New-Onset Diabetes After Transplantation in Renal Recipients: A Pilot Comparative Study of Immediate vs. Extended-Release Tacrolimus Formulation
Abstract
1. Introduction
2. Results
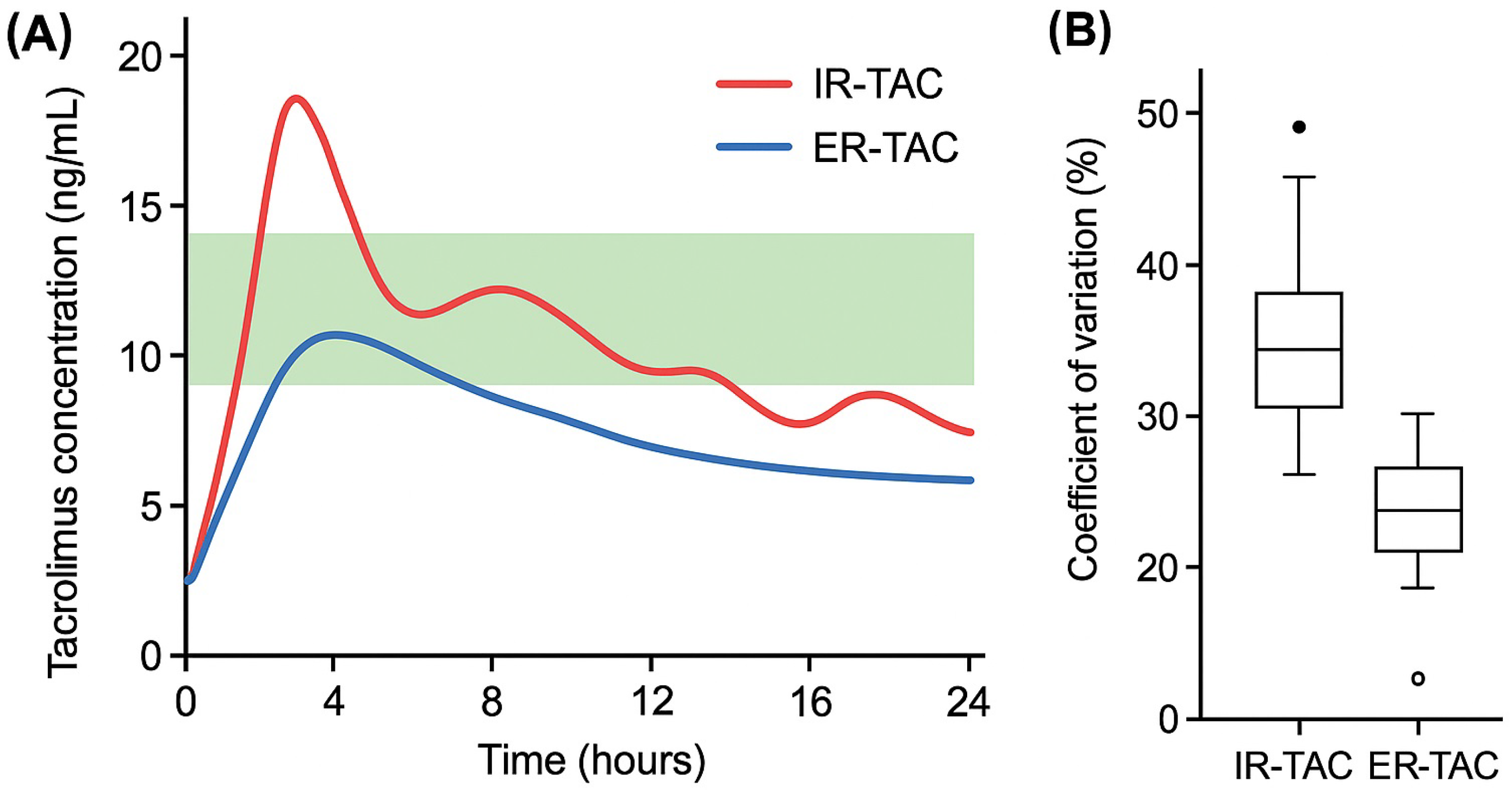
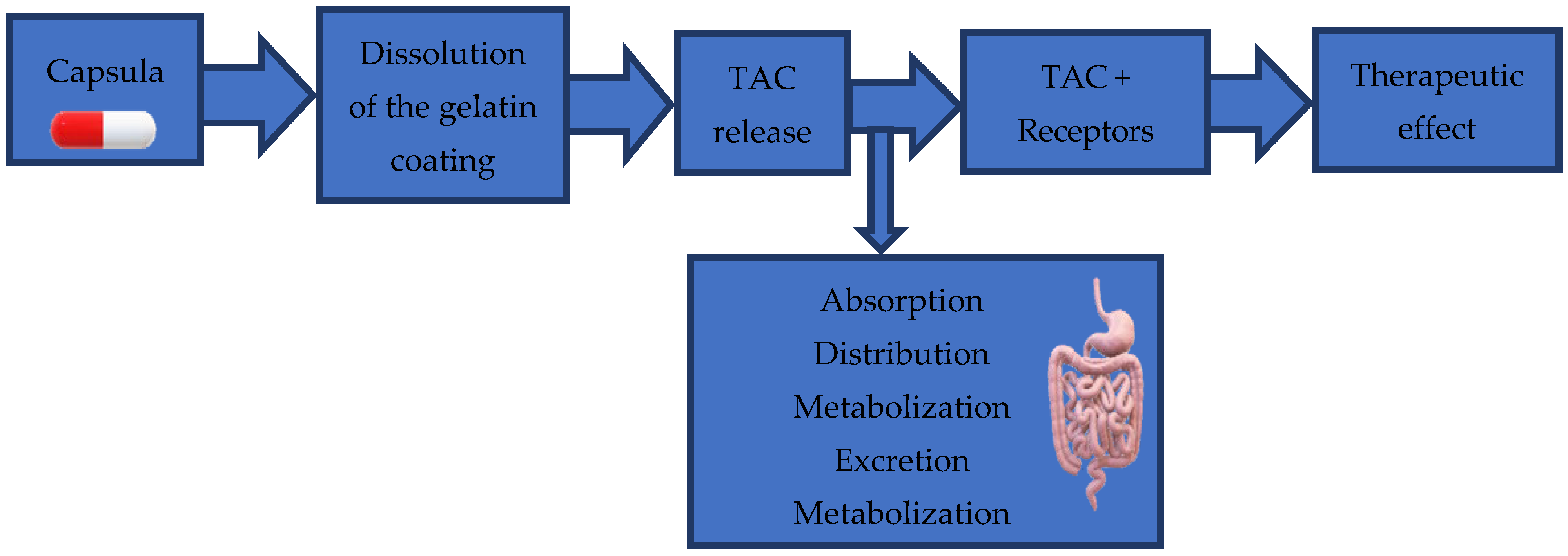
2.1. Dissolution Test and Tacrolimus Dosing
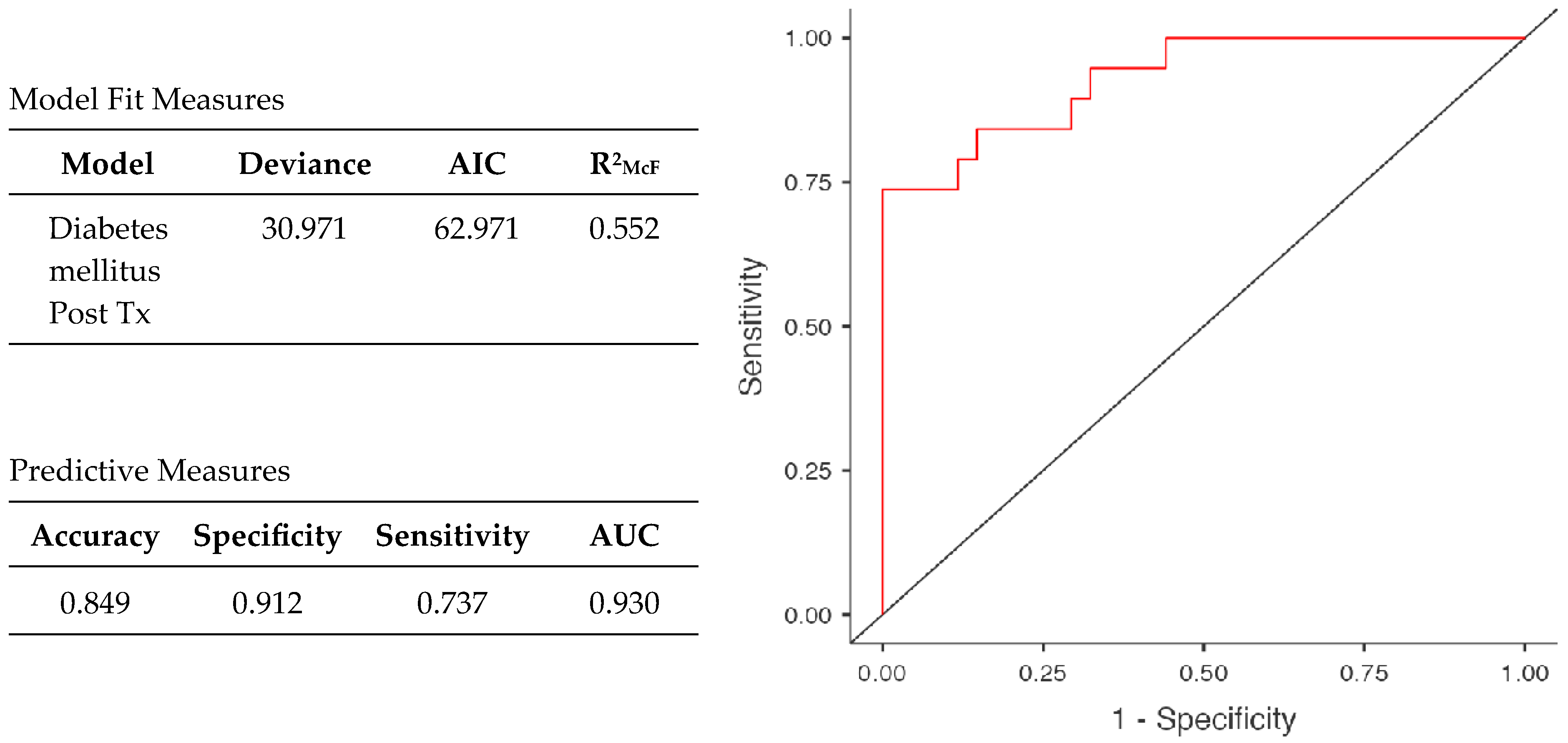
2.2. Clinical Impact Study
3. Discussion
3.1. NODAT After Renal Transplantation
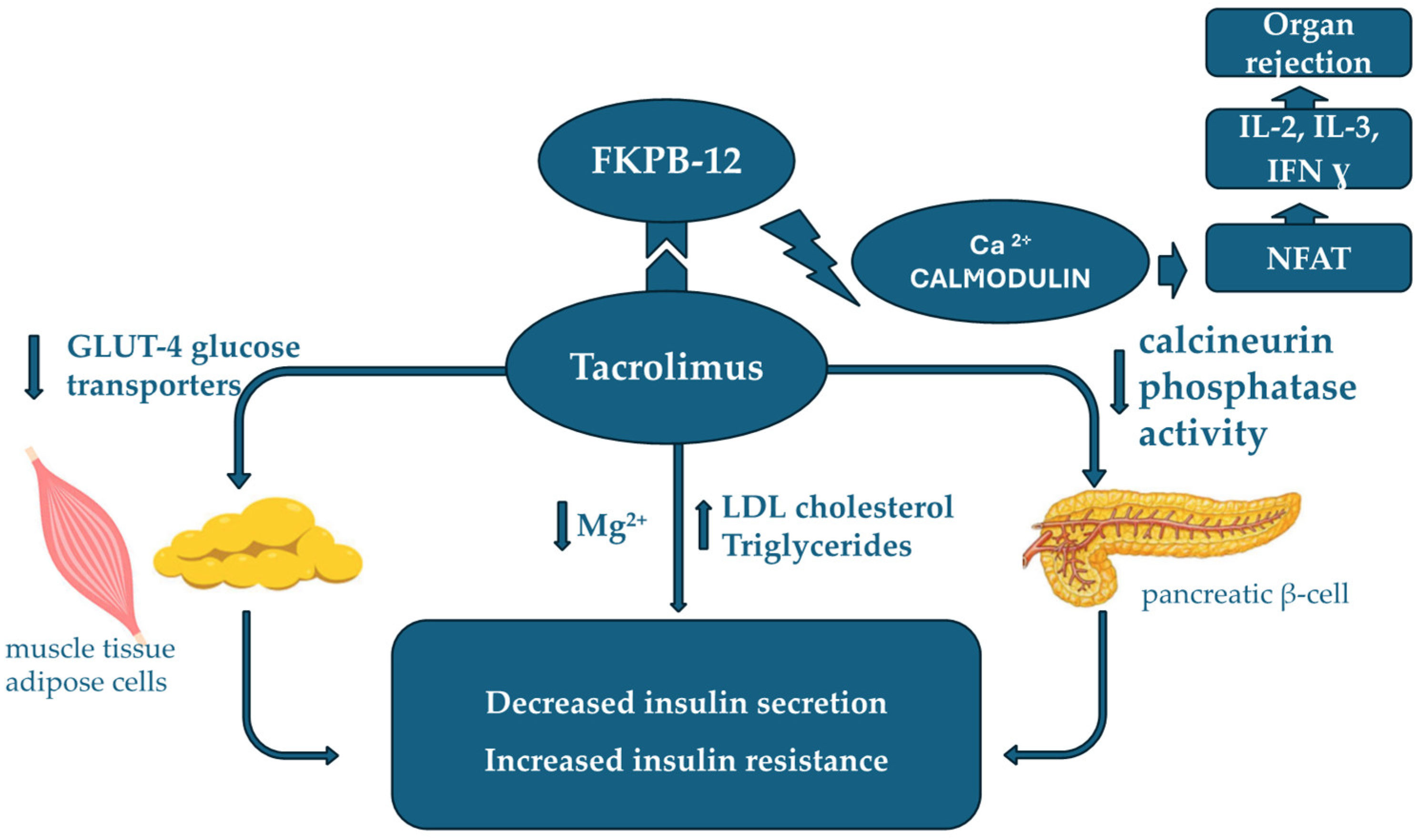
3.2. Tacrolimus Metabolism and Side Effects in Renal Transplantation
4. Materials and Methods
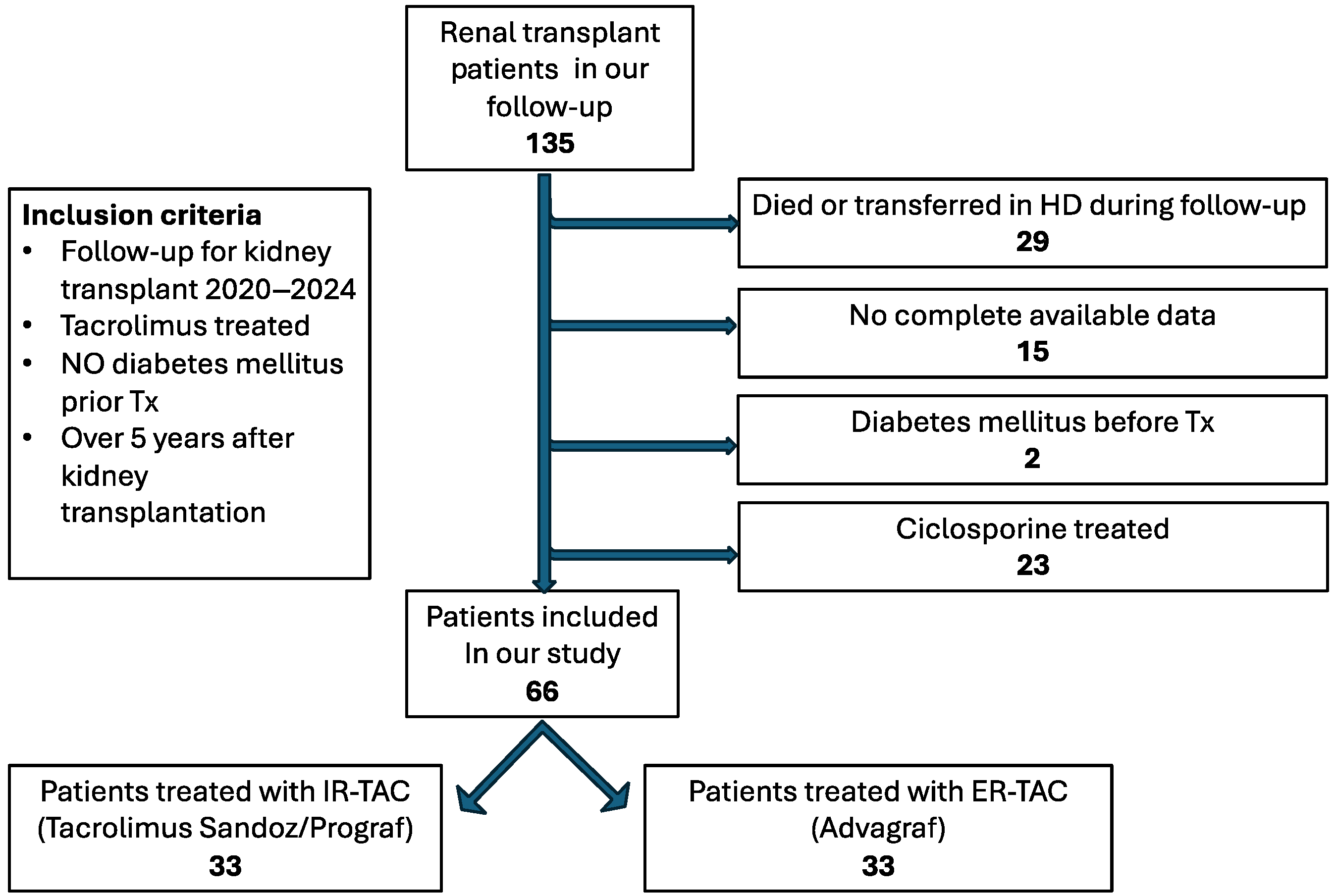
4.1. Study Design
4.2. Study Objectives
4.3. Data Collection
- (a)
- Demographic data: age, age after transplantation, and gender;
- (b)
- Data related to transplant procedure: type of donator and acute rejection episodes;
- (c)
- Biometric data: body mass index (BMI);
- (d)
- Treatment data: type of tacrolimus formulation, median tacrolimus dosage, the use of corticotherapy, and type of antidiabetic treatment, e.g., insulin, oral antidiabetics, diet;
- (e)
- Laboratory analyses: creatinine (0.7–1.3 mg/dL), eGFR, fasting glucose (74.00–100.00 mg/dL), Hb A1c (4–5.7%), hs-CRP (0–5 mg/dL), AST (11–34 U/L), triglycerides (0–150.00 mg/dL), total cholesterol (0–200.00 mg/dL) and LDL cholesterol (10.00–100.00 mg/dL), calcium (8.5–10.2), magnesium (1.6–2.6 mg/dL), uric acid (3.7–7.7 mg/dL), and proteinuria; the glomerular filtration rate (eGFR) was estimated by applying the CKD-EPI 2021 formula according to the KDIGO 2024 recommendation.
4.4. Tacrolimus Formulations and Dosing Protocols
4.4.1. Dissolution Test
4.4.2. Tacrolimus Dosing
4.4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixidó-Trujillo, S.; Porrini, E.; Menéndez-Quintanal, L.M.; Torres-Ramírez, A.; Fumero, C.; Rodríguez-Rodríguez, A.E. Induction of diabetes by Tacrolimus in a phenotypic model of obesity and metabolic syndrome. Front. Endocrinol. 2024, 15, 1388361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharif, A.; Chakkera, H.; de Vries, A.P.J.; Eller, K.; Guthoff, M.; Haller, M.C.; Hornum, M.; Nordheim, E.; Kautzky-Willer, A.; Krebs, M.; et al. International consensus on post-transplantation diabetes mellitus. Nephrol. Dial. Transplant. 2024, 39, 531–549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tremblay, S.; Nigro, V.; Weinberg, J.; Woodle, E.S.; Alloway, R.R. A Steady-State Head to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am. J. Transplant. 2017, 17, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Oberbauer, R.; Bestard, O.; Furian, L.; Maggiore, U.; Pascual, J.; Rostaing, L.; Budde, K. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant. Rev. 2020, 34, 100531. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Cassuto, E.; Piotti, G.; Govoni, M.; Ciurlia, G.; Geraci, S.; Poli, G.; Nicolini, G.; Mariat, C.; Essig, M.; et al. Pharmacokinetics of Prolonged-Release Once-Daily Formulations of Tacrolimus in de Novo Kidney Transplant Recipients: A Randomized, Parallel-Group, Open-Label, Multicenter Study. Adv. Ther. 2019, 36, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Song, T.; Jiang, Y.; Li, X.; Fan, Y.; Lin, T. Tacrolimus Trough Level at the First Month May Predict Renal Transplantation Outcomes Among Living Chinese Kidney Transplant Patients: A Propensity Score–Matched Analysis. Ther. Drug Monit. 2019, 41, 308–316. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, N.; Kim, M.G.; Yun, H.-Y.; Lee, S.; Bae, E.; Kim, Y.S.; Kim, I.-W.; Oh, J.M. Increased Exposure of Tacrolimus by Co-administered Mycophenolate Mofetil: Population Pharmacokinetic Analysis in Healthy Volunteers. Sci. Rep. 2018, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.M.; Li, Y.; De Winter, B.C.M.; Shi, Y.Y.; Baan, C.C.; Van Gelder, T.; Hesselink, D.A. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Mendoza Rojas, A.; Hesselink, D.A.; van Besouw, N.M.; Baan, C.C.; van Gelder, T. Impact of low tacrolimus exposure and high tacrolimus intra-patient variability on the development of de novo anti-HLA donor-specific antibodies in kidney transplant recipients. Expert Rev. Clin. Immunol. 2019, 15, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.O.; Alloway, R.R.; Bodziak, K.; Kaplan, B.; Bunnapradist, S. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): A phase 2 trial of stable renal transplant recipients. Transplantation 2013, 96, 191–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, H.T., Jr.; Yang, H.C.; Meier-Kriesche, H.U.; Croy, R.; Holman, J.; Fitzsimmons, W.E.; First, M.R. Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 2014, 97, 636–641. [Google Scholar] [CrossRef]
- Torres, A.; Rodríguez-Adanero, C.; Fernández-Rivera, C.; Marrero-Miranda, D.; de Bonis-Redondo, E.; Rodríguez-Hernández, A.P.; Pérez-Tamajón, L.; González-Rinne, A.; Álvarez-Sosa, D.; Álvarez-González, A.; et al. Efficacy of the once-daily tacrolimus formulation LCPT compared to the immediate-release formulation in preventing early post-transplant diabetes in high-risk kidney transplant patients: A randomized, controlled, open-label pilot study. J. Clin. Med. 2024, 13, 7802. [Google Scholar] [CrossRef] [PubMed]
- Krämer, B.K.; Charpentier, B.; Bäckman, L.; Silva, H.T., Jr.; Mondragon-Ramirez, G.; Cassuto-Viguier, E.; Mourad, G.; Sola, R.; Rigotti, P.; Mirete, J.O.; et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: A randomized phase III study. Am. J. Transplant. 2010, 10, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.; Banas, B.; Klempnauer, J.L.; Glyda, M.; Viklicky, O.; Kamar, N.; Optimising immunoSuppression After Kidney transplantation with ADVAGRAF Study Group. OSAKA trial: A randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013, 96, 897–903. [Google Scholar] [CrossRef]
- Rostaing, L.; Bunnapradist, S.; Grinyó, J.M.; Ciechanowski, K.; Denny, J.E.; Silva, H.T., Jr.; Budde, K.; Envarsus Study Group. Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial. Am. J. Kidney Dis. 2016, 67, 648–659. [Google Scholar] [CrossRef]
- Kumar, J.; Reccia, I.; Virdis, F.; Podda, M.; Sharma, A.K.; Halawa, A. Belatacept in renal transplantation in comparison to tacrolimus and molecular understanding of resistance pattern: Meta-analysis and systematic review. World J. Transplant. 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Zidan, A.S.; Rahman, Z.; Sayeed, V.; Raw, A.; Yu, L.; Khan, M.A. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. Int. J. Pharm. 2012, 423, 341–350. [Google Scholar] [CrossRef]
- Tsunashima, D.; Yamashita, K.; Ogawara, K.; Sako, K.; Higaki, K. Preparation of extended release solid dispersion formulations of tacrolimus using ethylcellulose and hydroxypropylmethylcellulose by solvent evaporation method. J. Pharm. Pharmacol. 2016, 68, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Enayatifard, R.; Saeedi, M.; Saghafi, M. Influence of Hydroxypropyl Methylcellulose Molecular Weight Grade on Water Uptake, Erosion and Drug Release Properties of Diclofenac Sodium Matrix Tablets. Trop. J. Pharm. Res. 2011, 10, 535–541. [Google Scholar] [CrossRef][Green Version]
- Everaerts, M.; Cools, L.; Adriaensens, P.; Reekmans, G.; Baatsen, P.; Van Den Mooter, G. Investigating the Potential of Ethyl Cellulose and a Porosity-Increasing Agent as a Carrier System for the Formulation of Amorphous Solid Dispersions. Mol. Pharm. 2022, 19, 2712–2724. [Google Scholar] [CrossRef]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e71–e174. [Google Scholar] [CrossRef]
- Hamed, R.; Mohamed, E.M.; Sediri, K.; Khan, M.A.; Rahman, Z. Development of stable amorphous solid dispersion and quantification of crystalline fraction of lopinavir by spectroscopic-chemometric methods. Int. J. Pharm. 2021, 602, 120657. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Siddiqui, A.; Bykadi, S.; Khan, M.A. Determination of tacrolimus crystalline fraction in the commercial immediate release amorphous solid dispersion products by a standardized X-ray powder diffraction method with chemometrics. Int. J. Pharm. 2014, 475, 462–470. [Google Scholar] [CrossRef]
- Ponnammal, P.; Kanaujia, P.; Yani, Y.; Ng, W.K.; Tan, R.B.H. Orally Disintegrating Tablets Containing Melt Extruded Amorphous Solid Dispersion of Tacrolimus for Dissolution Enhancement. Pharmaceutics 2018, 10, 35. [Google Scholar] [CrossRef]
- Luan, F.L.; Steffick, D.E.; Ojo, A.O. New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation 2011, 91, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.A.; Cheungpasitporn, W.; Bunnapradist, S.; Schnitzler, M.A.; Xiao, H.; McAdams-DeMarco, M.; Caliskan, Y.; Bae, S.; Ahn, J.B.; Segev, D.L.; et al. Posttransplant Diabetes Mellitus and Immunosuppression Selection in Older and Obese Kidney Recipients. Kidney Med. 2021, 4, 100377. [Google Scholar] [CrossRef]
- Bamgbola, O. Metabolic consequences of modern immunosuppressive agents in solid organ transplantation. Ther. Adv. Endocrinol. Metab. 2016, 7, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.; Matas, A.J. Diabetes Mellitus after Kidney Transplantation in the United States. Am. J. Transplant. 2003, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, L.A.; Smidt, K.; Mortensen, D.M.; Carstens, J.; Jorgensen, K.A.; Rungby, J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br. J. Pharmacol. 2010, 162, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Triñanes, J.; Rodriguez-Rodriguez, A.E.; Brito-Casillas, Y.; Wagner, A.; De Vries, A.P.J.; Cuesto, G.; Acebes, A.; Salido, E.; Torres, A.; Porrini, E.; et al. Deciphering Tacrolimus-Induced Toxicity in Pancreatic β Cells. Arab. Archaeol. Epigr. 2017, 17, 2829–2840. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Kudva, Y.; Kaplan, B. Calcineurin Inhibitors: Pharmacologic Mechanisms Impacting Both Insulin Resistance and Insulin Secretion Leading to Glucose Dysregulation and Diabetes Mellitus. Clin. Pharmacol. Ther. 2017, 101, 114–120. [Google Scholar] [CrossRef]
- Chmielnicka, K.; Heleniak, Z.; Dębska-Ślizień, A. Dyslipidemia in Renal Transplant Recipients. Transplantology 2022, 3, 188–199. [Google Scholar] [CrossRef]
- Ratiu, I.; Moisa, C.; Marc, L.; Olariu, N.; Ratiu, C.; Bako, G.; Ratiu, A.; Fratila, S.; Teusdea, A.; Ganea, M.; et al. The impact of hypomagnesemia on the long term evolution after kidney transplantation. Nutrients 2025, 17, 50. [Google Scholar] [CrossRef]
- Moisa, C.; Cadar, O.; Barabas, R.; Vicaș, L.; Hoaghia, A.; Levei, E.; Jurca, C.; Berce, C. Influence of magnesium compounds on sodium, potassium and calcium levels in different mice organs. Farmacia 2019, 67, 274–281. [Google Scholar] [CrossRef]
- Higgins, R.; Ramaiyan, K.; Dasgupta, T.; Kanji, H.; Fletcher, S.; Lam, F.; Kashi, H. Hyponatraemia and hyperkalaemia are more frequent in renal transplant recipients treated with tacrolimus than with cyclosporin. Further evidence for differences between cyclosporin and tacrolimus nephrotoxicities. Nephrol. Dial. Transplant. 2004, 19, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Solhjoo, M.; Kumar, S.C. New Onset Diabetes After Transplant. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bayés, B.; Lauzurica, R.; Granada, M.L.; Serra, A.; Bonet, J.; Fontseré, N.; Salinas, I.; Romero, R. Adiponectin and risk of new-onset diabetes mellitus after kidney transplantation. Transplantation 2004, 78, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.; Gatault, P.; Doute, C.; Büchler, M.; Al-Najjar, A.; Barbet, C.; Chatelet, V.; Marlière, J.F.; Nivet, H.; Lebranchu, Y.; et al. Immunosuppressive medications, clinical and metabolic parameters in new-onset diabetes mellitus after kidney transplantation. Transpl. Int. 2008, 21, 523–530. [Google Scholar] [CrossRef]
- Bayer, N.D.; Cochetti, P.T.; Anil Kumar, M.S.; Teal, V.; Huan, Y.; Doria, C.; Bloom, R.D.; Rosas, S.E. Association of metabolic syndrome with development of new-onset diabetes after transplantation. Transplantation 2010, 90, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Van Biesen, W.; Verbeke, F.; De Bacquer, D.; Peeters, P.; Vanholder, R. Postransplantation hypomagnesemia and its realtion with immunosuppression as predictors of new-onset diabetes after transplantation. Am. J. Transplant. 2009, 9, 2140–2149. [Google Scholar] [CrossRef]
- Roland, M.; Gatault, P.; Al-Naijjar, A.; Doute, C.; Barbet, C.; Chatelet, V.; Laouad, I.; Marlière, J.F.; Nivet, H.; Büchler, M.; et al. Early pulse pressure and lowgrade proteinuria as independent long-term risk factors for new-onset diabetes after kidney transplantation. Am. J. Transplant. 2008, 8, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.T.; Pham, P.M.; Pham, S.V.; Pham, P.A.; Pham, P.C. New onset diabetes after transplantation (NODAT): An overview. Diabetes Metab. Syndr. Obes. 2011, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Ghisdal, L.; Laecke, S.V.; Abramowicz, M.J.; Vanholder, R.; Abramowicz, D. New-Onset Diabetes After Renal Transplantation: Risk assessment and management. Diabetes Care 2011, 35, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lancia, P.; Adam de Beaumais, T.; Elie, V.; Garaix, F.; Fila, M.; Nobili, F.; Ranchin, B.; Testevuide, P.; Ulinski, T.; Zhao, W.; et al. Pharmacogenetics of post-transplant diabetes mellitus in children with renal transplantation treated with tacrolimus. Pediatr. Nephrol. 2018, 33, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Kim, D.H.; Lee, S.M.; Han, N.Y.; Oh, J.M.; Ha, J.; Kim, Y.S. Pharmacokinetics of tacrolimus according to body composition in recipients of kidney transplants. Kidney Res. Clin. Pract. 2012, 31, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, T. Drug interactions with tacrolimus. Drug Saf. 2002, 25, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef]
- Roy, J.N.; Barama, A.; Poirier, C.; Vinet, B.; Roger, M. Cyp3A4, Cyp3A5, and MDR-1 Genetic Influences on Tacrolimus Pharmacokinetics in Renal Transplant Recipients. Pharmacogenet. Genom. 2006, 16, 659–665. [Google Scholar] [CrossRef]
- Vidal-Alabró, A.; Colom, H.; Fontova, P.; Cerezo, G.; Melilli, E.; Montero, N.; Coloma, A.; Manonelles, A.; Favà, A.; Cruzado, J.M.; et al. Tools for a Personalized Tacrolimus Dose Adjustment in the Follow-up of Renal Transplant Recipients. Metabolizing Phenotype According to CYP3A Genetic Polymorphisms versus Concentration-Dose Ratio. Nefrol. (Engl. Ed.) 2024, 44, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Thölking, G.; Fortmann, C.; Koch, R.; Gerth, H.U.; Pabst, D.; Pavenstädt, H.; Kabar, I.; Hüsing, A.; Wolters, H.; Reuter, S.; et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS ONE 2014, 9, e111128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giral, M.; Grimbert, P.; Morin, B.; Bouvier, N.; Buchler, M.; Dantal, J.; Garrigue, V.; Bertrand, D.; Kamar, N.; Malvezzi, P.; et al. Impact of Switching From Immediate- or Prolonged-Release to Once-Daily Extended-Release Tacrolimus (LCPT) on Tremor in Stable Kidney Transplant Recipients: The Observational ELIT Study. Transpl. Int. 2024, 37, 11571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suwelack, B.; Bunnapradist, S.; Meier-Kriesche, U.; Stevens, D.R.; Procaccianti, C.; Morganti, R.; Budde, K. Effect of Concentration/Dose Ratio in De Novo Kidney Transplant Recipients Receiving LCP-Tacrolimus or Immediate-Release Tacrolimus: Post Hoc Analysis of a Phase 3 Clinical Trial. Ann. Transplant. 2020, 25, e923278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandez Rivera, C.; Calvo Rodríguez, M.; Poveda, J.L.; Pascual, J.; Crespo, M.; Gomez, G.; Cabello Pelegrin, S.; Paul, J.; Lauzurica, R.; Perez Mir, M.; et al. Better study. Bioavailability of once-daily tacrolimus formulations used in clinical practice in the management of De Novo kidney transplant recipients: The better study. Clin. Transplant. 2022, 36, e14550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thölking, G.; Filensky, B.; Jehn, U.; Schütte-Nütgen, K.; Koch, R.; Kurschat, C.; Pavenstädt, H.; Suwelack, B.; Reuter, S.; Kuypers, D. Increased renal function decline in fast metabolizers using extended-release tacrolimus after kidney transplantation. Sci. Rep. 2021, 11, 15606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowicka, M.; Górska, M.; Nowicka, Z.; Edyko, K.; Edyko, P.; Wiślicki, S.; Zawiasa-Bryszewska, A.; Strzelczyk, J.; Matych, J.; Kurnatowska, I. Tacrolimus: Influence of the Posttransplant Concentration/Dose Ratio on Kidney Graft Function in a Two-Year Follow-Up. Kidney Blood Press Res. 2019, 44, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Alloway, R.R. Clinical Evaluation of Modified Release and Immediate Release Tacrolimus Formulations. AAPS J. 2017, 19, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Jouve, T.; Fonrose, X.; Noble, J.; Janbon, B.; Fiard, G.; Malvezzi, P.; Stanke-Labesque, F.; Rostaing, L. The TOMATO Study (Tacrolimus Metabolization in Kidney Transplantation): Impact of the Concentration-Dose Ratio on Death-censored Graft Survival. Transplantation 2020, 104, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A.; Rathbone, M.J. Overview of Controlled Release Mechanisms; Springer: Boston, MA, USA, 2012; pp. 19–43. [Google Scholar]
- Moisa, C.; Hoaghia, M.; Simedru, D.; Cadar, O. Influence of tablet formulation in vitro release of magnesium. Stud. UBB Chem. 2016, 2, 441–449. Available online: http://chem.ubbcluj.ro/~studiachemia/issues/chemia2016_3/tom2/15_Moisa_etal_441_449.pdf (accessed on 23 October 2024).
- Moisa, C.; Cadar, O.; Levei, E.; Mureșan, L.; Ganea, M.; Nemeth, S.; Cavalu, S.; Dobjanschi, L.; Zdrȋncă, M.; Barabas, R.; et al. Compatibility study between magnesium orotate and various excipients in their physical mixtures. Farmacia 2022, 70, 465–476. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Hadgraft, J.; Roberts, M.S.; Lane, M.E. Modified-Release Drug Delivery Technology, 2nd ed.; Informa Healthcare: London, UK, 2008; Volume 2. [Google Scholar]
- Mehta, R.; Teckoe, J.; Schoener, C.; Workentine, S.; Ferrizzi, D.; Rajabi-Siahoboomi, A. Investigation into effect of ethylcellulose viscosity variation on the drug release of metoprolol tartrate and acetaminophen extended release multiparticulates—Part I. AAPS PharmSciTech 2016, 17, 1366–1375. [Google Scholar] [CrossRef][Green Version]
- Mehta, R.Y.; Missaghi, S.; Tiwari, S.B.; Rajabi-Siahboomi, A.R. Application of ethylcellulose coating to hydrophilic matrices: A strategy to modulate drug release profile and reduce drug release variability. AAPS PharmSciTech 2014, 15, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Ho, M.J.; Kim, S.R.; Im, S.H.; Kim, C.H.; Lee, S.; Kang, M.J.; Choi, Y.W. Formulation and in vivo pharmacokinetic evaluation of ethyl cellulose-coated sustained release multiple-unit system of tacrolimus. Int. J. Biol. Macromol. 2018, 1, 544–550. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009; pp. 262–267. [Google Scholar]
- Kuypers, D.R.; Peeters, P.C.; Sennesael, J.J.; Kianda, M.N.; Vrijens, B.; Kristanto, P.; Dobbels, F.; Vanrenterghem, Y.; Kanaan, N.; ADMIRAD Study Team. Improved Adherence to Tacrolimus Once-Daily Formulation in Renal Recipients: A Randomized Controlled Trial Using Electronic Monitoring. Transplantation 2013, 95, 333–340. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Clinical Pharmacokinetics of Once-Daily Tacrolimus in Solid-Organ Transplant Patients. Clin. Pharmacokinet. 2015, 54, 993–1025. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.R. What do we know about tacrolimus pharmacogenetics in transplant recipients? Pharmacogenomics 2018, 19, 593–597. [Google Scholar] [CrossRef]
- Berggren, S.; Gall, C.; Wollnitz, N.; Ekelund, M.; Karlbom, U.; Hoogstraate, J.; Schrenk, D.; Lennernäs, H. Gene and Protein Expression of P-Glycoprotein, MRP1, MRP2, and CYP3A4 in the Small and Large Human Intestine. Mol. Pharm. 2007, 4, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Bauer, S.; Brakemeier, S.; Burgwinkel, P.; Glander, P.; Kreutz, R.; Lorkowski, C.; Slowinski, T.; Neumayer, H.; Budde, K. Differential impact of the CYP3A5*1 and CYP3A5*3 alleles on pre-dose concentrations of two tacrolimus formulations. Pharm. Genom. 2010, 21, 179–184. [Google Scholar] [CrossRef]
- Satoh, S.; Niioka, T.; Kagaya, H.; Numakura, K.; Inoue, T.; Saito, M.; Komine, N.; Narita, S.; Tsuchiya, N.; Habuchi, T.; et al. Pharmacokinetic and CYP3A5 pharmacogenetic differences between once- and twice-daily tacrolimus from the first dosing day to 1 year after renal transplantation. Pharmacogenomics 2014, 15, 1495–1506. [Google Scholar] [CrossRef]
- Barraclough, K.A.; Isbel, N.M.; Campbell, S.; Barraclough, K.A.; Johnson, D.W.; Staatz, C.E. Once-Versus Twice-Daily Tacrolimus: Are the Formulations Truly Equivalent? Drugs 2011, 71, 1561–1577. [Google Scholar] [CrossRef]

| Product Name | Advagraf (Capsules) | Tacrolimus (Capsules) | Prograf (Capsules) |
|---|---|---|---|
| Active substance | Tacrolimus monohydrate | Tacrolimus monohydrate | Tacrolimus monohydrate |
| Excipients | Hypromellose Ethylcellulose Lactose monohydrate Magnesium stearate | Hypromellose Croscarmellose sodium Lactose monohydrate Magnesium stearate | Hypromellose Croscarmellose sodium Lactose monohydrate Magnesium stearate |
| Release mode of active substance | Extended | Immediate | Immediate |
| Time | Prograf | Tacrolimus | Advagraf | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 1.2 | pH 4.5 | pH 6.8 | pH 1.2 | pH 4.5 | pH 6.8 | pH 1.2 | pH 4.5 | pH 6.8 | |
| 15 min | 38% | 35% | 30% | 36% | 30% | 29% | 5% | 4% | 3% |
| 30 min | 66% | 62% | 60% | 65% | 60% | 58% | 8% | 6% | 5% |
| 60 min | 83% | 81% | 80% | 80% | 78% | 78% | 20% | 18% | 18% |
| 90 min | 85% | 83% | 80% | 82% | 80% | 80% | 35% | 33% | 33% |
| 120 min | 95% | 93% | 92% | 91% | 90% | 90% | 40% | 40% | 40% |
| 360 min | 100% | 100% | 100% | 100% | 100% | 100% | 45% | 46% | 48% |
| Patients Characteristics M ± SD/Median | Entire Cohort n = 66 | IR-TAC n = 33 | ER-TAC n = 33 | p |
|---|---|---|---|---|
| Age (years) | 50.273 ± 10.14/50 | 50.121 ± 11.683/49 | 50.424 ± 8.511/50 | 0.905 |
| Gender (female) | 31 (47%) | 17 (25.8%) | 14 (21.2%) | 0.62 |
| Tx vintage | 14 ± 6.919/12.5 | 14.365 ± 5.079/14 | 13.636 ± 8.437/10 | 0.673 |
| BMI | 26.285 ± 3.503/26.15 | 26.709 ± 3.013/26.2 | 25.861 ± 3.934/25.3 | 0.329 |
| Cadaveric donor | 52 (78.8%) | 28 (84.84%) | 24 (72.72%) | 0.366 |
| Documented acute rejection | 16 (24.2%) | 8 (12.1%) | 8 (12.1%) | 0.773 |
| Corticotherapy | 60 (90.9%) | 33 (100%) | 27 (81.81%) | 0.024 |
| TAC mean dose | 3.97 ± 1.637/4 | 4.227 ± 1.663/4 | 3.727 ± 1.596/4 | 0.217 |
| Creatinine | 1.468 ± 0.686/1.25 | 1.515 ± 0.807/1.3 | 1.421 ± 0.549/1.2 | 0.579 |
| eGFR (mL/min) | 61.98 ± 28.045/59 | 61.697 ± 30.424/61 | 62.273 ± 25.93/57 | 0.934 |
| Glucose | 96.318 ± 20.795/90 | 95.303 ± 18.437/90 | 97.333 ± 23.159/87 | 0.695 |
| Hb A1c | 5.668 ± 0.720/5.45 | 5.7 ± 0.663/5.9 | 5.573 ± 0.744/5.2 | 0.723 |
| NODAT | 27 (40.9%) | 13 (39.39%) | 14 (42.42%) | 1 |
| hs-CRP | 4.814 ± 4.192/3.5 | 4.773 ± 4.137/3.5 | 4.855 ± 4.31/3.5 | 0.938 |
| AST | 29.698 ± 39.53/23 | 24.406 ± 8.257/23 | 35.161 ± 55.67/23 | 0.620 |
| T cholesterol | 188.11 ± 46.998/191.5 | 179.077 ± 38.428/180 | 196.5 ± 53.075/209 | 0.176 |
| Triglycerides | 156.354 ± 50.059/160 | 150.606 ± 54.006/143 | 162.281 ± 45.73/170 | 0.351 |
| Calcium | 8.808 ± 1.176/8.9 | 8.887 ± 0.802/8.9 | 8.718 ± 1.506/8.95 | 0.582 |
| Magnesium | 1.697 ± 0.158/1.7 | 1.686 ±0.162/1.7 | 1.708 ± 0.156/1.7 | 0.569 |
| Uric acid | 6.395 ± 1.277/6.7 | 6.512 ± 1.002/7 | 6.267 ± 1.532/6.65 | 0.451 |
| Proteinuria g/24 h | 0.656 ± 0.838/0.425 | 0.536 ± 0.765/0.32 | 0.775 ± 0.902/0.5 | 0.251 |
| Patients Characteristics M ± SD/Me | NODAT n = 27 | Non-NODAT n = 39 | OR (Univariable) | p |
|---|---|---|---|---|
| Age (years) | 49.111 ± 8.541/50 | 51.077 ± 11.155/51 | 1.00 (0.94–1.06, p = 0.881) | 0.583 |
| Gender (female) | 13 (48%) | 18 (46.15%) | 1.59 (0.51–5.01, p = 0.423) | 0.873 |
| BMI | 27.619 ± 3.459/27.3 | 25.362 ± 3.266/24.8 | 1.20 (1.01–1.45, p = 0.047) | 0.008 |
| Tx vintage | 10.519 ± 3.469/10 | 16.410 ± 7.687/16 | 0.97 (0.89–1.04, p = 0.414) | 0.070 |
| Cadaveric donor | 21 (77.77%) | 31 (79.48%) | 0.867 | |
| Documented acute rejection | 6 (22.22%) | 10 (25.64%) | 0.99 (0.26–3.48, p = 0.990) | 0.750 |
| Corticotherapy | 25 (92.59%) | 35 (89.74%) | 1.74 (0.21–36.67, p = 0.642) | 0.692 |
| TAC mean dose | 3.593 ± 1.494/3.5 | 4.244 ± 1.697/4 | 0.70 (0.46–1.01, p = 0.072) | 0.147 |
| ADV | 14 (51.85%) | 19 (48.71%) | 0.802 | |
| TAC | 13 (48.14%) | 20 (51.28%) | 0.802 | |
| Creatinine | 1.481 ± 0.636/1.3 | 1.459 ± 0.627/1.2 | 0.73 (0.27-.73, p = 0.498) | 0.615 |
| eGFR (mL/min) | 60.222 ± 26.56/57 | 63.205 ± 29.308/62 | 1.00 (0.98–1.02, p = 0.943) | 0.730 |
| Glucose | 113.296 ± 22.35/115 | 84 ± 7.155/84 | 1.12 (1.06–1.1, p < 0.001 | <0.001 |
| HbA1c | 6.326 ± 0.43/6.3 | 5.159 ± 0.381/5.1 | <0.001 | |
| hs-CRP | 5.867 ± 5.197/4 | 4.085 ± 3.20/3 | 1.13 (0.98–1.34, p = 0.111) | 0.112 |
| AST | 26.480 ± 9.55/24 | 24 ± 8.829/23 | 1.05 (0.98–1.14, p = 0.199 | 0.569 |
| T cholesterol | 201.631 ± 50.81/219 | 180.771 ± 43.804/190 | 1.01 (1.00–1.03, p = 0.103) | 0.118 |
| LDL cholesterol | 126.37 ± 49.65/115 | 114.49 ± 34.104/116 | 1.01 (1–1.03, p = 0.074) | 0.447 |
| Triglycerides | 184.111 ± 42.95/189 | 136.632 ± 45.563/130 | 1.03 (1.01–1.05, p = 0.001) | <0.001 |
| T proteins | 6.938 ± 0.404/6.9 | 6.663 ± 1.184/6.9 | 1.44 (0.73–4.84, p = 0.456) | 0.624 |
| Calcium | 8.7 ± 1.52/8.9 | 8.886 ± 0.868/8.9 | 0.87 (0.51–1.39, p = 0.539) | 0.976 |
| Magnesium | 1.618 ± 0.148/1.6 | 1.752 ± 0.143/1.74 | 0.01 (0.00–0.37, p = 0.030) | <0.001 |
| Uric acid | 6.319 ± 1.705/7 | 6.449 ± 0.886/6.7 | 0.82 (0.52–1.27, p = 0.380) | 0.594 |
| Proteinuria g/24 h | 0.883 ± 0.73/0.6 | 0.498 ± 0.876 | 1.66 (0.89–3.29, p = 0.115) | <0.001 |
| NODAT patients Characteristics M ± SD/Median | ER-TAC n = 14 | IR-TAC n = 13 | p |
|---|---|---|---|
| Age (years) | 51.786 ± 5.563/50 | 46.231 ± 10.35/47 | 0.103 |
| Gender (female) | 7 (50%) | 6 (46.15%) | 1 |
| BMI | 28.35 ± 3.6/28.45 | 26.831 ± 3.255/26.2 | 0.174 |
| Tx vintage | 8.429 ± 2.344/8 | 13.077 ± 3.095/13 | <0.001 |
| Cadaveric donor | 7 (50%) | 3 (23.07%) | 0.236 |
| Documented acute rejection | 3 (21.42%) | 3 (23.07%) | 1 |
| Corticotherapy | 12 (85.71%) | 13 (100%) | 0.481 |
| TAC mean dose | 3.143 ± 1.365/3 | 4.132 ± 1.770 | 0.213 |
| Creatinine | 1.512 ± 0.574/1.3 | 1.448 ± 0.718/1.3 | 0.697 |
| eGFR (mL/min) | 56 ± 24.019/55.5 | 64.769 ± 29.329/61 | 0.512 |
| Glucose | 116 ± 24.58/120.5 | 110.385 ± 20.259/110 | 0.436 |
| HbA1c | 6.457 ± 0.386/6.4 | 6.346 ± 0.35/6.3 | 0.338 |
| hs-CRP | 6,621 ± 5.535/4.8 | 5.054 ± 4.893/4 | 0.243 |
| AST | 29.308 ± 11.056/28 | 23.417 ± 7.948/20.5 | 0.157 |
| T cholesterol | 207.636 ± 61.74/210 | 193.375 ± 32.53/200 | 0.431 |
| Triglycerides | 196.429 ± 26.795/191 | 170.846 ± 53.412/167 | 0.174 |
| Calcium | 8.467 ± 2.016/8.95 | 8.915 ± 0.891/8.7 | 0.604 |
| Magnesium | 1.614 ± 0.143/1.6 | 1,622 ± 0.159/1.64 | 0.921 |
| Uric acid | 6.2 ± 2.146/7 | 6.438 ± 1.192/7 | 0.857 |
| Proteinuria g/24 h | 1.105 ± 0.695/0.85 | 0.643 ± 0.736/0.45 | 0.003 |
| Insulin | 5 (35.71%) | 1 | 0.164 |
| Oral antidiabetics | 4 | 5 | 0.694 |
| Diet | 4 | 7 | 0.251 |
| 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | Z | p | Odds Ratio | Lower | Upper |
| Age | 0.068 | 0.073 | 0.941 | 0.347 | 1.071 | 0.929 | 1.234 |
| Gender (female) | −0.407 | 1.336 | −0.305 | 0.760 | 0.665 | 0.048 | 9.132 |
| Deceased donor | −0.086 | 1.440 | −0.060 | 0.952 | 0.918 | 0.055 | 15.428 |
| Tx vintage | −0.291 | 0.142 | −2.048 | 0.041 | 0.748 | 0.566 | 0.988 |
| Acute rejection: | 2.566 | 1.850 | 1.387 | 0.165 | 13.009 | 0.347 | 488.337 |
| ER tacrolimus | −0.027 | 1.265 | −0.021 | 0.983 | 0.974 | 0.082 | 11.612 |
| Corticotherapy | 1.435 | 6.244 | 0.230 | 0.818 | 4.199 | 0.000 | 866,526.459 |
| eGFR | 0.014 | 0.027 | 0.524 | 0.601 | 1.014 | 0.963 | 1.068 |
| BMI | 0.205 | 0.232 | 0.884 | 0.377 | 1.228 | 0.779 | 1.935 |
| hs CRP | −0.145 | 0.178 | −0.815 | 0.415 | 0.865 | 0.611 | 1.225 |
| T cholesterol | −0.021 | 0.018 | −1.153 | 0.249 | 0.979 | 0.945 | 1.015 |
| Triglycerides | 0.052 | 0.021 | 2.449 | 0.014 | 1.053 | 1.010 | 1.098 |
| Magnesium | −7.275 | 6.301 | −1.155 | 0.248 | 0.001 | 0.000 | 159.894 |
| Uric acid | −0.934 | 0.600 | −1.558 | 0.119 | 0.393 | 0.121 | 1.273 |
| Proteinuria | 0.532 | 0.765 | 0.696 | 0.487 | 1.703 | 0.380 | 7.625 |
| Dependent: DM Mean (SD) | Non-NODAT | NODAT | OR (Univariable) | OR (Multivariable) |
|---|---|---|---|---|
| Age | 50.0 (11.2) | 49.6 (7.3) | 1.00 (0.94–1.06, p = 0.881) | 1.07 (0.94–1.26, p = 0.347) |
| Gender (female) | 14 (58.3) | 10 (41.7) | 1.59 (0.51–5.01, p = 0.423) | 0.67 (0.04–9.69, p = 0.760) |
| DD | 27 (64.3) | 15 (35.7) | 0.97 (0.25–4.21, p = 0.968) | 0.92 (0.05–21.96, p = 0.952) |
| Tx VINTAGE | 16.4 (8.1) | 10.6 (3.5) | 0.83 (0.71–0.94, p = 0.009) | 0.75 (0.54–0.95, p = 0.041) |
| Acute rejection | 9 (64.3) | 5 (35.7) | 0.99 (0.26–3.48, p = 0.990) | 13.01 (0.46–1297.03, p = 0.165) |
| ER tacrolimus | 16 (59.3) | 11 (40.7) | 1.55 (0.50–4.93, p = 0.450) | 0.97 (0.07–13.83, p = 0.983) |
| Corticotherapy | 31 (63.3) | 18 (36.7) | 1.74 (0.21–36.67, p = 0.642) | 4.20 (0.00–102,701.30, p = 0.818) |
| eGFR | 61.1 (29.5) | 61.6 (26.5) | 1.00 (0.98–1.02, p = 0.943) | 1.01 (0.96–1.08, p = 0.601) |
| BMI | 25.3 (3.4) | 27.4 (3.4) | 1.20 (1.01–1.45, p = 0.047) | 1.23 (0.75–1.96, p = 0.377) |
| CRP | 3.9 (3.2) | 5.9 (5.1) | 1.13 (0.98–1.34, p = 0.111) | 0.87 (0.55–1.22, p = 0.415) |
| T-chol | 179.3 (43.5) | 201.6 (50.8) | 1.01 (1.00–1.03, p = 0.103) | 0.98 (0.94–1.01, p = 0.249) |
| Triglyceride | 140.3 (43.9) | 191.5 (38.3) | 1.03 (1.01–1.05, p = 0.001) | 1.05 (1.02–1.12, p = 0.014) |
| Magnesium | 1.7 (0.1) | 1.6 (0.2) | 0.01 (0.00–0.37, p = 0.030) | 0.00 (0.00–14.01, p = 0.248) |
| Uric acid | 6.5 (0.9) | 6.1 (1.9) | 0.82 (0.52–1.27, p = 0.380) | 0.39 (0.08–1.05, p = 0.119) |
| Proteinuria | 0.6 (0.9) | 1.0 (0.8) | 1.66 (0.89–3.29, p = 0.115) | 1.70 (0.39–9.50, p = 0.487) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratiu, I.A.; Bănică, F.; Moisa, C.; Pașca, B.; Gîtea, D.; Grosu, I.D.; Bako, G.C.; Voștinaru, O.; Abu Dayyih, W.; Filip, L. New-Onset Diabetes After Transplantation in Renal Recipients: A Pilot Comparative Study of Immediate vs. Extended-Release Tacrolimus Formulation. Pharmaceuticals 2025, 18, 1532. https://doi.org/10.3390/ph18101532
Ratiu IA, Bănică F, Moisa C, Pașca B, Gîtea D, Grosu ID, Bako GC, Voștinaru O, Abu Dayyih W, Filip L. New-Onset Diabetes After Transplantation in Renal Recipients: A Pilot Comparative Study of Immediate vs. Extended-Release Tacrolimus Formulation. Pharmaceuticals. 2025; 18(10):1532. https://doi.org/10.3390/ph18101532
Chicago/Turabian StyleRatiu, Ioana Adela, Florin Bănică, Corina Moisa, Bianca Pașca, Daniela Gîtea, Iulia Dana Grosu, Gabriel Cristian Bako, Oliviu Voștinaru, Wael Abu Dayyih, and Lorena Filip. 2025. "New-Onset Diabetes After Transplantation in Renal Recipients: A Pilot Comparative Study of Immediate vs. Extended-Release Tacrolimus Formulation" Pharmaceuticals 18, no. 10: 1532. https://doi.org/10.3390/ph18101532
APA StyleRatiu, I. A., Bănică, F., Moisa, C., Pașca, B., Gîtea, D., Grosu, I. D., Bako, G. C., Voștinaru, O., Abu Dayyih, W., & Filip, L. (2025). New-Onset Diabetes After Transplantation in Renal Recipients: A Pilot Comparative Study of Immediate vs. Extended-Release Tacrolimus Formulation. Pharmaceuticals, 18(10), 1532. https://doi.org/10.3390/ph18101532








