Predictive Value of Baseline Left Ventricular Global Longitudinal Strain for Cardiac Dysfunction in Patients with Moderate to High Risk of Cancer Therapy-Related Cardiovascular Toxicity
Abstract
1. Introduction
The Research Question
2. Results
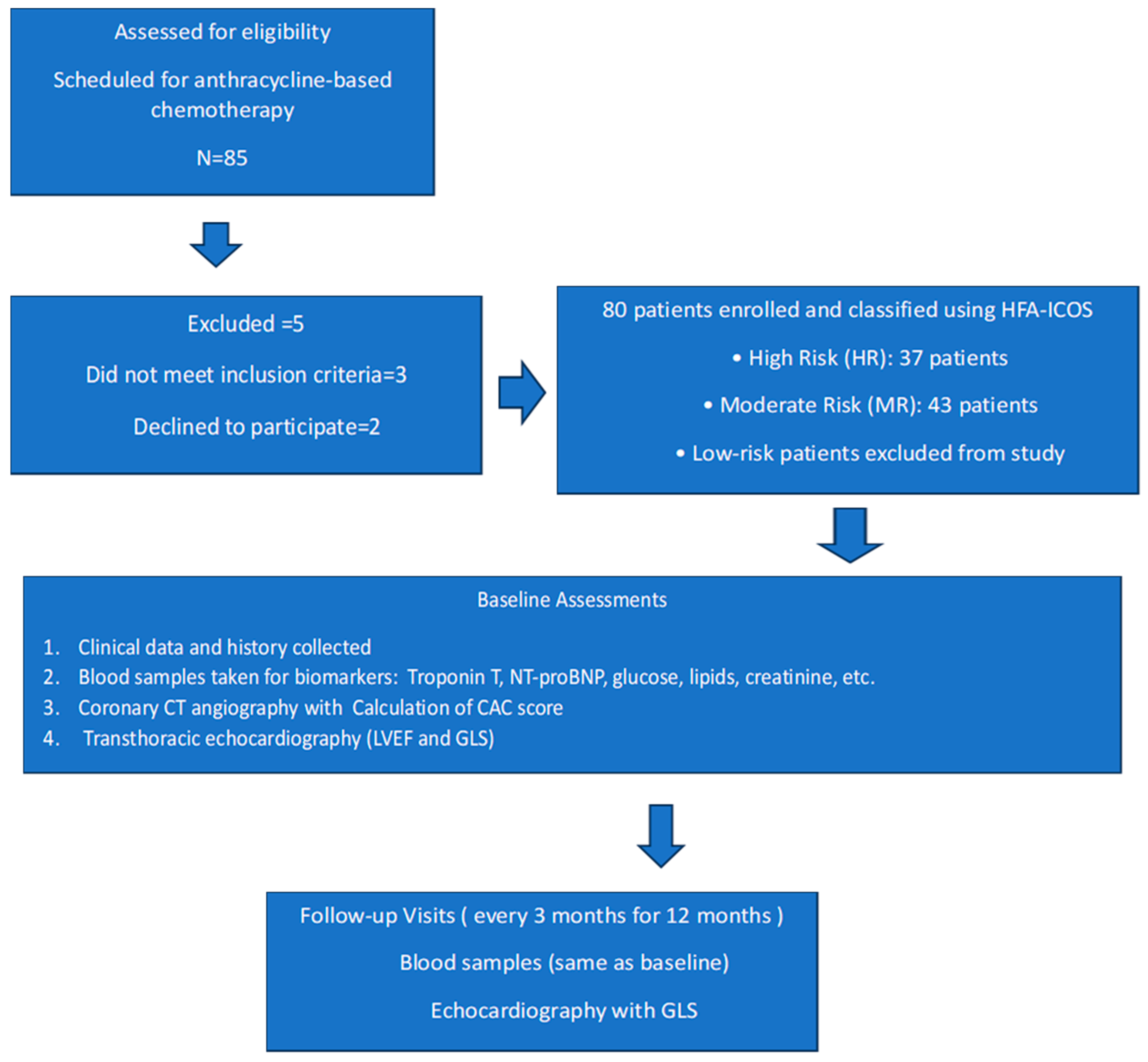
2.1. Baseline Characteristics of the Patients
2.2. Associations Between Global Longitudinal Strain of the Left Ventricle and Clinical Parameters
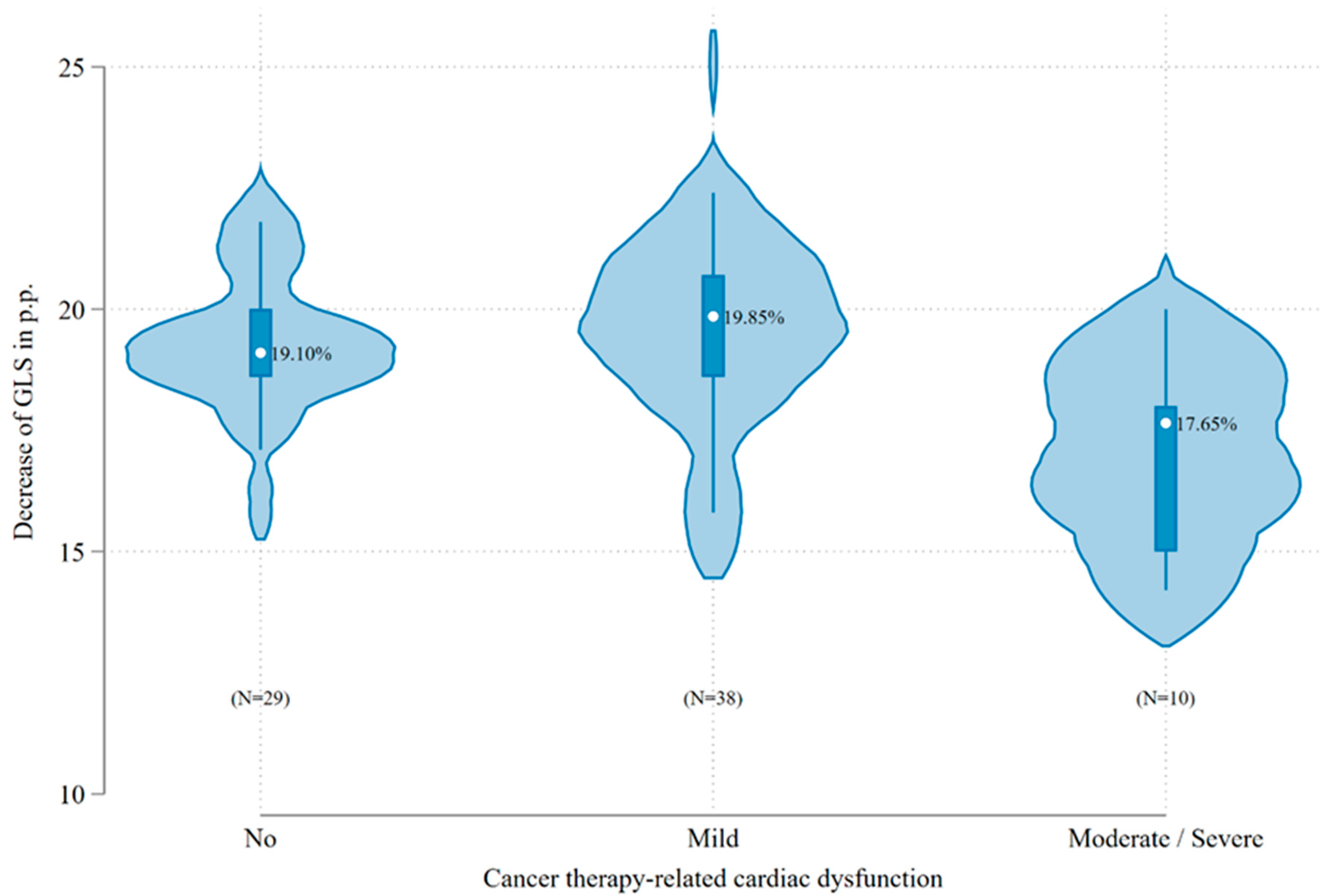
2.3. Incidence of CTRCD
2.4. Univariate and Multivariate Analysis
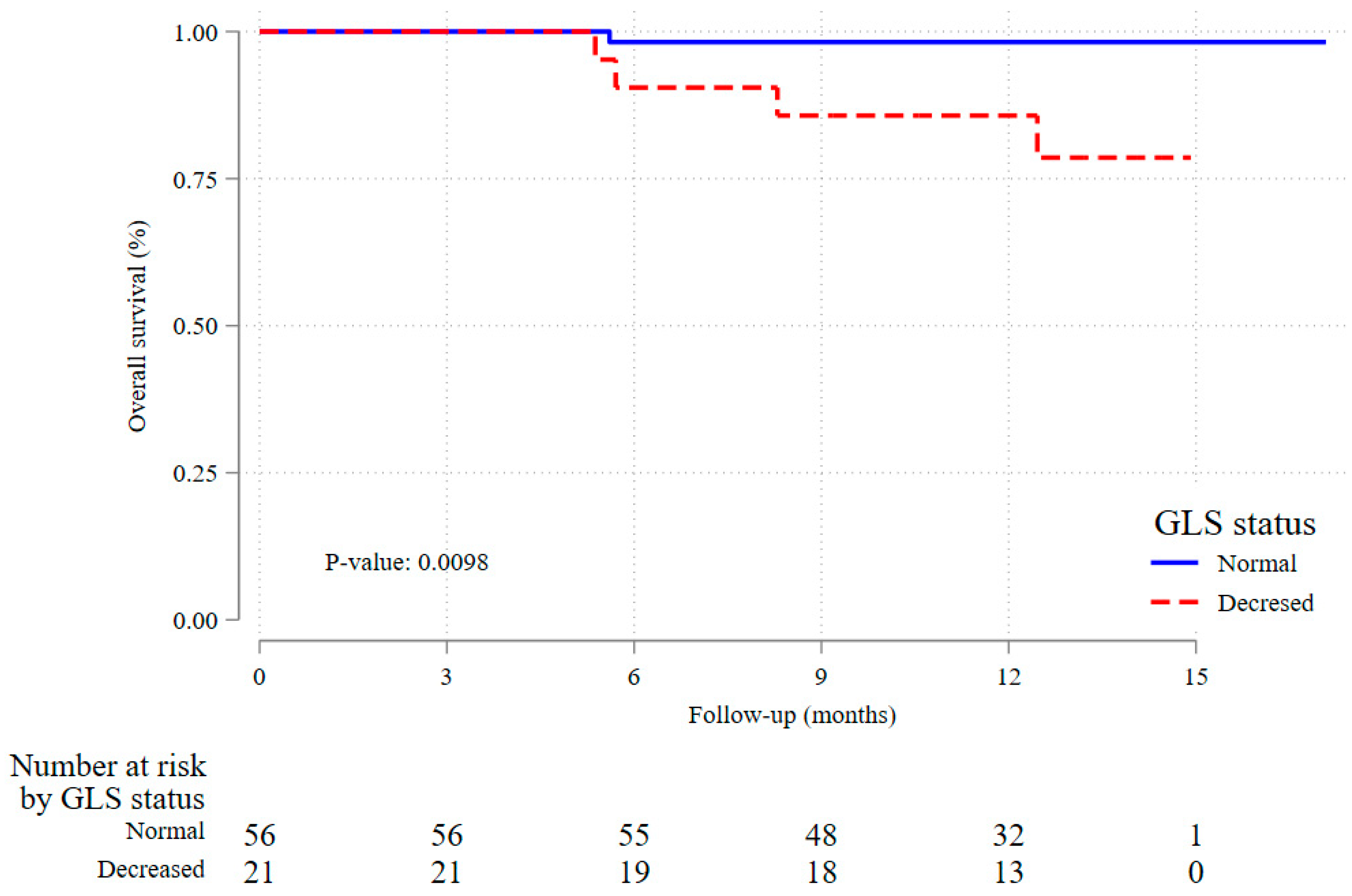
2.5. Secondary End Point of All-Cause Mortality
3. Discussion
3.1. Impact of Baseline Left Ventricular GLS on CTRCD
3.2. Impact of Decreased Baseline GLS on All-Cause Mortality
3.3. Limitations
4. Materials and Methods
4.1. Study Structure
4.2. Patient Classification and Echocardiographic Assessment
4.3. Primary Endpoint
4.4. Secondary Endpoint
4.5. Data Acquisition and Statistical Analysis
4.5.1. Data Acquisition and Quality Control
4.5.2. Sample Size Calculations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTRCD | cancer therapy-related cardiac dysfunction |
| CTR-CVT | cancer therapy-related cardiovascular toxicity |
| HFA-ICOS | Heart Failure Association-International Cardio-Oncology Society |
| GLS | left ventricular peak systolic global longitudinal strain |
| hs-cTnT | high-sensitivity cardiac troponin T |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| eCRF | electronic case report forms |
| HIS | Hospital Information System |
| LVEF | left ventricle ejection fraction |
| CVD | cardiovascular disease |
References
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Byth, K.; Stuart, K.; Clarke, J.L.; Thomas, L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: A comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur. J. Cancer 2013, 49, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Gutierrez, R.; Chitturi, K.R.; Xu, J.; Wang, Y.; Kinder, E.; Senapati, A.; Chebrolu, L.B.; Kassi, M.; Trachtenberg, B.H. Baseline global longitudinal strain predictive of anthracycline-induced cardiotoxicity. Cardio-Oncology 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Assuncao, B.L.; Denduluri, S.; McCurdy, S.; Luger, S.; Lefebvre, B.; Carver, J.; Scherrer-Crosbie, M. Symptomatic Heart Failure in Acute Leukemia Patients Treated with Anthracyclines. JACC CardioOncol. 2019, 1, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, K.; Tanaka, H.; Nonaka, A.; Takada, H.; Soga, F.; Hatani, Y.; Matsuzoe, H.; Shimoura, H.; Ooka, J.; Sano, H.; et al. Baseline Global Longitudinal Strain as a Predictor of Left Ventricular Dysfunction and Hospitalization for Heart Failure of Patients with Malignant Lymphoma After Anthracycline Therapy. Circ. J. 2018, 82, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Charbonnel, C.; Convers-Domart, R.; Rigaudeau, S.; Taksin, A.L.; Baron, N.; Lambert, J.; Ghez, S.; Georges, J.-L.; Farhat, H.; Lambert, J.; et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Narayan, H.K.; French, B.; Khan, A.M.; Plappert, T.; Hyman, D.; Bajulaiye, A.; Domchek, S.; DeMichele, A.; Clark, A.; Matro, J.; et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics–Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2016, 9, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Dewar, E.; Nolan, M.; Shirazi, M.; Dias, P.; Wright, L.; Fitzgerald, B.; Kearney, L.; Srivastava, P.; Atherton, J.; et al. Strain surveillance during chemotherapy to improve cardiovascular outcomes: The SUCCOUR-MRI trial. Eur. Heart J. 2024, 45, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Goto, S.; Himeno, Y.; Katsumata, Y.; Hashimoto, M.; MacRae, C.A.; Deo, R.C. Artificial intelligence-enabled prediction of chemotherapy-induced cardiotoxicity from baseline electrocardiograms. Nat. Commun. 2024, 15, 2536. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, A.; Ozdowska, P.; Rosinska, M.; Jagiello-Gruszfeld, A.; Jasek, S.; Waniewska, J.; Kotowicz, B.; Kosela-Paterczyk, H.; Lampka, E.; Makowka, A.; et al. Prognostic value of coronary atherosclerosis and CAC score for the risk of chemotherapy-related cardiac dysfunction (CTRCD): The protocol of ANTEC study. PLoS ONE 2023, 18, e0288146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. 2020, 2, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.D.; Edvardsen, T.; Abraham, T.; Appadurai, V.; Badano, L.; Banchs, J.; Cho, G.Y.; Cosyns, B.; Delgado, V.; Donal, E.; et al. Clinical Applications of Strain Echocardiography: A Clinical Consensus Statement From the American Society of Echocardiography Developed in Collaboration with the European Association of Cardiovascular Imaging of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2025, S0894-7317(25)00395-5, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Factor | Normal GLS, n = 56 | Decreased GLS, n = 21 | Total, n = 77 | p-Values | |
|---|---|---|---|---|---|
| Age group (years) | <65 | 33 (58.9%) | 8 (38.1%) | 41 (53.2%) | 0.10 |
| ≥65 | 23 (41.1%) | 13 (61.9%) | 36 (46.8%) | ||
| Cancer | breast | 42 (75.0%) | 17 (81.0%) | 59 (76.6%) | 0.72 |
| lymphoma | 6 (10.7%) | 1 (4.8%) | 7 (9.1%) | ||
| sarcoma | 8 (14.3%) | 3 (14.3%) | 11 (14.3%) | ||
| Risk group | High | 24 (42.9%) | 13 (61.9%) | 37 (48.1%) | 0.14 |
| Moderate | 32 (57.1%) | 8 (38.1%) | 40 (51.9%) | ||
| BMI (kg/m2) | <30 | 40 (71.4%) | 14 (66.7%) | 54 (70.1%) | 0.68 |
| ≥30 | 16 (28.6%) | 7 (33.3%) | 23 (29.9%) | ||
| SBP (mmHg)-mean (sd) | 137.1 (14.0) | 140.4 (18.1) | 138.0 (15.2) | 0.36 | |
| DBP (mmHg)-mean (sd) | 83.6 (7.8) | 84.1 (10.5) | 83.7 (8.5) | 0.53 | |
| NYHA scale | 1 | 42 (75.0%) | 12 (57.1%) | 54 (70.1%) | 0.13 |
| 2 | 14 (25.0%) | 9 (42.9%) | 23 (29.9%) | ||
| ECOG score | 0 | 43 (76.8%) | 12 (57.1%) | 55 (71.4%) | 0.09 |
| 1 | 13 (23.2%) | 8 (38.1%) | 21 (27.3%) | ||
| 2 | 0 (0.0%) | 1 (4.8%) | 1 (1.3%) | ||
| Coexisting conditions | Hypertension | 41 (73.2%) | 19 (90.5%) | 60 (77.9%) | 0.10 |
| Hyperlipidemia | 41 (73.2%) | 17 (81.0%) | 58 (75.3%) | 0.48 | |
| Ever smoking | 26 (46.4%) | 11 (52.4%) | 37 (48.1%) | 0.64 | |
| Diabetes | 8 (14.3%) | 3 (14.3%) | 11 (14.3%) | 0.99 | |
| Chronic kidney disease | 8 (14.3%) | 4 (19.0%) | 12 (15.6%) | 0.61 | |
| Concomitant medications | Beta-blockers | 23 (41.1%) | 10 (47.6%) | 33 (42.9%) | 0.61 |
| ACE-I | 21 (37.5%) | 13 (61.9%) | 34 (44.2%) | 0.06 | |
| Statins | 15 (26.8%) | 8 (38.1%) | 23 (29.9%) | 0.33 | |
| Anthracycline dose (mg/m2) | <250 | 43 (76.8%) | 19 (90.5%) | 62 (80.5%) | 0.18 |
| ≥250 | 13 (23.2%) | 2 (9.5%) | 15 (19.5%) | ||
| Troponin T (ng/L) | ≤14 | 52 (92.9%) | 19 (90.5%) | 71 (92.2%) | 0.73 |
| >14 | 4 (7.1%) | 2 (9.5%) | 6 (7.8%) | ||
| NT-proBNP (pg/mL) | ≤125 | 39 (69.6%) | 9 (42.9%) | 48 (62.3%) | 0.031 |
| >125 | 17 (30.4%) | 12 (57.1%) | 29 (37.7%) | ||
| LVEF (%)-mean (sd) | 62.2 (2.9) | 60.0 (4.5) | 61.6 (3.5), 62 (50–69) | 0.07 |
| Factor | No/Mild CTRCD, n = 67 | Moderate/Severe CTRCD, n = 10 | Total, n = 77 | p-Values | |
|---|---|---|---|---|---|
| Age group (years) | <65 | 38 (56.7%) | 3 (30.0%) | 41 (53.2%) | 0.11 |
| ≥65 | 29 (43.3%) | 7 (70.0%) | 36 (46.8%) | ||
| Age (years), mean | 59.7 (12.5) | 65.2 (9.4) | 60.5 (12.2) | 0.18 | |
| Cancer | breast | 52 (77.6%) | 7 (70.0%) | 59 (76.6%) | 0.42 |
| lymphoma | 5 (7.5%) | 2 (20.0%) | 7 (9.1%) | ||
| sarcoma | 10 (14.9%) | 1 (10.0%) | 11 (14.3%) | ||
| Risk group | HR | 29 (43.3%) | 8 (80.0%) | 37 (48.1%) | 0.03 |
| MR | 38 (56.7%) | 2 (20.0%) | 40 (51.9%) | ||
| BMI (kg/m2) | <30 | 49 (73.1%) | 5 (50.0%) | 54 (70.1%) | 0.14 |
| ≥30 | 18 (26.9%) | 5 (50.0%) | 23 (29.9%) | ||
| BMI (kg/m2), mean | 27.6 (4.7) | 29.6 (8.0) | 27.9 (5.2) | 0.59 | |
| NYHA scale | 1 | 48 (71.6%) | 6 (60.0%) | 54 (70.1%) | 0.45 |
| 2 | 19 (28.4%) | 4 (40.0%) | 23 (29.9%) | ||
| ECOG score | 0 | 49 (73.1%) | 6 (60.0%) | 55 (71.4%) | 0.59 |
| 1 | 17 (25.4%) | 4 (40.0%) | 21 (27.3%) | ||
| 2 | 1 (1.5%) | 0 (0.0%) | 1 (1.3%) | ||
| Coexisting conditions: | |||||
| Hypertension | Yes | 51 (76.1%) | 9 (90.0%) | 60 (77.9%) | 0.32 |
| Hyperlipidemia | Yes | 50 (74.6%) | 8 (80.0%) | 58 (75.3%) | 0.71 |
| Ever smoking | Yes | 33 (49.3%) | 4 (40.0%) | 37 (48.1%) | 0.59 |
| Diabetes | Yes | 10 (14.9%) | 1 (10.0%) | 11 (14.3%) | 0.68 |
| Chronic kidney disease | Yes | 10 (14.9%) | 2 (20.0%) | 12 (15.6%) | 0.68 |
| Medications: | |||||
| Beta-blockers | Yes | 27 (40.3%) | 6 (60.0%) | 33 (42.9%) | 0.24 |
| ACE-I | Yes | 28 (41.8%) | 6 (60.0%) | 34 (44.2%) | 0.28 |
| Statins | Yes | 18 (26.9%) | 5 (50.0%) | 23 (29.9%) | 0.14 |
| Anthracycline dose (mg/m2) | <250 | 54 (80.6%) | 8 (80.0%) | 62 (80.5%) | 0.97 |
| ≥250 | 13 (19.4%) | 2 (20.0%) | 15 (19.5%) | ||
| Anthracycline dose (mg/m2), median [IQR] | 240.0 [240.0–240.0] | 240.0 [240.0–240.0] | 240.0 [240.0–240.0] | 0.80 | |
| Troponin T (ng/L) | ≤14 | 62 (92.5%) | 9 (90.0%) | 71 (92.2%) | 0.78 |
| >14 | 5 (7.5%) | 1 (10.0%) | 6 (7.8%) | ||
| Troponin T (ng/L), median [IQR] | 6.6 [5.2–10.0] | 8.9 [7.2–10.6] | 7 [5.4–10] | 0.06 | |
| NT-proBNP (pg/mL) | ≤125 | 44 (65.7%) | 4 (40.0%) | 48 (62.3%) | 0.12 |
| >125 | 23 (34.3%) | 6 (60.0%) | 29 (37.7%) | ||
| NT-proBNP (pg/mL), median [IQR] | 84.0 [36.4–156.0] | 196.5 [49.0–399.0] | 94 [38.5–180] | 0.10 | |
| GLS (%) | <−18% | 54 (80.6%) | 2 (20.0%) | 56 (72.7%) | <0.001 |
| ≥−18% | 13 (19.4%) | 8 (80.0%) | 21 (27.3%) | ||
| GLS (%), mean | 19.4 (1.9) | 17.1 (2.1) | 19.1 (2.0) | 0.002 | |
| LVEF (%), mean | 62.0 (3.0) | 59.0 (5.3) | 61.6 (3.5) | 0.11 |
| Factor | Univariate Risk Ratio for Moderate/Severe CTRCD, 95% CI | Univariable p-Value | Multivariable Risk Ratio for Moderate/Severe CTRCD, 95% CI | Multivariable p-Value | |
|---|---|---|---|---|---|
| Sex | Female | Ref. | Ref. | ||
| Male | 1.6 (0.25–10.2) | 0.62 | 0.22 (0.02–2.7) | 0.23 | |
| Age group | <65 | Ref. | Ref. | ||
| ≥65 | 2.7 (0.7–9.5) | 0.13 | 2.1 (0.31–14.8) | 0.44 | |
| Age (years) | per year increase | 1.05 (0.98–1.10) | 0.98 | - | |
| Risk group | HR | 4.3 (1.0–19.1) | 0.05 | 3.9 (0.4–35.8) | 0.23 |
| MR | Ref. | Ref. | |||
| BMI (kg/m2) | <30 | Ref. | Ref. | ||
| ≥30 | 2.3 (0.8–7.3) | 0.14 | 2.3 (0.7–7.7) | 0.20 | |
| Hypertension | No | Ref. | Ref. | ||
| Yes | 2.5 (0.3–18.7) | 0.36 | 0.3 (0.03–3.9) | 0.39 | |
| Hyperlipidemia | No | Ref. | Ref. | ||
| Yes | 1.3 (0.3–5.6) | 0.72 | 0.6 (0.09–3.7) | 0.57 | |
| Ever smoking | No | Ref. | Ref. | ||
| Yes | 0.7 (0.2–2.4) | 0.59 | 0.4 (0.08–2.3) | 0.33 | |
| Diabetes | No | Ref. | Ref. | ||
| Yes | 0.7 (0.1–4.8) | 0.69 | 1.8 (0.22–14.2) | 0.59 | |
| Chronic kidney disease | No | Ref. | Ref. | ||
| Yes | 1.4 (0.3–5.6) | 0.68 | 0.3 (0.03–2.4) | 0.24 | |
| Troponin T (ng/L) | ≤14 | Ref. | Ref. | ||
| >14 | 1.3 (0.2–8.7) | 0.78 | 0.6 (0.07–5.3) | 0.64 | |
| Troponin T (ng/L) | per unit increase on log scale | 2.8 (0.8–9.5) | 0.10 | - | |
| NT-proBNP (pg/mL) | ≤125 | Ref. | Ref. | ||
| >125 | 2.5 (0.8–8.1) | 0.13 | 0.5 (0.1–2.0) | 0.32 | |
| NT-proBNP (pg/mL) | per unit increase on log scale | 1.8 (1.0–3.3) | 0.047 | ||
| GLS (%) | <−18% | Ref. | Ref. | ||
| ≥−18% | 10.7 (2.5–46.2) | 0.002 | 12.0 (2.0–71.9) | 0.007 | |
| GLS (%) | per unit increase | 0.58 (0.49–0.68) | <0.001 | ||
| LVEF (%) | ≥55% | Ref. | Ref. | ||
| 50–54% | 7.8 (3.2–19.3) | <0.001 | 4.5 (0.7–28.5) | 0.11 | |
| LVEF (%) | per unit increase | 0.82 (0.77–0.87) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiec, A.; Ozdowska, P.; Rosinska, M.; Zebrowska, A.M.; Jasek, S.; Kotowicz, B.; Kosela-Paterczyk, H.; Lampka, E.; Nowecki, Z.; Walewski, J. Predictive Value of Baseline Left Ventricular Global Longitudinal Strain for Cardiac Dysfunction in Patients with Moderate to High Risk of Cancer Therapy-Related Cardiovascular Toxicity. Pharmaceuticals 2025, 18, 1530. https://doi.org/10.3390/ph18101530
Borowiec A, Ozdowska P, Rosinska M, Zebrowska AM, Jasek S, Kotowicz B, Kosela-Paterczyk H, Lampka E, Nowecki Z, Walewski J. Predictive Value of Baseline Left Ventricular Global Longitudinal Strain for Cardiac Dysfunction in Patients with Moderate to High Risk of Cancer Therapy-Related Cardiovascular Toxicity. Pharmaceuticals. 2025; 18(10):1530. https://doi.org/10.3390/ph18101530
Chicago/Turabian StyleBorowiec, Anna, Patrycja Ozdowska, Magdalena Rosinska, Agnieszka Maria Zebrowska, Slawomir Jasek, Beata Kotowicz, Hanna Kosela-Paterczyk, Elzbieta Lampka, Zbigniew Nowecki, and Jan Walewski. 2025. "Predictive Value of Baseline Left Ventricular Global Longitudinal Strain for Cardiac Dysfunction in Patients with Moderate to High Risk of Cancer Therapy-Related Cardiovascular Toxicity" Pharmaceuticals 18, no. 10: 1530. https://doi.org/10.3390/ph18101530
APA StyleBorowiec, A., Ozdowska, P., Rosinska, M., Zebrowska, A. M., Jasek, S., Kotowicz, B., Kosela-Paterczyk, H., Lampka, E., Nowecki, Z., & Walewski, J. (2025). Predictive Value of Baseline Left Ventricular Global Longitudinal Strain for Cardiac Dysfunction in Patients with Moderate to High Risk of Cancer Therapy-Related Cardiovascular Toxicity. Pharmaceuticals, 18(10), 1530. https://doi.org/10.3390/ph18101530






