Preliminary Data Regarding the Alleviating Effects of Haloperidol and Risperidone on the Short-Term Memory and Associative Learning in a Zebrafish Model of Schizophrenia
Abstract
1. Introduction
2. Results
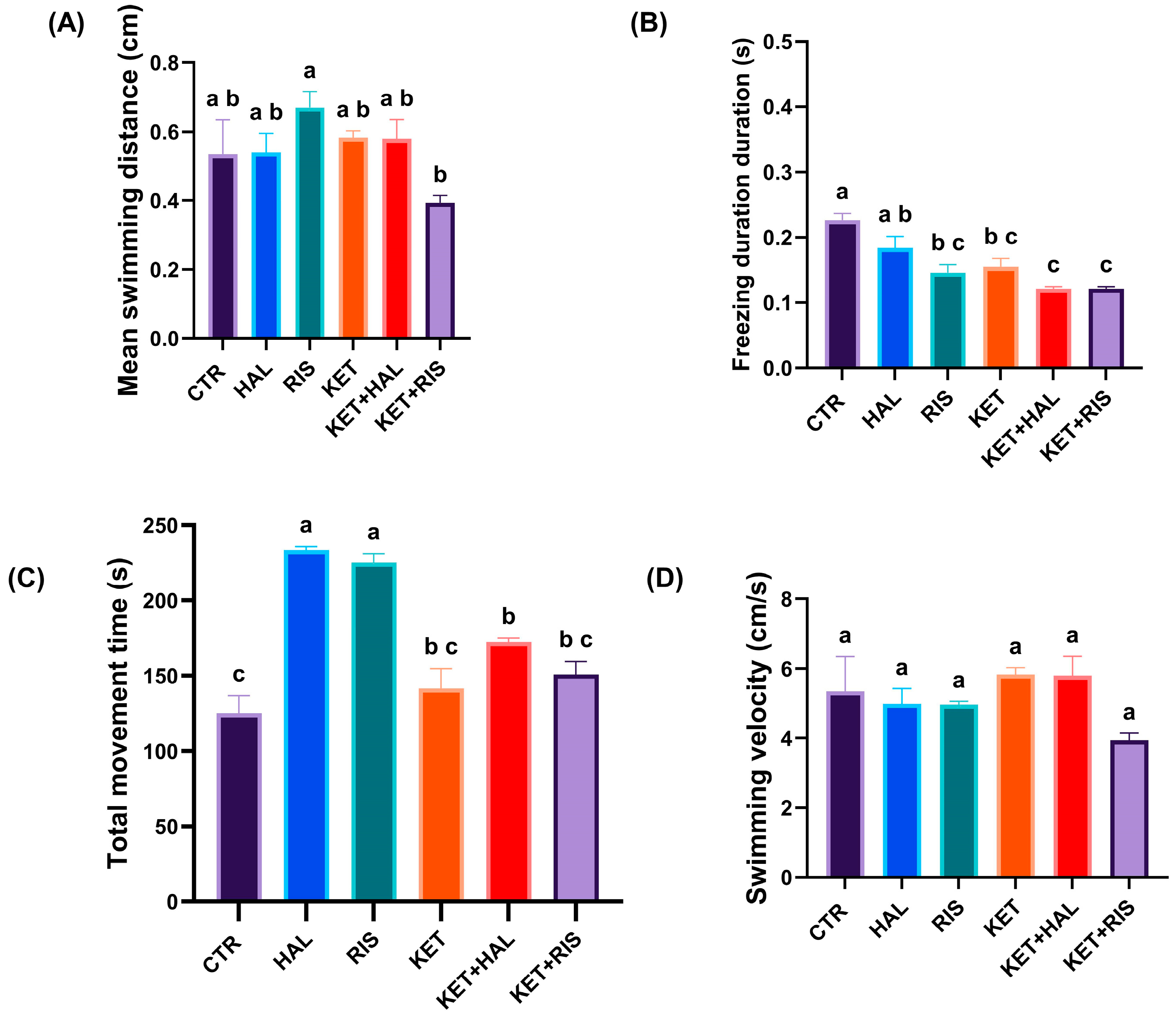
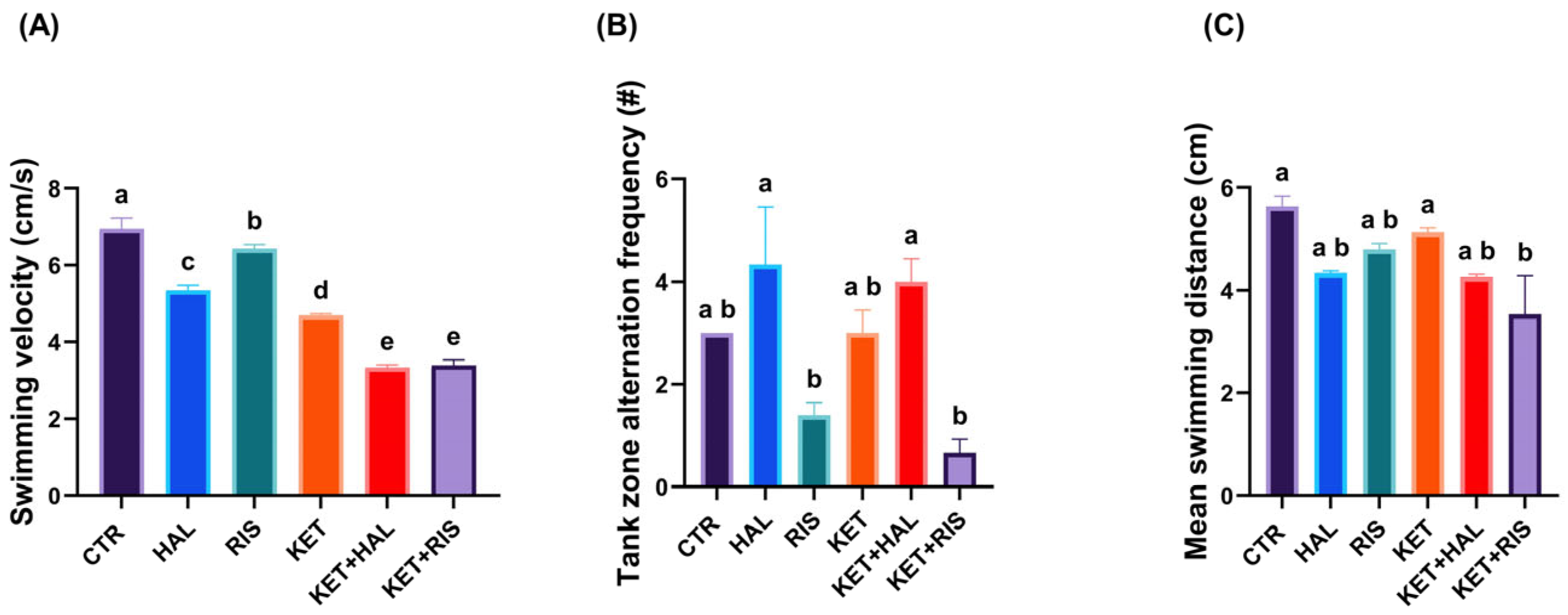
2.1. T-Maze Memory Test
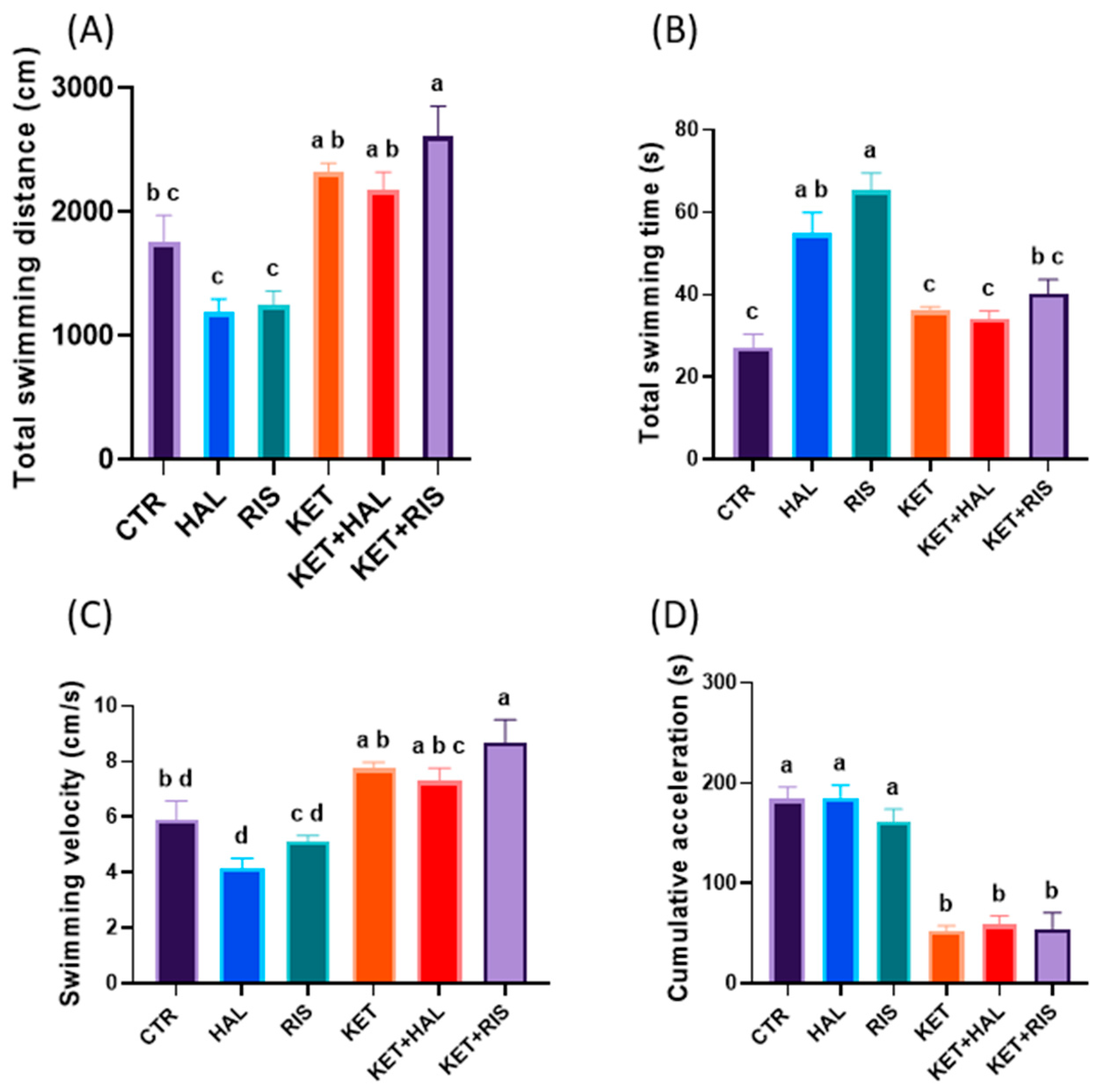
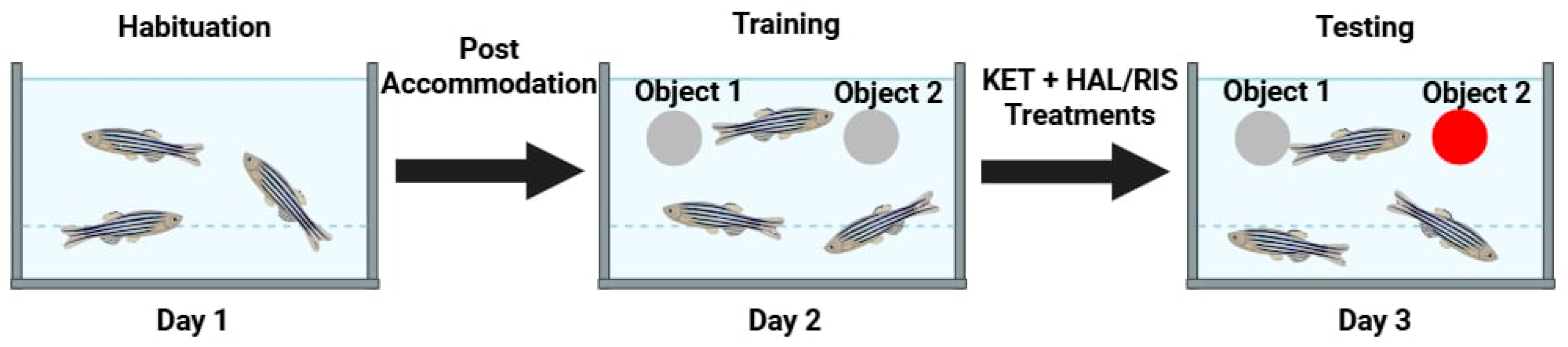
2.2. NOR Test
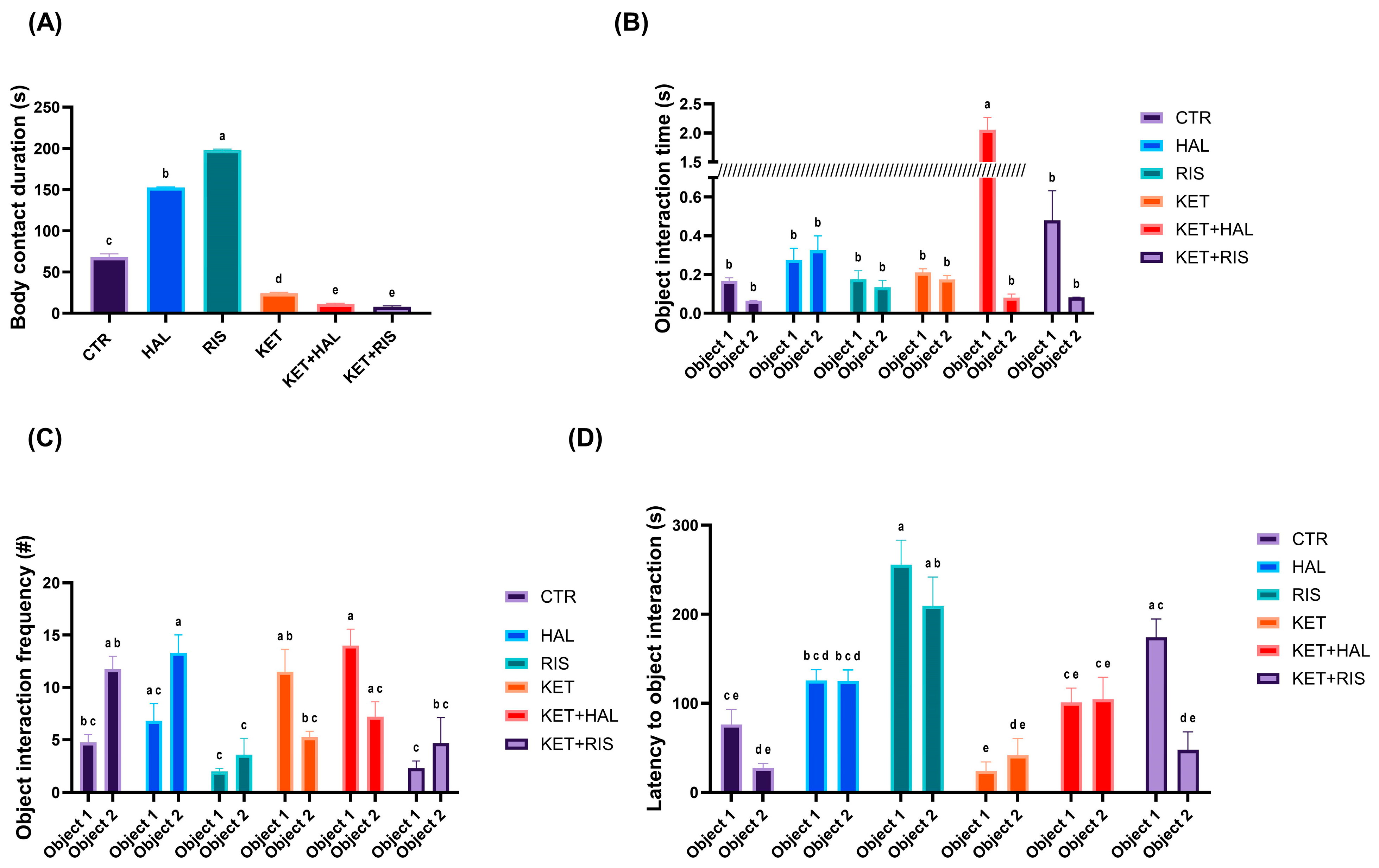
2.3. Social Interaction Test
3. Discussion
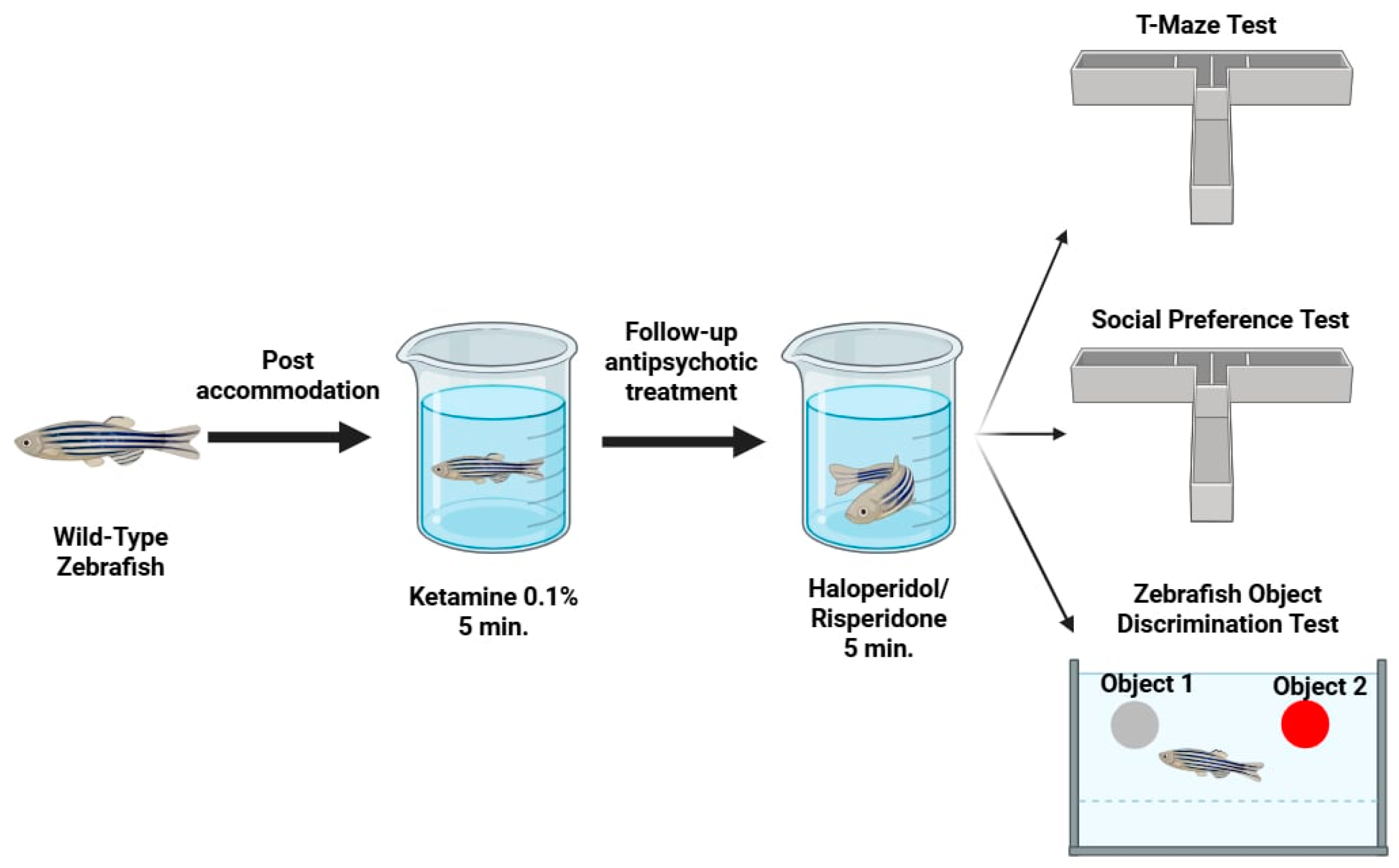
4. Materials and Methods
4.1. Animals
4.2. Ethical Note
4.3. Substances and Treatments
4.4. Behavioural Tests
4.4.1. T-Maze Test
4.4.2. Novel Object Recognition (NOR) Test
4.4.3. The Social Preference Test
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vita, A.; Barlati, S.; Cavallaro, R.; Mucci, A.; Riva, M.A.; Rocca, P.; Rossi, A.; Galderisi, S. Definition, Assessment and Treatment of Cognitive Impairment Associated with Schizophrenia: Expert Opinion and Practical Recommendations. Front. Psychiatry 2024, 15, 1451832. [Google Scholar] [CrossRef]
- Fadgyas Stanculete, M.; Capatina, O. The Fog of Schizophrenia: Cognitive Impairments and Their Impact on Daily Life. In The Impact of Psychosis on Mental Health; IntechOpen: London, UK, 2025. [Google Scholar]
- Vita, A.; Nibbio, G.; Barlati, S. Pharmacological Treatment of Cognitive Impairment Associated with Schizophrenia: State of the Art and Future Perspectives. Schizophr. Bull. Open 2024, 5, sgae013. [Google Scholar] [CrossRef]
- MacKenzie, N.E.; Kowalchuk, C.; Agarwal, S.M.; Costa-Dookhan, K.A.; Caravaggio, F.; Gerretsen, P.; Chintoh, A.; Remington, G.J.; Taylor, V.H.; Müeller, D.J.; et al. Antipsychotics, Metabolic Adverse Effects, and Cognitive Function in Schizophrenia. Front. Psychiatry 2018, 9, 420384. [Google Scholar] [CrossRef]
- Lungu, P.F.; Lungu, C.M.; Ciobica, A.; Balmus, I.M.; Vitalaru, R.; Mavroudis, I.; Dobrin, R.; Cimpeanu, M.; Gurzu, I.L. The Effect of Antipsychotics on Cognition in Schizophrenia—A Current Narrative Review. Brain Sci. 2024, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, K.; Takahashi, T.; Morinobu, S. Association of Typical versus Atypical Antipsychotics with Symptoms and Quality of Life in Schizophrenia. PLoS ONE 2012, 7, e37087. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Gadaleta, E. Contrasting Typical and Atypical Antipsychotic Drugs. Focus 2021, 19, 3–13. [Google Scholar] [CrossRef]
- Selva-Vera, G.; Balanzá-Martínez, V.; Salazar-Fraile, J.; Sánchez-Moreno, J.; Martinez-Aran, A.; Correa, P.; Vieta, E.; Tabarés-Seisdedos, R. The Switch from Conventional to Atypical Antipsychotic Treatment Should Not Be Based Exclusively on the Presence of Cognitive Deficits. A Pilot Study in Individuals with Schizophrenia. BMC Psychiatry 2010, 10, 47. [Google Scholar] [CrossRef]
- Dold, M.; Samara, M.T.; Li, C.; Tardy, M.; Leucht, S. Haloperidol versus First-Generation Antipsychotics for the Treatment of Schizophrenia and Other Psychotic Disorders. Cochrane Database Syst. Rev. 2015, 2017, CD009831. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Hufeisen, S.J.; Popadak, B.A.; Renock, S.M.; Steinberg, S.; Ernsberger, P.; Jayathilake, K.; Meltzer, H.Y.; Roth, B.L. H1-Histamine Receptor Affinity Predicts Short-Term Weight Gain for Typical and Atypical Antipsychotic Drugs. Neuropsychopharmacology 2003, 28, 519–526. [Google Scholar] [CrossRef]
- Haloperidol: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00502 (accessed on 1 August 2024).
- Houthoofd, S.A.M.K.; Morrens, M.; Sabbe, B.G.C. Cognitive and Psychomotor Effects of Risperidone in Schizophrenia and Schizoaffective Disorder. Clin. Ther. 2008, 30, 1565–1589. [Google Scholar] [CrossRef]
- Germann, D.; Kurylo, N.; Han, F. Chapter 8—Risperidone. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, pp. 313–361. ISBN 1871-5125. [Google Scholar]
- Cohen, L.J. Risperidone. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1994, 14, 253–265. [Google Scholar] [CrossRef]
- Leysen, J.E.; Janssen, P.M.; Gommeren, W.; Wynants, J.; Pauwels, P.J.; Janssen, P.A. In Vitro and in Vivo Receptor Binding and Effects on Monoamine Turnover in Rat Brain Regions of the Novel Antipsychotics Risperidone and Ocaperidone. Mol. Pharmacol. 1992, 41, 494–508. [Google Scholar] [CrossRef]
- Grone, B.P.; Baraban, S.C. Animal Models in Epilepsy Research: Legacies and New Directions. Nat. Neurosci. 2015, 18, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Evolutionary Conservation, Translational Relevance and Cognitive Function: The Future of Zebrafish in Behavioral Neuroscience. Neurosci. Biobehav. Rev. 2020, 116, 426–435. [Google Scholar] [CrossRef]
- Oliveira, R.F. Mind the Fish: Zebrafish as a Model in Cognitive Social Neuroscience. Front. Neural Circuits 2013, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, M.; Michalak, A.; Skalicka-Woźniak, K.; Zykubek, M.; Ciszewski, A.; Budzyńska, B. Zebrafish and Mouse Models for Anxiety Evaluation—A Comparative Study with Xanthotoxin as a Model Compound. Brain Res. Bull. 2020, 165, 139–145. [Google Scholar] [CrossRef]
- Ang, M.J.; Lee, S.; Kim, J.-C.; Kim, S.-H.; Moon, C. Behavioral Tasks Evaluating Schizophrenia-like Symptoms in Animal Models: A Recent Update. Curr. Neuropharmacol. 2021, 19, 641. [Google Scholar] [CrossRef] [PubMed]
- Langova, V.; Vales, K.; Horka, P.; Horacek, J. The Role of Zebrafish and Laboratory Rodents in Schizophrenia Research. Front. Psychiatry 2020, 11, 549232. [Google Scholar] [CrossRef]
- Seibt, K.J.; Piato, A.L.; da Luz Oliveira, R.; Capiotti, K.M.; Vianna, M.R.; Bonan, C.D. Antipsychotic Drugs Reverse MK-801-Induced Cognitive and Social Interaction Deficits in Zebrafish (Danio rerio). Behav. Brain Res. 2011, 224, 135–139. [Google Scholar] [CrossRef]
- Zakhary, S.M.; Ayubcha, D.; Ansari, F.; Kamran, K.; Karim, M.; Leheste, J.R.; Horowitz, J.M.; Torres, G. A Behavioral and Molecular Analysis of Ketamine in Zebrafish. Synapse 2011, 65, 160. [Google Scholar] [CrossRef]
- Riehl, R.; Kyzar, E.; Allain, A.; Green, J.; Hook, M.; Monnig, L.; Rhymes, K.; Roth, A.; Pham, M.; Razavi, R.; et al. Behavioral and Physiological Effects of Acute Ketamine Exposure in Adult Zebrafish. Neurotoxicol Teratol. 2011, 33, 658–667. [Google Scholar] [CrossRef]
- Moghaddam, B. Bringing Order to the Glutamate Chaos in Schizophrenia. Neuron 2003, 40, 881–884. [Google Scholar] [CrossRef]
- Curpan, A.-S.; Savuca, A.; Hritcu, L.D.; Solcan, C.; Nicoara, M.N.; Luca, A.-C.; Ciobica, A.-S. A New Approach to Explore the Correlation between Declarative Memory and Anxiety in Animal Models of Schizophrenia and Microplastic Pollution. Behav. Brain Res. 2024, 458, 114742. [Google Scholar] [CrossRef]
- Leheste, J.R.; Curcio, C.; Baldinger, L.; Sarwar, S.; Zakhary, S.M.; Hallas, B.H.; Horowitz, J.M.; Torres, G. Glutamate-Based Drugs for the Treatment of Clinical Depression. Cent. Nerv. Syst. Agents Med. Chem. 2012, 8, 170–176. [Google Scholar] [CrossRef]
- Sanacora, G.; Kendell, S.F.; Levin, Y.; Simen, A.A.; Fenton, L.R.; Coric, V.; Krystal, J.H. Preliminary Evidence of Riluzole Efficacy in Antidepressant-Treated Patients with Residual Depressive Symptoms. Biol. Psychiatry 2007, 61, 822–825. [Google Scholar] [CrossRef]
- Ross, C.A.; Margolis, R.L.; Reading, S.A.J.; Pletnikov, M.; Coyle, J.T. Neurobiology of Schizophrenia. Neuron 2006, 52, 139–153. [Google Scholar] [CrossRef]
- Diwadkar, V.A.; Flaugher, B.; Jones, T.; Zalányi, L.; Ujfalussy, B.; Keshavan, M.S.; Érdi, P. Impaired Associative Learning in Schizophrenia: Behavioral and Computational Studies. Cogn. Neurodyn 2008, 2, 207. [Google Scholar] [CrossRef]
- Benvenutti, R.; Gallas-Lopes, M.; Sachett, A.; Marcon, M.; Strogulski, N.R.; Reis, C.G.; Chitolina, R.; Piato, A.; Herrmann, A.P. How Do Zebrafish (Danio rerio) Respond to MK-801 and Amphetamine? Relevance for Assessing Schizophrenia-Related Endophenotypes in Alternative Model Organisms. J. Neurosci. Res. 2021, 99, 2844–2859. [Google Scholar] [CrossRef] [PubMed]
- Lungu, F. A Short and Modern Perspective on Using Zebrafish as a Possible Animal Model for Studying the Cognitive Functions in Schizophrenia. Bul. De. Psihiatr. Integr. 2022, 94, 65–68. [Google Scholar] [CrossRef]
- Vinnakota, C.; Schroeder, A.; Du, X.; Ikeda, K.; Ide, S.; Mishina, M.; Hudson, M.; Charles Jones, N.; Sundram, S.; Anne Hill, R.; et al. Understanding the Role of the NMDA Receptor Subunit, GluN2D, in Mediating NMDA Receptor Antagonist-Induced Behavioral Disruptions in Male and Female Mice. J. Neurosci. Res. 2023, 102, e25257. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Wang, P.; Zhang, R.; Valenzuela, R.K.; Shang, S.; Wan, T.; Ma, J. Schizophrenia-Like Deficits and Impaired Glutamate/Gamma-Aminobutyric Acid Homeostasis in Zfp804a Conditional Knockout Mice. Schizophr. Bull. 2024, 50, 1411–1426. [Google Scholar] [CrossRef]
- Benvenutti, R.; Gallas-Lopes, M.; Marcon, M.; Reschke, C.R.; Herrmann, A.P.; Piato, A. Glutamate NMDA Receptor Antagonists with Relevance to Schizophrenia: A Review of Zebrafish Behavioral Studies. Curr. Neuropharmacol. 2022, 20, 494–509. [Google Scholar] [CrossRef]
- Zink, M.; Schmitt, A.; May, B.; Müller, B.; Demirakca, T.; Braus, D.F.; Henn, F.A. Differential Effects of Long-Term Treatment with Clozapine or Haloperidol on GABAA Receptor Binding and GAD67 Expression. Schizophr. Res. 2004, 66, 151–157. [Google Scholar] [CrossRef]
- Cheng, W.J.; Chen, C.H.; Chen, C.K.; Huang, M.C.; Pietrzak, R.H.; Krystal, J.H.; Xu, K. Similar Psychotic and Cognitive Profile between Ketamine Dependence with Persistent Psychosis and Schizophrenia. Schizophr. Res. 2018, 199, 313–318. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, K.S.; Park, A.R.; Min, T.J. Adding Preferred Color to a Conventional Reward Method Improves the Memory of Zebrafish in the T-Maze Behavior Model. Anim. Cells Syst. 2017, 21, 374–381. [Google Scholar] [CrossRef]
- Savuca, A.; Chelaru, I.A.; Balmus, I.M.; Curpan, A.S.; Nicoara, M.N.; Ciobica, A.S. Toxicological Response of Zebrafish Exposed to Cocktails of Polymeric Materials and Valproic Acid. Sustainability 2024, 16, 2057. [Google Scholar] [CrossRef]
- Seibt, K.J.; da Oliveira, R.L.; Zimmermann, F.F.; Capiotti, K.M.; Bogo, M.R.; Ghisleni, G.; Bonan, C.D. Antipsychotic Drugs Prevent the Motor Hyperactivity Induced by Psychotomimetic MK-801 in Zebrafish (Danio rerio). Behav. Brain Res. 2010, 214, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Robea, M.A.; Petrovici, A.; Ureche, D.; Nicoara, M.; Ciobica, A.S. Histopathological and Behavioral Impairments in Zebrafish (Danio rerio) Chronically Exposed to a Cocktail of Fipronil and Pyriproxyfen. Life 2023, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Ilie, O.-D.; Duta, R.; Jijie, R.; Nita, I.-B.; Nicoara, M.; Faggio, C.; Dobrin, R.; Mavroudis, I.; Ciobica, A.; Doroftei, B. Assessing Anti-Social and Aggressive Behavior in a Zebrafish (Danio rerio) Model of Parkinson’s Disease Chronically Exposed to Rotenone. Brain Sci. 2022, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Ayehu, M.; Shibre, T.; Milkias, B.; Fekadu, A. Movement Disorders in Neuroleptic-Naïve Patients with Schizophrenia Spectrum Disorders. BMC Psychiatry 2014, 14, 280. [Google Scholar] [CrossRef]
- Ward, K.M.; Citrome, L. Antipsychotic-Related Movement Disorders: Drug-Induced Parkinsonism vs. Tardive Dyskinesia—Key Differences in Pathophysiology and Clinical Management. Neurol. Ther. 2018, 7, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Karnik, V.; Osland, S.; Barnes, T.R.E.; Pringsheim, T.M. Movement Disorders Associated with Antipsychotic Medication in People with Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis. Can. J. Psychiatry 2018, 63, 730–739. [Google Scholar] [CrossRef]
- Nasim, R.; Nawaz, S.; Nasim, M.T. The Effects of Antipsychotic Drugs and Non-Pharmacological Therapies on Schizophrenia. Targets 2025, 3, 10. [Google Scholar] [CrossRef]
- Güzey, C.; Scordo, M.G.; Spina, E.; Landsem, V.M.; Spigset, O. Antipsychotic-Induced Extrapyramidal Symptoms in Patients with Schizophrenia: Associations with Dopamine and Serotonin Receptor and Transporter Polymorphisms. Eur. J. Clin. Pharmacol. 2007, 63, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.W.; Shyu, B.C.; Hsiao, S. Dose-Dependent Dissociable Effects of Haloperidol on Locomotion, Appetitive Responses, and Consummatory Behavior in Water-Deprived Rats. Pharmacol. Biochem. Behav. 2010, 95, 285–291. [Google Scholar] [CrossRef]
- Hershenberg, R.; Gros, D.F.; Brawman-Mintzer, O. Role of Atypical Antipsychotics in the Treatment of Generalized Anxiety Disorder. CNS Drugs 2014, 28, 519–533. [Google Scholar] [CrossRef]
- Miller, D.D. Atypical Antipsychotics: Sleep, Sedation, and Efficacy. Prim. Care Companion J. Clin. Psychiatry 2004, 6, 3–7. [Google Scholar] [PubMed]
- Crapanzano, C.; Casolaro, I.; Damiani, S.; Amendola, C. Efficacy of Olanzapine in Anxiety Dimension of Schizophrenia: A Systematic Review of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2022, 20, 592–599. [Google Scholar] [CrossRef]
- Idalencio, R.; Kalichak, F.; Gabriel Santos Rosa, J.; Acosta de Oliveira, T.; Koakoski, G.; Gusso, D.; Sander de Abreu, M.; Cristina Varrone Giacomini, A.; Helena de Alcântara Barcellos, H.; Piato, A.L.; et al. Waterborne Risperidone Decreases Stress Response in Zebrafish. PLoS ONE 2015, 10, e0140800. [Google Scholar] [CrossRef]
- Chen, P.A.; Wang, H.Y.; Sun, C.L.; Chen, M.L.; Chen, Y.C. Neurobehavioral Differences of Valproate and Risperidone on MK-801 Inducing Acute Hyperlocomotion in Mice. Behav. Neurol. 2022, 2022, 1048463. [Google Scholar] [CrossRef]
- Arruda, M.D.O.V.; Soares, P.M.; Honório, J.E.R.; Lima, R.C.d.S.; Chaves, E.M.C.; Lobato, R.D.F.G.; Martin, A.L.d.A.R.; Sales, G.T.M.; Carvalho, K.D.M.; Assreuy, A.M.S.; et al. Activities of the Antipsychotic Drugs Haloperidol and Risperidone on Behavioural Effects Induced by Ketamine in Mice. Sci. Pharm. 2008, 76, 673–687. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the Dopamine System in the Pathophysiology of Schizophrenia and Depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III--The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Coyle, J.T. NMDA Receptor and Schizophrenia: A Brief History. Schizophr. Bull. 2012, 38, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Haney, J.R.; Parikshak, N.N.; Leppa, V.; Ramaswami, G.; Hartl, C.; Schork, A.J.; Appadurai, V.; Buil, A.; Werge, T.M.; et al. Shared Molecular Neuropathology across Major Psychiatric Disorders Parallels Polygenic Overlap. Science 2018, 359, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, C.F.; Yang, J.Y.; Guo, T. Differential Effects of Haloperidol, Clozapine and Olanzapine on Learning and Memory Functions in Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1486–1495. [Google Scholar] [CrossRef]
- Gaspary, K.V.; Reolon, G.K.; Gusso, D.; Bonan, C.D. Novel Object Recognition and Object Location Tasks in Zebrafish: Influence of Habituation and NMDA Receptor Antagonism. Neurobiol. Learn. Mem. 2018, 155, 249–260. [Google Scholar] [CrossRef]
- Magyary, I. Floating Novel Object Recognition in Adult Zebrafish: A Pilot Study. Cogn. Process 2019, 20, 359–362. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Rogóż, Z.; Kamińska, K. The Effect of Combined Treatment with Escitalopram and Risperidone on the MK-801-Induced Changes in the Object Recognition Test in Mice. Pharmacol. Rep. 2016, 68, 116–120. [Google Scholar] [CrossRef]
- Samizadeh, M.-A.; Abdollahi-Keyvani, S.-T.; Fallah, H.; Beigi, B.; Motamedi-Manesh, A.; Adibian, S.; Vaseghi, S. Sex Difference Alters the Behavioral and Cognitive Performance in a Rat Model of Schizophrenia Induced by Sub-Chronic Ketamine. J. Psychiatr. Res. 2024, 178, 180–187. [Google Scholar] [CrossRef]
- Javitt, D.C. Glutamatergic Theories of Schizophrenia. Isr. J. Psychiatry Relat. Sci. 2010, 47, 4–16. [Google Scholar]
- Lisman, J.E.; Coyle, J.T.; Green, R.W.; Javitt, D.C.; Benes, F.M.; Heckers, S.; Grace, A.A. Circuit-Based Framework for Understanding Neurotransmitter and Risk Gene Interactions in Schizophrenia. Trends Neurosci. 2008, 31, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Avdesh, A.; Martin-Iverson, M.T.; Mondal, A.; Chen, M.; Askraba, S.; Morgan, N.; Lardelli, M.; Groth, D.M.; Verdile, G.; Martins, R.N. Evaluation of Color Preference in Zebrafish for Learning and Memory. J. Alzheimer’s Dis. 2012, 28, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, J.H. The Zebrafish Visual System: From Circuits to Behavior. Annu. Rev. Vis. Sci. 2019, 5, 269–293. [Google Scholar] [CrossRef]
- Michelotti, P.; Quadros, V.A.; Pereira, M.E.; Rosemberg, D.B. Ketamine Modulates Aggressive Behavior in Adult Zebrafish. Neurosci. Lett. 2018, 684, 164–168. [Google Scholar] [CrossRef]
- Pinheiro-da-Silva, J.; Silva, P.F.; Nogueira, M.B.; Luchiari, A.C. Sleep Deprivation Effects on Object Discrimination Task in Zebrafish (Danio rerio). Anim. Cogn. 2017, 20, 159–169. [Google Scholar] [CrossRef]
- Giacomini, N.J.; Rose, B.; Kobayashi, K.; Guo, S. Antipsychotics Produce Locomotor Impairment in Larval Zebrafish. Neurotoxicol Teratol. 2006, 28, 245–250. [Google Scholar] [CrossRef]
- De Donatis, D.; Porcelli, S.; De Ronchi, D.; Merlo Pich, E.; Kas, M.J.; Bilderbeck, A.; Serretti, A. Social Withdrawal and Neurocognitive Correlates in Schizophrenia. Int. Clin. Psychopharmacol. 2022, 37, 102–109. [Google Scholar] [CrossRef]
- Du, N.; Meng, X.; Li, J.; Shi, L.; Zhang, X. Decline in Working Memory in Stable Schizophrenia May Be Related to Attentional Impairment: Mediating Effects of Negative Symptoms, a Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2024, 20, 149–158. [Google Scholar] [CrossRef]
- Kalichak, F.; de Alcantara Barcellos, H.H.; Idalencio, R.; Koakoski, G.; Soares, S.M.; Pompermaier, A.; Rossini, M.; Barcellos, L.J.G. Persistent and Transgenerational Effects of Risperidone in Zebrafish. Environ. Sci. Pollut. Res. 2019, 26, 26293–26303. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Cascino, G.; Monteleone, A.M.; Rocca, P.; Rossi, A.; Bertolino, A.; Aguglia, E.; Amore, M.; Collantoni, E.; Corrivetti, G.; et al. Prevalence of Antipsychotic-Induced Extrapyramidal Symptoms and Their Association with Neurocognition and Social Cognition in Outpatients with Schizophrenia in the “Real-Life”. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110250. [Google Scholar] [CrossRef] [PubMed]
- Kucharska-Pietura, K.; Mortimer, A. Can Antipsychotics Improve Social Cognition in Patients with Schizophrenia? CNS Drugs 2013, 27, 335–343. [Google Scholar] [CrossRef]
- Nakazawa, K.; Zsiros, V.; Jiang, Z.; Nakao, K.; Kolata, S.; Zhang, S.; Belforte, J.E. GABAergic Interneuron Origin of Schizophrenia Pathophysiology. Neuropharmacology 2012, 62, 1574–1583. [Google Scholar] [CrossRef]
- Barch, D.M.; Ceaser, A. Cognition in Schizophrenia: Core Psychological and Neural Mechanisms. Trends Cogn. Sci. 2012, 16, 27–34. [Google Scholar] [CrossRef]
- Celikyurt, I.K.; Akar, F.Y.; Ulak, G.; Mutlu, O.; Erden, F. Effects of Risperidone on Learning and Memory in Naive and MK-801-Treated Rats. Pharmacology 2011, 87, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sergi, M.J.; Green, M.F.; Widmark, C.; Reist, C.; Erhart, S.; Braff, D.L.; Kee, K.S.; Marder, S.R.; Mintz, J. Cognition and Neurocognition: Effects of Risperidone, Olanzapine, and Haloperidol. Am. J. Psychiatry 2007, 164, 1585–1592. [Google Scholar] [CrossRef]
- Sampedro-Viana, D.; Cañete, T.; Sanna, F.; Oliveras, I.; Lavín, V.; Torrecilla, P.; Río-Álamos, C.; Tapias-Espinosa, C.; Sánchez-González, A.; Tobeña, A.; et al. Atypical Antipsychotics Attenuate MK801-Induced Social Withdrawal and Hyperlocomotion in the RHA Rat Model of Schizophrenia-Relevant Features. Psychopharmacology 2023, 240, 1931–1945. [Google Scholar] [CrossRef]
- Griffiths, K.; Velichkova, N.; Quadt, L.; Berni, J. Can Atypical Antipsychotics Alleviate Deficits in Psychosocial Impairments in Patients with a Diagnosis of Borderline Personality? A Systematic Review and Meta-Analysis. Psychiatry Res. Commun. 2024, 4, 100187. [Google Scholar] [CrossRef]
- Terry, A.V.; Gearhart, D.A.; Warner, S.E.; Zhang, G.; Bartlett, M.G.; Middlemore, M.L.; Beck, W.D.; Mahadik, S.P.; Waller, J.L. Oral Haloperidol or Risperidone Treatment in Rats: Temporal Effects on Nerve Growth Factor Receptors, Cholinergic Neurons, and Memory Performance. Neuroscience 2007, 146, 1316–1332. [Google Scholar] [CrossRef]
- Abril-de-Abreu, R.; Cruz, J.; Oliveira, R.F. Social Eavesdropping in Zebrafish: Tuning of Attention to Social Interactions. Sci. Rep. 2015, 5, 12678. [Google Scholar] [CrossRef]
- Zaki, H.; Lushi, E.; Severi, K.E. Larval Zebrafish Exhibit Collective Circulation in Confined Spaces. Front. Phys. 2021, 9, 678600. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish Models in Neuropsychopharmacology and CNS Drug Discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Marino Gammazza, A. The Zebrafish Model in Animal and Human Health Research. Int. J. Mol. Sci. 2025, 26, 1945. [Google Scholar] [CrossRef] [PubMed]
- Jarema, K.A.; Hunter, D.L.; Hill, B.N.; Olin, J.K.; Britton, K.N.; Waalkes, M.R.; Padilla, S. Developmental Neurotoxicity and Behavioral Screening in Larval Zebrafish with a Comparison to Other Published Results. Toxics 2022, 10, 256. [Google Scholar] [CrossRef]
- Leonardos, M.; Georgalis, C.; Sergiou, G.; Leonardos, D.; Lakkas, L.; Alexiou, G.A. The Developmental Toxicity of Haloperidol on Zebrafish (Danio rerio) Embryos. Biomedicines 2025, 13, 1794. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, P.F.; Hritcu, L.D.; Nicoara, M.-N.; Savuca, A.; Curpan, A.-S.; Chelaru, A.I.; Lungu, C.M.; Gurzu, B.; Balmus, I.-M.; Ciobica, A.; et al. Preliminary Data Regarding the Alleviating Effects of Haloperidol and Risperidone on the Short-Term Memory and Associative Learning in a Zebrafish Model of Schizophrenia. Pharmaceuticals 2025, 18, 1548. https://doi.org/10.3390/ph18101548
Lungu PF, Hritcu LD, Nicoara M-N, Savuca A, Curpan A-S, Chelaru AI, Lungu CM, Gurzu B, Balmus I-M, Ciobica A, et al. Preliminary Data Regarding the Alleviating Effects of Haloperidol and Risperidone on the Short-Term Memory and Associative Learning in a Zebrafish Model of Schizophrenia. Pharmaceuticals. 2025; 18(10):1548. https://doi.org/10.3390/ph18101548
Chicago/Turabian StyleLungu, Petru Fabian, Luminita Diana Hritcu, Mircea-Nicusor Nicoara, Alexandra Savuca, Alexandrina-Stefania Curpan, Alexandru Ionut Chelaru, Corina Miruna Lungu, Bogdan Gurzu, Ioana-Miruna Balmus, Alin Ciobica, and et al. 2025. "Preliminary Data Regarding the Alleviating Effects of Haloperidol and Risperidone on the Short-Term Memory and Associative Learning in a Zebrafish Model of Schizophrenia" Pharmaceuticals 18, no. 10: 1548. https://doi.org/10.3390/ph18101548
APA StyleLungu, P. F., Hritcu, L. D., Nicoara, M.-N., Savuca, A., Curpan, A.-S., Chelaru, A. I., Lungu, C. M., Gurzu, B., Balmus, I.-M., Ciobica, A., & Plavan, G.-I. (2025). Preliminary Data Regarding the Alleviating Effects of Haloperidol and Risperidone on the Short-Term Memory and Associative Learning in a Zebrafish Model of Schizophrenia. Pharmaceuticals, 18(10), 1548. https://doi.org/10.3390/ph18101548









