Immunomodulatory Activities of Emerging Rare Ginsenosides F1, Rg5, Rk1, Rh1, and Rg2: From Molecular Mechanisms to Therapeutic Applications
Abstract
1. Introduction
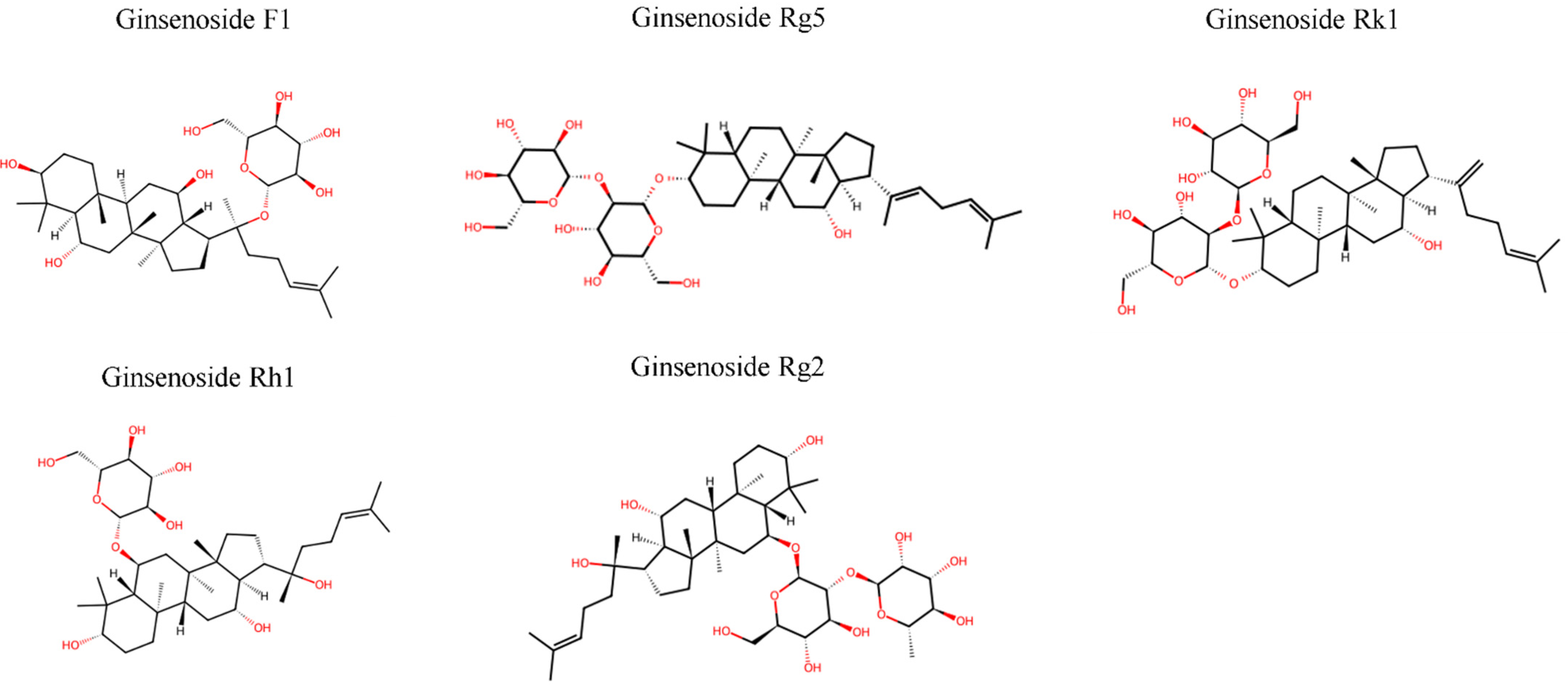
2. Chemical Structures and Pharmacological Properties
2.1. Structure, Biosynthesis, and Production
2.1.1. Classification and Basic Structures
2.1.2. Formation Pathways and Production Technologies
2.1.3. Production Technologies
2.1.4. Structure–Function Relationships
2.2. SAR for Immunomodulation
2.2.1. Sugar Position and Immune Activity
2.2.2. Anti-Inflammatory Mechanisms and Sugar Moiety Effects
2.2.3. Stereochemistry and Biological Activity
2.2.4. Molecular Weight and Membrane Permeability
2.2.5. Receptor Interactions and Signal Transduction
2.2.6. Structure-Based Design Implications
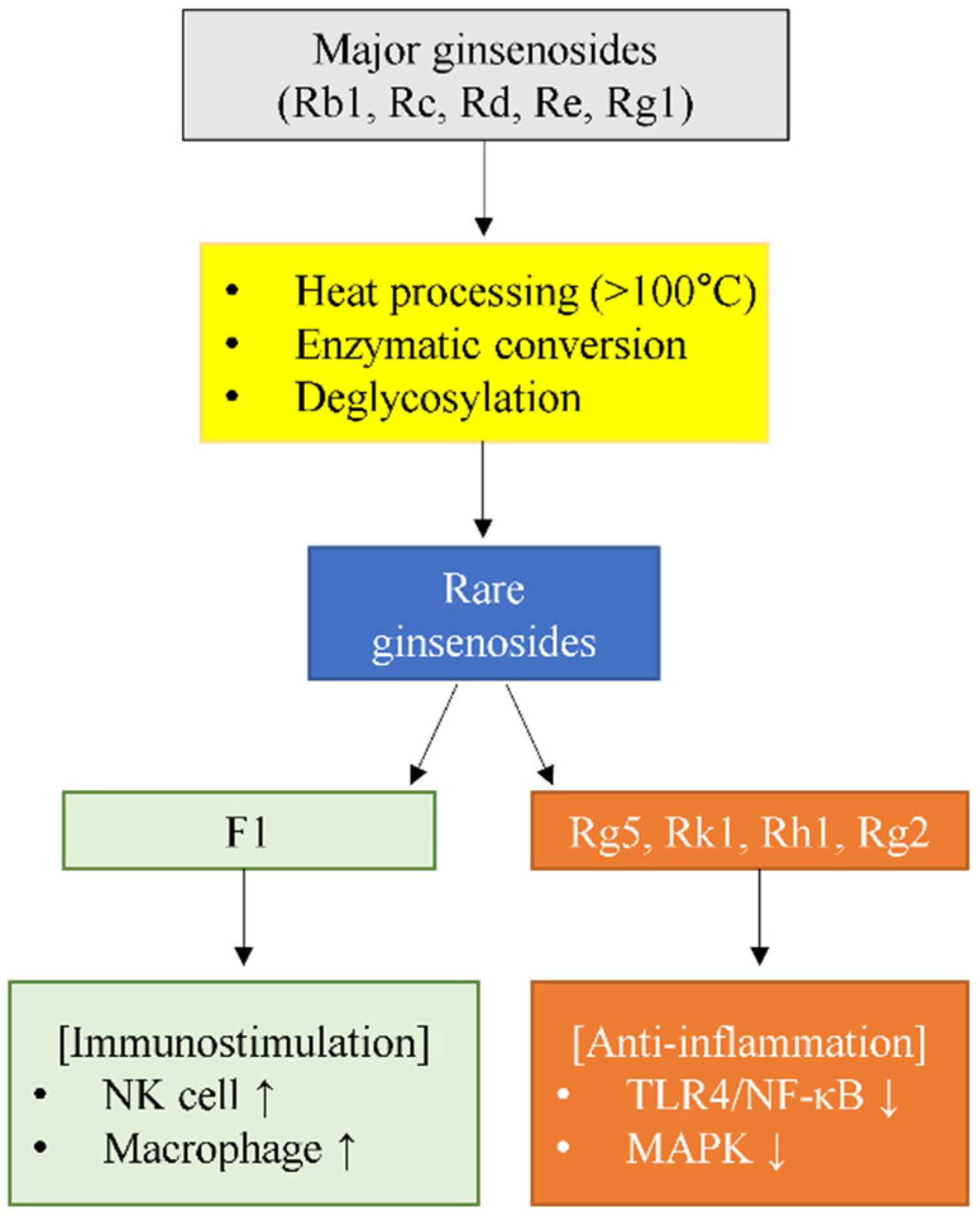
3. Individual Ginsenoside Profiles: Unique Immunomodulatory Mechanisms
3.1. Ginsenoside F1: The Exceptional Immune Enhancer
3.1.1. Unique Immunostimulatory Profile Among Rare Ginsenosides
3.1.2. NK Cell Activation Mechanisms
3.1.3. In Vivo Efficacy in Cancer and Inflammatory Models
3.1.4. Bioavailability Enhancement and Clinical Applications
3.2. Ginsenoside Rg5: Direct TLR4 Antagonist
3.2.1. TLR4-Mediated Anti-Inflammatory Mechanisms
3.2.2. HMGB1-Mediated Septic Response Inhibition
3.2.3. Synergistic Anti-Inflammatory Effects
3.2.4. Additional Activities and Clinical Translation
3.3. Ginsenoside Rk1: Multi-Pathway Modulator
3.3.1. Broadest Pathway Coverage Among Rare Ginsenosides
3.3.2. Triple Pathway Inhibition: NF-κB, p38 MAPK, and STAT Signaling
3.3.3. Additional Therapeutic Targets
3.3.4. Clinical Development and Combination Strategies
3.4. Ginsenoside Rh1: Anti-Allergic Specialist
3.4.1. Unique Anti-Allergic Profile Among Rare Ginsenosides
3.4.2. Mast Cell Stabilization and Histamine Release Inhibition
3.4.3. Modulation of Th2-Mediated Allergic Responses
3.4.4. Pharmaceutical Optimization and Safety Profile
3.5. Ginsenoside Rg2: Dual-Compartment Immunomodulator
3.5.1. Neuroprotective Mechanisms in Central Nervous System
3.5.2. Peripheral Organ Protection and Systemic Therapeutic Effects
3.5.3. Anti-Inflammatory and Immune Modulation
3.5.4. Clinical Translation Potential and Safety Profile
4. Comparative Analysis and SAR
4.1. Standardized Indirect Comparison
4.2. Mechanistic Pathway Mapping
4.3. Structural and Mechanistic Basis for F1’s Unique Immunostimulatory Activity
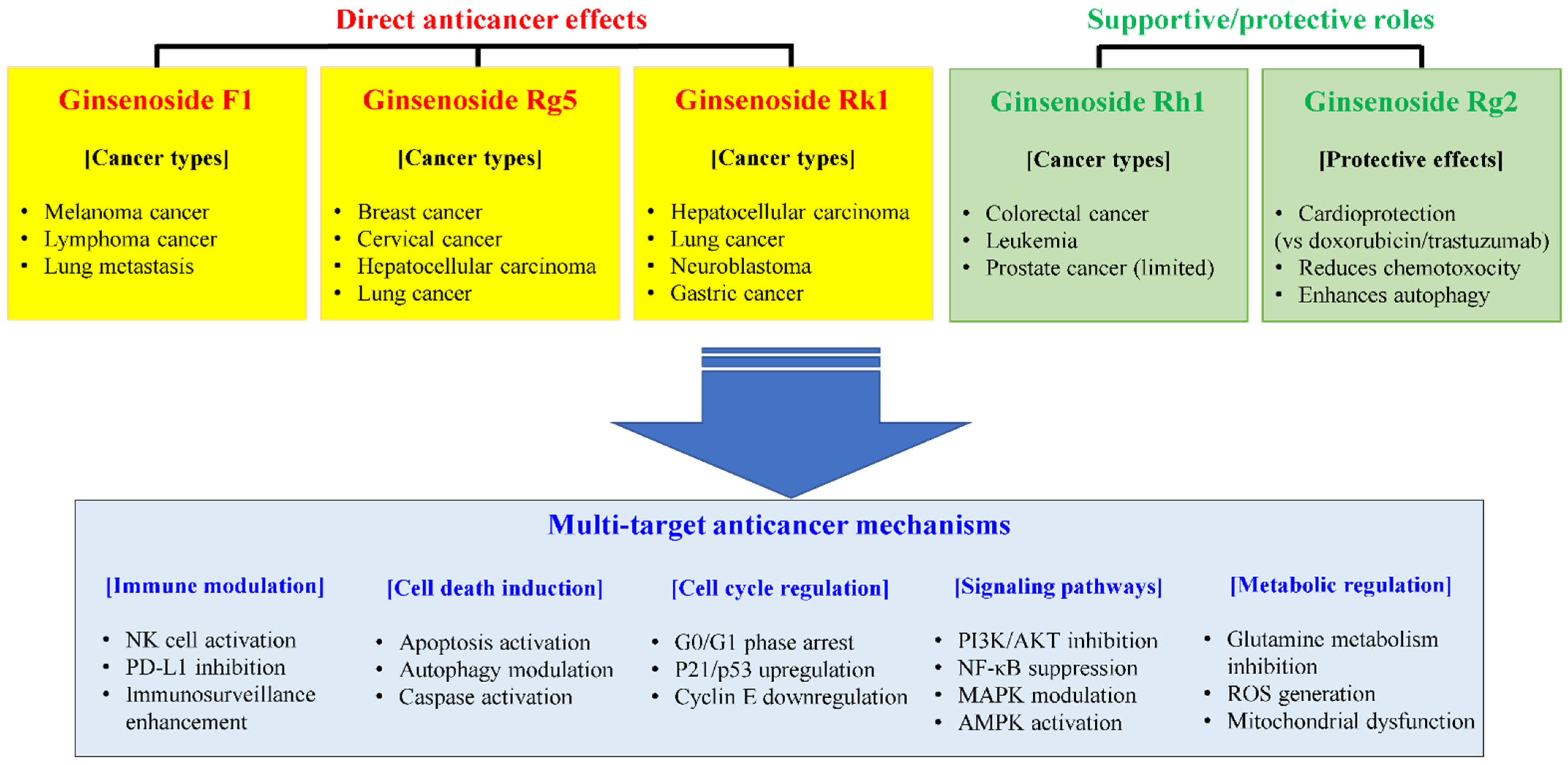
5. Cancer Immunotherapy Applications
5.1. F1: Multitarget Anticancer Mechanisms
5.2. Rk1’s Multifaceted Anticancer Mechanisms
5.3. Strategic Combination Approaches for Ginsenoside-Based Cancer Therapy
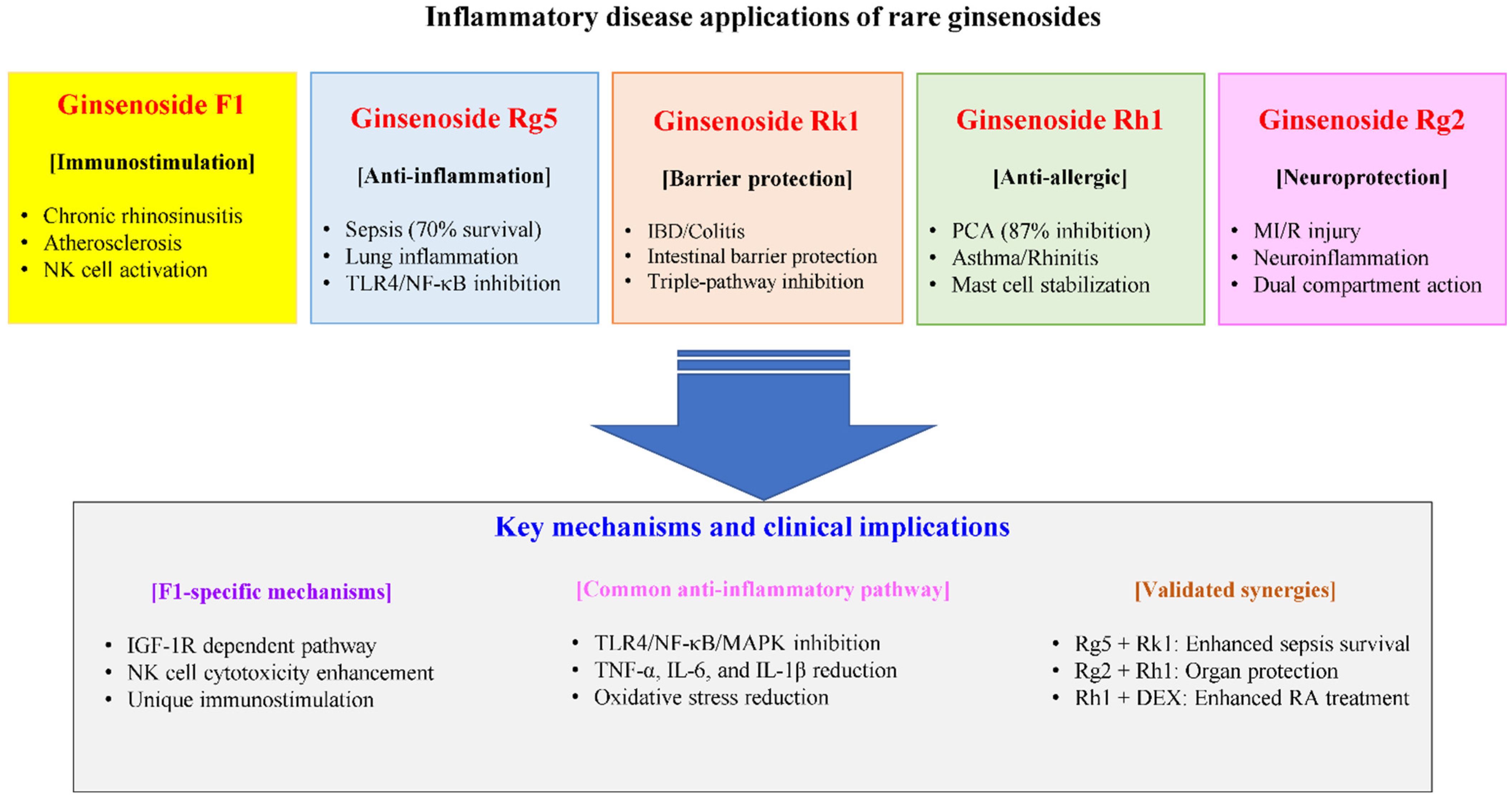
6. Applications in Inflammatory and Autoimmune Diseases
6.1. Allergic Diseases—Rh1 Dominance
6.1.1. Mechanisms of Anti-Allergic Action
6.1.2. Disease Model Efficacy
6.2. Inflammatory Disease—Rk1’s Intestinal Focus
6.2.1. Anti-Inflammatory Mechanisms
6.2.2. Barrier Function Restoration
6.3. Sepsis—Individual Mechanisms
6.4. Synergistic Effects and Combination Strategies
7. From Bench to Bedside: Translational Challenges and Opportunities
7.1. Current Translational Status
7.2. Pharmacokinetic Challenges and Solutions
7.3. Critical Assessment of Delivery Systems: Benefits, Limitations, and Enhancement Strategies
7.4. Pharmaceutical Applications and Safety Profiles
7.5. Future Opportunities and Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25-OCH3-PPD | 25-methoxy-protopanaxadiol |
| A20 | Tumor necrosis factor-α-induced protein 3 |
| AA | Arachidonic acid |
| AGS-H | Heat-processed American ginseng saponins |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator protein-1 |
| ApoE | Apolipoprotein E |
| APPswe | Amyloid precursor protein Swedish mutation |
| ARE | Antioxidant response element |
| ASC | Apoptosis-associated speck-like protein containing a caspase recruitment domain |
| AST | Aspartate aminotransferase |
| ATP | Adenosine triphosphate |
| Bax | Bcl-2-associated x protein |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| BMP-2 | Bone morphogenetic protein-2 |
| Brn-3a | Brain-specific homeobox/POU domain protein 3a |
| BUN | Blood urea nitrogen |
| C17SCV | C17 side-chain varied |
| cAMP | cyclic adenosine monophosphate |
| CD | Cluster of differentiation |
| c-Fos | c-Fos proto-oncogene |
| c-Jun | Cellular Jun proto-oncogen |
| c-Myc | Cellular myelocytomatosis oncogene |
| COL1A1 | Collagen type I alpha 1 |
| COVID-19 | Coronavirus disease 2019 |
| COX | Cyclooxygenase |
| cPLA2 | Cytosolic phospholipase A2 |
| CREB | cyclic adenosine monophosphate response element-binding protein |
| CYP3A4 | Cytochrome P450 3A4 |
| CYP450 | Cytochrome P450 |
| EC50 | Half maximal effective concentration |
| EGCG | Epigallocatechin-3-gallate |
| EMT | Epithelial to mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| Egr-1 | Early growth response 1 |
| ERK | Extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| Foxp3 | Forkhead box P3 |
| FUNDC1 | FUN14 domain containing 1 |
| GABA | Gamma-aminobutyric acid |
| G-CSF | Granulocyte colony-stimulating factor |
| GLS1 | Glutaminase 1 |
| Glu | Glutamate |
| GLUT1 | Glucose transporter 1 |
| GM1 | Monosialotetrahexosylganglioside 1 |
| GRAS | Generally recognized as safe |
| GSH | Glutathione |
| GTPase | Guanosine triphosphatase |
| GZMB | Granzyme B |
| HaCaT | Human keratinocyte |
| HDAC2 | Histone deacetylase 2 |
| HMGB1 | High mobility group box 1 |
| HO | Heme oxygenase |
| hP2X7 | Human P2X purinoceptor 7 |
| HSP70 | Heat shock protein 70 |
| IBD | Inflammatory bowel disease |
| IC50 | Half maximal inhibitory concentration |
| ICAM-1 | Intercellular adhesion molecule-1 |
| ICU | Intensive care unit |
| IDE | Insulin-degrading enzyme |
| IFN-γ | Interferon-gamma |
| Ig | Immunoglobulin |
| IGF-1 | Insulin-like growth factor-1 |
| IGF-1R | Insulin-like growth factor-1 receptor |
| IκBα | Inhibitor of nuclear factor kappa b alpha |
| IKKβ | Inhibitor of nuclear factor kappa b kinase subunit beta |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MD2 | Myeloid differentiation factor 2 |
| MDA | Malondialdehyde |
| MI/R | Myocardial ischemia/reperfusion |
| MIP-1δ | Macrophage inflammatory protein-1 delta |
| MMP | Matrix metalloproteinase |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRL/lpr | Murphy Roths Large/lymphoproliferation |
| mTOR | Mammalian target of rapamycin |
| MW | Molecular weight |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NEP | Neprilysin |
| NETosis | neutrophil extracellular traps formation |
| NeuN | Neuronal nuclei |
| NFATc1 | Nuclear factor of activated T-cells, cytoplasmic 1 |
| NF-κB | Nuclear factor-kappa B |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NGF | Nerve growth factor |
| NK | Natural killer |
| NKG2D | Natural killer group 2 member D |
| NLC | Nanostructured lipid carrier |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor protein 3 |
| NMDA | N-methyl-D-aspartate |
| NOX1 | Nicotinamide adenine dinucleotide phosphate oxidase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OA | Oleanolic acid |
| p-ACC | Phosphoacetyl-CoA carboxylase |
| PAM | Positive allosteric modulator |
| PCA | Passive cutaneous anaphylaxis |
| PD-L1 | Programmed death-ligand 1 |
| PEGylated | Polyethylene glycol-conjugated |
| PGE | Prostaglandin E |
| P-gp | P-glycoprotein |
| PI3K/AKT | Phosphatidylinositol 3-kinase/protein kinase B |
| PKC | Protein kinase C |
| PKCδ | Protein kinase C delta |
| PLA2 | Phospholipase A2 |
| PLCγ | Phospholipase Cγ |
| PLD | Phospholipase D |
| PPD | Protopanaxadiol |
| PPT | Protopanaxatriol |
| PSEFS | Peanut sprout extracts cultivated with fermented sawdust medium |
| PSEN1dE9 | presenilin 1 deletion of exon 9 |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RC50 | Half maximal response concentration |
| Rho | Ras homolog |
| RIP | Receptor-interacting protein |
| ROS | Reactive oxygen species |
| Runx2 | Runt-related transcription factor 2 |
| SAR | Structure–activity relationship |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SIRT1 | Sirtuin 1 |
| Smad | Smad family proteins |
| SMEDDS | Self-microemulsifying drug delivery systems |
| SOD | Superoxide dismutase |
| STAT | Signal transducer and activator of transcription |
| Syk | Spleen tyrosine kinase |
| TAK1 | Transforming growth factor-beta-activated kinase 1 |
| t-BHP | Tertiary-butyl hydroperoxide |
| TGF-β1 | Transforming growth factor-β1 |
| Th | T helper |
| TLR4 | Toll-like receptor 4 |
| TME | Tumor microenvironment |
| TNBS | Trinitrobenzenesulfonic acid |
| TNF-α | Tumor necrosis factor alpha |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| UVB | Ultraviolet B |
| VEGF | Vascular endothelial growth factor |
| ZO-1 | Zonula occludens-1 |
| α-MSH | Alpha-melanocyte stimulating hormone |
| α-SMA | Alpha-smooth muscle actin |
| γδ T cells | Epidermal gamma delta T cells |
References
- Chang, Y.S.; Seo, E.K.; Gyllenhaal, C.; Block, K.I. Panax ginseng: A role in cancer therapy? Integr. Cancer Ther. 2003, 2, 13–33. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.S.; Park, H.W.; Ahn, N.G.; Cho, B.G.; Cho, Y.L.; Kwak, Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Kim, D.H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Carabin, I.G.; Burdock, G.A.; Chatzidakis, C. Safety Assessment of Panax ginseng. Int. J. Toxicol. 2000, 19, 293–301. [Google Scholar] [CrossRef]
- Ogawa-Ochiai, K.; Kawasaki, K. Panax ginseng for Frailty-Related Disorders: A Review. Front. Nutr. 2018, 5, 140. [Google Scholar] [CrossRef]
- Kim, S.; Kim, N.; Jeong, J.; Lee, S.; Kim, W.; Ko, S.-G.; Kim, B. Anti-Cancer Effect of Panax ginseng and Its Metabolites: From Traditional Medicine to Modern Drug Discovery. Processes 2021, 9, 1344. [Google Scholar] [CrossRef]
- Lee, I.S.; Kang, K.S.; Kim, S.Y. Panax ginseng Pharmacopuncture: Current Status of the Research and Future Challenges. Biomolecules 2019, 10, 33. [Google Scholar] [CrossRef]
- Xu, J.; Chu, Y.; Liao, B.; Xiao, S.; Yin, Q.; Bai, R.; Su, H.; Dong, L.; Li, X.; Qian, J.; et al. Panax ginseng genome examination for ginsenoside biosynthesis. Gigascience 2017, 6, gix093. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.A.; Son, J.S.; Awais, M.; Ko, J.H.; Yang, D.C.; Jung, S.K. β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering 2023, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.S.; Hong, Y.D.; Kim, Y.; Sung, N.Y.; Yang, S.; Lee, K.M.; Park, J.Y.; Park, J.S.; Rho, H.S.; Shin, S.S.; et al. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J. Ginseng Res. 2015, 39, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Yi, Y.S.; Kim, D.; Kim, M.H.; Park, J.G.; Kim, E.; Lee, S.Y.; Yoon, K.; Kim, J.H.; Park, J.; et al. Nuclear factor kappa-B- and activator protein-1-mediated immunostimulatory activity of compound K in monocytes and macrophages. J. Ginseng Res. 2017, 41, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Liu, H.; Guo, L.; Liu, Y.; Ma, Z. Panax ginseng abuse exhibits a pro-inflammatory effect by activating the NF-κB pathway. Food Sci. Nutr. 2023, 11, 2130–2140. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yang, T.J.; Kim, S.-U.; Park, S.U. Biochemical and molecular analysis of Ginsenoside biosynthesis in Panax ginseng during flower and berry development. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 27–34. [Google Scholar] [CrossRef]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in Saponin Diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Yu, Z.; Lv, G.; Huang, X.; Lin, H.; Ma, C.; Lin, Z.; Qu, P. Functional Mechanism of Ginsenoside Compound K on Tumor Growth and Metastasis. Integr. Cancer Ther. 2022, 21, 15347354221101203. [Google Scholar] [CrossRef]
- Morshed, M.N.; Akter, R.; Karim, M.R.; Iqbal, S.; Kang, S.C.; Yang, D.C. Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases. Curr. Issues Mol. Biol. 2024, 46, 2320–2342. [Google Scholar] [CrossRef]
- Chu, L.L.; Hanh, N.T.Y.; Quyen, M.L.; Nguyen, Q.H.; Lien, T.T.P.; Do, K.V. Compound K Production: Achievements and Perspectives. Life 2023, 13, 1565. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, M.Y.; Wu, Y.X.; Wang, Y.Z.; Li, F.T.; Han, M.X.; Dai, Y.L.; Yue, H. Biotransformation of Ginsenosides (Rb(1), Rb(2), Rb(3), Rc) in Human Intestinal Bacteria and Its Effect on Intestinal Flora. Chem. Biodivers. 2021, 18, e2100296. [Google Scholar] [CrossRef]
- Upadhyaya, J.; Kim, M.J.; Kim, Y.H.; Ko, S.R.; Park, H.W.; Kim, M.K. Enzymatic formation of compound-K from ginsenoside Rb1 by enzyme preparation from cultured mycelia of Armillaria mellea. J. Ginseng Res. 2016, 40, 105–112. [Google Scholar] [CrossRef]
- Kim, G.O.; Kim, N.; Song, G.Y.; Bae, J.S. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2022, 23, 10836. [Google Scholar] [CrossRef]
- Hu, J.N.; Xu, X.Y.; Jiang, S.; Liu, Y.; Liu, Z.; Wang, Y.P.; Gong, X.J.; Li, K.K.; Ren, S.; Li, W. Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells. Kaohsiung J. Med. Sci. 2020, 36, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, L.; Wang, L. A narrative review of the pharmacology of ginsenoside compound K. Ann. Transl. Med. 2022, 10, 234. [Google Scholar] [CrossRef]
- Yun, Y.J.; Park, B.H.; Hou, J.; Oh, J.P.; Han, J.H.; Kim, S.C. Ginsenoside F1 Protects the Brain against Amyloid Beta-Induced Toxicity by Regulating IDE and NEP. Life 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Lee, H.; Choi, G.E.; Kwon, S.J.; Song, A.Y.; Kim, S.J.; Choi, W.S.; Hwang, S.H.; Kim, S.C.; Kim, H.S. Ginsenoside F1 Promotes Cytotoxic Activity of NK Cells via Insulin-like Growth Factor-1-Dependent Mechanism. Front. Immunol. 2018, 9, 2785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Huang, M.; Chen, M.; Zhang, D.; Luo, L.; Ye, G.; Deng, L.; Peng, Y.; Wu, X.; et al. Ginsenoside F1 promotes angiogenesis by activating the IGF-1/IGF1R pathway. Pharmacol. Res. 2019, 144, 292–305. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.; Choi, W.S.; Kim, H.J.; Kim, M.Y.; Kim, S.C.; Kim, H.S. Ginsenoside F1 Attenuates Eosinophilic Inflammation in Chronic Rhinosinusitis by Promoting NK Cell Function. J. Ginseng Res. 2021, 45, 695–705. [Google Scholar] [CrossRef]
- Liu, L.; Lyu, J.; Yang, L.; Gao, Y.; Zhao, B. Using Pharmacokinetic-Pharmacodynamic Modeling to Study the Main Active Substances of the Anticancer Effect in Mice from Panax ginseng–Ophiopogon japonicus. Molecules 2024, 29, 334. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Yu, K.; Zhang, X.; Shi, Y. Effect of Ginsenoside Rh1 on Proliferation, Apoptosis, and Oxidative Stress in Vascular Endothelial Cells by Regulation of the Nuclear Erythroid 2-related Factor-2/Heme Oxygenase-1 Signaling Pathway. J. Cardiovasc. Pharmacol. 2022, 79, 335–341. [Google Scholar] [CrossRef]
- Liu, G.; Qi, X.; Li, X.; Sun, F. Ginsenoside Rg2 protects cardiomyocytes against trastuzumab-induced toxicity by inducing autophagy. Exp. Ther. Med. 2021, 21, 473. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Kim, J.H.; Kim, N.; Lee, J. Ginsenoside Rg2 inhibits osteoclastogenesis by downregulating the NFATc1, c-Fos, and MAPK pathways. BMB Rep. 2023, 56, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Gou, D.; Pei, X.; Wang, J.; Wang, Y.; Hu, C.; Song, C.; Cui, S.; Zhou, Y. Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride-induced arrhythmias without oral toxicity. J. Ginseng Res. 2020, 44, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Azike, C.G.; Charpentier, P.A.; Lui, E.M. Stimulation and suppression of innate immune function by American ginseng polysaccharides: Biological relevance and identification of bioactives. Pharm. Res. 2015, 32, 876–897. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, Y.S.; Jeong, T.C.; Joe, C.O.; Shin, H.J.; Lee, Y.H.; Nam, K.Y.; Park, J.D. Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta Med. 2001, 67, 122–126. [Google Scholar] [CrossRef]
- Kim, J.S. Investigation of Phenolic, Flavonoid, and Vitamin Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Townsend, A.R. Ginsenoside Rg3: Potential Molecular Targets and Therapeutic Indication in Metastatic Breast Cancer. Mededicines 2019, 6, 17. [Google Scholar] [CrossRef]
- Oh, J.; Kim, J.S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016, 7, 4506–4515. [Google Scholar] [CrossRef]
- Yu, H.; Gong, J.; Zhang, C.; Jin, F. Purification and characterization of ginsenoside-alpha-L-rhamnosidase. Chem. Pharm. Bull. 2002, 50, 175–178. [Google Scholar] [CrossRef]
- Jin, S.; Jeon, J.H.; Lee, S.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Choi, M.K.; Song, I.S. Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract. Molecules 2019, 24, 2618. [Google Scholar] [CrossRef]
- Li, L.; Shin, S.Y.; Lee, S.J.; Moon, J.S.; Im, W.T.; Han, N.S. Production of Ginsenoside F2 by Using Lactococcus lactis with Enhanced Expression of β-Glucosidase Gene from Paenibacillus mucilaginosus. J. Agric. Food Chem. 2016, 64, 2506–2512. [Google Scholar] [CrossRef]
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.E.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Med. 2018, 84, 139–152. [Google Scholar] [CrossRef]
- Huynh, D.T.N.; Baek, N.; Sim, S.; Myung, C.S.; Heo, K.S. Minor Ginsenoside Rg2 and Rh1 Attenuates LPS-Induced Acute Liver and Kidney Damages via Downregulating Activation of TLR4-STAT1 and Inflammatory Cytokine Production in Macrophages. Int. J. Mol. Sci. 2020, 21, 6656. [Google Scholar] [CrossRef]
- Bae, E.A.; Shin, J.E.; Kim, D.H. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol. Pharm. Bull. 2005, 28, 1903–1908. [Google Scholar] [CrossRef]
- Wang, Y.; Choi, K.D.; Yu, H.; Jin, F.; Im, W.T. Production of ginsenoside F1 using commercial enzyme Cellulase KN. J. Ginseng Res. 2016, 40, 121–126. [Google Scholar] [CrossRef]
- Jin, Y.; Jung, S.Y.; Kim, Y.J.; Lee, D.Y.; Min, J.W.; Wang, C.; Yang, D.C. Microbial ketonization of ginsenosides F1 and C-K by Lactobacillus brevis. Antonie Van. Leeuwenhoek 2014, 106, 1215–1221. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, N.Y.; Han, S.B.; Kim, J.M.; Kwon, S.W.; Kim, H.J.; Park, M.K.; Park, J.H. Three new dammarane glycosides from heat processed ginseng. Arch. Pharm. Res. 2002, 25, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.R.; Lee, S.H.; Jang, G.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 2014, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.N.; Lin, H.S.; Koh, H.L. Quantification of ginsenosides Rh4 and Rk3 in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pre-clinical pharmacokinetic study. J. Mass. Spectrom. 2012, 47, 1510–1517. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Liu, C.; Yang, J.; Xu, L.; Li, G.; Song, J.; Jin, F. Conversion of Ginsenoside Rb1 into Six Types of Highly Bioactive Ginsenoside Rg3 and Its Derivatives by FeCl3 Catalysis. Chem. Pharm. Bull. 2018, 66, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Song, B.K.; Kim, K.M.; Choi, K.D.; Im, W.T. Production of the Rare Ginsenoside Rh2-MIX (20(S)-Rh2, 20(R)-Rh2, Rk2, and Rh3) by Enzymatic Conversion Combined with Acid Treatment and Evaluation of Its Anti-Cancer Activity. J. Microbiol. Biotechnol. 2017, 27, 1233–1241. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhou, R.X.; Sun, C.K.; Jin, Y.H.; Yu, H.S.; Zhang, T.Y.; Xu, L.Q.; Jin, F.X. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g.848. J. Ginseng Res. 2015, 39, 221–229. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Cui, C.H.; Park, S.K.; Han, N.S.; Kim, S.C.; Im, W.T. Comparative analysis of the expression level of recombinant ginsenoside-transforming β-glucosidase in GRAS hosts and mass production of the ginsenoside Rh2-Mix. PLoS ONE 2017, 12, e0176098. [Google Scholar] [CrossRef]
- Lin, S.K.; Zhou, J.; Lu, Y.; Guo, L.; Huang, J.J.; Lin, J.F. Computer-Guided Engineered Endo- and Exocleaving Glycosidase for Significantly Improving Production of Ginsenoside F1. J. Agric. Food Chem. 2024, 72, 26294–26304. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Kim, T.H.; Choi, J.H.; Oh, D.K. Complete Biotransformation of Protopanaxadiol-Type Ginsenosides to 20-O-β-Glucopyranosyl-20(S)-protopanaxadiol Using a Novel and Thermostable β-Glucosidase. J. Agric. Food Chem. 2018, 66, 2822–2829. [Google Scholar] [CrossRef]
- Wang, P.; Tang, C.; Liu, Y.; Yang, J.; Fan, D. Biotransformation of High Concentrations of Ginsenoside Substrate into Compound K by β-glycosidase from Sulfolobus solfataricus. Genes 2023, 14, 897. [Google Scholar] [CrossRef]
- Li, R.; Wu, Z.; Liu, X.; Chen, H.; Li, X.; Fan, D.; Wu, Z. Increasing Multienzyme Cascade Efficiency and Stability of MOF via Partitioning Immobilization. ACS Appl. Mater. Interfaces 2024, 16, 33235–33245. [Google Scholar] [CrossRef]
- Yue, J.; Li, Z.; Liu, X.; Wu, Z.; Wang, J.; Tu, M.; Shi, H.; Fan, D.; Li, Y. Green and Fast Synthesis of NiCo-MOF for Simultaneous Purification-Immobilization of Bienzyme to Catalyze the Synthesis of Ginsenoside Rh2. ACS Appl. Mater. Interfaces 2024, 16, 61725–61738. [Google Scholar] [CrossRef]
- Cao, S.; Li, R.; Tian, F.; Liu, X.; Fan, D.; Wu, Z. Construction of a hollow MOF with high sedimentation performance and co-immobilization of multiple-enzymes for preparing rare ginsenoside CK. React. Chem. Eng. 2023, 8, 2804–2817. [Google Scholar] [CrossRef]
- Oh, H.B.; Lee, J.W.; Lee, D.E.; Na, S.C.; Jeong, D.E.; Hwang, D.I.; Kim, Y.S.; Park, C.B. Characteristics of Black Ginseng (Panax ginseng C.A. Mayer) Production Using Ginseng Stored at Low Temperature after Harvest. Metabolites 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, X.; Xu, Z.; Wang, Y.; Shen, X.; Zhang, L.; Zhou, Z.; Wang, P. Pathway elucidation of bioactive rhamnosylated ginsenosides in Panax ginseng and their de novo high-level production by engineered Saccharomyces cerevisiae. Commun. Biol. 2022, 5, 775. [Google Scholar] [CrossRef] [PubMed]
- Wonhwa, L.; Kim, J.-E.; Yang, S.; Lee, B.-S.; Cho, S.-H.; Lee, J.-H.; Choi, G.-E.; Park, E.K.; Song, G.-Y.; Bae, J.-S. Suppressive activity of RGX-365 on HMGB1-mediated septic responses. Braz. J. Pharm. Sci. 2022, 58, e19473. [Google Scholar] [CrossRef]
- Kim, E.O.; Cha, K.H.; Lee, E.H.; Kim, S.M.; Choi, S.W.; Pan, C.H.; Um, B.H. Bioavailability of ginsenosides from white and red ginsengs in the simulated digestion model. J. Agric. Food Chem. 2014, 62, 10055–10063. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Ko, E.-S.; Jeong, J.H.; Park, C.-O.; Seo, J.H.; Jang, Y.-S. Enzymatic bioconversion of ginseng powder increases the content of minor ginsenosides and potentiates immunostimulatory activity. J. Ginseng Res. 2022, 46, 304–314. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.S.; Jung, J.S.; Kim, D.H.; Kim, H.S. Anti-inflammatory effect of ginsenoside Rg5 in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Sci. 2013, 14, 9820–9833. [Google Scholar] [CrossRef]
- Cho, Y.L.; Hur, S.M.; Kim, J.Y.; Kim, J.H.; Lee, D.K.; Choe, J.; Won, M.H.; Ha, K.S.; Jeoung, D.; Han, S.; et al. Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J. Biol. Chem. 2015, 290, 467–477. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, W.; Yang, S.; Cho, S.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive effects of rare ginsenosides, Rk1 and Rg5, on HMGB1-mediated septic responses. Food Chem. Toxicol. 2019, 124, 45–53. [Google Scholar] [CrossRef]
- Xue, Q.; Yu, T.; Wang, Z.; Fu, X.; Li, X.; Zou, L.; Li, M.; Cho, J.Y.; Yang, Y. Protective effect and mechanism of ginsenoside Rg2 on atherosclerosis. J. Ginseng Res. 2023, 47, 237–245. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Wang, X.; Lin, Z.; Lin, H.; Lin, Z. Ginsenoside—A promising natural active ingredient with steroidal hormone activity. Food Funct. 2024, 15, 1825–1839. [Google Scholar] [CrossRef]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.Y.; Li, L.; Ma, H. Cellular stress response mechanisms as therapeutic targets of ginsenosides. Med. Res. Rev. 2018, 38, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, Y.; Zhu, D.; Ji, Y. Pharmacokinetic Comparison of 20(R)- and 20(S)-Ginsenoside Rh1 and 20(R)- and 20(S)-Ginsenoside Rg3 in Rat Plasma following Oral Administration of Radix Ginseng Rubra and Sheng-Mai-San Extracts. Evid. Based Complement. Altern. Med. 2017, 2017, 6451963. [Google Scholar] [CrossRef]
- Murugesan, M.; Mathiyalagan, R.; Boopathi, V.; Kong, B.M.; Choi, S.K.; Lee, C.S.; Yang, D.C.; Kang, S.C.; Thambi, T. Production of Minor Ginsenoside CK from Major Ginsenosides by Biotransformation and Its Advances in Targeted Delivery to Tumor Tissues Using Nanoformulations. Nanomaterials 2022, 12, 3427. [Google Scholar] [CrossRef]
- Ryu, J.S.; Lee, H.J.; Bae, S.H.; Kim, S.Y.; Park, Y.; Suh, H.J.; Jeong, Y.H. The bioavailability of red ginseng extract fermented by Phellinus linteus. J. Ginseng Res. 2013, 37, 108–116. [Google Scholar] [CrossRef]
- Han, M.; Sha, X.; Wu, Y.; Fang, X. Oral absorption of ginsenoside Rb1 using in vitro and in vivo models. Planta Med. 2006, 72, 398–404. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Chen, G.; Wu, Y.; Xie, L.; Han, X.; Zhang, G.; Tan, Z.; Ding, W.; Fan, H.; et al. Anti-Inflammatory Effects of Ginsenoside Rb3 in LPS-Induced Macrophages Through Direct Inhibition of TLR4 Signaling Pathway. Front. Pharmacol. 2022, 13, 714554. [Google Scholar] [CrossRef]
- Chung, H.; Bak, S.H.; Shin, E.; Park, T.; Kim, J.; Jeong, H.; Jung, H.; Yoon, S.R.; Noh, J.Y. Resveratrol from Peanut Sprout Extract Promotes NK Cell Activation and Antitumor Activity. Biomol. Ther. 2025, 33, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Liu, N.; Yao, M.; Zhang, Y.; Yao, Z.; Feng, Y.; Liu, J.; Zhou, G. A Review of Neuroprotective Effects and Mechanisms of Ginsenosides From Panax ginseng in Treating Ischemic Stroke. Front. Pharmacol. 2022, 13, 946752. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, E.; Zhou, Y.; Liao, Z.; Wang, D. Therapeutic potential of natural products in ischemic stroke: Targeting angiogenesis. Front. Pharmacol. 2025, 16, 1579172. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Choi, Y.; Cui, C.H.; Lee, D.; Kim, S.C.; Kim, H.M. Pro-angiogenic Ginsenosides F1 and Rh1 Inhibit Vascular Leakage by Modulating NR4A1. Sci. Rep. 2019, 9, 4502. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, E.J.; Lee, E.J.; Yeom, M.H.; Park, J.S.; Lee, K.W.; Kang, N.J. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp. Dermatol. 2015, 24, 150–152. [Google Scholar] [CrossRef]
- Han, J.; Oh, J.P.; Yoo, M.; Cui, C.H.; Jeon, B.M.; Kim, S.C.; Han, J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol. Brain 2019, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Luo, Y.; Lu, S.; Sun, J.; Yang, K.; Sun, G.; Sun, X. Ginsenoside F1 Ameliorates Endothelial Cell Inflammatory Injury and Prevents Atherosclerosis in Mice through A20-Mediated Suppression of NF-kB Signaling. Front. Pharmacol. 2017, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Feng, R.; Sun, Y.; Chu, S.; Chen, J.; Ma, C.; Fu, J.; Zhao, Z.; Huang, M.; Shou, J.; et al. Simultaneous quantification of ginsenoside Rg1 and its metabolites by HPLC-MS/MS: Rg1 excretion in rat bile, urine and feces. Acta Pharm. Sin. B 2016, 6, 593–599. [Google Scholar] [CrossRef]
- Han, J.; Lee, E.; Kim, E.; Yeom, M.H.; Kwon, O.; Yoon, T.H.; Lee, T.R.; Kim, K. Role of epidermal γδ T-cell-derived interleukin 13 in the skin-whitening effect of Ginsenoside F1. Exp. Dermatol. 2014, 23, 860–862. [Google Scholar] [CrossRef]
- Kang, T.; Suh, S.; Jo, H.E.; Choi, K.O. Physical, chemical, and biological characterization of ginsenoside F1 incorporated in nanostructured lipid carrier. J. Food Biochem. 2021, 45, e13860. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Chen, Y.; Zhao, X. Screening SIRT1 Activators from Medicinal Plants as Bioactive Compounds against Oxidative Damage in Mitochondrial Function. Oxid. Med. Cell Longev. 2016, 2016, 4206392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.; Liu, S.; Chen, C.; Li, Q.; Qin, M.; Ren, L. Ginsenoside F1 attenuates pirarubicin-induced cardiotoxicity by modulating Nrf2 and AKT/Bcl-2 signaling pathways. J. Ginseng Res. 2023, 47, 106–116. [Google Scholar] [CrossRef]
- Kim, T.W.; Joh, E.H.; Kim, B.; Kim, D.H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int. Immunopharmacol. 2012, 12, 110–116. [Google Scholar] [CrossRef]
- Hong, M.; Kim, S.W.; Han, S.H.; Kim, D.J.; Suk, K.T.; Kim, Y.S.; Kim, M.J.; Kim, M.Y.; Baik, S.K.; Ham, Y.L. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS ONE 2015, 10, e0117451. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Zhang, J.; Jimenez Perez, Z.E.; Kim, Y.J.; Yang, D.C. Ginsenoside Rg5:Rk1 attenuates TNF-α/IFN-γ-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. Vitr. Cell Dev. Biol. Anim. 2016, 52, 287–295. [Google Scholar] [CrossRef]
- Heo, H.; Kim, Y.; Cha, B.; Brito, S.; Kim, H.; Kim, H.; Fatombi, B.M.; Jung, S.Y.; Lee, S.M.; Lei, L.; et al. A systematic exploration of ginsenoside Rg5 reveals anti-inflammatory functions in airway mucosa cells. J. Ginseng Res. 2023, 47, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, F.; Wang, Z.; Yang, L.; Zhou, Y. Ginsenoside Rg5 inhibits cancer cell migration by inhibiting the nuclear factor-κB and erythropoietin-producing hepatocellular receptor A2 signaling pathways. Oncol. Lett. 2021, 21, 452. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Mora-Poblete, F. Saponins: Research Progress and Their Potential Role in the Post-COVID-19 Pandemic Era. Pharmaceutics 2023, 15, 348. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.S.; Li, W.; Kwon, E.B.; Chung, H.S.; Go, Y.; Choi, J.G. Ginsenoside Rg5, a potent agonist of Nrf2, inhibits HSV-1 infection-induced neuroinflammation by inhibiting oxidative stress and NF-κB activation. J. Ginseng Res. 2024, 48, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Liu, F.; Gao, Y.L.; Yin, J.N.; Yan, W.Q.; Liu, J.G.; Li, H.J. Pharmacological activities of ginsenoside Rg5 (Review). Exp. Ther. Med. 2021, 22, 840. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.N.; Yan, M.H.; Xing, J.J.; Liu, W.C.; Li, W. Caspase-Mediated Anti-Apoptotic Effect of Ginsenoside Rg5, a Main Rare Ginsenoside, on Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wan, H.; Shan, G.Y.; Cheng, J.Y.; Gao, Z.C.; Liu, Y.Y.; Shi, W.N.; Sun, Z.J.; Li, H.J. Ginsenoside Rg5 modulates the TLR4 and BCL-2 pathways by inhibiting NOX1, thereby alleviating inflammation, apoptosis and pyroptosis in hyperuricemia nephropathy. J. Ginseng Res. 2025, 49, 426–437. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, G.; Xie, X.; Liu, H.; Liao, J.; Shi, H.; Chen, M.; Lai, S.; Wang, Z.; Wu, X. Ginsenoside Rg5 allosterically interacts with P2RY(12) and ameliorates deep venous thrombosis by counteracting neutrophil NETosis and inflammatory response. Front. Immunol. 2022, 13, 918476. [Google Scholar] [CrossRef]
- Shao, J.; Zheng, X.; Qu, L.; Zhang, H.; Yuan, H.; Hui, J.; Mi, Y.; Ma, P.; Fan, D. Ginsenoside Rg5/Rk1 ameliorated sleep via regulating the GABAergic/serotoninergic signaling pathway in a rodent model. Food Funct. 2020, 11, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Yesmin Simu, S.; Ahn, S.; Castro-Aceituno, V.; Yang, D.C. Ginsenoside Rg5: Rk1 Exerts an Anti-obesity Effect on 3T3-L1 Cell Line by the Downregulation of PPARγ and CEBPα. Iran. J. Biotechnol. 2017, 15, 252–259. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.J.; Veerappan, K.; Yang, D.U.; Yang, D.C. Stimulative effect of ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother. Res. 2014, 28, 1447–1455. [Google Scholar] [CrossRef]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, H.; Zhu, C.; Deng, J.; Fan, D. Hypoglycemic Effect of Ginsenoside Rg5 Mediated Partly by Modulating Gut Microbiota Dysbiosis in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 5107–5117. [Google Scholar] [CrossRef]
- Xiao, N.; Yang, L.L.; Yang, Y.L.; Liu, L.W.; Li, J.; Liu, B.; Liu, K.; Qi, L.W.; Li, P. Ginsenoside Rg5 Inhibits Succinate-Associated Lipolysis in Adipose Tissue and Prevents Muscle Insulin Resistance. Front. Pharmacol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.E.; Jung, K.; Kang, K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef]
- Ha, Y.; Jo, H.S.; Kwon, T.W.; Jeon, S.H.; Moon, S.K.; Jung, J.H.; Kim, M.S.; Nah, S.Y.; Lee, J.K.; Cho, I.H. Korean black ginseng extract alleviates Alzheimer’s disease-related cognitive impairment by activating the Nrf2/HO-1 pathway and suppressing the p38 MAPK/NF-κB/STAT3 pathways and NLRP3 inflammasome via TLR2 and TLR4 modulation. J. Ginseng Res. 2025, 49, 294–305. [Google Scholar] [CrossRef]
- Elshafay, A.; Tinh, N.X.; Salman, S.; Shaheen, Y.S.; Othman, E.B.; Elhady, M.T.; Kansakar, A.R.; Tran, L.; Van, L.; Hirayama, K.; et al. Ginsenoside Rk1 bioactivity: A systematic review. PeerJ 2017, 5, e3993. [Google Scholar] [CrossRef]
- Kim, H.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Park, Y.T.; Choi, S.J.; Yoon, C.H.; Lim, W.C.; Ko, H.; et al. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J. Ginseng Res. 2021, 45, 134–148. [Google Scholar] [CrossRef]
- Shin, J.H.; Kwon, H.W.; Irfan, M.; Rhee, M.H.; Lee, D.H. Ginsenoside Rk1 suppresses platelet mediated thrombus formation by downregulation of granule release and α(IIb)β(3) activation. J. Ginseng Res. 2021, 45, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qu, L.; Wan, S.; Li, Y.; Fan, D. Ginsenoside Rk1 Prevents UVB Irradiation-Mediated Oxidative Stress, Inflammatory Response, and Collagen Degradation via the PI3K/AKT/NF-κB Pathway In Vitro and In Vivo. J. Agric. Food Chem. 2022, 70, 15804–15817. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yin, H.; Qu, L.; Ma, X.; Fu, R.; Fan, D. Ginsenoside Rk1 regulates glutamine metabolism in hepatocellular carcinoma through inhibition of the ERK/c-Myc pathway. Food Funct. 2022, 13, 3793–3811. [Google Scholar] [CrossRef]
- Maeng, Y.S.; Maharjan, S.; Kim, J.H.; Park, J.H.; Suk Yu, Y.; Kim, Y.M.; Kwon, Y.G. Rk1, a ginsenoside, is a new blocker of vascular leakage acting through actin structure remodeling. PLoS ONE 2013, 8, e68659. [Google Scholar] [CrossRef]
- Ju, H.K.; Lee, J.G.; Park, M.K.; Park, S.J.; Lee, C.H.; Park, J.H.; Kwon, S.W. Metabolomic investigation of the anti-platelet aggregation activity of ginsenoside Rk1 reveals attenuated 12-HETE production. J. Proteome Res. 2012, 11, 4939–4946. [Google Scholar] [CrossRef]
- Chen, C.; Lv, Q.; Li, Y.; Jin, Y.H. The Anti-Tumor Effect and Underlying Apoptotic Mechanism of Ginsenoside Rk1 and Rg5 in Human Liver Cancer Cells. Molecules 2021, 26, 3926. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, J.; Yan, K.; Guo, J. Ginsenoside Rk1 protects human melanocytes from H2O2-induced oxidative injury via regulation of the PI3K/AKT/Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 24, 821. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, D.; Ding, Y.; Wei, N. Ginsenoside Rk1 prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease via activating silence information regulator 3-mediated Nrf2/HO-1 signaling pathway. Hum. Exp. Toxicol. 2023, 42, 09603271231220610. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tang, Z.; Pu, X.; Wang, T.; Gao, F.; Li, C. A novel cabazitaxel liposomes modified with ginsenoside Rk1 for cancer targeted therapy. Acupunct. Herb. Med. 2024, 4, 113–121. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Fan, A.; Li, G.; Liu, Q. Pharmacokinetics and bioavailability study of ginsenoside Rk1 in rat by liquid chromatography/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4580. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, M.K.; Kim, Y.K.; Jung, E.Y.; Park, C.S.; Woo, M.J.; Lee, S.H.; Kim, J.S.; Suh, H.J. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J. Ethnopharmacol. 2011, 133, 710–717. [Google Scholar] [CrossRef]
- Ginosyan, A.; Prazyan, A.; Davinyan, A.; Areg, H. Effect of γ-Cyclodextrin on the Dissolution of Ginsenosides Rg5 and Rk1 from Red Ginseng Chewable Tablets. Glob. J. Pharmaceu Sci. 2022, 9, 555766. [Google Scholar] [CrossRef]
- Vu-Huynh, K.L.; Le, T.H.V.; Nguyen, H.T.; Kim, H.M.; Kang, K.S.; Park, J.H.; Nguyen, M.D. Increase in Protective Effect of Panax vietnamensis by Heat Processing on Cisplatin-Induced Kidney Cell Toxicity. Molecules 2019, 24, 4627. [Google Scholar] [CrossRef]
- Hu, J.N.; Xu, X.Y.; Li, W.; Wang, Y.M.; Liu, Y.; Wang, Z.; Wang, Y.P. Ginsenoside Rk1 ameliorates paracetamol-induced hepatotoxicity in mice through inhibition of inflammation, oxidative stress, nitrative stress and apoptosis. J. Ginseng Res. 2019, 43, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Yu, H.; Van Le, T.H.; Qin, X.; Dong, X.; Cao, G.; Meng, Z.; Wang, Z.; Sohail, A.; et al. An Efficient Method to Separate the Main Components from Black Ginseng of Panax quinquefolium L. by Recycling and Consecutive High-Speed Counter-Current Chromatography Coupled with Evaporative Light Scattering Detection. J. Sep. Sci. 2025, 48, e70105. [Google Scholar] [CrossRef]
- Park, E.K.; Choo, M.K.; Han, M.J.; Kim, D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jeong, Y.; Song, J.; Ji, G.E. Oral administration of ginsenoside Rh1 inhibits the development of atopic dermatitis-like skin lesions induced by oxazolone in hairless mice. Int. Immunopharmacol. 2011, 11, 511–518. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, J.J.; Eun, S.H.; Kim, D.H. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur. J. Pharmacol. 2015, 762, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Ro, J.Y.; Lee, C.H. 20(S)-Protopanaxatriol inhibits release of inflammatory mediators in immunoglobulin E-mediated mast cell activation. J. Ginseng Res. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Chai, J.; Yang, J.; Dai, L.; Yang, Y.; Zhang, Y.; Jin, Y.; Wang, C.; Yan, G. Ginsenoside Rh1 Alleviates Allergic Rhinitis by Mediating Mitochondrial Autophagy via Activation of the AMPK/ULK1/FUNDC1 Pathway. Food Sci. Nutr. 2025, 13, e70464. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, S.H.; Lee, K.S.; Yoon, H.K.; Yoo, Y.C.; Lee, J.; Choi, J.E.; Kim, P.H.; Park, S.R. Ginsenoside Rg1 and 20(S)-Rg3 Induce IgA Production by Mouse B Cells. Immune Netw. 2015, 15, 331–336. [Google Scholar] [CrossRef]
- Li, J.; Du, J.; Liu, D.; Cheng, B.; Fang, F.; Weng, L.; Wang, C.; Ling, C. Ginsenoside Rh1 potentiates dexamethasone’s anti-inflammatory effects for chronic inflammatory disease by reversing dexamethasone-induced resistance. Arthritis Res. Ther. 2014, 16, R106. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Cheng, S.; Wang, X.; Meng, X.; Li, L.; Du, J.; Liu, Q.; Guo, Y.; Meng, Y.; et al. Ginsenoside Rh1 Improves the Effect of Dexamethasone on Autoantibodies Production and Lymphoproliferation in MRL/lpr Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 727650. [Google Scholar] [CrossRef]
- Jung, J.S.; Lee, S.Y.; Kim, D.H.; Kim, H.S. Protopanaxatriol Ginsenoside Rh1 Upregulates Phase II Antioxidant Enzyme Gene Expression in Rat Primary Astrocytes: Involvement of MAP Kinases and Nrf2/ARE Signaling. Biomol. Ther. 2016, 24, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xue, J.; Lee, M.; Yu, J.; Sung, C. Long-term administration of ginsenoside Rh1 enhances learning and memory by promoting cell survival in the mouse hippocampus. Int. J. Mol. Med. 2014, 33, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Kim, D.H. Effects of Red and Fermented Ginseng and Ginsenosides on Allergic Disorders. Biomolecules 2020, 10, 634. [Google Scholar] [CrossRef]
- Bae, E.-A.; Trinh, H.-T.; Lee, Y.-C.; Kim, S.-W.; Kim, D.-H. Inhibitory Effect of Fermented Red Ginseng against Passive Cutaneous Anaphylaxis Reaction and Scratching behaviors in Mice. J. Ginseng Res. 2008, 32, 33–38. [Google Scholar] [CrossRef][Green Version]
- Park, K.-H.; Kim, Y.-S.; Jeong, J.-H. Inhibitory Effects of Ginseng Extracts on Histamine-release from Rat’s Mast Cell. Korean J. Plant Res. 2011, 24, 98–104. [Google Scholar] [CrossRef][Green Version]
- McLeod, J.J.; Baker, B.; Ryan, J.J. Mast cell production and response to IL-4 and IL-13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, C.; Wang, G.; Zhou, J.; Li, W.; Xie, L.; Shi, Z. Ginsenoside Rh1 attenuates ovalbumin-induced asthma by regulating Th1/Th2 cytokines balance. Biosci. Biotechnol. Biochem. 2021, 85, 1809–1817. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, J.; Hu, X.; Yu, S.K.; Liu, C.; Pan, R.; Chang, Q.; Liu, X.; Liao, Y. Preparation and evaluation of self-microemulsions for improved bioavailability of ginsenoside-Rh1 and Rh2. Drug Deliv. Transl. Res. 2017, 7, 731–737. [Google Scholar] [CrossRef]
- Choi, J.H.; Cho, S.H.; Yun, J.J.; Yu, Y.B.; Cho, C.W. Ethosomes and Transfersomes for Topical Delivery of Ginsenoside Rhl from Red Ginseng: Characterization and In Vitro Evaluation. J. Nanosci. Nanotechnol. 2015, 15, 5660–5662. [Google Scholar] [CrossRef]
- Bi, Y.; Li, Q.; Tao, W.; Tang, J.; You, G.; Yu, L. Ginsenoside Rg1 and ginsenoside Rh1 prevent liver injury induced by acetaminophen in mice. J. Food Biochem. 2021, 45, e13816. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Qian, L.; Zhang, S. Ginsenoside Rh1 Alleviates HK-2 Apoptosis by Inhibiting ROS and the JNK/p53 Pathways. Evid. Based Complement. Altern. Med. 2020, 2020, 3401067. [Google Scholar] [CrossRef]
- Gong, S.; Chen, H.; Fang, S.; Li, M.; Hu, J.; Li, Y.; Yu, B.; Kou, J.; Li, F. Ginsenoside Rh1 mitigates mitochondrial dysfunction induced by myocardial ischaemia through its novel role as a sirtuin 3 activator. Br. J. Pharmacol. 2025, 182, 3017–3035. [Google Scholar] [CrossRef]
- Lu, C.; Lv, J.; Dong, L.; Jiang, N.; Wang, Y.; Fan, B.; Wang, F. Neuroprotective Effect of Ginsenoside Rh1 on Scopolamine-Induced Cognitive Dysfunctions. Neuropsychiatry 2018, 8, 749–760. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, G.; Wang, S.; Li, M.; Zheng, Q.; Jia, X. Ginsenoside RH1 Improves Neurological Function By Inhibiting Oxidative Stress and Promoting the Expression of Neurotrophic Factors in Cerebral Ischemia-Reperfusion Rats. Asian J. Pharm. Res. Dev. 2024, 11, 7–12. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, F.; Zhang, X.; Li, Y.; Yin, W. Neuroprotection and mechanisms of ginsenosides in nervous system diseases: Progress and perspectives. IUBMB Life 2024, 76, 862–882. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, A.; Zhou, Y.; San, X.; Jin, T.; Jin, Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J. Ethnopharmacol. 2008, 115, 441–448. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, N.; Rocchi, A.; Zhang, W.; Vassar, R.; Zhou, Y.; He, C. Identification of natural products with neuronal and metabolic benefits through autophagy induction. Autophagy 2017, 13, 41–56. [Google Scholar] [CrossRef]
- Lu, Y.W.; Wang, Y.J.; Wang, Z.; Ren, S.; Gong, X.J.; Hu, J.N.; Zhang, J.T.; Li, W. Ginsenoside Rg2 alleviates astrocyte inflammation and ameliorates the permeability of the Alzheimer’s disease related blood-brain barrier. Phytomedicine 2024, 135, 156063. [Google Scholar] [CrossRef]
- Sala, F.; Mulet, J.; Choi, S.; Jung, S.Y.; Nah, S.Y.; Rhim, H.; Valor, L.M.; Criado, M.; Sala, S. Effects of ginsenoside Rg2 on human neuronal nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2002, 301, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, J.L.; Zhang, X.; Wang, H.; Ye, Y.; Song, L.; Wang, Y.J.; Tu, M.J.; Wang, W.W.; Yang, L.; et al. Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res. Bull. 2017, 134, 211–219. [Google Scholar] [CrossRef]
- Yin, P.; Wang, H.; Xue, T.; Yu, X.; Meng, X.; Mi, Q.; Song, S.; Xiong, B.; Bi, Y.; Yu, L. Four-Dimensional Label-Free Quantitative Proteomics of Ginsenoside Rg2 Ameliorated Scopolamine-Induced Memory Impairment in Mice through the Lysosomal Pathway. J. Agric. Food Chem. 2024, 72, 14640–14652. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Y.J.; Wei, K.; Li, W.L.; Liu, Y.B.; Hu, J.N.; Chang, W.G.; Zhang, W.X.; Chen, L.; Li, W. Ginsenoside Rg2 Alleviates HFD/STZ-Induced Diabetic Nephropathy by Inhibiting Pyroptosis via NF-κB/NLRP3 Signaling Pathways. Am. J. Chin. Med. 2025, 53, 909–930. [Google Scholar] [CrossRef]
- Li, Y.; Hao, H.; Yu, H.; Yu, L.; Ma, H.; Zhang, H. Ginsenoside Rg2 Ameliorates Myocardial Ischemia/Reperfusion Injury by Regulating TAK1 to Inhibit Necroptosis. Front. Cardiovasc. Med. 2022, 9, 824657. [Google Scholar] [CrossRef]
- He, Z.; Chen, S.; Pan, T.; Li, A.; Wang, K.; Lin, Z.; Liu, W.; Wang, Y.; Wang, Y. Ginsenoside Rg2 Ameliorating CDAHFD-Induced Hepatic Fibrosis by Regulating AKT/mTOR-Mediated Autophagy. J. Agric. Food Chem. 2022, 70, 1911–1922. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yi, E.S.; Kang, C.H.; Liu, Y.; Lee, Y.G.; Choi, H.S.; Jang, H.B.; Huo, Y.; Baek, N.I.; Yang, D.C.; et al. Whitening and inhibiting NF-κB-mediated inflammation properties of the biotransformed green ginseng berry of new cultivar K1, ginsenoside Rg2 enriched, on B16 and LPS-stimulated RAW 264.7 cells. J. Ginseng Res. 2021, 45, 631–641. [Google Scholar] [CrossRef]
- Fu, W.; Xu, H.; Yu, X.; Lyu, C.; Tian, Y.; Guo, M.; Sun, J.; Sui, D. 20(S)-Ginsenoside Rg2 attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation: Role of SIRT1. RSC Adv. 2018, 8, 23947–23962. [Google Scholar] [CrossRef]
- Qiu, B.; Mao, M.; Ma, Z.; Deng, B.; Shen, L.; Zhou, D.; Zheng, W.; Wei, Y. Ginsenoside Rg2 Attenuates Doxorubicin-induced Cardiomyocyte Apoptosis via the PI3K/Akt Pathway. Rev. Bras. Farmacogn. 2022, 32, 433–439. [Google Scholar] [CrossRef]
- Ma, L.Y.; Zhou, Q.L.; Yang, X.B.; Wang, H.P.; Yang, X.W. Metabolism of 20(S)-Ginsenoside Rg2 by Rat Liver Microsomes: Bioactivation to SIRT1-Activating Metabolites. Molecules 2016, 21, 757. [Google Scholar] [CrossRef]
- Park, S.A.; Hwang, D.; Kim, J.H.; Lee, S.Y.; Lee, J.; Kim, H.S.; Kim, K.A.; Lim, B.; Lee, J.E.; Jeon, Y.H.; et al. Formulation of lipid nanoparticles containing ginsenoside Rg2 and protopanaxadiol for highly efficient delivery of mRNA. Biomater. Sci. 2024, 12, 6299–6309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cecarini, V.; Cuccioloni, M.; Bonfili, L.; Gong, C.; Angeletti, M.; Eleuteri, A.M. Ginsenosides Rg1 and Rg2 Activate Autophagy and Attenuate Oxidative Stress in Neuroblastoma Cells Overexpressing Aβ(1-42). Antioxidants 2024, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-S.; Song, J.H.; Choi, P.; Lee, J.H.; Kim, S.-Y.; Shin, K.-S.; Ham, J.; Kang, K.S. Stimulation of Innate Immune Function by Panax ginseng after Heat Processing. J. Agric. Food Chem. 2018, 66, 4652–4659. [Google Scholar] [CrossRef]
- Li, C.; Zhao, B.; Xiong, J.; Li, L.; Pang, D.; Unger, K.; Jung, M.; Lyu, J.; Kuang, H.; Liang, L.; et al. Ginsenoside Rg5 alleviates radiation-induced acute lung vascular endothelium injury by reducing mitochondrial apoptosis via Sirt1. J. Ginseng Res. 2025, 49, 260–270. [Google Scholar] [CrossRef]
- Kotsifaki, A.; Maroulaki, S.; Karalexis, E.; Stathaki, M.; Armakolas, A. Decoding the Role of Insulin-like Growth Factor 1 and Its Isoforms in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 9302. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Hwang, Y.C.; Lee, E.J.; Choi, I. Therapeutic Applications of Ginseng Natural Compounds for Health Management. Int. J. Mol. Sci. 2023, 24, 17290. [Google Scholar] [CrossRef]
- Tung, N.H.; Quang, T.H.; Son, J.H.; Koo, J.E.; Hong, H.J.; Koh, Y.S.; Song, G.Y.; Kim, Y.H. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2011, 34, 681–685. [Google Scholar] [CrossRef]
- Junaid, M.; Butt, A.; Ali, Z.; Hussain, Y. Ginsenosides: An overview of its antiplatelet effects and its underlying mechanisms. PHYTONutrients 2023, 1, 19–47. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Maswikiti, E.P.; Yu, Y.; Song, K.; Ma, C.; Han, X.; Ma, H.; Deng, X.; Yu, R.; et al. AMPK-a key factor in crosstalk between tumor cell energy metabolism and immune microenvironment? Cell Death Discov. 2024, 10, 237. [Google Scholar] [CrossRef]
- Liu, J.; Shimizu, K.; Yu, H.; Zhang, C.; Jin, F.; Kondo, R. Stereospecificity of hydroxyl group at C-20 in antiproliferative action of ginsenoside Rh2 on prostate cancer cells. Fitoterapia 2010, 81, 902–905. [Google Scholar] [CrossRef]
- Darsandhari, S.; Shrestha, B.; Pandey, R.P.; Lee, S.; Jung, H.J.; Kim, Y.J.; Sohng, J.K. Enzymatically Synthesized Ginsenoside Exhibits Antiproliferative Activity in Various Cancer Cell Lines. Appl. Sci. 2019, 9, 893. [Google Scholar] [CrossRef]
- Piyasirananda, W.; Beekman, A.; Ganesan, A.; Bidula, S.; Stokes, L. Insights into the Structure-Activity Relationship of Glycosides as Positive Allosteric Modulators Acting on P2X7 Receptors. Mol. Pharmacol. 2021, 99, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.A.; Qin, J.J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R. Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Zaib, S.; Jannat, S.; Khan, I.; Rahman, M.M.; Park, S.K.; Chang, M.S. Inhibition of Aldose Reductase by Ginsenoside Derivatives via a Specific Structure Activity Relationship with Kinetics Mechanism and Molecular Docking Study. Molecules 2022, 27, 2134. [Google Scholar] [CrossRef] [PubMed]
- Rentería, M.E.; Gandhi, N.S.; Vinuesa, P.; Helmerhorst, E.; Mancera, R.L. A comparative structural bioinformatics analysis of the insulin receptor family ectodomain based on phylogenetic information. PLoS ONE 2008, 3, e3667. [Google Scholar] [CrossRef]
- Hsu, C.H.; Hung, S.C.; Wu, C.Y.; Wong, C.H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 11872–11923. [Google Scholar] [CrossRef]
- Yoo, D.-S.; Rho, H.-S.; Lee, Y.-G.; Yeom, M.-H.; Kim, D.-H.; Lee, S.-J.; Hong, S.-Y.; Lee, J.-H.; Cho, J.-Y. Ginsenoside F1 Modulates Cellular Responses of Skin Melanoma Cells. J. Ginseng Res. 2011, 35, 86–91. [Google Scholar] [CrossRef]
- Lee, E.H.; Cho, S.Y.; Kim, S.J.; Shin, E.S.; Chang, H.K.; Kim, D.H.; Yeom, M.H.; Woe, K.S.; Lee, J.; Sim, Y.C.; et al. Ginsenoside F1 protects human HaCaT keratinocytes from ultraviolet-B-induced apoptosis by maintaining constant levels of Bcl-2. J. Invest. Dermatol. 2003, 121, 607–613. [Google Scholar] [CrossRef]
- Lu, L.M.; Zavitz, C.C.; Chen, B.; Kianpour, S.; Wan, Y.; Stämpfli, M.R. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J. Immunol. 2007, 178, 936–943. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, T.M.; Di, L. Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10827. [Google Scholar] [CrossRef]
- Nath, L.R.; Sethi, G.; Liju, V.B. Editorial: Immunomodulatory molecules of natural origin: Innovative strategies for combatting cancer. Front. Oncol. 2025, 15, 1568568. [Google Scholar] [CrossRef]
- Li, Z.M.; Shao, Z.J.; Qu, D.; Huo, X.H.; Hua, M.; Chen, J.B.; Lu, Y.S.; Sha, J.Y.; Li, S.S.; Sun, Y.S. Transformation Mechanism of Rare Ginsenosides in American Ginseng by Different Processing Methods and Antitumour Effects. Front. Nutr. 2022, 9, 833859. [Google Scholar] [CrossRef]
- Ren, G.; Shi, Z.; Teng, C.; Yao, Y. Antiproliferative Activity of Combined Biochanin A and Ginsenoside Rh2 on MDA-MB-231 and MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 2908. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Ling, C.; Zhang, P.; Han, R. Potential of Dietary HDAC2i in Breast Cancer Patients Receiving PD-1/PD-L1 Inhibitors. Nutrients 2023, 15, 3984. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ma, Z.; Luo, X.; Wang, Y.; Liu, X.; Tang, M.; Chen, J.; Tu, L.; Ouyang, D.; Zheng, J.; et al. Self-Assembled Micelles Based on Ginsenoside Rg5 for the Targeted Treatment of PTX-Resistant Tumors. Mol. Pharm. 2024, 21, 3502–3512. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.H.; Park, D.J.; Zhang, H.; Noh, M.; Kim, Y.; Kim, Y.S.; Kim, H.; Kim, Y.M.; Ha, S.J.; et al. CU06-1004-Induced Vascular Normalization Improves Immunotherapy by Modulating Tumor Microenvironment via Cytotoxic T Cells. Front. Immunol. 2020, 11, 620166. [Google Scholar] [CrossRef]
- Sijisha, K.S.; Anusha, R.; Priya, S. Synergistic effects of epoxyazadiradione (EAD) and paclitaxel against triple-negative breast cancer cells. Fundam. Clin. Pharmacol. 2024, 38, 758–766. [Google Scholar] [CrossRef]
- Webb, M.J.; Kukard, C. A Review of Natural Therapies Potentially Relevant in Triple Negative Breast Cancer Aimed at Targeting Cancer Cell Vulnerabilities. Integr. Cancer Ther. 2020, 19, 1534735420975861. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, J.; Thilakan, R.C.; Gopalakrishnan, R.M.; Vijayapoopathi, S.; Dorschel, A.; Venugopal, B. Ginsenoside Rg5 Sensitizes Paclitaxel-Resistant Human Cervical-Adeno-Carcinoma Cells to Paclitaxel-And Enhances the Anticancer Effect of Paclitaxel. Genes 2022, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Duan, Z.; Deng, J.; Zhang, Z.; Fu, R.; Zhu, C.; Fan, D. Ginsenoside Rh4 inhibits colorectal cancer via the modulation of gut microbiota-mediated bile acid metabolism. J. Adv. Res. 2025, 72, 37–52. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef]
- Liang, L.D.; He, T.; Du, T.W.; Fan, Y.G.; Chen, D.S.; Wang, Y. Ginsenoside-Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol. Med. Rep. 2015, 11, 940–946. [Google Scholar] [CrossRef]
- Ko, H.; Kim, Y.J.; Park, J.S.; Park, J.H.; Yang, H.O. Autophagy inhibition enhances apoptosis induced by ginsenoside Rk1 in hepatocellular carcinoma cells. Biosci. Biotechnol. Biochem. 2009, 73, 2183–2189. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, J.; Im, W.T.; Chun, S. Ginsenoside Rk1 Induces Apoptosis in Neuroblastoma Cells Through Loss of Mitochondrial Membrane Potential and Activation of Caspases. Int. J. Mol. Sci. 2019, 20, 1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, J.; Sun, F.; Ma, J.; Qi, X. Ginsenoside Rg2 Attenuated Trastuzumab-Induced Cardiotoxicity in Rats. Biomed. Res. Int. 2022, 2022, 8866660. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tangchang, W.; Kwon, O.S.; Lee, J.Y.; Heo, K.S.; Son, H.Y. Ginsenoside Rh1 ameliorates the asthma and allergic inflammation via inhibiting Akt, MAPK, and NF-κB signaling pathways in vitro and in vivo. Life Sci. 2023, 321, 121607. [Google Scholar] [CrossRef]
- Jung, J.H.; Kang, T.K.; Oh, J.H.; Jeong, J.U.; Ko, K.P.; Kim, S.T. The Effect of Korean Red Ginseng on Symptoms and Inflammation in Patients with Allergic Rhinitis. Ear Nose Throat J. 2021, 100, 712s–719s. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-J.; Kim, E.-J.; Lee, Y.-H.; Park, S.-D.; Shim, J.-J.; Lee, J.-L.; Lee, J.-H. Fermented Cultured Wild Ginseng Roots (Panax ginseng C.A. Meyer) Using Limosilactobacillus fermentum HY7303 Enhances the Intestinal Barrier by Bioconversion of Ginsenosides and Extracellular Vesicle Production. Fermentation 2024, 10, 362. [Google Scholar] [CrossRef]
- Jang, W.Y.; Hwang, J.Y.; Cho, J.Y. Ginsenosides from Panax ginseng as Key Modulators of NF-κB Signaling Are Powerful Anti-Inflammatory and Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 6119. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Zhou, R.; Li, W.; Sama, A.E. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev. Mol. Med. 2008, 10, e32. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, M.; Jiang, D.; Su, Q.; Shi, J. The role of inflammation in autoimmune disease: A therapeutic target. Front. Immunol. 2023, 14, 1267091. [Google Scholar] [CrossRef]
- Lee, T.K.; Johnke, R.M.; Allison, R.R.; O’Brien, K.F.; Dobbs, L.J., Jr. Radioprotective potential of ginseng. Mutagenesis 2005, 20, 237–243. [Google Scholar] [CrossRef]
- You, L.; Cha, S.; Kim, M.Y.; Cho, J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J. Ginseng Res. 2022, 46, 711–721. [Google Scholar] [CrossRef]
- Yoon, S.-h.; Nam, Y.-m.; Hong, J.-t.; Kim, S.-j.; Ko, S.-k. Modification of ginsenoside composition in red ginseng (Panax ginseng) by ultrasonication. J. Ginseng Res. 2016, 40, 300–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.Y.; Choi, P.; Lee, D.; Kim, T.; Jung, E.B.; Hwang, B.S.; Kang, K.S.; Ham, J. Effect of Amino Acids on the Generation of Ginsenoside Rg3 Epimers by Heat Processing and the Anticancer Activities of Epimers in A2780 Human Ovarian Cancer Cells. Evid. Based Complement. Altern. Med. 2016, 2016, 3146402. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, T.K.; Kim, Y.H.; Lee, J.; Moon, J.M.; Park, Y.S.; Sung, C.M. Pharmacokinetics of Ginsenoside Rb1, Rg3, Rk1, Rg5, F2, and Compound K from Red Ginseng Extract in Healthy Korean Volunteers. Evid. Based Complement. Altern. Med. 2022, 2022, 8427519. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-assembled targeted nanoparticles: Evolution of technologies and bench to bedside translation. Acc. Chem. Res. 2011, 44, 1123–1134. [Google Scholar] [CrossRef]
- Park, H.H.; Kim, H.; Lee, H.S.; Seo, E.U.; Kim, J.E.; Lee, J.H.; Mun, Y.H.; Yoo, S.Y.; An, J.; Yun, M.Y.; et al. PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses. Biomaterials 2021, 273, 120827. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Lee, G.M.; Liu, K.H.; Jung, D.H. Effect of Korean Red Ginseng on Plasma Ceramide Levels in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Metabolites 2021, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Duan, L.; Zhang, Y.H.; Jin, D.; Zhao, S.; Zhou, R.R.; Duan, Y.; Lian, F.; Tong, X. The three syndromes and six Chinese patent medicine study during the recovery phase of COVID-19. Chin. Med. 2021, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef]
- Rahimi, S.; van Leeuwen, D.; Roshanzamir, F.; Pandit, S.; Shi, L.; Sasanian, N.; Nielsen, J.; Esbjörner, E.K.; Mijakovic, I. Ginsenoside Rg3 Reduces the Toxicity of Graphene Oxide Used for pH-Responsive Delivery of Doxorubicin to Liver and Breast Cancer Cells. Pharmaceutics 2023, 15, 391. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Ding, M.; Xin, Y.; Xuan, Y.; Zhao, Y. Genotoxicity and subchronic toxicological study of a novel ginsenoside derivative 25-OCH3-PPD in beagle dogs. J. Ginseng Res. 2019, 43, 562–571. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, D.; Yoo, D.; Jang, E.J.; Jun, J.B.; Sung, Y.K. Korean Red Ginseng exhibits no significant adverse effect on disease activity in patients with rheumatoid arthritis: A randomized, double-blind, crossover study. J. Ginseng Res. 2018, 42, 144–148. [Google Scholar] [CrossRef]
- Shen, W.; Wei, Y.; Tang, D.; Jia, X.; Chen, B. Metabolite profiles of ginsenosides Rk1 and Rg5 in zebrafish using ultraperformance liquid chromatography/quadrupole-time-of-flight MS. J. Ginseng Res. 2017, 41, 78–84. [Google Scholar] [CrossRef]
- Chen, C.; Xia, J.; Ren, H.; Wang, A.; Zhu, Y.; Zhang, R.; Gan, Z.; Wang, J. Effect of the structure of ginsenosides on the in vivo fate of their liposomes. Asian J. Pharm. Sci. 2022, 17, 219–229. [Google Scholar] [CrossRef]
- Lai, L.; Hao, H.; Liu, Y.; Zheng, C.; Wang, Q.; Wang, G.; Chen, X. Characterization of pharmacokinetic profiles and metabolic pathways of 20(S)-ginsenoside Rh1 in vivo and in vitro. Planta Med. 2009, 75, 797–802. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.H.; Kim, J.E.; Kim, Y.S.; Ryu, C.H.; Lee, H.J.; Kim, H.M.; Jeon, H.; Won, H.J.; Lee, J.Y.; et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 2018, 42, 361–369. [Google Scholar] [CrossRef]
- Hong, C.; Liang, J.; Xia, J.; Zhu, Y.; Guo, Y.; Wang, A.; Lu, C.; Ren, H.; Chen, C.; Li, S.; et al. One Stone Four Birds: A Novel Liposomal Delivery System Multi-functionalized with Ginsenoside Rh2 for Tumor Targeting Therapy. Nano-Micro Lett. 2020, 12, 129. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, L.; Wei, N.; Chen, R.; Wang, Y.; Wu, L.; Li, W.; Yan, Z.; Chen, L.; Chen, Z. Comparative pharmacokinetics of 11 major bioactive components between two dosage forms of Qixue Shuangbu Prescription in rats by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2024, 47, e2300677. [Google Scholar] [CrossRef]
- Lu, Y.; Qi, J.; Wu, W. Absorption, disposition and pharmacokinetics of nanoemulsions. Curr. Drug Metab. 2012, 13, 396–417. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zhang, J.; Wang, J.; Le, Y. Ginsenoside Drug Nanocomposites Prepared by the Aerosol Solvent Extraction System for Enhancing Drug Solubility and Stability. Pharmaceutics 2018, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, G.; Wang, W.; Chen, C.; Jiao, L.; Wu, W. Preparation, Characterization, and Bioavailability of Host-Guest Inclusion Complex of Ginsenoside Re with Gamma-Cyclodextrin. Molecules 2021, 26, 7227. [Google Scholar] [CrossRef]
- Baek, J.S.; Yeon, W.G.; Lee, C.A.; Hwang, S.J.; Park, J.S.; Kim, D.C.; Cho, C.W. Preparation and characterization of mucoadhesive enteric-coating ginsenoside-loaded microparticles. Arch. Pharm. Res. 2015, 38, 761–768. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Duong, V.A.; Maeng, H.J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Nie, L.; Zhu, S.; Zhang, X. Nanovesicles-Mediated Drug Delivery for Oral Bioavailability Enhancement. Int. J. Nanomed. 2022, 17, 4861–4877. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Feng, C.; Jiang, X.; Xu, X.; Wang, J. Ginsenoside compound K-based multifunctional liposomes for the treatment of rheumatoid arthritis. Drug Deliv. 2025, 32, 2464190. [Google Scholar] [CrossRef]
- Yang, L.Q.; Wang, B.; Gan, H.; Fu, S.T.; Zhu, X.X.; Wu, Z.N.; Zhan, D.W.; Gu, R.L.; Dou, G.F.; Meng, Z.Y. Enhanced oral bioavailability and anti-tumour effect of paclitaxel by 20(s)-ginsenoside Rg3 in vivo. Biopharm. Drug Dispos. 2012, 33, 425–436. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, S.; Zhang, R.; Wang, A.; Zhu, Y.; Dong, M.; Ma, S.; Hong, C.; Liu, S.; Wang, D.; et al. Targeting therapy and tumor microenvironment remodeling of triple-negative breast cancer by ginsenoside Rg3 based liposomes. J. Nanobiotechnol. 2022, 20, 414. [Google Scholar] [CrossRef]
- Byun, J.; Lee, D.Y.; Jeong, C.-W.; Kim, Y.; Rhee, H.Y.; Moon, K.W.; Heo, J.; Hong, Y.; Kim, W.J.; Nam, S.-J.; et al. Analysis of treatment pattern of anti-dementia medications in newly diagnosed Alzheimer’s dementia using OMOP CDM. Sci. Rep. 2022, 12, 4451. [Google Scholar] [CrossRef]
- Varadharajan, A.; Davis, A.D.; Ghosh, A.; Jagtap, T.; Xavier, A.; Menon, A.J.; Roy, D.; Gandhi, S.; Gregor, T. Guidelines for pharmacotherapy in Alzheimer’s disease—A primer on FDA-approved drugs. J. Neurosci. Rural. Pract. 2023, 14, 566–573. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Grossberg, G.T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Devel Ther. 2016, 10, 3267–3279. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, K.H.; Yun, J.; Kim, S.H.; Kim, H.J.; Lee, S.C.; Bae, S.B.; Kim, C.K.; Lee, N.S.; Lee, K.T.; et al. Phase II Clinical Trial of Genexol® (Paclitaxel) and Carboplatin for Patients with Advanced Non-small Cell Lung Cancer. Cancer Res. Treat. 2011, 43, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.O.; Coskun, U.; Ozkan, M.; Sevinc, A.; Yilmaz, A.U.; Gumus, M.; Unal, O.U.; Ozdemir, N.Y.; Alici, S.; Berk, V.; et al. Paclitaxel plus doxorubicin chemotherapy as second-line therapy in patients with advanced urothelial carcinoma pretreated with platinum plus gemcitabine chemotherapy. Onkologie 2012, 35, 576–580. [Google Scholar] [CrossRef]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yi Koo, G.P.; Dutta, A.; Zhang, Y.H. Unusual case of concurrent metformin-associated lactic acidosis and euglycaemic ketoacidosis. Proc. Singap. Healthc. 2023, 32, 20101058231204703. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, Z.; Liu, R.; Shi, P.; Qian, S.; Qu, X.; Zhu, L.; Zhang, W.; Wang, J. Prospective Study of Transcatheter Arterial Chemoembolization (TACE) with Ginsenoside Rg3 versus TACE Alone for the Treatment of Patients with Advanced Hepatocellular Carcinoma. Radiology 2016, 280, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Cheng, Y.; Chen, Q.; Tan, H.; Son, D.; Chang, D.; Bian, Z.; Fang, H.; Xu, H. Safety and antifatigue effect of Korean Red Ginseng: A randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2019, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Deng, Y.; Feng, Y.; Liu, Y.; Yang, L.; Huang, Y.; Sun, J.; Liang, W.; Guan, Y. Pharmacokinetics and safety of ginsenoside Rd following a single or multiple intravenous dose in healthy Chinese volunteers. J. Clin. Pharmacol. 2010, 50, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Song, H.; Zhang, C.; Wang, A.; Zhang, B.; Xiong, C.; Zhuang, X.; Zang, Y.; Li, C.; Fang, Q.; et al. Efficacy and Safety of Panax notoginseng Saponins in the Treatment of Adults with Ischemic Stroke in China: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2317574. [Google Scholar] [CrossRef]
- Shen, L.; Gwak, S.R.; Joo, J.C.; Song, B.K.; Cha, S.W.; Song, Y.U.; Pyo, M.K.; Park, S.J. Effectiveness and Safety of Panax ginseng Extract on Hepatic Dysfunction: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Evid. Based Complement. Altern. Med. 2020, 2020, 2689565. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Huang, J.; Wang, Y.; Yang, G.; Tan, Z.; Wang, Y.; Zhou, G.; Liao, J.; Ouyang, D. Single- and Multiple-Dose Trials to Determine the Pharmacokinetics, Safety, Tolerability, and Sex Effect of Oral Ginsenoside Compound K in Healthy Chinese Volunteers. Front. Pharmacol. 2017, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, T.; Zhou, P.; Zhang, J.; Guan, G.; Zhang, H.; Ling, X.; Li, W.; Meng, F.; Liu, G.; et al. Post-marketing safety surveillance and re-evaluation of Xueshuantong injection. BMC Complement. Altern. Med. 2018, 18, 277. [Google Scholar] [CrossRef]
- Ahn, C.M.; Hong, S.J.; Choi, S.C.; Park, J.H.; Kim, J.S.; Lim, D.S. Red ginseng extract improves coronary flow reserve and increases absolute numbers of various circulating angiogenic cells in patients with first ST-segment elevation acute myocardial infarction. Phytother. Res. 2011, 25, 239–249. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.; Kim, M.J.; Kim, M.S.; Kim, J.; Park, C.W.; Seo, D.; Shin, S.S.; Oh, S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: A 12-wk randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2018, 42, 90–97. [Google Scholar] [CrossRef]
- Baek, H.I.; Ha, K.C.; Park, Y.K.; Kim, T.Y.; Park, S.J. Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 1952. [Google Scholar] [CrossRef]
- Fu, R.J.; Wang, W.X.; Tao, H.J.; Wang, M.; Chen, Y.Y.; Gao, H.; Yue, S.J.; Tang, Y.P. Quantitative evaluation of Danqi tablet by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry integrated with bioassay. J. Sep. Sci. 2021, 44, 1552–1563. [Google Scholar] [CrossRef]
- Ikeuchi, S.; Minamida, M.; Nakamura, T.; Konishi, M.; Kamioka, H. Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance. Nutrients 2022, 14, 1185. [Google Scholar] [CrossRef] [PubMed]
| Ginsenoside | Type | Key Immunomodulatory Effects | Main Mechanisms | Ref. |
|---|---|---|---|---|
| F1 | PPT |
|
| [25,27,63] |
| Rg5 | PPD |
|
| [64,65] |
| Rk1 | PPD |
|
| [66] |
| Rh1 | PPT |
|
| [41,42] |
| Rg2 | PPT |
|
| [42,67] |
| Ginsenoside | Primary Effect | Key Mechanisms | Target Diseases | Ref. |
|---|---|---|---|---|
| F1 | Immunostimulation |
|
| [25,63] |
| Rg5 | Anti-inflammation |
|
| [66,103] |
| Rk1 | Multi-pathway inhibition |
|
| [66,117] |
| Rh1 | Anti-allergic |
|
| [42,130] |
| Rg2 | Neuroimmune regulation |
|
| [42,162] |
| Model | Ginsenoside | Dose | Route | Duration | Key Outcome | Ref. |
|---|---|---|---|---|---|---|
| Cancer models | ||||||
| CT26 colon cancer (mice) | F1 | 20 mg/kg | i.p. | 14 days | 67% tumor growth inhibition | [25] |
| RMA-s lymphoma (mice) | F1 | 25–50 mg/kg | i.p. | 3 days pretreatment | 70% rejection vs. 20% control at 6 h | [25] |
| B16F10 melanoma (mice) | F1 | 50 mg/kg | i.p. | 3 days pre + 3×/week | Reduced lung metastases | [25] |
| Sepsis models | ||||||
| CLP-induced sepsis (mice) | Rg5/Rk1 | 0.061 mg/kg | i.v. | 12 h & 50 h post-CLP | 70% survival vs. 20% control | [66] |
| HMGB1-induced sepsis (mice) | Rg5/Rk1 | 0.031–0.061 mg/kg | i.v. | Single | Reduced HMGB1, TNF-α, IL-6 | [66] |
| Inflammatory models | ||||||
| LPS-induced inflammation (mice) | Rg2/Rh1 | Not specified | Not specified | Not specified | Synergistic reduction in liver/kidney damage | [42] |
| CIA (mice) | Rh1 + DEX | 10 mg/kg (Rh1) + 1 mg/kg (DEX) | i.p. | 10 days | Enhanced anti-inflammatory effects vs. DEX alone | [130] |
| Neurological models | ||||||
| MCAO stroke (rats) | F1 | 50 mg/kg | p.o. | 14 days | Increased MVD, improved cerebral perfusion | [26] |
| Sleep deprivation (mice) | Rg5/Rk1 | 30–60 mg/kg | p.o. | 7 days | Increased sleep duration, reduced latency | [99] |
| Ginsenoside | Species /Model | Bioavailability /PK Parameters | Enhancement Strategy | Improved Outcome | Ref |
|---|---|---|---|---|---|
| F1 | Caco-2 cells | <5% (estimated); 26.0% permeability | Nanostructured lipid carrier | 39.2% permeability; 90% encapsulation | [85] |
| Rat (as Rg1 metabolite) | Detected in feces (parent Rg1: 40.11%) | – | – | [83] | |
| Rg5 | In vitro | Low | Cyclodextrin complexation | 1.8-fold increase | [121] |
| Zebrafish | Low; 7 metabolites identified | – | – | [218] | |
| Rk1 | In vivo | <3% oral absorption | Liposome (97.24% encapsulation) | >50% tumor reduction | [117] |
| Rat | 2.87–4.23% (T1/2: 3.09–3.40 h) | – | – | [118] | |
| Zebrafish | Low; 4 metabolites | – | – | [218] | |
| Rh1 | In vitro/rat | 12.92% | SMEDDS | 33.25% (2.6-fold increase) | [139] |
| Rat | 1.01% (T1/2β: 0.41 h) | – | – | [220] | |
| Rg2 | In vitro | Low | Lipid nanoparticles | Enhanced mRNA delivery (81.9% encapsulation) | [162] |
| Rat microsomes | Low; metabolites M1, M3, M4 | – | – | [161] | |
| Human plasma | Not detected as parent | – | – | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.-E.; Lyu, S.-Y. Immunomodulatory Activities of Emerging Rare Ginsenosides F1, Rg5, Rk1, Rh1, and Rg2: From Molecular Mechanisms to Therapeutic Applications. Pharmaceuticals 2025, 18, 1529. https://doi.org/10.3390/ph18101529
Hong C-E, Lyu S-Y. Immunomodulatory Activities of Emerging Rare Ginsenosides F1, Rg5, Rk1, Rh1, and Rg2: From Molecular Mechanisms to Therapeutic Applications. Pharmaceuticals. 2025; 18(10):1529. https://doi.org/10.3390/ph18101529
Chicago/Turabian StyleHong, Chang-Eui, and Su-Yun Lyu. 2025. "Immunomodulatory Activities of Emerging Rare Ginsenosides F1, Rg5, Rk1, Rh1, and Rg2: From Molecular Mechanisms to Therapeutic Applications" Pharmaceuticals 18, no. 10: 1529. https://doi.org/10.3390/ph18101529
APA StyleHong, C.-E., & Lyu, S.-Y. (2025). Immunomodulatory Activities of Emerging Rare Ginsenosides F1, Rg5, Rk1, Rh1, and Rg2: From Molecular Mechanisms to Therapeutic Applications. Pharmaceuticals, 18(10), 1529. https://doi.org/10.3390/ph18101529








