Intermedin Inhibits DNA Damage-Promoted Senescent Phenotype Transition of Vascular Smooth Muscle Cells in Aorta by Activating NAMPT/PARP1 in Mice
Abstract
1. Introduction
2. Results
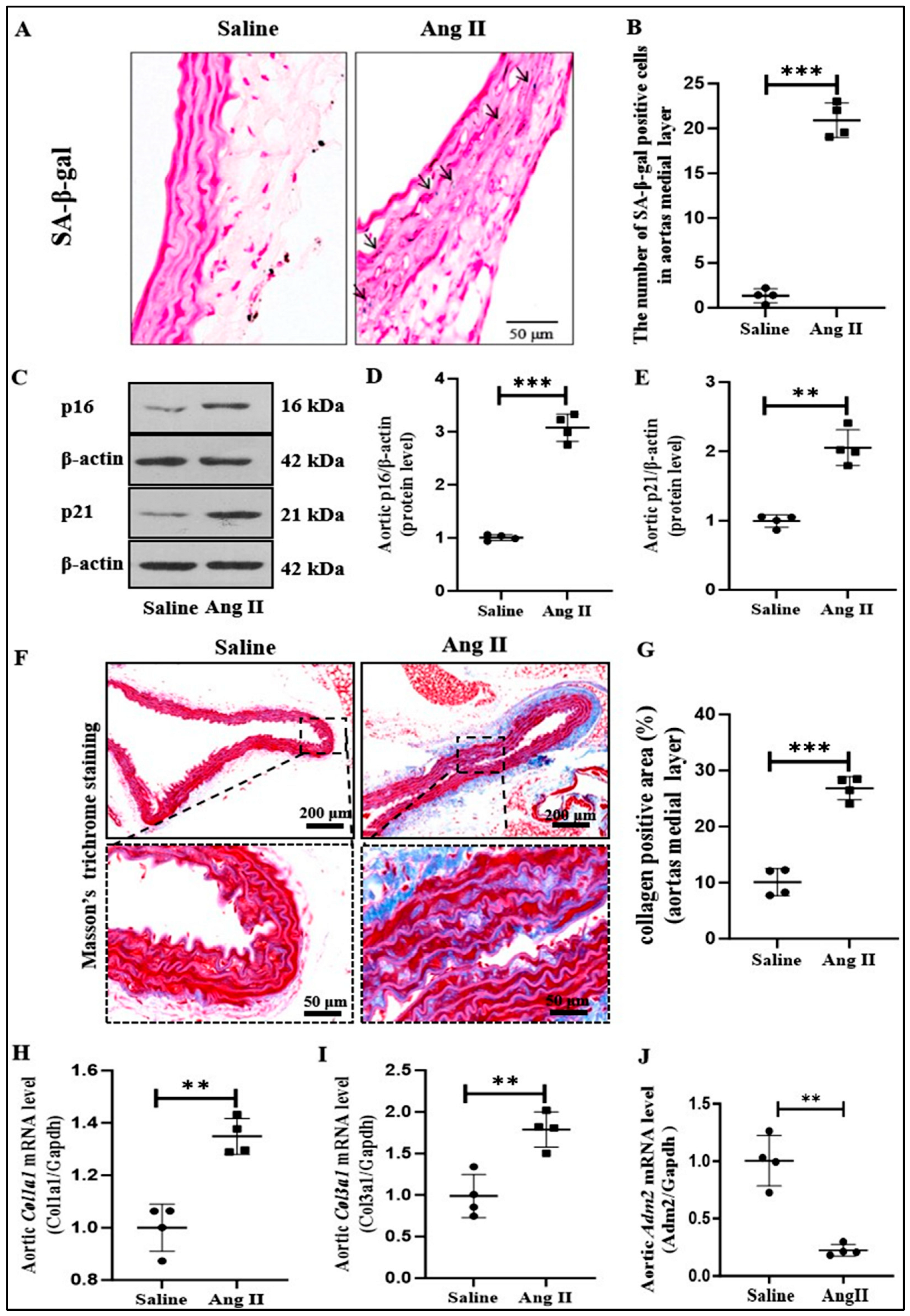
2.1. IMD Expression and Ang II-Induced Senescent Phenotype Transition of VSMCs in Aorta of Mice
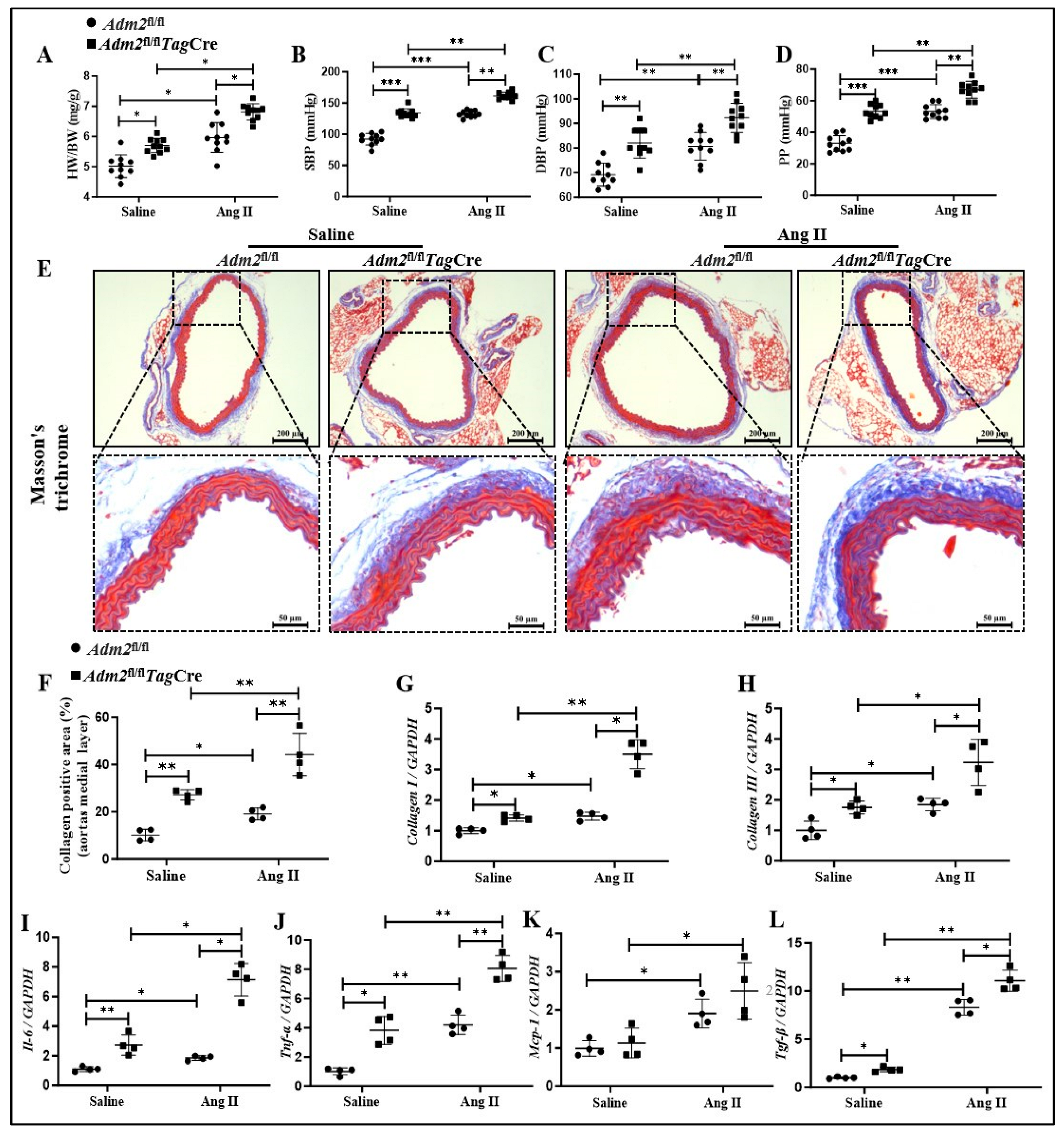
2.2. Deficiency in Endogenous IMD Exacerbated Ang II-Induced Senescent Phenotype Transition of VSMCs in Aorta
2.3. Deficiency in Endogenous IMD Exacerbated Ang II-Induced Vascular Fibrosis and Prosenescent Factors Expression
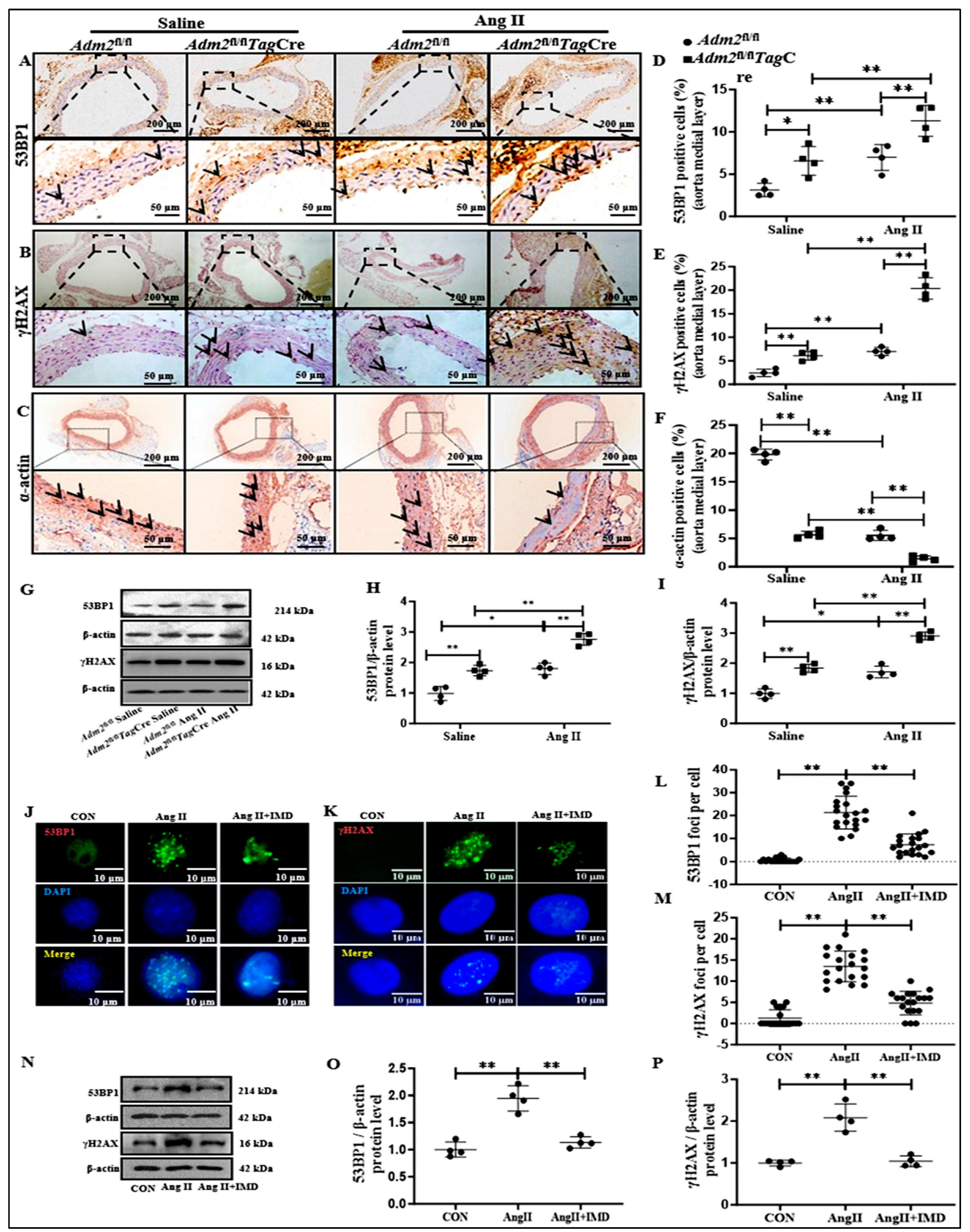
2.4. IMD Alleviated DNA Damage in Ang II-Treated Aortas
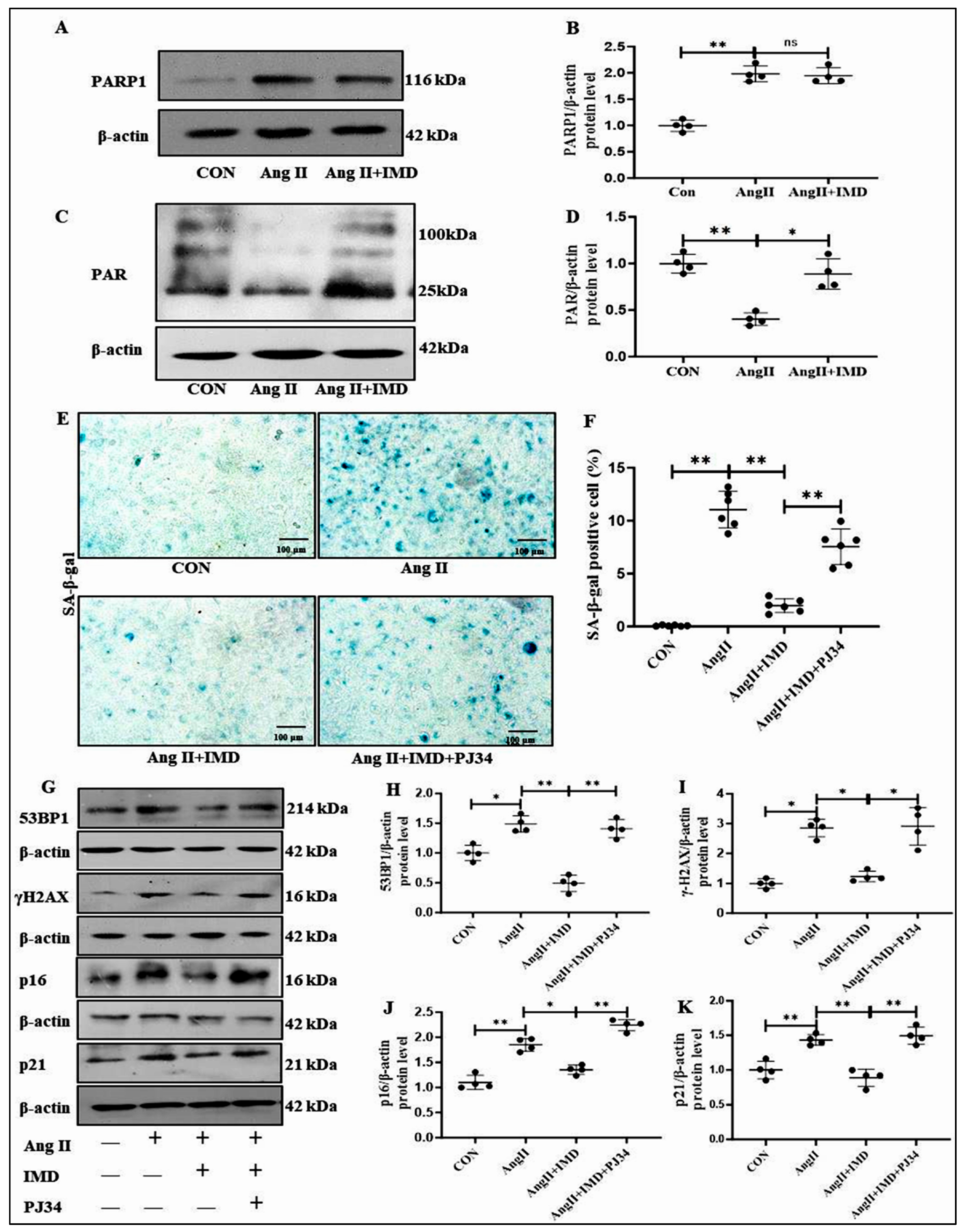
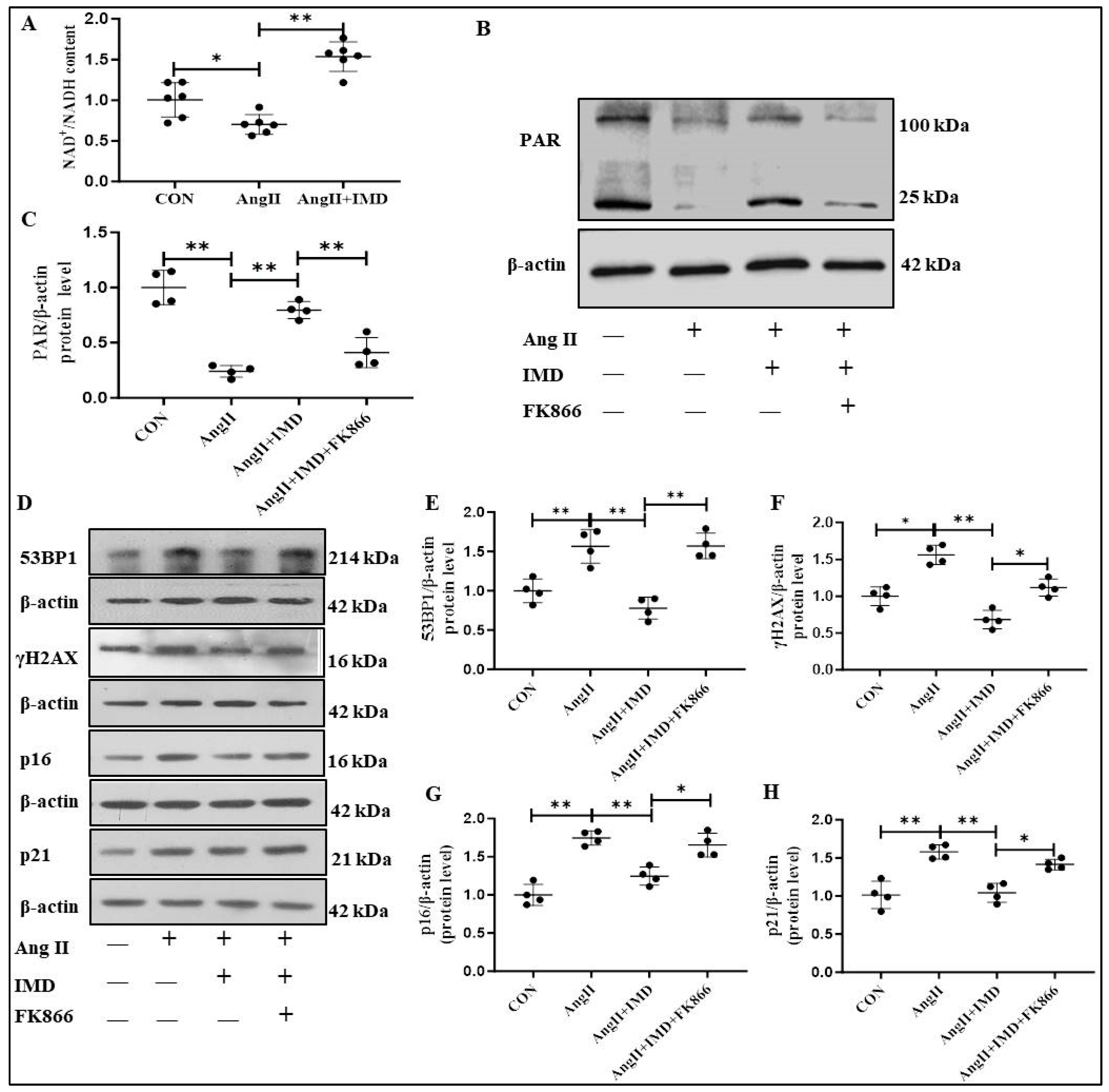
2.5. IMD Inhibited DNA Damage and the Senescent Phenotype Transition of VSMCs by Activating PARP1
2.6. IMD Increased PARP1 Activity by Activating NAMPT
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Senescence-Associated β-Galactosidase Staining
4.4. Masson’s Trichrome Staining
4.5. Immunostaining
4.6. Real-Time (RT) PCR Analysis
4.7. Western Blot Analysis
4.8. Cell Culture
4.9. Intracellular NAD+/NADH Content
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; Siddik, A.B. Anatomy, Arterioles. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Avolio, A.P. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovasc. Res. 2018, 114, 513–528. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Nicoletti, A.; Li, Z.; Michel, J.-B. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012, 95, 194–204. [Google Scholar] [CrossRef]
- Gan, L.; Liu, D.; Liu, J.; Chen, E.; Chen, C.; Liu, L.; Hu, H.; Guan, X.; Ma, W.; Zhang, Y.; et al. CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice. Signal Transduct. Target. Ther. 2021, 6, 223. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of vascular aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Ou, H.-L.; Schumacher, B. DNA damage responses and p53 in the aging process. Blood 2018, 131, 488–495. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; DeMaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Chang, C.L.; Bhalla, A.; Klein, C.; Hsu, S.Y.T. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J. Biol. Chem. 2004, 279, 7264–7274. [Google Scholar] [CrossRef]
- Takei, Y.; Inoue, K.; Ogoshi, M.; Kawahara, T.; Bannai, H.; Miyano, S. Identification of novel adrenomedullin in mammals: A potent cardiovascular and renal regulator. FEBS Lett. 2004, 556, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Hay, D.L.; Quirion, R.; Poyner, D.R. The pharmacology of adrenomedullin 2/intermedin. Br. J. Pharmacol. 2012, 166, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.R.; Guo, J.; Wang, Y.; Hou, Y.L.; Lu, W.W.; Zhang, J.S.; Yu, Y.R.; Xu, M.J.; Liu, X.Y.; Wang, X.J.; et al. Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho. Kidney Int. 2016, 89, 586–600. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.-S.; Ren, J.-L.; Zhang, Y.-R.; Wu, N.; Jia, M.-Z.; Yu, Y.-R.; Ning, Z.-P.; Tang, C.-S.; Qi, Y.-F. Intermedin(1-53) attenuates aging-associated vascular calcification in rats by upregulating sirtuin 1. Aging 2020, 12, 5651–5674. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-Q.; Zhang, Y.-R.; Jia, L.-X.; Lu, W.-W.; Zhu, Q.; Ren, J.-L.; Chen, Y.; Zhang, L.-S.; Liu, X.; Yu, Y.-R.; et al. Inhibition of Notch1-mediated inflammation by intermedin protects against abdominal aortic aneurysm via PI3K/Akt signaling pathway. Aging 2021, 13, 5164–5184. [Google Scholar] [CrossRef]
- Kunieda, T.; Minamino, T.; Nishi, J.-I.; Tateno, K.; Oyama, T.; Katsuno, T.; Miyauchi, H.; Orimo, M.; Okada, S.; Takamura, M.; et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 2006, 114, 953–960. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Liu, Y.; Chen, Y.; Ren, J.-L.; Zhang, Y.-R.; Yu, Y.-R.; Jia, M.-Z.; Ning, Z.-P.; Du, J.; Tang, C.-S.; et al. Intermedin alleviates pathological cardiac remodeling by upregulating klotho. Pharmacol. Res. 2020, 159, 104926. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Turinetto, V.; Giachino, C. Multiple facets of histone variant H2AX: A DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015, 43, 2489–2498. [Google Scholar] [CrossRef]
- Sharma, A.; Alswillah, T.; Singh, K.; Chatterjee, P.; Willard, B.; Venere, M.; Summers, M.K.; Almasan, A. USP14 regulates DNA damage repair by targeting RNF168-dependent ubiquitination. Autophagy 2018, 14, 1976–1990. [Google Scholar] [CrossRef]
- Chaudhuri, A.R.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Takagi, G.; Asai, K.; Resuello, R.G.; Natividad, F.F.; Vatner, D.E.; Vatner, S.F.; Lakatta, E.G. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 2003, 41, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiao, C.; Long, F.; Wu, W.; Huang, M.; Qu, L.; Zhu, Y. Fra-1 plays a critical role in angiotensin II-induced vascular senescence. FASEB J. 2019, 33, 7603–7614. [Google Scholar] [CrossRef]
- Nagashima, H.; Sakomura, Y.; Aoka, Y.; Uto, K.; Kameyama, K.-I.; Ogawa, M.; Aomi, S.; Koyanagi, H.; Ishizuka, N.; Naruse, M.; et al. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan’s syndrome. Circulation 2001, 104, I-282. [Google Scholar] [CrossRef]
- Ren, J.-L.; Chen, Y.; Zhang, L.-S.; Zhang, Y.-R.; Liu, S.-M.; Yu, Y.-R.; Jia, M.-Z.; Tang, C.-S.; Qi, Y.-F.; Lu, W.-W. Intermedin(1-53) attenuates atherosclerotic plaque vulnerability by inhibiting CHOP-mediated apoptosis and inflammasome in macrophages. Cell Death Dis. 2021, 12, 436. [Google Scholar] [CrossRef]
- Qi, Y.F.; Lu, W.-W.; Jia, L.-X.; Ni, X.-Q.; Zhao, L.; Chang, J.-R.; Zhang, J.-S.; Hou, Y.-L.; Zhu, Y.; Guan, Y.-F.; et al. Intermedin1-53 attenuates abdominal aortic aneurysm by inhibiting oxidative stress. Arter. Thromb. Vasc. Biol. 2016, 36, 2176–2190. [Google Scholar] [CrossRef]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; Von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, Q.; Liu, X.; Wei, L.; Liu, L.; Li, Y.; Wang, X. Dynamic transcriptome profiling in DNA damage-induced cellular senescence and transient cell-cycle arrest. Genomics 2020, 112, 1309–1317. [Google Scholar] [CrossRef]
- Herbig, U.; Ferreira, M.; Condel, L.; Carey, D.; Sedivy, J.M. Cellular senescence in aging primates. Science 2006, 311, 1257. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Horikawa, I.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M.; Barrett, J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004, 6, 168–170. [Google Scholar] [CrossRef]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef]
- Anderson, L.; Henderson, C.; Adachi, Y. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol. Cell. Biol. 2001, 21, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Rappold, I.; Iwabuchi, K.; Date, T.; Chen, J. Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J. Cell Biol. 2001, 153, 613–620. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Lavrik, O.I. Poly(ADP-ribosyl)ation by PARP1: Reaction mechanism and regulatory proteins. Nucleic Acids Res. 2019, 47, 3811–3827. [Google Scholar] [CrossRef]
- Mak, J.P.; Ma, H.T.; Poon, R.Y. Synergism between ATM and PARP1 inhibition involves DNA damage and abrogating the G(2) DNA damage checkpoint. Mol. Cancer Ther. 2020, 19, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Demin, A.A.; Hirota, K.; Tsuda, M.; Adamowicz, M.; Hailstone, R.; Brazina, J.; Gittens, W.; Kalasova, I.; Shao, Z.; Zha, S.; et al. XRCC1 prevents toxic PARP1 trapping during DNA base excision repair. Mol. Cell 2021, 81, 3018–3030.e5. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Tian, X.; Van Meter, M.; Ke, Z.; Gorbunova, V.; Seluanov, A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc. Natl. Acad. Sci. USA 2012, 109, 11800–11805. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Gopinath, R.K.; Hsueh, Y.-A.; Shieh, S.-Y. CHK2-mediated regulation of PARP1 in oxidative DNA damage response. Oncogene 2019, 38, 1166–1182. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.R.; Chang, R.; Liu, S.M.; Zhang, Y.R.; Zhao, J.; Yu, Y.R.; Qi, Y.F. Intermedin1–53 improves aging-associated cardiac remodeling and dysfunction via mitochondrial SIRT3-mediated SOD2 deacetylation. Mol. Cell Cardiol. 2025, 205, 86–98. [Google Scholar] [CrossRef] [PubMed]

| Targets | Sequence | Temp (°C) | |
|---|---|---|---|
| Adm2 | Forward | 5-CTTGCCAGCTGTCTCCAGAT-3′ | 60 |
| Reverse | 5′-CAGGTAGAGGAGGCTGATGC-3′ | ||
| Col1a1 | Forward | 5′-GCTCCTCTTAGGGGCCACT-3′ | 60 |
| Reverse | 5′-CCACGTCTCACCATTGGGG-3′ | ||
| Col3a1 | Forward | 5′-CTGTAACATGGAAACTGGGGAAA-3′ | 60 |
| Reverse | 5′-CCATAGCTGAACTGAAAACCACC-3′ | ||
| Tgfb1 | Forward | 5′-GGCACCATCCATGACATGAACCG-3′ | 60 |
| Reverse | 5′-GCCGTACACAGCAGTTCTTCTCTG-3′ | ||
| Il-6 | Forward | 5′-AGGAGTGGCTAAGGACCAAGACC-3′ | 60 |
| Reverse | 5′-TGCCGAGTAGACCTCATAGTGACC-3′ | ||
| Mcp-1 | Forward | 5′-CTATGCAGGTCTCTGTCACGCTTC-3′ | 60 |
| Reverse | 5′-CCAGTGAATGAGTAGCAGCAGGTG-3′ | ||
| Tnf-α | Forward | 5′-GCATGATCCGAGATGTGGAACTGG-3′ | 60 |
| Reverse | 5′-CGCCACGAGCAGGAATGAGAAG-3′ | ||
| Gapdh | Forward | 5′-ACTTTGTCAAGCTCATTTCC-3′ | 60 |
| Reverse | 5′-TGCAGCGAACTTTATTGATG-3′ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, D.-R.; Chen, Y.; Zhu, H.-X.; Liu, S.-M.; Wu, N.; Zhang, Y.-R.; Zhao, J.; Yu, Y.-R.; Jia, M.-Z.; Han, L.; et al. Intermedin Inhibits DNA Damage-Promoted Senescent Phenotype Transition of Vascular Smooth Muscle Cells in Aorta by Activating NAMPT/PARP1 in Mice. Pharmaceuticals 2025, 18, 1503. https://doi.org/10.3390/ph18101503
Ji D-R, Chen Y, Zhu H-X, Liu S-M, Wu N, Zhang Y-R, Zhao J, Yu Y-R, Jia M-Z, Han L, et al. Intermedin Inhibits DNA Damage-Promoted Senescent Phenotype Transition of Vascular Smooth Muscle Cells in Aorta by Activating NAMPT/PARP1 in Mice. Pharmaceuticals. 2025; 18(10):1503. https://doi.org/10.3390/ph18101503
Chicago/Turabian StyleJi, Deng-Ren, Yao Chen, Han-Xu Zhu, Shi-Meng Liu, Ning Wu, Ya-Rong Zhang, Jie Zhao, Yan-Rong Yu, Mo-Zhi Jia, Ling Han, and et al. 2025. "Intermedin Inhibits DNA Damage-Promoted Senescent Phenotype Transition of Vascular Smooth Muscle Cells in Aorta by Activating NAMPT/PARP1 in Mice" Pharmaceuticals 18, no. 10: 1503. https://doi.org/10.3390/ph18101503
APA StyleJi, D.-R., Chen, Y., Zhu, H.-X., Liu, S.-M., Wu, N., Zhang, Y.-R., Zhao, J., Yu, Y.-R., Jia, M.-Z., Han, L., Tang, C.-S., Chen, L.-L., Zhou, Y.-B., & Qi, Y.-F. (2025). Intermedin Inhibits DNA Damage-Promoted Senescent Phenotype Transition of Vascular Smooth Muscle Cells in Aorta by Activating NAMPT/PARP1 in Mice. Pharmaceuticals, 18(10), 1503. https://doi.org/10.3390/ph18101503






