Thermogenic Targets for Obesity Management in the Era of Incretin-Based Therapies
Abstract
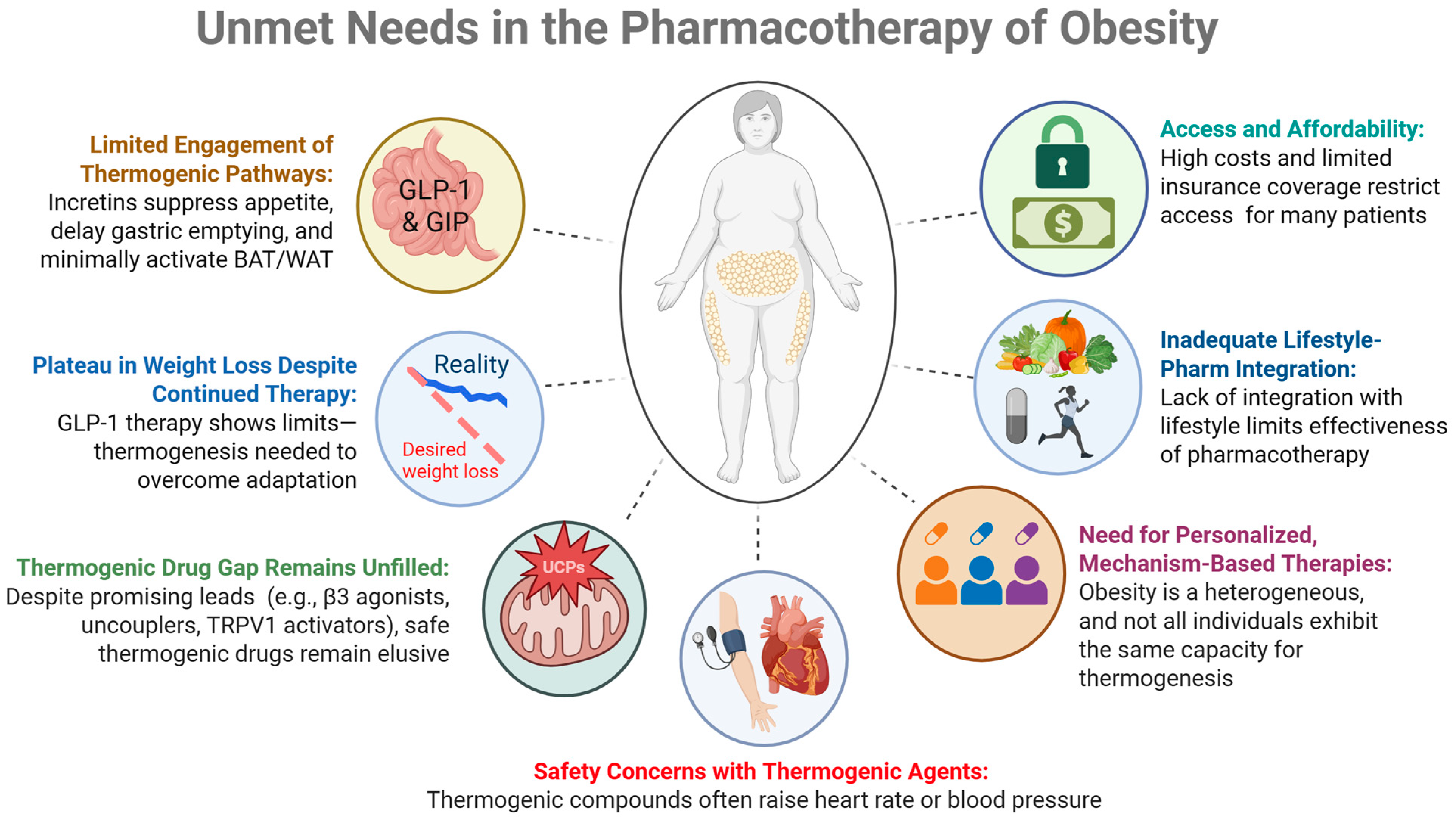
1. Introduction: Unmet Needs in Obesity Pharmacotherapy
1.1. Plateau in Weight Loss Despite Continued Incretin Therapy
1.2. Limited Engagement of Thermogenic Pathways During Incretin Monotherapies
1.3. Therapeutic Void (Lack of FDA-Approved Agents Specifically Targeting Thermogenesis)
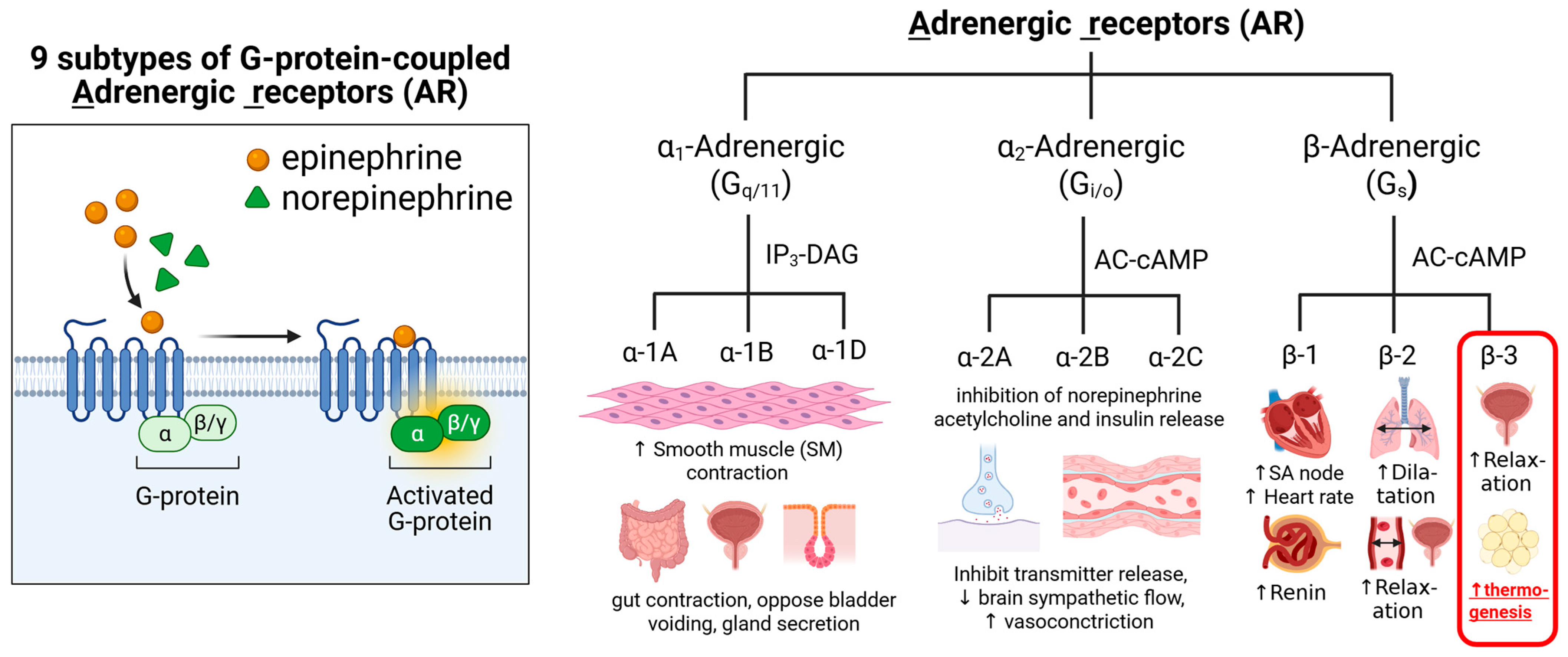
1.3.1. β3-Adrenergic Receptor Agonists
1.3.2. Mitochondrial Uncouplers
1.3.3. TRPV1 Agonists
1.3.4. Pharmacological Activation of Thyroid Hormone Receptors
1.3.5. Centrally Acting Sympathomimetics
1.4. Safety Concerns About Thermogenic Agents
1.5. Need for Personalized and Mechanism-Based Therapies
1.6. Inadequate Integration with Lifestyle Interventions
1.7. Access and Affordability
2. β3-Adrenergic Receptors and the Mechanism of Thermogenesis
3. β3-Adrenergic Receptor Agonists: FDA-Approved Indications
4. Preclinical vs. Clinical Studies
4.1. Preclinical Studies
4.2. Clinical Studies
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenylyl cyclase |
| AMPK | 5′adenosine monophosphate-activated protein kinase |
| AR | Adrenergic receptors |
| ATGL | Adipose triglyceride lipase |
| ATP | Adenosine triphosphate |
| BAT | Brown adipose tissue |
| cAMP | Cyclic adenosine monophosphate |
| CIDEA | Cell death–inducing DFFA-like effector A |
| CREB | cAMP-responsive element-binding protein |
| DNP | 2,4-dinitrophenol |
| EE | Energy expenditure |
| FDA | U.S. Food and Drug Administration |
| FFA | Free fatty acid |
| GCPR | G protein-coupled receptors |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GIPR | GIP receptor |
| GLP–1 RA | GLP-1 receptor agonist |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1R | GLP-1 receptor |
| Gs | Stimulatory G protein |
| HSL | Hormone-sensitive lipase |
| MGL | Monoacylglycerol lipase |
| PGC | 1α-Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKA | Protein kinase A |
| RMR | Resting metabolic rate |
| SNS | Sympathetic nervous system |
| TAG | Triacylglycerol |
| TH | Thyroid hormone |
| THR | Thyroid hormone receptor |
| TMEM26 | Transmembrane protein 26 |
| UCP1 | Uncoupling protein 1 |
| WAT | White adipose tissue |
| WHO | World Health Organization |
| β3-AR | Beta-3 adrenergic receptors |
| 18F-FDG | 18F-fluorodeoxyglucose |
| 18F-FDG PET/CT | 18F-fluorodeoxyglucose positron emission tomography/computerized tomography |
References
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2024; pp. 1–10. [Google Scholar]
- Islam, A.S.; Sultana, H.; Refat, N.H.; Farhana, Z.; Kamil, A.A.; Rahman, M.M. The global burden of overweight-obesity and its association with economic status, benefiting from STEPs survey of WHO member states: A meta-analysis. Prev. Med. Rep. 2024, 46, 102882. [Google Scholar] [CrossRef]
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health—NCI. Cancer Causes and Prevention. Risk Factors. Obesity and Cancer. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet (accessed on 3 October 2025).
- Ward, Z.J.; Willett, W.C.; Hu, F.B.; Pacheco, L.S.; Long, M.W.; Gortmaker, S.L. Excess mortality associated with elevated body weight in the USA by state and demographic subgroup: A modelling study. eClinicalMedicine 2022, 48, 101429. [Google Scholar] [CrossRef]
- Holst, J.J. GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat. Metab. 2024, 6, 1866–1885. [Google Scholar] [CrossRef]
- Véniant, M.M.; Lu, S.-C.; Atangan, L.; Komorowski, R.; Stanislaus, S.; Cheng, Y.; Wu, B.; Falsey, J.R.; Hager, T.; Thomas, V.A.; et al. A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat. Metab. 2024, 6, 290–303. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, H.; Bi, Y.; Li, H.; Tian, J.; Liu, D.; Zhao, Y.; Qiu, W.; Huang, C.; Chen, L.; et al. Once-Weekly Mazdutide in Chinese Adults with Obesity or Overweight. N. Engl. J. Med. 2025, 392, 2215–2225. [Google Scholar] [CrossRef]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning Through Hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Choi, Y.; Yu, L. Natural Bioactive Compounds as Potential Browning Agents in White Adipose Tissue. Pharm. Res. 2021, 38, 549–567. [Google Scholar] [CrossRef]
- NN8022-1807 Investigators; Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.E.J.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012, 36, 843–854. [Google Scholar] [CrossRef]
- Lundgren, J.R.; Janus, C.; Jensen, S.B.; Juhl, C.R.; Olsen, L.M.; Christensen, R.M.; Svane, M.S.; Bandholm, T.; Bojsen-Møller, K.N.; Blond, M.B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. [Google Scholar] [CrossRef]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef]
- Williams, D.L. Minireview: Finding the sweet spot: Peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology 2009, 150, 2997–3001. [Google Scholar] [CrossRef]
- Campbell, J.E. Targeting the GIPR for obesity: To agonize or antagonize? Potential mechanisms. Mol. Metab. 2021, 46, 101139. [Google Scholar] [CrossRef]
- NamKoong, C.; Kim, M.S.; Jang, B.-T.; Lee, Y.H.; Cho, Y.-M.; Choi, H.J. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem. Biophys. Res. Commun. 2017, 490, 247–252. [Google Scholar] [CrossRef]
- Sparre-Ulrich, A.H.; Hansen, L.S.; Svendsen, B.; Christensen, M.; Knop, F.K.; Hartmann, B.; Holst, J.J.; Rosenkilde, M.M. Species-specific action of (Pro3)GIP—A full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. Br. J. Pharmacol. 2016, 173, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Rosenkilde, M.M. GIP as a Therapeutic Target in Diabetes and Obesity: Insight From Incretin Co-agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2710–e2716. [Google Scholar] [CrossRef]
- Xu, F.; Lin, B.; Zheng, X.; Chen, Z.; Cao, H.; Xu, H.; Liang, H.; Weng, J. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 2016, 59, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Dong, C.; Zhao, B.; Zhou, B.; Yang, L. Glucagon-like peptide-1 receptor agonist regulates fat browning by altering the gut microbiota and ceramide metabolism. MedComm 2023, 4, e416. [Google Scholar] [CrossRef]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef]
- López, M.; Diéguez, C.; Nogueiras, R. Hypothalamic GLP-1: The control of BAT thermogenesis and browning of white fat. Adipocyte 2015, 4, 141–145. [Google Scholar] [CrossRef]
- Challa, T.D.; Beaton, N.; Arnold, M.; Rudofsky, G.; Langhans, W.; Wolfrum, C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 2012, 287, 6421–6430. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, H.; Ma, X.; Zhang, Y.; Lu, S.; Wang, Y.; Zong, C.; Qin, D.; Wang, Y.; Yang, Y.Y.; et al. GLP-1/GLP-1R Signaling in Regulation of Adipocyte Differentiation and Lipogenesis. Cell Physiol. Biochem. 2017, 42, 1165–1176. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Q.; Zhang, H.; Zhang, Y.; Yang, G.; Ban, B.; Li, Y.; Zhang, M. The effects of glucagon-like peptide-1 receptor agonists on adipose tissues in patients with type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS ONE 2022, 17, e0270899. [Google Scholar] [CrossRef]
- Ross, S.A.; Dupre, J. Effects of ingestion of triglyceride or galactose on secretion of gastric inhibitory polypeptide and on responses to intravenous glucose in normal and diabetic subjects. Diabetes 1978, 27, 327–333. [Google Scholar] [CrossRef]
- Yip, R.G.; Wolfe, M.M. GIP biology and fat metabolism. Life Sci. 2000, 66, 91–103. [Google Scholar] [CrossRef]
- Beaudry, J.L.; Kaur, K.D.; Varin, E.M.; Baggio, L.L.; Cao, X.; Mulvihill, E.E.; Bates, H.E.; Campbell, J.E.; Drucker, D.J. Physiological roles of the GIP receptor in murine brown adipose tissue. Mol. Metab. 2019, 28, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Beaudry, J.L.; Svendsen, B.; Baggio, L.L.; Gordon, A.N.; Ussher, J.R.; Wong, C.K.; Gribble, F.M.; D’aLessio, D.A.; Reimann, F.; et al. GIPR Is Predominantly Localized to Nonadipocyte Cell Types Within White Adipose Tissue. Diabetes 2022, 71, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, S.; Funcke, J.-B.; Straub, L.G.; Pirro, V.; Emont, M.P.; Droz, B.A.; Collins, K.A.; Joung, C.; Pearson, M.J.; et al. The GIP receptor activates futile calcium cycling in white adipose tissue to increase energy expenditure and drive weight loss in mice. Cell Metab. 2025, 37, 187–204.e7. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.E.; Donnelly, D.; Wabitsch, M.; Grant, P.J.; Balmforth, A.J. Functional expression of glucose-dependent insulinotropic polypeptide receptors is coupled to differentiation in a human adipocyte model. Int. J. Obes. 2008, 32, 1705–1711. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Genchi, V.A.; Palma, G.; Sorice, G.P.; D’oRia, R.; Caccioppoli, C.; Marrano, N.; Biondi, G.; Caruso, I.; Cignarelli, A.; Natalicchio, A.; et al. Pharmacological modulation of adaptive thermogenesis: New clues for obesity management? J. Endocrinol. Investig. 2023, 46, 2213–2236. [Google Scholar] [CrossRef]
- Satheesan, A.; Kumar, J.; Leela, K.V.; Lathakumari, R.H.; Angelin, M.; Murugesan, R.; Chaithanya, V. The multifaceted regulation of white adipose tissue browning and their therapeutic potential. J. Physiol. Biochem. 2025. ahead of print. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Heaton, J.M. The distribution of brown adipose tissue in the human. J. Anat. 1972, 112 Pt 1, 35–39. [Google Scholar]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Cohade, C.; Osman, M.; Pannu, H.K.; Wahl, R.L. Uptake in supraclavicular area fat (“USA-Fat”): Description on 18F-FDG PET/CT. J. Nucl. Med. 2003, 44, 170–176. [Google Scholar] [PubMed]
- Yeung, H.W.D.; Grewal, R.K.; Gonen, M.; Schöder, H.; Larson, S.M. Patterns of (18)F-FDG uptake in adipose tissue and muscle: A potential source of false-positives for PET. J. Nucl. Med. 2003, 44, 1789–1796. [Google Scholar]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Lidell, M.E.; Oikonen, V.; Noponen, T.; Viljanen, T.; Scheinin, M.; Taittonen, M.; Niemi, T.; Enerbäck, S.; et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011, 14, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Labbé, S.M.; Blondin, D.P.; Phoenix, S.; Guérin, B.; Haman, F.; Turcotte, E.E.; Richard, D.; Carpentier, A.C. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Investig. 2012, 122, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Jespersen, N.Z.; Larsen, T.J.; Peijs, L.; Daugaard, S.; Homøe, P.; Loft, A.; de Jong, J.; Mathur, N.; Cannon, B.; Nedergaard, J.; et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013, 17, 798–805. [Google Scholar] [CrossRef]
- Sacks, H.; Symonds, M.E. Anatomical locations of human brown adipose tissue: Functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013, 62, 1783–1790. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J. Luxuskonsumption, diet-induced thermogenesis and brown fat: The case in favour. Clin. Sci. 1983, 64, 19–23. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue-A Translational Perspective. Endocr. Rev. 2023, 44, 143–192. [Google Scholar] [CrossRef]
- Arch, J.R. Challenges in β(3)-Adrenoceptor Agonist Drug Development. Ther. Adv. Endocrinol. Metab. 2011, 2, 59–64. [Google Scholar] [CrossRef]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.-H.; Cypess, A.M. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 2021, 6, e139160. [Google Scholar] [CrossRef]
- Warner, A.; Kjellstedt, A.; Carreras, A.; Böttcher, G.; Peng, X.-R.; Seale, P.; Oakes, N.; Lindén, D. Activation of β3-adrenoceptors increases in vivo free fatty acid uptake and utilization in brown but not white fat depots in high-fat-fed rats. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E901–E910. [Google Scholar] [CrossRef]
- Ursino, M.G.; Vasina, V.; Raschi, E.; Crema, F.; De Ponti, F. The β3-adrenoceptor as a therapeutic target: Current perspectives. Pharmacol. Res. 2009, 59, 221–234. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Farah, C.; Balligand, J.L. The Beta3 Adrenergic Receptor in Healthy and Pathological Cardiovascular Tissues. Cells 2020, 9, 2584. [Google Scholar] [CrossRef]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-dinitrophenol (DNP): A weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef]
- Hermetet, C.; Jourdan, M.; Baert, A.; Gheddar, L.; Ameline, A.; Kintz, P.; Bouvet, R. Case report: Fatal long-term intoxication by 2,4-dinitrophenol and anabolic steroids in a young bodybuilder with muscle dysmorphia. Front. Public Health 2024, 12, 1452196. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Jiang, J.K.; Huang, W.D. Clinical features and treatment in patients with acute 2,4-dinitrophenol poisoning. J. Zhejiang Univ. Sci. B 2011, 12, 189–192. [Google Scholar] [CrossRef]
- Okamoto, T.; Shimada, T.; Matsumura, C.; Minoshima, H.; Ban, T.; Itotani, M.; Shinohara, T.; Fujita, S.; Matsuda, S.; Sato, S.; et al. New Approach to Drug Discovery of a Safe Mitochondrial Uncoupler: OPC-163493. ACS Omega 2021, 6, 16980–16988. [Google Scholar] [CrossRef]
- Xiong, G.; Zhang, K.; Ma, Y.; Song, Y.; Zhang, W.; Qi, T.; Qiu, H.; Shi, J.; Kan, C.; Zhang, J.; et al. BAM15 as a mitochondrial uncoupler: A promising therapeutic agent for diverse diseases. Front. Endocrinol. 2023, 14, 1252141. [Google Scholar] [CrossRef] [PubMed]
- Danielak, A.; Magierowski, M. Obesity and mitochondrial uncoupling—An opportunity for the carbon monoxide-based pharmacology of metabolic diseases. Pharmacol. Res. 2025, 215, 107741. [Google Scholar] [CrossRef]
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell. Neurosci. 2020, 14, 612480. [Google Scholar] [CrossRef]
- Christie, S.; Wittert, G.A.; Li, H.; Page, A.J. Involvement of TRPV1 Channels in Energy Homeostasis. Front. Endocrinol. 2018, 9, 420. [Google Scholar] [CrossRef]
- Krishnan, V.; Baskaran, P.; Thyagarajan, B. Troglitazone activates TRPV1 and causes deacetylation of PPARγ in 3T3-L1 cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 445–453. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.; Yen, P.M. Thermogenesis in Adipose Tissue Activated by Thyroid Hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Martagon, A.J.; Cimini, S.L.; Gonzalez, D.D.; Tinkey, D.W.; Biter, A.; Baxter, J.D.; Webb, P.; Gustafsson, J.Å.; Hartig, S.M.; et al. Pharmacological Activation of Thyroid Hormone Receptors Elicits a Functional Conversion of White to Brown Fat. Cell Rep. 2015, 13, 1528–1537. [Google Scholar] [CrossRef]
- Saponaro, F.; Sestito, S.; Runfola, M.; Rapposelli, S.; Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Front. Med. 2020, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef]
- Bianco, A.C.; McAninch, E.A. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013, 1, 250–258. [Google Scholar] [CrossRef]
- Lesses, M.F.; Myerson, A. Human autonomic pharmacology. XVI. Benzedrine sulfate as an aid in the treatment of obesity. 1938. Obes Res. 1994, 2, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Casimirri, F.; Melchionda, N.; Grossi, G.; Bortoluzzi, L.; Labate, A.M.M.; Stefanini, C.; Raitano, A. Effects of chronic administration of ephedrine during very-low-calorie diets on energy expenditure, protein metabolism and hormone levels in obese subjects. Clin. Sci. 1992, 82, 85–92. [Google Scholar] [CrossRef]
- Lorello, C.; Goldfield, G.S.; Doucet, E. Methylphenidate hydrochloride increases energy expenditure in healthy adults. Obesity 2008, 16, 470–472. [Google Scholar] [CrossRef]
- Jones, J.R.; Caul, W.F.; Hill, J.O. The effects of amphetamine on body weight and energy expenditure. Physiol. Behav. 1992, 51, 607–611. [Google Scholar] [CrossRef]
- Lang, S.S.; Danforth, E., Jr.; Lien, E.L. Anorectic drugs which stimulate thermogenesis. Life Sci. 1983, 33, 1269–1275. [Google Scholar] [CrossRef]
- Bushnell, P.J. Differential effects of amphetamine and related compounds on locomotor activity and metabolic rate in mice. Pharmacol. Biochem. Behav. 1986, 25, 161–170. [Google Scholar] [CrossRef]
- Arch, J.R. The contribution of increased thermogenesis to the effect of anorectic drugs on body composition in mice. Am. J. Clin. Nutr. 1981, 34, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.D.; Trevaskis, J.L.; Wilson, J.; Lei, C.; Athanacio, J.; Mack, C.; Kesty, N.C.; Coffey, T.; Weyer, C.; Parkes, D.G. Antiobesity effects of the β-cell hormone amylin in combination with phentermine or sibutramine in diet-induced obese rats. Int. J. Obes. 2008, 32, 1201–1210. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. 2025, 31, 951–962. [Google Scholar] [CrossRef]
- Oeckl, J.; Janovska, P.; Adamcova, K.; Bardova, K.; Brunner, S.; Dieckmann, S.; Ecker, J.; Fromme, T.; Funda, J.; Gantert, T.; et al. Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat. Mol. Metab. 2022, 61, 101499. [Google Scholar] [CrossRef] [PubMed]
- Bunk, J.; Hussain, M.F.; Delgado-Martin, M.; Samborska, B.; Ersin, M.; Shaw, A.; Rahbani, J.F.; Kazak, L. The Futile Creatine Cycle powers UCP1-independent thermogenesis in classical BAT. Nat. Commun. 2025, 16, 3221. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Tadrous, M.; Matta, R.; Greaves, S.; Herschorn, S.; Mamdani, M.M.; Juurlink, D.N.; Gomes, T. Association of Mirabegron With the Risk of Arrhythmia in Adult Patients 66 Years or Older—A Population-Based Cohort Study. JAMA Intern. Med. 2019, 179, 1436–1439. [Google Scholar] [CrossRef]
- Hoffman, V.; Hallas, J.; Linder, M.; Margulis, A.V.; Suehs, B.T.; Arana, A.; Phiri, K.; Enger, C.; Horter, L.; Odsbu, I.; et al. Cardiovascular Risk in Users of Mirabegron Compared with Users of Antimuscarinic Treatments for Overactive Bladder: Findings from a Non-Interventional, Multinational, Cohort Study. Drug Saf. 2021, 44, 899–915. [Google Scholar] [CrossRef]
- Hou, J.; Xu, F.; Du, H.; Li, N. Adverse events associated with mirabegron 50mg versus placebo: A systematic review and meta-analysis. Prog. Urol. 2021, 31, 627–633. [Google Scholar] [CrossRef]
- Slattery, J.D.; Rambousek, J.R.; Tsui, E.; Honeycutt, M.K.; Goldberg, M.; Graham, J.L.; Wietecha, T.A.; Wolden-Hanson, T.; Williams, A.L.; O’bRien, K.D.; et al. Effects of systemic oxytocin and beta-3 receptor agonist (CL 316243) treatment on body weight and adiposity in male diet-induced obese rats. Front. Endocrinol. 2025, 16, 1503096. [Google Scholar] [CrossRef]
- Tavernier, G.; Toumaniantz, G.; Erfanian, M.; Heymann, M.; Laurent, K.; Langin, D.; Gauthier, C. beta3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human beta3-adrenergic receptor. Cardiovasc. Res. 2003, 59, 288–296. [Google Scholar] [CrossRef]
- Kopec, K.T.; Kim, T.; Mowry, J.; Aks, S.; Kao, L. Role of dantrolene in dinitrophenol (DNP) overdose: A continuing question? Am. J. Emerg. Med. 2019, 37, 1216.e1–1216.e2. [Google Scholar] [CrossRef]
- Scott, H.C.; Gold, M.; Bechtel, A.; Spitzer, J.J. Influence of 2,4-dinitrophenol on myocardial metabolism and hemodynamics. Metabolism 1968, 17, 370–376. [Google Scholar] [CrossRef]
- Peng, J.; Li, Y.-J. The vanilloid receptor TRPV1: Role in cardiovascular and gastrointestinal protection. Eur. J. Pharmacol. 2010, 627, 1–7. [Google Scholar] [CrossRef]
- Patanè, S.; Marte, F.; La Rosa, F.C.; La Rocca, R. Capsaicin and arterial hypertensive crisis. Int. J. Cardiol. 2010, 144, e26–e27. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Jiang, D.-L.; Jia, X.-T.; Fu, L.-Y.; Tian, H.; Liu, K.-L.; Qi, J.; Kang, Y.-M.; Yu, X.-J. Capsaicin improves hypertension and cardiac hypertrophy via SIRT1/NF-κB/MAPKs pathway in the hypothalamic paraventricular nucleus. Phytomedicine 2023, 118, 154951. [Google Scholar] [CrossRef]
- Sayin, M.R.; Karabag, T.; Dogan, S.M.; Akpinar, I.; Aydin, M. A case of acute myocardial infarction due to the use of cayenne pepper pills. Wien. Klin. Wochenschr. 2012, 124, 285–287. [Google Scholar] [CrossRef]
- Dore, R.; Mittag, J. Thyroid Hormone Receptors in Control of Heart Rate. Endocrinology 2024, 165, bqae093. [Google Scholar] [CrossRef]
- Ratziu, V.; Scanlan, T.S.; Bruinstroop, E. Thyroid hormone receptor-β analogues for the treatment of metabolic dysfunction-associated steatohepatitis (MASH). J. Hepatol. 2025, 82, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Gadde, K.M.; Garvey, W.T.; Peterson, C.A.; Schwiers, M.L.; Najarian, T.; Tam, P.Y.; Troupin, B.; Day, W.W. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obesity 2012, 20, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Allison, D.B.; Ryan, D.H.; Peterson, C.A.; Troupin, B.; Schwiers, M.L.; Day, W.W. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Sargeant, J.A.; Henson, J.; King, J.A.; Yates, T.; Khunti, K.; Davies, M.J. A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans. Endocrinol. Metab. 2019, 34, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Madsen, L.W.; Andersen, S.; Almholt, K.; de Boer, A.S.; Drucker, D.J.; Gotfredsen, C.; Egerod, F.L.; Hegelund, A.C.; Jacobsen, H.; et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010, 151, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.C.; Eneli, I.; Osganian, S.K.; Wagner, B.E.; Waldrop, S.W.; Kelly, A.S. Pediatric Obesity Pharmacotherapy: State of the Science, Research Gaps, and Opportunities. Pediatrics 2024, 154, e2024067858. [Google Scholar] [CrossRef]
- Skinner, A.C.; Skelton, S.N.R.J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018, 141, e20173459, Erratum in Pediatrics 2018, 142, e20181916. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Lobstein, T.B.H. Atlas of Childhood Obesity. 2019. Available online: https://www.worldobesity.org/membersarea/global-atlas-on-childhood-obesity (accessed on 3 October 2025).
- Garnett, S.P.; Baur, L.A.; Jones, A.M.D.; Hardy, L.L. Trends in the Prevalence of Morbid and Severe Obesity in Australian Children Aged 7–15 Years, 1985–2012. PLoS ONE 2016, 11, e0154879. [Google Scholar] [CrossRef]
- Spinelli, A.; Buoncristiano, M.; Kovacs, V.A.; Yngve, A.; Spiroski, I.; Obreja, G.; Starc, G.; Pérez, N.; Rito, A.I.; Kunešová, M.; et al. Prevalence of Severe Obesity among Primary School Children in 21 European Countries. Obes. Facts 2019, 12, 244–258. [Google Scholar] [CrossRef]
- Ogden, C.L.; Fryar, C.D.; Hales, C.M.; Carroll, M.D.; Aoki, Y.; Freedman, D.S. Differences in Obesity Prevalence by Demographics and Urbanization in US Children and Adolescents, 2013–2016. JAMA 2018, 319, 2410–2418. [Google Scholar] [CrossRef]
- Ogden, C.L.; Fryar, C.D.; Martin, C.B.; Freedman, D.S.; Carroll, M.D.; Gu, Q.; Hales, C.M. Trends in Obesity Prevalence by Race and Hispanic Origin-1999–2000 to 2017–2018. JAMA 2020, 324, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Marín, A.M.; Iguacel, I.; De Miguel-Etayo, P.; Moreno, L.A. Consideration of Social Disadvantages for Understanding and Preventing Obesity in Children. Front. Public Health 2020, 8, 423. [Google Scholar] [CrossRef]
- Vazquez, C.E.; Cubbin, C. Socioeconomic Status and Childhood Obesity: A Review of Literature from the Past Decade to Inform Intervention Research. Curr. Obes. Rep. 2020, 9, 562–570. [Google Scholar] [CrossRef]
- Guo, S.S.; Roche, A.F.; Chumlea, W.C.; Gardner, J.D.; Siervogel, R.M. The predictive value of childhood body mass index values for overweight at age 35 y. Am. J. Clin. Nutr. 1994, 59, 810–819. [Google Scholar] [CrossRef]
- Parsons, T.J.; Power, C.; Logan, S.; Summerbell, C.D. Childhood predictors of adult obesity: A systematic review. Int. J. Obes. Relat. Metab. Disord. 1999, 23 (Suppl. S8), S1–S107. [Google Scholar]
- Ward, Z.J.; Long, M.W.; Resch, S.C.; Giles, C.M.; Cradock, A.L.; Gortmaker, S.L. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N. Engl. J. Med. 2017, 377, 2145–2153. [Google Scholar] [CrossRef]
- Rundle, A.G.; Factor-Litvak, P.; Suglia, S.F.; Susser, E.S.; Kezios, K.L.; Lovasi, G.S.; Cirillo, P.M.; Cohn, B.A.; Link, B.G. Tracking of Obesity in Childhood into Adulthood: Effects on Body Mass Index and Fat Mass Index at Age 50. Child. Obes. 2020, 16, 226–233. [Google Scholar] [CrossRef]
- Rudolf, M. Predicting babies’ risk of obesity. Arch. Dis. Child. 2011, 96, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Hulman, A.; Narayan, K.M.V.; Cunningham, S.A. Body Mass Index Trajectories From Childhood to Adulthood and Age at Onset of Overweight and Obesity: The Influence of Parents’ Weight Status. Am. J. Epidemiol. 2022, 191, 1877–1885. [Google Scholar] [CrossRef]
- Mead, E.; Batterham, A.M.; Atkinson, G.; Ells, L.J. Predicting future weight status from measurements made in early childhood: A novel longitudinal approach applied to Millennium Cohort Study data. Nutr. Diabetes 2016, 6, e200. [Google Scholar] [CrossRef] [PubMed]
- Buscot, M.-J.; Thomson, R.J.; Juonala, M.; Sabin, M.A.; Burgner, D.P.; Lehtimäki, T.; Hutri-Kähönen, N.; Viikari, J.S.A.; Jokinen, E.; Tossavainen, P.; et al. BMI Trajectories Associated With Resolution of Elevated Youth BMI and Incident Adult Obesity. Pediatrics 2018, 141, e20172003. [Google Scholar] [CrossRef]
- Barlow, S.E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), S164–S192. [Google Scholar] [CrossRef]
- Reilly, J.L.; Arora, S.; Chatman, K.; Cooper, Z.; Hsia, D.S. New directions in childhood obesity treatment—A path forward or wishful thinking? J. Clin. Lipidol. 2025, 19, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Bensignor, M.O.; Hsia, D.S.; Shoemaker, A.H.; Shih, W.; Peterson, C.; Varghese, S.T. Phentermine/Topiramate for the Treatment of Adolescent Obesity. NEJM Evid. 2022, 1, EVIDoa2200014. [Google Scholar] [CrossRef] [PubMed]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Edwards, K.C.A.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060643. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Adolescent BMI Collaborators. Global, regional, and national prevalence of child and adolescent overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S.; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S. Once-Weekly Semaglutide in Adolescents with Obesity. N. Engl. J. Med. 2022, 387, 2245–2257. [Google Scholar] [CrossRef]
- Fox, C.K. Managing Pediatric Obesity Using Advanced Therapies: Practical Guide for Pediatric Health Care Providers; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Jenssen, B.P.; Kelly, M.K.; Powell, M.; Bouchelle, Z.; Mayne, S.L.; Fiks, A.G. COVID-19 and Changes in Child Obesity. Pediatrics 2021, 147, e2021050123. [Google Scholar] [CrossRef]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.R.; Resnicow, K.; Koebnick, C. Changes in Body Mass Index Among Children and Adolescents During the COVID-19 Pandemic. JAMA 2021, 326, 1434–1436. [Google Scholar] [CrossRef]
- Löffler, M.C.; Betz, M.J.; Blondin, D.P.; Augustin, R.; Sharma, A.K.; Tseng, Y.-H.; Scheele, C.; Zimdahl, H.; Mark, M.; Hennige, A.M.; et al. Challenges in tackling energy expenditure as obesity therapy: From preclinical models to clinical application. Mol. Metab. 2021, 51, 101237. [Google Scholar] [CrossRef]
- Farrell, E.; le Roux, C.W.; Hollmann, E.; Nadglowski, J.; McGillicuddy, D. Patient perspectives on personalised medicine for obesity: An IMI2 SOPHIA Study. Obes. Res. Clin. Pract. 2024, 18, 216–221. [Google Scholar] [CrossRef]
- Price, S.; Le, Q.N.; White, N.D. Lifestyle and Pharmacotherapy for Weight Loss in Preventing or Delaying Diabetes. Am. J. Lifestyle Med. 2018, 12, 34–37. [Google Scholar] [CrossRef]
- Rahelić, V.; Perković, T.; Romić, L.; Perković, P.; Klobučar, S.; Pavić, E.; Rahelić, D. The Role of Behavioral Factors on Chronic Diseases-Practice and Knowledge Gaps. Healthcare 2024, 12, 2520. [Google Scholar] [CrossRef] [PubMed]
- Washington, T.B.; Johnson, V.R.; Kendrick, K.; Ibrahim, A.A.; Tu, L.; Sun, K.; Stanford, F.C. Disparities in Access and Quality of Obesity Care. Gastroenterol. Clin. North. Am. 2023, 52, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, L.; Zhao, S. Ligands of Adrenergic Receptors: A Structural Point of View. Biomolecules 2021, 11, 936. [Google Scholar] [CrossRef]
- Hein, L.; Kobilka, B.K. Adrenergic Receptors From Molecular Structure to in vivo function. Trends Cardiovasc. Med. 1997, 7, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Matsunaga, T.; Adachi, T.; Aoki, N.; Tsujimoto, G.; Tsuda, K. Adrenergic receptor polymorphisms and autonomic nervous system function in human obesity. Trends Endocrinol. Metab. 2006, 17, 269–275. [Google Scholar] [CrossRef]
- Szentirmai, É.; Kapás, L. The role of the brown adipose tissue in β3-adrenergic receptor activation-induced sleep, metabolic and feeding responses. Sci. Rep. 2017, 7, 958. [Google Scholar] [CrossRef]
- Bragg, R.; Hebel, D.; Vouri, S.M.; Pitlick, J.M. Mirabegron: A Beta-3 agonist for overactive bladder. Consult. Pharm. 2014, 29, 823–837. [Google Scholar] [CrossRef]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about β(3)-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L. Thematic review series: Adipocyte Biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose tissue lipid metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Ricquier, D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: A historical perspective. Front. Endocrinol. 2011, 2, 85. [Google Scholar] [CrossRef]
- De Jong, K.A.; Siddig, S.; Pfeifer, A.; Nikolaev, V.O. The role of compartmentalized β-AR/cAMP signaling in the regulation of lipolysis in white and brown adipocytes. FEBS J. 2025, 292, 261–271. [Google Scholar] [CrossRef]
- Michel, M.C.; Ochodnicky, P.; Summers, R.J. Tissue functions mediated by beta(3)-adrenoceptors-findings and challenges. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 382, 103–108. [Google Scholar] [CrossRef]
- Andersson, K.E.; Martin, N.; Nitti, V. Selective β3-adrenoceptor agonists for the treatment of overactive bladder. J. Urol. 2013, 190, 1173–1180. [Google Scholar] [CrossRef]
- Nayak, V.; Holla, S.N.; Shenoy, S.; H, H.; Raveendran, A.V. Exploring the therapeutic potential of beta-3 adrenergic agonists in overactive bladder: A comprehensive review. Med. J. Armed Forces India 2025, in press. [Google Scholar] [CrossRef]
- Kennelly, M.; Wielage, R.; Shortino, D.; Thomas, E.; Mudd, P.N., Jr. Long-term efficacy and safety of vibegron versus mirabegron and anticholinergics for overactive bladder: A systematic review and network meta-analysis. Drugs Context 2022, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Bientinesi, R. Mirabegron: A review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 2012, 4, 315–324. [Google Scholar] [CrossRef]
- Dąbrowska, A.M.; Dudka, J. Mirabegron, a Selective β3-Adrenergic Receptor Agonist, as a Potential Anti-Obesity Drug. J. Clin. Med. 2023, 12, 6897. [Google Scholar] [CrossRef]
- van Gelderen, M.; Tretter, R.; Meijer, J.; Dorrepaal, C.; Gangaram-Panday, S.; Brooks, A.; Krauwinkel, W.; Dickinson, J. Absence of clinically relevant cardiovascular interaction upon add-on of mirabegron or tamsulosin to an established tamsulosin or mirabegron treatment in healthy middle-aged to elderly men. Int. J. Clin. Pharmacol. Ther. 2014, 52, 693–701. [Google Scholar] [CrossRef]
- Balachandran, A.A.; Duckett, J.R. The risk and severity of developing symptomatic palpitations when prescribed mirabegron for overactive bladder. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 187, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Loh, R.K.C.; Formosa, M.F.; La Gerche, A.; Reutens, A.T.; Kingwell, B.A.; Carey, A.L. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes Obes. Metab. 2019, 21, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Kuwamoto, K.; Kato, D.; Kuroishi, K. Real-world cardiovascular assessment of mirabegron treatment in patients with overactive bladder and concomitant cardiovascular disease: Results of a Japanese post-marketing study. Int. J. Urol. 2016, 23, 1009–1015. [Google Scholar] [CrossRef]
- White, W.B.; Siddiqui, E.; Tat, T.; Franks, B.; Schermer, C.R. Cardiovascular safety of mirabegron: Analysis of an integrated clinical trial database of patients with overactive bladder syndrome. J. Am. Soc. Hypertens. 2018, 12, 768–778 e1. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Chapple, C.; Gratzke, C.; Herschorn, S.; Robinson, D.; Frankel, J.; Ridder, A.; Stoelzel, M.; Paireddy, A.; van Maanen, R.; et al. Cardiovascular Safety of the β3-Adrenoceptor Agonist Mirabegron and the Antimuscarinic Agent Solifenacin in the SYNERGY Trial. J. Clin. Pharmacol. 2018, 58, 1084–1091. [Google Scholar] [CrossRef]
- Malik, M.; van Gelderen, E.M.; Lee, J.H.; Kowalski, D.L.; Yen, M.; Goldwater, R.; Mujais, S.K.; Schaddelee, M.P.; de Koning, P.; Kaibara, A.; et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: A randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin. Pharmacol. Ther. 2012, 92, 696–706. [Google Scholar] [CrossRef]
- van Gelderen, M.; Stölzel, M.; Meijer, J.; Kerbusch, V.; Collins, C.; Korstanje, C. An Exploratory Study in Healthy Male Subjects of the Mechanism of Mirabegron-Induced Cardiovascular Effects. J. Clin. Pharmacol. 2017, 57, 1534–1544. [Google Scholar] [CrossRef]
- Mo, W.; Michel, M.C.; Lee, X.W.; Kaumann, A.J.; Molenaar, P. The β3-adrenoceptor agonist mirabegron increases human atrial force through β1-adrenoceptors: An indirect mechanism? Br. J. Pharmacol. 2017, 174, 2706–2715. [Google Scholar] [CrossRef]
- Chastek, B.; Carrera, A.; Landis, C.; Snyder, D.; Abedinzadeh, L.; Bancroft, T.; Nesheim, J.; Kennelly, M.; Staskin, D. Comparative analysis of real-world adherence and persistence patterns with vibegron, mirabegron, and anticholinergics in patients with overactive bladder: A retrospective claims study. Neurourol. Urodyn. 2024, 43, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kusabuka, H.; Matsuzawa, A.; Maruyama, I.; Yamazaki, T. Vibegron shows high selectivity and potent agonist activity for β3-adrenoceptors, irrespective of receptor density. PLoS ONE 2023, 18, e0290685. [Google Scholar] [CrossRef] [PubMed]
- Brucker, B.M.; King, J.; Mudd, P.N.; McHale, K. Selectivity and Maximum Response of Vibegron and Mirabegron for β3-Adrenergic Receptors. Curr. Ther. Res. Clin. Exp. 2022, 96, 100674. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Huang, G.; Tian, Y.; Sun, Q.; Liu, X. Efficacy and safety of vibegron compared with mirabegron for overactive bladder: A systematic review and network meta-analysis. Low. Urin. Tract. Symptoms 2023, 15, 80–88. [Google Scholar] [CrossRef]
- Hao, L.; Scott, S.; Abbasi, M.; Zu, Y.; Khan, S.H.; Yang, Y.; Wu, D.; Zhao, L.; Wang, S. Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice. J. Pharmacol. Exp. Ther. 2019, 369, 419–427. [Google Scholar] [CrossRef]
- Weyer, C.; Tataranni, P.A.; Snitker, S.; Danforth, E.; Ravussin, E. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes 1998, 47, 1555–1561. [Google Scholar] [CrossRef]
- Larsen, T.M.; Toubro, S.; van Baak, M.A.; Gottesdiener, K.M.; Larson, P.; Saris, W.H.; Astrup, A. Effect of a 28-d treatment with L-796568, a novel β3-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am. J. Clin. Nutr. 2002, 76, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; de Jonge, L.; Fang, X.; Gamlin, B.; Recker, D.; Greenway, F.L.; Smith, S.R.; Ravussin, E. Lack of an Effect of a Novel β3-Adrenoceptor Agonist, TAK-677, on Energy Metabolism in Obese Individuals: A Double-Blind, Placebo-Controlled Randomized Study. J. Clin. Endocrinol. Metab. 2007, 92, 527–531. [Google Scholar] [CrossRef]
- Dehvari, N.; Sato, M.; Bokhari, M.H.; Kalinovich, A.; Ham, S.; Gao, J.; Nguyen, H.T.M.; Whiting, L.; Mukaida, S.; Merlin, J.; et al. The metabolic effects of mirabegron are mediated primarily by β-3-adrenoceptors. Pharmacol. Res. Perspect. 2020, 8, e00643. [Google Scholar] [CrossRef]
- da Silva, C.P.V.; Calmasini, F.; Alexandre, E.C.; Raposo, H.F.; Delbin, M.A.; Monica, F.Z.; Zanesco, A. The effects of mirabegron on obesity-induced inflammation and insulin resistance are associated with brown adipose tissue activation but not beiging in the subcutaneous white adipose tissue. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, Y.; Lin, W.; Lian, H.; Meng, Z. A microneedle patch realizes weight loss through photothermal induction of fat browning. Biomater. Sci. 2024, 12, 1726–1737. [Google Scholar] [CrossRef]
- Calmasini, F.B.; de Oliveira, M.G.; Alexandre, E.C.; da Silva, F.H.; da Silva, C.P.; Candido, T.Z.; Antunes, E.; Mónica, F.Z. Long-term treatment with the beta-3 adrenoceptor agonist, mirabegron ameliorates detrusor overactivity and restores cyclic adenosine monophosphate (cAMP) levels in obese mice. Neurourol. Urodyn. 2017, 36, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Riis-Vestergaard, M.J.; Richelsen, B.; Bruun, J.M.; Li, W.; Hansen, J.B.; Pedersen, S.B. Beta-1 and Not Beta-3 Adrenergic Receptors May Be the Primary Regulator of Human Brown Adipocyte Metabolism. J. Clin. Endocrinol. Metab. 2020, 105, e994–e1005. [Google Scholar] [CrossRef]
- Baskin, A.S.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Anflick-Chames, E.; Cero, C.; Johnson, J.W.; O’mAra, A.E.; Fletcher, L.A.; Leitner, B.P.; et al. Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a β3-Adrenergic Receptor Agonist. Diabetes 2018, 67, 2113–2125. [Google Scholar] [CrossRef]
- Nahon, K.J.; Janssen, L.G.M.; Mishre, A.S.D.S.; Bilsen, M.P.; van der Eijk, J.A.; Botani, K.; Overduin, L.A.; Ruiz, J.R.; Burakiewicz, J.; Dzyubachyk, O.; et al. The effect of mirabegron on energy expenditure and brown adipose tissue in healthy lean South and Europidmen. Diabetes Obes. Metab. 2020, 22, 2032–2044. [Google Scholar] [CrossRef]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by β2-AR Stimulation. Cell Metab. 2020, 32, 287–300.e7. [Google Scholar] [CrossRef]
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human adipose beiging in response to cold and mirabegron. JCI Insight 2018, 3, e121510. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020, 130, 2319–2331. [Google Scholar] [CrossRef]

| Compound Name | Mechanism of Action | Observed Side Effects | Development Stage (and/or FDA) Status |
|---|---|---|---|
| Mirabegron Vibegron | β3-AR agonist | Increased heart rate [88], hypertension [89], urinary tract infections [90] | FDA-approved for overactive bladder |
| CL-316,243 | β3-AR agonist | Decrease in motor activity [91] decreased cardiac contractility [92] | Preclinical stage, not approved for human use |
| DNP * (2,4-Dinitrophenol) | Mitochondrial uncoupler | Hyperthermia [93], tachycardia [94], fatal toxicity [60] | Not approved by the FDA, banned due to safety concerns |
| /TRPV1 agonists * | TRPV1 receptor- activation stimulates sympathetic system | Inflammation of GI tract [95], hyper- [96] or hypotension [97] myocardial infarction [98] | Qutenza (8% capsaicin) is FDA-approved for neuropathic pain |
| Thyroid hormone analogs (e.g., GC-1) * | TH receptor activation leads to ↑ metabolism & UCPs | Tachycardia [99], arrhythmias [99], muscle and bone mass loss [100] | Sobetirome (GC-1) drug development halted due to cardiac risks |
| Centrally acting sympathomimetics * | ↑ hypothalamic catecholamine release | Dry mouth [101], constipation, insomnia [102] | Phentermine is FDA-approved for short-term weight management |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.; Andrews-Dickert, R.; Rocic, P.; Mitov, M. Thermogenic Targets for Obesity Management in the Era of Incretin-Based Therapies. Pharmaceuticals 2025, 18, 1519. https://doi.org/10.3390/ph18101519
Soliman S, Andrews-Dickert R, Rocic P, Mitov M. Thermogenic Targets for Obesity Management in the Era of Incretin-Based Therapies. Pharmaceuticals. 2025; 18(10):1519. https://doi.org/10.3390/ph18101519
Chicago/Turabian StyleSoliman, Sahar, Rebecca Andrews-Dickert, Petra Rocic, and Mihail Mitov. 2025. "Thermogenic Targets for Obesity Management in the Era of Incretin-Based Therapies" Pharmaceuticals 18, no. 10: 1519. https://doi.org/10.3390/ph18101519
APA StyleSoliman, S., Andrews-Dickert, R., Rocic, P., & Mitov, M. (2025). Thermogenic Targets for Obesity Management in the Era of Incretin-Based Therapies. Pharmaceuticals, 18(10), 1519. https://doi.org/10.3390/ph18101519








