Synthesis, Spectroscopic Characterization, and Biological Evaluation of a Novel Acyclic Heterocyclic Compound: Anticancer, Antioxidant, Antifungal, and Molecular Docking Studies
Abstract
1. Introduction
2. Results
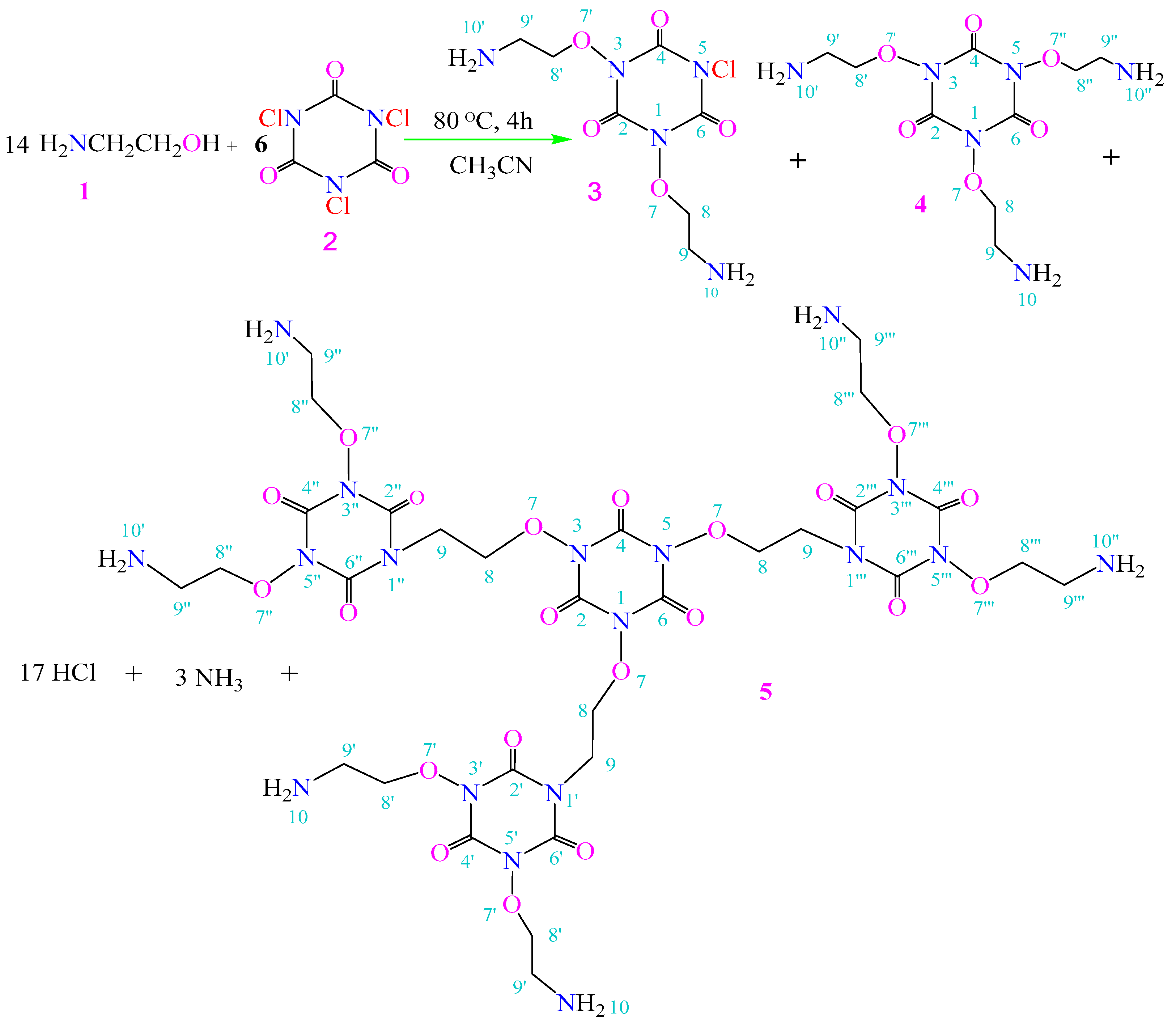
2.1. Chemistry
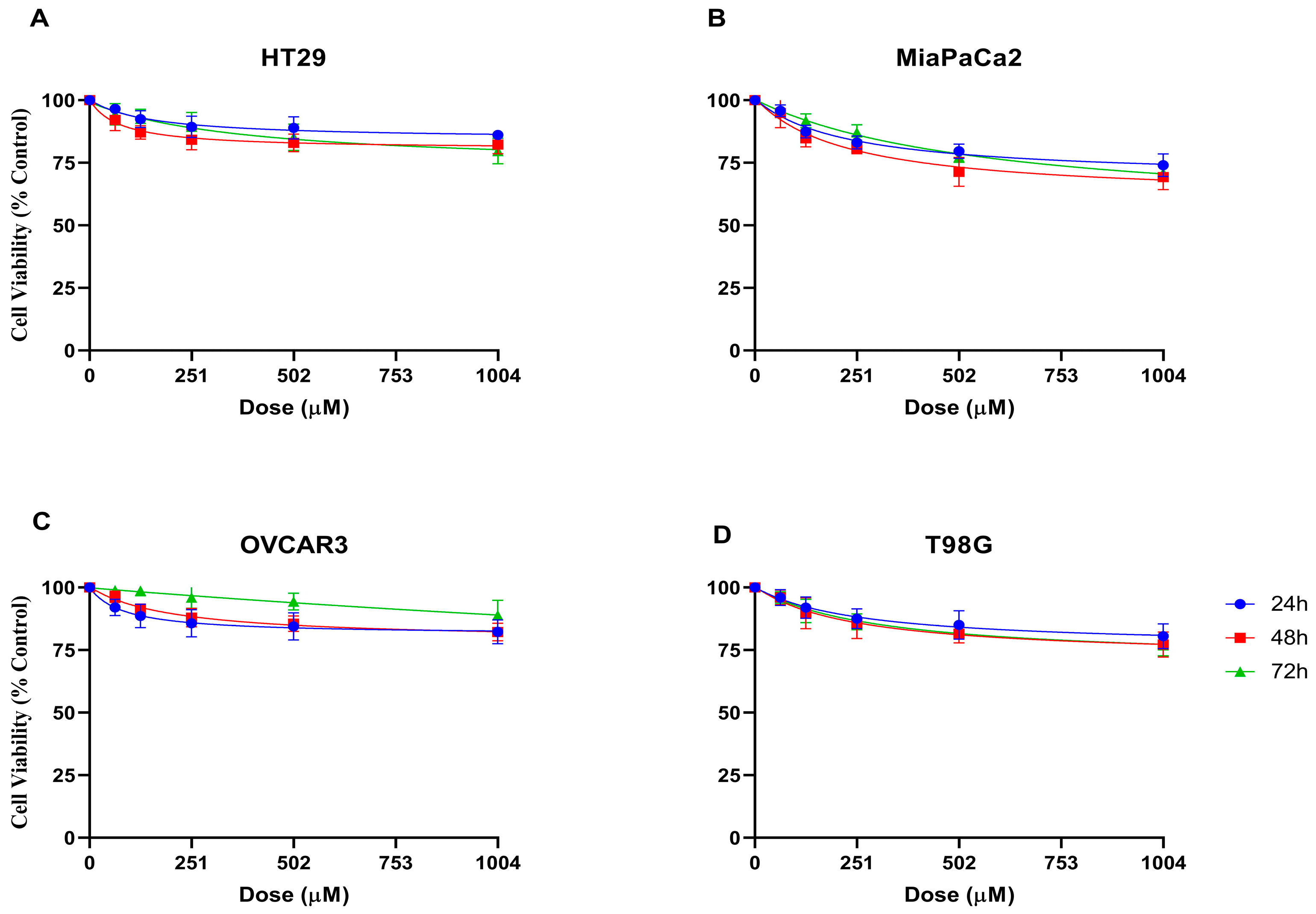
2.2. Anticancer Activity
2.3. Antioxidant Activity
2.4. Antifungal Activity
2.5. Molecular Docking Studies
2.5.1. Molecular Docking of Compound 5 into the Active Site of Thymidylate Synthase
2.5.2. Molecular Docking Analysis into the CYP51 Active Site
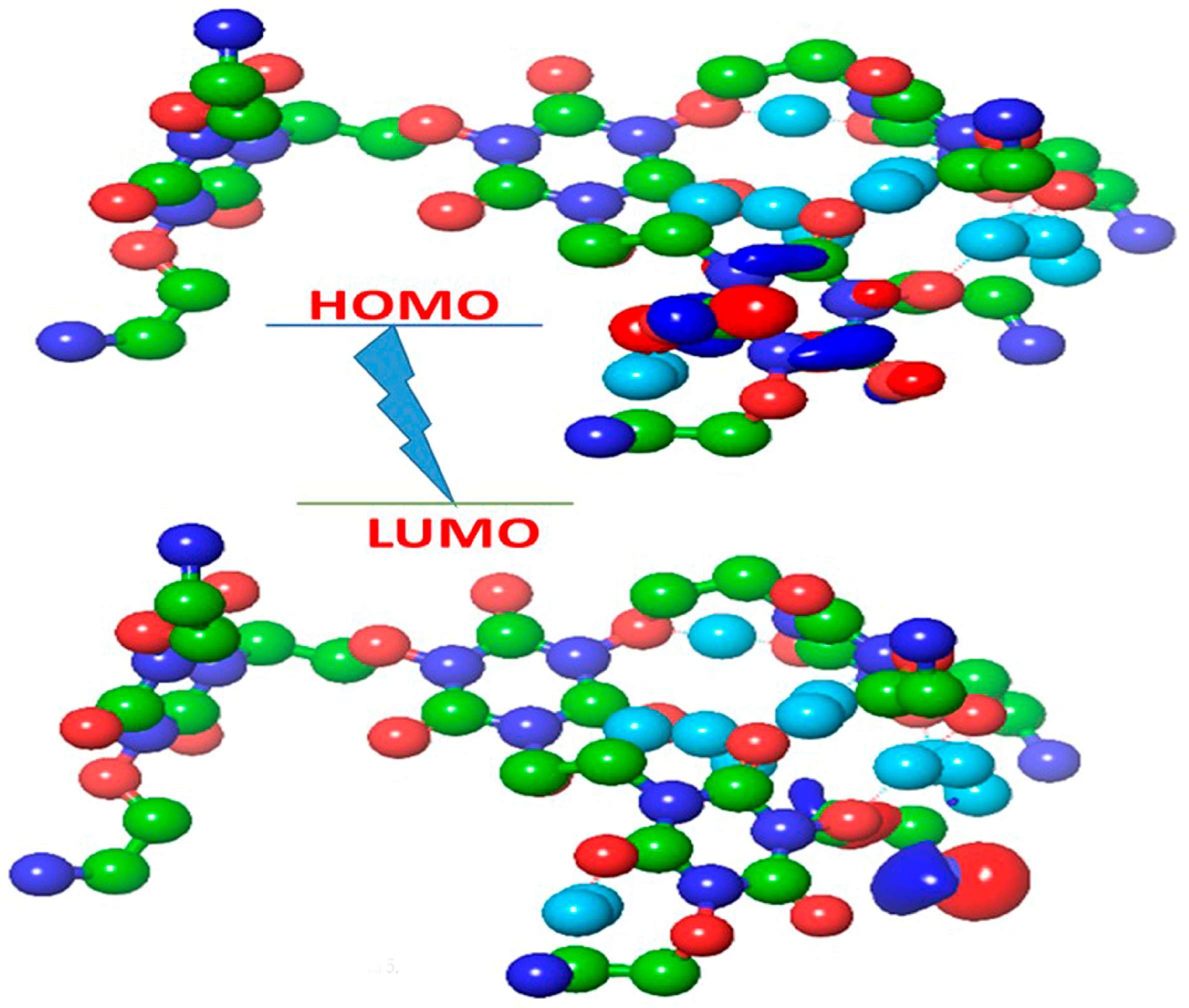
2.6. Quantum Chemical Analysis: Electronic Structure and Reactivity
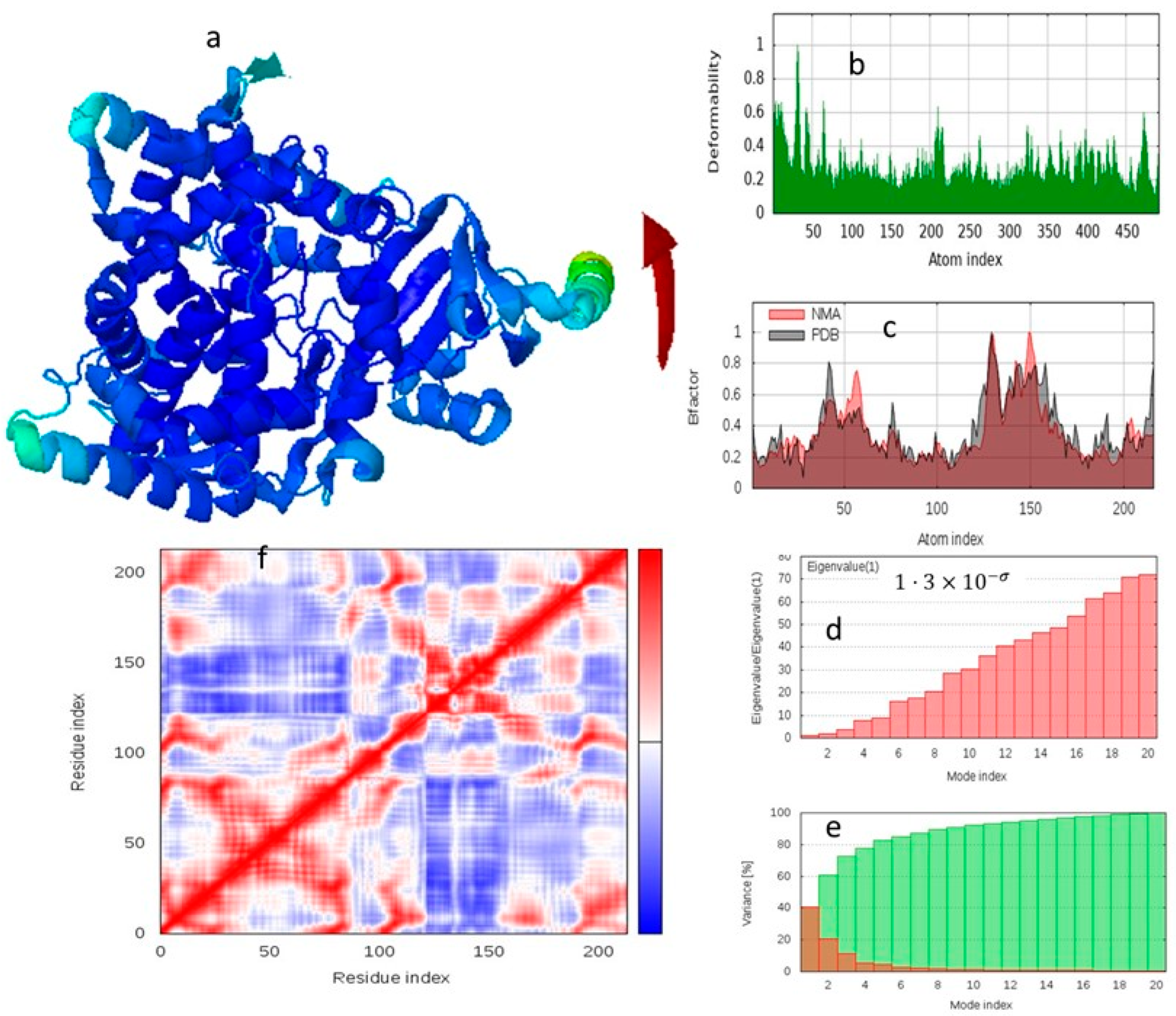
2.7. Protein Dynamics and Mechanism of Inhibition: A Normal Mode Analysis (NMA) Perspective
2.7.1. NMA of the Compound 5-1HZW Complex
2.7.2. NMA of the Compound 5-CYP51 (5V5Z) Complex
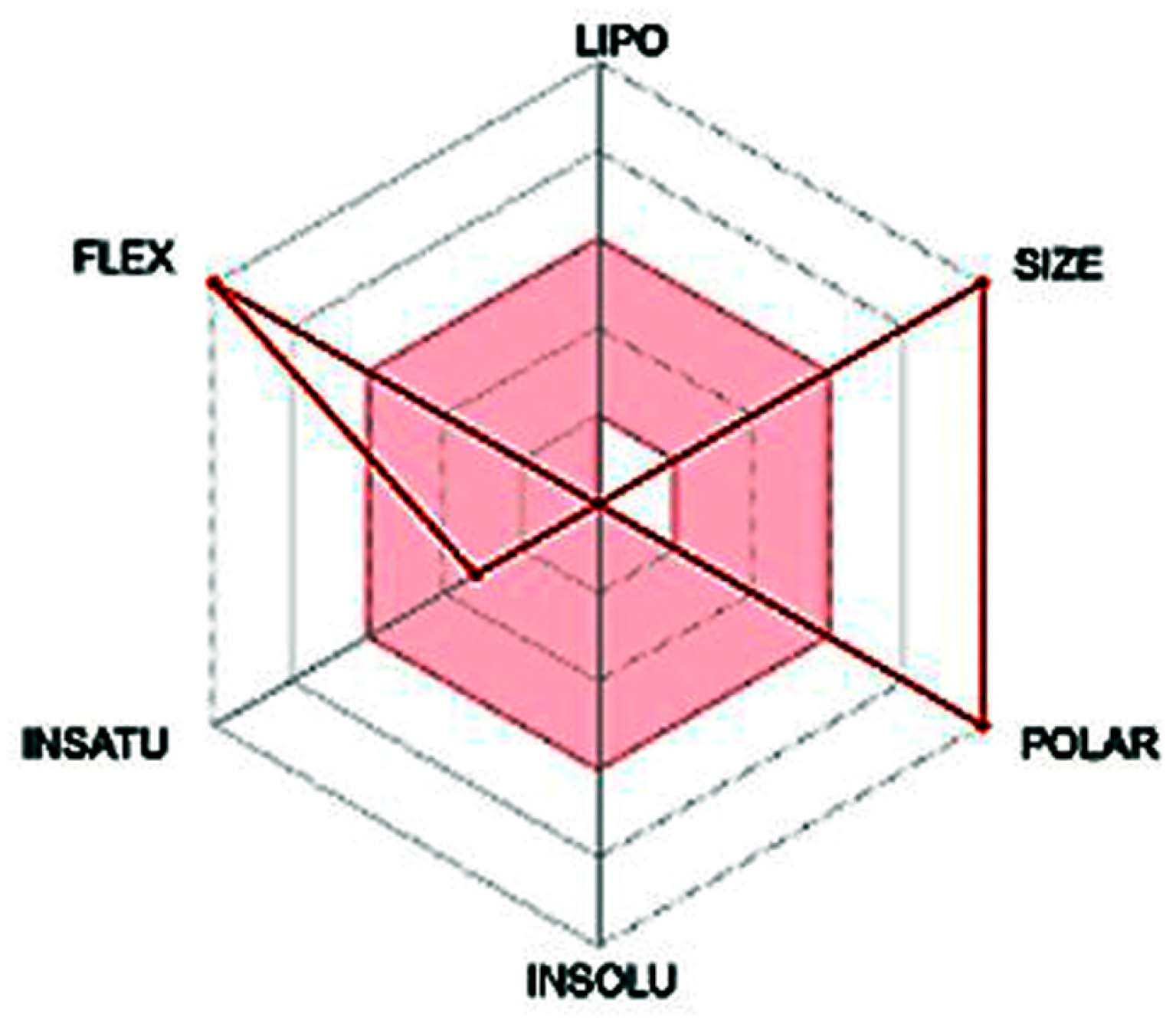
2.8. In Silico ADMET and Drug-likeness Prediction
3. Discussion
3.1. Chemistry
3.1.1. Confirmation of the Structure of Compound 5
The Fingerprint of Symmetry: Deconstructing the Structure of Compound 4
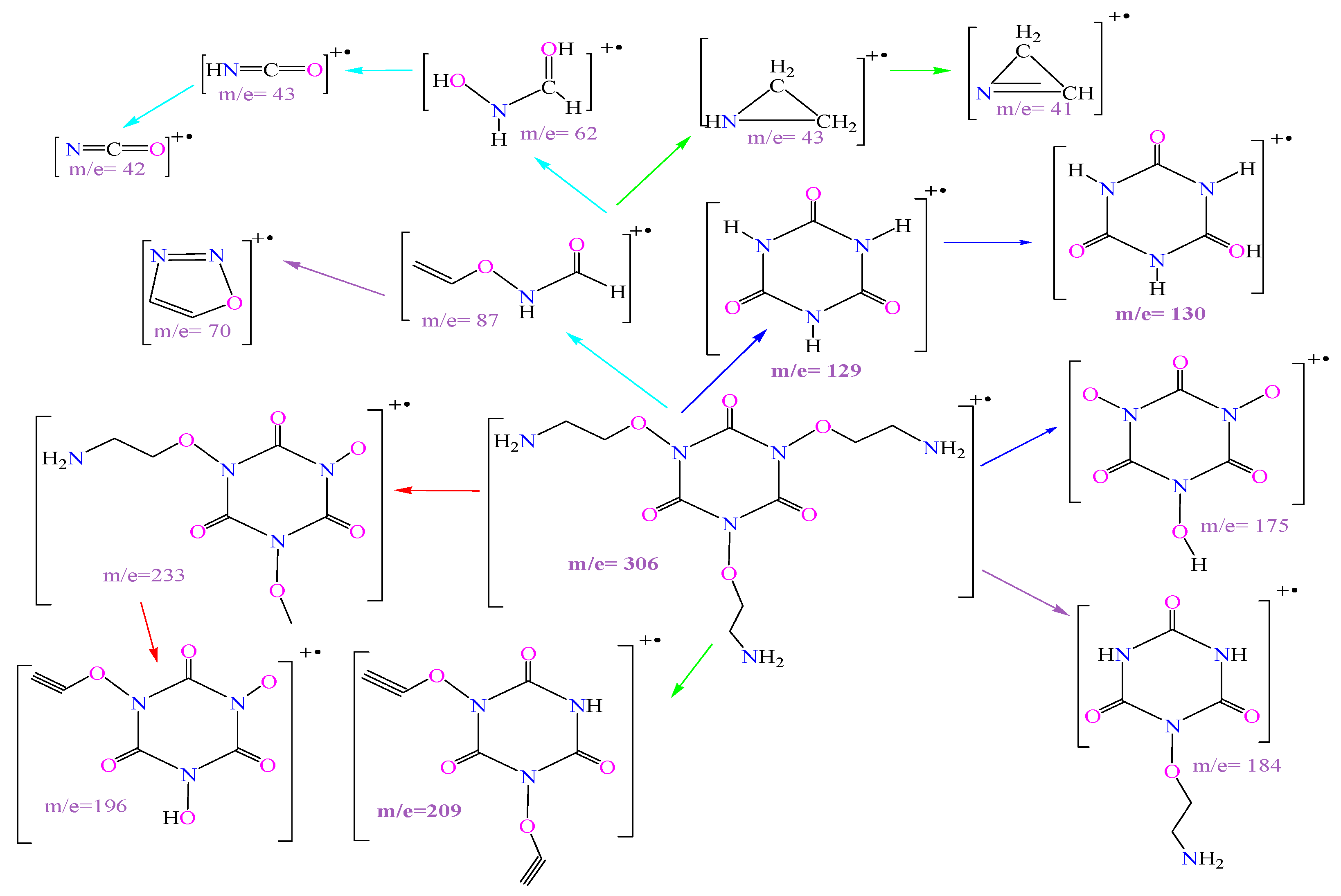
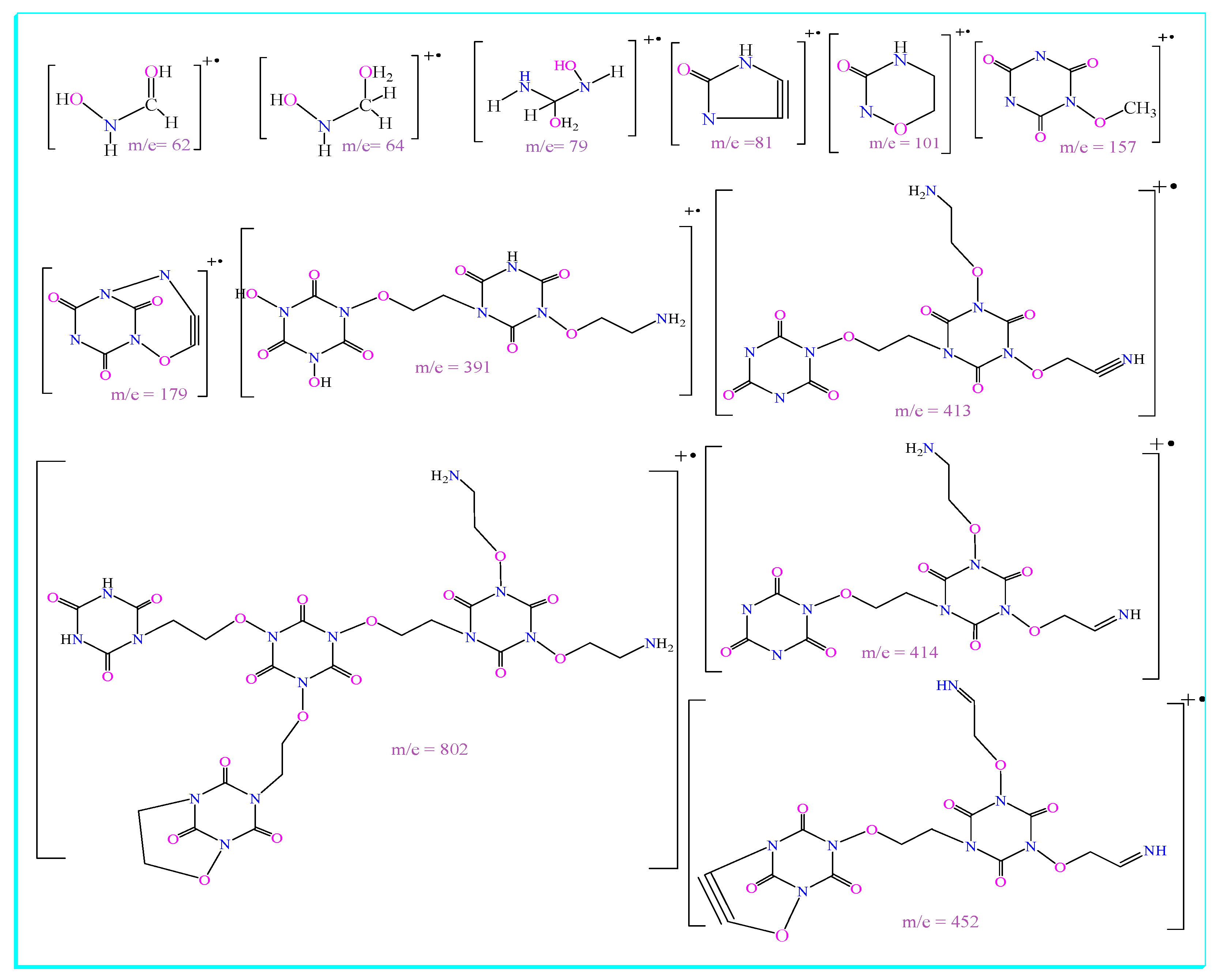
Liquid Chromatography Mass Spectrum (LC/Q-TOF/MS) (Figure S5)
Infrared (IR) Spectroscopy
Nuclear Magnetic Resonance (NMR) Spectroscopy (Figures S2–S4)
3.2. Anticancer Activity
3.3. Antioxidant Activity
3.4. Antifungal Activity
3.5. Molecular Docking Studies
3.5.1. Molecular Docking of Compound 5 into the Active Site of TS
3.5.2. Molecular Docking Analysis into the CYP51 Active Site
3.6. Quantum Chemical Analysis: Electronic Structure and Reactivity
3.7. Protein Dynamics and Mechanism of Inhibition: A Normal Mode Analysis (NMA) Perspective
3.7.1. NMA of the Compound 5–1HZW Complex
3.7.2. NMA of the Compound 5-CYP51 (5V5Z) Complex
3.8. In Silico ADMET and Drug-likeness Prediction
4. Materials and Methods
4.1. Chemistry
4.2. Anticancer Studies
4.3. Antioxidant Studies
4.3.1. DPPH Method
4.3.2. CUPRAC Method
4.4. Antifungal Studies
4.5. Molecular Docking Studies
4.5.1. Computational Methods
4.5.2. Molecular Docking Methodology
4.5.3. Normal Mode Analysis (NMA) and Protein Dynamics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Eissa, A.E.; Abdelsalam, M.; Tharwat, N.; Zaki, M. Detection of Saprolegnia parasitica in eggs of angelfish Pterophyllum scalare (Cuvier–Valenciennes) with a history of decreased hatchability. Int. J. Vet. Sci. Med. 2013, 1, 7–14. [Google Scholar] [CrossRef]
- Manju, M.; Suresh, S.; Vivekanand, P.A.; Gunasekaran, S.; Srinivasan, S.; Biju, C.S. Vibrational spectroscopic investigation and antibacterial activity studies on Trichloroisocyanuric acid. Mater. Today Proc. 2021, 36, 857–862. [Google Scholar] [CrossRef]
- Paseta, L.; Simón-Gaudó, E.; Gracia-Gorría, F.; Coronas, J. Encapsulation of essential oils in porous silica and MOFs for trichloroisocyanuric acid tablets used for water treatment in swimming pools. Chem. Eng. J. 2016, 292, 28–34. [Google Scholar] [CrossRef]
- Phakhodee, W.; Yamano, D.; Pattarawarapan, M. ultrasound-assisted synthesis of n-acylcyanamides and n-acyl-substituted ımidazolones from carboxylic acids by using trichloroisocyanuric acid/triphenylphosphine. Synlett 2020, 31, 703–707. [Google Scholar] [CrossRef]
- Moraes, A.M.; da Silva, T.L.; de Mattos, M.C.S. An eco-friendly synthesis of 5-aminotetrazoles using trichloroisocyanuric acid as desulfurization agent of thioureas. J. Heterocycl. Chem. 2023, 60, 1625–1632. [Google Scholar] [CrossRef]
- Sun, N.; Zheng, K.; Sun, P.; Chen, Y.; Jin, L.; Hu, B.; Shen, Z.; Hu, X. Trichloroisocyanuric acid-promoted synthesis of arylselenides and aryltellurides from diorganyl dichalcogenides and arylboronic acids at ambient temperature. Adv. Synth. Catal. 2021, 363, 3577–3584. [Google Scholar] [CrossRef]
- Gambacorta, G.; Baxendale, I.R. Continuous-flow hofmann rearrangement using trichloroisocyanuric acid for the preparation of 2-benzoxazolinone. Org. Process. Res. Dev. 2022, 26, 422–430. [Google Scholar] [CrossRef]
- Blödorn, G.B.; Duarte, L.F.B.; Roehrs, J.A.; Silva, M.S.; Neto, J.S.S.; Alves, D. Trichloroisocyanuric acid (TCCA): A suitable reagent for the synthesis of selanyl-benzo[b]chalcogenophenes. Eur. J. Org. Chem. 2022, 2022, e202200775. [Google Scholar] [CrossRef]
- Talha, A.; Favreau, C.; Bourgoin, M.; Robert, G.; Auberger, P.; ELAmmari, L.; Saadi, M.; Benhida, R.; Martin, A.R.; Bougrin, K. Ultrasound-assisted one-pot three-component synthesis of new isoxazolines bearing sulfonamides and their evaluation against hematological malignancies. Ultrason. Sonochem. 2021, 78, 105748. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, J.; Liu, C.; Gong, H.; Qi, B.; Zhu, R.; Xia, L.; Li, L.; Liu, S.; Jiang, Q.; et al. Application of trichloroisocyanuric acid in controlling kiwifruit bacterial canker disease demonstrates its promising potential as an eco-friendly bactericide. Chem. Biol. Technol. Agric. 2025, 12, 3. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, J.; Wang, R.; Zhang, J.; Yang, Z.; Tian, F.; Hu, Y.; Wu, Z. Green and efficient depolymerization and recycling of polyester/cotton blended fabrics by ethanolamine. Sustain. Chem. Pharm. 2025, 45, 102011. [Google Scholar] [CrossRef]
- Brodie, C.N.; Goodfellow, A.S.; Andrews, M.J.; Owen, A.E.; Bühl, M.; Kumar, A. Direct synthesis of partially ethoxylated branched polyethylenimine from ethanolamine. Nat. Commun. 2024, 15, 6253. [Google Scholar] [CrossRef]
- Geldiev, Y.; Turaev, K.; Umbarov, I.; Eshmurodov, K. Effects of different factors on the kinetics of modification of polysilicic acids with ethanolamine. Int. J. Eng. Trends Technol. 2022, 70, 447–452. [Google Scholar] [CrossRef]
- Bartolucci, S.; Mari, M.; Bedini, A.; Piersanti, G.; Spadoni, G. Iridium-catalyzed direct synthesis of tryptamine derivatives from indoles: Exploiting N-protected β-amino alcohols as alkylating agents. J. Org. Chem. 2015, 80, 3217–3222. [Google Scholar] [CrossRef]
- Ramanivas, T.; Parameshwar, M.; Gayatri, G.; Nanubolu, J.B.; Srivastava, A.K. Asymmetric synthesis of functionalized 2,5-pyrrolidinediones and β-lactams through diastereospecific cycloisomerization/rearrangement of chiral ethanolamine-derived ugi adducts. Eur. J. Org. Chem. 2017, 2017, 2245–2257. [Google Scholar] [CrossRef]
- Melnyk, S.; Melnyk, Y.; Mahorivska, H.; Fuchyla, O. KInetic aspects of catalytic interactions involving pentyl acetate and ethanolamine. Chem. Chem. Technol. 2023, 17, 820–828. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, X.; Wang, K.X.; Zou, L.J.; Ji, P.; Yin, Y.L. Ethanolamine enhances intestinal functions by altering gut microbiome and mucosal anti-stress capacity in weaned rats. Br. J. Nutr. 2018, 120, 241–249. [Google Scholar] [CrossRef]
- Augimeri, G.; Bonofiglio, D. Promising effects of N-docosahexaenoyl ethanolamine in breast cancer: Molecular and cellular ınsights. Molecules 2023, 28, 3694. [Google Scholar] [CrossRef]
- Alhilal, M.; Alhilal, S.; Gomha, S.M.; Farag, B.; Sabancilar, I.; Ouf, S.A. Biological evaluation and molecular docking studies of novel aza-acyclic nucleosides as putative antimicrobial, anticancer, and antioxidant agents. J. BMC Chem. 2025, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Nazreen, S.; Elbehairi, S.E.I.; Alfarsi, A.; Elhenawy, A.A.; Shati, A.A.; Alfaifi, M.Y.; Malebari, A.M.; Mohamed, S.A.; Asad, M.; et al. Synthesis, characterization and biological evaluation of thymol-triazole conjugates as antiproliferative agents. J. Indian Chem. Soc. 2025, 102, 102077. [Google Scholar] [CrossRef]
- McGuire, J.J. Anticancer antifolates: Current status and future directions. Curr. Pharm. Des. 2003, 9, 2593–2613. [Google Scholar] [CrossRef] [PubMed]
- Burdelski, C.; Strauss, C.; Tsourlakis, M.C.; Kluth, M.; Hube-Magg, C.; Melling, N.; Lebok, P.; Minner, S.; Koop, C.; Graefen, M.; et al. Overexpression of thymidylate synthase (TYMS) is associated with aggressive tumor features and early PSA recurrence in prostate cancer. Oncotarget 2015, 6, 8377–8387. [Google Scholar] [CrossRef] [PubMed]
- Zsidó, B.Z.; Hetényi, C. The role of water in ligand binding. Curr. Opin. Struct. Biol. 2021, 67, 1–8. [Google Scholar] [CrossRef]
- Bahar, I.; Lezon, T.R.; Bakan, A.; Shrivastava, I.H. Normal mode analysis of biomolecular structures: Functional mechanisms of membrane proteins. Chem. Rev. 2010, 110, 1463–1497. [Google Scholar] [CrossRef]
- Alhilal, S.; Alhilal, M.; Gomha, S.M.; Ouf, S.A. Synthesis and biological evaluation of new aza-acyclic nucleosides and their hydrogen complexes from indole. Res. Chem. Intermed. 2022, 48, 3567–3587. [Google Scholar] [CrossRef]
- Alhilal, M.; Sulaiman, Y.A.M.; Alhilal, S.; Gomha, S.M.; Ouf, S.A. Synthesis of novel acyclic nucleoside analogue starting from 6-aminouracil as potent antimicrobial agent. Polycycl. Aromat. Compd. 2022, 42, 6463–6474. [Google Scholar] [CrossRef]
- Benedetti, S.; Catalani, S.; Canonico, B.; Nasoni, M.G.; Luchetti, F.; Papa, S.; Potenza, L.; Palma, F. The effects of acyclovir administration to NCI-H1975 non-small cell lung cancer cells. Toxicol. Vitr. 2022, 79, 105301. [Google Scholar] [CrossRef]
- Wawrzyniak, D.; Framski, G.; Januszczyk, P.; Ostrowski, T.; Baraniak, D.; Jahnz-Wechmann, Z.; Fogt, J.; Manikowski, A.; Baranowski, D.; Rolle, K.; et al. 7-(β-D-Ribofuranosyl)guanine and its analogues modified in the sugar portion: Synthesis and antiglioma properties. Chem. Sel. 2020, 5, 13370–13375. [Google Scholar] [CrossRef]
- Devineni, S.R.; Golla, M.; Shaik, T.B.; Avilala, J.; Saddala, M.S.; Golla, N.; Chamarthi, N.R. Ethyl phosphoramidates of acyclovir: Design, synthesis, molecular docking (HN Protein), and evaluation of antiviral and antioxidant activities. Med. Chem. Res. 2017, 26, 999–1009. [Google Scholar] [CrossRef]
- Diopan, V.; Babula, P.; Shestivska, V.; Adam, V.; Zemlicka, M.; Dvorska, M.; Hubalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Electrochemical and spectrometric study of antioxidant activity of pomiferin, isopomiferin, osajin and catalposide. J. Pharm. Biomed. Anal. 2008, 48, 127–133. [Google Scholar] [CrossRef]
- Alhilal, M.; Erol, H.S.; Yildirim, S.; Cakir, A.; Koc, M.; Celebi, D.; Halici, M.B. Osajin from Maclura pomifera alleviates sepsis-induced liver injury in rats: Biochemical, histopathological and immunohistochemical estimation. J. Taibah Univ. Sci. 2023, 17, 2201250. [Google Scholar] [CrossRef]
- Alhilal, M.; Erol, H.S.; Yildirim, S.; Cakir, A.; Koc, M.; Alhilal, S.; Dereli, E.; Alkanoglu, O.; Ay, V.; Can, I.; et al. Medicinal evaluation and molecular docking study of osajin as an anti-inflammatory, antioxidant, and antiapoptotic agent against sepsis-associated acute kidney injury in rats. Ren. Fail. 2024, 46, 2379008. [Google Scholar] [CrossRef]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.G.; Endo, E.H.; Filho, B.P.D. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef]
- Mostafa, A.A.F.; Al-Askar, A.A.; Yassin, M.T. Anti-saprolegnia potency of some plant extracts against Saprolegnia diclina, the causative agent of saprolengiasis. Saudi J. Biol. Sci. 2020, 27, 1482–1487. [Google Scholar] [CrossRef]
- Bailey, T.A. Effects of twenty-five compounds on four species of aquatic fungi (Saprolegniales) pathogenic to fish. Aquaculture 1984, 38, 97–104. [Google Scholar] [CrossRef]
- Trösken, E.R.; Adamska, M.; Arand, M.; Zarn, J.A.; Patten, C.; Völkel, W.; Lutz, W.K. Comparison of lanosterol-14α-demethylase (CYP51) of human and Candida albicans for inhibition by different antifungal azoles. Toxicology 2006, 228, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and autodocktools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio Visualizer, v21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2020.
- Bakan, A.; Meireles, L.M.; Bahar, I. ProDy: Protein dynamics ınferred from theory and experiments. Bioinformatics 2011, 27, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, A.R.; Durell, S.R.; Jernigan, R.L.; Demirel, M.C.; Keskin, O.; Bahar, I. Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys. J. 2001, 80, 505–515. [Google Scholar] [CrossRef] [PubMed]




| Compound | Molecular Weight (g/mol) | Molecular Formula | Yield (%) | M.P. (°C) | Rf (Tolu:MeOH) |
|---|---|---|---|---|---|
| 3 | 281 | C7H12N5O5Cl | 11.32 | Dec. 252–258 | 0.28 (1:1) |
| 4 | 306 | C9H18N6O6 | 11.32 | Dec. 252–258 | 0.28 (1:1) |
| 5 | 996 | C30H48N18O21 | 84.19 | Dec. 245 | 0.20 (2:1) |
| m/z | Relative Abundance (%) | m/z | Relative Abundance (%) | m/z | Relative Abundance (%) |
|---|---|---|---|---|---|
| 71 | 28.68 | 132 | 2.96 | 281 | 4.96 |
| 70 | 27.96 | 113 | 13.64 | 279 | 6.03 |
| 69 | 1.87 | 112 | 6.44 | 253 | 1.12 |
| 57 | 46.83 | 105 | 4.81 | 178 | 1.58 |
| 56 | 10.00 | 104 | 8.60 | 168 | 2.78 |
| 55 | 24.56 | 84 | 4.74 | 167 | 35.61 |
| 43 | 26.37 | 83 | 11.85 | 163 | 1.66 |
| 42 | 6.12 | 76 | 5.68 | 150 | 9.92 |
| 41 | 29.76 | 72 | 9.50 | 149 | 100.00 |
| m/z | Relative Abundance (%) | m/z | Relative Abundance (%) | m/z | Relative Abundance (%) |
|---|---|---|---|---|---|
| 70 | 14.26 | 233 | 0.26 | 389 | 0.32 |
| 63 | 1.11 | 209 | 0.23 | 379 | 0.50 |
| 62 | 2.69 | 196 | 0.51 | 370 | 0.50 |
| 48 | 3.73 | 184 | 0.53 | 365 | 0.57 |
| 44 | 75.69 | 175 | 0.43 | 363 | 0.46 |
| 43 | 80.81 | 130 | 2.76 | 352 | 0.34 |
| 42 | 16.99 | 129 | 100.00 | 324 | 0.78 |
| 41 | 2.87 | 87 | 1.47 | 310 | 0.08 |
| 40 | 7.93 | 86 | 16.64 | 308 | 0.17 |
| m/z | Relative Abundance (%) | m/z | Relative Abundance (%) | m/z | Relative Abundance (%) |
|---|---|---|---|---|---|
| 62 | 87.00 | 284 | 1.44 | 453 | 6.11 |
| 63 | 1.83 | 286 | 1.48 | 491 | 1.82 |
| 64 | 54.13 | 391 | 7.59 | 512 | 5.14 |
| 79 | 77.10 | 392 | 1.78 | 513 | 1.62 |
| 80 | 1.83 | 413 | 100.00 | 540 | 2.40 |
| 81 | 35.62 | 414 | 23.68 | 803 | 25.86 |
| 101 | 87.69 | 415 | 3.22 | 804 | 12.85 |
| 102 | 3.38 | 429 | 3.62 | 805 | 3.29 |
| 117 | 1.93 | 452 | 23.98 | 983.7 | 1.34 |
| 140 | 3.81 | 984.7 | 0.82 | ||
| 150 | 2.05 | 997.69 | 1.43 | ||
| 157 | 17.13 | 998.69 | 0.88 |
| Exposure Time (Minutes) | 3 | 5 | 10 |
|---|---|---|---|
| Dose (µM) | Inhibition (%) | Inhibition (%) | Inhibition (%) |
| 20 | 8.42 ± 2.11 | 12.42 ± 3.51 | 18.42 ± 2.11 |
| 40 | 12.71 ± 3.23 | 18.21 ± 4.25 | 32.75 ± 3.23 |
| 60 | 27.02 ± 4.51 | 41.20 ± 7.43 | 65.22 ± 5.61 |
| 100 | 53.4 ± 5.22 | 88.5 ± 7.3 | 100 |
| Control 1 (Negative control; water) | 0.0 | 0.0 | 0.0 |
| Control 2 (Positive control: formaline) | 9.3 ± 2.6 | 11.3 ± 2.1 | 14.2 ± 2.6 |
| BE | rmsd | E_Int | E_H.B | |
|---|---|---|---|---|
| 5V5Z | ||||
| 5 | −9.874 | 1.125 | −45.263 | −30.698 |
| 1YN | −9.551 | 1.514 | −107.02 | −38.643 |
| 1HZW | ||||
| 5 | −5.454 | 1.534 | −26.2266 | −2.1429 |
| Raltitrexed | −4.153 | 1.689 | −13.209 | −4.347 |
| dUMP | −3.984 | 1.159 | −13.314 | −3.390 |
| Parameter | Compound 3 | Compound 4 | Compound 5 |
|---|---|---|---|
| EHOMO | −6.91 | −6.62 | −5.42 |
| ELUMO | −1.88 | −1.65 | −3.82 |
| Energy Gap (ΔƐ) | 5.03 | 4.97 | 1.60 |
| Hardness (η) | 2.52 | 2.49 | 0.80 |
| Softness (σ) | 0.397 | 0.402 | 1.25 |
| Electronegativity (χ) | 4.40 | 4.14 | 4.62 |
| Electrophilicity (ω) | 3.84 | 3.44 | 13.34 |
| Property/Predictor | Compound 5 | Interpretation |
|---|---|---|
| Physicochemical Properties | ||
| Molecular Weight (g/mol) | 996.81 | High |
| Num. H-bond acceptors | 27 | High |
| Num. H-bond donors | 6 | Moderate |
| Molar Refractivity | 221.41 | High |
| TPSA (Å2) | 503.19 | Very High |
| Pharmacokinetics | ||
| GI absorption | Low | Poor oral absorption expected |
| BBB permeant | No | Unlikely to cross BBB |
| P-glycoprotein substrate | Yes | Subject to efflux |
| CYP Inhibitor (1A2, 2C19, 2C9, 2D6, 3A4) | No | Low risk of CYP-mediated drug interactions |
| Skin Permeability (log Kp, cm/s) | −18.34 | Very low (unlikely to penetrate skin) |
| Drug-likeness | ||
| Lipinski (Rule of 5) | 3 violations (MW, HBA, Log P) | Not drug-like by strict oral standards |
| Ghose | 4 violations | |
| Veber | 2 violations | (Rot. bonds, TPSA) |
| Egan | 1 violation | (TPSA) |
| Muegge | 6 violations | |
| Bioavailability Score | 0.17 | Low |
| Medicinal Chemistry | ||
| PAINS alerts | 0 | No pan-assay interference substructures |
| Brenk alerts | 0 | No problematic functional groups |
| Leadlikeness | 2 violations | Not lead-like (MW, Rot. bonds) |
| Synthetic Accessibility | 6.95 | Very complex to synthesize |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhilal, M.; Alhilal, S.; Sabancilar, I.; Gomha, S.M.; Elhenawy, A.A.; Ouf, S.A. Synthesis, Spectroscopic Characterization, and Biological Evaluation of a Novel Acyclic Heterocyclic Compound: Anticancer, Antioxidant, Antifungal, and Molecular Docking Studies. Pharmaceuticals 2025, 18, 1533. https://doi.org/10.3390/ph18101533
Alhilal M, Alhilal S, Sabancilar I, Gomha SM, Elhenawy AA, Ouf SA. Synthesis, Spectroscopic Characterization, and Biological Evaluation of a Novel Acyclic Heterocyclic Compound: Anticancer, Antioxidant, Antifungal, and Molecular Docking Studies. Pharmaceuticals. 2025; 18(10):1533. https://doi.org/10.3390/ph18101533
Chicago/Turabian StyleAlhilal, Mohammad, Suzan Alhilal, Ilhan Sabancilar, Sobhi M. Gomha, Ahmed A. Elhenawy, and Salama A. Ouf. 2025. "Synthesis, Spectroscopic Characterization, and Biological Evaluation of a Novel Acyclic Heterocyclic Compound: Anticancer, Antioxidant, Antifungal, and Molecular Docking Studies" Pharmaceuticals 18, no. 10: 1533. https://doi.org/10.3390/ph18101533
APA StyleAlhilal, M., Alhilal, S., Sabancilar, I., Gomha, S. M., Elhenawy, A. A., & Ouf, S. A. (2025). Synthesis, Spectroscopic Characterization, and Biological Evaluation of a Novel Acyclic Heterocyclic Compound: Anticancer, Antioxidant, Antifungal, and Molecular Docking Studies. Pharmaceuticals, 18(10), 1533. https://doi.org/10.3390/ph18101533









