Discovery of Personalized Treatment for Immuno-Metabolic Depression—Focus on 11beta Hydroxysteroid Dehydrogenase Type 2 (11betaHSD2) and Toll-like Receptor 4 (TLR4) Inhibition with Enoxolone
Abstract
1. Introduction
2. Blood Pressure
3. Renin–Angiotensin–Aldosterone System
What Leads to an Increase in Aldosterone?
4. Inflammation
RAAS and Inflammation
5. Role of Obesity and Metabolic Parameters
6. Clinical Relevance
7. Brain Imaging Studies
8. The Role of Childhood Trauma
9. Possible Therapeutic Interventions
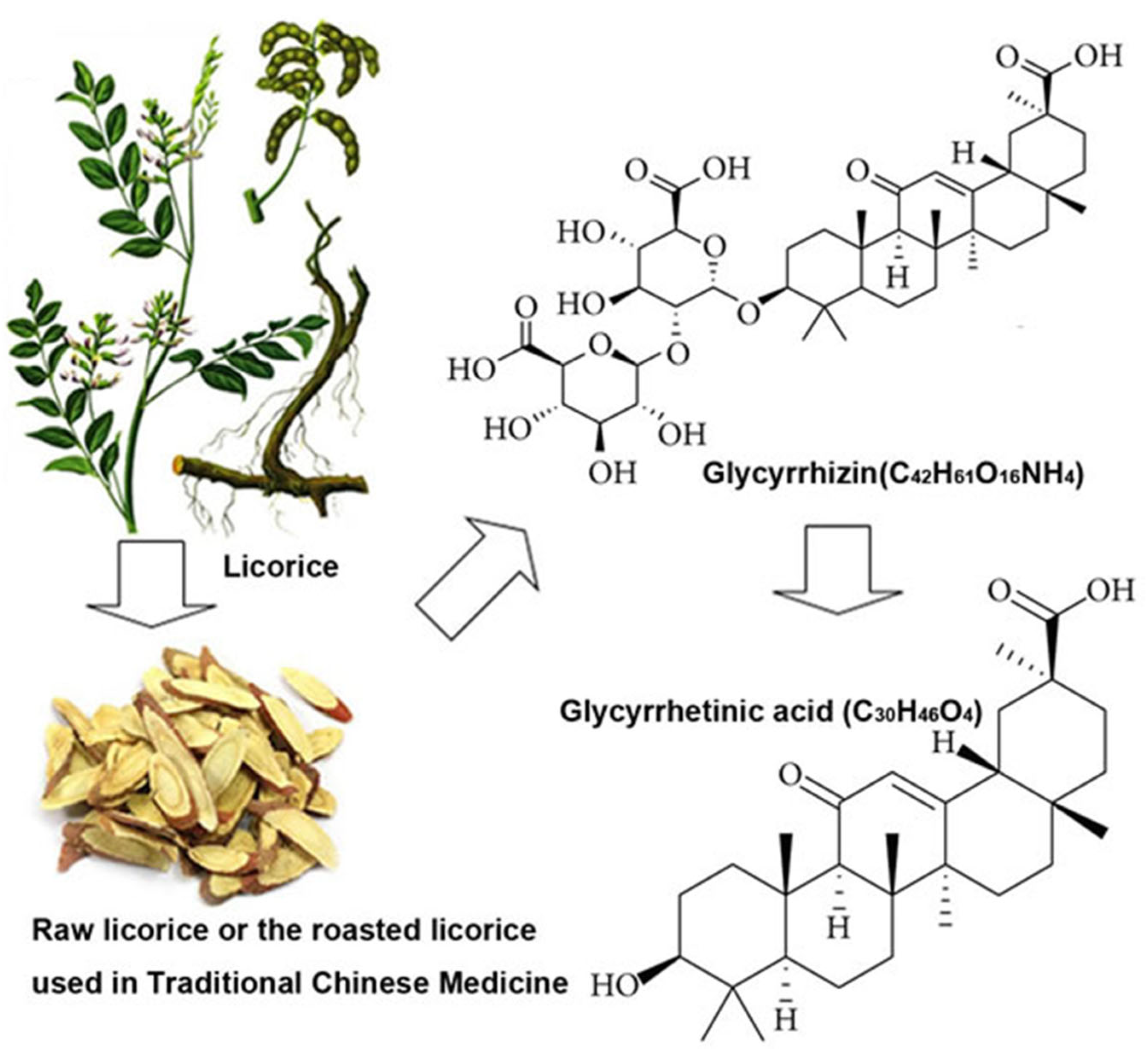
9.1. Glycyrrhizin/Enoxolone
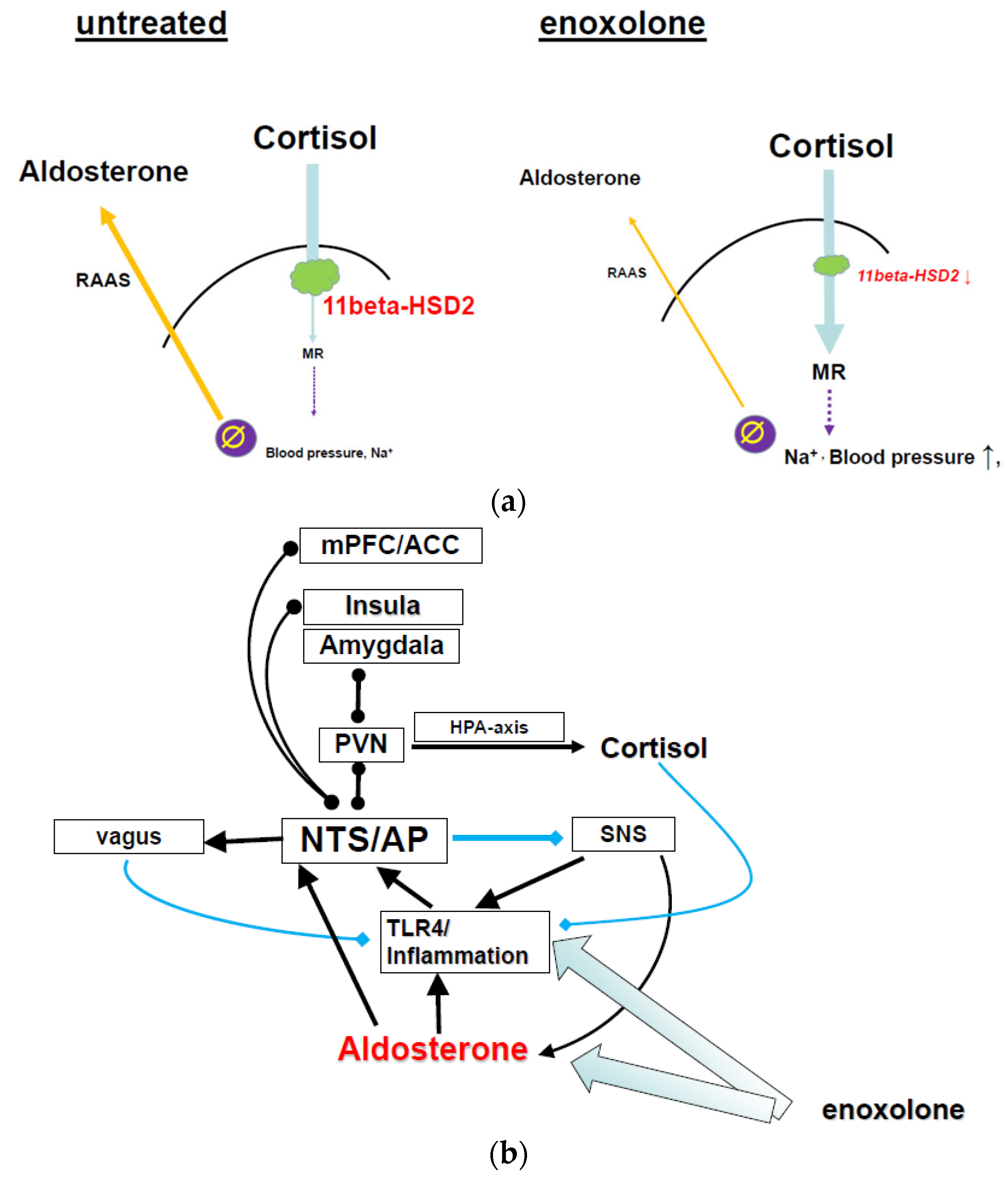
9.1.1. Glycyrrhizin/Enoxolone Reduces Aldosterone via Inhibition of the 11betaHSD2
9.1.2. Glycyrrhizin Reduces Inflammation via TLR4 Inhibition
9.1.3. Behavioral Effects of Glycyrrhizin/Enoxolone
9.1.4. Effect of Glycyrrhizin/Enoxolone on Metabolic Parameters
9.1.5. Glycyrrhizin Reverses Catecholamine Depletion—Autonomic Activity as Primary Driver?
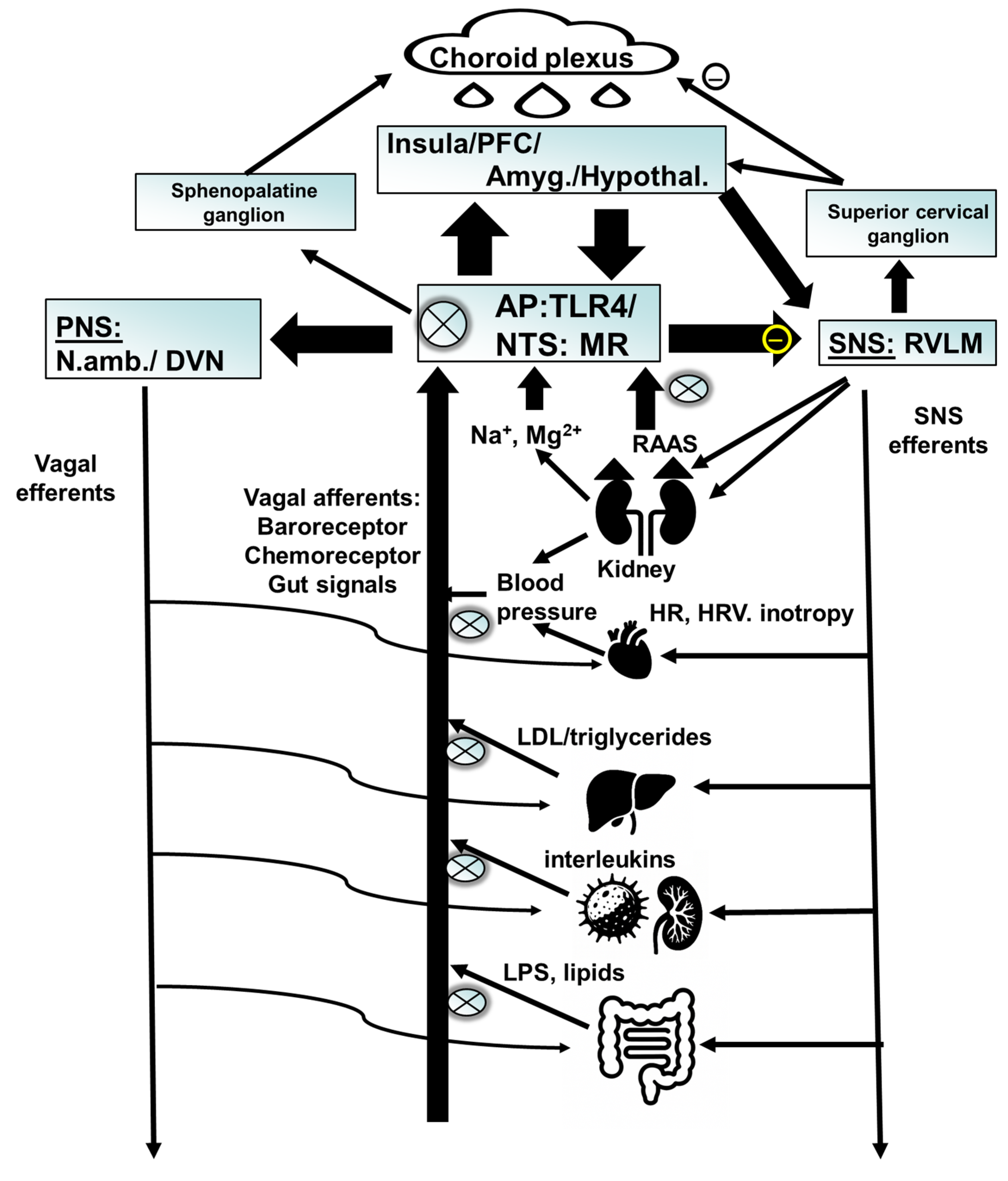
9.1.6. Clinical Studies
9.1.7. Safety
10. Limitation
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Fava, M.; Rush, A.J.; Alpert, J.E.; Balasubramani, G.K.; Wisniewski, S.R.; Carmin, C.N.; Biggs, M.M.; Zisook, S.; Leuchter, A.; Howland, R.; et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: A STAR*D report. Am. J. Psychiatry 2008, 165, 342–351. [Google Scholar] [CrossRef]
- Stewart, J.W.; McGrath, P.J.; Fava, M.; Wisniewski, S.R.; Zisook, S.; Cook, I.; Nierenberg, A.A.; Trivedi, M.H.; Balasubramani, G.K.; Warden, D.; et al. Do atypical features affect outcome in depressed outpatients treated with citalopram? Int. J. Neuropsychopharmacol. 2010, 13, 15–30. [Google Scholar] [CrossRef]
- Murck, H. Atypical depression spectrum disorder—Neurobiology and treatment. Acta Neuropsychiatr. 2003, 15, 227–241. [Google Scholar] [CrossRef]
- Lamers, F.; Bot, M.; Jansen, R.; Chan, M.K.; Cooper, J.D.; Bahn, S.; Penninx, B.W. Serum proteomic profiles of depressive subtypes. Transl. Psychiatry 2016, 6, e851. [Google Scholar] [CrossRef]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; de Jonge, P.; Beekman, A.T.; Penninx, B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef]
- Gold, P.W.; Wong, M.L. Re-assessing the catecholamine hypothesis of depression: The case of melancholic depression. Mol. Psychiatry 2021, 26, 6121–6124. [Google Scholar] [CrossRef]
- Wong, M.L.; Kling, M.A.; Munson, P.J.; Listwak, S.; Licinio, J.; Prolo, P.; Karp, B.; McCutcheon, I.E.; Geracioti, T.D., Jr.; DeBellis, M.D.; et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. USA 2000, 97, 325–330. [Google Scholar] [CrossRef]
- Schildkraut, J.J. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am. J. Psychiatry 1965, 122, 509–522. [Google Scholar] [CrossRef]
- Shelton, R.C.; Pencina, M.J.; Barrentine, L.W.; Ruiz, J.A.; Fava, M.; Zajecka, J.M.; Papakostas, G.I. Association of obesity and inflammatory marker levels on treatment outcome: Results from a double-blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs. J. Clin. Psychiatry 2015, 76, 1635–1641. [Google Scholar] [CrossRef]
- Vreijling, S.R.; Chin Fatt, C.R.; Williams, L.M.; Schatzberg, A.F.; Usherwood, T.; Nemeroff, C.B.; Rush, A.J.; Uher, R.; Aitchison, K.J.; Kohler-Forsberg, O.; et al. Features of immunometabolic depression as predictors of antidepressant treatment outcomes: Pooled analysis of four clinical trials. Br. J. Psychiatry 2024, 224, 89–97. [Google Scholar] [CrossRef]
- Penninx, B.; Lamers, F.; Jansen, R.; Berk, M.; Khandaker, G.M.; De Picker, L.; Milaneschi, Y. Immuno-metabolic depression: From concept to implementation. Lancet Reg. Health Eur. 2025, 48, 101166. [Google Scholar] [CrossRef]
- Licht, C.M.; de Geus, E.J.; Penninx, B.W. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2484–2493. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Duivis, H.E.; Beekman, A.T.; Kluft, C.; Neuteboom, J.; Hoogendijk, W.; Smit, J.H.; de Jonge, P.; Penninx, B.W. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 2012, 2, e79. [Google Scholar] [CrossRef]
- Angst, J.; Gamma, A.; Benazzi, F.; Silverstein, B.; Ajdacic-Gross, V.; Eich, D.; Rossler, W. Atypical depressive syndromes in varying definitions. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 256, 44–54. [Google Scholar] [CrossRef]
- Licht, C.M.; de Geus, E.J.; Seldenrijk, A.; van Hout, H.P.; Zitman, F.G.; van Dyck, R.; Penninx, B.W. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension 2009, 53, 631–638. [Google Scholar] [CrossRef]
- Montano, D. Depressive symptoms and blood pressure. J. Psychophysiol. 2020, 34, 123–135. [Google Scholar] [CrossRef]
- Hildrum, B.; Mykletun, A.; Stordal, E.; Bjelland, I.; Dahl, A.A.; Holmen, J. Association of low blood pressure with anxiety and depression: The Nord-Trondelag Health Study. J. Epidemiol. Community Health 2007, 61, 53–58. [Google Scholar] [CrossRef]
- Schaare, H.L.; Blochl, M.; Kumral, D.; Uhlig, M.; Lemcke, L.; Valk, S.L.; Villringer, A. Associations between mental health, blood pressure and the development of hypertension. Nat. Commun. 2023, 14, 1953. [Google Scholar] [CrossRef]
- Stroup-Benham, C.A.; Markides, K.S.; Black, S.A.; Goodwin, J.S. Relationship between low blood pressure and depressive symptomatology in older people. J. Am. Geriatr. Soc. 2000, 48, 250–255. [Google Scholar] [CrossRef]
- Newton, J.L.; Sheth, A.; Shin, J.; Pairman, J.; Wilton, K.; Burt, J.A.; Jones, D.E. Lower ambulatory blood pressure in chronic fatigue syndrome. Psychosom. Med. 2009, 71, 361–365. [Google Scholar] [CrossRef]
- Halls Dally, J.F. Nervous exhaustion and low blood pressure. Br. Med. J. 1925, 534–535. [Google Scholar]
- Herrmann-Lingen, C.; Meyer, T.; Bosbach, A.; Chavanon, M.L.; Hassoun, L.; Edelmann, F.; Wachter, R. Cross-Sectional and Longitudinal Associations of Systolic Blood Pressure with Quality of Life and Depressive Mood in Older Adults with Cardiovascular Risk Factors: Results from the Observational DIAST-CHF Study. Psychosom. Med. 2018, 80, 468–474. [Google Scholar] [CrossRef]
- Berendes, A.; Meyer, T.; Hulpke-Wette, M.; Herrmann-Lingen, C. Association of elevated blood pressure with low distress and good quality of life: Results from the nationwide representative German Health Interview and Examination Survey for Children and Adolescents. Psychosom. Med. 2013, 75, 422–428. [Google Scholar] [CrossRef]
- Hassoun, L.; Herrmann-Lingen, C.; Hapke, U.; Neuhauser, H.; Scheidt-Nave, C.; Meyer, T. Association between chronic stress and blood pressure: Findings from the German Health Interview and Examination Survey for Adults 2008–2011. Psychosom. Med. 2015, 77, 575–582. [Google Scholar] [CrossRef]
- Dworkin, B.R.; Filewich, R.J.; Miller, N.E.; Craigmyle, N.; Pickering, T.G. Baroreceptor activation reduces reactivity to noxious stimulation: Implications for hypertension. Science 1979, 205, 1299–1301. [Google Scholar] [CrossRef]
- Rau, H.; Elbert, T. Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol. Psychol. 2001, 57, 179–201. [Google Scholar] [CrossRef]
- Jeon, S.W.; Chang, Y.; Lim, S.W.; Cho, J.; Kim, H.N.; Kim, K.B.; Kim, J.; Kim, Y.H.; Shin, D.W.; Oh, K.S.; et al. Bidirectional association between blood pressure and depressive symptoms in young and middle-age adults: A cohort study. Epidemiol. Psychiatr. Sci. 2020, 29, e142. [Google Scholar] [CrossRef]
- Patel, J.S.; Berntson, J.; Polanka, B.M.; Stewart, J.C. Cardiovascular Risk Factors as Differential Predictors of Incident Atypical and Typical Major Depressive Disorder in US Adults. Psychosom. Med. 2018, 80, 508–514. [Google Scholar] [CrossRef]
- Buttner, M.; Jezova, D.; Greene, B.; Konrad, C.; Kircher, T.; Murck, H. Target-based biomarker selection—Mineralocorticoid receptor-related biomarkers and treatment outcome in major depression. J. Psychiatr. Res. 2015, 66–67, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.; Murck, H.; Wagner, S.; Zillich, L.; Streit, F.; Herzog, D.P.; Braus, D.F.; Tadic, A.; Lieb, K.; Muller, M.B. Routinely accessible parameters of mineralocorticoid receptor function, depression subtypes and response prediction: A post-hoc analysis from the early medication change trial in major depressive disorder. World J. Biol. Psychiatry 2022, 23, 631–642. [Google Scholar] [CrossRef]
- Luppino, F.S.; Bouvy, P.F.; Giltay, E.J.; Penninx, B.W.; Zitman, F.G. The metabolic syndrome and related characteristics in major depression: Inpatients and outpatients compared: Metabolic differences across treatment settings. Gen. Hosp. Psychiatry 2014, 36, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, M.; Sunbul, E.A.; Kosker, S.D.; Durmus, E.; Kivrak, T.; Ileri, C.; Oguz, M.; Sari, I. Depression and anxiety are associated with abnormal nocturnal blood pressure fall in hypertensive patients. Clin. Exp. Hypertens. 2014, 36, 354–358. [Google Scholar] [CrossRef]
- Zhao, S.; Fu, S.; Ren, J.; Luo, L. Poor sleep is responsible for the impaired nocturnal blood pressure dipping in elderly hypertensive: A cross-sectional study of elderly. Clin. Exp. Hypertens. 2018, 40, 582–588. [Google Scholar] [CrossRef]
- Okajima, K.; Yamanaka, G.; Oinuma, S.; Kikichi, T.; Yamanaka, T.; Otsuka, K.; Cornelissen, G. Even mild depression is associated with among-day blood pressure variability, including masked non-dipping assessed by 7-d/24-h ambulatory blood pressure monitoring. Clin. Exp. Hypertens. 2015, 37, 426–432. [Google Scholar] [CrossRef]
- Lederbogen, F.; Gernoth, C.; Hamann, B.; Kniest, A.; Heuser, I.; Deuschle, M. Circadian blood pressure regulation in hospitalized depressed patients and non-depressed comparison subjects. Blood Press. Monit. 2003, 8, 71–76. [Google Scholar] [CrossRef]
- Fang, J.T.; Huang, C.C. Midodrine hydrochloride in patients on hemodialysis with chronic hypotension. Ren. Fail. 1996, 18, 253–260. [Google Scholar] [CrossRef]
- Medow, M.S.; Stewart, J.M.; Sanyal, S.; Mumtaz, A.; Sica, D.; Frishman, W.H. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol. Rev. 2008, 16, 4–20. [Google Scholar] [CrossRef]
- Gracie, J.; Newton, J.L.; Norton, M.; Baker, C.; Freeston, M. The role of psychological factors in response to treatment in neurocardiogenic (vasovagal) syncope. Europace 2006, 8, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Hinkelmann, K.; Moritz, S.; Yassouridis, A.; Jahn, H.; Wiedemann, K.; Kellner, M. Modulation of the mineralocorticoid receptor as add-on treatment in depression: A randomized, double-blind, placebo-controlled proof-of-concept study. J. Psychiatr. Res. 2010, 44, 339–346. [Google Scholar] [CrossRef]
- Czajkowska, J.; Ozhog, S.; Smith, E.; Perlmuter, L.C. Cognition and hopelessness in association with subsyndromal orthostatic hypotension. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 873–879. [Google Scholar] [CrossRef]
- Perlmuter, L.C.; Sarda, G.; Casavant, V.; O’Hara, K.; Hindes, M.; Knott, P.T.; Mosnaim, A.D. A review of orthostatic blood pressure regulation and its association with mood and cognition. Clin. Auton. Res. 2012, 22, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Schussler, P.; Steiger, A. Renin-angiotensin-aldosterone system: The forgotten stress hormone system: Relationship to depression and sleep. Pharmacopsychiatry 2012, 45, 83–95. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Van Acker, S.A.; Sibug, R.M.; Oitzl, M.S.; Meijer, O.C.; Rahmouni, K.; de Jong, W. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 2000, 57, 1329–1336. [Google Scholar] [CrossRef]
- Selye, H. Stress and inflammation. Am. J. Proctol. 1953, 4, 229–230. [Google Scholar]
- Hlavacova, N.; Wes, P.D.; Ondrejcakova, M.; Flynn, M.E.; Poundstone, P.K.; Babic, S.; Murck, H.; Jezova, D. Subchronic treatment with aldosterone induces depression-like behaviours and gene expression changes relevant to major depressive disorder. Int. J. Neuropsychopharmacol. 2012, 15, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Joels, M.; de Kloet, E.R. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: The brain mineralocorticoid receptor: A saga in three episodes. J. Endocrinol. 2017, 234, T49–T66. [Google Scholar] [CrossRef]
- Goldstein, D.S. Stress and the “extended” autonomic system. Auton. Neurosci. 2021, 236, 102889. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.P. Mineralocorticoid receptors in the brain and cardiovascular regulation: Minority rule? Trends Endocrinol. Metab. 2011, 22, 179–187. [Google Scholar] [CrossRef]
- Franklin, M.; Bermudez, I.; Hlavacova, N.; Babic, S.; Murck, H.; Schmuckermair, C.; Singewald, N.; Gaburro, S.; Jezova, D. Aldosterone increases earlier than corticosterone in new animal models of depression: Is this an early marker? J. Psychiatr. Res. 2012, 46, 1394–1397. [Google Scholar] [CrossRef]
- Franklin, M.; Bermudez, I.; Murck, H.; Singewald, N.; Gaburro, S. Sub-chronic dietary tryptophan depletion—An animal model of depression with improved face and good construct validity. J. Psychiatr. Res. 2012, 46, 239–247. [Google Scholar] [CrossRef]
- Franklin, M.; Hlavacova, N.; Babic, S.; Pokusa, M.; Bermudez, I.; Jezova, D. Aldosterone Signals the Onset of Depressive Behaviour in a Female Rat Model of Depression along with SSRI Treatment Resistance. Neuroendocrinology 2015, 102, 274–287. [Google Scholar] [CrossRef]
- Gideon, A.; Sauter, C.; Fieres, J.; Berger, T.; Renner, B.; Wirtz, P.H. Kinetics and Interrelations of the Renin Aldosterone Response to Acute Psychosocial Stress: A Neglected Stress System. J. Clin. Endocrinol. Metab. 2020, 105, e762–e773. [Google Scholar] [CrossRef]
- Makatsori, A.; Duncko, R.; Moncek, F.; Loder, I.; Katina, S.; Jezova, D. Modulation of neuroendocrine response and non-verbal behavior during psychosocial stress in healthy volunteers by the glutamate release-inhibiting drug lamotrigine. Neuroendocrinology 2004, 79, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Held, K.; Ziegenbein, M.; Kunzel, H.; Koch, K.; Steiger, A. The renin-angiotensin-aldosterone system in patients with depression compared to controls—A sleep endocrine study. BMC Psychiatry 2003, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Geroldi, D.; Minoretti, P.; Coen, E.; Politi, P. Increased plasma aldosterone in patients with clinical depression. Arch. Med. Res. 2005, 36, 544–548. [Google Scholar] [CrossRef]
- Nowacki, J.; Wingenfeld, K.; Kaczmarczyk, M.; Chae, W.R.; Salchow, P.; Abu-Tir, I.; Piber, D.; Hellmann-Regen, J.; Otte, C. Cardiovascular risk and steroid hormone secretion after stimulation of mineralocorticoid and NMDA receptors in depressed patients. Transl. Psychiatry 2020, 10, 109. [Google Scholar] [CrossRef]
- Izakova, L.; Hlavacova, N.; Segeda, V.; Kapsdorfer, D.; Morovicsova, E.; Jezova, D. Salivary aldosterone, cortisol, and their morning to evening slopes in patients with depressive disorder and healthy subjects: Acute episode and follow-up six months after reaching remission. Neuroendocrinology 2020, 110, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Segeda, V.; Izakova, L.; Hlavacova, N.; Bednarova, A.; Jezova, D. Aldosterone concentrations in saliva reflect the duration and severity of depressive episode in a sex dependent manner. J. Psychiatr. Res. 2017, 91, 164–168. [Google Scholar] [CrossRef]
- Murck, H.; Adolf, C.; Schneider, A.; Schlageter, L.; Heinrich, D.; Ritzel, K.; Sturm, L.; Quinkler, M.; Beuschlein, F.; Reincke, M.; et al. Differential effects of reduced mineralocorticoid receptor activation by unilateral adrenalectomy vs mineralocorticoid antagonist treatment in patients with primary aldosteronism—Implications for depression and anxiety. J. Psychiatr. Res. 2021, 137, 376–382. [Google Scholar] [CrossRef]
- Kunzel, H.E. Psychopathological symptoms in patients with primary hyperaldosteronism–possible pathways. Horm. Metab. Res. 2012, 44, 202–207. [Google Scholar] [CrossRef]
- Bondy, B.; Baghai, T.C.; Zill, P.; Schule, C.; Eser, D.; Deiml, T.; Zwanzger, P.; Ella, R.; Rupprecht, R. Genetic variants in the angiotensin I-converting-enzyme (ACE) and angiotensin II receptor (AT1) gene and clinical outcome in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Schlageter, L.; Schneider, A.; Adolf, C.; Heinrich, D.; Quinkler, M.; Beuschlein, F.; Reincke, M.; Kunzel, H. The potential pathophysiological role of aldosterone and the mineralocorticoid receptor in anxiety and depression—Lessons from primary aldosteronism. J. Psychiatr. Res. 2020, 130, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Shekhtman, E.; Geerling, J.C.; Loewy, A.D. Aldosterone-sensitive neurons of the nucleus of the solitary tract: Multisynaptic pathway to the nucleus accumbens. J. Comp. Neurol. 2007, 501, 274–289. [Google Scholar] [CrossRef]
- Forstenpointner, J.; Maallo, A.M.S.; Elman, I.; Holmes, S.; Freeman, R.; Baron, R.; Borsook, D. The solitary nucleus connectivity to key autonomic regions in humans. Eur. J. Neurosci. 2022, 56, 3938–3966. [Google Scholar] [CrossRef]
- Critchley, H.D.; Harrison, N.A. Visceral influences on brain and behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Geerling, J.C.; Loewy, A.D. Vagal innervation of the aldosterone-sensitive HSD2 neurons in the NTS. Brain Res. 2009, 1249, 135–147. [Google Scholar] [CrossRef]
- Geerling, J.C.; Loewy, A.D. Sodium depletion activates the aldosterone-sensitive neurons in the NTS independently of thirst. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1338–R1348. [Google Scholar] [CrossRef]
- Goldstein, P.; Leshem, M. Dietary sodium, added salt, and serum sodium associations with growth and depression in the U.S. general population. Appetite 2014, 79, 83–90. [Google Scholar] [CrossRef]
- Ileri-Gurel, E.; Pehlivanoglu, B.; Dogan, M. Effect of acute stress on taste perception: In relation with baseline anxiety level and body weight. Chem. Senses 2013, 38, 27–34. [Google Scholar] [CrossRef]
- Heath, T.P.; Melichar, J.K.; Nutt, D.J.; Donaldson, L.F. Human taste thresholds are modulated by serotonin and noradrenaline. J. Neurosci. 2006, 26, 12664–12671. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Stornetta, R.L.; Souza, G.; Abbott, S.B.G.; Brooks, V.L. Neuronal Networks in Hypertension: Recent Advances. Hypertension 2020, 76, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.D.; Leuenberger, U.A.; Ray, C.A. Aldosterone impairs baroreflex sensitivity in healthy adults. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H190–H197. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.S.; Brognara, F.; Castania, J.A.; Talbot, J.; Cunha, T.M.; Cunha, F.Q.; Ulloa, L.; Kanashiro, A.; Dias, D.P.; Salgado, H.C. Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Brain Behav. Immun. 2015, 49, 140–147. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Kang, Y.M.; Yu, Y.; Wei, S.G.; Schmidt, T.J.; Johnson, A.K.; Felder, R.B. 11beta-hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension 2006, 48, 127–133. [Google Scholar] [CrossRef]
- Csecs, J.L.L.; Dowell, N.G.; Savage, G.K.; Iodice, V.; Mathias, C.J.; Critchley, H.D.; Eccles, J.A. Variant connective tissue (joint hypermobility) and dysautonomia are associated with multimorbidity at the intersection between physical and psychological health. Am. J. Med. Genet. C Semin. Med. Genet. 2021, 187, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Cleare, A.J.; Papadopoulos, A.S.; Poon, L.; Lightman, S.; Pariante, C.M. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology 2006, 189, 225–235. [Google Scholar] [CrossRef]
- Juruena, M.F.; Pariante, C.M.; Papadopoulos, A.S.; Poon, L.; Lightman, S.; Cleare, A.J. The role of mineralocorticoid receptor function in treatment-resistant depression. J. Psychopharmacol. 2013, 27, 1169–1179. [Google Scholar] [CrossRef]
- Vogt, W.; Fischer, I.; Ebenroth, S.; Appel, S.; Knedel, M.; Lucker, P.W.; Rennekamp, H. Pharmacokinetics of 9-fluorhydrocortisone. Arzneimittelforschung 1971, 21, 1133–1143. [Google Scholar]
- Fluharty, S.J.; Epstein, A.N. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineralocorticoids. Behav. Neurosci. 1983, 97, 746–758. [Google Scholar] [CrossRef]
- Wolf, G.; Handal, P.J. Aldosterone-induced sodium appetite: Dose-response and specificity. Endocrinology 1966, 78, 1120–1124. [Google Scholar] [CrossRef]
- Sakai, R.R.; McEwen, B.S.; Fluharty, S.J.; Ma, L.Y. The amygdala: Site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000, 57, 1337–1345. [Google Scholar] [CrossRef]
- Sakai, R.R.; Nicolaidis, S.; Epstein, A.N. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am. J. Physiol. 1986, 251, R762–R768. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2020, 12, CD004022. [Google Scholar] [CrossRef] [PubMed]
- Leshem, M. Low dietary sodium is anxiogenic in rats. Physiol. Behav. 2011, 103, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Moffitt, J.A.; Beltz, T.G.; Johnson, A.K. Reduced hedonic behavior and altered cardiovascular function induced by mild sodium depletion in rats. Behav. Neurosci. 2006, 120, 1133–1143. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kadota, K.; Koyamatsu, J.; Yamanashi, H.; Nagayoshi, M.; Noda, M.; Nishimura, T.; Tayama, J.; Nagata, Y.; Maeda, T. Salt intake and mental distress among rural community-dwelling Japanese men. J. Physiol. Anthropol. 2015, 34, 26. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Calvird, M.; Gordon, R.D.; Taylor, P.J.; Ward, G.; Pimenta, E.; Young, R.; Stowasser, M. Effects of two selective serotonin reuptake inhibitor antidepressants, sertraline and escitalopram, on aldosterone/renin ratio in normotensive depressed male patients. J. Clin. Endocrinol. Metab. 2011, 96, 1039–1045. [Google Scholar] [CrossRef]
- Vivas, L.; Godino, A.; Dalmasso, C.; Caeiro, X.E.; Macchione, A.F.; Cambiasso, M.J. Neurochemical Circuits Subserving Fluid Balance and Baroreflex: A Role for Serotonin, Oxytocin, and Gonadal Steroids. In Neurobiology of Body Fluid Homeostasis: Transduction and Integration; De Luca, L.A., Jr., Menani, J.V., Johnson, A.K., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Charloux, A.; Gronfier, C.; Lonsdorfer-Wolf, E.; Piquard, F.; Brandenberger, G. Aldosterone release during the sleep-wake cycle in humans. Am. J. Physiol. 1999, 276, E43–E49. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Lim, Q.H.; Chai, C.S.; Goh, S.L.; Lim, L.L.; Yee, A.; Sukor, N. Influence and implications of the renin-angiotensin-aldosterone system in obstructive sleep apnea: An updated systematic review and meta-analysis. J. Sleep. Res. 2023, 32, e13726. [Google Scholar] [CrossRef]
- Opstad, P.K.; Oktedalen, O.; Aakvaag, A.; Fonnum, F.; Lund, P.K. Plasma renin activity and serum aldosterone during prolonged physical strain. The significance of sleep and energy deprivation. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 1–6. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Luborsky, L.; McKay, J.R.; Rosenthal, R.; Houldin, A.; Tax, A.; McCorkle, R.; Seligman, D.A.; Schmidt, K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav. Immun. 2001, 15, 199–226. [Google Scholar] [CrossRef]
- Arteaga-Henriquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Muller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation and depression but where does the inflammation come from? Curr. Opin. Psychiatry 2019, 32, 422–428. [Google Scholar] [CrossRef]
- Kenney, M.J.; Ganta, C.K. Autonomic nervous system and immune system interactions. Compr. Physiol. 2014, 4, 1177–1200. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Bay-Richter, C.; Janelidze, S.; Hallberg, L.; Brundin, L. Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav. Brain Res. 2011, 222, 193–199. [Google Scholar] [CrossRef]
- Frenois, F.; Moreau, M.; O’Connor, J.; Lawson, M.; Micon, C.; Lestage, J.; Kelley, K.W.; Dantzer, R.; Castanon, N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 2007, 32, 516–531. [Google Scholar] [CrossRef]
- Yirmiya, R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996, 711, 163–174. [Google Scholar] [CrossRef]
- Vedder, H.; Schreiber, W.; Schuld, A.; Kainz, M.; Lauer, C.J.; Krieg, J.C.; Holsboer, F.; Pollmacher, T. Immune-endocrine host response to endotoxin in major depression. J. Psychiatr. Res. 2007, 41, 280–289. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Yang, J.; Wise, L.; Fukuchi, K.I. TLR4 Cross-Talk with NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front Immunol. 2020, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cao, F.S.; Feng, J.; Chen, H.W.; Wan, J.R.; Lu, Q.; Wang, J. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience 2017, 343, 77–84. [Google Scholar] [CrossRef]
- Nakano, Y.; Furube, E.; Morita, S.; Wanaka, A.; Nakashima, T.; Miyata, S. Astrocytic TLR4 expression and LPS-induced nuclear translocation of STAT3 in the sensory circumventricular organs of adult mouse brain. J. Neuroimmunol. 2015, 278, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T.; Okuma, Y.; Matsuda, T.; Nomura, Y. Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Auton. Neurosci. 2005, 120, 104–107. [Google Scholar] [CrossRef]
- Garcia Bueno, B.; Caso, J.R.; Madrigal, J.L.; Leza, J.C. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci. Biobehav. Rev. 2016, 64, 134–147. [Google Scholar] [CrossRef]
- Wisor, J.P.; Clegern, W.C.; Schmidt, M.A. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep 2011, 34, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, T.; Shirato, K.; Sakurai, T.; Ogasawara, J.E.; Oh-ishi, S.; Matsuoka, T.; Izawa, T.; Imaizumi, K.; Haga, S.; Ohno, H. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol. Immunol. 2009, 46, 1195–1203. [Google Scholar] [CrossRef]
- Glezer, I.; Zekki, H.; Scavone, C.; Rivest, S. Modulation of the innate immune response by NMDA receptors has neuropathological consequences. J. Neurosci. 2003, 23, 11094–11103. [Google Scholar] [CrossRef]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef]
- Lai, S.; Wu, G.; Jiang, Z. Glycyrrhizin Treatment Facilitates Extinction of Conditioned Fear Responses After a Single Prolonged Stress Exposure in Rats. Cell Physiol. Biochem. 2018, 45, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, Y.; Chen, K.; Long, Z.; Zou, J. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biol. Pharm. Bull 2017, 40, 1260–1267. [Google Scholar] [CrossRef]
- Garate, I.; Garcia-Bueno, B.; Madrigal, J.L.; Bravo, L.; Berrocoso, E.; Caso, J.R.; Mico, J.A.; Leza, J.C. Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J. Neuroinflamm. 2011, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Garate, I.; Garcia-Bueno, B.; Madrigal, J.L.; Caso, J.R.; Alou, L.; Gomez-Lus, M.L.; Mico, J.A.; Leza, J.C. Stress-induced neuroinflammation: Role of the Toll-like receptor-4 pathway. Biol. Psychiatry 2013, 73, 32–43. [Google Scholar] [CrossRef]
- Xia, Y.; Yamagata, K.; Krukoff, T.L. Differential expression of the CD14/TLR4 complex and inflammatory signaling molecules following i.c.v. administration of LPS. Brain Res. 2006, 1095, 85–95. [Google Scholar] [CrossRef]
- Garate, I.; Garcia-Bueno, B.; Madrigal, J.L.; Caso, J.R.; Alou, L.; Gomez-Lus, M.L.; Leza, J.C. Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J. Neuroinflamm. 2014, 11, 8. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Allers, K.A.; Beekman, A.T.F.; Giltay, E.J.; Keller, S.; Schoevers, R.A.; Sussmuth, S.D.; Niessen, H.G.; Penninx, B. The association between plasma tryptophan catabolites and depression: The role of symptom profiles and inflammation. Brain Behav. Immun. 2021, 97, 167–175. [Google Scholar] [CrossRef]
- Corona, A.W.; Norden, D.M.; Skendelas, J.P.; Huang, Y.; O’Connor, J.C.; Lawson, M.; Dantzer, R.; Kelley, K.W.; Godbout, J.P. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain. Behav. Immun. 2013, 31, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zunich, S.M.; O’Connor, J.C.; Kavelaars, A.; Dantzer, R.; Kelley, K.W. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J. Neuroinflamm. 2010, 7, 43. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef]
- Brooks, A.K.; Lawson, M.A.; Smith, R.A.; Janda, T.M.; Kelley, K.W.; McCusker, R.H. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. J. Neuroinflamm. 2016, 13, 98. [Google Scholar] [CrossRef]
- Luo, F.; Liu, L.; Guo, M.; Liang, J.; Chen, L.; Shi, X.; Liu, H.; Cheng, Y.; Du, Y. Deciphering and Targeting the ESR2-miR-10a-5p-BDNF Axis in the Prefrontal Cortex: Advancing Postpartum Depression Understanding and Therapeutics. Research 2024, 7, 0537. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, K.; Yang, C. The Intersection between Tryptophan-Kynurenine Pathway Metabolites and Immune Inflammation, Hormones, and Gut Microbiota in Perinatal Depression. Actas Esp. Psiquiatr. 2024, 52, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hlavacova, N.; Kerlik, J.; Radikova, Z.; Izakova, L.; Jezova, D. Measurement of salivary aldosterone: Validation by low-dose ACTH test and gender differences. Endocr. Regul. 2013, 47, 201–204. [Google Scholar] [CrossRef]
- Barrett Mueller, K.; Lu, Q.; Mohammad, N.N.; Luu, V.; McCurley, A.; Williams, G.H.; Adler, G.K.; Karas, R.H.; Jaffe, I.Z. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology 2014, 155, 4461–4472. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Vance, K.M.; Ribnicky, D.M.; Hermann, G.E.; Rogers, R.C. St. John’s Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition 2014, 30, S37–S42. [Google Scholar] [CrossRef]
- Sartor, D.M.; Verberne, A.J. The role of NMDA and non-NMDA receptors in the NTS in mediating three distinct sympathoinhibitory reflexes. Naunyn. Schmiedebergs Arch. Pharmacol. 2007, 376, 241–252. [Google Scholar] [CrossRef]
- Cooper, J.N.; Tepper, P.; Barinas-Mitchell, E.; Woodard, G.A.; Sutton-Tyrrell, K. Serum aldosterone is associated with inflammation and aortic stiffness in normotensive overweight and obese young adults. Clin. Exp. Hypertens. 2012, 34, 63–70. [Google Scholar] [CrossRef]
- Cooper, J.N.; Fried, L.; Tepper, P.; Barinas-Mitchell, E.; Conroy, M.B.; Evans, R.W.; Mori Brooks, M.; Woodard, G.A.; Sutton-Tyrrell, K. Changes in serum aldosterone are associated with changes in obesity-related factors in normotensive overweight and obese young adults. Hypertens. Res. 2013, 36, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Bay-Richter, C.; Hallberg, L.; Ventorp, F.; Janelidze, S.; Brundin, L. Aldosterone synergizes with peripheral inflammation to induce brain IL-1beta expression and depressive-like effects. Cytokine 2012, 60, 749–754. [Google Scholar] [CrossRef]
- Sanchez-Lemus, E.; Murakami, Y.; Larrayoz-Roldan, I.M.; Moughamian, A.J.; Pavel, J.; Nishioku, T.; Saavedra, J.M. Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology 2008, 149, 5177–5188. [Google Scholar] [CrossRef] [PubMed]
- Benicky, J.; Sanchez-Lemus, E.; Honda, M.; Pang, T.; Orecna, M.; Wang, J.; Leng, Y.; Chuang, D.M.; Saavedra, J.M. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 2011, 36, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Libianto, R.; Hu, J.; Chee, M.R.; Hoo, J.; Lim, Y.Y.; Shen, J.; Li, Q.; Young, M.J.; Fuller, P.J.; Yang, J. A Multicenter Study of Neutrophil-to-Lymphocyte Ratio in Primary Aldosteronism. J. Endocr. Soc. 2020, 4, bvaa153. [Google Scholar] [CrossRef]
- Herrada, A.A.; Contreras, F.J.; Marini, N.P.; Amador, C.A.; Gonzalez, P.A.; Cortes, C.M.; Riedel, C.A.; Carvajal, C.A.; Figueroa, F.; Michea, L.F.; et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J. Immunol. 2010, 184, 191–202. [Google Scholar] [CrossRef]
- Odermatt, A.; Kratschmar, D.V. Tissue-specific modulation of mineralocorticoid receptor function by 11beta-hydroxysteroid dehydrogenases: An overview. Mol. Cell. Endocrinol. 2012, 350, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Kloiber, S.; Ising, M.; Reppermund, S.; Horstmann, S.; Dose, T.; Majer, M.; Zihl, J.; Pfister, H.; Unschuld, P.G.; Holsboer, F.; et al. Overweight and obesity affect treatment response in major depression. Biol. Psychiatry 2007, 62, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Jantaratnotai, N.; Mosikanon, K.; Lee, Y.; McIntyre, R.S. The interface of depression and obesity. Obes. Res. Clin. Pract. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Lasserre, A.M.; Glaus, J.; Vandeleur, C.L.; Marques-Vidal, P.; Vaucher, J.; Bastardot, F.; Waeber, G.; Vollenweider, P.; Preisig, M. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: A prospective, population-based study. JAMA Psychiatry 2014, 71, 880–888. [Google Scholar] [CrossRef]
- Lasserre, A.M.; Strippoli, M.F.; Glaus, J.; Gholam-Rezaee, M.; Vandeleur, C.L.; Castelao, E.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Preisig, M. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Mol. Psychiatry 2017, 22, 1026–1034. [Google Scholar] [CrossRef]
- Miller, G.E.; Freedland, K.E.; Carney, R.M.; Stetler, C.A.; Banks, W.A. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav. Immun. 2003, 17, 276–285. [Google Scholar] [CrossRef]
- van Reedt Dortland, A.K.; Vreeburg, S.A.; Giltay, E.J.; Licht, C.M.; Vogelzangs, N.; van Veen, T.; de Geus, E.J.; Penninx, B.W.; Zitman, F.G. The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology 2013, 38, 209–218. [Google Scholar] [CrossRef] [PubMed]
- van Reedt Dortland, A.K.; Giltay, E.J.; van Veen, T.; van Pelt, J.; Zitman, F.G.; Penninx, B.W. Associations between serum lipids and major depressive disorder: Results from the Netherlands Study of Depression and Anxiety (NESDA). J. Clin. Psychiatry 2010, 71, 729–736. [Google Scholar] [CrossRef]
- van Reedt Dortland, A.K.; Giltay, E.J.; van Veen, T.; Zitman, F.G.; Penninx, B.W. Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosom. Med. 2013, 75, 83–89. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Li, X.; Zhang, Y.; Gulbins, E.; Zhang, Y. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 2016, 7, 73229–73241. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflamm. 2014, 2014, 689360. [Google Scholar] [CrossRef]
- Duprez, D.A. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: A clinical review. J. Hypertens. 2006, 24, 983–991. [Google Scholar] [CrossRef]
- de Kloet, A.D.; Pioquinto, D.J.; Nguyen, D.; Wang, L.; Smith, J.A.; Hiller, H.; Sumners, C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol. Behav. 2014, 136, 31–38. [Google Scholar] [CrossRef]
- Pires, P.W.; McClain, J.L.; Hayoz, S.F.; Dorrance, A.M. Mineralocorticoid receptor antagonism prevents obesity-induced cerebral artery remodeling and reduces white matter injury in rats. Microcirculation 2018, 25, e12460. [Google Scholar] [CrossRef]
- Irani, H.; Abiri, B.; Khodami, B.; Yari, Z.; Lafzi Ghazi, M.; Hosseinzadeh, N.; Saidpour, A. Effect of time restricted feeding on anthropometric measures, eating behavior, stress, serum levels of BDNF and LBP in overweight/obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2024, 27, 577–589. [Google Scholar] [CrossRef]
- Law, S.; Dong, S.; Zhou, F.; Zheng, D.; Wang, C.; Dong, Z. Bariatric surgery and mental health outcomes: An umbrella review. Front. Endocrinol. 2023, 14, 1283621. [Google Scholar] [CrossRef]
- Peterhansel, C.; Petroff, D.; Klinitzke, G.; Kersting, A.; Wagner, B. Risk of completed suicide after bariatric surgery: A systematic review. Obes. Rev. 2013, 14, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Zade, D.; Beiser, A.; McGlinchey, R.; Au, R.; Seshadri, S.; Palumbo, C.; Wolf, P.A.; DeCarli, C.; Milberg, W. Apolipoprotein epsilon 4 allele modifies waist-to-hip ratio effects on cognition and brain structure. J. Stroke Cerebrovasc. Dis. 2013, 22, 119–125. [Google Scholar] [CrossRef]
- Willette, A.A.; Kapogiannis, D. Does the brain shrink as the waist expands? Ageing Res. Rev. 2015, 20, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Pontiroli, A.E.; Merlotti, C.; Veronelli, A.; Lombardi, F. Effect of weight loss on sympatho-vagal balance in subjects with grade-3 obesity: Restrictive surgery versus hypocaloric diet. Acta Diabetol. 2013, 50, 843–850. [Google Scholar] [CrossRef]
- Hassenstab, J.J.; Sweet, L.H.; Del Parigi, A.; McCaffery, J.M.; Haley, A.P.; Demos, K.E.; Cohen, R.A.; Wing, R.R. Cortical thickness of the cognitive control network in obesity and successful weight loss maintenance: A preliminary MRI study. Psychiatry Res. 2012, 202, 77–79. [Google Scholar] [CrossRef]
- Dreimuller, N.; Lieb, K.; Tadic, A.; Engelmann, J.; Wollschlager, D.; Wagner, S. Body mass index (BMI) in major depressive disorder and its effects on depressive symptomatology and antidepressant response. J. Affect. Disord. 2019, 256, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Novick, J.S.; Stewart, J.W.; Wisniewski, S.R.; Cook, I.A.; Manev, R.; Nierenberg, A.A.; Rosenbaum, J.F.; Shores-Wilson, K.; Balasubramani, G.K.; Biggs, M.M.; et al. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: Preliminary findings from STAR*D. J. Clin. Psychiatry 2005, 66, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Matza, L.S.; Revicki, D.A.; Davidson, J.R.; Stewart, J.W. Depression with atypical features in the National Comorbidity Survey: Classification, description, and consequences. Arch. Gen. Psychiatry 2003, 60, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Quitkin, F.M.; Stewart, J.W.; McGrath, P.J.; Tricamo, E.; Rabkin, J.G.; Ocepek-Welikson, K.; Nunes, E.; Harrison, W.; Klein, D.F. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br. J. Psychiatry Suppl. 1993, 163 (Suppl. 21), 30–34. [Google Scholar] [CrossRef]
- Mannel, M.; Kuhn, U.; Schmidt, U.; Ploch, M.; Murck, H. St. John’s wort extract LI160 for the treatment of depression with atypical features—A double-blind, randomized, and placebo-controlled trial. J. Psychiatr. Res. 2010, 44, 760–767. [Google Scholar] [CrossRef]
- Murck, H.; Fava, M.; Alpert, J.; Nierenberg, A.A.; Mischoulon, D.; Otto, M.W.; Zajecka, J.; Mannel, M.; Rosenbaum, J.F. Hypericum extract in patients with MDD and reversed vegetative signs: Re-analysis from data of a double-blind, randomized trial of hypericum extract, fluoxetine, and placebo. Int. J. Neuropsychopharmacol. 2005, 8, 215–221. [Google Scholar] [CrossRef]
- Schroeder, C.; Tank, J.; Goldstein, D.S.; Stoeter, M.; Haertter, S.; Luft, F.C.; Jordan, J. Influence of St John’s wort on catecholamine turnover and cardiovascular regulation in humans. Clin. Pharmacol. Ther. 2004, 76, 480–489. [Google Scholar] [CrossRef]
- Bekhbat, M.; Li, Z.; Dunlop, B.W.; Treadway, M.T.; Mehta, N.D.; Revill, K.P.; Lucido, M.J.; Hong, C.; Ashchi, A.; Wommack, E.C.; et al. Sustained effects of repeated levodopa (L-DOPA) administration on reward circuitry, effort-based motivation, and anhedonia in depressed patients with higher inflammation. Brain Behav. Immun. 2025, 125, 240–248. [Google Scholar] [CrossRef]
- Roitman, M.F.; Patterson, T.A.; Sakai, R.R.; Bernstein, I.L.; Figlewicz, D.P. Sodium depletion and aldosterone decrease dopamine transporter activity in nucleus accumbens but not striatum. Am. J. Physiol. 1999, 276, R1339–R1345. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.X.; Penninx, B.; de Geus, E.J.C.; Lamers, F.; Kuan, D.C.; Wright, A.G.C.; Marsland, A.L.; Muldoon, M.F.; Manuck, S.B.; Gianaros, P.J. Associations of immunometabolic risk factors with symptoms of depression and anxiety: The role of cardiac vagal activity. Brain Behav. Immun. 2018, 73, 493–503. [Google Scholar] [CrossRef]
- Licht, C.M.; Vreeburg, S.A.; van Reedt Dortland, A.K.; Giltay, E.J.; Hoogendijk, W.J.; DeRijk, R.H.; Vogelzangs, N.; Zitman, F.G.; de Geus, E.J.; Penninx, B.W. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J. Clin. Endocrinol. Metab. 2010, 95, 2458–2466. [Google Scholar] [CrossRef]
- Geerling, J.J.; Boon, M.R.; Kooijman, S.; Parlevliet, E.T.; Havekes, L.M.; Romijn, J.A.; Meurs, I.M.; Rensen, P.C. Sympathetic nervous system control of triglyceride metabolism: Novel concepts derived from recent studies. J. Lipid Res. 2014, 55, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Warne, J.P.; Foster, M.T.; Horneman, H.F.; Pecoraro, N.C.; Ginsberg, A.B.; Akana, S.F.; Dallman, M.F. Afferent signalling through the common hepatic branch of the vagus inhibits voluntary lard intake and modifies plasma metabolite levels in rats. J. Physiol. 2007, 583, 455–467. [Google Scholar] [CrossRef]
- Windham, B.G.; Fumagalli, S.; Ble, A.; Sollers, J.J.; Thayer, J.F.; Najjar, S.S.; Griswold, M.E.; Ferrucci, L. The Relationship between Heart Rate Variability and Adiposity Differs for Central and Overall Adiposity. J. Obes. 2012, 2012, 149516. [Google Scholar] [CrossRef]
- Ziegler, D.; Strom, A.; Kupriyanova, Y.; Bierwagen, A.; Bonhof, G.J.; Bodis, K.; Mussig, K.; Szendroedi, J.; Bobrov, P.; Markgraf, D.F.; et al. Association of Lower Cardiovagal Tone and Baroreflex Sensitivity with Higher Liver Fat Content Early in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 1130–1138. [Google Scholar] [CrossRef]
- Hua, K.; Usichenko, T.; Cummings, M.; Bernatik, M.; Willich, S.N.; Brinkhaus, B.; Dietzel, J. Effects of auricular stimulation on weight- and obesity-related parameters: A systematic review and meta-analysis of randomized controlled clinical trials. Front. Neurosci. 2024, 18, 1393826. [Google Scholar] [CrossRef]
- Apovian, C.M.; Shah, S.N.; Wolfe, B.M.; Ikramuddin, S.; Miller, C.J.; Tweden, K.S.; Billington, C.J.; Shikora, S.A. Two-Year Outcomes of Vagal Nerve Blocking (vBloc) for the Treatment of Obesity in the ReCharge Trial. Obes. Surg. 2017, 27, 169–176. [Google Scholar] [CrossRef]
- Catinis, A.M.; Hinojosa, A.J.; Leonardi, C.; Cook, M.W. Hepatic Vagotomy in Patients with Obesity Leads to Improvement of the Cholesterol to High-Density Lipoprotein Ratio. Obes. Surg. 2023, 33, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Lubaczeuski, C.; Balbo, S.L.; Ribeiro, R.A.; Vettorazzi, J.F.; Santos-Silva, J.C.; Carneiro, E.M.; Bonfleur, M.L. Vagotomy ameliorates islet morphofunction and body metabolic homeostasis in MSG-obese rats. Braz. J. Med. Biol. Res. 2015, 48, 447–457. [Google Scholar] [CrossRef]
- Zou, J.; Li, J.; Wang, X.; Tang, D.; Chen, R. Neuroimmune modulation in liver pathophysiology. J. Neuroinflamm. 2024, 21, 188. [Google Scholar] [CrossRef]
- Murck, H. Neuroendocrine and Autonomic Dysregulation in Affective Disorders. In Handbook of the Biology and Pathology of Mental Disorders; Springer: Cham, Switzerland, 2025; pp. 1–23. [Google Scholar]
- Otte, C.; Chae, W.R.; Dogan, D.Y.; Piber, D.; Roepke, S.; Cho, A.B.; Trumm, S.; Kaczmarczyk, M.; Brasanac, J.; Wingenfeld, K.; et al. Simvastatin as Add-On Treatment to Escitalopram in Patients with Major Depression and Obesity: A Randomized Clinical Trial. JAMA Psychiatry 2025, 82, 759–767. [Google Scholar] [CrossRef]
- Murck, H.; Luerweg, B.; Hahn, J.; Braunisch, M.; Jezova, D.; Zavorotnyy, M.; Konrad, C.; Jansen, A.; Kircher, T. Ventricular volume, white matter alterations and outcome of major depression and their relationship to endocrine parameters—A pilot study. World J. Biol. Psychiatry 2020, 22, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Fava, M.; Cusin, C.; Fatt, C.C.; Trivedi, M. Brain ventricle and choroid plexus morphology as predictor of treatment response in major depression: Findings from the EMBARC study. Brain Behav. Immun. Health 2024, 35, 100717. [Google Scholar] [CrossRef]
- Fleischer, V.; Gonzalez-Escamilla, G.; Ciolac, D.; Albrecht, P.; Kury, P.; Gruchot, J.; Dietrich, M.; Hecker, C.; Muntefering, T.; Bock, S.; et al. Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2025000118. [Google Scholar] [CrossRef]
- Hagenberg, J.; Be, C.S.G.; Bruckl, T.M.; Erhart, M.; Kopf-Beck, J.; Kodel, M.; Rehawi, G.; Roh-Karamihalev, S.; Sauer, S.; OPTIMA Study Group; et al. Dissecting depression symptoms: Multi-omics clustering uncovers immune-related subgroups and cell-type specific dysregulation. Brain Behav. Immun. 2025, 123, 353–369. [Google Scholar] [CrossRef]
- Bravi, B.; Melloni, E.M.T.; Paolini, M.; Palladini, M.; Calesella, F.; Servidio, L.; Agnoletto, E.; Poletti, S.; Lorenzi, C.; Colombo, C.; et al. Choroid plexus volume is increased in mood disorders and associates with circulating inflammatory cytokines. Brain Behav. Immun. 2023, 116, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lizano, P.; Deng, G.; Sun, H.; Zhou, X.; Xie, H.; Zhan, Y.; Mu, J.; Long, X.; Xiao, H.; et al. Brain-derived subgroups of bipolar II depression associate with inflammation and choroid plexus morphology. Psychiatry Clin. Neurosci. 2023, 77, 613–621. [Google Scholar] [CrossRef]
- Althubaity, N.; Schubert, J.; Martins, D.; Yousaf, T.; Nettis, M.A.; Mondelli, V.; Pariante, C.; Harrison, N.A.; Bullmore, E.T.; Dima, D.; et al. Choroid plexus enlargement is associated with neuroinflammation and reduction of blood brain barrier permeability in depression. Neuroimage Clin. 2022, 33, 102926. [Google Scholar] [CrossRef]
- Turner, C.A.; Thompson, R.C.; Bunney, W.E.; Schatzberg, A.F.; Barchas, J.D.; Myers, R.M.; Akil, H.; Watson, S.J. Altered choroid plexus gene expression in major depressive disorder. Front. Hum. Neurosci. 2014, 8, 238. [Google Scholar] [CrossRef]
- Carvajal, C.A.; Herrada, A.A.; Castillo, C.R.; Contreras, F.J.; Stehr, C.B.; Mosso, L.M.; Kalergis, A.M.; Fardella, C.E. Primary aldosteronism can alter peripheral levels of transforming growth factor beta and tumor necrosis factor alpha. J. Endocrinol. Investig. 2009, 32, 759–765. [Google Scholar] [CrossRef]
- Macmaster, F.P.; Carrey, N.; Marie Langevin, L. Corpus callosal morphology in early onset adolescent depression. J. Affect. Disord. 2013, 145, 256–259. [Google Scholar] [CrossRef]
- Murck, H.; Lehr, L.; Jezova, D. A viewpoint on aldosterone and BMI related brain morphology in relation to treatment outcome in patients with major depression. J. Neuroendocrinol. 2023, 35, e13219. [Google Scholar] [CrossRef]
- Dempster, K.S.; O’Leary, D.D.; MacNeil, A.J.; Hodges, G.J.; Wade, T.J. Linking the hemodynamic consequences of adverse childhood experiences to an altered HPA axis and acute stress response. Brain Behav. Immun. 2021, 93, 254–263. [Google Scholar] [CrossRef]
- Terock, J.; Hannemann, A.; Klinger-Konig, J.; Janowitz, D.; Grabe, H.J.; Murck, H. The neurobiology of childhood trauma-aldosterone and blood pressure changes in a community sample. World J. Biol. Psychiatry 2022, 23, 622–630. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Zisk, A. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 185–222. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Keshavan, M.S.; Shifflett, H.; Iyengar, S.; Beers, S.R.; Hall, J.; Moritz, G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: A sociodemographically matched study. Biol. Psychiatry 2002, 52, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lizano, P.; Li, M.; Colic, L.; Chand, T.; Javaheripour, N.; Sun, H.; Deng, G.; Zhou, X.; Long, X. Associations among bipolar II depression white matter subgroups, inflammation, symptoms and childhood maltreatment. Nat. Ment. Health 2025, 3, 724–734. [Google Scholar]
- Nelson, J.; Klumparendt, A.; Doebler, P.; Ehring, T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br. J. Psychiatry 2017, 210, 96–104. [Google Scholar] [CrossRef]
- Lippard, E.T.C.; Nemeroff, C.B. The Devastating Clinical Consequences of Child Abuse and Neglect: Increased Disease Vulnerability and Poor Treatment Response in Mood Disorders. Am. J. Psychiatry 2020, 177, 20–36. [Google Scholar] [CrossRef]
- Noetel, M.; Sanders, T.; Gallardo-Gomez, D.; Taylor, P.; Del Pozo Cruz, B.; van den Hoek, D.; Smith, J.J.; Mahoney, J.; Spathis, J.; Moresi, M.; et al. Effect of exercise for depression: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2024, 384, e075847. [Google Scholar] [CrossRef]
- Rethorst, C.D.; Tu, J.; Carmody, T.J.; Greer, T.L.; Trivedi, M.H. Atypical depressive symptoms as a predictor of treatment response to exercise in Major Depressive Disorder. J. Affect. Disord. 2016, 200, 156–158. [Google Scholar] [CrossRef]
- Toni, G.; Belvederi Murri, M.; Piepoli, M.; Zanetidou, S.; Cabassi, A.; Squatrito, S.; Bagnoli, L.; Piras, A.; Mussi, C.; Senaldi, R.; et al. Physical Exercise for Late-Life Depression: Effects on Heart Rate Variability. Am. J. Geriatr. Psychiatry 2016, 24, 989–997. [Google Scholar] [CrossRef]
- Baffour-Awuah, B.; Man, M.; Goessler, K.F.; Cornelissen, V.A.; Dieberg, G.; Smart, N.A.; Pearson, M.J. Effect of exercise training on the renin-angiotensin-aldosterone system: A meta-analysis. J. Hum. Hypertens. 2024, 38, 89–101. [Google Scholar] [CrossRef]
- Berney, M.; Vakilzadeh, N.; Maillard, M.; Faouzi, M.; Grouzmann, E.; Bonny, O.; Favre, L.; Wuerzner, G. Bariatric Surgery Induces a Differential Effect on Plasma Aldosterone in Comparison to Dietary Advice Alone. Front. Endocrinol. 2021, 12, 745045. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Xu, M.; Cai, W.; Zhu, Q.; Qian, L.; Zhang, Y.E.; Yuan, K.; Liu, J.; Li, Q.; et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int. J. Obes. 2016, 40, 1558–1565. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert, P. Final report on the safety assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, Trisodium Glycyrrhizate, Methyl Glycyrrhizate, and Potassium Glycyrrhizinate. Int. J. Toxicol. 2007, 26 (Suppl. S2), 79–112. [Google Scholar] [CrossRef]
- Yamamura, Y.; Kawakami, J.; Santa, T.; Kotaki, H.; Uchino, K.; Sawada, Y.; Tanaka, N.; Iga, T. Pharmacokinetic profile of glycyrrhizin in healthy volunteers by a new high-performance liquid chromatographic method. J. Pharm. Sci. 1992, 81, 1042–1046. [Google Scholar] [CrossRef]
- Murck, H.; Lehr, L.; Hahn, J.; Braunisch, M.C.; Jezova, D.; Zavorotnyy, M. Adjunct Therapy with Glycyrrhiza Glabra Rapidly Improves Outcome in Depression-A Pilot Study to Support 11-Beta-Hydroxysteroid Dehydrogenase Type 2 Inhibition as a New Target. Front. Psychiatry 2020, 11, 605949. [Google Scholar] [CrossRef]
- Huang, Q.C.; Wang, M.J.; Chen, X.M.; Yu, W.L.; Chu, Y.L.; He, X.H.; Huang, R.Y. Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget 2016, 7, 1193–1202. [Google Scholar] [CrossRef]
- Sigurjonsdottir, H.A.; Franzson, L.; Manhem, K.; Ragnarsson, J.; Sigurdsson, G.; Wallerstedt, S. Liquorice-induced rise in blood pressure: A linear dose-response relationship. J. Hum. Hypertens. 2001, 15, 549–552. [Google Scholar] [CrossRef]
- Forslund, T.; Fyhrquist, F.; Froseth, B.; Tikkanen, I. Effects of licorice on plasma atrial natriuretic peptide in healthy volunteers. J. Intern. Med. 1989, 225, 95–99. [Google Scholar] [CrossRef]
- Epstein, M.T.; Espiner, E.A.; Donald, R.A.; Hughes, H. Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. Br. Med. J. 1977, 1, 488–490. [Google Scholar] [CrossRef]
- Armanini, D.; Lewicka, S.; Pratesi, C.; Scali, M.; Zennaro, M.C.; Zovato, S.; Gottardo, C.; Simoncini, M.; Spigariol, A.; Zampollo, V. Further studies on the mechanism of the mineralocorticoid action of licorice in humans. J. Endocrinol. Investig. 1996, 19, 624–629. [Google Scholar] [CrossRef]
- Kurtz, A. Renin release: Sites, mechanisms, and control. Annu. Rev. Physiol. 2011, 73, 377–399. [Google Scholar] [CrossRef]
- Hubert, C.; Gasc, J.M.; Berger, S.; Schutz, G.; Corvol, P. Effects of mineralocorticoid receptor gene disruption on the components of the renin-angiotensin system in 8-day-old mice. Mol. Endocrinol. 1999, 13, 297–306. [Google Scholar] [CrossRef]
- Krahenbuhl, S.; Hasler, F.; Frey, B.M.; Frey, F.J.; Brenneisen, R.; Krapf, R. Kinetics and dynamics of orally administered 18 beta-glycyrrhetinic acid in humans. J. Clin. Endocrinol. Metab. 1994, 78, 581–585. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- van Gelderen, C.E.; Bijlsma, J.A.; van Dokkum, W.; Savelkoul, T.J. Glycyrrhizic acid: The assessment of a no effect level. Hum. Exp. Toxicol. 2000, 19, 434–439. [Google Scholar] [CrossRef]
- Af Geijerstam, P.; Joelsson, A.; Radholm, K.; Nystrom, F.H. A low dose of daily licorice intake affects renin, aldosterone, and home blood pressure in a randomized crossover trial. Am. J. Clin. Nutr. 2024, 119, 682–691. [Google Scholar] [CrossRef]
- Walker, B.R.; Connacher, A.A.; Webb, D.J.; Edwards, C.R. Glucocorticoids and blood pressure: A role for the cortisol/cortisone shuttle in the control of vascular tone in man. Clin. Sci. 1992, 83, 171–178. [Google Scholar] [CrossRef]
- Hautaniemi, E.J.; Tahvanainen, A.M.; Koskela, J.K.; Tikkakoski, A.J.; Kahonen, M.; Uitto, M.; Sipila, K.; Niemela, O.; Mustonen, J.; Porsti, I.H. Voluntary liquorice ingestion increases blood pressure via increased volume load, elevated peripheral arterial resistance, and decreased aortic compliance. Sci. Rep. 2017, 7, 10947. [Google Scholar] [CrossRef]
- Tabuchi, M.; Imamura, S.; Kawakami, Z.; Ikarashi, Y.; Kase, Y. The blood-brain barrier permeability of 18beta-glycyrrhetinic acid, a major metabolite of glycyrrhizin in Glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell Mol. Neurobiol. 2012, 32, 1139–1146. [Google Scholar] [CrossRef]
- Iigaya, K.; Onimaru, H.; Ikeda, K.; Iizuka, M.; Izumizaki, M. Cellular mechanisms of synchronized rhythmic burst generation in the ventromedial hypothalamus. Pflugers Arch. 2025, 477, 131–145. [Google Scholar] [CrossRef]
- Broncel, A.; Bocian, R.; Klos-Wojtczak, P.; Konopacki, J. Hippocampal theta rhythm induced by vagal nerve stimulation: The effect of modulation of electrical coupling. Brain Res. Bull. 2019, 152, 236–245. [Google Scholar] [CrossRef]
- Hisaoka-Nakashima, K.; Tomimura, Y.; Yoshii, T.; Ohata, K.; Takada, N.; Zhang, F.F.; Nakamura, Y.; Liu, K.; Wake, H.; Nishibori, M.; et al. High-mobility group box 1-mediated microglial activation induces anxiodepressive-like behaviors in mice with neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 347–362. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, C.R.; Zeng, W.F.; Xu, H.J.; Li, J.M.; Zhang, T.; Deng, G.H.; Wang, Y.X. Inhibition of Connexin 36 attenuates HMGB1-mediated depressive-like behaviors induced by chronic unpredictable mild stress. Brain Behav. 2022, 12, e2470. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, F.; Li, W.; Li, W.; Hu, Y.C.; Li, S.; Zhu, J.H.; Zhou, M.; Hang, C.H. Glycyrrhizic acid confers neuroprotection after subarachnoid hemorrhage via inhibition of high mobility group box-1 protein: A hypothesis for novel therapy of subarachnoid hemorrhage. Med. Hypotheses 2013, 81, 681–685. [Google Scholar] [CrossRef]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef]
- Vitali, R.; Palone, F.; Cucchiara, S.; Negroni, A.; Cavone, L.; Costanzo, M.; Aloi, M.; Dilillo, A.; Stronati, L. Dipotassium Glycyrrhizate Inhibits HMGB1-Dependent Inflammation and Ameliorates Colitis in Mice. PLoS ONE 2013, 8, e66527. [Google Scholar] [CrossRef]
- Wu, C.X.; He, L.X.; Guo, H.; Tian, X.X.; Liu, Q.; Sun, H. Inhibition effect of glycyrrhizin in lipopolysaccharide-induced high-mobility group box 1 releasing and expression from RAW264.7 cells. Shock 2015, 43, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Liu, L.; Zhang, W.; Zhang, Y.; Liu, Y.Z.; Shen, X.L.; Gong, H.; Yang, Y.Y.; Bi, X.Y.; Jiang, C.L.; et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J. Psychiatr. Res. 2015, 64, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Weng, Z.; Zhou, T.; Feng, T.; Lin, Y. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain Res. 2014, 1582, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Fang, Y.; Deng, S.; Li, X.; Zhou, Y.; Gong, Y.; Zhu, H.; Wang, W. Glycyrrhizin Protects Rats from Sepsis by Blocking HMGB1 Signaling. BioMed Res. Int. 2017, 2017, 9719647. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, C.; Cui, L.; Xing, H.; Liu, J.; Liao, W.; Liao, H.; Yu, Y. HMGB1 contributes to the irradiation-induced endothelial barrier injury through receptor for advanced glycation endproducts (RAGE). J. Cell. Physiol. 2018, 233, 6714–6721. [Google Scholar] [CrossRef]
- Akamatsu, H.; Komura, J.; Asada, Y.; Niwa, Y. Mechanism of anti-inflammatory action of glycyrrhizin: Effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991, 57, 119–121. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Babele, P.; Sadhu, S.; Madan, U.; Tripathy, M.R.; Goswami, S.; Mani, S.; Kumar, S.; Awasthi, A.; Dikshit, M. Prophylactic treatment of Glycyrrhiza glabra mitigates COVID-19 pathology through inhibition of pro-inflammatory cytokines in the hamster model and NETosis. Front. Immunol. 2022, 13, 945583. [Google Scholar] [CrossRef]
- Bordbar, N.; Karimi, M.H.; Amirghofran, Z. The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell Immunol. 2012, 280, 44–49. [Google Scholar] [CrossRef]
- Chen, X.; Fang, D.; Li, L.; Chen, L.; Li, Q.; Gong, F.; Fang, M. Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol. Res. 2017, 65, 666–680. [Google Scholar] [CrossRef]
- Ma, C.; Wang, F.; Zhu, J.; Wang, S.; Liu, Y.; Xu, J.; Zhao, Q.; Qin, Y.; Si, W.; Zhang, J. 18Beta-Glycyrrhetinic Acid Attenuates H(2)O(2)-Induced Oxidative Damage and Apoptosis in Intestinal Epithelial Cells via Activating the PI3K/Akt Signaling Pathway. Antioxidants 2024, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, M.M.; Zhang, M.H.; Wang, R.S.; Tan, X.B.; Song, J.; Ding, S.M.; Jia, X.B.; Hu, S.Y. Protection of glycyrrhizic acid against AGEs-induced endothelial dysfunction through inhibiting RAGE/NF-kappaB pathway activation in human umbilical vein endothelial cells. J. Ethnopharmacol. 2013, 148, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lian, Y.J.; Dong, X.; Peng, W.; Liu, L.L.; Su, W.J.; Gong, H.; Zhang, T.; Jiang, C.L.; Li, J.S.; et al. Glycyrrhizic acid ameliorates the kynurenine pathway in association with its antidepressant effect. Behav. Brain Res. 2018, 353, 250–257. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujigaki, H.; Kato, K.; Yamazaki, K.; Fujigaki, S.; Kunisawa, K.; Yamamoto, Y.; Mouri, A.; Oda, A.; Nabeshima, T.; et al. Selective and competitive inhibition of kynurenine aminotransferase 2 by glycyrrhizic acid and its analogues. Sci. Rep. 2019, 9, 10243. [Google Scholar] [CrossRef]
- Ainsah, O.; Nabishah, B.M.; Osman, C.B.; Khalid, B.A. Short- and long-term effects of glycyrrhizic acid in repetitive stress. Clin. Exp. Pharmacol. Physiol. 1999, 26, 444–448. [Google Scholar] [CrossRef]
- Gupta, G.L.; Sharma, L.; Sharma, M. 18beta-Glycyrrhetinic Acid Ameliorates Neuroinflammation Linked Depressive Behavior Instigated by Chronic Unpredictable Mild Stress via Triggering BDNF/TrkB Signaling Pathway in Rats. Neurochem. Res. 2023, 48, 551–569. [Google Scholar] [CrossRef]
- Ding, W.; Yao, M.; Liang, X.; Wong, W.; Yao, W.; Zhang, J.C. The serum levels of polyunsaturated fatty acids contribute to the antidepressant-like effects of 18beta-Glycyrrhetinic acid in mice. Psychopharmacology 2025, 102, 10858–10863. [Google Scholar] [CrossRef]
- Murck, H.; Karailiev, P.; Karailievova, L.; Puhova, A.; Jezova, D. Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression. Nutrients 2024, 16, 515. [Google Scholar] [CrossRef]
- Jezova, D.; Karailiev, P.; Karailievova, L.; Puhova, A.; Murck, H. Food Enrichment with Glycyrrhiza glabra Extract Suppresses ACE2 mRNA and Protein Expression in Rats-Possible Implications for COVID-19. Nutrients 2021, 13, 2321. [Google Scholar] [CrossRef]
- Li, Y. Effect of Xiaoyaosan on brain volume and microstructure diffusion changes to exert antidepressant-like effects in mice with chronic social defeat stress. Front. Psychiatry 2024, 15, 1414295. [Google Scholar] [CrossRef] [PubMed]
- Kamisli, S.; Ciftci, O.; Taslidere, A.; Basak Turkmen, N.; Ozcan, C. The beneficial effects of 18beta-glycyrrhetinic acid on the experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mouse model. Immunopharmacol. Immunotoxicol. 2018, 40, 344–352. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cai, T.T.; Shen, Y.; Zhou, X.B.; Chen, T.; Xu, Q. Si-Ni-San, a traditional Chinese prescription, and its active ingredient glycyrrhizin ameliorate experimental colitis through regulating cytokine balance. Int. Immunopharmacol. 2009, 9, 1437–1443. [Google Scholar] [CrossRef]
- Visvanathan, R.; Houghton, M.J.; Williamson, G. Impact of Glucose, Inflammation and Phytochemicals on ACE2, TMPRSS2 and Glucose Transporter Gene Expression in Human Intestinal Cells. Antioxidants 2025, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Singhal, T.; Sharma, H. Cardioprotective effect of ammonium glycyrrhizinate against doxorubicin-induced cardiomyopathy in experimental animals. Indian J. Pharmacol. 2014, 46, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, X.; Mao, B.; Zhang, Q.; Zhao, J.; Cui, S.; Chen, W. Ethanol Extract of Licorice Alleviates HFD-Induced Liver Fat Accumulation in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Obesity Mice. Nutrients 2022, 14, 4180. [Google Scholar] [CrossRef]
- Park, M.; Lee, J.H.; Choi, J.K.; Hong, Y.D.; Bae, I.H.; Lim, K.M.; Park, Y.H.; Ha, H. 18beta-glycyrrhetinic acid attenuates anandamide-induced adiposity and high-fat diet induced obesity. Mol. Nutr. Food Res. 2014, 58, 1436–1446. [Google Scholar] [CrossRef]
- Zhou, L.; Yi, Y.; Lin, B.; Qiu, Z.; Wang, C.; Li, Y. Glycyrrhizic acid mitigates hepatocyte steatosis and inflammation through ACE2 stabilization via dual modulation of AMPK activation and MDM2 inhibition. Eur. J. Pharmacol. 2025, 1002, 177817. [Google Scholar] [CrossRef]
- Seseke, F.G.; Gardemann, A.; Jungermann, K. Signal propagation via gap junctions, a key step in the regulation of liver metabolism by the sympathetic hepatic nerves. FEBS Lett. 1992, 301, 265–270. [Google Scholar] [CrossRef]
- Rowe, P.C.; Bou-Holaigah, I.; Kan, J.S.; Calkins, H. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet 1995, 345, 623–624. [Google Scholar] [CrossRef]
- Otari, K.S.; Shete, R.V.; Bhutada, R.N.; Upasani, C.D. Effect of ammonium glycyrrhizinate on haloperidol- and reserpine- induced neurobehavioral alterations in experimental paradigms. Orient Pharm. Exp. Med. 2011, 11, 153–160. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 449–454. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Haredy, S.A.; Niazy, R.M.; Linhardt, R.J.; Warda, M. Dose-dependent neuroprotective effect of oriental phyto-derived glycyrrhizin on experimental neuroterminal norepinephrine depletion in a rat brain model. Chem. Biol. Interact. 2019, 308, 279–287. [Google Scholar] [CrossRef]
- Komiyama, M.; Ozaki, Y.; Wada, H.; Yamakage, H.; Satoh-Asahara, N.; Yasoda, A.; Sunagawa, Y.; Morimoto, T.; Tamaki, S.; Masahiro, S.; et al. Randomized double-blind placebo-controlled multicenter trial for the effects of a polyherbal remedy, Yokukansan (YiganSan), in smokers with depressive tendencies. BMC Complement. Med. Ther. 2022, 22, 311. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Mizoguchi, K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol. Ther. 2016, 166, 84–95. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Liu, Y.Z.; Li, J.M.; Ruan, Y.M.; Yan, W.J.; Zhong, S.Y.; Zhang, T.; Liu, L.L.; Wu, R.; Wang, B.; et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J. Affect. Disord. 2020, 265, 247–254. [Google Scholar] [CrossRef]
- Murck, H.; Mahmoudi, S.T.; Jezova, D. Inhibition of the 11beta-Hydroxysteroid-Dehydrogenase Type 2 (11betaHSD2) Reduces Aldosterone/Cortisol Ratio and Affects Treatment Outcome Depending on Childhood Trauma History and Inflammation. Biol. Psychiatry 2025, 97, S213. [Google Scholar] [CrossRef]
- The National Archives and Records Administration. CFR—Code of Federal Regulations Title 21—Subchapter B—Food for Human Consumption—Subpart B-Listing of Specific Substances Affirmed as GRAS-Sec. 184.1408 Licorice and Licorice Derivatives. 2017. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-184/subpart-B/section-184.1408 (accessed on 8 October 2025).
- MacKenzie, M.A.; Hoefnagels, W.H.; Jansen, R.W.; Benraad, T.J.; Kloppenborg, P.W. The influence of glycyrrhetinic acid on plasma cortisol and cortisone in healthy young volunteers. J. Clin. Endocrinol. Metab. 1990, 70, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, B.; Mensinga, T.; Sips, A.; Deerenberg, C.; Meulenbelt, J.; DeJongh, J. A population physiologically based pharmacokinetic/pharmacodynamic model for the inhibition of 11-beta-hydroxysteroid dehydrogenase activity by glycyrrhetic acid. Toxicol. Appl. Pharmacol. 2001, 170, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Uehlinger, D.E.; Ferrari, P.; Dick, B.; Frey, B.M.; Frey, F.J.; Vogt, B. Glycyrrhetinic acid decreases plasma potassium concentrations in patients with anuria. J. Am. Soc. Nephrol. 2002, 13, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Xiao, J.; Zhang, K.; Li, Q.; Chen, H.; Liu, F. 18 beta-glycyrrhetinic acid ameliorates the cognitive functions and decreases the recurrence rate of pituitary adenomas patients. EXCLI J. 2018, 17, 753–761. [Google Scholar] [CrossRef]
- Farese, S.; Kruse, A.; Pasch, A.; Dick, B.; Frey, B.M.; Uehlinger, D.E.; Frey, F.J. Glycyrrhetinic acid food supplementation lowers serum potassium concentration in chronic hemodialysis patients. Kidney Int. 2009, 76, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Chen, C.; Xu, W.; Guo, T.; Pan, H.; Gao, S. Study on Antiviral Activities of Glycyrrhizin. Int. J. Biomed. Eng. Clin. Sci. 2020, 6, 68–70. [Google Scholar] [CrossRef]
- Ding, H.; Deng, W.; Ding, L.; Ye, X.; Yin, S.; Huang, W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID-19 patients. J. Med. Virol. 2020, 92, 2200–2204. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J. 11beta-Hydroxysteroid dehydrogenase and the brain: Not (yet) lost in translation. J. Intern. Med. 2024, 295, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.A.; Conca, T.J.; Morris, D.J. The effects of the licorice derivative, glycyrrhetinic acid, on hepatic 3 alpha- and 3 beta-hydroxysteroid dehydrogenases and 5 alpha- and 5 beta-reductase pathways of metabolism of aldosterone in male rats. Steroids 1990, 55, 52–58. [Google Scholar] [CrossRef]
- Baker, M.E. Licorice and enzymes other than 11 beta-hydroxysteroid dehydrogenase: An evolutionary perspective. Steroids 1994, 59, 136–141. [Google Scholar] [CrossRef]
- Ulmann, A.; Menard, J.; Corvol, P. Binding of glycyrrhetinic acid to kidney mineralocorticoid and glucocorticoid receptors. Endocrinology 1975, 97, 46–51. [Google Scholar] [CrossRef]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef]
- Du, Y.M.; Xia, C.K.; Zhao, N.; Dong, Q.; Lei, M.; Xia, J.H. 18beta-Glycyrrhetinic acid preferentially blocks late Na current generated by DeltaKPQ Nav1.5 channels. Acta Pharmacol. Sin. 2012, 33, 752–760. [Google Scholar] [CrossRef]
- Fu, X.X.; Du, L.L.; Zhao, N.; Dong, Q.; Liao, Y.H.; Du, Y.M. 18beta-Glycyrrhetinic acid potently inhibits Kv1.3 potassium channels and T cell activation in human Jurkat T cells. J. Ethnopharmacol. 2013, 148, 647–654. [Google Scholar] [CrossRef]
- Nabekura, T.; Yamaki, T.; Ueno, K.; Kitagawa, S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother. Pharmacol. 2008, 62, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, K.; Morinaga, O.; Yoshino, T.; Mitamura, M.; Hirasawa, A.; Maki, Y.; Tashita, Y.; Kondo, T.; Ogawa, K.; Lian, F.; et al. Identification of an Alternative Glycyrrhizin Metabolite Causing Liquorice-Induced Pseudohyperaldosteronism and the Development of ELISA System to Detect the Predictive Biomarker. Front Pharmacol. 2021, 12, 688508. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kanaoka, M.; Yano, S.; Kobayashi, M. 3-Monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism. J. Clin. Endocrinol. Metab. 1995, 80, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Makino, T. 3-Monoglucuronyl glycyrrhretinic acid is a possible marker compound related to licorice-induced pseudoaldosteronism. Biol. Pharm. Bull. 2014, 37, 898–902. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murck, H. Discovery of Personalized Treatment for Immuno-Metabolic Depression—Focus on 11beta Hydroxysteroid Dehydrogenase Type 2 (11betaHSD2) and Toll-like Receptor 4 (TLR4) Inhibition with Enoxolone. Pharmaceuticals 2025, 18, 1517. https://doi.org/10.3390/ph18101517
Murck H. Discovery of Personalized Treatment for Immuno-Metabolic Depression—Focus on 11beta Hydroxysteroid Dehydrogenase Type 2 (11betaHSD2) and Toll-like Receptor 4 (TLR4) Inhibition with Enoxolone. Pharmaceuticals. 2025; 18(10):1517. https://doi.org/10.3390/ph18101517
Chicago/Turabian StyleMurck, Harald. 2025. "Discovery of Personalized Treatment for Immuno-Metabolic Depression—Focus on 11beta Hydroxysteroid Dehydrogenase Type 2 (11betaHSD2) and Toll-like Receptor 4 (TLR4) Inhibition with Enoxolone" Pharmaceuticals 18, no. 10: 1517. https://doi.org/10.3390/ph18101517
APA StyleMurck, H. (2025). Discovery of Personalized Treatment for Immuno-Metabolic Depression—Focus on 11beta Hydroxysteroid Dehydrogenase Type 2 (11betaHSD2) and Toll-like Receptor 4 (TLR4) Inhibition with Enoxolone. Pharmaceuticals, 18(10), 1517. https://doi.org/10.3390/ph18101517






