Surface-Modified Nanocarriers Encapsulating Brucine and Nigella Sativa Oil: A Novel Approach to Solid Tumor Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Developed BR-Loaded NE
2.1.1. Assessment of Particle Size, Size Distribution and Zeta Potential
2.1.2. Assessment of Viscosity
2.1.3. Assessment of Drug Content
2.2. TEM Analysis
2.3. Studying In Vitro Release of BR from the Developed NE
2.4. Studying In Vitro Release of BR from the Developed NE Following Serum Incubation
2.5. Quantitative Determination of Serum Protein Adsorbed onto the Surface of NE
2.6. Hemolytic Activity
2.7. In Vitro Cytotoxicity Study: MTT Assay
2.8. In Vivo Studies
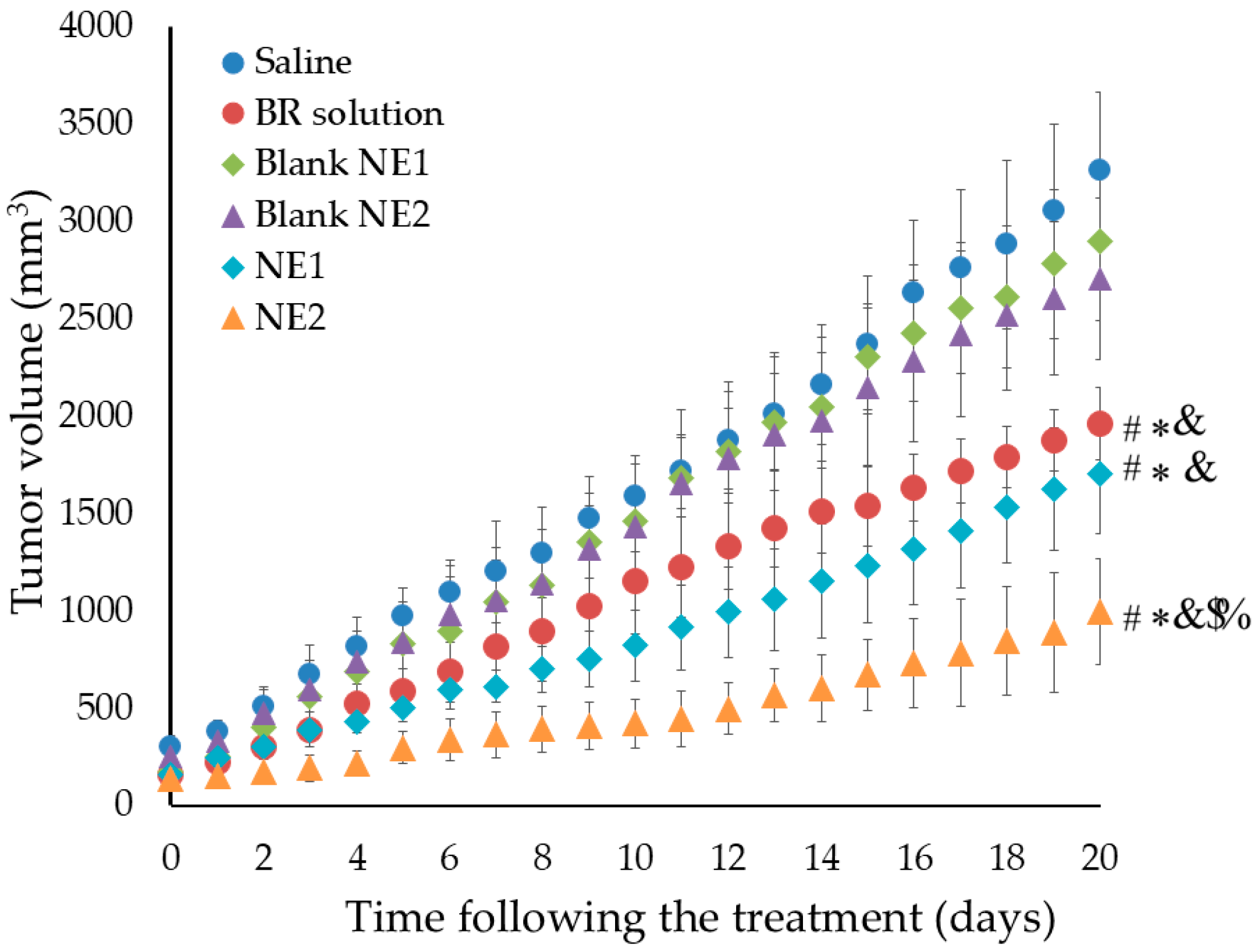
Evaluating the Anti-Tumor Activity of BR-Loaded NE
3. Materials and Methods
3.1. Materials
3.2. Formulation of NE
3.3. Characterization of the Developed BR-Loaded NE
3.3.1. Assessment of Particle Size, Size Distribution, and Zeta Potential
3.3.2. Assessment of Viscosity
3.3.3. Assessment of Drug Content
3.4. Transmission Electron Microscopy (TEM Analysis)
3.5. Studying In Vitro Release of BR from the Developed NE
3.6. Studying In Vitro Release of BR from the Developed NE Following Serum Incubation
3.7. Quantitative Determination of Serum Protein Adsorbed onto the Surface of NE
3.8. Hemolytic Activity
3.9. In Vitro Cytotoxicity Study
3.9.1. Cell Line
3.9.2. MTT Assay
3.10. Animals
3.10.1. Declaration of Ethical Approval
3.10.2. Experimental Animals
3.11. In Vivo Studies
Evaluating the Anti-Tumor Activity of BR-Loaded NEs
3.12. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Mani, K.; Deng, D.; Lin, C.; Wang, M.; Hsu, M.L.; Zaorsky, N.G. Causes of death among people living with metastatic cancer. Nat. Commun. 2024, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Vegunta, S.; Lester, S.P.; Pruthi, S.; Mussallem, D.M. Effects of Major Lifestyle Factors on Breast Cancer Risk: Impact of Weight, Nutrition, Physical Activity, Alcohol and Tobacco. Breast Cancer Manag. 2020, 9, BMT51. [Google Scholar] [CrossRef]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene–Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef]
- Adhikary, S.; Pathak, S.; Palani, V.; Acar, A.; Banerjee, A.; Al-Dewik, N.I.; Essa, M.M.; Mohammed, S.G.A.A.; Qoronfleh, M.W. Current Technologies and Future Perspectives in Immunotherapy towards a Clinical Oncology Approach. Biomedicines 2024, 12, 217. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Xu, Y.M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Shi, P.; Cheng, Z.; Zhao, K.; Chen, Y.; Zhang, A.; Gan, W.; Zhang, Y. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J. Nanobiotechnol. 2023, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.M.; Emeka, P.M.; Badger-Emeka, L.I.; Elsewedy, H.S. Date palm extract (Phoenix dactylifera) PEGylated nanoemulsion: Development, optimization and cytotoxicity evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef]
- Shehata, T.; Kono, Y.; Higaki, K.; Kimura, T.; Ogawara, K.-I. In vivo distribution characteristics and anti-tumor effects of doxorubicin encapsulated in PEG-modified niosomes in solid tumor-bearing mice. J. Drug Deliv. Sci. Technol. 2023, 80, 104122. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Shehata, T.M.; Almostafa, M.M.; Soliman, W.E. Hypolipidemic Activity of Olive Oil-Based Nanostructured Lipid Carrier Containing Atorvastatin. Nanomaterials 2022, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Nayik, G.A.; Ercisli, S.; Marc, R.A.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Elsewedy, H.S. Insights of Nanoemulsion as a Drug Delivery System: An Overview of Current Trends and Applications. Ind. J. Pharm. Edu. Res. 2025, 59, 472–492. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Alshahrani, S.M. A judicious review on the applications of chemotherapeutic loaded nanoemulsions in cancer management. J. Drug Deliv. Sci. Technol. 2022, 68, 103085. [Google Scholar] [CrossRef]
- Lu, L.; Huang, R.; Wu, Y.; Jin, J.M.; Chen, H.Z.; Zhang, L.J.; Luan, X. Brucine: A Review of Phytochemistry, Pharmacology, and Toxicology. Front. Pharmacol. 2020, 11, 377. [Google Scholar] [CrossRef]
- Seshadri, V.D. Brucine promotes apoptosis in cervical cancer cells (ME-180) via suppression of inflammation and cell proliferation by regulating PI3K/AKT/mTOR signaling pathway. Environ. Toxicol. 2021, 36, 1841–1847. [Google Scholar] [CrossRef]
- Yan, W.; Zeng, Z.; Qin, F.; Xu, J.; Liao, Z.; Ouyang, M. Effects of brucine on mitochondrial apoptosis and expression of HSP70 in prostate cancer cells. Transl. Cancer Res. 2022, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Elsewedy, H.S.; Shehata, T.M.; Tratrat, C.; Al Dhubiab, B.E.; Venugopala, K.N.; Almostafa, M.M.; Kochkar, H.; Elnahas, H.M. Significant of injectable brucine PEGylated niosomes in treatment of MDA cancer cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103322. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Aldhubiab, B.E.; Mahdy, M.A.; Elnahas, H.M. Brucine PEGylated nanoemulsion: In vitro and in vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125618. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Dhubiab, B.E.A.; Mahdy, M.A.; Elnahas, H.M. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Deliv. 2020, 27, 1134–1146. [Google Scholar] [CrossRef]
- Ismail, T.A.; Shehata, T.M.; Mohamed, D.I.; Elsewedy, H.S.; Soliman, W.E. Quality by Design for Development, Optimization and Characterization of Brucine Ethosomal Gel for Skin Cancer Delivery. Molecules 2021, 26, 3454. [Google Scholar] [CrossRef]
- Jain, B.; Jain, N.; Jain, S.; Teja, P.K.; Chauthe, S.K.; Jain, A. Exploring brucine alkaloid: A comprehensive review on pharmacology, therapeutic applications, toxicity, extraction and purification techniques. Phytomed. Plus 2023, 3, 100490. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th ed.; Merck: Whitehouse Station, NJ, USA, 1996. [Google Scholar]
- Michel, M.C.; Staskin, D. Study Designs for Evaluation of Combination Treatment: Focus on Individual Patient Benefit. Biomedicines 2022, 10, 270. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Mostofa, A.; Hossain, M.K.; Basak, D.; Bin Sayeed, M.S. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017, 8, 295. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, H.C.; Tania, M.; Zhang, D.Z. Anticancer activities of Nigella sativa (black cumin). Afr. J. Tradit. Complement. Altern. Med. 2011, 8 (Suppl. 5), 226–232. [Google Scholar] [CrossRef]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.E.; Wani, G.; Wani, A.A. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer 2005, 117, 409–417. [Google Scholar] [CrossRef]
- Ait Mbarek, L.; Ait Mouse, H.; Elabbadi, N.; Bensalah, M.; Gamouh, A.; Aboufatima, R.; Benharref, A.; Chait, A.; Kamal, M.; Dalal, A.; et al. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Braz. J. Med. Biol. Res. 2007, 40, 839–847. [Google Scholar] [CrossRef]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular Mechanisms of Thymoquinone as Anticancer Agent. Comb. Chem. High. Throughput Screen. 2021, 24, 1644–1653. [Google Scholar] [CrossRef]
- Chen, E.; Chen, B.-M.; Su, Y.-C.; Chang, Y.-C.; Cheng, T.-L.; Barenholz, Y.; Roffler, S.R. Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin via Formation of the Membrane Attack Complex. ACS Nano 2020, 14, 7808–7822. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.A.; Kelmann, R.G.; Araújo, B.V.; Finatto, R.B.; Teixeira, H.F.; Koester, L.S. Development and characterization of parenteral nanoemulsions containing thalidomide. Eur. J. Pharm. Sci. 2011, 42, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Đoković, J.B.; Savić, S.M.; Mitrović, J.R.; Nikolic, I.; Marković, B.D.; Randjelović, D.V.; Antic-Stankovic, J.; Božić, D.; Cekić, N.D.; Stevanović, V.; et al. Curcumin Loaded PEGylated Nanoemulsions Designed for Maintained Antioxidant Effects and Improved Bioavailability: A Pilot Study on Rats. Int. J. Mol. Sci. 2021, 22, 7991. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Preeti Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Séguy, L.; Groo, A.C.; Goux, D.; Hennequin, D.; Malzert-Fréon, A. Design of Non-Haemolytic Nanoemulsions for Intravenous Administration of Hydrophobic APIs. Pharmaceutics 2020, 12, 1141. [Google Scholar] [CrossRef]
- Samiun, W.S.; Ashari, S.E.; Salim, N.; Ahmad, S. Optimization of processing parameters of nanoemulsion containing aripiprazole using response surface methodology. Int. J. Nanomed. 2020, 2020, 1585–1594. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Shah, J.; Nair, A.B. Innovations in Nanoemulsion Technology: Enhancing Drug Delivery for Oral, Parenteral, and Ophthalmic Applications. Pharmaceutics 2024, 16, 1333. [Google Scholar] [CrossRef]
- Buszello, K.; Harnisch, S.; Müller, R.H.; Müller, B.W. The influence of alkali fatty acids on the properties and the stability of parenteral O/W emulsions modified with solutol HS 15. Eur. J. Pharm. Biopharm. 2000, 49, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.L.; Anraku, Y.; Sakuma, I.; Akagi, Y. Effect of PEGylation on the Drug Release Performance and Hemocompatibility of Photoresponsive Drug-Loading Platform. Int. J. Mol. Sci. 2022, 23, 6686. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.P.; Boyd, C.; Bubeck, D. Molecular cell biology of complement membrane attack. Semin. Cell Dev. Biol. 2017, 72, 124–132. [Google Scholar] [CrossRef]
- Ellsworth, C.R.; Chen, Z.; Xiao, M.T.; Qian, C.; Wang, C.; Khatun, M.S.; Liu, S.; Islamuddin, M.; Maness, N.J.; Halperin, J.A.; et al. Enhanced complement activation and MAC formation accelerates severe COVID-19. Cell. Mol. Life Sci. 2024, 81, 405. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.; Kimura, T.; Higaki, K.; Ogawara, K.I. In-vivo disposition characteristics of PEG niosome and its interaction with serum proteins. Int. J. Pharm. 2016, 512, 322–328. [Google Scholar] [CrossRef]
- Gonzalez Solveyra, E.; Thompson, D.H.; Szleifer, I. Proteins Adsorbing onto Surface-Modified Nanoparticles: Effect of Surface Curvature, pH, and the Interplay of Polymers and Proteins Acid–Base Equilibrium. Polymers 2022, 14, 739. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Li, X.; El-Nabarawi, M.; Elassasy, A.; Jasti, B. Effect of fixed aqueous layer thickness of polymeric stabilizers on zeta potential and stability of aripiprazole nanosuspensions. Pharm. Dev. Technol. 2013, 18, 730–735. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Shehata, T.; Ogawara, K.-I.; Higaki, K.; Kimura, T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. Int. J. Pharm. 2008, 359, 272–279. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Z.; Wang, Y.; Feng, J. Brucine induces the apoptosis of U266 multiple myeloma cells by phosphorylation of c-Jun. Mol. Med. Rep. 2013, 7, 481–484. [Google Scholar] [CrossRef]
- Li, M.; Jiang, S.; Simon, J.; Paßlick, D.; Frey, M.-L.; Wagner, M.; Mailänder, V.; Crespy, D.; Landfester, K. Brush Conformation of Polyethylene Glycol Determines the Stealth Effect of Nanocarriers in the Low Protein Adsorption Regime. Nano Lett. 2021, 21, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Elsewedy, H.S.; Alqahtani, N.K.; Soliman, W.E.; Mohamed, H.; Khalil, H.E. Nanoemulsion based hydrogel: A promising platform for potential anti-inflammatory action of date palm extract. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134657. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, F.; Ke, Y. Design and characterization of oil-in-water nanoemulsion for enhanced oil recovery stabilized by amphiphilic copolymer, nonionic surfactant, and LAPONITE® RD. RSC Adv. 2021, 11, 1952–1959. [Google Scholar] [CrossRef]
- Shehata, T.M.; Khalil, H.E.; Elsewedy, H.S.; Soliman, W.E. Myrrh essential oil-based nanolipid formulation for enhancement of the antihyperlipidemic effect of atorvastatin. J. Drug Deliv. Sci. Technol. 2021, 61, 102277. [Google Scholar] [CrossRef]
- Kirkby, M.; Moffatt, K.; Rogers, A.M.; McCague, P.J.; McElnay, J.C.; Quinn, C.; Donnelly, R.F. A Drug Content, Stability Analysis, and Qualitative Assessment of Pharmacists’ Opinions of Two Exemplar Extemporaneous Formulations. Molecules 2020, 25, 3078. [Google Scholar] [CrossRef] [PubMed]
- Musallam, A.A.; Aldeeb, R.A.; Mansour, R.M.; Kassem MAE-k Saeed, D.F.; Mahdy, M.A.; Ibrahim, T.M. Development of a Novel Drug Delivery System “Nanoemulfoam” for Topical Delivery of Terbinafine Hydrochloride as a Repurposed Therapy in Skin Cancer: Formulation, Optimization, In Vitro Characterization, Ex Vivo Transdermal Permeability, Cytotoxicity Studies, and In Silico Assessment. Pharmaceuticals 2025, 18, 972. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Barth, C.; Spreen, H.; Mulac, D.; Keuter, L.; Behrens, M.; Humpf, H.U.; Langer, K. Spacer length and serum protein adsorption affect active targeting of trastuzumab-modified nanoparticles. Biomater. Biosyst. 2022, 5, 100032. [Google Scholar] [CrossRef]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Dong, T.; Shen, J.; Gao, X.; Zhou, J. Naringenin has a chemoprotective effect in MDA-MB-231 breast cancer cells via inhibition of caspase-3 and -9 activities. Oncol. Lett. 2019, 17, 1217–1222. [Google Scholar]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control Release 2005, 103, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Un, K.; Minato, K.; Tanaka, K.; Higaki, K.; Kimura, T. Determinants for in vivo anti-tumor effects of PEG liposomal doxorubicin: Importance of vascular permeability within tumors. Int. J. Pharm. 2008, 359, 234–240. [Google Scholar] [CrossRef] [PubMed]
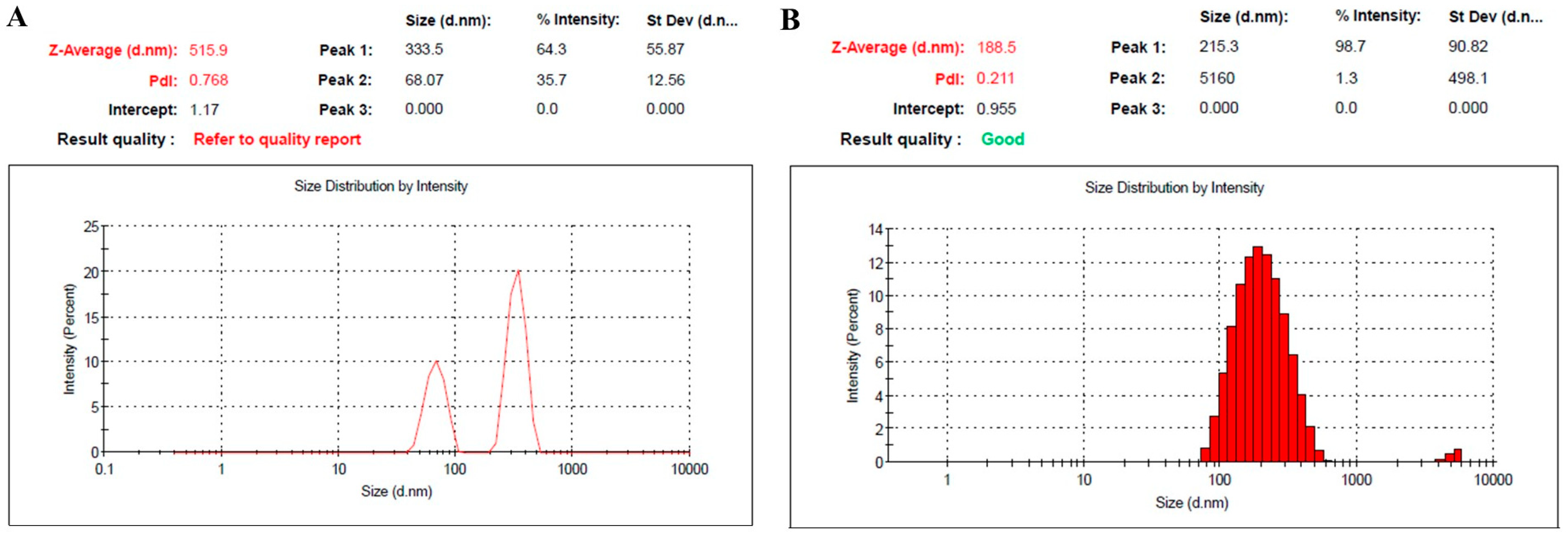

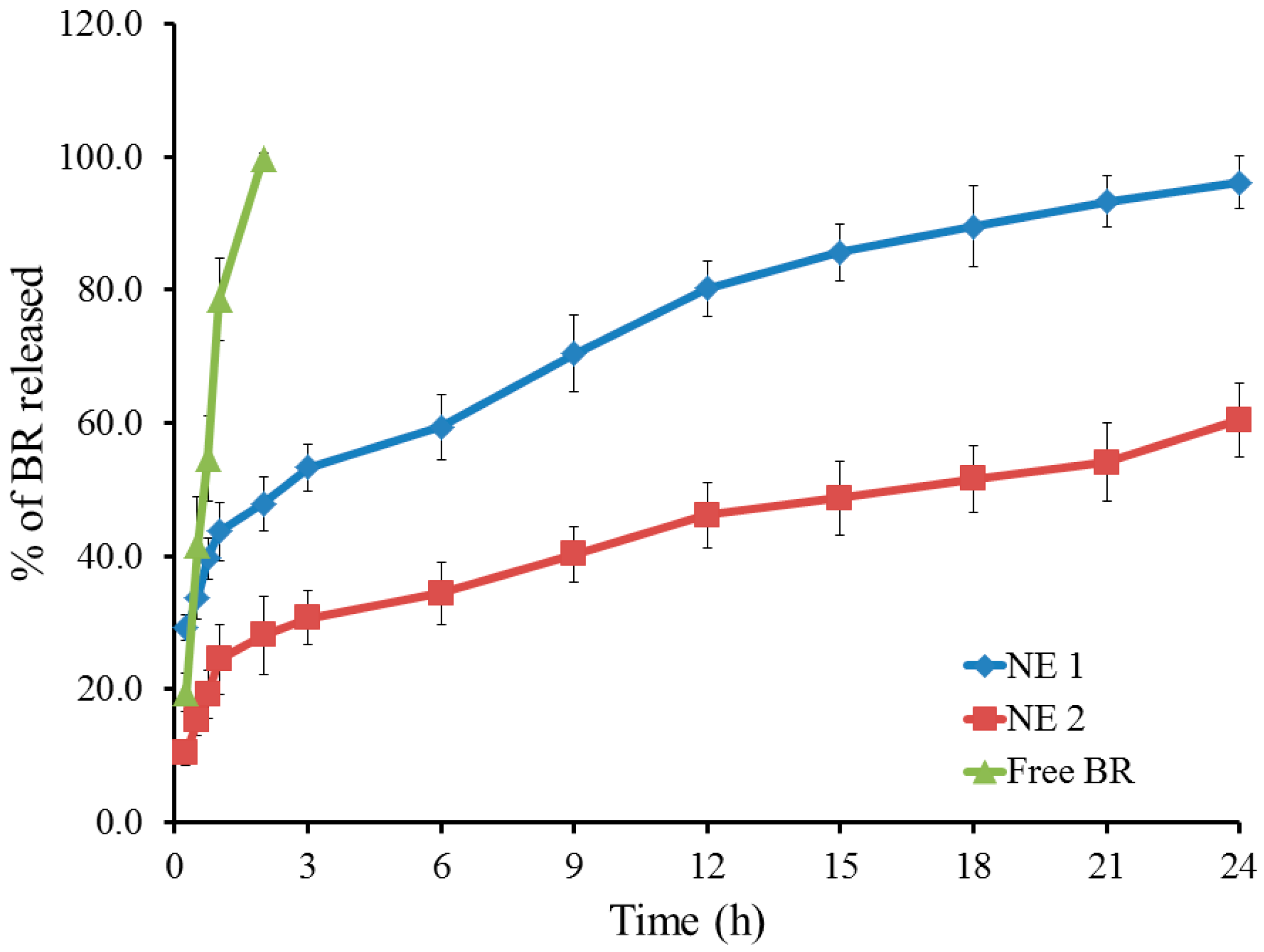
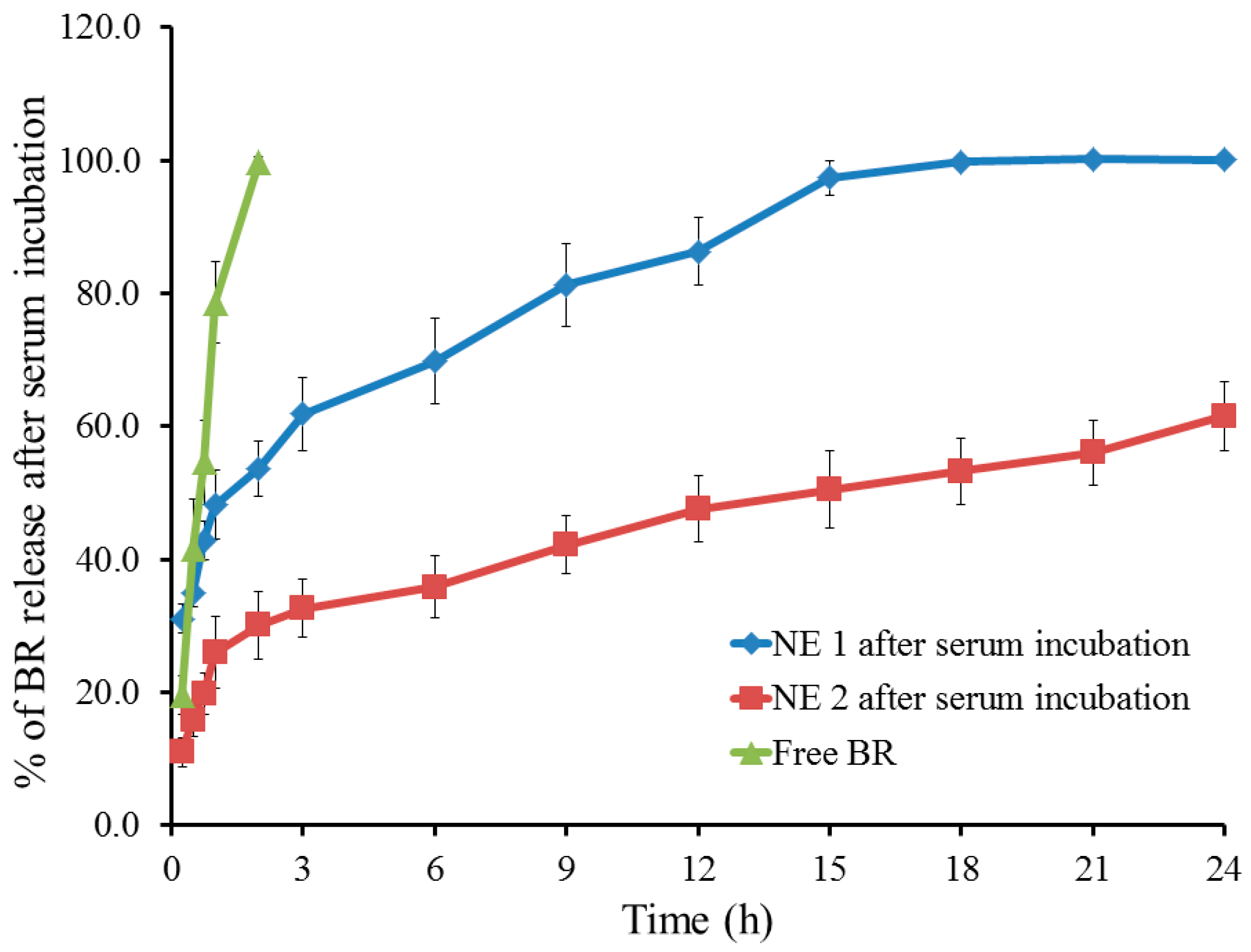


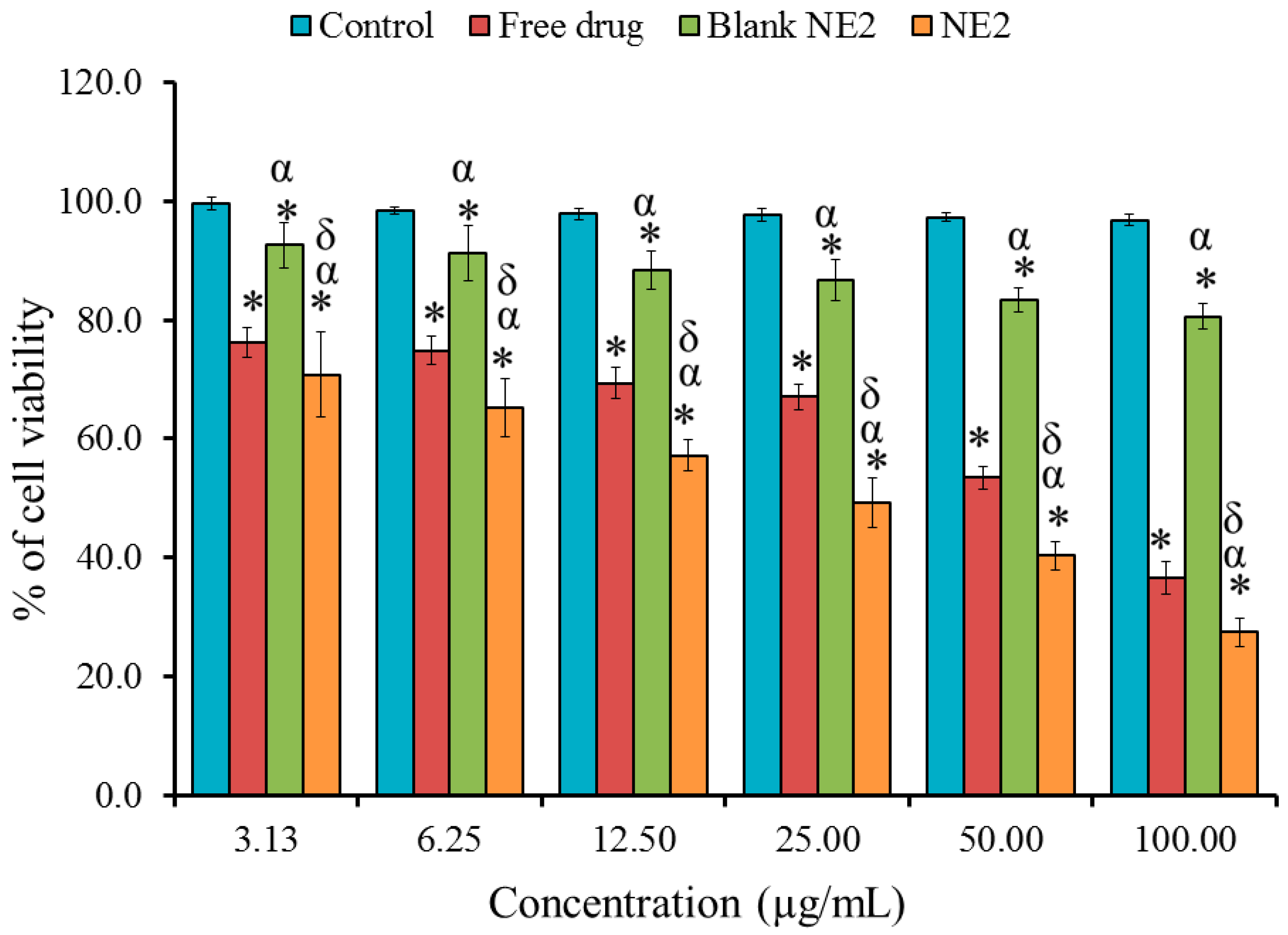
| Formulation | Particle Size (nm) | PDI | Viscosity (cp) | Zeta Potential (mV) | Drug Content % |
|---|---|---|---|---|---|
| NE1 | 515.9 ± 5.2 | 0.768 | 2.9 ± 0.30 | −20.0 ± 1.15 | 98.6 ± 0.7 |
| NE2 | 188.5 ± 3.3 | 0.211 | 3.4 ± 0.45 | −1.61 ± 0.82 | 99.0 ± 1.0 |
| Constituents | BR (%w/w) | NS Oil (%w/w) | Cholesterol (%w/w) | Oleic Acid (%w/w) | PEG-DSPE (%w/w) | EPC (%w/w) | Dist. H2O to (%w/w) |
|---|---|---|---|---|---|---|---|
| NE1 (Naked NE) | 0.5 | 15 | 0.4 | 0.3 | 0 | 0.12 | 100 |
| NE2 (PEGylated NE) | 0.5 | 15 | 0.4 | 0.3 | 0.5 | 0.12 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsewedy, H.S.; Shehata, T.M. Surface-Modified Nanocarriers Encapsulating Brucine and Nigella Sativa Oil: A Novel Approach to Solid Tumor Therapy. Pharmaceuticals 2025, 18, 1495. https://doi.org/10.3390/ph18101495
Elsewedy HS, Shehata TM. Surface-Modified Nanocarriers Encapsulating Brucine and Nigella Sativa Oil: A Novel Approach to Solid Tumor Therapy. Pharmaceuticals. 2025; 18(10):1495. https://doi.org/10.3390/ph18101495
Chicago/Turabian StyleElsewedy, Heba S., and Tamer M. Shehata. 2025. "Surface-Modified Nanocarriers Encapsulating Brucine and Nigella Sativa Oil: A Novel Approach to Solid Tumor Therapy" Pharmaceuticals 18, no. 10: 1495. https://doi.org/10.3390/ph18101495
APA StyleElsewedy, H. S., & Shehata, T. M. (2025). Surface-Modified Nanocarriers Encapsulating Brucine and Nigella Sativa Oil: A Novel Approach to Solid Tumor Therapy. Pharmaceuticals, 18(10), 1495. https://doi.org/10.3390/ph18101495







