Study on the Mechanism and Dose–Effect Relationship of Flavonoids in Different Extracts of Radix Hedysari Against Gastrointestinal Injury Induced by Chemotherapy
Abstract
1. Introduction
2. Results
2.1. Determination of Principal Components in Different Extracts
2.2. Results of the Network Pharmacology Analysis
2.2.1. Component Screening and Target Prediction
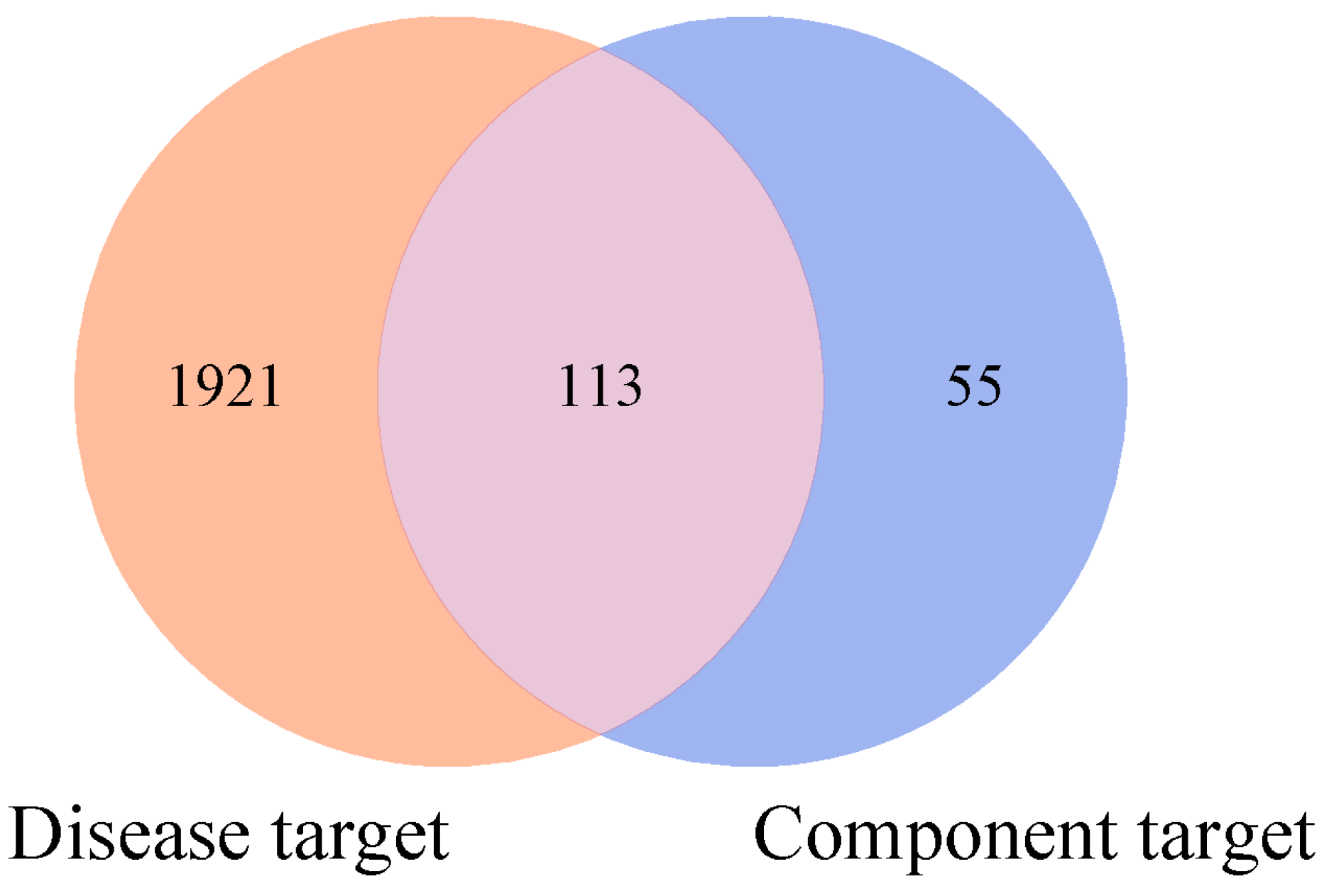
2.2.2. Acquisition of Disease Targets and Their Intersection Mapping with Component Targets
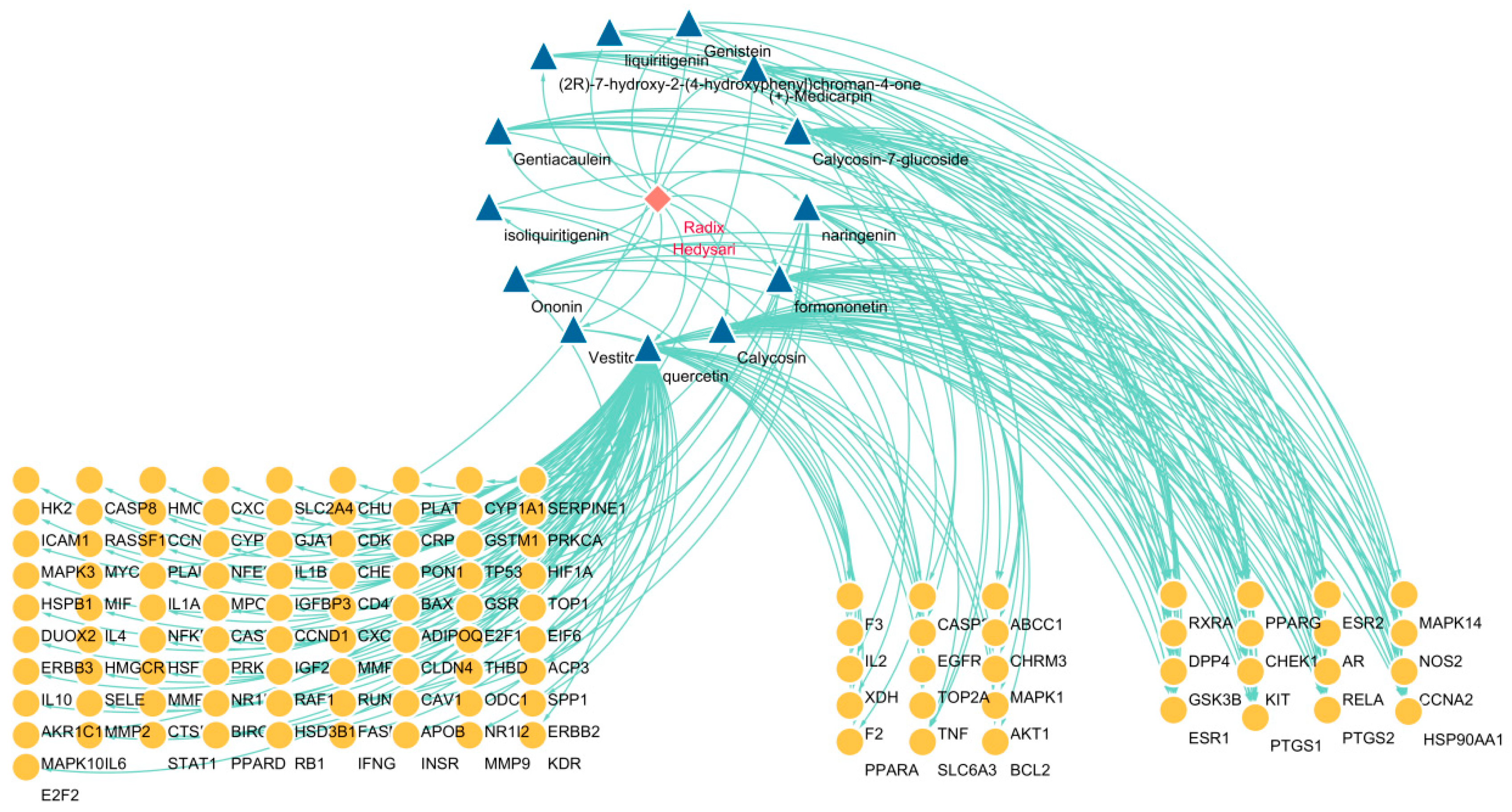
2.2.3. Construction of “Drug–Component–Target” Network
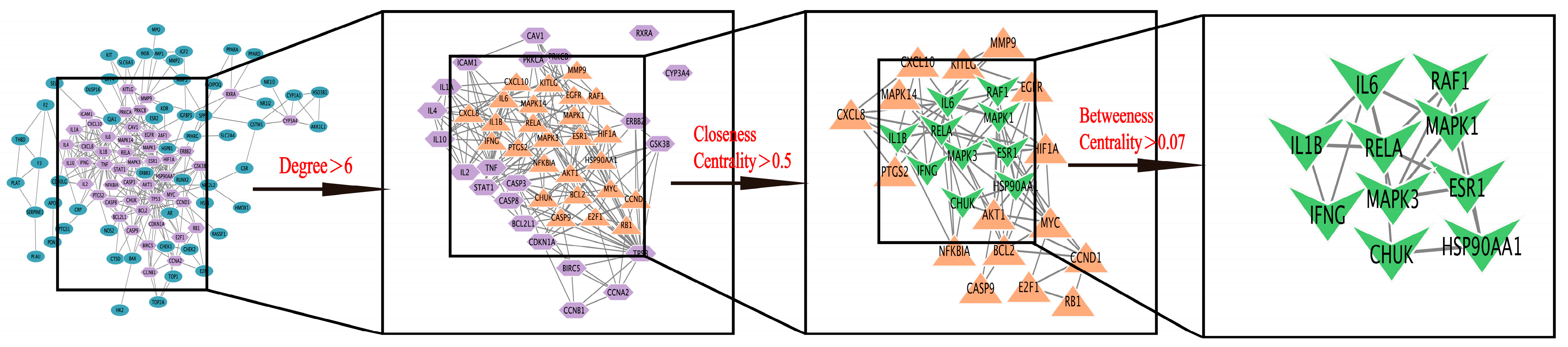
2.2.4. Construction of PPI Protein Interaction Network and Screening of Key Targets
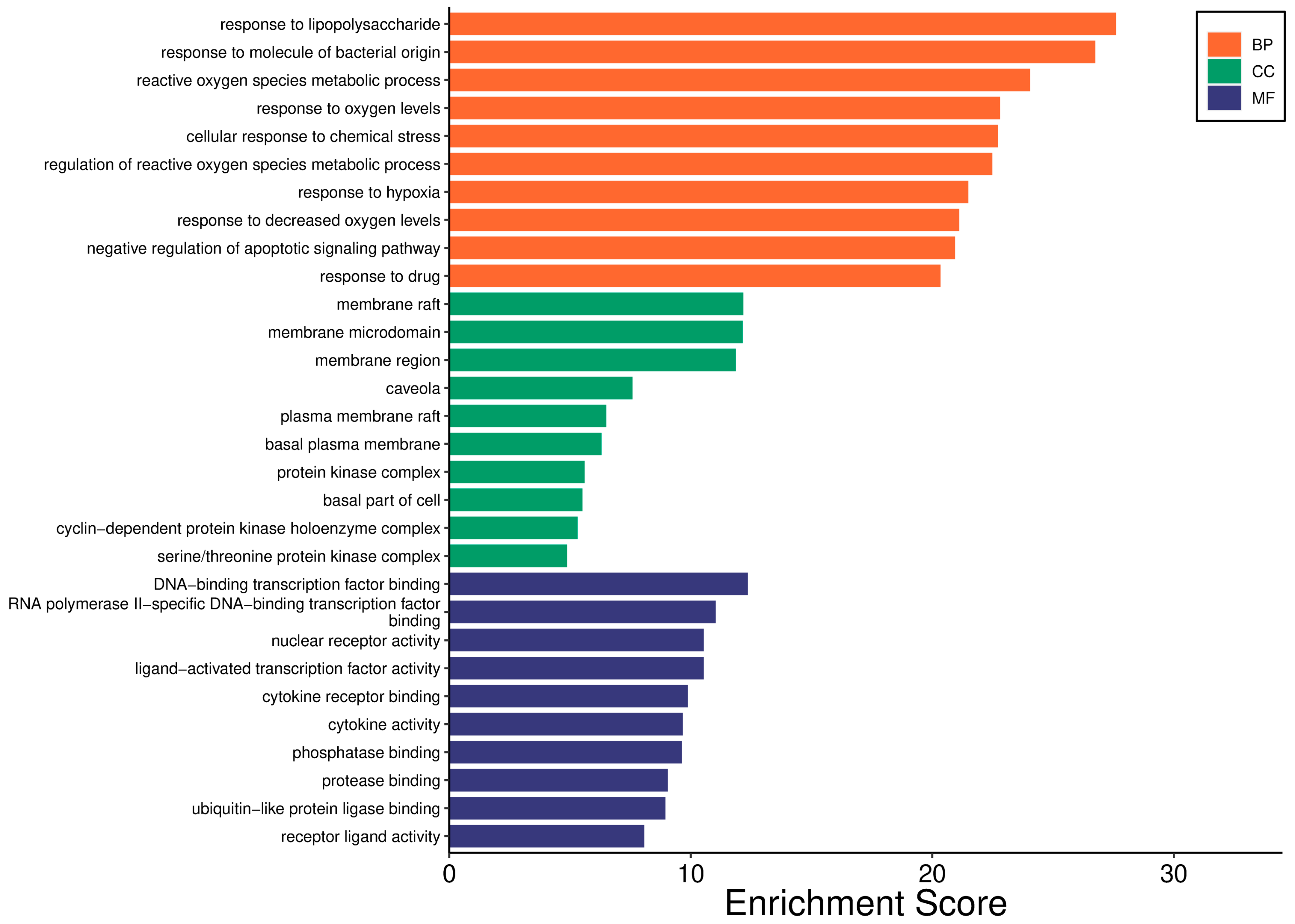
2.2.5. GO Analysis
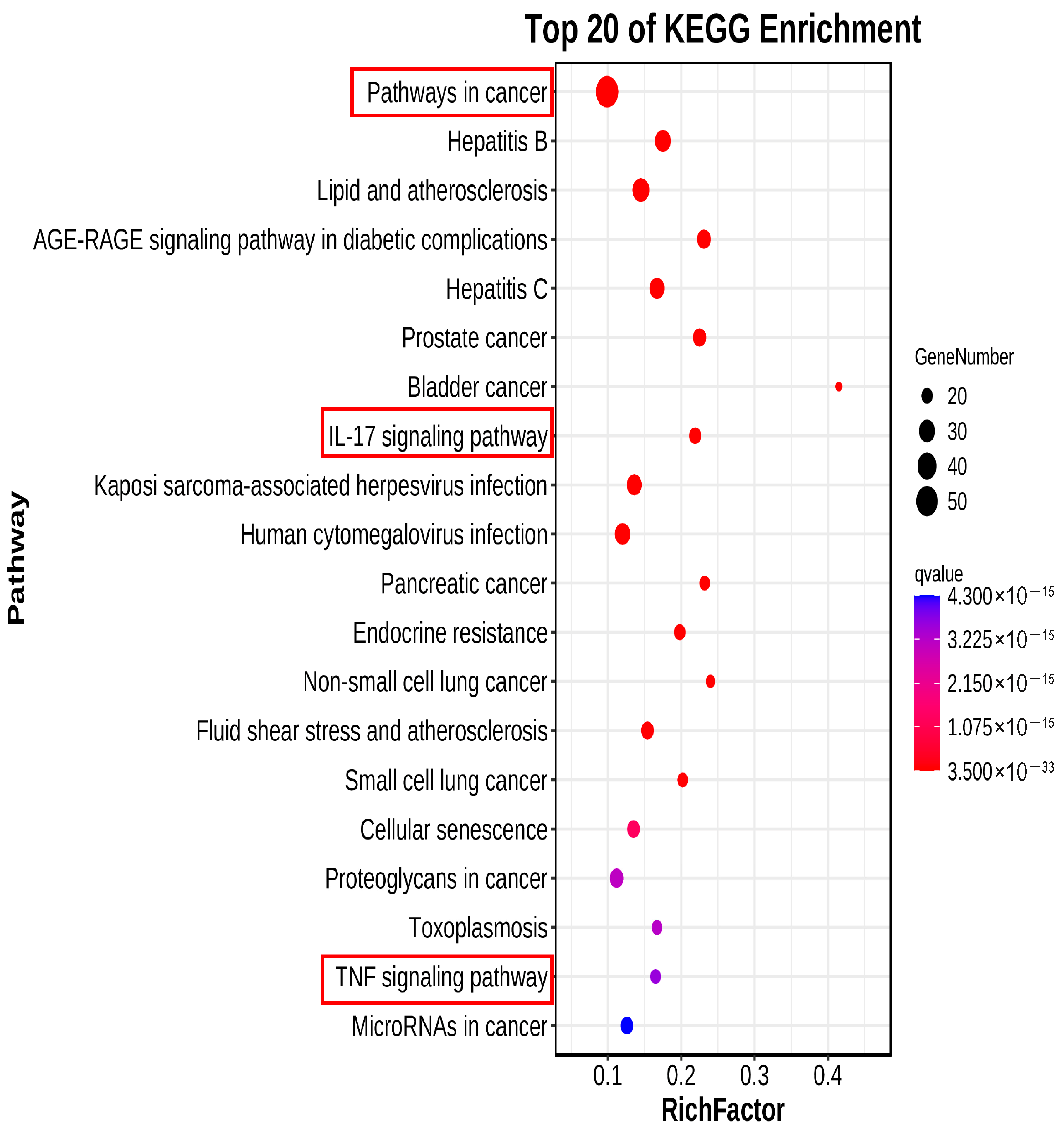
2.2.6. KEGG Pathway Analysis
2.3. Screening of Effective Fractions and Study on Mechanism of Action
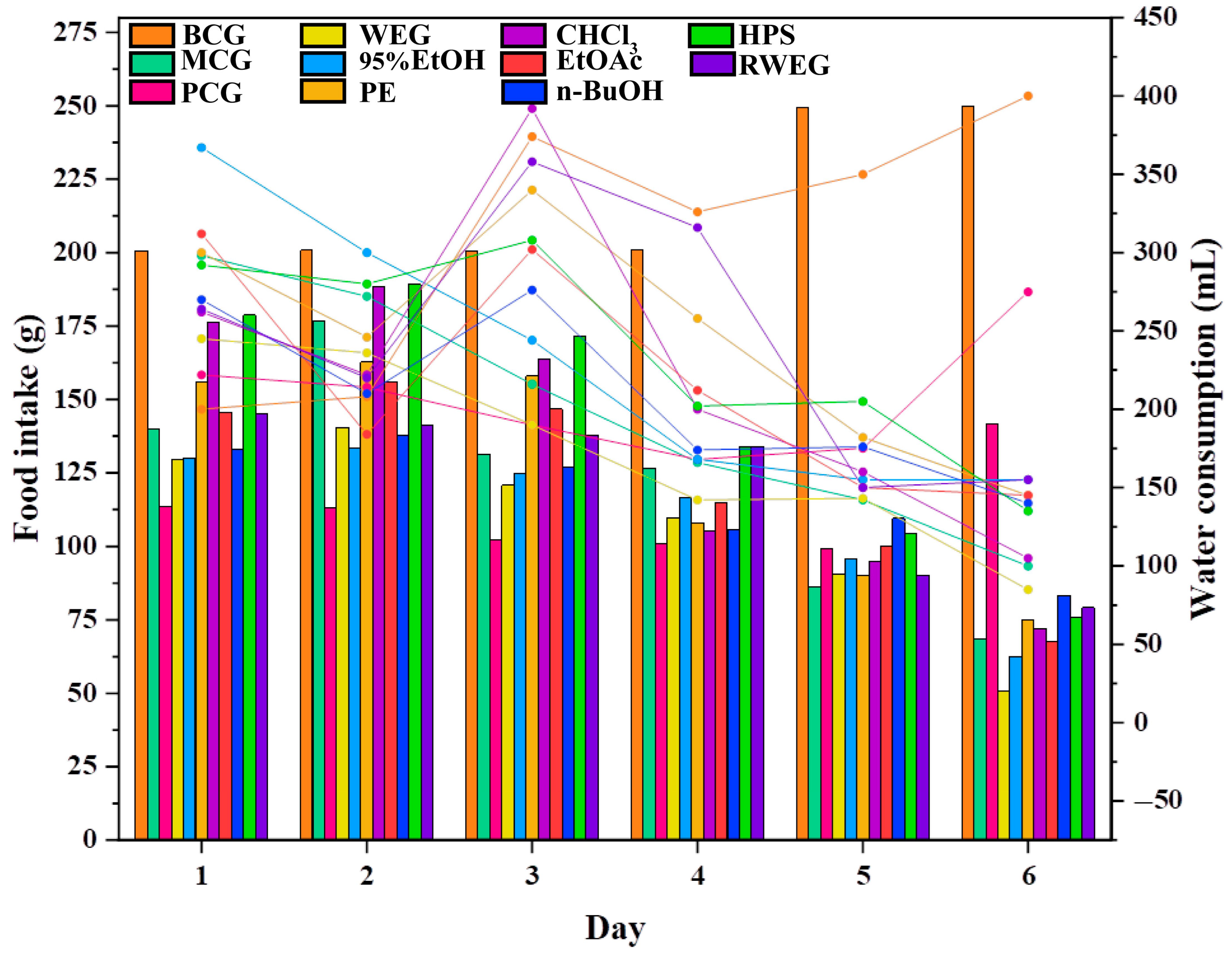
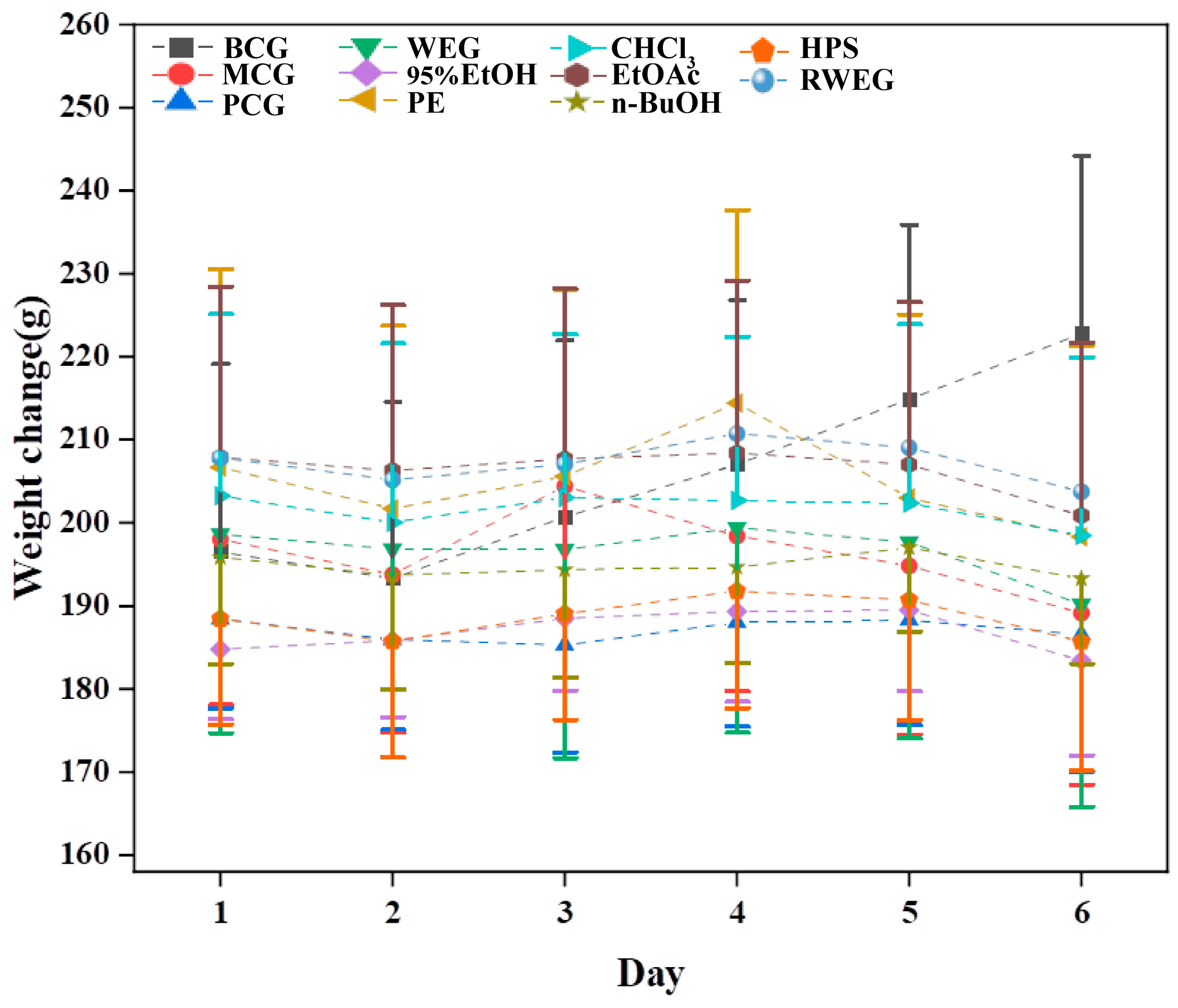
2.3.1. General Behavioral Monitoring of Rats
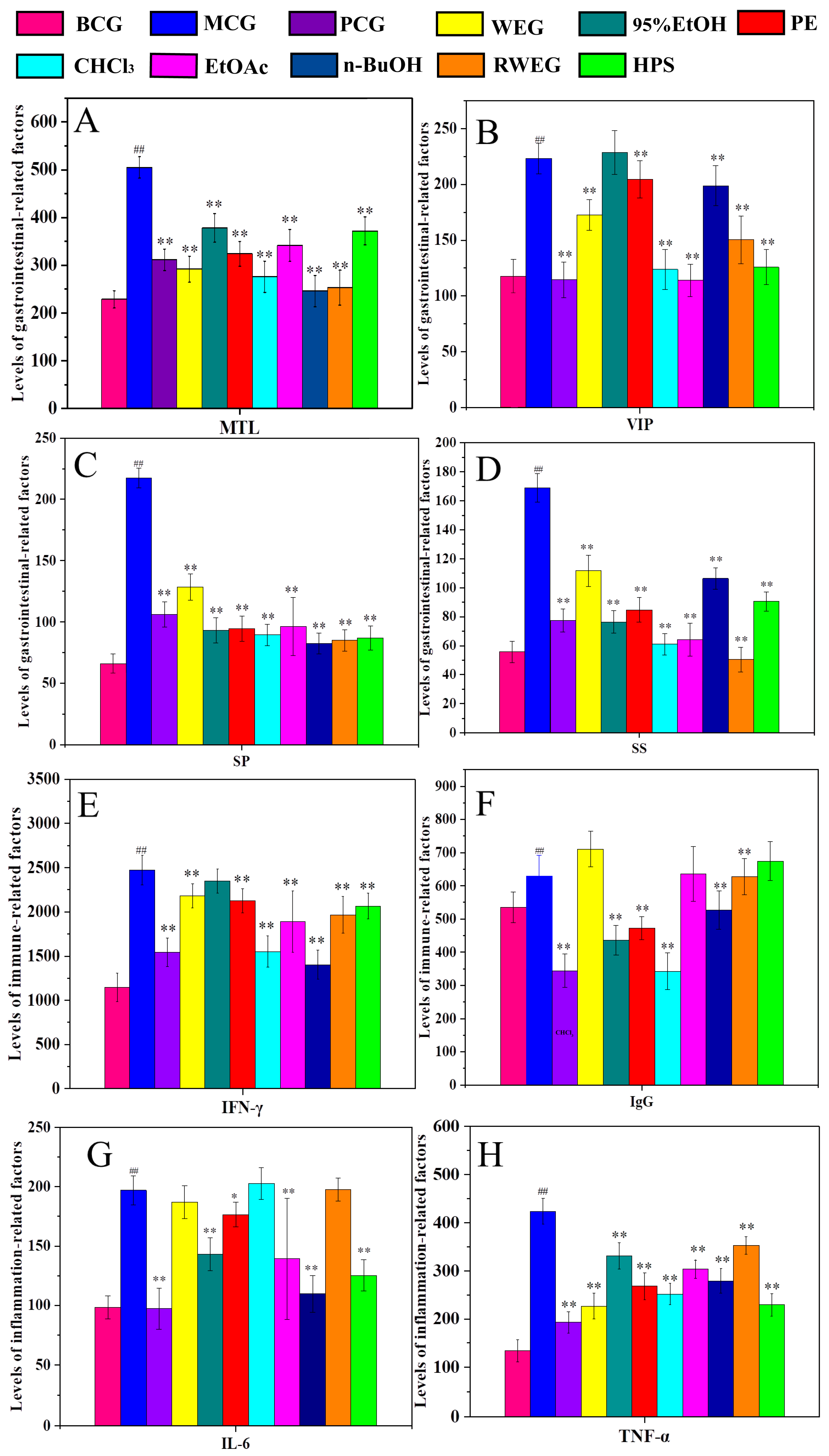
2.3.2. Detection of Serum Factors in Rats
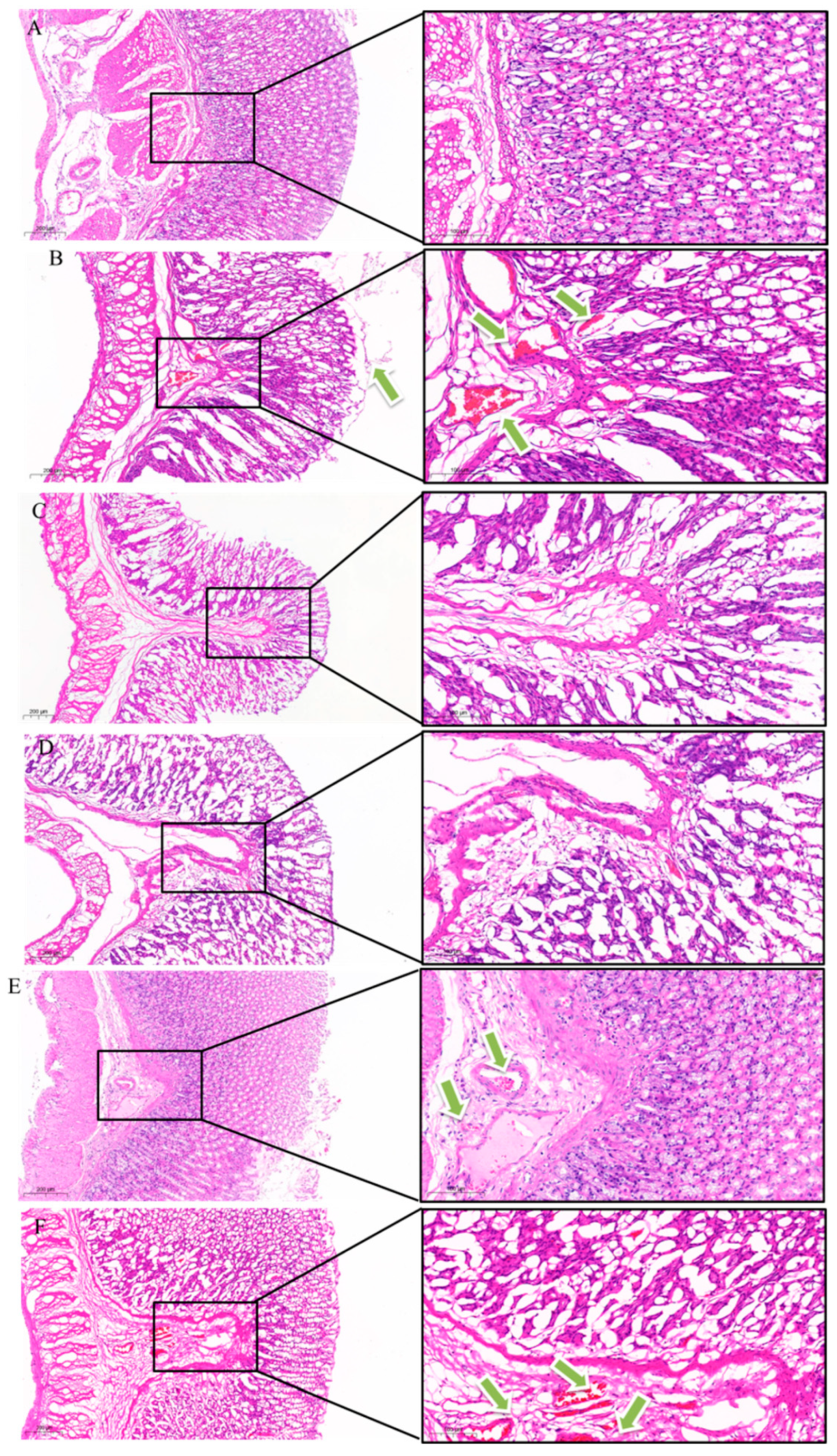
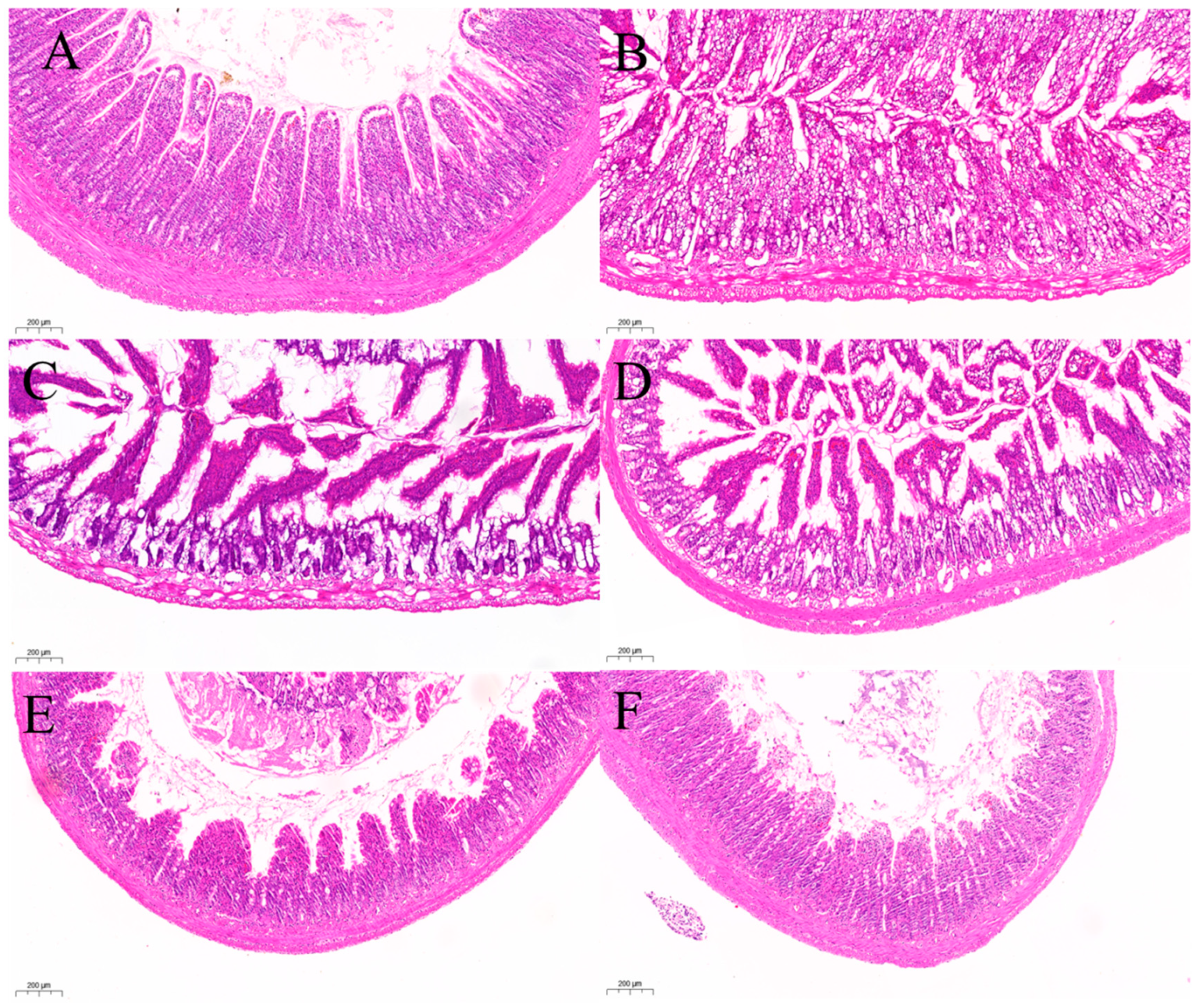
2.3.3. Histopathological Observation
H&E Staining of Gastric Antrum
H&E Staining of Ileum
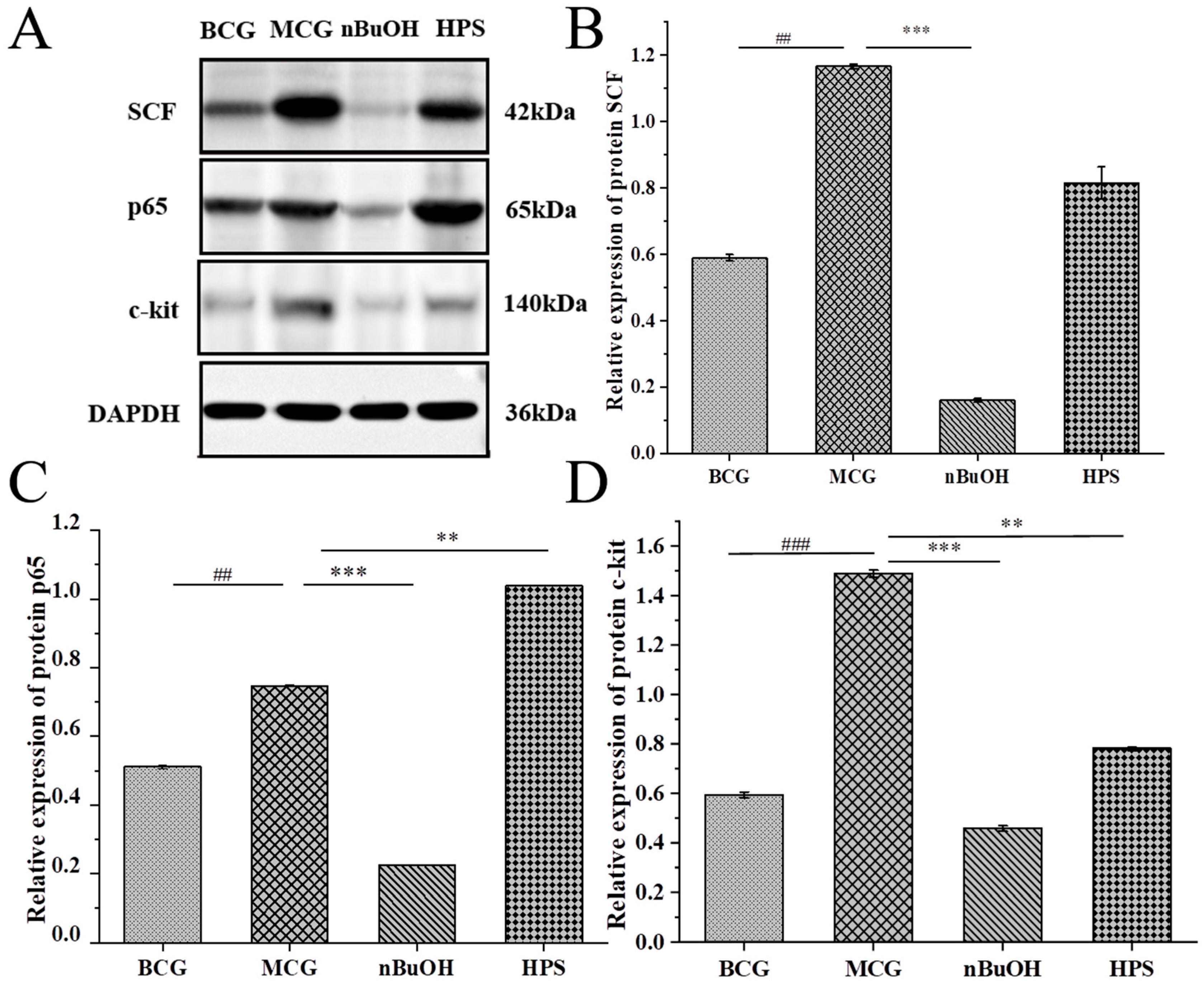
2.3.4. Detection of Signal Pathway Protein Molecules
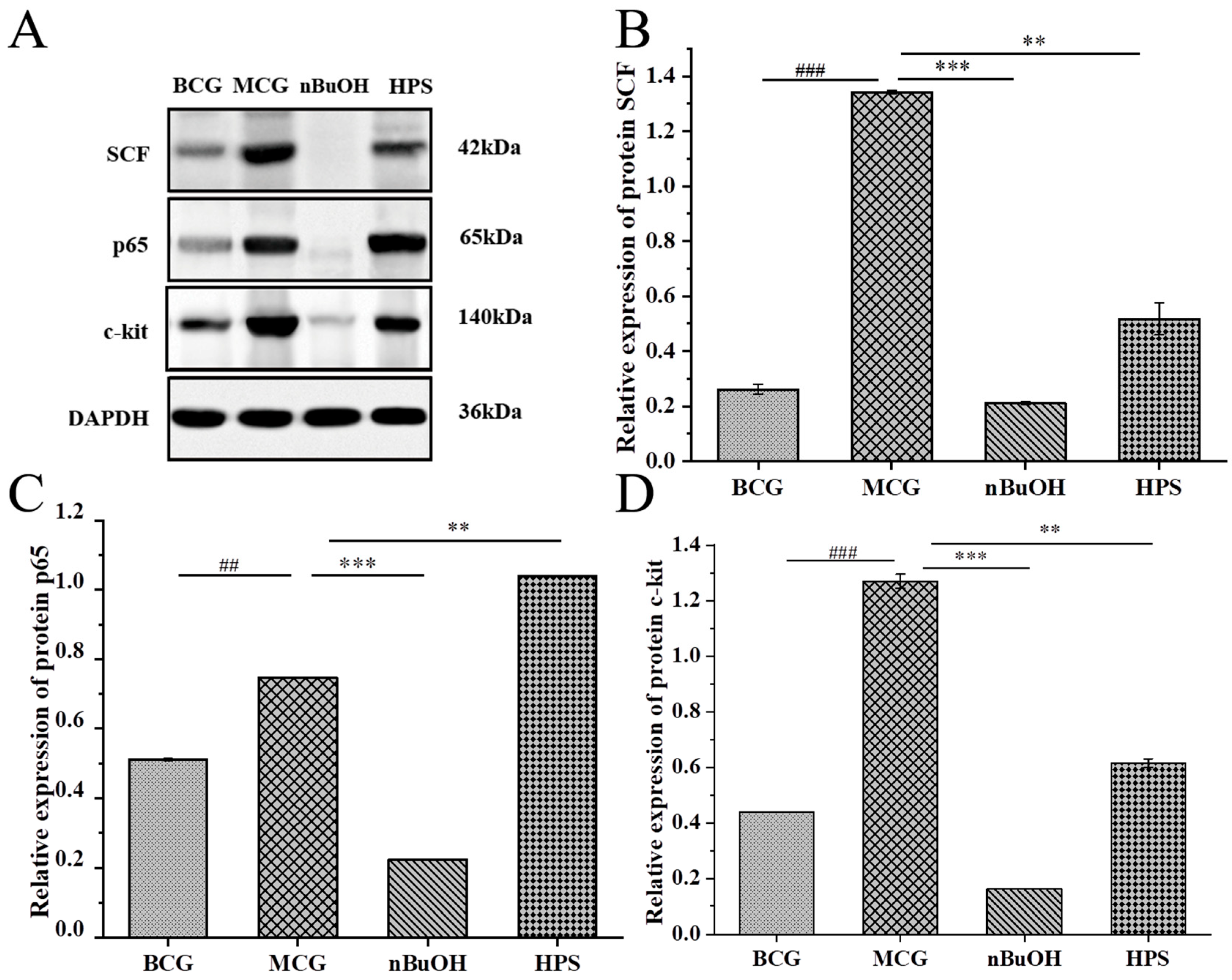
Molecular Detection of Gastric Protein
Molecular Detection of Intestinal Protein
2.4. Correlation Analysis of “Dose–Effect”
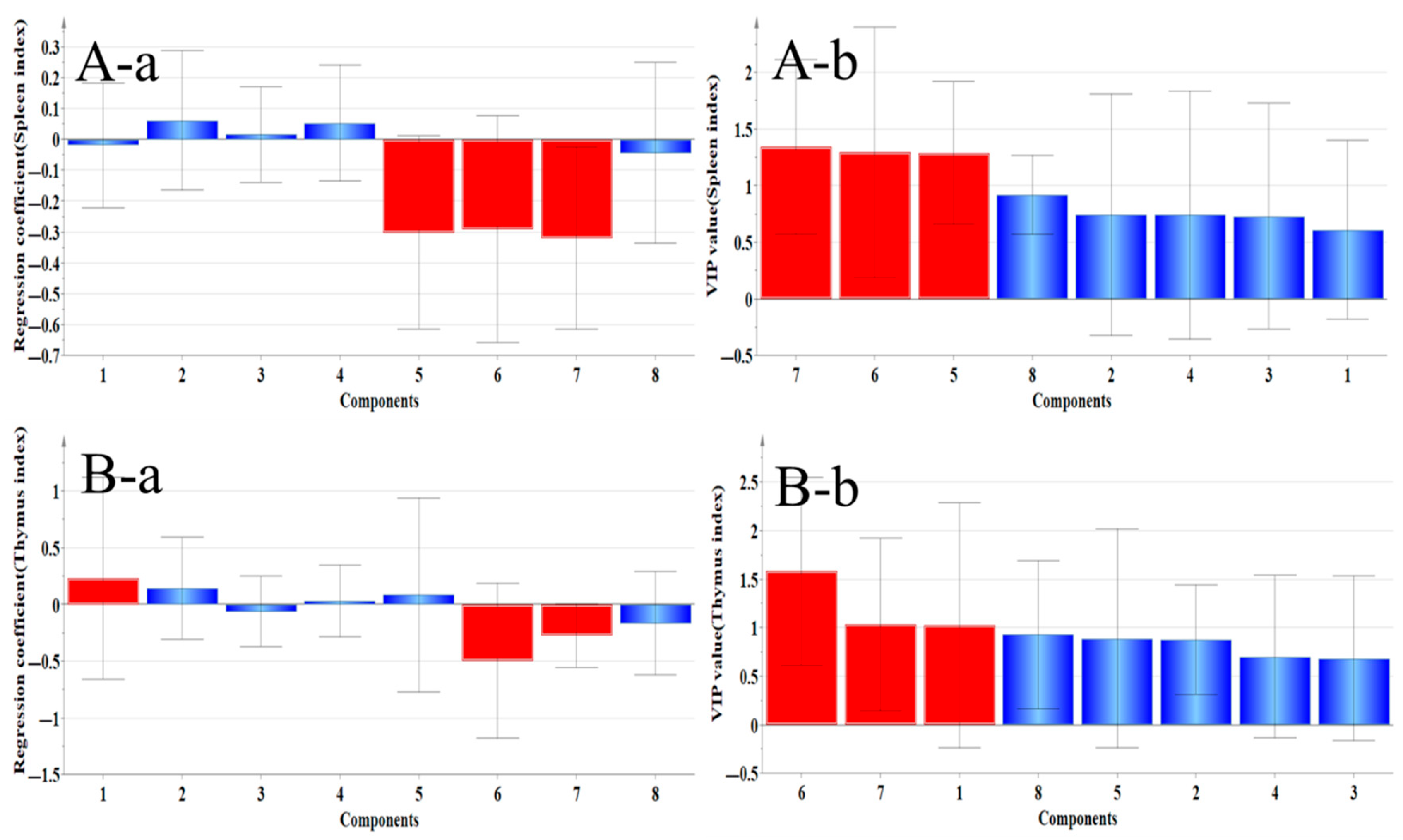
2.4.1. Correlation Analysis Between Organ Index and Main Ingredients
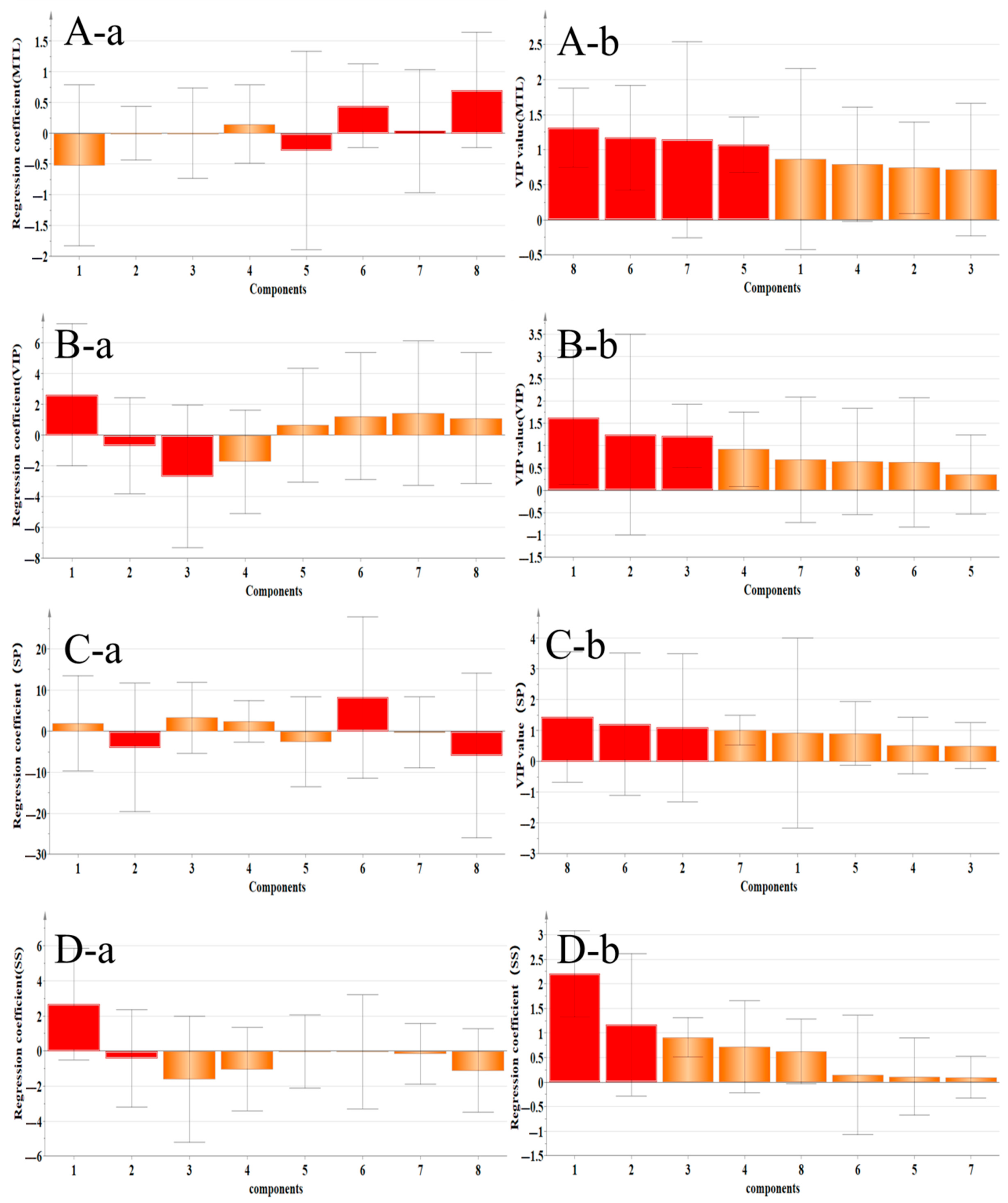
2.4.2. Correlation Analysis Between Gastrointestinal–Related Factors and Main Components
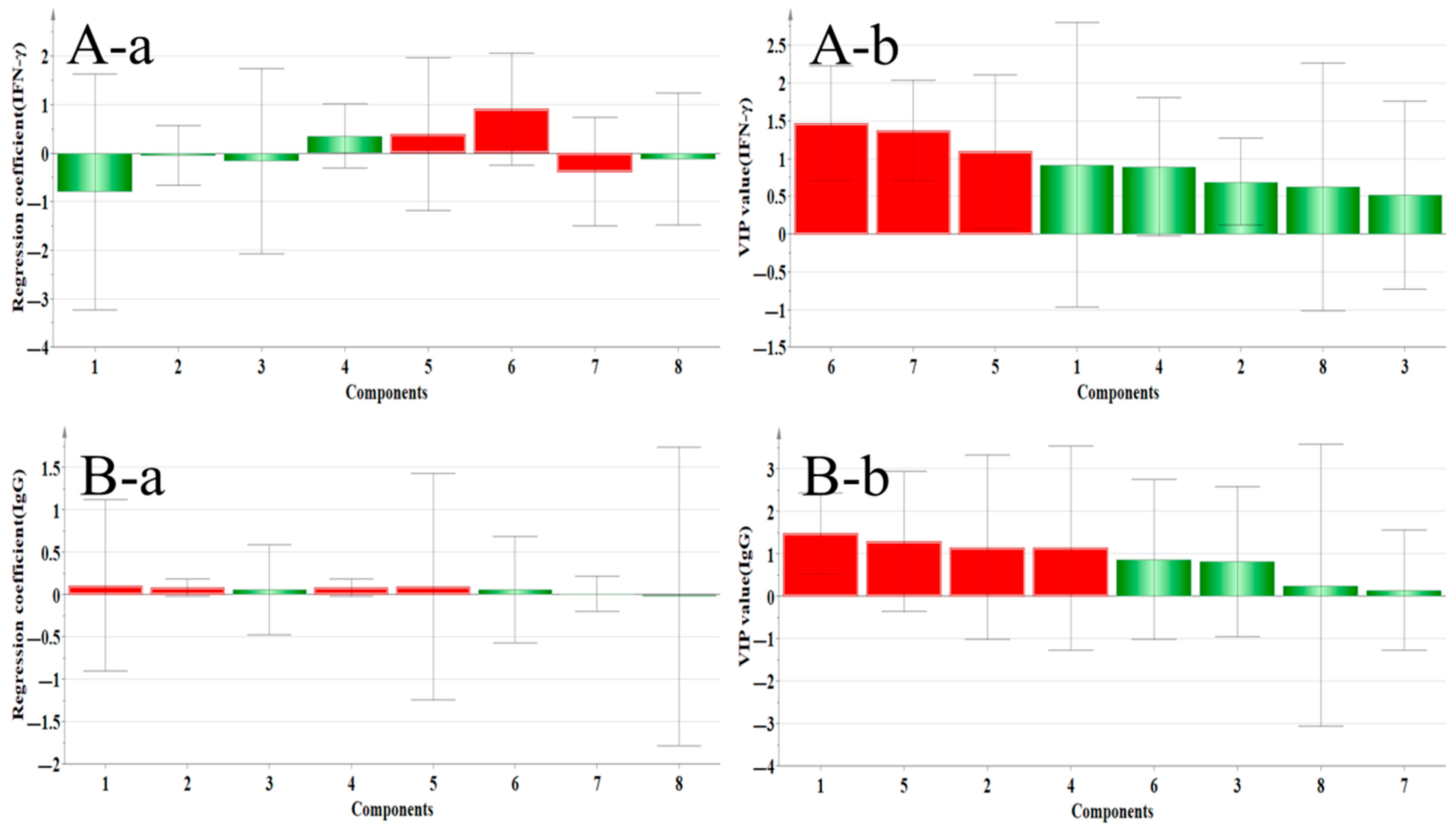
2.4.3. Correlation Analysis Between Immune Factors and Main Components
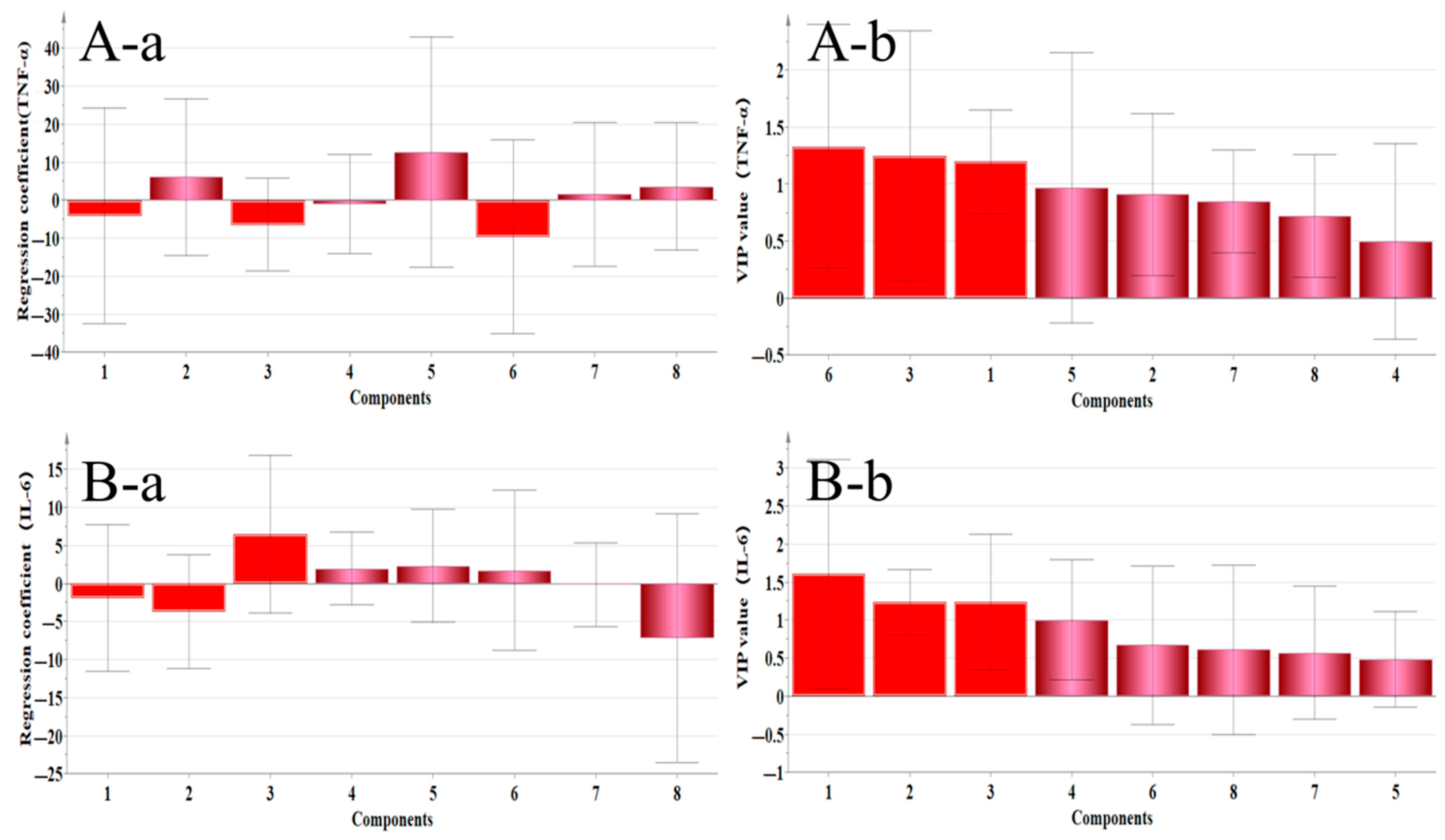
2.4.4. Correlation Analysis Between Inflammatory Factors and Main Active Components
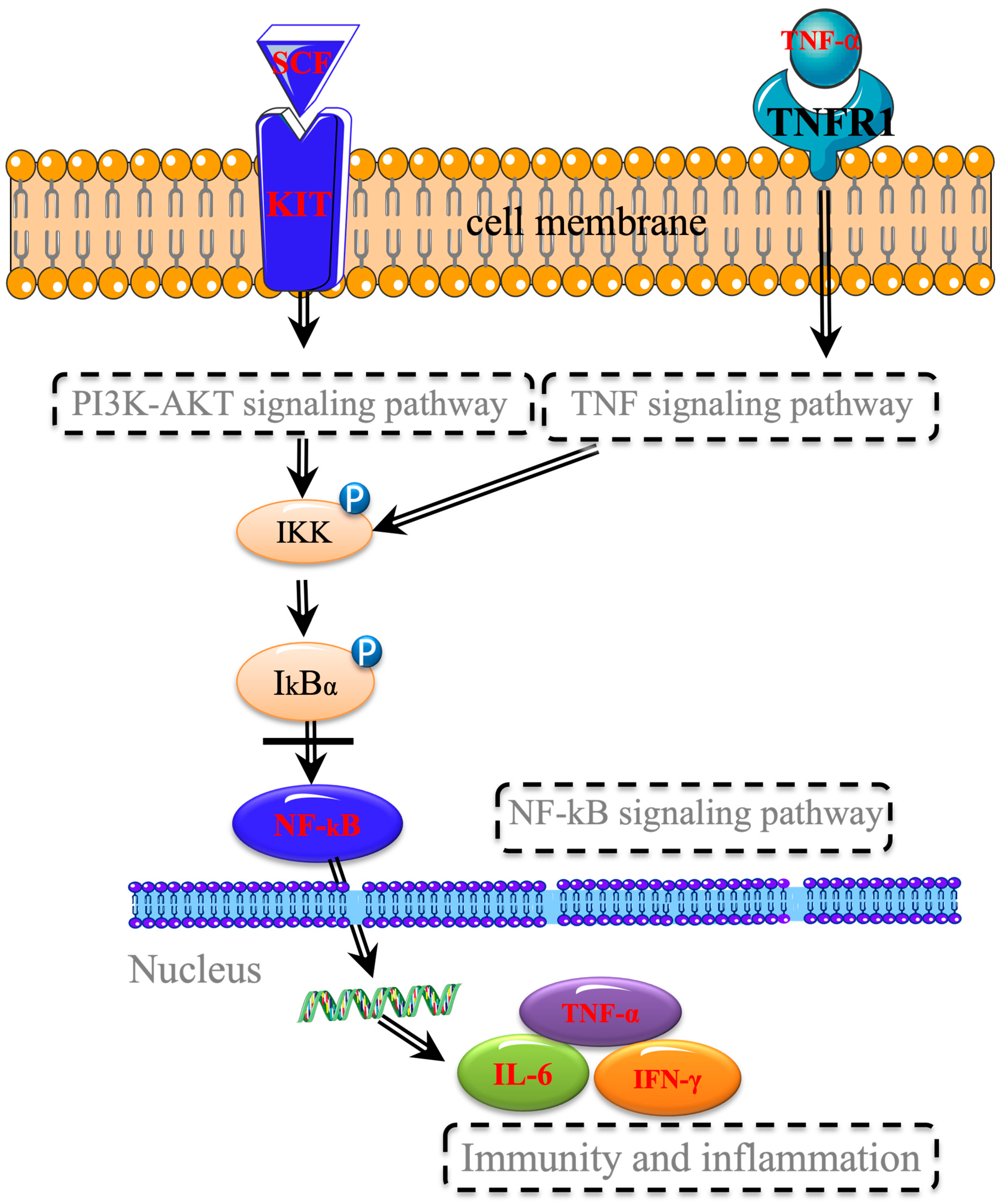
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Different Extracts
4.3. HPLC Quantification of Flavonoids
4.3.1. HPLC Conditions
4.3.2. Preparation of Reference Substance and Sample Solution
4.4. Prediction of “Target–Protein–Pathway” of RH Against Gastrointestinal Injury Induced by Chemotherapy [39,40]
4.4.1. Network Pharmacology Workflow
4.4.2. Construction of “Drug–Compound–Target” Network
4.4.3. Construction of PPI Protein Interaction Network and Screening of Key Targets
4.4.4. GO and KEGG Analysis
4.5. Screening of Effective Fractions and Study on Mechanism of Action [42]
4.5.1. Animals
4.5.2. Monitoring of General Behavior
4.5.3. Collection of Serum and Specimens
4.5.4. Detection of Serum Factor
4.5.5. Pathological Detection by H&E Staining
4.5.6. Western Blot Analysis
4.6. Correlation Analysis of “Dose–Effect”
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sharma, A.; Jasrotia, S.; Kumar, A. Effects of Chemotherapy on the Immune System: Implications for Cancer Treatment and Patient Outcomes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2551–2566. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Mallepally, N.; Goldstein, R.; Chen, E.; Tang, T.; Dike, U.K.; Al-Asadi, M.; Westin, S.; Halperin, D.; Wang, Y. Gastrointestinal toxic effects in patients with cancer receiving platinum–based therapy. J. Cancer 2020, 11, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; He, D.H.; Cheng, Y.X. Protection against chemotherapy– and radiotherapy–induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Salama, S.A.; Youssef, M.E. Advances in understanding cisplatin–induced toxicity: Molecular mechanisms and protective strategies. Eur. J. Pharm. Sci. 2024, 203, 106939. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fox, M.; Cong, Y.; Chu, H.; Zheng, X.; Long, Y.; Fried, M.; Dai, N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: The roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment. Pharmacol. Ther. 2014, 39, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wei, Y.; Cheng, W.; Zhang, L. Sulfated modification, characterization and antitumor activities of Radix hedysari polysaccharide. Int. J. Biol. Macromol. 2012, 51, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Shi, G.; Fang, Y.; Liu, X.; Zhou, X.; Feng, S.; Zhao, L. Protective effect of polysaccharides from Radix Hedysari on gastric ulcers induced by acetic acid in rats. Food Funct. 2019, 10, 3965–3976. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhao, L.; Wang, D.; Chen, X.; Liu, D.; Liu, X.; Feng, S. Structural characterization of a polysaccharide from Radix Hedysari and its protective effects against H(2)O(2)–induced injury in human gastric epithelium cells. Int. J. Biol. Macromol. 2021, 189, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Niu, J.; Zhang, S.; Si, X.; Bian, T.T.; Wu, H.; Li, D.; Sun, Y.; Jia, J.; Xin, E.; et al. Comparative study on the gastrointestinal– and immune– regulation functions of Hedysari Radix Paeparata Cum Melle and Astragali Radix Praeparata cum Melle in rats with spleen–qi deficiency, based on fuzzy matter–element analysis. Pharm. Biol. 2022, 60, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, D.; Xue, Z.; Zhou, X.; Zhao, L. The amelioration of ulcerative colitis induced by Dinitrobenzenesulfonic acid with Radix Hedysari. J. Food Biochem. 2020, 4, e13421. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Barros, H.R.D.M.; García-Villalba, R.; Tomás-Barberán, F.A.; Genovese, M.I. Evaluation of the distribution and metabolism of polyphenols derived from cupuassu (Theobroma grandiflorum) in mice gastrointestinal tract by UPLC–ESI–QTOF. J. Funct. Foods 2016, 22, 477–489. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.Y.; Li, J.T.; Zhang, Y.; Li, X.Y.; Liu, Y.Q.; Jing, M.; Sun, S.B.; Su, Y.; Liu, G.W. Effect of flavonoids from hedysari radix on pulmonary functions of pulmonary fibrosis rat. Chin. Med. Mate 2013, 36, 771–775. (In Chinese) [Google Scholar]
- Wang, Y.L.; Luo, Y.L.; Chen, C.; Li, N.L.; She, Y.L.; Zhang, L. The influence of the total flavonoids of Hedysarum polybotry on the proliferation, cell cycle, and expressions of p21Ras and proliferating cell nuclear antigen gene in erythroleukemia cell line K562. Chin. J. Integr. Med. 2012, 18, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liu, X.; Wang, Z.W. Study on the effect of different extracts from Radix Hedysari on neurological function. Pharmacol. Clin. Chin. Mater. Clin. Med. 2018, 34, 104–107. (In Chinese) [Google Scholar] [CrossRef]
- Shao, J.; Wang, Z.W.; Du, L.D. Study of effect on immunocompromised mice induced by cyclophosphamide and the relative compound content of different extracts of Radix hedysari. Chin. J. Clin. Pharmacol. 2018, 34, 698–702. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, S.S.; He, H.; Wang, Z.Y.; Xing, Y.Y.; Ren, Y.; Shao, J. Study on the relationship of target organ injury of mechanism and “structure–effect–dose” of Hedysari Radix during radiotherapy–chemotherapy induced. Chin. Pharmacol. Bull. 2024, 40, 371–380. (In Chinese) [Google Scholar]
- Zhang, S.; Yang, P.; Li, X.; Wang, X.; Song, J.; Peng, W.; Wu, C. Comparative Researches of Semen Arecae and Charred Semen Arecae on Gastrointestinal Motility, Motilin, Substance P, and CCK in Chronically Stressed Rats. Evid. Based Complement. Alternat Med. 2017, 2017, 1273561. [Google Scholar] [CrossRef] [PubMed]
- Geissler, E.N.; Liao, M.; Brook, J.D.; Martin, F.H.; Zsebo, K.M.; Housman, D.E.; Galli, S.J. Stem cell factor (SCF), a novel hematopoietic growth factor and ligand for c–kit tyrosine kinase receptor, maps on human chromosome 12 between 12q14.3 and 12qter. Somat. Cell Mol. Genet. 1991, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.S.; Sy, E.D.; Tsai, M.L.; Tang, L.Y.; Li, P.S.; Lin, P.W. Effects of somatostatin prophylaxis after pylorus–preserving pancreaticoduodenectomy: Increased delayed gastric emptying and reduced plasma motilin. World J. Surg. 2005, 29, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.J.; Wu, Z.J.; Sun, Y.P.; Xu, G.; Xu, K.G.; Tian, G.Q. Changes of somatostatin (SS) in stomach and duodenum of rats after +Gz exposure. Space Med. Med. Eng. 2005, 18, 148–150. (In Chinese) [Google Scholar] [CrossRef]
- Lin, R.; Lin, Y.; Wang, J.; Peng, L. Regulation of mouse digestive function, intestinal mucosal barrier function, and inflammatory reaction by lycium barbarum polysaccharide pathway through myosin light chain kinase. Heliyon 2024, 10, e29795. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, G.; Yu, Z.; Sun, H.; Wang, L. Role of interferon–gamma (IFN–γ) and IFN–γ receptor 1/2 (IFNγR1/2) in regulation of immunity, infection, and cancer development: IFN–γ–dependent or independent pathway. Biomed. Pharmacother. 2022, 155, 113683. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and significance of c–KIT receptor tyrosine kinase in cancer: A review. Bosn. J. Basic. Med. Sci. 2022, 22, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, D.; He, Y.; Wang, X.; Gao, L.; Li, J.; Yan, M.; Liu, D.; Wang, Y.; Zhang, L.; et al. The impact of inflammatory cells in malignant ascites on small intestinal ICCs’ morphology and function. J. Cell Mol. Med. 2015, 19, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Santana, L.F.; Baker, S.A. Interstitial cells of Cajal–pacemakers of the gastrointestinal tract. J. Physiol. 2023, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xing, F.; Tao, Y.; Liu, H.; Huang, K.; Peng, Y.; Feng, N.; Liu, C. Xiaozhang Tie Improves Intestinal Motility in Rats With Cirrhotic Ascites by Regulating the Stem Cell Factor/c–kit Pathway in Interstitial Cells of Cajal. Front. Pharmacol. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Qiu, H.; Wang, K.; He, J.W.; Chen, H.; Kong, L.W.; Zou, Y. Changes in esophagus interstitial cells of Cajal in response to acute stress. Scand. J. Gastroenterol. 2022, 57, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.T.; Ma, B.; Wei, W.; Xu, Y.; Wang, Y.; Tang, H.T.; Xia, Z.F. Sustained activation of nuclear factor–kappaB by reactive oxygen species is involved in the pathogenesis of stress–induced gastric damage in rats. Crit. Care Med. 2007, 35, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.M.; Beuers, U.; Vujasinovic, M.; Alvaro, D.; Frøkjær, J.B.; Buttgereit, F.; Capurso, G.; Culver, E.L.; de-Madaria, E.; Della-Torre, E.; et al. European Guideline on IgG4–related digestive disease–UEG and SGF evidence–based recommendations. United Eur. Gastroenterol. J. 2020, 8, 637–666. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.; Hwang, S.J.; Kim, W.J.; Youn, H.J.; Lee, H.J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF–kB. Biomed. Pharmacother. 2018, 99, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhan, X.; Tu, J.; Xu, K.; Sun, X.; Liu, C.; Ke, C.; Cao, G.; Zhou, Z.; Liu, Y. Atractylodes oil alleviates diarrhea–predominant irritable bowel syndrome by regulating intestinal inflammation and intestinal barrier via SCF/c–kit and MLCK/MLC2 pathways. J. Ethnopharmacol. 2021, 272, 113925. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Huang, Y.; Tang, H.; Tu, X.; He, J.; Wang, T.; Zhang, Q.; Xiong, F.; Li, D.; Qiu, Z. Role of stem cell growth factor/c–Kit in the pathogenesis of irritable bowel syndrome. Exp. Ther. Med. 2017, 13, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Mao-mao; Shao, M.-M.; Wang, M.-C. Therapeutic potential of natural flavonoids in pulmonary arterial hypertension: A review. Phytomedicine 2024, 128, 155535. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhou, Y.; Guo, L.; Chen, C. MFE–DDI: A multi–view feature encoding framework for drug–drug interaction prediction. Comput. Struct. Biotechnol. J. 2025, 27, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T. Unveiling secrets of traditional Chinese medicine: Cutting–edge techniques in component analysis. Chin. Herbal. Med. 2025, 17, 484–487. [Google Scholar] [CrossRef]
- Naota, M.; Nozaki, Y.; Mikashima, F.; Higashiyama, G. Key considerations for dose selection in first–in–human studies of cell and gene therapy products. Cytotherapy 2025. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Chen, C.; Ding, N.; Zheng, P.; Chen, X.; Ma, S.; Yang, M. TCMNPAS: A comprehensive analysis platform integrating network formulaology and network pharmacology for exploring traditional Chinese medicine. Chin. Med. 2024, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, H.; Wang, X.; Kang, J.; Guo, W.; Zhou, L.; Liu, H.; Wang, M.; Jia, R.; Du, X.; et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 2022, 95, 153837. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, M.; Yang, X.; Tu, L.; Liu, J.; Yang, Y.; Li, R.; Tang, X.; Hu, Y.; Zhang, G.; et al. Total alkaloids in Fritillaria cirrhosa D. Don alleviate OVA–induced allergic asthma by inhibiting M2 macrophage polarization. J. Ethnopharmacol. 2025, 337 Pt 3, 118935. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, H.; Chen, L.; Yuan, H.; Peng, C.; Wang, W. Distinct Changes in Metabolic Profile and Sensory Quality with Different Varieties of Chrysanthemum (Juhua) Tea Measured by LC–MS–Based Untargeted Metabolomics and Electronic Tongue. Foods 2024, 13, 1080. [Google Scholar] [CrossRef] [PubMed]
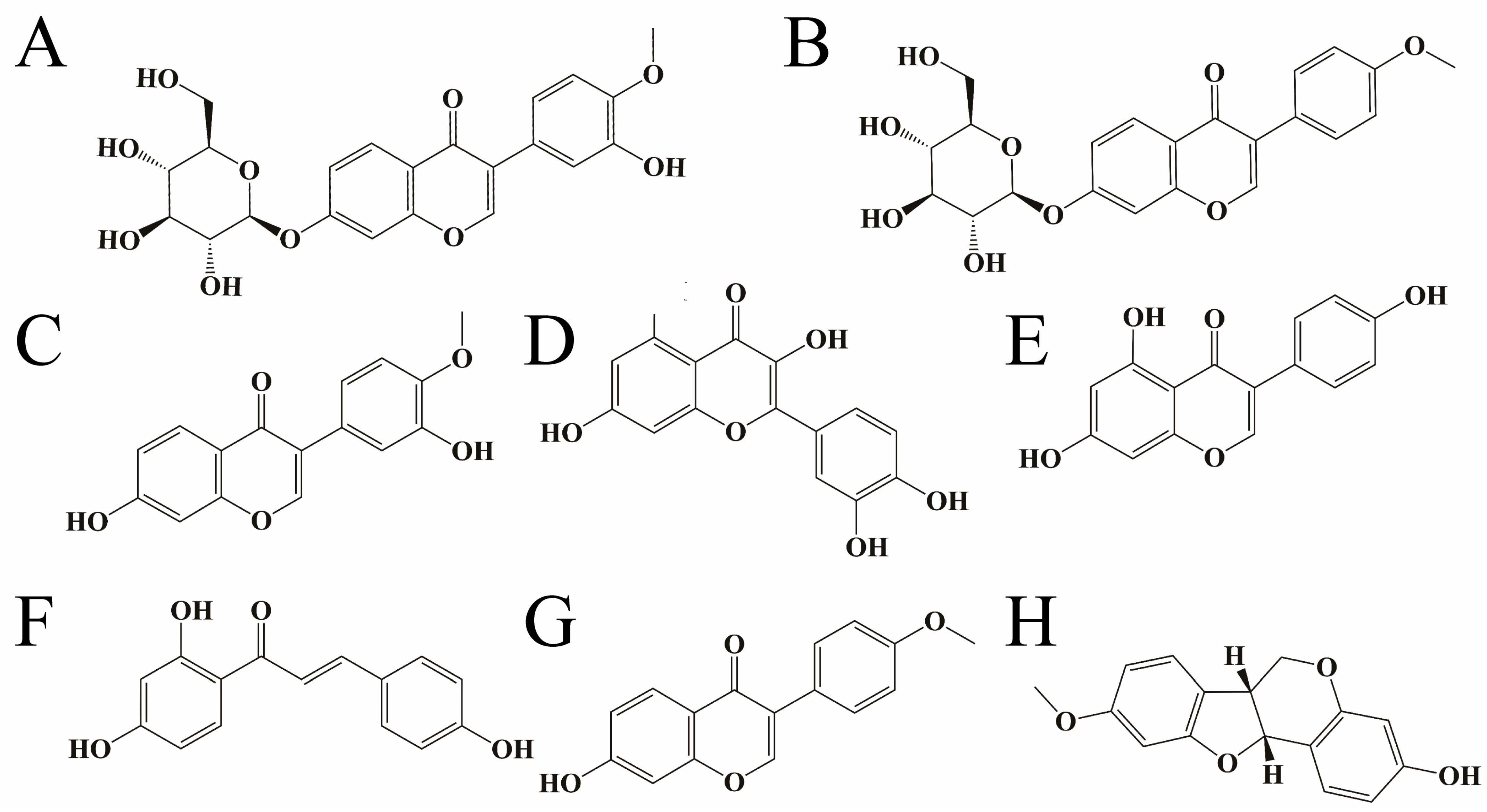

| WE | 95% EtOH | EtOAc | CHCl3 | PE | n–BuOH | RWE | |
|---|---|---|---|---|---|---|---|
| Calycosin–7–glucoside | 3.0149 | 2.3015 | 9.1728 | 0.1366 | 0.0128 | 6.8075 | 0.0965 |
| Ononin | 1.6171 | 2.8848 | 22.2778 | 1.2574 | 0.1193 | 6.5518 | 0.1669 |
| Calycosin | 1.2201 | 1.2348 | 7.8777 | 2.0956 | 0.0100 | 0.9642 | 0.0930 |
| Quercetin | 0.4948 | 0.6551 | 8.0639 | 0.6411 | 0.0088 | 0.8874 | —— |
| Genistein | 0.4624 | 0.8606 | 0.7293 | 0.1659 | —— | 0.2242 | 0.5757 |
| Isoliquiritigenin | 0.8077 | 1.0296 | 0.8593 | 0.2022 | —— | —— | —— |
| Formononetin | 0.6932 | 1.1765 | 0.6604 | 0.3986 | 0.0136 | 0.2949 | 0.2626 |
| Medicarpin | 0.5689 | 1.5039 | 1.6939 | 0.7397 | 0.3220 | 0.4956 | 0.2055 |
| Total content | 8.8791 | 11.6468 | 51.3351 | 5.6371 | 0.4865 | 16.2256 | 1.4002 |
| Target Name | Protein | Degree | Closeness Centrality | Betweenness Centrality |
|---|---|---|---|---|
| IL6 | Interleukin 6 | 20 | 0.613 | 0.88 |
| HSP90AA1 | Heat shock protein HSP 90–alpha | 19 | 0.49 | 0.84 |
| MAPK3 | MAP kinase–activated protein kinase 3 | 17 | 0.77 | 0.53 |
| MAPK1 | Mitogen–activated protein kinase 1 | 17 | 0.75 | 0.64 |
| ESR1 | Estrogen receptor | 17 | 0.55 | 0.23 |
| IL1B | Interleukin–1 beta | 15 | 0.49 | 0.30 |
| IFNG | Interferon gamma | 12 | 0.47 | 0.05 |
| RELA | Transcription factor p65 | 11 | 1.00 | 0.09 |
| RAF1 | RAF proto–oncogene serine | 8 | 0.60 | 0.14 |
| CHUK | Inhibitor of nuclear factor kappa–B kinase subunit alpha | 7 | 0.40 | 0.06 |
| Group | Spleen Index | Thymus Index |
|---|---|---|
| BCG | 2.59 ± 0.35 a | 2.15 ± 0.69 a |
| MCG | 2.14 ± 0.32 bc | 1.25 ± 0.64 bc |
| PCG | 2.31 ± 0.24 ab | 1.65 ± 0.50 b |
| WEG | 2.12 ± 0.22 bc | 1.10 ± 0.32 c |
| 95% EtOH | 2.16 ± 0.23 bc | 1.18 ± 0.45 bc |
| PE | 2.27 ± 0.41 ab | 1.24 ± 0.47 bc |
| CHCl3 | 2.26 ± 0.29 bc | 1.23 ± 0.37 bc |
| EtOAc | 2.18 ± 0.19 bc | 1.20 ± 0.41 bc |
| n–BuOH | 2.27 ± 0.31 ab | 1.42 ± 0.48 ab |
| HPS | 2.05 ± 0.22683 c | 1.27 ± 0.71 bc |
| RWEG | 2.23 ± 0.25 bc | 1.41 ± 0.36 ab |
| Regression Coefficient > 0 | VIP > 1 | Positive Correlation Component | |
|---|---|---|---|
| Spleen index | Ononin, Quercetin, and Calycosin | Genistein, Isoliquiritigenin, and Formononetin | No statistical significance |
| Thymus index | Calycosin–7–glucoside, Ononin, Genistein, and Quercetin | Isoliquiritigenin, Formononetin, and Calycosin–7–glucoside | Calycosin–7–glucoside |
| VIP | Calycosin–7–glucoside, Isoliquiritigenin, Medicarpin, Formononetin, and Isoliquiritigenin | Calycosin–7–glucoside, Ononin, and Calycosin | Calycosin–7–glucoside |
| SS | Calycosin–7–glucoside | Calycosin–7–glucoside and Ononin | Calycosin–7–glucoside |
| MTL | Medicarpin, Isoliquiritigenin, Formononetin, and Quercetin | Medicarpin, Isoliquiritigenin, Formononetin, and Genistein | Medicarpin, Isoliquiritigenin, and Formononetin |
| SP | Isoliquiritigenin, Calycosin, Calycosin–7–glucoside, and Quercetin | Ononin, Isoliquiritigenin, and Medicarpin | Isoliquiritigenin |
| IFN–γ | Quercetin, Genistein, and Isoliquiritigenin | Genistein, Isoliquiritigenin, and Formononetin | Isoliquiritigenin and Genistein |
| IgG | Calycosin–7–glucoside, Ononin, Calycosin, Genistein, Quercetin, and Isoliquiritigenin | Calycosin–7–glucoside, Ononin, Genistein, and Quercetin | Calycosin–7–glucoside, Ononin, Genistein, and Quercetin |
| TNF–α | Ononin, Genistein, Formononetin, and Medicarpin | Calycosin–7–glucoside, Ononin, and Calycosin | No statistical significance |
| IL–6 | Calycosin, Isoliquiritigenin, Quercetin, and Genistein | Calycosin–7–glucoside, Ononin, and Calycosin | Calycosin |
| Extract | Weight of Extract (g) | Yield (%) |
|---|---|---|
| WE | 115.65 | 23.13 |
| 95% EtOH | 76.30 | 15.26 |
| PE | 0.42 | 0.05 |
| CHCl3 | 0.76 | 0.10 |
| EtOAc | 0.69 | 0.09 |
| n–BuOH | 19.9 | 2.49 |
| RWE | 23.30 | 2.91 |
| HPS | 3.94 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Yang, M.; Yang, Z.; He, H.; Wang, Z.; Zhu, X.; Cui, Z.; Shao, J. Study on the Mechanism and Dose–Effect Relationship of Flavonoids in Different Extracts of Radix Hedysari Against Gastrointestinal Injury Induced by Chemotherapy. Pharmaceuticals 2025, 18, 1072. https://doi.org/10.3390/ph18071072
Zhao S, Yang M, Yang Z, He H, Wang Z, Zhu X, Cui Z, Shao J. Study on the Mechanism and Dose–Effect Relationship of Flavonoids in Different Extracts of Radix Hedysari Against Gastrointestinal Injury Induced by Chemotherapy. Pharmaceuticals. 2025; 18(7):1072. https://doi.org/10.3390/ph18071072
Chicago/Turabian StyleZhao, Shasha, Miaomiao Yang, Zimu Yang, Hai He, Ziyang Wang, Xinyu Zhu, Zhijia Cui, and Jing Shao. 2025. "Study on the Mechanism and Dose–Effect Relationship of Flavonoids in Different Extracts of Radix Hedysari Against Gastrointestinal Injury Induced by Chemotherapy" Pharmaceuticals 18, no. 7: 1072. https://doi.org/10.3390/ph18071072
APA StyleZhao, S., Yang, M., Yang, Z., He, H., Wang, Z., Zhu, X., Cui, Z., & Shao, J. (2025). Study on the Mechanism and Dose–Effect Relationship of Flavonoids in Different Extracts of Radix Hedysari Against Gastrointestinal Injury Induced by Chemotherapy. Pharmaceuticals, 18(7), 1072. https://doi.org/10.3390/ph18071072






