From AI-Assisted In Silico Computational Design to Preclinical In Vivo Models: A Multi-Platform Approach to Small Molecule Anti-IBD Drug Discovery
Abstract
1. Introduction
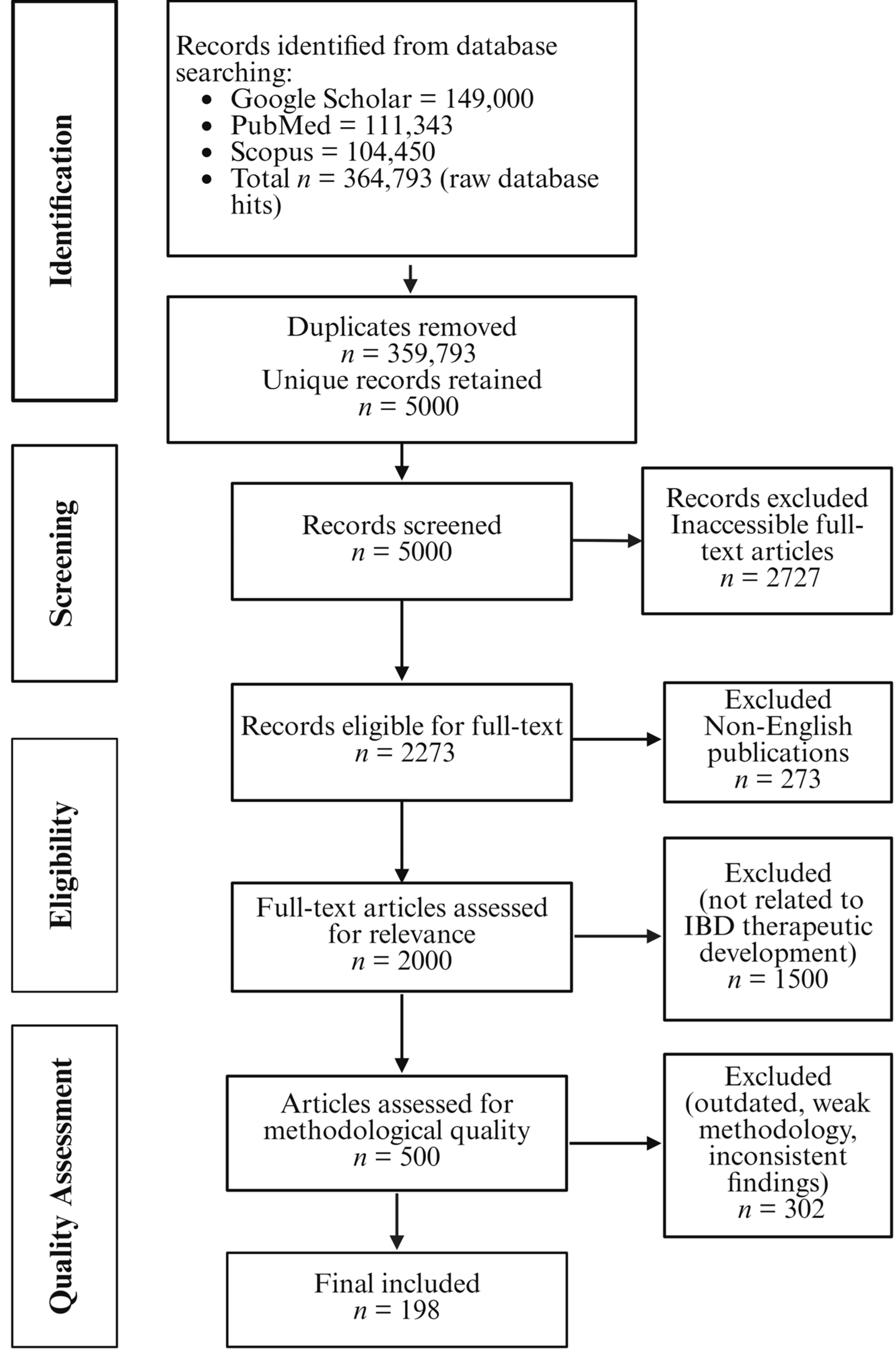
2. Review Methodology
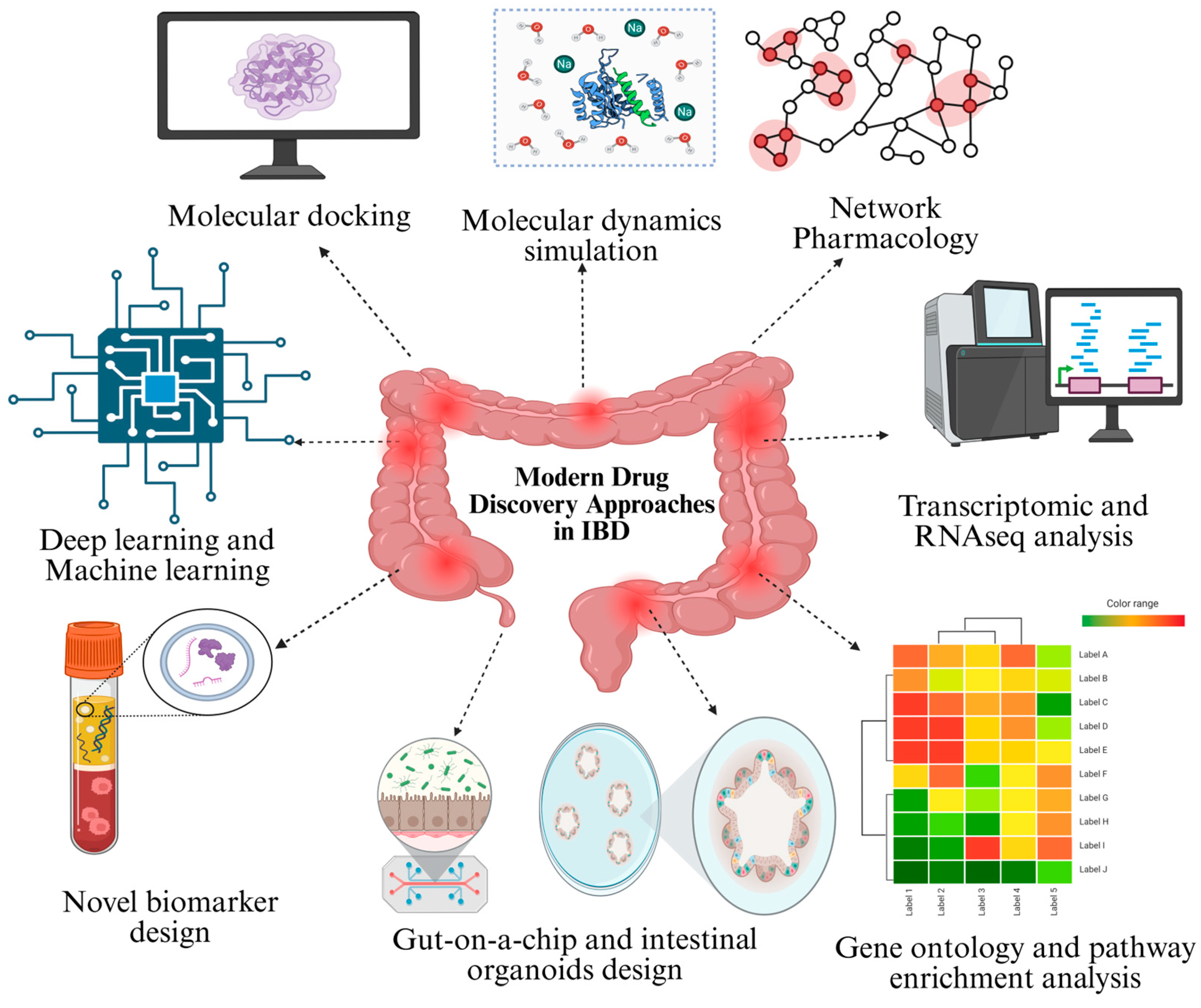
3. Modern Drug Discovery Approaches in IBD Research
3.1. Discovery of Novel Biomarkers in IBD
3.1.1. Diagnostic, Predictive, and Monitoring Biomarkers
3.1.2. Digital Health Tools and Computational Approaches in Biomarker Discovery
3.2. In Vivo Studies in Small-Molecule Drug Discovery
3.2.1. Lead Identification via Cheminformatics and CADD
3.2.2. Mathematical Modelling in IBD
3.2.3. Molecular Docking: An Essential Molecular Modelling Tool
3.2.4. Target Identification Through Bioinformatics Approach: Network Pharmacology and Systems-Biology
3.2.5. Other Bioinformatics Approaches in IBD Research
3.2.6. Software and Databases Supporting Bioinformatics and In Silico Approaches for Small Molecule Drug Discovery in IBD
3.2.7. Artificial Intelligence and Advancement in In Silico Techniques in IBD Research
3.2.8. Advantages and Limitations of Applying Computational Models in the Early Stage of Drug Discovery
4. In Vitro Models Using Human Cells in IBD Research
4.1. 2D Cell Culture Models (Immunological Monoculture and Co-Culture Models)
4.1.1. Caco-2 (Cancer-Coli-2) Cell Line
4.1.2. Human Colorectal Adenocarcinoma (HT29) Cell Line
4.1.3. Caco-2/HT29-MTX Cell Line
4.1.4. T84 Cell Line
4.1.5. Peripheral Blood Mononuclear Cells (PBMCs)
4.1.6. Human Acute Monocytic Leukemia Cell Line (THP-1)
4.1.7. RAW 246.7 Cell Line
4.2. Ex Vivo Three-Dimensional (3D) Culture Model
4.2.1. Organ-on-a-Chip (OoC) Model
4.2.2. 3D Tissue Culture, Stem Cells, and Organoids Design
4.2.3. Single-Cell RNA Sequencing (scRNA-Seq) and Whole-Genome Sequencing (WGS) in IBD Research
5. Animal Models in IBD: Connecting In Vitro Insights with In Vivo Outcomes
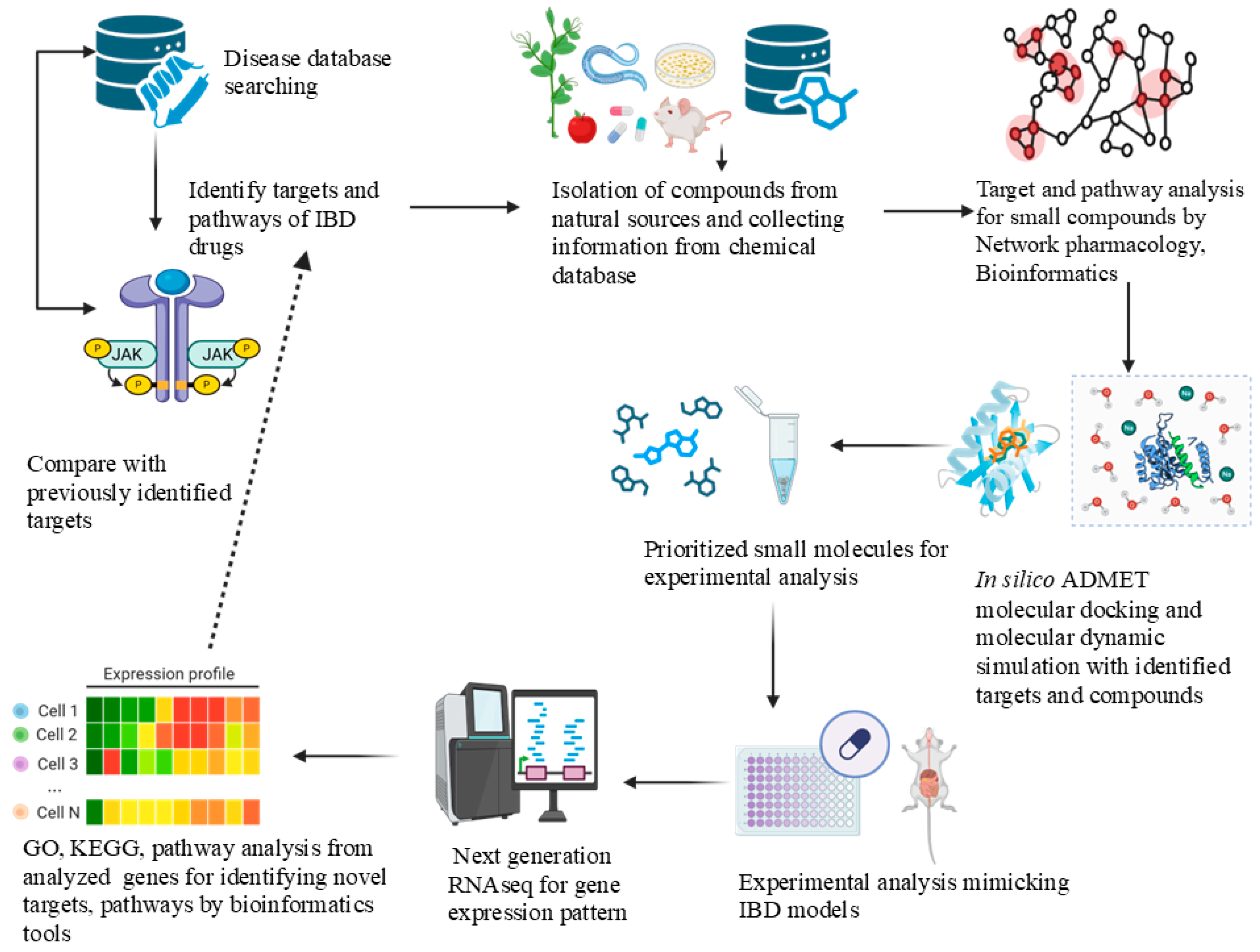
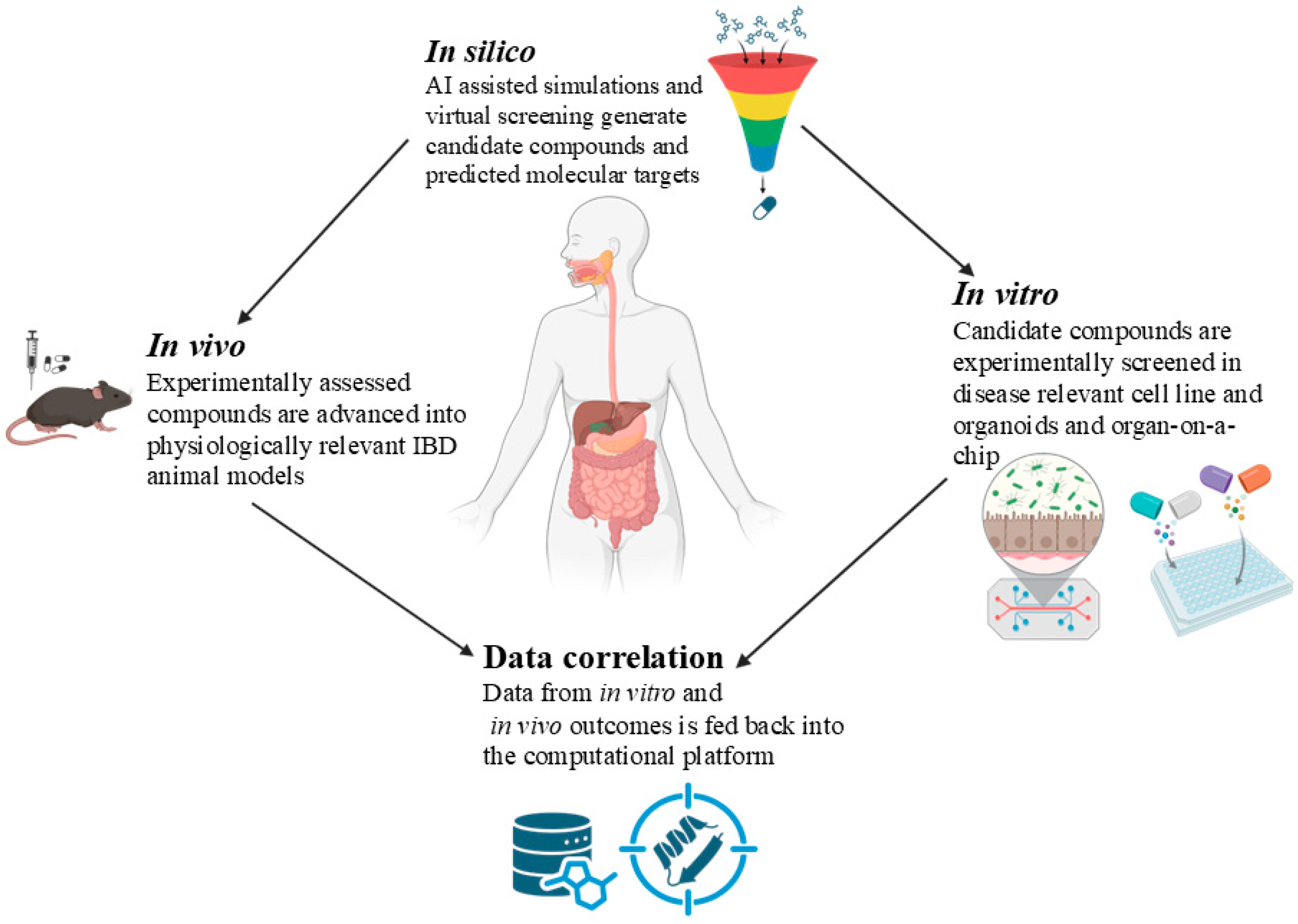
6. Integrating Computational, Cell Culture, and Animal Models: Approaches to Overcome Limitations of Conventional Drug Discovery
7. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Burisch, J.; Claytor, J.; Hernandez, I.; Hou, J.K.; Kaplan, G.G. The cost of inflammatory bowel disease care: How to make it sustainable. Clin. Gastroenterol. Hepatol. 2025, 23, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, A.; Zagaria, A.; Baccini, F.; Micheli, F.; Di Nardo, G.; Lombardo, C. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Med. Rev. 2021, 60, 101518. [Google Scholar] [CrossRef]
- Agrawal, M.; Spencer, E.A.; Colombel, J.-F.; Ungaro, R.C. Approach to the management of recently diagnosed inflammatory bowel disease patients: A user’s guide for adult and pediatric gastroenterologists. Gastroenterology 2021, 161, 47–65. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinović, A.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Odze, R. Diagnostic problems and advances in inflammatory bowel disease. Mod. Pathol. 2003, 16, 347–358. [Google Scholar] [CrossRef]
- Calvez, V.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Bartocci, B.; Murgiano, M.; Iaccarino, J.; Parand, E.; Napolitano, D.; Pugliese, D.; et al. Novel Insights into the pathogenesis of inflammatory bowel diseases. Biomedicines 2025, 13, 305. [Google Scholar] [CrossRef]
- Imbrizi, M.; Magro, F.; Coy, C.S.R. Pharmacological therapy in inflammatory bowel diseases: A narrative review of the past 90 years. Pharmaceuticals 2023, 16, 1272. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Bolinger, A.A.; Chen, H.; Liu, Z.; Cong, Y.; Brasier, A.R.; Pinchuk, I.V.; Tian, B.; Zhou, J. Target-based small molecule drug discovery towards novel therapeutics for inflammatory bowel diseases. Inflamm. Bowel Dis. 2021, 27, S38–S62. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational approaches streamlining drug discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Chen, J.; Lin, A.; Luo, P. Advancing pharmaceutical research: A comprehensive review of cutting-edge tools and technologies. Curr. Pharm. Anal. 2024, 21, 1–19. [Google Scholar] [CrossRef]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Applications to targets and beyond. Br. J. Pharmacol. 2007, 152, 21–37. [Google Scholar] [CrossRef]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational methods in drug discovery. Pharmacol. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef]
- Joshi, A.; Soni, A.; Acharya, S. In vitro models and ex vivo systems used in inflammatory bowel disease. Vitr. Model. 2022, 1, 213–227. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory bowel disease: A review of pre-clinical murine models of human disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, J.P.; Korcsmaros, T.; Gul, L. Integrating multi-omics to unravel host-microbiome interactions in inflammatory bowel disease. Cell Rep. Med. 2024, 5, 101738. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, D.; Xu, J.; Hu, J.; Wang, Y. Complex in vitro model: A transformative model in drug development and precision medicine. Clin. Transl. Sci. 2024, 17, e13695. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, P.; Mansouri, P.; Najafipour, S.; Kouhpayeh, S.A.; Farjadfar, A.; Behmard, E. Comprehensive computational strategies for multi-target drug discovery in inflammatory bowel disease utilizing bioactive compounds. Sci. Rep. 2025, 15, 15542. [Google Scholar] [CrossRef] [PubMed]
- Duarte, Y.; Márquez-Miranda, V.; Miossec, M.J.; González-Nilo, F. Integration of target discovery, drug discovery and drug delivery: A review on computational strategies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1554. [Google Scholar] [CrossRef]
- Quinn, T.A.; Kohl, P. Combining wet and dry research: Experience with model development for cardiac mechano-electric structure-function studies. Cardiovasc. Res. 2013, 97, 601–611. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, W.; Tan, X.; Bao, Y.-J. Network-based multi-omics integrative analysis methods in drug discovery: A systematic review. BioData Min. 2025, 18, 27. [Google Scholar] [CrossRef]
- Wagatsuma, K.; Yokoyama, Y.; Nakase, H. Role of biomarkers in the diagnosis and treatment of inflammatory bowel disease. Life 2021, 11, 1375. [Google Scholar] [CrossRef]
- Clough, J.; Colwill, M.; Poullis, A.; Pollok, R.; Patel, K.; Honap, S. Biomarkers in inflammatory bowel disease: A practical guide. Therap. Adv. Gastroenterol. 2024, 17, 17562848241251600. [Google Scholar] [CrossRef]
- Sakurai, T.; Saruta, M. Positioning and usefulness of biomarkers in inflammatory bowel disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef]
- Vila, A.V.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Ruigrok, R.A.; Abu-Ali, G.; Giallourakis, C.; et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023, 72, 1472–1485. [Google Scholar] [CrossRef]
- Aldars-Garcia, L.; Gisbert, J.P.; Chaparro, M. Metabolomics insights into inflammatory bowel disease: A comprehensive review. Pharmaceuticals 2021, 14, 1190. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.J.; Lekkerkerker, A.N.; Casavant, E.P.; Hackney, J.A.; Nguyen, A.; McBride, J.M.; Mathews, W.R.; Anania, V.G. Comprehensive profiling of the human fecal proteome from IBD patients with DIA-MS enables evaluation of disease-relevant proteins. Proteom.-Clin. Appl. 2024, 18, 2300075. [Google Scholar] [CrossRef] [PubMed]
- Shajari, E.; Gagné, D.; Malick, M.; Brunet, M.; Delisle, M.; Boisvert, F.; Beaulieu, J. A260 Advancing inflammatory bowel disease diagnosis through stool proteomic signatures obtained via DIA-MASS spectrometry and machine learning. J. Can. Assoc. Gastroenterol. 2024, 7, 209–210. [Google Scholar] [CrossRef]
- James, J.P.; Riis, L.B.; Malham, M.; Høgdall, E.; Langholz, E.; Nielsen, B.S. MicroRNA biomarkers in IBD—Differential diagnosis and prediction of colitis-associated cancer. Int. J. Mol. Sci. 2020, 21, 7893. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum biomarkers for inflammatory bowel disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef]
- Perez, K.; Ngollo, M.; Rabinowitz, K.; Hammoudi, N.; Seksik, P.; Xavier, R.J.; Daly, M.J.; Dotan, I.; Le Bourhis, L.; Allez, M. Meta-analysis of IBD gut samples gene expression identifies specific markers of ileal and colonic diseases. Inflamm. Bowel Dis. 2022, 28, 775–782. [Google Scholar] [CrossRef]
- Mortensen, J.H.; Sinkeviciute, D.; Manon-Jensen, T.; Domislović, V.; McCall, K.; Thudium, C.S.; Brinar, M.; Önnerfjord, P.; Goodyear, C.S.; Krznarić, Ž.; et al. A specific calprotectin neo-epitope [CPa9-HNE] in serum from inflammatory bowel disease patients is associated with neutrophil activity and endoscopic severity. J. Crohn’s Colitis 2022, 16, 1447–1460. [Google Scholar] [CrossRef]
- Khan, F.; Abdulla, N.; du Plessis, T.-L.; Karlsson, K.; Barrow, P.; Bebington, B.; Gu, L.; Kaur, M. Identification and validation of biomarkers to predict early diagnosis of inflammatory bowel disease and its progression to colorectal cancer. Biochem. Genet. 2024, 68, 1–27. [Google Scholar] [CrossRef]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Lee, J.; Yoo, J.; Oh, S.; Park, S.; Park, D.; Bae, J.; Lee, C. OP18 Multi-analytical approaches reveal robust gut microbial biomarkers for inflammatory bowel disease diagnosis: A large-scale cohort study. J. Crohn’s Colitis 2025, 19, i36–i37. [Google Scholar] [CrossRef]
- Bencardino, S.; D’Amico, F.; Zilli, A.; Parigi, T.L.; Allocca, M.; Fiorino, G.; Danese, S.; Furfaro, F. Fecal, blood, and urinary biomarkers in inflammatory bowel diseases. Transl Gastroenterol. 2024, 2, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Huang, C.; Wang, X.; Zhou, R.; Hao, W.; Lin, Q. The Reg protein family: Potential new targets for the treatment of inflammatory bowel disease and colorectal cancer. Front. Gastroenterol. 2024, 3, 1386069. [Google Scholar] [CrossRef]
- Harindranath, S.; Desai, D. Wearable technology in inflammatory bowel disease: Current state and future direction. Expert Rev. Med. Devices 2025, 22, 121–126. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-assisted detection of biomarkers by sensors and biosensors for early diagnosis and monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Hudu, S.A.; Alshrari, A.S.; Abu-Shoura, E.a.J.I.; Osman, A.; Jimoh, A.O. A critical review of the prospect of integrating artificial intelligence in infectious disease diagnosis and prognosis. Interdiscip. Perspect. Infect. Dis. 2025, 2025, 6816002. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, B.; Lin, K.-C.; Pali, M.; Sankhala, D.; Muthukumar, S.; Prasad, S. A sweat-based wearable enabling technology for real-time monitoring of IL-1β and CRP as potential markers for inflammatory bowel disease. Inflamm. Bowel Dis. 2020, 26, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Xu, C.; Kumar, A. Advanced computational tools, artificial intelligence and machine-learning approaches in gut microbiota and biomarker identification. Front. Med. Technol. 2025, 6, 1434799. [Google Scholar]
- Yu, S.; Kalinin, A.A.; Paraskevopoulou, M.D.; Maruggi, M.; Cheng, J.; Tang, J.; Icke, I.; Luo, Y.; Wei, Q.; Scheibe, D.; et al. Integrating inflammatory biomarker analysis and artificial-intelligence-enabled image-based profiling to identify drug targets for intestinal fibrosis. Cell Chem. Biol. 2023, 30, 1169–1182.e1168. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Niu, R.; Xiong, S.; He, J.; Wang, Y.; Zhang, P.; Su, F.; Liu, Z.; Zhou, L.; et al. Multi-Omics biomarkers for predicting efficacy of biologic and small-molecule therapies in adults with inflammatory bowel disease: A systematic review. United Eur. Gastroenterol. J. 2025, 13, 517–530. [Google Scholar] [CrossRef]
- Mak, K.-K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef]
- Garcia-Domenech, R.; Gálvez-Llompart, M.; Zanni, R.; Recio, M.C.; Galvez, J. QSAR methods for the discovery of new inflammatory bowel disease drugs. Expert Opin. Drug Discov. 2013, 8, 933–949. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, X.-g.; Lv, W.; Xue, Y. Prediction of novel and selective TNF-alpha converting enzyme (TACE) inhibitors and characterization of correlative molecular descriptors by machine learning approaches. J. Mol. Graph. Model. 2009, 28, 236–244. [Google Scholar] [CrossRef]
- Kilian, C.; Ulrich, H.; Zouboulis, V.A.; Sprezyna, P.; Schreiber, J.; Landsberger, T.; Büttner, M.; Biton, M.; Villablanca, E.J.; Huber, S.; et al. Longitudinal single-cell data informs deterministic modelling of inflammatory bowel disease. npj Syst. Biol. Appl. 2024, 10, 69. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Verstockt, B.; Adamina, M.; Allin, K.H.; Allocca, M.; Bourgonje, A.R.; Burisch, J.; Doherty, G.; Dulai, P.S.; El-Hussuna, A.; et al. ECCO topical review on predictive models on inflammatory bowel disease disease course and treatment response. J. Crohn’s Colitis 2025, 19, jjaf073. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, A.; Altomare, A.; Boscarino, T.; Gori, M.; Balestrieri, P.; Putignani, L.; Del Chierico, F.; Carotti, S.; Cicala, M.; Guarino, M.P.L.; et al. Mathematical modelling of vedolizumab treatment’s effect on microbiota and intestinal permeability in inflammatory bowel disease patients. Bioengineering 2024, 11, 710. [Google Scholar] [CrossRef]
- Jansen, J.E.; Gaffney, E.A.; Wagg, J.; Coles, M.C. Combining mathematical models with experimentation to drive novel mechanistic insights into macrophage function. Front. Immunol. 2019, 10, 1283. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.; Ezeh, E.; Ofoke, I.; Ogbu, C.; Ugwuja, E.I.; Aja, P. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.-H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein–protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and its limits in rigid body protein-protein docking. Structure 2020, 28, 1071–1081.e1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dokholyan, N.V. MedusaDock 2.0: Efficient and accurate protein–ligand docking with constraints. J. Chem. Inf. Model. 2019, 59, 2509–2515. [Google Scholar] [CrossRef]
- Bouchouireb, Z.; Olivier-Jimenez, D.; Jaunet-Lahary, T.; Thany, S.H.; Le Questel, J.-Y. Navigating the complexities of docking tools with nicotinic receptors and acetylcholine binding proteins in the realm of neonicotinoids. Ecotoxicol. Environ. Saf. 2024, 281, 116582. [Google Scholar] [CrossRef]
- Sehnal, D.; Svobodová Vařeková, R.; Berka, K.; Pravda, L.; Navrátilová, V.; Banáš, P.; Ionescu, C.-M.; Otyepka, M.; Koča, J. MOLE 2.0: Advanced approach for analysis of biomacromolecular channels. J. Cheminform. 2013, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carmona, S.; Alvarez-Garcia, D.; Foloppe, N.; Garmendia-Doval, A.B.; Juhos, S.; Schmidtke, P.; Barril, X.; Hubbard, R.E.; Morley, S.D. rDock: A fast, versatile and open source program for docking ligands to proteins and nucleic acids. PLoS Comput. Biol. 2014, 10, e1003571. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.; Boelens, R.; Bonvin, A.M. HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 2003, 125, 1731–1737. [Google Scholar] [CrossRef]
- Pedotti, M.; Simonelli, L.; Livoti, E.; Varani, L. Computational docking of antibody-antigen complexes, opportunities and pitfalls illustrated by influenza hemagglutinin. Int. J. Mol. Sci. 2011, 12, 226–251. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein–ligand docking using GOLD. Proteins Struct. Funct. Bioinf. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Moawadh, M.S. Molecular docking analysis of natural compounds as TNF-α inhibitors for Crohn’s disease management. Bioinformation 2023, 19, 716. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Butt, S.S.; Badshah, Y.; Shabbir, M.; Rafiq, M. Molecular docking using chimera and autodock vina software for nonbioinformaticians. JMIR Bioinform. Biotechnol. 2020, 1, e14232. [Google Scholar] [CrossRef]
- Johansson, M.U.; Zoete, V.; Michielin, O.; Guex, N. Defining and searching for structural motifs using DeepView/Swiss-PdbViewer. BMC Bioinform. 2012, 13, 173. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Bibi, M.; Baboo, I.; Majeed, H.; Kumar, S.; Lackner, M. Molecular docking of key compounds from Acacia Honey and Nigella sativa oil and experimental validation for Colitis treatment in albino mice. Biology 2024, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Lexa, K.W.; Carlson, H.A. Protein flexibility in docking and surface mapping. Q. Rev. Biophys. 2012, 45, 301–343. [Google Scholar] [CrossRef]
- Kamenik, A.S.; Singh, I.; Lak, P.; Balius, T.E.; Liedl, K.R.; Shoichet, B.K. Energy penalties enhance flexible receptor docking in a model cavity. Proc. Natl. Acad. Sci. USA 2021, 118, e2106195118. [Google Scholar] [CrossRef]
- Cozzini, P.; Dottorini, T. Is it possible docking and scoring new ligands with few experimental data? Preliminary results on estrogen receptor as a case study. Eur. J. Med. Chem. 2004, 39, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Brunsteiner, M.; Petukhov, P.A. Insights from comprehensive multiple receptor docking to HDAC8. J. Mol. Model. 2012, 18, 3927–3939. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Ding, F.; Dokholyan, N.V. Incorporating backbone flexibility in MedusaDock improves ligand-binding pose prediction in the CSAR2011 docking benchmark. J. Chem. Inf. Model. 2013, 53, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor–ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef]
- Zheng, S.; Xue, T.; Wang, B.; Guo, H.; Liu, Q. Application of network pharmacology in the study of mechanism of Chinese medicine in the treatment of ulcerative colitis: A review. Front. Bioinform. 2022, 2, 928116. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, L.; Yang, L.; He, C.; He, Y.; Chen, L.; Dong, Q.; Zhang, H.; Chen, S.; Li, P. Network pharmacology: A bright guiding light on the way to explore the personalized precise medication of traditional Chinese medicine. Chin. Med. 2023, 18, 146. [Google Scholar] [CrossRef]
- Sadegh, S.; Skelton, J.; Anastasi, E.; Bernett, J.; Blumenthal, D.B.; Galindez, G.; Salgado-Albarrán, M.; Lazareva, O.; Flanagan, K.; Cockell, S.; et al. Network medicine for disease module identification and drug repurposing with the NeDRex platform. Nat. Commun. 2021, 12, 6848. [Google Scholar] [CrossRef] [PubMed]
- Balbas-Martinez, V.; Ruiz-Cerdá, L.; Irurzun-Arana, I.; Gonzalez-Garcia, I.; Vermeulen, A.; Gómez-Mantilla, J.D.; Trocóniz, I.F. A systems pharmacology model for inflammatory bowel disease. PLoS ONE 2018, 13, e0192949. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Peng, W.; Luo, Q.; Zhao, W.; Dai, W.; Zeng, H.; Wong, H.L.X.; Hu, X. Exploring the mechanism of Suxin Hugan Fang in treating ulcerative colitis based on network pharmacology. Sci. Rep. 2024, 14, 27196. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Li, G. Integrated bioinformatics and network pharmacology to identify the therapeutic target and molecular mechanisms of Huangqin decoction on ulcerative Colitis. Sci. Rep. 2022, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Xiao, G.; Yang, M.; Huang, X.; Cai, D.; Xie, C.; Chen, Z.; Bi, X.; Xu, A. Integrated network pharmacology and metabolomics reveal the mechanisms of Jasminum elongatum in anti-ulcerative colitis. Sci. Rep. 2023, 13, 22449. [Google Scholar] [CrossRef]
- Deng, L.; Feng, Z.; Li, X.; Fan, L.; Wu, X.; Tavakoli, S.; Zhu, Y.; Ye, H.; Wu, K. Exploring the potential mechanism of B-phycoerythrin on DSS-induced colitis and colitis-associated bone loss based on network pharmacology, molecular docking, and experimental validation. Sci. Rep. 2025, 15, 5455. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Wan, T.; Mei, Y.; Wang, Z.; Xue, J.; Luo, Y.; Li, M.; Fang, S.; Pan, H.; et al. Systems pharmacology approach uncovers Ligustilide attenuates experimental colitis in mice by inhibiting PPARγ-mediated inflammation pathways. Cell Biol. Toxicol. 2021, 37, 113–128. [Google Scholar] [CrossRef]
- Thomas, J.P.; Modos, D.; Korcsmaros, T.; Brooks-Warburton, J. Network biology approaches to achieve precision medicine in inflammatory bowel disease. Front. Genet. 2021, 12, 760501. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Darzi, Y.; Vieira-Silva, S.; Falony, G.; Raes, J.; Joossens, M. Meta-omics in inflammatory bowel disease research: Applications, challenges, and guidelines. J. Crohn’s Colitis 2016, 10, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Franzosa, E.A.; Lloyd-Price, J.; McIver, L.J.; Schwager, R.; Poon, T.W.; Ananthakrishnan, A.N.; Andrews, E.; Barron, G.; Lake, K.; et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol. 2018, 3, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Garand, M.; Al Khodor, S. Integrating omics for a better understanding of Inflammatory Bowel Disease: A step towards personalized medicine. J. Transl. Med. 2019, 17, 419. [Google Scholar] [CrossRef]
- Xu, C.; Shao, J. High-throughput omics technologies in inflammatory bowel disease. Clin. Chim. Acta 2024, 555, 117828. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Asadzadeh Aghdaei, H.; Nazemalhosseini-Mojarad, E.; Nadalian, B.; Nadalian, B.; Houri, H. Overrepresentation of Enterobacteriaceae and Escherichia coli is the major gut microbiome signature in Crohn’s disease and ulcerative colitis; a comprehensive metagenomic analysis of IBDMDB datasets. Front. Cell. Infect. Microbiol. 2022, 12, 1015890. [Google Scholar] [CrossRef]
- Assadsangabi, A.; Evans, C.A.; Corfe, B.M.; Lobo, A. Application of proteomics to inflammatory bowel disease research: Current status and future perspectives. Gastroenterol. Res. Pract. 2019, 2019, 1426954. [Google Scholar]
- Luo, Y.; Zhao, C.; Chen, F. Multiomics research: Principles and challenges in integrated analysis. BioDesign Res. 2024, 6, 59. [Google Scholar] [CrossRef]
- Drobin, K.; Assadi, G.; Hong, M.-G.; Andersson, E.; Fredolini, C.; Forsström, B.; Reznichenko, A.; Akhter, T.; Ek, W.E.; Bonfiglio, F.; et al. Targeted analysis of serum proteins encoded at known inflammatory bowel disease risk loci. Inflamm. Bowel Dis. 2019, 25, 306–316. [Google Scholar] [CrossRef]
- Noecker, C.; Eng, A.; Muller, E.; Borenstein, E. MIMOSA2: A metabolic network-based tool for inferring mechanism-supported relationships in microbiome-metabolome data. Bioinformatics 2022, 38, 1615–1623. [Google Scholar]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Gao, X.; Sun, R.; Jiao, N.; Liang, X.; Li, G.; Gao, H.; Wu, X.; Yang, M.; Chen, C.; Sun, X.; et al. Integrative multi-omics deciphers the spatial characteristics of host-gut microbiota interactions in Crohn’s disease. Cell Rep. Med. 2023, 4, 101050. [Google Scholar] [CrossRef]
- Kong, L.; Subramanian, S.; Segerstolpe, A.; Tran, V.; Shih, A.R.; Carter, G.T.; Kunitake, H.; Twardus, S.W.; Li, J.; Gandhi, S.; et al. Single-cell and spatial transcriptomics of stricturing Crohn’s disease highlights a fibrosis-associated network. Nat. Genet. 2025, 57, 1742–1753. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Oler, E.; Peters, H.; Guo, A.; Girod, S.; Han, S.; Saha, S.; Lui, V.W.; LeVatte, M.; Gautam, V.; et al. MiMeDB: The human microbial metabolome database. Nucleic Acids Res. 2023, 51, D611–D620. [Google Scholar] [CrossRef]
- Yan, D.; Zheng, G.; Wang, C.; Chen, Z.; Mao, T.; Gao, J.; Yan, Y.; Chen, X.; Ji, X.; Yu, J.; et al. HIT 2.0: An enhanced platform for Herbal Ingredients’ Targets. Nucleic Acids Res. 2022, 50, D1238–D1243. [Google Scholar] [CrossRef]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, D514–D517. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef]
- Irwin, J.J.; Tang, K.G.; Young, J.; Dandarchuluun, C.; Wong, B.R.; Khurelbaatar, M.; Moroz, Y.S.; Mayfield, J.; Sayle, R.A. ZINC20—A free ultralarge-scale chemical database for ligand discovery. J. Chem. Inf. Model. 2020, 60, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Fong, D.; Gao, C.; Churas, C.; Pillich, R.; Lenkiewicz, J.; Pratt, D.; Pico, A.R.; Hanspers, K.; Xin, Y.; et al. Cytoscape Web: Bringing network biology to the browser. Nucleic Acids Res. 2025, 53, gkaf365. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef]
- Tian, S.; Wu, L.; Zheng, H.; Zhong, X.; Yu, X.; Wu, W. Identification of autophagy-related genes in neuropathic pain through bioinformatic analysis. Hereditas 2023, 160, 8. [Google Scholar] [CrossRef]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Freddolino, L.; Zhang, Y. BioLiP2: An updated structure database for biologically relevant ligand–protein interactions. Nucleic Acids Res. 2024, 52, D404–D412. [Google Scholar] [CrossRef] [PubMed]
- Dundas, J.; Ouyang, Z.; Tseng, J.; Binkowski, A.; Turpaz, Y.; Liang, J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006, 34, W116–W118. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Bell, E.W.; Zhang, Y. I-TASSER gateway: A protein structure and function prediction server powered by XSEDE. Future Gener. Comput. Syst. 2019, 99, 73–85. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Di Felice, R.; Mayes, M.L.; Richard, R.M.; Williams-Young, D.B.; Chan, G.K.-L.; de Jong, W.A.; Govind, N.; Head-Gordon, M.; Hermes, M.R.; Kowalski, K.; et al. A perspective on sustainable computational chemistry software development and integration. J. Chem. Theory Comput. 2023, 19, 7056–7076. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Yu, D.; Li, H.; Liu, Y.; Yang, X.; Yang, W.; Fu, Y.; Zuo, Y.-A.; Huang, X. Application of the molecular dynamics simulation GROMACS in food science. Food Res. Int. 2024, 190, 114653. [Google Scholar] [CrossRef]
- Cheng, C.; Hua, J.; Tan, J.; Qian, W.; Zhang, L.; Hou, X. Identification of differentially expressed genes, associated functional terms pathways, and candidate diagnostic biomarkers in inflammatory bowel diseases by bioinformatics analysis. Exp. Ther. Med. 2019, 18, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, F.; Yang, N.; Zhan, X.; Liao, J.; Mai, S.; Huang, Z. In silico methods for identification of potential therapeutic targets. Interdiscip. Sci. Comput. life Sci. 2022, 14, 285–310. [Google Scholar] [CrossRef]
- Gorelov, S.; Titov, A.; Tolicheva, O.; Konevega, A.; Shvetsov, A. Determination of hydrogen bonds in GROMACS: A new implementation to overcome memory limitation. J. Chem. Inf. Model. 2024, 64, 6241–6246. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Tao, H.; Feng, W.; Ren, C. Network pharmacology integrated with molecular docking and molecular dynamics simulations to explore the mechanism of Tongxie Yaofang in the treatment of ulcerative colitis. Medicine 2024, 103, e39569. [Google Scholar] [CrossRef]
- Fiocchi, C. Integrating omics: The future of IBD? Dig. Dis. 2014, 32, 96–102. [Google Scholar] [CrossRef]
- Caballero Mateos, A.M.; Canadas de la Fuente, G.A.; Gros, B. Paradigm shift in inflammatory bowel disease management: Precision medicine, artificial intelligence, and emerging therapies. J. Clin. Med. 2025, 14, 1536. [Google Scholar] [CrossRef]
- Sahoo, D.; Swanson, L.; Sayed, I.M.; Katkar, G.D.; Ibeawuchi, S.-R.; Mittal, Y.; Pranadinata, R.F.; Tindle, C.; Fuller, M.; Stec, D.L.; et al. Artificial intelligence guided discovery of a barrier-protective therapy in inflammatory bowel disease. Nat. Commun. 2021, 12, 4246. [Google Scholar] [CrossRef] [PubMed]
- Sedano, R.; Solitano, V.; Vuyyuru, S.K.; Yuan, Y.; Hanžel, J.; Ma, C.; Nardone, O.M.; Jairath, V. Artificial intelligence to revolutionize IBD clinical trials: A comprehensive review. Therap. Adv. Gastroenterol. 2025, 18, 17562848251321915. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Patel, M.; Shah, M.; Patel, M.; Prajapati, M. Computational transformation in drug discovery: A comprehensive study on molecular docking and quantitative structure activity relationship (QSAR). Int. Pharm. 2024, 2, 589–595. [Google Scholar] [CrossRef]
- Chang, Y.; Hawkins, B.A.; Du, J.J.; Groundwater, P.W.; Hibbs, D.E.; Lai, F. A guide to In Silico drug design. Pharmaceutics 2023, 15, 49. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Carneiro, A.S. AI-Driven Drug Discovery: A Comprehensive Review. ACS Omega 2025, 10, 23889–23903. [Google Scholar] [CrossRef]
- Selo, M.A.; Sake, J.A.; Kim, K.-J.; Ehrhardt, C. In vitro and ex vivo models in inhalation biopharmaceutical research—Advances, challenges and future perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113862. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; Barros, A.S.; Leite-Pereira, C.; Viegas, J.; das Neves, J.; Nunes, R.; Sarmento, B. Trends in 3D models of inflammatory bowel disease. Biochim. Biophys. Acta—Mol. Basis Dis. 2024, 1870, 167042. [Google Scholar] [CrossRef]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylan hydrolyzates as immunomodulators in Caco-2 and HT-29 colon cancer cell lines. Food Funct. 2017, 8, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Le, N.P.K.; Altenburger, M.J.; Lamy, E. Development of an inflammation-triggered in vitro “leaky gut” model using Caco-2/HT29-MTX-E12 combined with macrophage-like THP-1 cells or primary human-derived macrophages. Int. J. Mol. Sci. 2023, 24, 7427. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.-Y.; Choi, S.-H.; Kim, H.-J.; Kim, D.-h.; Bae, J.-H.; Kwon, G.-S.; Lee, D.-h.; Hwang, J.-H.; Kim, D.-K.; Baek, M.-C.; et al. Hemp-derived nanovesicles protect leaky gut and liver injury in dextran sodium sulfate-induced colitis. Int. J. Mol. Sci. 2022, 23, 9955. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Nagy, L.I.; Berkó, A.; Hoffmann, A.; Fehér, L.Z.; Bagyánszki, M.; Kari, B.; Balog, J.A.; Hackler, L., Jr.; Kanizsai, I.; et al. The anti-inflammatory role of mannich curcuminoids; special focus on colitis. Molecules 2019, 24, 1546. [Google Scholar] [CrossRef]
- Garcia, I.; Pouzet, C.; Brulas, M.; Bauza, E.; Botto, J.; Domloge, N. Evaluation of THP-1 cell line as an in vitro model for long-term safety assessment of new molecules. Int. J. Cosmet. Sci. 2013, 35, 568–574. [Google Scholar] [CrossRef]
- Mao, N.; Yu, Y.; Lu, X.; Yang, Y.; Liu, Z.; Wang, D. Preventive effects of matrine on LPS-induced inflammation in RAW 264.7 cells and intestinal damage in mice through the TLR4/NF-κB/MAPK pathway. Int. Immunopharmacol. 2024, 143, 113432. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Tataru, C.; Livni, M.; Marean-Reardon, C.; Franco, M.C.; David, M. Cytokine induced inflammatory bowel disease model using organ-on-a-chip technology. PLoS ONE 2023, 18, e0289314. [Google Scholar] [CrossRef]
- Kaden, T.; Alonso-Román, R.; Stallhofer, J.; Gresnigt, M.S.; Hube, B.; Mosig, A.S. Leveraging Organ-on-Chip models to investigate host–microbiota dynamics and targeted therapies for inflammatory bowel disease. Adv. Healthc. Mater. 2024, 14, 2402756. [Google Scholar] [CrossRef]
- Beaurivage, C.; Kanapeckaite, A.; Loomans, C.; Erdmann, K.S.; Stallen, J.; Janssen, R.A. Development of a human primary gut-on-a-chip to model inflammatory processes. Sci. Rep. 2020, 10, 21475. [Google Scholar] [CrossRef]
- Liu, J.; Lu, R.; Zheng, X.; Hou, W.; Wu, X.; Zhao, H.; Wang, G.; Tian, T. Establishment of a gut-on-a-chip device with controllable oxygen gradients to study the contribution of Bifidobacterium bifidum to inflammatory bowel disease. Biomater. Sci. 2023, 11, 2504–2517. [Google Scholar] [CrossRef] [PubMed]
- Beaurivage, C.; Naumovska, E.; Chang, Y.X.; Elstak, E.D.; Nicolas, A.; Wouters, H.; van Moolenbroek, G.; Lanz, H.L.; Trietsch, S.J.; Joore, J.; et al. Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int. J. Mol. Sci. 2019, 20, 5661. [Google Scholar] [CrossRef]
- Shin, W.; Hackley, L.A.; Kim, H.J. “Good fences make good neighbors”: How does the human gut microchip unravel mechanism of intestinal inflammation? Gut Microbes 2020, 11, 581–586. [Google Scholar] [CrossRef]
- Okamoto, R.; Shimizu, H.; Suzuki, K.; Kawamoto, A.; Takahashi, J.; Kawai, M.; Nagata, S.; Hiraguri, Y.; Takeoka, S.; Sugihara, H.Y.; et al. Organoid-based regenerative medicine for inflammatory bowel disease. Regen. Ther. 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Dotti, I.; Salas, A. Potential use of human stem cell–derived intestinal organoids to study inflammatory bowel diseases. Inflamm. Bowel Dis. 2018, 24, 2501–2509. [Google Scholar]
- Tindle, C.; Fonseca, A.G.; Taheri, S.; Katkar, G.D.; Lee, J.; Maity, P.; Sayed, I.M.; Ibeawuchi, S.-R.; Vidales, E.; Pranadinata, R.F.; et al. A living organoid biobank of patients with Crohn’s disease reveals molecular subtypes for personalized therapeutics. Cell Rep. Med. 2024, 5, 101748. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Wang, J.; Li, H. Current applications of intestinal organoids: A review. Stem Cell Res. Ther. 2024, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Flood, P.; Hanrahan, N.; Nally, K.; Melgar, S. Human intestinal organoids: Modeling gastrointestinal physiology and immunopathology—Current applications and limitations. Eur. J. Immunol. 2024, 54, 2250248. [Google Scholar] [CrossRef]
- Yao, Q.; Cheng, S.; Pan, Q.; Yu, J.; Cao, G.; Li, L.; Cao, H. Organoids: Development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm 2024, 5, e735. [Google Scholar] [CrossRef]
- Ren, J.; Huang, S. Intestinal organoids in inflammatory bowel disease: Advances, applications, and future directions. Front. Cell Dev. Biol. 2025, 13, 1517121. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Liang, G.; Yu, X.; Zhang, A.; Luo, X.; Liu, Y.; Tang, Z.; Wu, B.; Song, Z.; Lan, D. Establishment of epithelial inflammatory injury model using intestinal organoid cultures. Stem Cells Int. 2023, 2023, 3328655. [Google Scholar] [CrossRef] [PubMed]
- Dotti, I.; Mayorgas, A.; Salas, A. Generation of human colon organoids from healthy and inflammatory bowel disease mucosa. PLoS ONE 2022, 17, e0276195. [Google Scholar] [CrossRef]
- Verstockt, B. OP29 Young researcher award: Breaking new ground in IBD research: Organoid models as the vanguard for personalized therapies. J. Crohns Colitis 2025, 19, i59–i60. [Google Scholar] [CrossRef]
- Bernardes, J.; Schulte-Schrepping, J.; Tran, F.; Lorant, A.; Soumyabrata, G.; Taubenheim, J.; Volk, V.; Yim, A.; Wang, X.; Aden, K.; et al. P0092 Advancing precision medicine in IBD: Systematic evaluation of single-cell transcriptomics protocols for intestinal biopsies. J. Crohn’s Colitis 2025, 19, i458–i459. [Google Scholar] [CrossRef]
- Leipner, M.; Rimmer, P.; Paun, A.; Cheesbrough, J.; Danilin, S.; Klein, A.; Scepanovic, P.; Sharma, N.; Trenkle, P.; Regan-Komito, D.; et al. OP37 Delineating molecular and microbial signatures across disease severity in inflammatory bowel disease in adult treatment naive patients through single-cell transcriptomic and microbiome profiling. J. Crohn’s Colitis 2025, 19, i73–i74. [Google Scholar] [CrossRef]
- Grant, S.; Johnson, K.; Thomas, T.; Honig, G.; Bielecki, P.; Dendrou, C.; Guo, S.; Kulicke, R.; Li, P.; L’Italien, L.; et al. Single cell RNA sequencing of ulcerative colitis and Crohn’s disease tissue samples informs the selection of TREM1 as a target for the treatment of inflammatory bowel disease. Inflamm. Bowel Dis. 2023, 29, S52–S53. [Google Scholar] [CrossRef]
- Harris, B.; Alegbe, T.; Ramirez-Navaro, L.; Tutert, M.; Krzak, M.; Ozols, M.; Ghouraba, M.; Strickland, M.; Wana, N.; Hu, M.; et al. OP03 Mapping the observable IBDverse: Using massive-scale single-cell RNA sequencing of gut and blood samples to nominate genes, cell types and pathways driving IBD susceptibility. J. Crohn’s Colitis 2025, 19, i5–i6. [Google Scholar] [CrossRef]
- Townsend, H.A.; Rosenberger, K.J.; Vanderlinden, L.A.; Inamo, J.; Zhang, F. Evaluating methods for integrating single-cell data and genetics to understand inflammatory disease complexity. Front. Immunol. 2024, 15, 1454263. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Liu, X.; Yan, Q.; Gao, Q.; Ni, K.; Yang, J.; Zhang, S.; Zhang, C.; Shan, C. Transcriptome analysis identifies genetic risk markers and explores the pathogenesis for inflammatory bowel disease. Biochim. Biophys. Acta (BBA)-Mol. Basis. Dis. 2024, 1870, 167013. [Google Scholar] [CrossRef]
- Lähnemann, D.; Köster, J.; Szczurek, E.; McCarthy, D.J.; Hicks, S.C.; Robinson, M.D.; Vallejos, C.A.; Campbell, K.R.; Beerenwinkel, N.; Mahfouz, A.; et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020, 21, 31. [Google Scholar] [CrossRef]
- Chen, G.; Ning, B.; Shi, T. Single-cell RNA-seq technologies and related computational data analysis. Front. Genet. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, F.; Jin, Y.; Ma, Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct Target Ther. 2022, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Merlin, D. Unveiling colitis: A journey through the dextran sodium sulfate-induced model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef]
- Mizoguchi, A. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 2012, 105, 263–320. [Google Scholar]
- Nakanishi, A.; Toyama, S.; Onozato, D.; Watanabe, C.; Hashita, T.; Iwao, T.; Matsunaga, T. Effects of human induced pluripotent stem cell-derived intestinal organoids on colitis-model mice. Regen. Ther. 2022, 21, 351–361. [Google Scholar] [CrossRef]
- Sugimoto, S.; Ohta, Y.; Fujii, M.; Matano, M.; Shimokawa, M.; Nanki, K.; Date, S.; Nishikori, S.; Nakazato, Y.; Nakamura, T.; et al. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. 2018, 22, 171–176.e175. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279. [Google Scholar] [CrossRef]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.-E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Lee, C.H.; Koh, S.-J.; Radi, Z.A.; Habtezion, A. Animal models of inflammatory bowel disease: Novel experiments for revealing pathogenesis of colitis, fibrosis, and colitis-associated colon cancer. Intestig. Res. 2023, 21, 295–305. [Google Scholar] [CrossRef]
- Meroni, E.; Stakenborg, N.; Gomez-Pinilla, P.J.; De Hertogh, G.; Goverse, G.; Matteoli, G.; Verheijden, S.; Boeckxstaens, G.E. Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS ONE 2018, 13, e0197487. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.; Padma, S.; Mukherjee, S. An improved method for experimental induction of ulcerative colitis in Sprague Dawley rats. MethodsX 2023, 10, 102158. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Joosse, M.E.; Liu, L.; Sun, Y.; Dong, Y.; Cai, C.; Song, Z.; Zhang, J.; Brant, S.R.; Lazarev, M.; et al. Deletion of IL-6 exacerbates colitis and induces systemic inflammation in IL-10-deficient mice. J. Crohn’s Colitis 2020, 14, 831–840. [Google Scholar] [CrossRef]
- Scheinin, T.; Butler, D.M.; Salway, F.; Scallon, B.; Feldmann, M. Validation of the interleukin-10 knockout mouse model of colitis: Antitumour necrosis factor-antibodies suppress the progression of colitis. Clin. Exp. Immunol. 2003, 133, 38–43. [Google Scholar] [CrossRef]
- Cominelli, F.; Arseneau, K.O.; Rodriguez-Palacios, A.; Pizarro, T.T. Uncovering pathogenic mechanisms of inflammatory bowel disease using mouse models of Crohn’s disease–like ileitis: What is the right model? Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 19–32. [Google Scholar] [CrossRef]
- Gray, S.M.; Moss, A.D.; Herzog, J.W.; Kashiwagi, S.; Liu, B.; Young, J.B.; Sun, S.; Bhatt, A.P.; Fodor, A.A.; Balfour Sartor, R. Mouse adaptation of human inflammatory bowel diseases microbiota enhances colonization efficiency and alters microbiome aggressiveness depending on the recipient colonic inflammatory environment. Microbiome 2024, 12, 147. [Google Scholar] [CrossRef]
- Li, D.; Cui, L.; Gao, Y.; Li, Y.; Tan, X.; Xu, H. Fecal microbiota transplantation improves intestinal inflammation in mice with ulcerative colitis by modulating intestinal flora composition and down-regulating NF-kB signaling pathway. Microb. Pathog. 2022, 173, 105803. [Google Scholar] [CrossRef] [PubMed]
- Baydi, Z.; Limami, Y.; Khalki, L.; Zaid, N.; Naya, A.; Mtairag, E.M.; Oudghiri, M.; Zaid, Y. An update of research animal models of inflammatory bowel disease. Sci. World J. 2021, 2021, 7479540. [Google Scholar] [CrossRef]
- Steinbach, E.C.; Gipson, G.R.; Sheikh, S.Z. Induction of murine intestinal inflammation by adoptive transfer of effector CD4+ CD45RBhigh T cells into immunodeficient mice. J. Vis. Exp. 2015, 98, 52533. [Google Scholar]
- He, Z.; Xu, X.; Chen, Y.; Huang, Y.; Wu, B.; Xu, Z.; Du, J.; Zhou, Q.; Cheng, X. Integrated network pharmacology and bioinformatics to identify therapeutic targets and molecular mechanisms of Huangkui Lianchang Decoction for ulcerative colitis treatment. BMC Complement. Med. Ther. 2024, 24, 280. [Google Scholar] [CrossRef]
- Fotis, C.; Antoranz, A.; Hatziavramidis, D.; Sakellaropoulos, T.; Alexopoulos, L.G. Network-based technologies for early drug discovery. Drug Discov. Today 2018, 23, 626–635. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational approaches in preclinical studies on drug discovery and development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Namour, F.; Diderichsen, P.M.; Cox, E.; Vayssière, B.; Van der Aa, A.; Tasset, C.; Van ‘t Klooster, G. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin. Pharmacokinet. 2015, 54, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, K.; Ramiro-Garcia, J.; Heinken, A.; Ullmann, P.; Bintener, T.; Pacheco, M.P.; Baginska, J.; Shah, P.; Frachet, A.; Halder, R.; et al. Integrated in vitro and in silico modeling delineates the molecular effects of a synbiotic regimen on colorectal-cancer-derived cells. Cell Rep. 2019, 27, 1621–1632.e1629. [Google Scholar] [CrossRef]
| Category | Biomarker Type | Indicators | Source | Application | Validation Status |
|---|---|---|---|---|---|
| Diagnostic | Metabolomic [27,28] | Short-chain fatty acids (SCFA), bile acids, sphingolipids, tryptophan metabolites | Fecal sample | Non-invasive diagnosis, disease severity | Clinical use |
| Proteomic [29,30] | Calprotectin, Lactoferrin, Calgranulin C, lipocalin-2. Blood: | Fecal and blood samples. | Disease severity assessment and non-invasive diagnosis. | Clinical use | |
| MicroRNA (miRNAs) [31,32] | Tissue: miR-192, miR-375, and miR-422b. Serum: miR-146a, miR-146b, miR-320a, miR-126, and let-7c. | Tissue and blood samples. | Differentiation of IBD subtypes (UC and CD) | Research/Early validation | |
| Inflammatory Biomarker [25] | Blood: CRP, ESR, ASCA, LRG, LCN-2 Feces: Fecal calprotectin, Lactoferrin, S100A12 | Blood, Fecal samples | Diagnosis, Subtype differentiation | Clinical use/Early Validation | |
| Predictive/Prognostic | Transcriptomic [33] | Ileal: FOLH1, CA2 Colonic: REG3α | Mucosal biopsies (ileum/colon) | Predicting histological and endoscopic healing | Research/Early validation |
| Serological [34] | CPa9-HNE | Serum | Assessment of endoscopic activity and neutrophil infiltration. | Research/Early validation | |
| Genetic [35] | RUNX1 | Colon tissue | IBD progression, risk of colorectal cancer. | Experimental/Preclinical | |
| Monitoring | Gut Microbial Biomarkers [36,37] | CRP, Fecal calprotectin (FC), Calgranulin-C (S100A12), Stool lactoferrin (SL) | Stool samples | Monitoring of mucosal healing, guidance for treatment decisions. | Clinical use |
| Myeloperoxidase [38] | Myeloperoxidase (MPO) | Fecal samples | Monitoring of neutrophilic inflammation and disease severity | Experimental/ Preclinical | |
| Transcriptomic [33] | Colonic: REG3α | Colon biopsies (ileum/colon) | Inflammatory burden, mucosal repair | Research/ Early validation |
| Category | Tool | Algorithm/Core Approach | Key Features | Accessibility | Limitations |
|---|---|---|---|---|---|
| Docking Engines | AutoDock [55,56] | Lamarckian genetic algorithm | Widely used, open-source, supports HTS | https://autodock.scripps.edu/ (accessed on 1 August 2025) | Limited protein flexibility, simplified scoring |
| SwissDock [57] | EADock DSS (evolutionary algorithm + local search) | Web-based Protein-ligand docking | http://www.swissdock.ch/ (accessed on 1 August 2025) | Limited to small molecules only. | |
| ZDOCK/M-ZDOCK [58,59] | Fast Fourier Transform (FFT) based correlation | Protein–protein docking. | https://zdock.wenglab.org/ (accessed on 1 August 2025) | Requires post-docking refinement. | |
| MedusaDock [60] | Monte Carlo-based docking | Flexible ligand docking. | https://dokhlab.med.psu.edu/cpi/ (accessed on 1 August 2025) | Limited to rotamer-based flexibility. | |
| LeDock [61] | Hybrid of simulated annealing + Genetic algorithm | High accuracy for small molecule docking. | https://www.lep har.com/software (accessed on 1 August 2025) | Time-consuming per ligand. | |
| MOLS 2.0 [62] | Mean-field optimization with scoring functions | Peptide and protein-ligand docking. | https://sourceforge.net/projects/mols2-0/ (accessed on 1 August 2025) | Docking accuracy needs improvement | |
| rDOCK [63] | Genetic + Monte Carlo search | Ligand and nucleic acid docking | https://rdock.github.io/ (accessed on 1 August 2025) | Linux-only | |
| HADDOCK [64,65] | Restraint-driven docking (ambiguous interaction restraints) | Integrates experimental data, protein–protein/nucleic acid docking | https://rascar.science.uu.nl/haddock2.4/ (accessed on 1 August 2025) | Requires experimental input; rigid assumptions | |
| ClusPro [59] | FFT-based+ Clustering | High-accuracy protein–protein docking | https://cluspro.org/help.php (accessed on 1 August 2025) | Relies on structural conformity | |
| Glide (Schrödinger) [66] | Hierarchical systematic search with scoring filters | Widely used for ligand-receptor docking. | https://www.schrodinger.com/ (accessed on 1 August 2025) | Poor results with bulky ligands. | |
| GOLD Dock [67] | Genetic algorithm | High receptor flexibility supports | https://www.ccdc.cam.ac.uk/solutions/software/gold/ (accessed on 1 August 2025) | Biased towards specific docking. | |
| Integrated Suites/Platforms | Biovia Discovery Studio [68] | Contains CDOCKER (CHARMm SA), LibDock (hotspot-based) | Protein prep, ligand interaction analysis | https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 1 August 2025) | Cannot perform docking calculations. |
| Molecular operating environment [69] | Lamarckian Genetic Algorithm | Structure and pharmacophore docking | https://www.chemcomp.com/en/Products.htm (accessed on 1 August 2025) | Commercial licensing required. | |
| Visualization and Modeling Tools | UCSF Chimera [70] | Visualization and external tool integration (not docking) | Ligand interaction analysis | https://www.cgl.ucsf.edu/chimera/ (accessed on 1 August 2025) | Does not perform docking. |
| Swiss-PdbViewer [71] | Genetic algorithm | Homology modelling and minimization | https://swissmodel.expasy.org/ https://spdbv.unil.ch/ (accessed on 1 August 2025) | One PDB at a time. | |
| PyMOL [72] | Molecular visualization (not docking) | Widely used for structural visualization and interaction analysis | https://www.pymol.org/ (accessed on 1 August 2025) | Limited features in the academic version. |
| Tool/Database | Application | Limitations | Website |
|---|---|---|---|
| STRING [90,103] | PPI network construction | Limited Gene Ontology classification | https://string-db.org/ (accessed on 3 August 2025) |
| STITCH [104] | Protein–small molecule interactions | Limited to known (430,000) Compounds | http://stitch.embl.de/ (accessed on 3 August 2025) |
| HMDB [105] | Human metabolite information. | Incomplete spectral data of many known compounds. | https://hmdb.ca/ (accessed on 3 August 2025) |
| MiMeDB [106] | Microbiome-derived small molecules | Requires frequent updates | https://mimedb.org/ (accessed on 3 August 2025) |
| HIT 2.0 [107] | Herb-target-compound data | Focused on herbal compounds only | http://hit2.badd-cao.net/ (accessed on 3 August 2025) |
| OMIM [108] | Human genes and phenotypes | Mainly Mendelian inheritance disorders | http://www.ncbi.nlm.nih.gov/omim/ (accessed on 3 August 2025) |
| GeneCards [109] | Gene-centric data from more than 200 sources | Human-only; no cross-species data is available. | http://www.genecards.org/ (accessed on 3 August 2025) |
| PubChem [110] | Largest compounds database on chemical and bioassay information. | Limited experimental protocol of bioassay data. | https://pubchem.ncbi.nlm.nih.gov/ (accessed on 3 August 2025) |
| UniProt [111] | Protein sequence, annotation, functional and structural information. | Lack of information about enzyme activity in biochemical reactions. | http://www.uniprot.org/ (accessed on 3 August 2025) |
| ChEMBL [112] | Drug–target activity data. | Limited information for novel compounds and targets. | https://www.ebi.ac.uk/chembl (accessed on 3 August 2025) |
| DrugBank [113] | Drug profiles, mechanism of action and pharmacokinetics. | May lacks information about investigational and experimental drugs. | https://go.drugbank.com/ (accessed on 3 August 2025) |
| ZINC [114] | Commercial small-molecule database. | Lacks full pharmacophore data. | https://zinc20.docking.org/ (accessed on 3 August 2025) |
| CytoScape [115] | Network visualization and analysis. | Memory-intensive. | https://cytoscape.org/ (accessed on 3 August 2025) |
| KEGG [116] | Pathway enrichment and maps analysis. | Depends on published articles, manually curated; slower updates. | https://www.kegg.jp/ (accessed on 3 August 2025) |
| DAVID [117,118] | Gene enrichment and annotation. | Sensitivity varies across background gene lists, scoring systems, tests. | https://davidbioinformatics.nih.gov/ (accessed on 3 August 2025) |
| R/RStudio [119] | Statistical and pathway analysis. | Requires coding knowledge, statistics and data analysis. | https://cran.r-project.org/ (accessed on 3 August 2025) |
| RCSB PDB [120] | Protein structure database | Some PDBx/mmCIF entries only files are not readable by docking tools. | https://www.rcsb.org/ (accessed on 3 August 2025) |
| BindingDB [120] | Protein–ligand affinity data. | Focuses binding affinities over other kinetics, allosteric effects, and complex structural information. | http://www.bindingdb.org/ (accessed on 3 August 2025) |
| BioLiP2 [121] | Protein-ligand interaction information. | Lacks RNA-ligand interaction application. | https://zhanggroup.org/BioLiP (accessed on 3 August 2025) |
| CastP [122] | Predicts protein binding sites. | Limited to annotated PDBs only. | http://sts.bioe.uic.edu/castp (accessed on 3 August 2025) |
| I-TASSER [123,124] | Protein 3D structure prediction, homology modelling | Accuracy depends on the templates of the protein. | https://zhanggroup.org/I-TASSER/ (accessed on 3 August 2025) |
| Gaussian [125] | Quantum energy calculations of small molecules. | High computational cost. | https://gaussian.com/ (accessed on 3 August 2025) |
| SwissADME [126] | ADME and drug-likeness prediction of small molecules. | Peptides not supported with SwissADME. | http://www.swissadme.ch/ (accessed on 3 August 2025) |
| pkCSM [127] | ADMET property prediction | Experimental validation needed. | https://biosig.lab.uq.edu.au/pkcsm/ (accessed on 3 August 2025) |
| ProTOX II [128] | Toxicity prediction models of small molecules. | Dependency on 2D input may lack the information of 3D structures. | http://tox.charite.de/protox_II (accessed on 3 August 2025) |
| GROMACS [129] | Molecular dynamics simulation. | Limited for large molecular weight protein. | https://www.gromacs.org/ (accessed on 3 August 2025) |
| Model Type | Induction Method | Disease Features | Advantages | Disadvantages |
|---|---|---|---|---|
| Dextran Sodium Sulfate (DSS) [175,180] | 3–5% DSS (C57BL/6 or BALB/c mice); 4% DSS in SD rats. | Ulceration, submucosal edema, bloody diarrhea | High reproducibility mimics human UC, cost-effective. | Excess dosing causes severe inflammation; lacks chronicity |
| TNBS (Trinitrobenzene Sulfonic Acid) [181,182,183] | Intrarectal TNBS (BALB/c mice, Wistar rats) | Bloody diarrhea, weight loss, IL-12 driven colitis | Suitable for anti-TNF-α drugs and conventional therapies. | Species-specific susceptibility; limited human relevance. |
| Oxazolone [183,184] | 1% oxazolone in BALB/c, SJL/J strains | Weight loss, goblet cell depletion, IL-9 upregulation | Models IL-4R and IL-9 pathways relevant in UC | Ineffective in C57BL/6 strain; short-lived inflammation |
| Acetic Acid-Induced Colitis [183,185] | 4% acetic acid in SD rats (1.5 mL) | Diarrhea, rectal bleeding, epithelial injury | Simple, rapid, cost-effective | Severe epithelial damage at high dose; poor chronic resemblance. |
| Adoptive T-cell Transfer [183,185] | CD4+CD45RBhigh T cells into Rag1 KO mice | Chronic colitis, loose stool, immune-driven inflammation | Insight into T-cell-mediated pathology | Requires an immune-deficient host; time-consuming |
| IL-10 Knockout [186,187] | IL-10 null C57BL/6 mice | Chronic inflammation, bowel thickening | Useful for understanding IL-10 signalling | Symptom onset variable; requires close monitoring |
| SAMP1/YitFc (Spontaneous) [188] | Genetically selected AKR/J mice | Ileitis, perianal fistulae, chronic lesions | Spontaneous CD-like pathology without intervention | Low breeding efficiency; variable disease onset |
| Microbiome-Induced (FMT) [189,190] | FMT into IL-10-/- or C57BL/6J mice | Microbiota-driven inflammation, weight loss | Allows study of host-microbe interactions | Contamination risk; strain-level microbial ID is difficult |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripa, J.D.; Ali, S.; Field, M.; Smithson, J.; Wangchuk, P. From AI-Assisted In Silico Computational Design to Preclinical In Vivo Models: A Multi-Platform Approach to Small Molecule Anti-IBD Drug Discovery. Pharmaceuticals 2025, 18, 1536. https://doi.org/10.3390/ph18101536
Ripa JD, Ali S, Field M, Smithson J, Wangchuk P. From AI-Assisted In Silico Computational Design to Preclinical In Vivo Models: A Multi-Platform Approach to Small Molecule Anti-IBD Drug Discovery. Pharmaceuticals. 2025; 18(10):1536. https://doi.org/10.3390/ph18101536
Chicago/Turabian StyleRipa, Joya Datta, Sarfaraz Ali, Matt Field, John Smithson, and Phurpa Wangchuk. 2025. "From AI-Assisted In Silico Computational Design to Preclinical In Vivo Models: A Multi-Platform Approach to Small Molecule Anti-IBD Drug Discovery" Pharmaceuticals 18, no. 10: 1536. https://doi.org/10.3390/ph18101536
APA StyleRipa, J. D., Ali, S., Field, M., Smithson, J., & Wangchuk, P. (2025). From AI-Assisted In Silico Computational Design to Preclinical In Vivo Models: A Multi-Platform Approach to Small Molecule Anti-IBD Drug Discovery. Pharmaceuticals, 18(10), 1536. https://doi.org/10.3390/ph18101536








