Daflon Enhances Morphine Analgesia and Mitigates Tolerance in a Rat Neuropathic Pain Model
Abstract
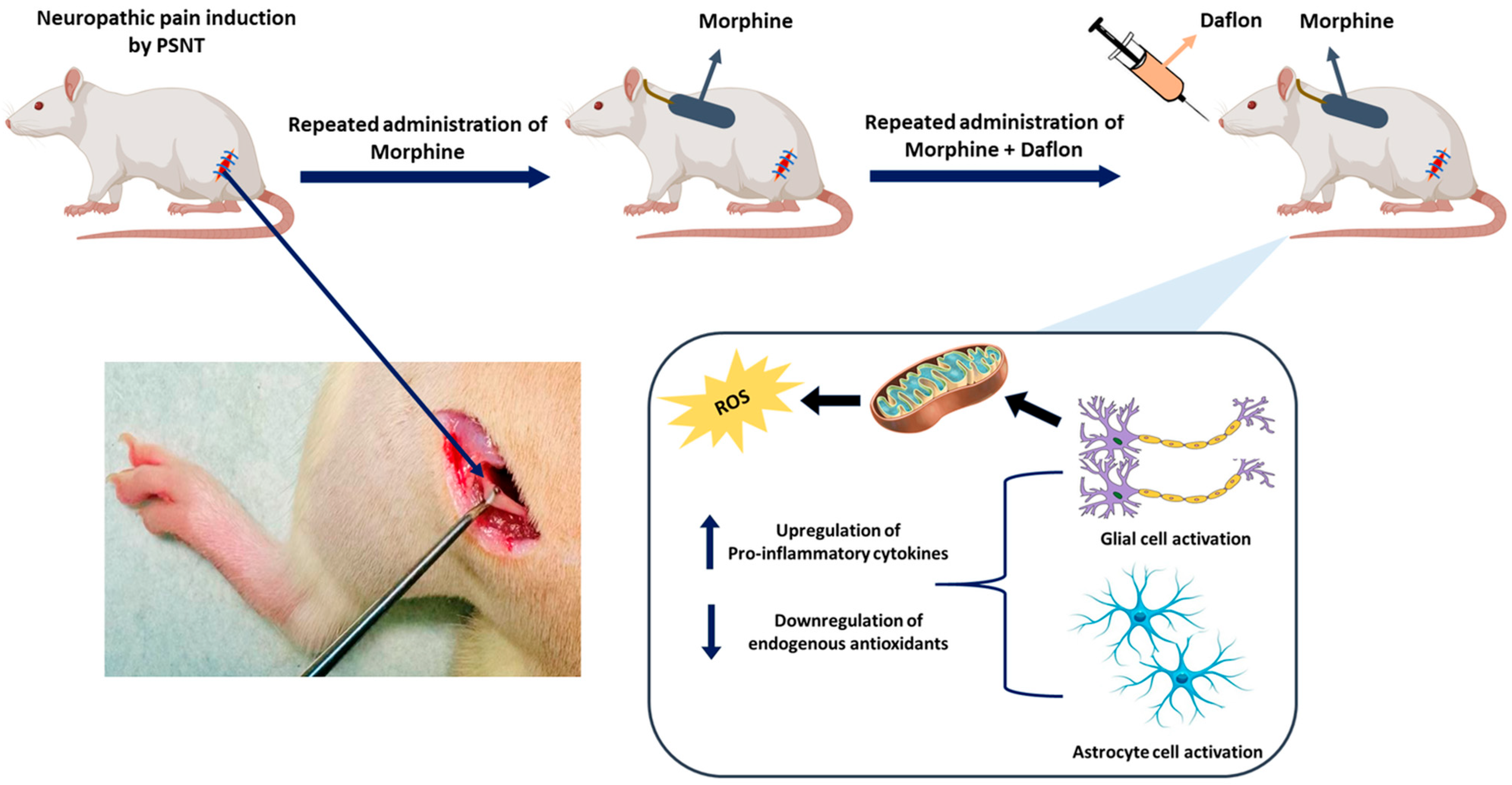
1. Introduction
2. Results
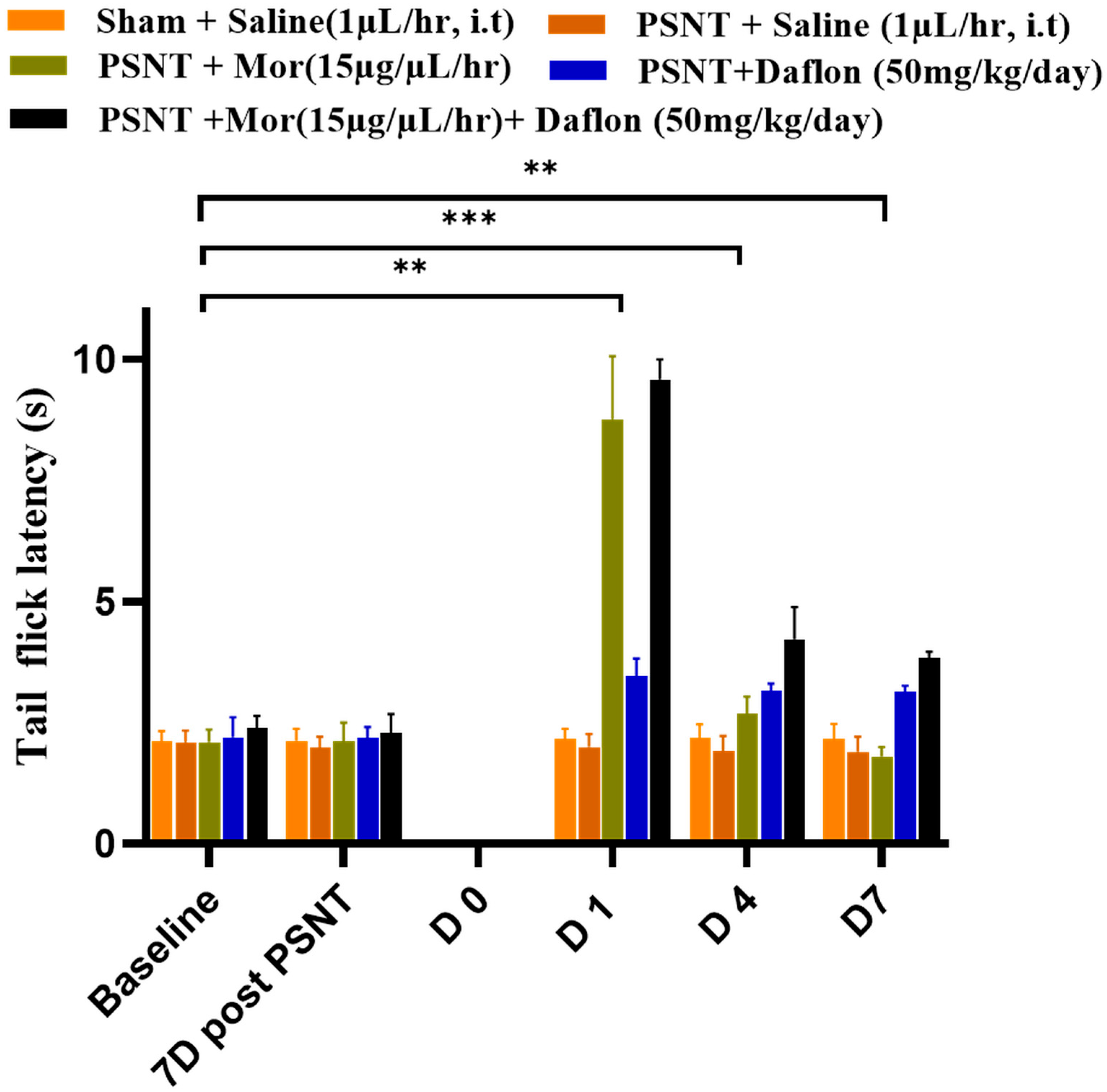
2.1. Antinociceptive Effects of Daflon and Morphine Against Acute Pain (Tail-Flick Test)
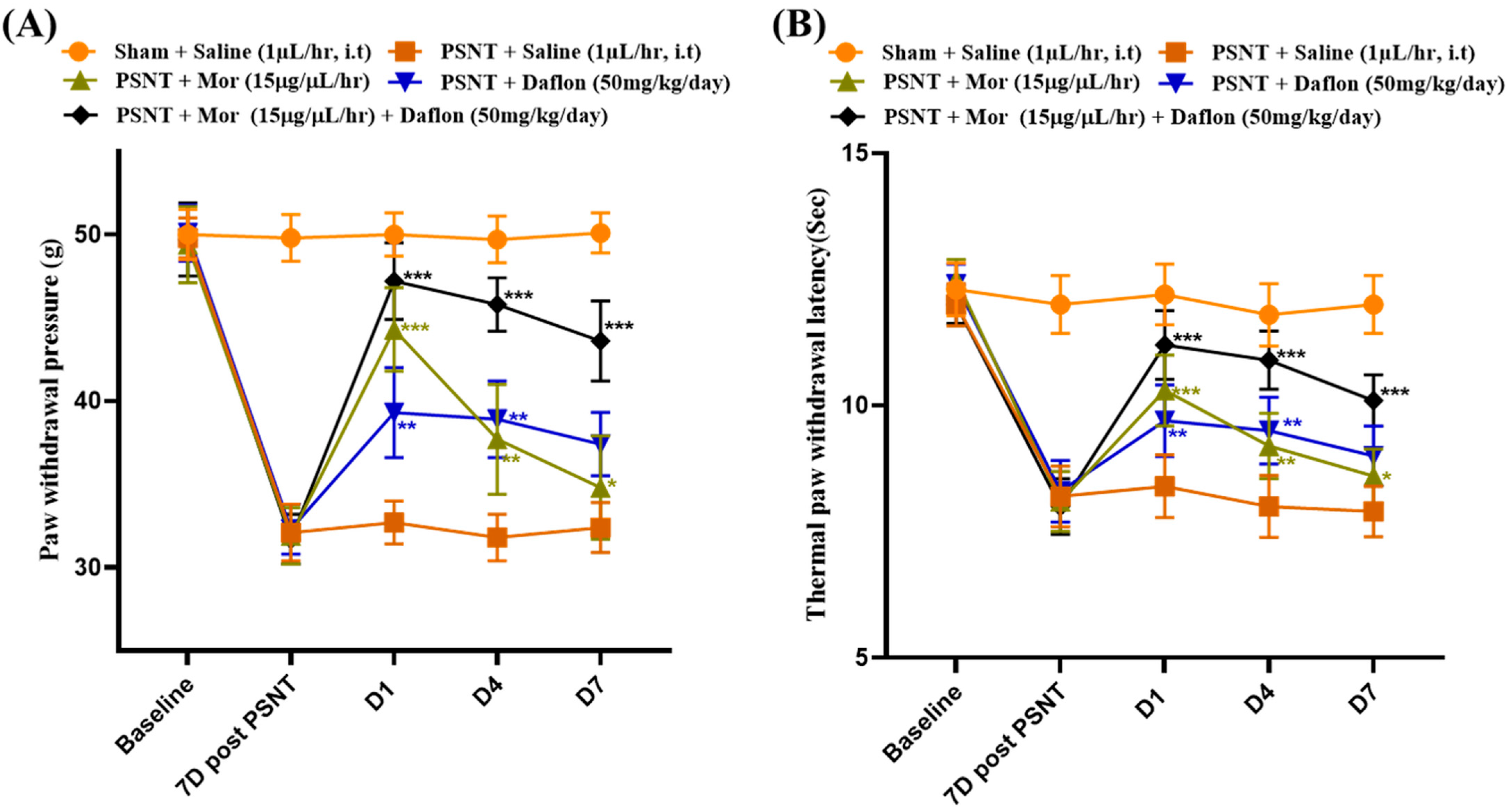
2.2. Daflon and Morphine Alleviated PSNT Induced Thermal Hyperalgesia and Mechanical Allodynia
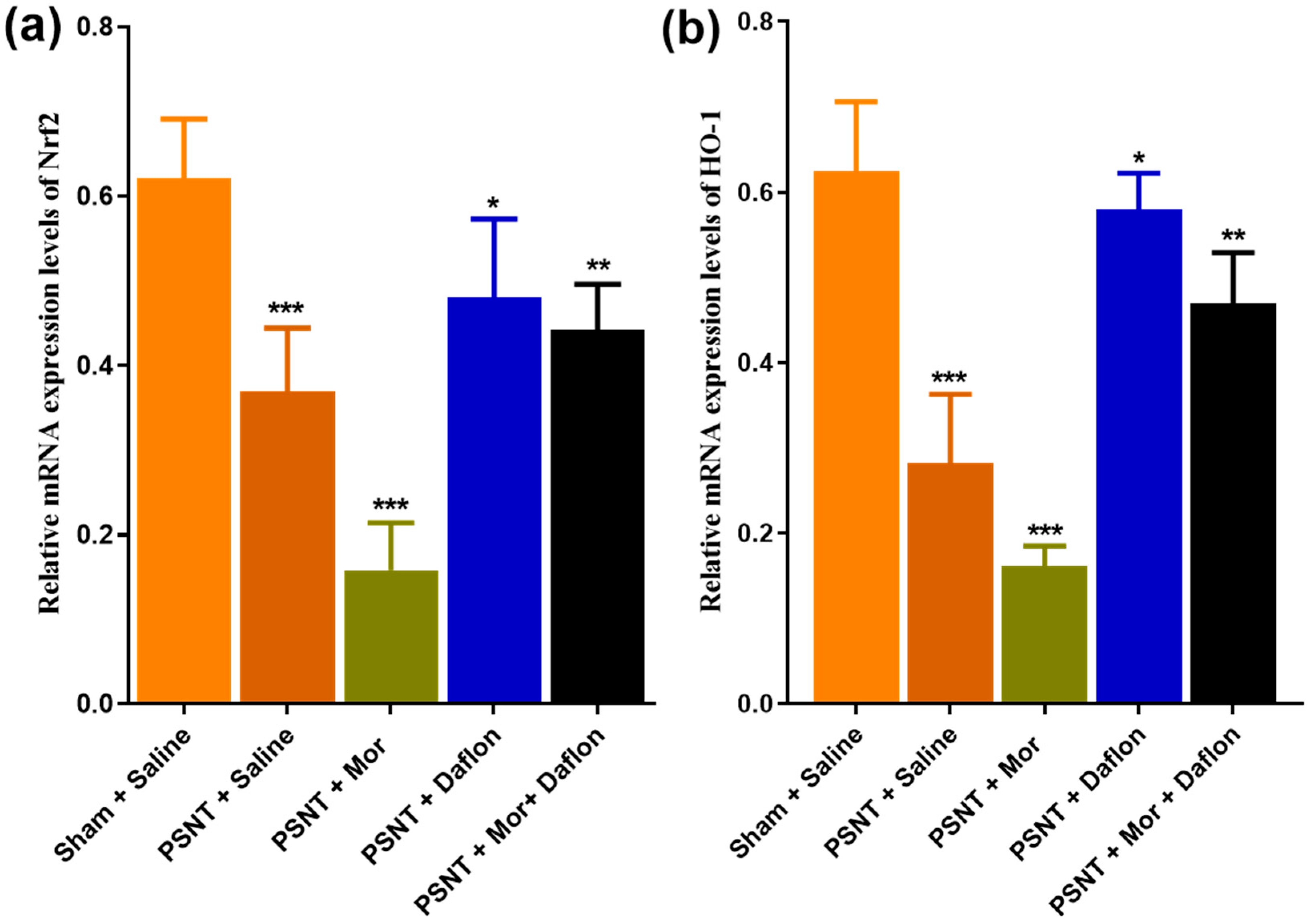
2.3. Daflon and Morphine-Induced Nrf-2 and HO-1 Expression in the Dorsal Horn of PSNT Rats
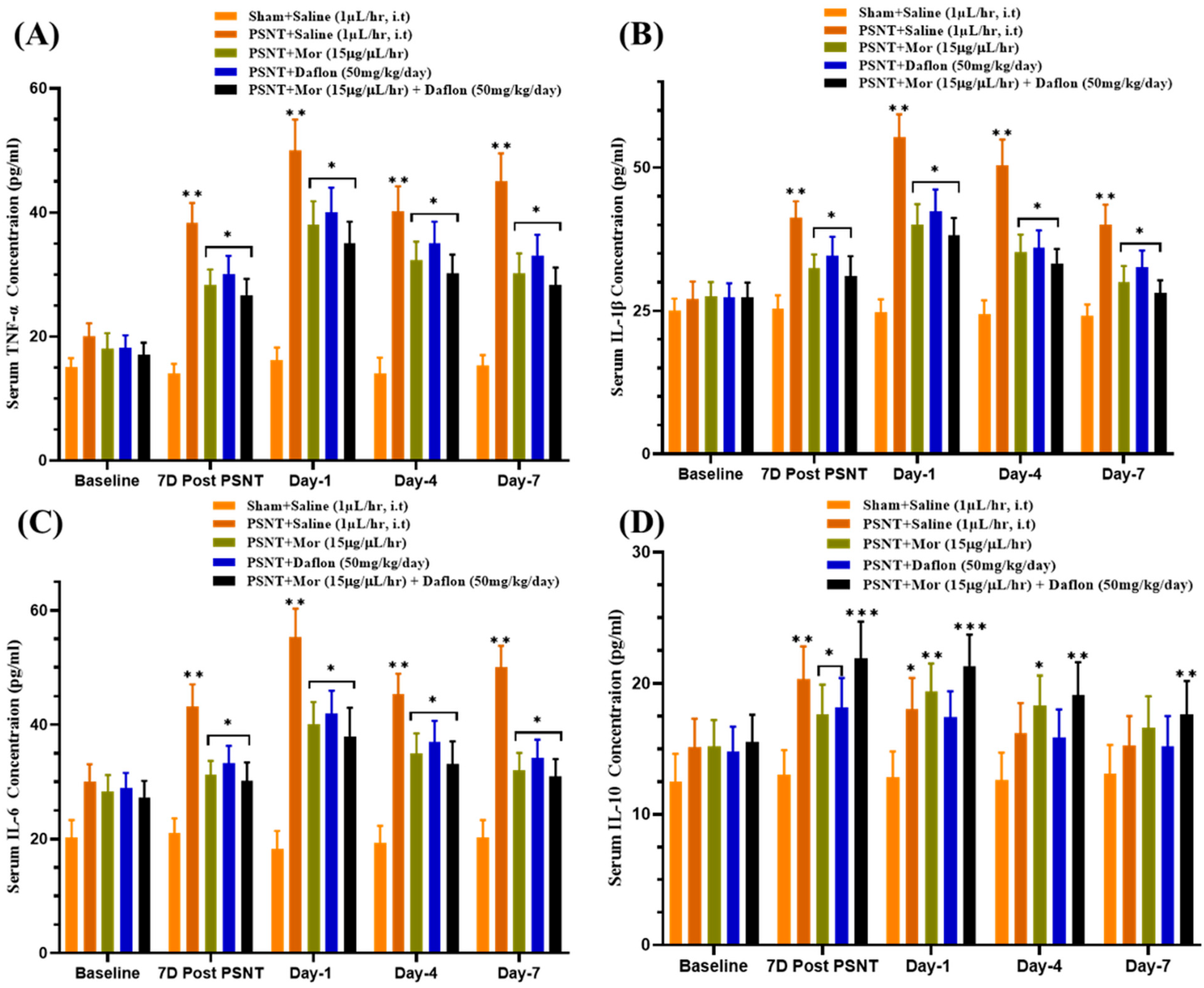
2.4. Effect of Daflon and Morphine on Serum Cytokine Levels in PSNT Rats
2.5. Mononucleate Cell Infiltration in the Dorsal Horn of Daflon and Morphine Induced PSNT Rats
2.6. GFAP Stained Activated Astrocytes in the Dorsal Horn of Daflon and Morphine Treated PSNT Rats
2.7. Iba-1 Stained Activated Microglia in the Dorsal Horn of Daflon and Morphine Treated PSNT Rats
3. Discussions
4. Materials and Methods
4.1. Establishment of the Neuropathic Pain Animal Model
4.2. Intrathecal Catheterization and Drug Administration
- Sham group (uninjured baseline, n = 6, saline, i.t.)
- PNST + Saline (injury control, n = 6, saline, i.t.)
- PSNT + Morphine group (n = 6), (15 μg/μL/h, i.t.),
- PSNT + Daflon group (n = 6), (50 mg/kg/day, oral),
- PSNT + Morphine + Daflon group (n = 6), (morphine 15 μg/μL/h, i.t. + oral Daflon 50 mg/kg/day).
4.3. Behavioral Measurements
4.4. Spinal Cord Sample Preparation
4.5. Measurement of Cytokines
4.6. H&E Staining for Mononucleate Cell Infiltration in Spinal Cord
4.7. Immunohistochemical Analysis for GFAP and Iba-1 in Spinal Cord
4.8. Real-Time Quantitative PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogucka-Kocka, A.; Woźniak, M.; Feldo, M.; Kocki, J.; Szewczyk, K. Diosmin—Isolation Techniques, Determination in Plant Material and Pharmaceutical Formulations, and Clinical Use. Nat. Prod. Commun. 2013, 8, 1934578X1300800435. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Mansilha, A.; Caldevilla, H.; Puskás, A.; Lucien, A.; Roby, L.; Kirienko, A. MPFF 1000 mg chewable once daily vs. MPFF 500 mg twice daily in chronic venous disease: The double-blind, randomized, non-inferiority CHEWY trial. Int. Angiol. 2022, 41, 464–475. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of Diosmin, a Citrus Flavone Glycoside: An Updated Review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 1–18. [Google Scholar] [CrossRef]
- Silvestro, L.; Tarcomnicu, I.; Dulea, C.; Attili, N.R.B.N.; Ciuca, V.; Peru, D.; Rizea Savu, S. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography–mass spectrometry and ion mobility mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 8295–8310. [Google Scholar] [CrossRef] [PubMed]
- Boisnic, S.; Branchet, M.-C.; Gouhier-Kodas, C.; Verriere, F.; Jabbour, V. Anti-inflammatory and antiradical effects of a 2% diosmin cream in a human skin organ culture as model. J. Cosmet. Dermatol. 2018, 17, 848–854. [Google Scholar] [CrossRef]
- Feldo, M.; Woźniak, M.; Wójciak-Kosior, M.; Sowa, I.; Kot-Waśik, A.; Aszyk, J.; Bogucki, J.; Zubilewicz, T.; Bogucka-Kocka, A. Influence of Diosmin Treatment on the Level of Oxidative Stress Markers in Patients with Chronic Venous Insufficiency. Oxid. Med. Cell. Longev. 2018, 2018, 2561705. [Google Scholar] [CrossRef]
- Berkoz, M. Diosmin suppresses the proinflammatory mediators in lipopolysaccharide-induced RAW264.7 macrophages via NF-κB and MAPKs signal pathways. Gen. Physiol. Biophys. 2019, 38, 315–324. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Iqbal, M.; Anwer, M.K.; Al Hoshani, A.R.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wan, R.; Yin, G.; Xiong, J.; Hu, Y.; Xing, M.; Cang, X.; Fan, Y.; Xiao, W.; Qiu, L.; et al. Diosmetin ameliorates the severity of cerulein-induced acute pancreatitis in mice by inhibiting the activation of the nuclear factor-κB. Int. J. Clin. Exp. Pathol. 2014, 7, 2133–2142. [Google Scholar]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential Therapeutic Anti-Inflammatory and Immunomodulatory Effects of Dihydroflavones, Flavones, and Flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef]
- Liao, W.; Ning, Z.; Chen, L.; Wei, Q.; Yuan, E.; Yang, J.; Ren, J. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J. Agric. Food Chem. 2014, 62, 8648–8654. [Google Scholar] [CrossRef]
- Wang, C.; Liao, Y.; Wang, S.; Wang, D.; Wu, N.; Xu, Q.; Jiang, W.; Qiu, M.; Liu, C. Cytoprotective effects of diosmetin against hydrogen peroxide-induced L02 cell oxidative damage via activation of the Nrf2-ARE signaling pathway. Mol. Med. Rep. 2018, 17, 7331–7338. [Google Scholar] [CrossRef]
- Mahgoub, S.; Sallam, A.O.; Sarhan, H.K.A.; Ammar, A.A.A.; Soror, S.H. Role of Diosmin in protection against the oxidative stress induced damage by gamma-radiation in Wistar albino rats. Regul. Toxicol. Pharmacol. 2020, 113, 104622. [Google Scholar] [CrossRef]
- Chen, Y.R.; Yang, K.C.; Lu, D.H.; Wu, W.T.; Wang, C.C.; Tsai, M.H. The chondroprotective effect of diosmin on human articular chondrocytes under oxidative stress. Phytother. Res. 2019, 33, 2378–2386. [Google Scholar] [CrossRef]
- Wen, Y.-R.; Tan, P.-H.; Cheng, J.-K.; Liu, Y.-C.; Ji, R.-R. Microglia: A Promising Target for Treating Neuropathic and Postoperative Pain, and Morphine Tolerance. J. Formos. Med. Assoc. 2011, 110, 487–494. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, X.; Jiang, L.; Hu, L.; Kong, H.; Han, Y.; Qian, C.; Song, C.; Qian, Y.; Liu, W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflamm. 2016, 13, 294. [Google Scholar] [CrossRef]
- Mika, J.; Wawrzczak-Bargiela, A.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain. Behav. Immun. 2009, 23, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, C.; Tang, J.; Wang, C.; Wu, P.; Zhang, G.; Liu, W.; Jamangulova, N.; Wu, X.; Song, X. Resveratrol reduces morphine tolerance by inhibiting microglial activation via AMPK signalling. Eur. J. Pain 2014, 18, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, M.A.; Parvardeh, S.; Ghafghazi, S.; Moini Zanjani, T.; Sabetkasaei, M. The Attenuating Effect of Curcumin on Morphine Dependence in Rats: The Involvement of Spinal Microglial Cells and Inflammatory Cytokines. Iran. J. Pharm. Res. 2019, 18, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Bloom, F.E. Cellular and molecular mechanisms of drug dependence. Science 1988, 242, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R.; Shippenberg, T.S. Modulation of morphine-induced sensitization by endogenous κ opioid systems in the rat. Neurosci. Lett. 1993, 153, 232–236. [Google Scholar] [CrossRef]
- Spanagel, R.; Almeida, O.F.X.; Shippenberg, T.S. Long lasting changes in morphine-induced mesolimbic dopamine release after chronic morphine exposure. Synapse 1993, 14, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Juhwan, K.; Suji, H.; Heeok, H.; Changjong, M.; Heh-In, I. Brain Reward Circuits in Morphine Addiction. Mol. Cells 2016, 39, 645–653. [Google Scholar] [CrossRef]
- Kuthati, Y.; Rao, V.N.; Busa, P.; Wong, C.S. Teneligliptin Exerts Antinociceptive Effects in Rat Model of Partial Sciatic Nerve Transection Induced Neuropathic Pain. Antioxidants 2021, 10, 1438. [Google Scholar] [CrossRef]
- Yang, C.-P.; Cherng, C.-H.; Wu, C.-T.; Huang, H.-Y.; Tao, P.-L.; Lee, S.-O.; Wong, C.-S. Intrathecal Ultra-Low Dose Naloxone Enhances the Antihyperalgesic Effects of Morphine and Attenuates Tumor Necrosis Factor-α and Tumor Necrosis Factor-α Receptor 1 Expression in the Dorsal Horn of Rats with Partial Sciatic Nerve Transection. Anesth. Analg. 2013, 117, 1493–1502. [Google Scholar] [CrossRef]
- Kuthati, Y.; Rao, V.N.; Huang, W.-H.; Busa, P.; Wong, C.-S. Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats. Antioxidants 2023, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated with Neuropathic Pain and Improved the Anti-allodynic Effects of Morphine in Mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Busa, P.; Lee, S.O.; Huang, N.; Kuthati, Y.; Wong, C.S. Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study. Antioxidants 2022, 11, 1209. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.I.; González-Trujano, M.E.; Pellicer, F.; Alvarado-Vásquez, N.; López-Muñoz, F.J. Central and peripheral anti-hyperalgesic effects of diosmin in a neuropathic pain model in rats. Biomed. Pharmacother. 2018, 97, 310–320. [Google Scholar] [CrossRef]
- Kuthati, Y.; Goutham Davuluri, V.N.; Yang, C.P.; Chang, H.C.; Chang, C.P.; Wong, C.S. Melatonin MT2 receptor agonist IIK-7 produces antinociception by modulation of ROS and suppression of spinal microglial activation in neuropathic pain rats. J. Pain Res. 2019, 12, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Lindenlaub, T.; Sommer, C. Partial sciatic nerve transection as a model of neuropathic pain: A qualitative and quantitative neuropathological study. Pain 2000, 89, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Abharian, N.; Naderi, N.; Nikray, N.; Khoramjouy, M.; Noori, S.; Shafaroodi, H.; Faizi, M. Effect of Micronized Purified Flavonoid Fraction Containing Hesperidin and Diosmin on Vincristine-Induced Neuropathy in Rats; the Role of Nitric Oxide Pathway. Iran. J. Pharm. Res. 2025, 24, e154455. [Google Scholar] [CrossRef]
- Tai, Y.H.; Wang, Y.H.; Wang, J.J.; Tao, P.L.; Tung, C.S.; Wong, C.S. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain 2006, 124, 77–86. [Google Scholar] [CrossRef]
- Tai, Y.H.; Wang, Y.H.; Tsai, R.Y.; Wang, J.J.; Tao, P.L.; Liu, T.M.; Wang, Y.C.; Wong, C.S. Amitriptyline preserves morphine’s antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain 2007, 129, 343–354. [Google Scholar] [CrossRef]
- Yu, S.M.; Kim, S.J. Withaferin A-Caused Production of Intracellular Reactive Oxygen Species Modulates Apoptosis via PI3K/Akt and JNKinase in Rabbit Articular Chondrocytes. J. Korean Med. Sci. 2014, 29, 1042–1053. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.X.; Pang, Z.Y.; Qi, G.B.; Hua, B.X.; Yan, Z.Q.; Yuan, H.F. Engeletin Protects Against TNF-alpha-Induced Apoptosis and Reactive Oxygen Species Generation in Chondrocytes and Alleviates Osteoarthritis in vivo. J. Inflamm. Res. 2021, 14, 745–760. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta—Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Deby-Dupont, G.P.; Reginster, J.Y.L.; Ayache, N.; Pujol, J.P.; Henrotin, Y.E. Regulation by reactive oxygen species of interleukin-1 beta, nitric oxide and prostaglandin E-2 production by human chondrocytes. Osteoarthr. Cartil. 2002, 10, 547–555. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mende, L.K.; Lee, M.-L.; Kuthati, Y.; Koh, S.-Y.; Wong, C.-S. Daflon Enhances Morphine Analgesia and Mitigates Tolerance in a Rat Neuropathic Pain Model. Pharmaceuticals 2025, 18, 1513. https://doi.org/10.3390/ph18101513
Mende LK, Lee M-L, Kuthati Y, Koh S-Y, Wong C-S. Daflon Enhances Morphine Analgesia and Mitigates Tolerance in a Rat Neuropathic Pain Model. Pharmaceuticals. 2025; 18(10):1513. https://doi.org/10.3390/ph18101513
Chicago/Turabian StyleMende, Lokesh Kumar, Meng-Lin Lee, Yaswanth Kuthati, Shu-Yi Koh, and Chih-Shung Wong. 2025. "Daflon Enhances Morphine Analgesia and Mitigates Tolerance in a Rat Neuropathic Pain Model" Pharmaceuticals 18, no. 10: 1513. https://doi.org/10.3390/ph18101513
APA StyleMende, L. K., Lee, M.-L., Kuthati, Y., Koh, S.-Y., & Wong, C.-S. (2025). Daflon Enhances Morphine Analgesia and Mitigates Tolerance in a Rat Neuropathic Pain Model. Pharmaceuticals, 18(10), 1513. https://doi.org/10.3390/ph18101513








