1. Introduction
Extracellular vesicles (EVs) are phospholipid bilayer-enclosed particles released by all cell types. These vesicles can be easily separated from tissue culture supernatants and biological fluids, including blood, saliva, breast milk, cerebrospinal fluid, and malignant ascites. EVs can be categorized into two distinct subtypes based on their biogenesis: exosomes and microvesicles (MVs). Exosomes are formed through the inward budding of the endosome limiting membrane, leading to the creation of multivesicular bodies that fuse with the plasma membrane and are subsequently released into the extracellular space. Extracellular MVs arise from plasma membrane invagination, forming neck-like structures that eventually undergo vesicle scission. Exosomes are typically 50–150 nm in size, while MVs range from 100 to 1000 nm in diameter. Distinguishing between exosomes and MVs can be challenging due to overlapping sizes, shared surface proteins, and the absence of specific markers [
1].
Cell activation and apoptosis are well-established stimuli for the formation and secretion of EVs, which serve as carriers for various biomolecules, including proteins, lipids, DNA, and different RNA species. The loading of EVs with molecular cargo occurs through an organized process that controls biomolecule sorting [
1]. The primary function of EVs is to transport and protect biomolecules from degradation by extracellular enzymes and other aggressive factors. Published evidence suggests that MVs can also transport and protect cell organelles, such as mitochondria. MVs possess intrinsic stability due to their overall negatively charged surface and expression of the surface immunoglobulin CD47 biomarker, which enables evasion from engulfment by mononuclear phagocytic cells [
2]. The migration direction patterns of EVs in the body are determined by the surface receptors they express [
3]. Recent reports have also demonstrated the efficient crossing of various biological cell membrane barriers, including the blood–brain barrier, by EVs [
1]. A low pH environment has been shown to stimulate EV fusion with cell membranes [
4].
Mesenchymal stem cells (MSCs) have been extensively studied and applied in regenerative medicine, with numerous clinical trials conducted for a wide range of diseases [
5]. Recently, research focus has shifted towards the investigation of EV preparations derived from MSCs. In particular, MSC-derived EVs have shown promising potential for in vivo tissue regeneration and the treatment of various diseases in experimental models, including respiratory, renal, hepatic, neurodegenerative, musculoskeletal, and cardiovascular disorders. These studies have highlighted the robust anti-inflammatory, antiapoptotic, pro-angiogenic, and immunomodulatory effects exhibited by MSC-derived EVs, similar to those displayed by MSCs in different disease models. Specifically, MSC-derived EVs from different sources have demonstrated renoprotective activity in experimental models of chronic kidney injury (CKI), diabetic nephropathy, and kidney fibrosis [
6]. Administration of these EVs has been found to significantly reduce serum markers of renal injury, such as blood urea nitrogen, creatinine, and transaminase levels, while improving necrotic lesions and ameliorating pronounced signs of tubular dilatation and cast formation [
7].
Currently, the treatment of CKI remains challenging and often fails to achieve the desired outcomes [
5,
8]. This report presents experimental data demonstrating, for the first time, that tumor cell-derived microvesicles (T-MVs) of various origins, similar to MSCs and MSC-derived microvesicles (MSC-MVs), effectively restore damaged kidney tissue and function in an experimental model of CKI. Furthermore, the observed regenerative effect may be partially attributed to the inhibition of pro-inflammatory immune reactivity.
3. Discussion
The tumor is known to possess pathological regenerative activity, capable of modulating the tumor microenvironment to facilitate its own growth, with tumor-derived EVs playing an important role in this process. These EVs contain a range of biologically active biomolecules that stimulate tumor growth upon entry into malignant cells. Additionally, tumor-derived EVs interact with immune cells, effectively inhibiting the development of anti-tumor cytotoxic immune reactivity and reprogramming systemic immunity to sustain tumor invasion [
13]. Importantly, both tumors and normal tissues use similar molecular mechanisms to regulate regenerative processes, often involving overlapping biomolecules [
14]. C. Waddington’s pioneering study suggested that innate natural regenerative mechanisms could act as regulators of cancer, reaching maximal efficiency in malignant cells [
15].
In this study, we conducted a comparative analysis of T-MVs, bone marrow-derived MSCs, and MSC-MVs to evaluate their impact on regenerative processes in a murine CKI model. MSCs and MSC-derived MVs have been extensively studied in experimental and clinical settings for their potential to treat various diseases. Notably, MSC-derived MVs exhibit a regenerative profile similar to MSCs themselves [
6] and have been shown to reduce reactive oxygen species (ROS) production in renal tubular epithelial cells in models of renal hypoxic injury [
16]. Moreover, MSCs and MSC-derived EVs have demonstrated efficacy in reducing serum markers of kidney failure (urea, creatinine, and transaminases) and promoting kidney regeneration in experimental models of CKI, diabetic nephropathy, and renal fibrosis [
7]. These effects were partly attributed to small non-coding microRNAs and transforming growth factor β, which enhance the functional activity of regulatory T cells [
6].
In this study, we modeled CKI using glycerol-induced kidney injury, a well-established and reproducible model of chronic kidney disease in mice. Its principal strength is that renal damage arises directly from the nephrotoxic action of glycerol on tubular structures, rather than from secondary systemic disease. This enables a clearer interpretation of kidney-specific regenerative processes. Although no animal model can fully replicate the complexity of human CKD, glycerol-induced injury reproduces key pathophysiological features—including tubular damage, inflammation, and impaired renal function—thus providing a clinically relevant context for evaluating regenerative interventions.
Our findings strongly support the notion that T-MVs possess significant regenerative activity in the kidney, comparable or even superior to that observed with MSCs or MSC-MVs. Notably, all T-MV preparations used in this study were of non-kidney origin, suggesting that T-MVs may exhibit universal regenerative properties similar to those of MSCs and MSC-MVs. Although both extracellular MVs and exosomes contribute to regenerative activity, we propose that MVs offer superior advantages. First, MVs, like cells, express receptor molecules on their surface that enable targeted interactions with recipient cells. Second, in contrast to exosomes, MVs can transfer not only biomolecules but also cellular organelles such as mitochondria, which are typically non-viable in the extracellular space. Experimental studies have demonstrated that MVs can mediate mitochondrial transfer and transfection into target cells [
17]. The transferred mitochondria enhance cellular energy production, likely contributing to the observed regenerative effects in injured organs [
18].
Our experiments demonstrated that T-MVs effectively improved kidney function not only in a CKI model but also in an acute kidney injury model, where the limited time for regenerative cell growth necessitates immediate reparative mechanisms [
19]. Under such conditions, we speculate that MV-mediated mitochondrial transfer provides additional energy support to the injured organ, playing a crucial role in maintaining functional activity and facilitating cellular regeneration.
The direct interaction of MVs with kidney cells plays a key role in initiating kidney regeneration [
3,
5,
8]. Beyond their direct regenerative effects, T-MVs exhibit immunomodulatory properties similar to those of MSCs. Published data suggest that MSCs, tumor cells, and their respective extracellular MVs possess immunosuppressive activity that inhibits cytodestructive immune responses, at least in part, through the enhancement of regulatory CD4+CD25+FoxP3+ T cell function. Regulatory T cells create an immunological cytoprotective microenvironment that benefits both normal and pathological (tumor) regenerative processes [
12,
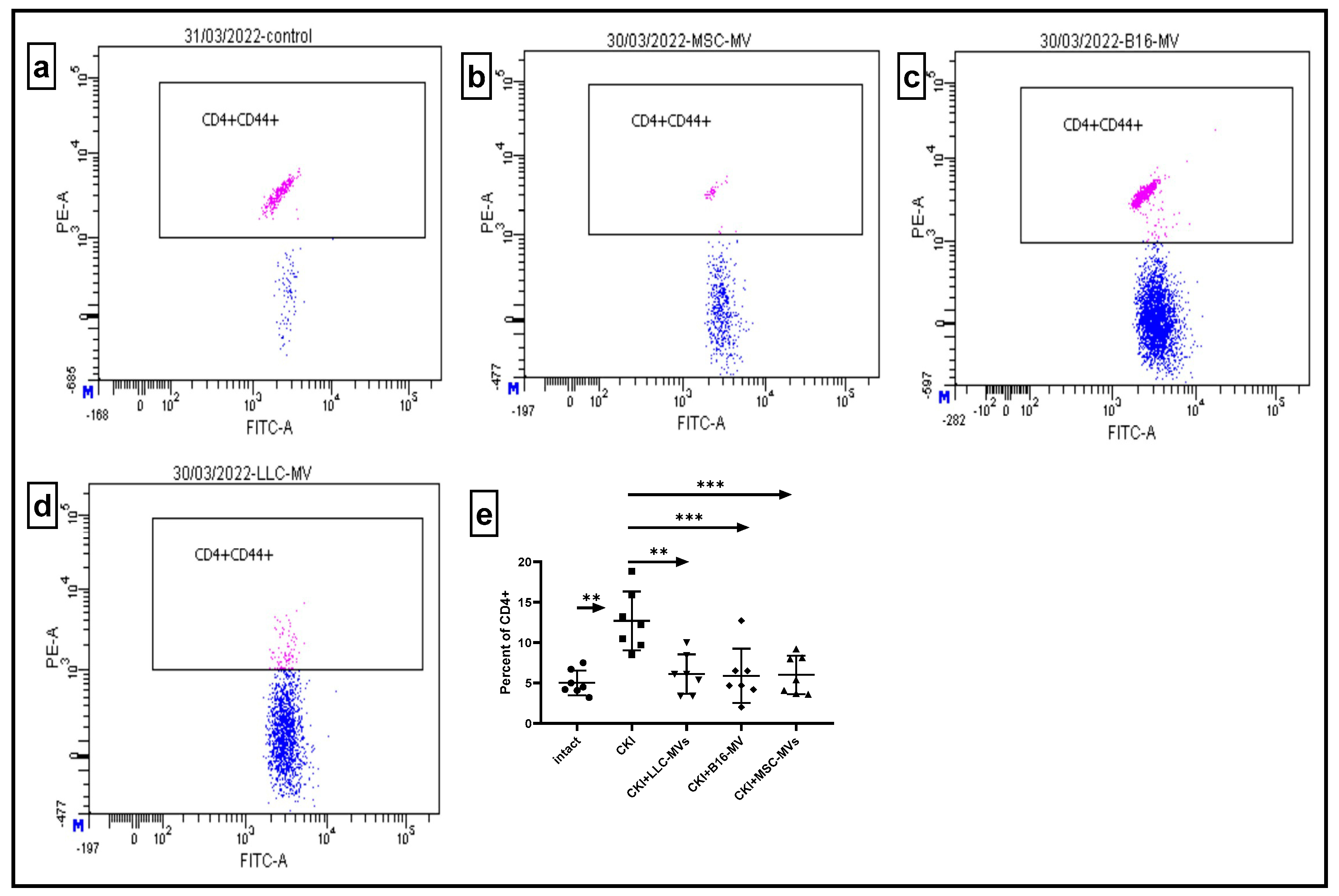
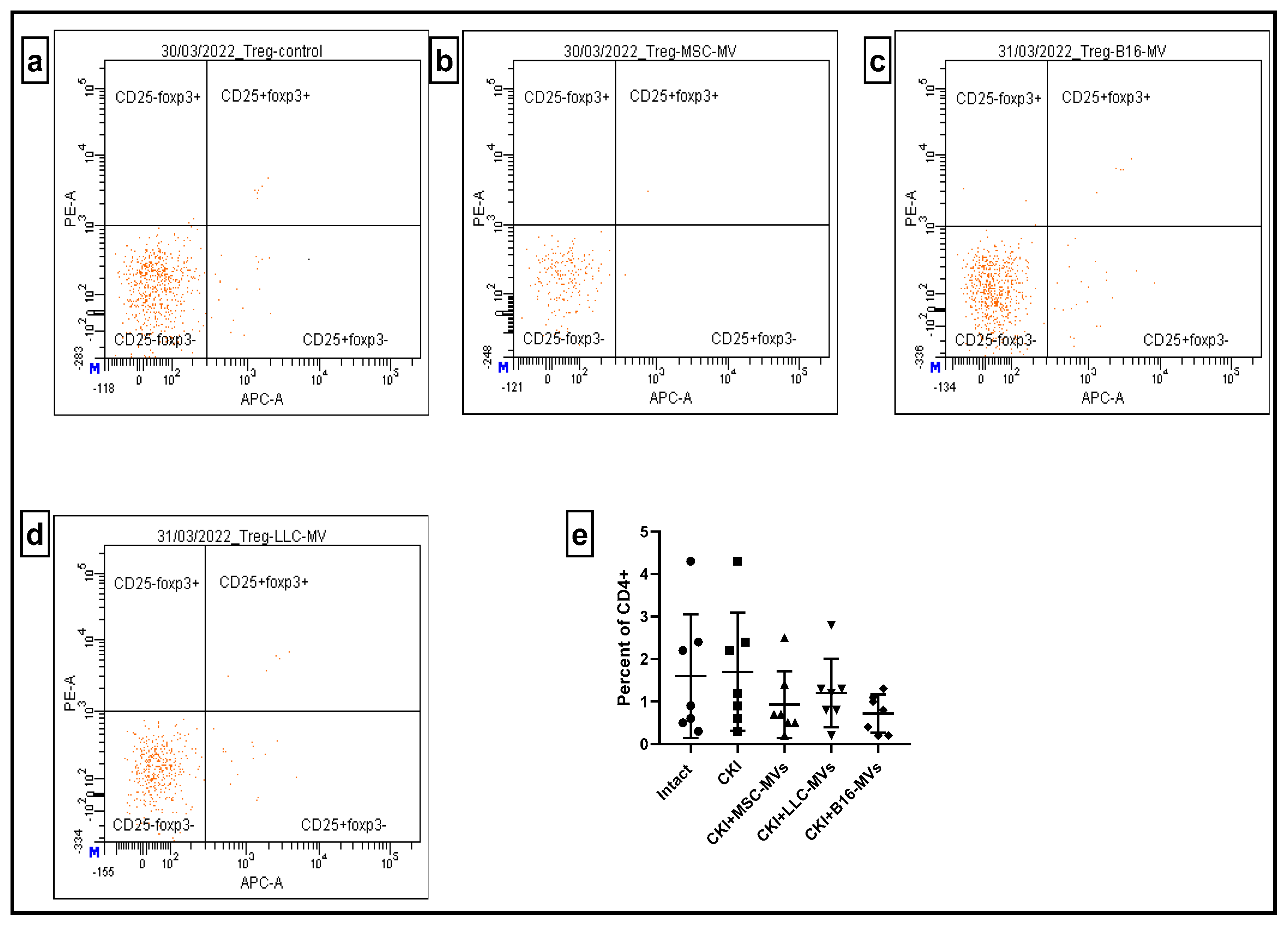
17]. Our study demonstrated that both MSC-MVs and T-MVs downregulated the relative content of pro-inflammatory CD4+CD44+ T cells in renal cell infiltrates and the spleens of CKI mice. Additionally, MVs restored (upregulated) the relative numbers of Tregs in the spleens of CKI mice. Although further studies are required, these data strongly suggest that immune-mediated modulation of inflammation could be one of the potential mechanisms underlying the regenerative effects of both MSC-MVs and T-MVs.
From a practical standpoint, generating MSCs is a laborious and technically demanding process requiring well-characterized donor cells. In contrast, tumor cells can be easily expanded in vitro without the need for donor material. Furthermore, T-MV isolation does not require the expensive and cumbersome ultracentrifugation or microfiltration steps necessary for exosome purification. Previous studies have demonstrated that xenogeneic MSC-derived EVs can influence the growth and functional activity of target cells [
20], suggesting that therapeutic T-MV-based drugs could be sourced from both human and animal cells. MV preparations also demonstrate stability in frozen and lyophilized forms over extended periods without significant loss of specific activity [
21]. These advantages make the large-scale production of T-MV-based regenerative drugs both feasible and cost-effective. Given their robust regenerative potential and immunomodulatory effects, T-MVs could fill a currently underrepresented niche in regenerative and immunotherapeutic medicine.
Despite their promising therapeutic profile, concerns remain regarding the potential oncogenic risks associated with T-MVs. Indeed, T-MVs have been shown to enhance tumor growth [
22]. However, it is important to note that T-MVs do not contain inherently mutagenic substances. Instead, they may accelerate the proliferation of cells that have already undergone malignant transformation rather than directly inducing carcinogenesis in healthy cells. In our study, a six-month follow-up of mice (n = 14) treated with T-MV preparations did not reveal an increased incidence of malignancy. At necropsy, no evidence of tumor formation or neoplastic involvement of internal organs was detected. Nevertheless, the long-term safety of repeated T-MV administration, particularly in chronic disorders, warrants further investigation.
For severe conditions, such as end-stage renal failure, the potential benefits of T-MV therapy may outweigh the oncogenic risks. Given their scalability and cost-effectiveness, T-MV-based treatments could represent a transformative approach for addressing various life-threatening conditions beyond kidney disease. Importantly, our experiments demonstrated that MVs derived from histologically different tumors had comparable regenerative effects on injured kidneys. However, it is plausible that the recovery of highly specialized organ functions could be influenced by the tumor origin of the MVs. From this perspective, MVs derived from kidney cancer cells might be optimal for renal therapy, while liver diseases may benefit more from liver cancer-derived MVs, and glioma-derived MVs could be most effective for neurological disorders. This hypothesis requires further experimental validation.
As for the limitations of this study, one potential concern is the possible contamination of MV preparations with apoptotic bodies. However, we maintain that apoptotic bodies, if present, are efficiently cleared by phagocytes and degraded within minutes to hours [
23], making it highly implausible that apoptotic debris could deliver sustained regenerative activity in vivo. We therefore consider their contribution negligible in the context of long-term regenerative outcomes. We emphasize that future studies aimed at a deeper characterization of MV content and purity—including the use of specific markers to distinguish apoptotic bodies from vesicles—will be essential to further strengthen the translational relevance of this approach.
Finally, our present study does not include direct mechanistic assays such as cytokine profiling or mitochondrial transfer experiments. At this stage, our conclusions are drawn from functional, morphological, and immunological outcomes rather than direct mechanistic proof. Indeed, our current research efforts are now focused on testing the mitochondrial transfer hypothesis in depth. Preliminary results are encouraging, but they remain inconclusive and raise additional questions that require careful investigation.
4. Materials and Methods
4.1. Mice
All experiments were conducted on male CBA mice aged between 4 and 6 months. The mice were provided with autoclaved food and boiled water. All procedures involving animals complied with the legislation of the Russian Federation and Directive 2010/63/EU of the European Parliament and Council of 22 September 2010 on the protection of animals used in scientific research. Euthanasia was performed using cervical dislocation.
4.2. Generation of Bone Marrow-Derived Mesenchymal Stromal Cells
Bone marrow was extracted from intact mice’s femoral and tibial bones using a glass homogenizer. The cells were then resuspended in cold RPMI 1640 medium. After washing, the cells were cultured in plastic flasks using a complete cell culture medium consisting of RPMI 1640 medium supplemented with 10% foetal calf serum, 2 mM L-glutamine, and antibiotics. All reagents were sourced from Sigma-Aldrich (St. Louis, MI, USA). Non-adherent cells were progressively removed starting from day 3. The spindle-shaped, plastic-adherent MSCs displayed a classical fibroblast-like phenotype and formed a complete monolayer by week 4. The MSCs were collected using a 0.25% versine–trypsin solution, followed by two washes in serum-free medium.
4.3. Separation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from blood using Ficoll–diatrizoate solution (density: 1.077 g/mL) gradient centrifugation at 1500 revolutions per minute for 40 min. Floating cells were collected, washed, counted, and utilised in subsequent experiments.
4.4. Tumor Cell Lines
The L929 murine fibroblast cell line, Lewis lung epidermoid carcinoma (LLC), and B16 melanoma cell lines were maintained in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, and antibiotics.
4.5. Generation, Isolation and Characterisation of Extracellular MVs
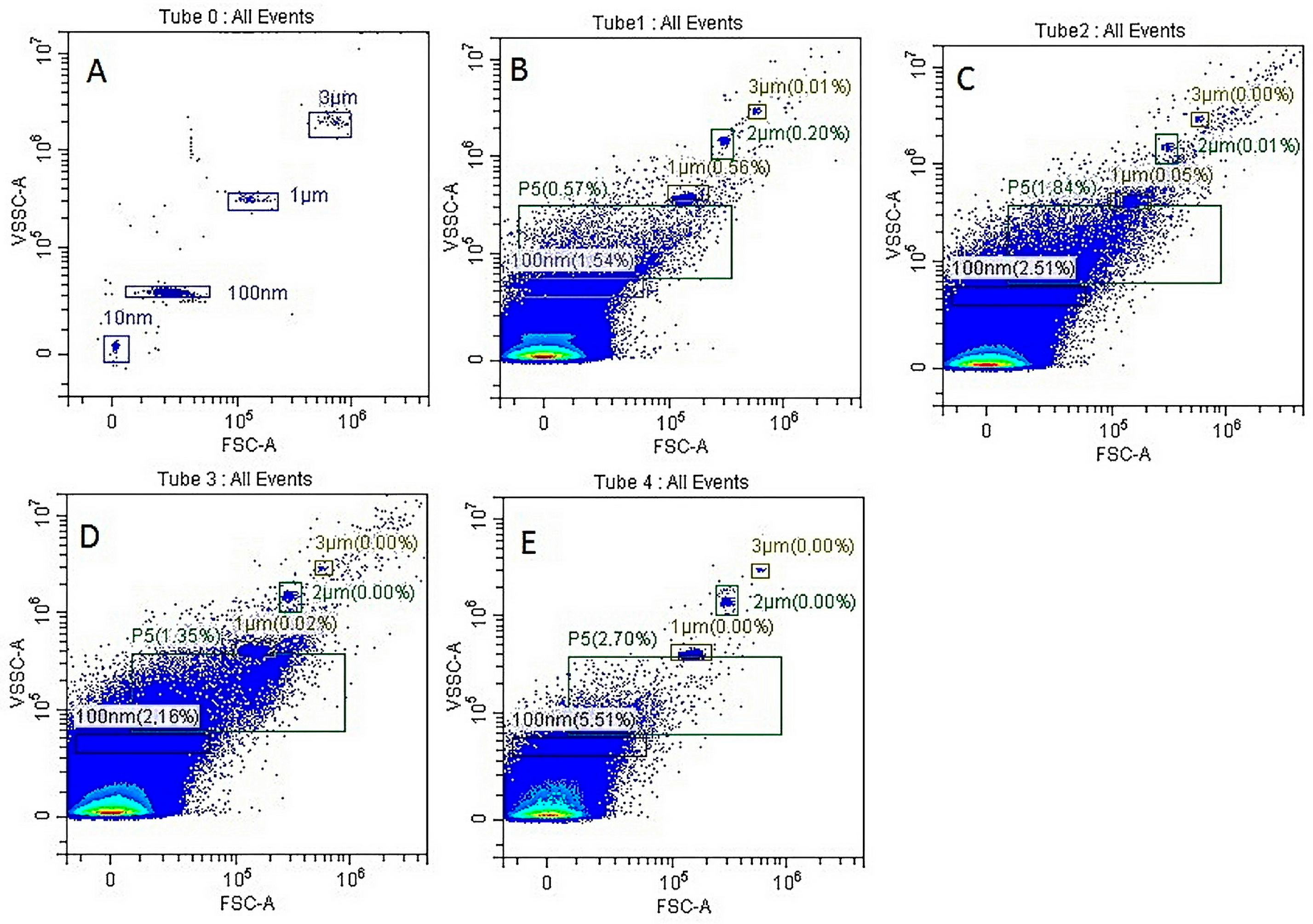
Apoptosis of MSCs, L929, LLC, B16 cells or PBMCs (1 × 106/mL) was induced by cultivating them under conditions of oxygen deprivation in serum-free medium for 24–48 h. After cultivation, cell debris was removed by centrifugation at 2000× g for 20 min, and the supernatants were subjected to a second centrifugation step at 12,000× g for 60 min at 4 °C. The MV pellet was resuspended in 100 μL of saline, and the size of Annexin V-positive MVs was determined by flow cytometry using a CytoFlex benchtop flow cytometer (Beckman Coulter Life Sciences, Indianapolis, IN, USA) with TruCOUNT nanoparticles (TC) for bead size calibration, strictly following the manufacturer’s instructions.
4.6. Induction of Chronic Kidney Injury
We induced rhabdomyolysis using glycerol, administered as a 50% glycerol solution intramuscularly into the hind limb muscles at a dose of 100 µL/mouse three times at weekly intervals. Glycerol-induced rhabdomyolysis exerted a mixed influence (ischemic, toxic, and retentional) on the kidneys, predominantly damaging the epithelium of the proximal convoluted tubules [
24,
25,
26,
27].
4.7. Treatment of CKI Mice with MSCs or MVs
MSCs (2 × 106/mouse) or MVs (derived from 2 × 106 cells or approximately 50–100 µg/mouse) were injected intravenously into the tail veins of the experimental mice 3 weeks after the last glycerol administration. The experiments were terminated 11 days after the administration of MSC/MV preparations. The study included seven groups, each consisting of ten male mice:
Control group (CKI mice without treatment).
CKI group treated with MSCs.
CKI group treated with MSC-MVs.
CKI group treated with L929 cell-derived MVs (L929-MVs).
CKI group treated with LLC-MVs.
CKI group treated with B16-MVs.
CKI group treated with PBMC-MVs
At least two independent identical experiments were performed.
4.8. Biochemical Analysis
Blood creatinine levels were measured in mg/dL using the Creatinine Parameter Assay Kit (R&D Systems, Minneapolis, MN, USA). Fatty acid binding protein-1 (FABP1) levels in the blood (ng/mL) were determined using the Mouse/Rat FABP1/L-FABP Quantikine® ELISA (R&D Systems, Minneapolis, MN, USA). Optical density units were converted to quantitative values using the calibration curve provided on the manufacturer’s website.
4.9. Histological Examinations
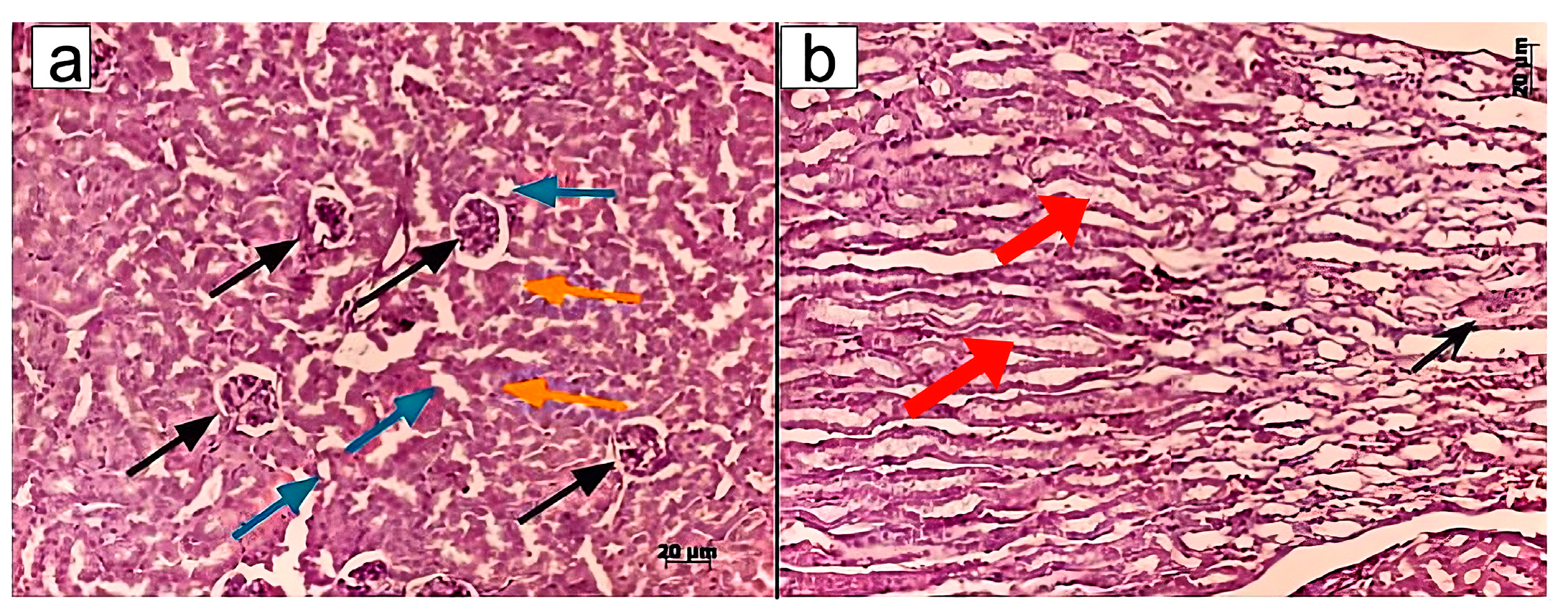
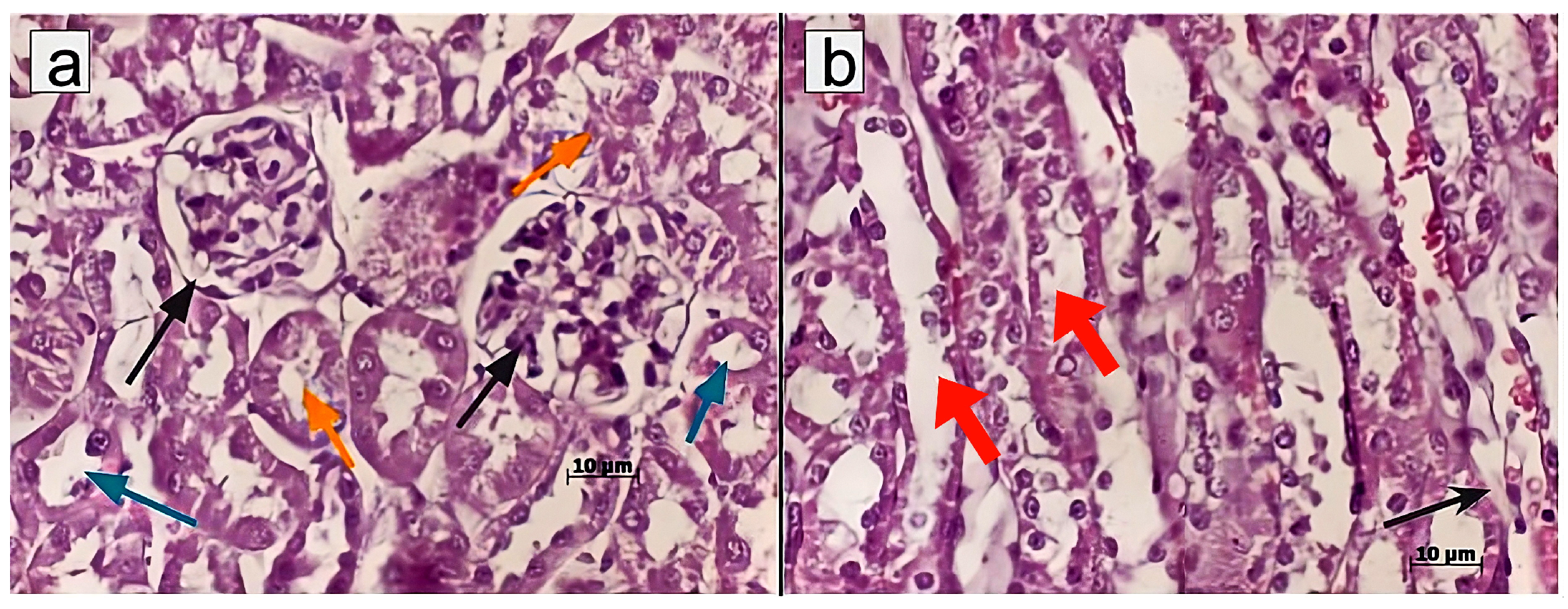
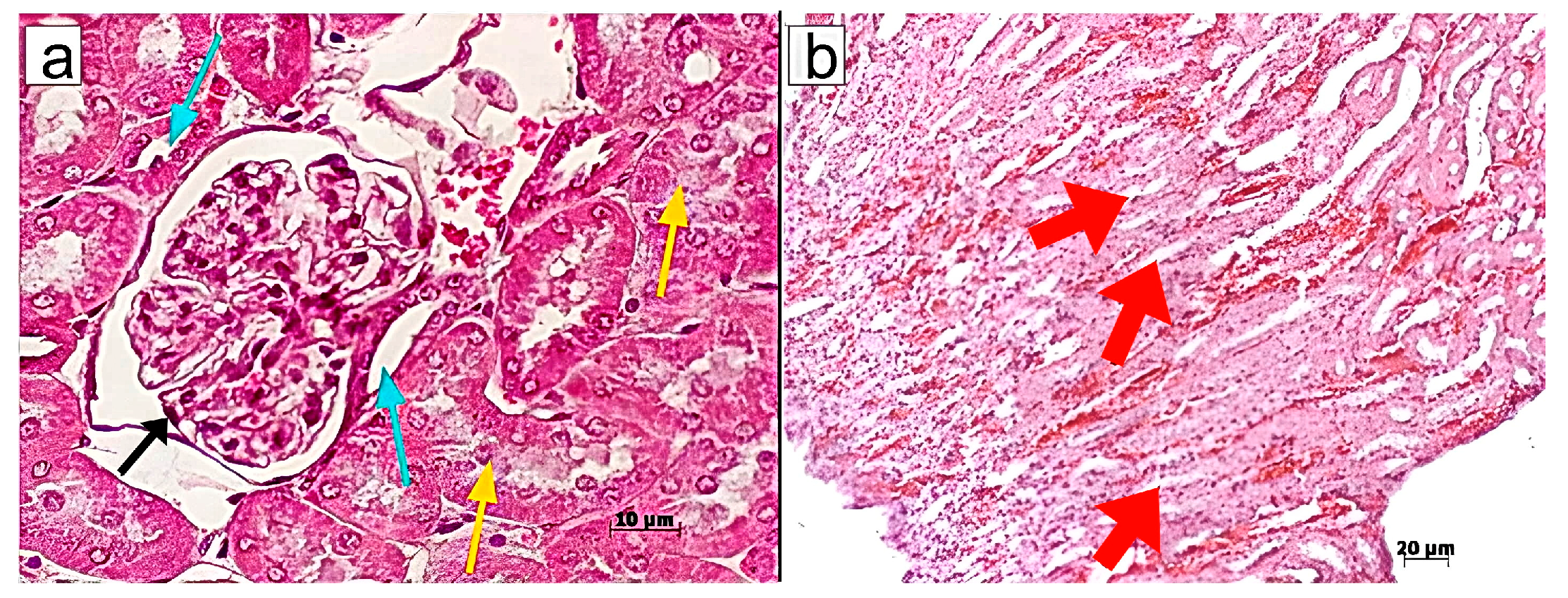
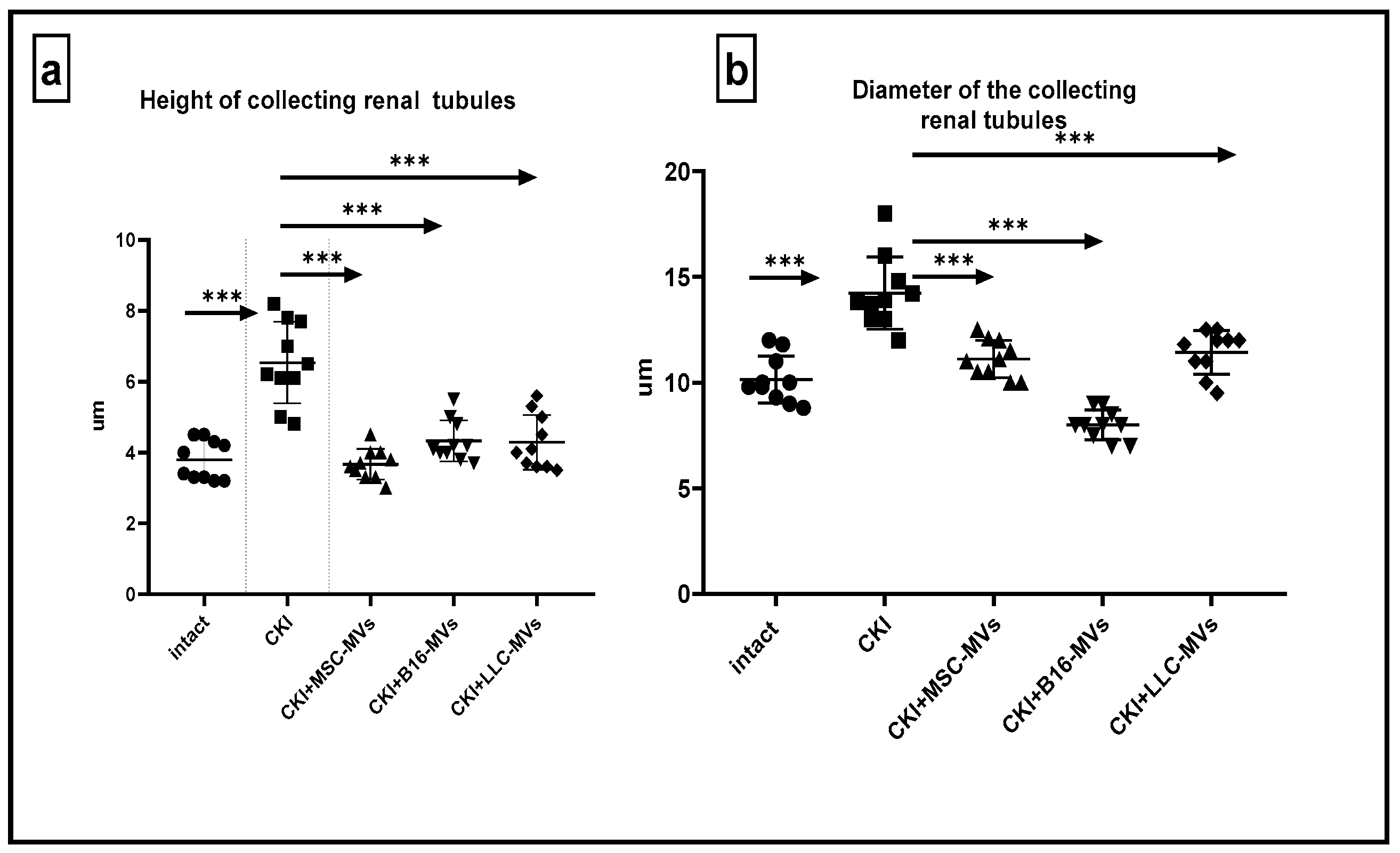
Murine kidneys were fixed in a 4% formalin solution, followed by standard steps of tissue processing for histopathology, including dehydration and paraffin embedding. Paraffin blocks were cut into 4–5 μm thick sections using a Rotary Microtome (Microm HM 340E; Carl Zeiss, Oberkochen, Germany) and stained with haematoxylin/eosin, Sirius red, or according to Mallory’s trichrome staining protocol. Light microscopy and microphotography were performed using a light microscope, Axioskop 40 (Carl Zeiss, Germany). Morphometric analysis of the histological kidney structure was conducted on paraffin sections by measuring the following morphometric parameters: diameters of superficial renal glomeruli, diameters of renal collecting tubules, and the size of cells in the middle third of the medullary region. Morphometric measurements were taken in one field of view (ocular lens, 10 × 25; objective lens 63×).
4.10. Flow Cytometry
Murine kidneys or spleens were cut into small pieces using scissors, followed by gentle homogenization in cold Versene solution using a glass homogenizer. The cell suspension was allowed to settle to remove large cell aggregates, after which density gradient centrifugation was performed in a Ficoll–diatrizoate solution (density: −1.082). After centrifugation, cells were collected, thoroughly washed, and counted. Flow cytometry analysis was conducted using a BD FACSCanto™ II Flow Cytometry System (BD International, Heidelberg, Germany) or CytoFLEX (Beckman Coulter, IN, USA) Flow cytometers with anti-murine CD4-FITC, CD44-PE, CD4-APC, CD25-FITC, and FoxP3-PE antibodies (BD Biosciences, San Jose, CA, USA), following the manufacturer’s instructions.
4.11. Statistics
Data analysis was performed using GraphPad Prism 8 and one-way ANOVA. Statistical significance of differences in biochemical parameters (creatinine and FABP1) and flow cytometry data (CD4+CD25+FoxP3+ and CD4+CD44+ cells) was assessed using Tukey’s multiple comparisons test, following confirmation of normality by the Shapiro–Wilk test. Outliers were identified and removed using the “Identify Outliers” function in GraphPad Prism 8 (ROUT, Q = 1%). For morphometric analyses, Sidak’s multiple comparisons test was applied. Data are presented as mean ± standard error of the mean (Mean ± SEM). The number of samples (n) analyzed is indicated in the figure legends. A p-value of <0.05 was considered statistically significant.
5. Conclusions
Our findings indicate that T-MVs, like MSC-MVs, effectively promote kidney regeneration in CKI. Unlike PBMC-MVs, which showed no regenerative potential, T-MVs demonstrated consistent therapeutic effects regardless of tumor origin. While the exact mechanisms remain to be fully defined, their regenerative activity may involve multiple factors, including mitochondrial transfer, bioactive molecule delivery, and immunomodulation. These results highlight T-MVs as a promising candidate for regenerative therapies, warranting further investigation into their clinical application and long-term safety.
The data presented here are not intended to provide an exhaustive dissection of the mechanisms underlying the regenerative activity of tumor-derived MVs, but rather to serve as a functional proof-of-concept and to map the direction for future targeted studies. Detailed analyses of cytokine networks, membrane molecules, antibody responses, and other biological mediators are indeed crucial next steps, and these will be the subject of future dedicated investigations.