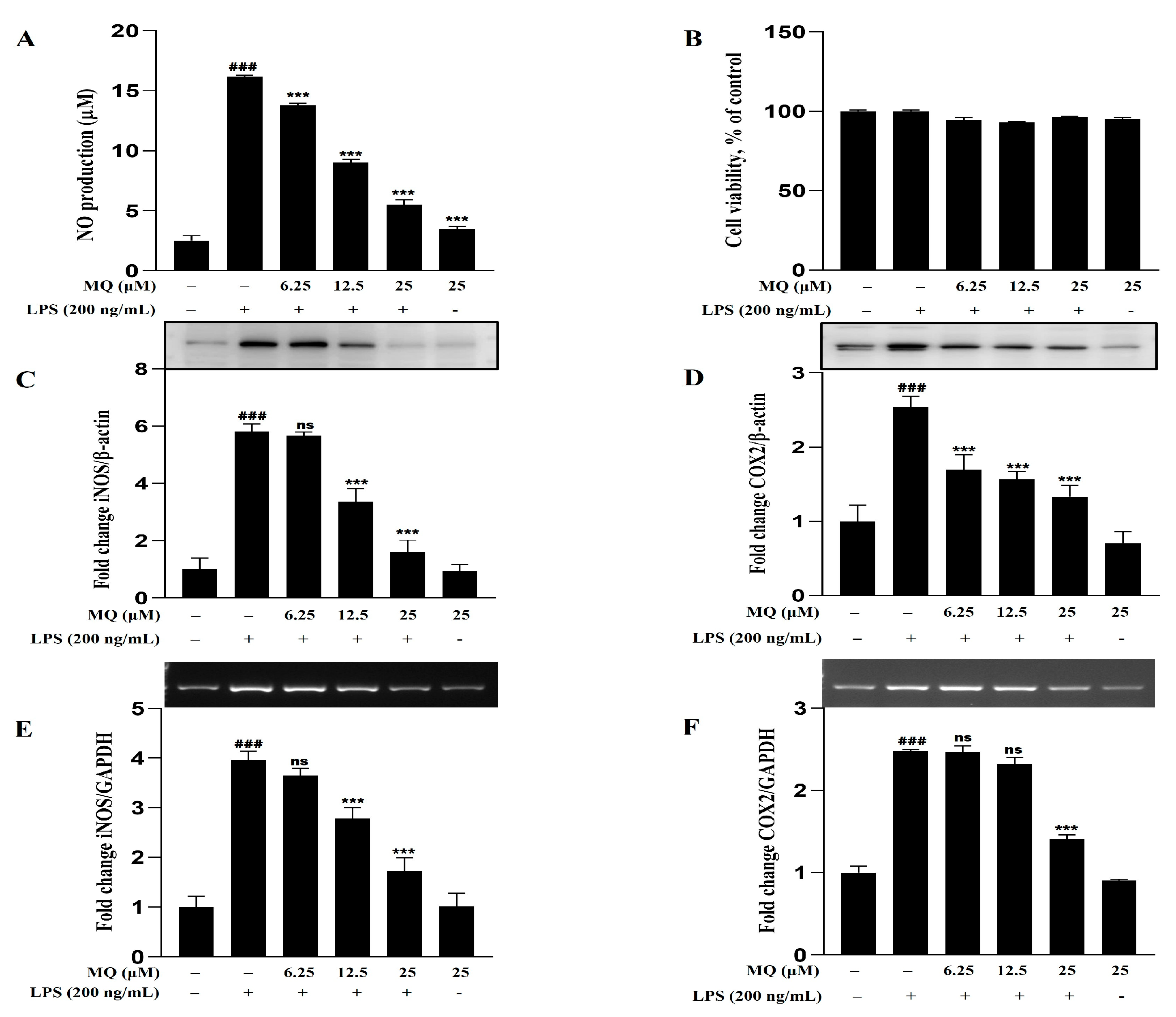
2.9. Anti-Inflammatory Effect of MQ in LPS-Stimulated BV-2 Microglia Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of R. occidentalis Extracts
4.3. Total Phenolic and Flavonoid Content
4.4. HPLC Analysis of MQ from ROE
4.4.1. Cell Culture and Treatment
4.4.2. Cell Viability and NO Assay
4.4.3. Total RNA Isolation and RT-PCR
4.4.4. Immunofluorescence
4.5. Animals
4.5.1. Animal Handling and Treatment
4.5.2. The Y-Maze Test
4.5.3. The MWM Test
4.5.4. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaugler, J.; James, B.; Johnson, T.; Reimer, J.; Solis, M.; Weuve, J.; Buckley, R.; Hohman, T. Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar]
- Balakrishnan, R.; Park, J.-Y.; Cho, D.-Y.; Ahn, J.-Y.; Yoo, D.-S.; Seol, S.-H.; Yoon, S.-H.; Choi, D.-K. AD−1 Small Molecule Improves Learning and Memory Function in Scopolamine-Induced Amnesic Mice Model through Regulation of CREB/BDNF and NF-κB/MAPK Signaling Pathway. Antioxidants 2023, 12, 648. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Feng, S.; Zhu, L.; Yang, L.; Liu, T.C.-Y.; Duan, R. Therapeutic Non-Invasive Brain Treatments in Alzheimer’s Disease: Recent Advances and Challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Wang, D.; Li, Y.; Xing, J.; Zeng, Y.; Liu, Z.; Zhou, X.; Fan, H. Neuroinflammation of microglial regulation in Alzheimer’s disease: Therapeutic approaches. Molecules 2024, 29, 1478. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, F.; Zhang, M. Therapeutic potential of plant-derived natural compounds in Alzheimer’s disease: Targeting microglia-mediated neuroinflammation. Biomed. Pharmacother. 2024, 178, 117235. [Google Scholar] [CrossRef]
- Lochner, M.; Thompson, A.J. The Muscarinic Antagonists Scopolamine and Atropine Are Competitive Antagonists at 5-HT 3 Receptors. Neuropharmacology 2016, 108, 220–228. [Google Scholar] [CrossRef]
- Falsafi, S.K.; Deli, A.; Höger, H.; Pollak, A.; Lubec, G. Scopolamine Administration Modulates Muscarinic, Nicotinic and NMDA Receptor Systems. PLoS ONE 2012, 7, e32082. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Kim, Y.S.; Kang, S.I.; Choi, D.K. Green oat cognitaven® attenuates mild cognitive impairment by activating the CREB/BDNF/Nrf2/HO-1 pathway and modulating NF-κB/MAPK signaling. Biomed. Pharmacother. 2025, 189, 118295. [Google Scholar] [CrossRef]
- Luo, Y.; Kuang, S.; Li, H.; Ran, D.; Yang, J. cAMP/PKA-CREB-BDNF Signaling Pathway in Hippocampus Mediates Cyclooxygenase 2-Induced Learning/Memory Deficits of Rats Subjected to Chronic Unpredictable Mild Stress. Oncotarget 2017, 8, 35558–35572. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.-Y.; Nam, Y.; Kang, H.-J.; Cho, H.-J.; Kim, J.; Hoe, H.-S. Ibrutinib Suppresses LPS-Induced Neuroinflammatory Responses in BV2 Microglial Cells and Wild-Type Mice. J. Neuroinflammation 2018, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Kula, M.; Krauze-Baranowska, M. Rubus Occidentalis: The Black Raspberry—Its Potential in the Prevention of Cancer. Nutr. Cancer 2016, 68, 18–28. [Google Scholar] [CrossRef]
- Meng, Q.; Manghwar, H.; Hu, W. Study on Supergenus rubus L.: Edible, Medicinal, and Phylogenetic Characterization. Plants 2022, 11, 1211. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.J.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Park, E.O.; Chae, S.W.; Lee, S.O.; Kwon, J.W.; You, J.H.; Kim, Y.G. Effects of unripe black raspberry extract supplementation on male climacteric syndrome and voiding dysfunction: A pilot, randomized, double-blind, placebo-controlled trial. Nutrients 2023, 15, 3313. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Turowski, P.; Ettcheto, M.; Duskey, J.T.; Tosi, G.; Sánchez-López, E.; García, M.L.; Camins, A.; Souto, E.B.; Ruiz, A.; et al. Nanomedicine-Based Technologies and Novel Biomarkers for the Diagnosis and Treatment of Alzheimer’s Disease: From Current to Future Challenges. J. Nanobiotechnol. 2021, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Trad. Compl. Alt. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of Microglia in Central Nervous System Infections. Clin. Microbiol. Rev. 2004, 17, 942–964. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The Effects of Microglia-Associated Neuroinflammation on Alzheimer’s Disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, C.; Zhang, Z.; Xue, Q. Anti-inflammatory effects of myristic acid mediated by the NF-κB pathway in lipopolysaccharide-induced BV-2 microglial cells. Mol. Omics 2003, 19, 726–734. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef]
- Tsuruta, K.; Sato, Y.; Nango, H.; Sakata, Y.; Ishikawa, H.; Tsuboi, M.; Miyagishi, H.; Kosuge, Y. Pentadecyl®, an odd-chain-rich triglyceride mixture derived from Aurantiochytrium oil, attenuates lipopolysaccharide-induced inflammatory cytokine production in BV-2 microglial cells. Int. Immunopharmacol. 2025, 158, 114810. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, W.; Liang, M.; Shi, Y.; Zhang, C.; Li, Q.; Liu, M.; Shou, Y.; Yin, H.; Zhu, X.; et al. (+)-JQ1 attenuated LPS-induced microglial inflammation via MAPK/NFκB signaling. Cell Biosci. 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Wang, H.; Dong, J.; Wu, Y.; Zhang, H.; Fu, L.; Zhang, J. Human umbilical cord mesenchymal stem cell—Derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF—κB/NLRP3 pathway. CNS Neurosci. Ther. 2024, 30, e14454. [Google Scholar] [CrossRef]
- Choi, J.W.; Jo, S.W.; Kim, D.E.; Paik, I.Y.; Balakrishnan, R. Aerobic exercise attenuates LPS-induced cognitive dysfunction by reducing oxidative stress, glial activation, and neuroinflammation. Redox Biol. 2024, 71, 103101. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Lee, D.; Park, C.; Choi, Y.H.; Choi, I.; Park, S.; Seo, S.; Lee, S.; Yea, S.S.; Ahn, S.; et al. Cilostazol Is Anti—Inflammatory in BV2 Microglial Cells by Inactivating Nuclear factor—kappaB and Inhibiting Mitogen—Activated Protein Kinases. Br. J. Pharmacol. 2010, 159, 1274–1285. [Google Scholar] [CrossRef]
- Hilliard, A.; Mendonca, P.; Soliman, K.F.A. Involvement of NFƙB and MAPK Signaling Pathways in the Preventive Effects of Ganoderma Lucidum on the Inflammation of BV-2 microglia Cells Induced by LPS. J. Neuroimmunol. 2020, 345, 577269. [Google Scholar] [CrossRef]
- Ahmed, T.; Zulfiqar, A.; Arguelles, S.; Rasekhian, M.; Nabavi, S.F.; Silva, A.S.; Nabavi, S.M. Map kinase signaling as therapeutic target for neurodegeneration. Pharmacol. Res. 2020, 160, 105090. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef]
- Dolanbay, S.N.; Şirin, S.; Aslim, B. Cocktail of three isoquinoline alkaloids derived from Glaucium grandiflorum Boiss. & A. Huet subsp. refractum (Nábelek) Mory inhibits the production of LPS-induced ROS, pro-inflammatory cytokines, and mediators through the down-regulation of p38 MAPK in BV-2 cells. Fitoterapia 2023, 170, 105652. [Google Scholar]
- Yu, Y.J.; Rahman, M.U.; Balakrishnan, R.; Kim, J.M.; Kim, J.H.; Choi, D.K. The novel peptide DBCH reduces LPS-stimulated NF-κB/MAPK signaling in BV-2 microglia and ameliorates cognitive impairment in scopolamine-treated mice by modulating BDNF/CREB. Neurochem. Int. 2025, 185, 105946. [Google Scholar] [CrossRef]
- Choi, J.W.; Im, J.H.; Balakrishnan, R. Paeoniflorin exercise-mimetic potential regulates the Nrf2/HO-1/BDNF/CREB and APP/BACE-1/NF-κB/MAPK signaling pathways to reduce cognitive impairments and neuroinflammation in amnesic mouse model. Biomed. Pharmacother. 2025, 189, 118299. [Google Scholar] [CrossRef] [PubMed]
- Thawkar, B.S.; Kaur, G. Betanin mitigates scopolamine-induced cognitive impairment by restoring cholinergic function, boosting brain antioxidative status, and increasing BDNF level in the zebrafish model. Fish Physiol. Biochem. 2023, 49, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr. Neurosci. 2022, 25, 485–501. [Google Scholar] [CrossRef]
- Chen, B.H.; Park, J.H.; Lee, T.K.; Song, M.; Kim, H.; Lee, J.C.; Kim, Y.M.; Lee, C.H.; Hwang, I.K.; Kang, I.J.; et al. Melatonin attenuates scopolamine-induced cognitive impairment via protecting against demyelination through BDNF-TrkB signaling in the mouse dentate gyrus. Chem. Biol. Interact. 2018, 285, 8–13. [Google Scholar] [CrossRef]
- Bartzokis, G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging 2004, 25, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and safety of Ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Kopec, A.M.; Carew, T.J. Growth Factor Signaling and Memory Formation: Temporal and Spatial Integration of a Molecular Network. Learn. Mem. 2013, 20, 531–539. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, X.; Moon, C.; Wang, H. Regulation of Brain-Derived Neurotrophic Factor Expression in Neurons. Int J Physiol Pathophysiol. Pharmacol. 2012, 4, 188–200. [Google Scholar] [PubMed]
- Balakrishnan, R.; Kim, Y.-S.; Kim, G.-W.; Kim, W.-J.; Hong, S.-M.; Kim, C.-G.; Choi, D.-K. Standardized Extract of Glehnia Littoralis Abrogates Memory Impairment and Neuroinflammation by Regulation of CREB/BDNF and NF-κB/MAPK Signaling in Scopolamine-Induced Amnesic Mice Model. Biomed. Pharmacother. 2023, 165, 115106. [Google Scholar] [CrossRef]
- Best, T.; Miller, J.; Teo, W.P. Neurocognitive effects a combined polyphenolic-rich herbal extract in healthy middle-aged adults–a randomised, double-blind, placebo-controlled study. Nutr. Neurosci. 2024, 27, 1293–1305. [Google Scholar]
- Pamies, D.; Sartori, C.; Schvartz, D.; González-Ruiz, V.; Pellerin, L.; Nunes, C.; Tavel, D.; Maillard, V.; Boccard, J.; Rudaz, S.; et al. Neuroinflammatory response to TNFα and IL1β cytokines is accompanied by an increase in glycolysis in human astrocytes in vitro. Int. J. Mol. Sci. 2012, 22, 4065. [Google Scholar] [CrossRef]
- Iqbal, S.; Shah, F.A.; Naeem, K.; Nadeem, H.; Sarwar, S.; Ashraf, Z.; Imran, M.; Khan, T.; Anwar, T.; Li, S. Succinamide derivatives ameliorate neuroinflammation and oxidative stress in scopolamine-induced neurodegeneration. Biomolecules 2020, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-α and IL-1β modulate blood-brain barrier permeability and decrease amyloid-β peptide efflux in a human blood-brain barrier model. Int. J. Mol. Sci. 2022, 23, 10235. [Google Scholar]
- Santos, M.C.; Soares, K.D.; Beltrame, B.M.; Toson, N.S.B.; Do Carmo, B.; Pimentel, M.; Bordignon, S.A.L.; Apel, M.A.; Mendez, A.S.L.; Henriques, A.T. Polyphenolic Composition and in Vitro Antihypertensive and Anti—Inflammatory Effects of Cuphea Lindmaniana and Cuphea Urbaniana. Chem. Biodivers. 2021, 18, e2100041. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Siberian Raspberries: LC-MS Profile, Seasonal Variation, Antioxidant Activity and, Thermal Stability of Rubus Matsumuranus Phenolome. Plants 2021, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sheng, Y.; Zhu, Y. Ginkgolide B Alleviates the Inflammatory Response and Attenuates the Activation of LPS—Induced BV2 Cells in Vitro and in Vivo. Exp. Ther. Med. 2021, 21, 586. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H.; Lee, O.H.; Kwon, J.W. Effect of immature Rubus occidentalis on postoperative pain in a rat model. Medicina 2023, 59, 264. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Kang, H.; Lee, O.H.; Ahn, E.J.; White, F.A.; Cho, Y.J.; Baek, C.W.; Jung, Y.H.; Kwon, J.W. Effectiveness of maturity of Rubus occidentalis on hyperalgesia induced by acidic saline injection in rats. BMC Complement. Med. Ther. 2022, 22, 12. [Google Scholar] [CrossRef] [PubMed]



| Samples | Total Polyphenol Content 1 | Total Flavonoid Content 2 | Samples | Total Polyphenol Content 1 | Total Flavonoid Content 2 |
|---|---|---|---|---|---|
| Mat; W | 61.116 ± 2.714 | 13.690 ± 0.412 | Pre; W | 115.243 ± 0.763 | 16.310 ± 0.412 |
| Mat; 30E | 48.683 ± 0.346 | 9.405 ± 0.412 | Pre; 30E | 91.593 ± 0.229 | 12.976 ± 0.412 |
| Mat; 50E | 49.556 ± 0.079 | 8.452 ± 0.412 | Pre; 50E | 103.497 ± 0.874 | 17.024 ± 0.412 |
| Mat; 70E | 48.656 ± 0.255 | 9.167 ± 0.412 | Pre; 70E | 175.548 ± 3.087 | 13.929 ± 0.000 |
| Mat; 100E | 51.884 ± 0.200 | 9.881 ± 0.412 | Pre; 100E | 186.063 ± 0.281 | 17.738 ± 0.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-W.; Kim, Y.-S.; Afroze Bondhon, T.; Balakrishnan, R.; Han, J.-H.; Kwon, J.-W.; Kim, W.-J.; Choi, D.-K. Rubus occidentalis Ethanol Extract Attenuates Neuroinflammation and Cognitive Impairment in Lipopolysaccharide-Stimulated Microglia and Scopolamine-Induced Amnesic Mice. Pharmaceuticals 2025, 18, 1557. https://doi.org/10.3390/ph18101557
Kim G-W, Kim Y-S, Afroze Bondhon T, Balakrishnan R, Han J-H, Kwon J-W, Kim W-J, Choi D-K. Rubus occidentalis Ethanol Extract Attenuates Neuroinflammation and Cognitive Impairment in Lipopolysaccharide-Stimulated Microglia and Scopolamine-Induced Amnesic Mice. Pharmaceuticals. 2025; 18(10):1557. https://doi.org/10.3390/ph18101557
Chicago/Turabian StyleKim, Ga-Won, Yon-Suk Kim, Tohmina Afroze Bondhon, Rengasamy Balakrishnan, Jun-Hyuk Han, Ji-Wung Kwon, Woo-Jung Kim, and Dong-Kug Choi. 2025. "Rubus occidentalis Ethanol Extract Attenuates Neuroinflammation and Cognitive Impairment in Lipopolysaccharide-Stimulated Microglia and Scopolamine-Induced Amnesic Mice" Pharmaceuticals 18, no. 10: 1557. https://doi.org/10.3390/ph18101557
APA StyleKim, G.-W., Kim, Y.-S., Afroze Bondhon, T., Balakrishnan, R., Han, J.-H., Kwon, J.-W., Kim, W.-J., & Choi, D.-K. (2025). Rubus occidentalis Ethanol Extract Attenuates Neuroinflammation and Cognitive Impairment in Lipopolysaccharide-Stimulated Microglia and Scopolamine-Induced Amnesic Mice. Pharmaceuticals, 18(10), 1557. https://doi.org/10.3390/ph18101557










