Mechanotransduction-Epigenetic Coupling in Pulmonary Regeneration: Multifunctional Bioscaffolds as Emerging Tools
Abstract
1. Introduction
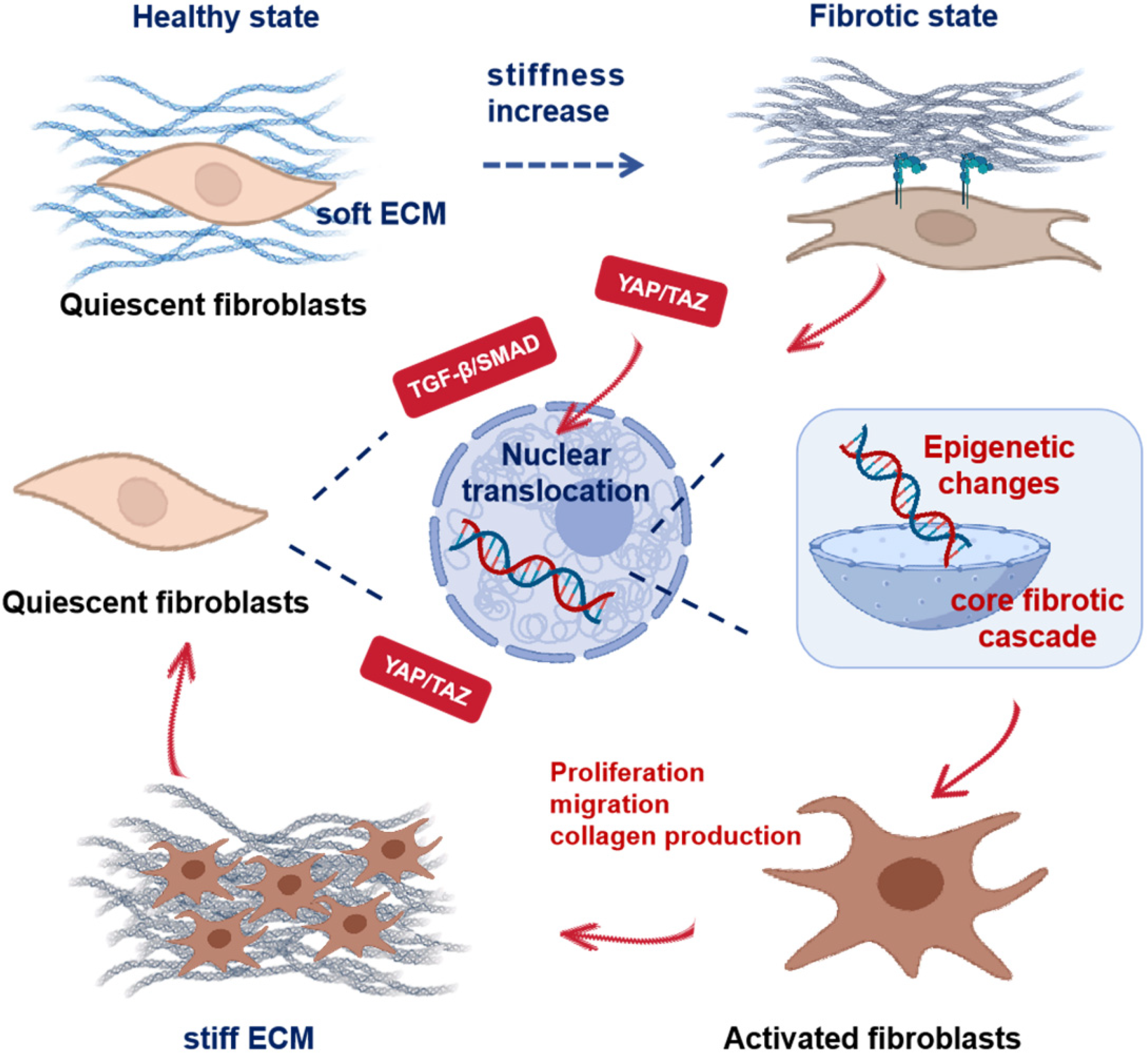
2. Mechanotransduction via Scaffold Mechanics
2.1. Scaffold Elastic Modulus and Stiffness Gradient Design
2.2. Dynamic Stretch and Aberrant YAP Activation
2.3. Focal Adhesions and Cytoskeletal Tension Transmission
2.4. Integrated Mechanosignaling in Fibrosis
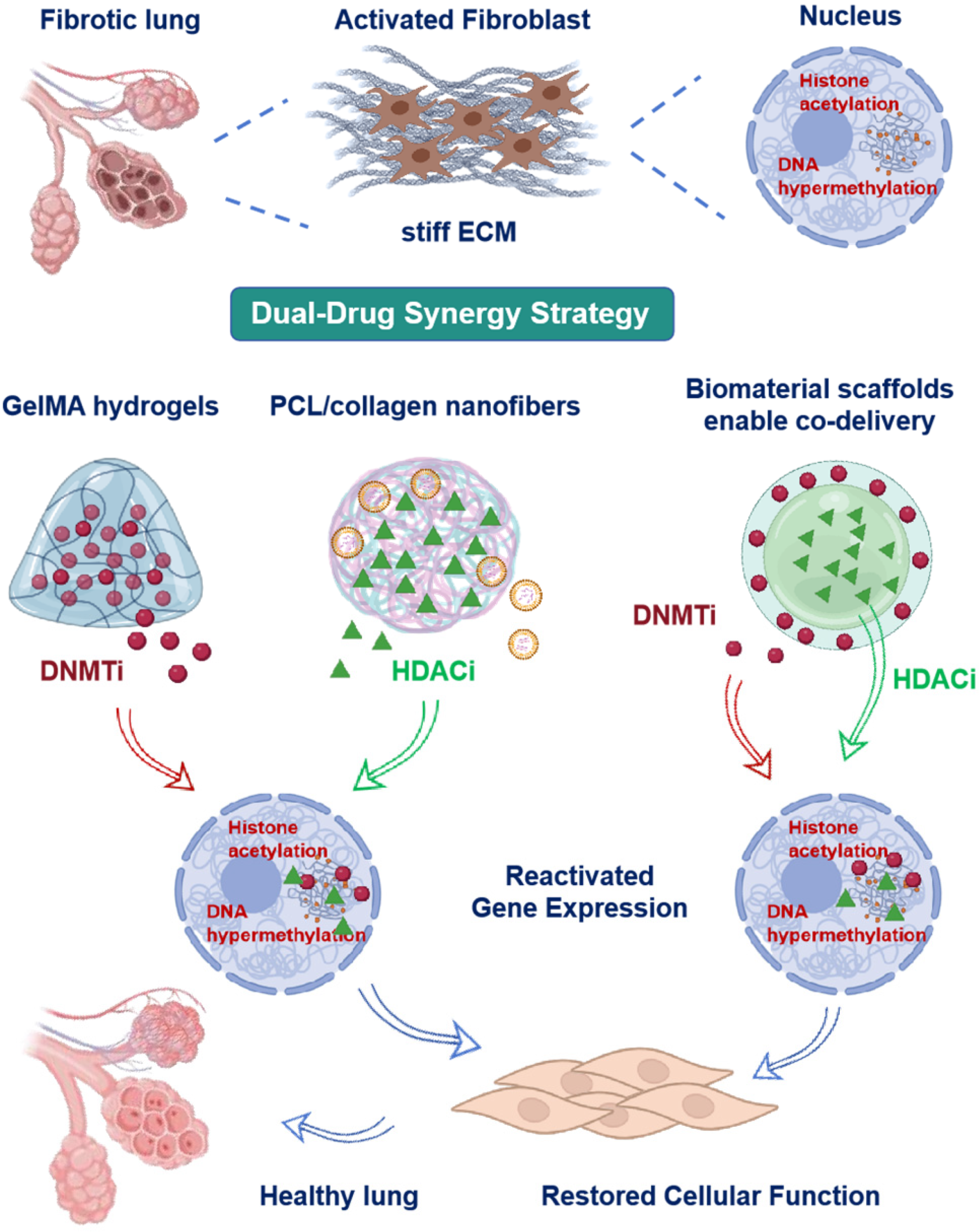
3. Epigenetic Drug Delivery via Scaffolds
3.1. DNMT Inhibitor Carrier Design
3.2. HDACi Carrier Design
3.3. Novel Epigenetic Targets for Scaffold-Based Intervention
3.4. Dual-Drug Synergy Strategy
4. Selected Pulmonary Fibrosis Models for Scaffold Application
4.1. Bleomycin-Induced Murine Model
4.2. Ex Vivo Lung Slices and Organoids
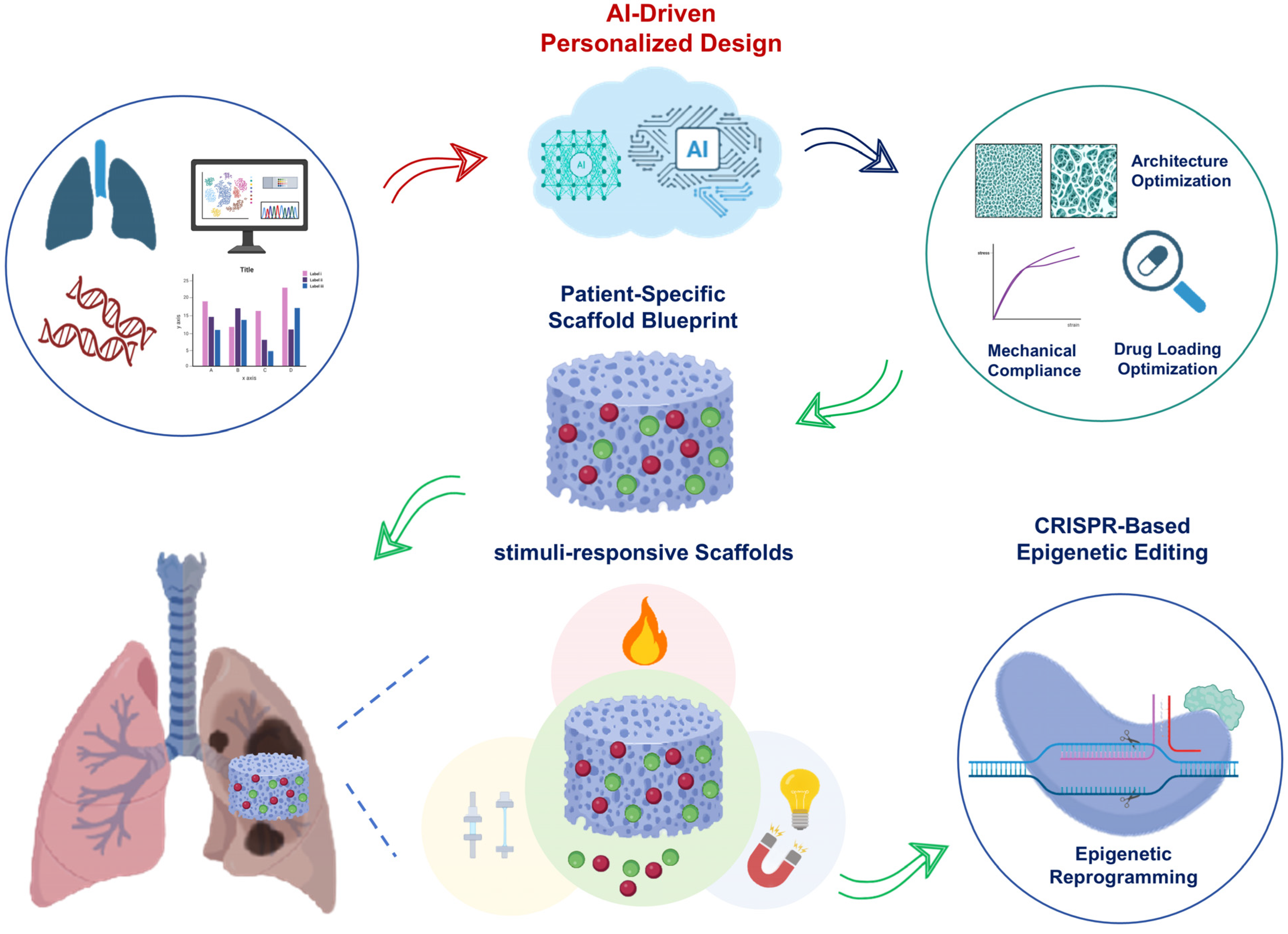
5. Discussion and Future Perspectives
5.1. Stimuli-Responsive Scaffolds
5.2. CRISPR-Based Epigenetic Editing
5.3. Artificial Intelligence-Driven Personalized Design
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Lettieri, S.; Bertuccio, F.R.; del Frate, L.; Perrotta, F.; Corsico, A.G.; Stella, G.M.; Madala, S.K. The Plastic Interplay between Lung Regeneration Phenomena and Fibrotic Evolution: Current Challenges and Novel Therapeutic Perspectives. Int. J. Mol. Sci. 2024, 25, 547. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, G.; Wang, H.; Mo, C. Comprehensive review of potential drugs with anti-pulmonary fibrosis properties. Biomed. Pharmacother. 2024, 173, 116282. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Gyoerfi, A.-H.; Ramanujam, M.; Whitfield, M.L.; Koenigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Long, Y.; Niu, Y.; Liang, K.; Du, Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022, 32, 70–90. [Google Scholar] [CrossRef]
- Strippoli, R.; Sandoval, P.; Moreno-Vicente, R.; Rossi, L.; Battistelli, C.; Terri, M.; Pascual-Anton, L.; Loureiro, M.; Matteini, F.; Calvo, E.; et al. Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 2020, 11, 647. [Google Scholar] [CrossRef]
- Zacharias, W.J.; Frank, D.B.; Zepp, J.A.; Morley, M.P.; Alkhaleel, F.A.; Kong, J.; Zhou, S.; Cantu, E.; Morrisey, E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018, 555, 251–255. [Google Scholar] [CrossRef]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ham, S.; Lee, Y.; Suh, G.Y.; Lee, Y.-S. TTC3 contributes to TGF-β1-induced epithelial-mesenchymal transition and myofibroblast differentiation, potentially through SMURF2 ubiquitylation and degradation. Cell Death Dis. 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wu, M.; Wang, Y.; Li, C.; Zeng, L.; Wang, Y.; Xiao, M.; Chen, X.; Geng, S.; Lai, P.; et al. Mesenchymal stem cells reversibly de-differentiate myofibroblasts to fibroblast-like cells by inhibiting the TGF-β-SMAD2/3 pathway. Mol. Med. 2023, 29, 59. [Google Scholar]
- Lv, Q.; Wang, J.; Xu, C.; Huang, X.; Ruan, Z.; Dai, Y. Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3β/β-catenin and TGF-β1/Smad2/3 signaling pathways. Mol. Med. 2020, 26, 49. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Chen, Q.; Zhou, Z. Notch signaling regulates pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1450038. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; DeMeo, D.L.; Glass, K.; Silverman, E.K.; Napoli, C. Epigenetics and pulmonary diseases in the horizon of precision medicine: A review. Eur. Respir. J. 2021, 57, 2003406. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chang, Y.-F.; Jiang, S.-H.; Li, X.-H.; Cheng, H.-P. DNA methylation modification in Idiopathic pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1416325. [Google Scholar] [CrossRef]
- Garitano, N.; Aguado-Alvaro, L.P.; Pelacho, B. Emerging Epigenetic Therapies for the Treatment of Cardiac Fibrosis. Biomedicine 2025, 13, 1170. [Google Scholar] [CrossRef]
- Toscano-Marquez, F.; Romero, Y.; Espina-Ordonez, M.; Cisneros, J. Absence of HDAC3 by Matrix Stiffness Promotes Chromatin Remodeling and Fibroblast Activation in Idiopathic Pulmonary Fibrosis. Cells 2023, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, Y.; Zheng, Q.; Ding, M.; Zhou, H.; Li, X. Epigenetic modification in liver fibrosis: Promising therapeutic direction with significant challenges ahead. Acta Pharm. Sin. B 2024, 14, 1009–1029. [Google Scholar] [CrossRef]
- Xue, T.; Qiu, X.; Liu, H.; Gan, C.; Tan, Z.; Xie, Y.; Wang, Y.; Ye, T. Epigenetic regulation in fibrosis progress. Pharmacol. Res. 2021, 173, 105910. [Google Scholar] [CrossRef]
- Ligresti, G.; Caporarello, N.; Meridew, J.A.; Jones, D.L.; Tan, Q.; Choi, K.M.; Haak, A.J.; Aravamudhan, A.; Roden, A.C.; Prakash, Y.S.; et al. CBX5/G9a/H3K9me-mediated gene repression is essential to fibroblast activation during lung fibrosis. JCI Insight 2019, 4, e127111. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Sun, M.; Sun, Y.; Feng, Z.; Kang, X.; Yang, W.; Wang, Y.; Luo, Y. New insights into the Hippo/YAP pathway in idiopathic pulmonary fibrosis. Pharmacol. Res. 2021, 169, 105635. [Google Scholar] [CrossRef]
- Lu, X.; Jin, H.; Quesada, C.; Farrell, E.C.; Huang, L.; Aliabouzar, M.; Kripfgans, O.D.; Fowlkes, J.B.; Franceschi, R.T.; Putnam, A.J.; et al. Spatially-directed cell migration in acoustically-responsive scaffolds through the controlled delivery of basic fibroblast growth factor. Acta Biomater. 2020, 113, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Quesada, C.; Aliabouzar, M.; Fowlkes, J.B.; Franceschi, R.T.; Liu, Z.; Putnam, A.J.; Fabiilli, M.L. Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds. Acta Biomater. 2021, 129, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, M.; Wentholt, S.; Li Vecchi, G.; de Vries, M.; Versteeg, E.M.M.; Boekema, B.K.H.L.; Choppin, A.; Barritault, D.; Chiappini, F.; van Kuppevelt, T.H.; et al. Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2. J. Funct. Biomater. 2025, 16, 51. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Chanamuangkon, T.; Nampuksa, K.; Monmaturapoj, N.; Sumrejkanchanakij, P.; Pimkhaokham, A.; Ampornaramveth, R.S. Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material. Cells 2022, 11, 3217. [Google Scholar] [CrossRef]
- Han, P.; Gomez, G.A.; Duda, G.N.; Ivanovski, S.; Poh, P.S.P. Scaffold geometry modulation of mechanotransduction and its influence on epigenetics. Acta Biomater. 2023, 163, 259–274. [Google Scholar] [CrossRef]
- Swanson, W.B.; Omi, M.; Zhang, Z.; Nam, H.K.; Jung, Y.; Wang, G.; Ma, P.X.; Hatch, N.E.; Mishina, Y. Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials 2021, 272, 120769. [Google Scholar] [CrossRef] [PubMed]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Healthc. Mater. 2023, 12, e2202766. [Google Scholar]
- Muzzio, N.; Moya, S.; Romero, G. Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine. Pharmaceutics 2021, 13, 792. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Chu, K.-A.; Wang, S.-Y.; Yeh, C.-C.; Fu, T.-W.; Fu, Y.-Y.; Ko, T.-L.; Chiu, M.-M.; Chen, T.-H.; Tsai, P.-J.; Fu, Y.-S. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics 2019, 9, 6646–6664. [Google Scholar] [CrossRef]
- Bailey, K.E.; Pino, C.; Lennon, M.L.; Lyons, A.; Jacot, J.G.; Lammers, S.R.; Konigshoff, M.; Magin, C.M. Embedding of Precision-Cut Lung Slices in Engineered Hydrogel Biomaterials Supports Extended Ex Vivo Culture. Am. J. Respir. Cell Mol. Biol. 2020, 62, 14–22. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Franks, N.A.; Cribbs, C.G.; Jacobs, D.J.; Dodson, E.L.; Knight, C.J.; Poulson, P.D.; Garfield, S.R.; Johnson, B.C.; Hemeyer, B.M.; et al. Soluble ECM promotes organotypic formation in lung alveolar model. Biomaterials 2022, 283, 121464. [Google Scholar] [CrossRef]
- Monaghan-Benson, E.; Aureille, J.; Guilluy, C. ECM stiffness regulates lung fibroblast survival through RasGRF1-dependent signaling. J. Biol. Chem. 2025, 301, 108161. [Google Scholar] [CrossRef] [PubMed]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Jandl, K.; Kwapiszewska, G. Stiffness of the Extracellular Matrix: A Regulator of Prostaglandins in Pulmonary Fibrosis? Am. J. Respir. Cell Mol. Biol. 2020, 63, 721–722. [Google Scholar] [CrossRef]
- Guo, T.; He, C.; Venado, A.; Zhou, Y. Extracellular Matrix Stiffness in Lung Health and Disease. Compr. Physiol. 2022, 12, 3523–3558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Qiao, Z.; Dou, B.; Xu, H.; Meng, F.; Huang, J. Polyacrylamide-Based Hydrogel with Biocompatibility and Tunable Stiffness for Three-Dimensional Cell Culture. ACS Appl. Bio Mater. 2025, 8, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, D.; Huang, D.; Li, X.; Duan, Y.; Chen, B. Biofabrication of Tunable 3D Hydrogel for Investigating the Matrix Stiffness Impact on Breast Cancer Chemotherapy Resistance. ACS Biomater. Sci. Eng. 2025, 11, 1417–1431. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Zhou, R.; Gao, H.; Wu, Y.; Wang, Y.; Wu, H.; Guan, C.; Wang, L.; Tang, L.; et al. Thermoplastic Elastomer-Reinforced Hydrogels with Excellent Mechanical Properties, Swelling Resistance, and Biocompatibility. Adv. Sci. 2025, 12, e2414339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ezzo, M.; Zondag, B.; Rakhshani, F.; Ma, Y.; Hinz, B.; Kumacheva, E. Intrafibrillar Crosslinking Enables Decoupling of Mechanical Properties and Structure of a Composite Fibrous Hydrogel. Adv. Mater. 2024, 36, e2305964. [Google Scholar] [CrossRef]
- Ibanez, R.I.R.; do Amaral, R.J.F.C.; Reis, R.L.; Marques, A.P.; Murphy, C.M.; O’Brien, F.J. 3D-Printed Gelatin Methacrylate Scaffolds with Controlled Architecture and Stiffness Modulate the Fibroblast Phenotype towards Dermal Regeneration. Polymers 2021, 13, 2510. [Google Scholar] [CrossRef]
- Farrell, E.; Aliabouzar, M.; Quesada, C.; Baker, B.M.; Franceschi, R.T.; Putnam, A.J.; Fabiilli, M.L. Spatiotemporal control of myofibroblast activation in acoustically-responsive scaffolds via ultrasound-induced matrix stiffening. Acta Biomater. 2022, 138, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ragazzini, S.; Scocozza, F.; Bernava, G.; Auricchio, F.; Colombo, G.I.; Barbuto, M.; Conti, M.; Pesce, M.; Garoffolo, G. Mechanosensor YAP cooperates with TGF- β 1 signaling to promote myofibroblast activation and matrix stiffening in a 3D model of human cardiac fibrosis. Acta Biomater. 2022, 152, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef]
- Tiskratok, W.; Chuinsiri, N.; Limraksasin, P.; Kyawsoewin, M.; Jitprasertwong, P. Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers 2025, 17, 822. [Google Scholar] [CrossRef]
- Soliman, B.G.; Chin, I.L.; Li, Y.; Ishii, M.; Ho, M.H.; Doan, V.K.; Cox, T.R.; Wang, P.Y.; Lindberg, G.C.J.; Zhang, Y.S.; et al. Droplet-based microfluidics for engineering shape-controlled hydrogels with stiffness gradient. Biofabrication 2024, 16, 045026. [Google Scholar] [CrossRef]
- Kuzucu, M.; Vera, G.; Beaumont, M.; Fischer, S.; Wei, P.; Shastri, V.P.; Forget, A. Extrusion-Based 3D Bioprinting of Gradients of Stiffness, Cell Density, and Immobilized Peptide Using Thermogelling Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 2192–2197. [Google Scholar] [CrossRef]
- Kersey, A.L.; Cheng, D.Y.; Deo, K.A.; Dubell, C.R.; Wang, T.-C.; Jaiswal, M.K.; Kim, M.H.; Murali, A.; Hargett, S.E.; Mallick, S.; et al. Stiffness assisted cell-matrix remodeling trigger 3D mechanotransduction regulatory programs. Biomaterials 2024, 306, 122473. [Google Scholar] [CrossRef]
- Chu, G.; Yuan, Z.; Zhu, C.; Zhou, P.; Wang, H.; Zhang, W.; Cai, Y.; Zhu, X.; Yang, H.; Li, B. Substrate stiffness- and topography-dependent differentiation of annulus fibrosus-derived stem cells is regulated by Yes-associated protein. Acta Biomater. 2019, 92, 254–264. [Google Scholar]
- Zhang, X.; Cao, D.; Xu, L.; Xu, Y.; Gao, Z.; Pan, Y.; Jiang, M.; Wei, Y.; Wang, L.; Liao, Y.; et al. Harnessing matrix stiffness to engineer a bone marrow niche for hematopoietic stem cell rejuvenation. Cell Stem Cell 2023, 30, 378–395. [Google Scholar] [CrossRef]
- Chen, G.-L.; Li, J.-Y.; Chen, X.; Liu, J.-W.; Zhang, Q.; Liu, J.-Y.; Wen, J.; Wang, N.; Lei, M.; Wei, J.-P.; et al. Mechanosensitive channels TMEM63A and TMEM63B mediate lung inflation-induced surfactant secretion. J. Clin. Investig. 2024, 134, e174508. [Google Scholar] [CrossRef]
- Kumar, V.; Madhurakkat Perikamana, S.K.; Tata, A.; Hoque, J.; Gilpin, A.; Tata, P.R.; Varghese, S. An In Vitro Microfluidic Alveolus Model to Study Lung Biomechanics. Front. Bioeng. Biotechnol. 2022, 10, 848699. [Google Scholar] [CrossRef]
- Sharifpoor, S.; Simmons, C.A.; Labow, R.S.; Santerre, J.P. A study of vascular smooth muscle cell function under cyclic mechanical loading in a polyurethane scaffold with optimized porosity. Acta Biomater. 2010, 6, 4218–4228. [Google Scholar] [CrossRef]
- Sencadas, V.; Sadat, S.; Silva, D.M. Mechanical performance of elastomeric PGS scaffolds under dynamic conditions. J. Mech. Behav. Biomed. Mater. 2020, 102, 103474. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Boujemaa-Paterski, R.; Winograd-Katz, S.E.; Venghateri, J.B.; Chung, W.-L.; Medalia, O. The Actin Network Interfacing Diverse Integrin-Mediated Adhesions. Biomolecules 2023, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Zhao, G.; Miao, Y.; Zhao, L.; Feng, J.; Zhang, H.; Miao, R.; Sun, L.; Gao, B.; et al. Programmable and Reversible Integrin-Mediated Cell Adhesion Reveals Hysteresis in Actin Kinetics that Alters Subsequent Mechanotransduction. Adv. Sci. 2023, 10, 2302421. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, H.; Okuda, S.; Nagayama, K.; Machiyama, H.; Kidoaki, S.; Kato, M.; Sokabe, M.; Miyata, T.; Hirata, H. Actin crosslinking by α-actinin averts viscous dissipation of myosin force transmission in stress fibers. iScience 2023, 26, 106090. [Google Scholar] [CrossRef]
- Amiri, S.; Muresan, C.; Shang, X.; Huet-Calderwood, C.; Schwartz, M.A.; Calderwood, D.A.; Murrell, M. Intracellular tension sensor reveals mechanical anisotropy of the actin cytoskeleton. Nat. Commun. 2023, 14, 8011. [Google Scholar] [CrossRef]
- Yang, Y.; Santos, D.M.; Pantano, L.; Knipe, R.; Abe, E.; Logue, A.; Pronzati, G.; Black, K.E.; Spinney, J.J.; Giacona, F.; et al. Screening for Inhibitors of YAP Nuclear Localization Identifies Aurora Kinase A as a Modulator of Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 67, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Blokland, K.E.C.; Nizamoglu, M.; Habibie, H.; Borghuis, T.; Schuliga, M.; Melgert, B.N.; Knight, D.A.; Brandsma, C.-A.; Pouwels, S.D.; Burgess, J.K. Substrate stiffness engineered to replicate disease conditions influence senescence and fibrotic responses in primary lung fibroblasts. Front. Pharmacol. 2022, 13, 989169. [Google Scholar] [CrossRef] [PubMed]
- Stancil, I.T.; Michalski, J.E.; Hennessy, C.E.; Hatakka, K.L.; Yang, I.V.; Kurche, J.S.; Rincon, M.; Schwartz, D.A. Interleukin-6-dependent epithelial fluidization initiates fibrotic lung remodeling. Sci. Transl. Med. 2022, 14, eabo5254. [Google Scholar] [CrossRef] [PubMed]
- Stancil, I.T.; Michalski, J.E.; Davis-Hall, D.; Chu, H.W.; Park, J.-A.; Magin, C.M.; Yang, I.V.; Smith, B.J.; Dobrinskikh, E.; Schwartz, D.A. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat. Commun. 2021, 12, 4566. [Google Scholar] [CrossRef]
- Papavassiliou, K.A.; Sofianidi, A.A.; Spiliopoulos, F.G.; Gogou, V.A.; Gargalionis, A.N.; Papavassiliou, A.G. YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis. Cells 2024, 13, 1519. [Google Scholar] [CrossRef]
- Digiovanni, G.T.; Han, W.; Sherrill, T.P.; Taylor, C.J.; Nichols, D.S.; Geis, N.M.; Singha, U.K.; Calvi, C.L.; McCall, A.S.; Dixon, M.M.; et al. Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury. JCI Insight 2023, 8, e173374. [Google Scholar] [CrossRef]
- Haak, A.J.; Kostallari, E.; Sicard, D.; Ligresti, G.; Choi, K.M.; Caporarello, N.; Jones, D.L.; Tan, Q.; Meridew, J.; Espinosa, A.M.D.; et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci. Transl. Med. 2019, 11, eaau6296. [Google Scholar] [CrossRef]
- Xiao, J.; Ang, J.W.; Zhong, X.; Wong, D.C.P.; Thivakar, T.; Yow, I.; Lee, C.J.M.; Foo, R.S.Y.; Kanchanawong, P.; Low, B.C. Coordination of Focal Adhesion Nanoarchitecture and Dynamics in Mechanosensing for Cardiomyoblast Differentiation. ACS Appl. Mater. Interfaces 2025, 17, 4463–4479. [Google Scholar] [CrossRef]
- Bachmann, M.; Skripka, A.; Weissenbruch, K.; Wehrle-Haller, B.; Bastmeyer, M. Phosphorylated paxillin and phosphorylated FAK constitute subregions within focal adhesions. J. Cell Sci. 2022, 135, jcs258764. [Google Scholar] [CrossRef]
- Ripamonti, M.; Wehrle-Haller, B.; de Curtis, I. Paxillin: A Hub for Mechano-Transduction from the β3 Integrin-Talin-Kindlin Axis. Front. Cell Dev. Biol. 2022, 10, 852016. [Google Scholar] [CrossRef]
- Cruz-Soca, M.; Faundez-Contreras, J.; Cordova-Casanova, A.; Gallardo, F.S.; Bock-Pereda, A.; Chun, J.; Carlos Casar, J.; Brandan, E. Activation of skeletal muscle FAPs by LPA requires the Hippo signaling via the FAK pathway. Matrix Biol. 2023, 119, 57–81. [Google Scholar] [CrossRef]
- Ren, Z.; Pan, X.; Li, J.; Dong, X.; Tu, X.; Pan, L.L.; Sun, J. G protein coupled receptor 41 regulates fibroblast activation in pulmonary fibrosis via Gαi/o and downstream Smad2/3 and ERK1/2 phosphorylation. Pharmacol. Res. 2023, 191, 106754. [Google Scholar] [CrossRef] [PubMed]
- Yeung, V.; Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Zieske, J.D.; Karamichos, D.; Ciolino, J.B. FAK Inhibition Attenuates Corneal Fibroblast Differentiation In Vitro. Biomolecules 2021, 11, 1682. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Lee, D.Y.; White, E.S.; Cui, Z.; Larios, J.M.; Chacon, R.; Horowitz, J.C.; Day, R.M.; Thomas, P.E. Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003, 278, 12384–12389. [Google Scholar] [CrossRef]
- Oakes, P.W.; Wagner, E.; Brand, C.A.; Probst, D.; Linke, M.; Schwarz, U.S.; Glotzer, M.; Gardel, M.L. Optogenetic control of RhoA reveals zyxin-mediated elasticity of stress fibres. Nat. Commun. 2017, 8, 15817. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Pastor, C.; Rubio-Moscardo, F.; Vogel-Gonzalez, M.; Serra, S.A.; Afthinos, A.; Mrkonjic, S.; Destaing, O.; Abenza, J.F.; Fernandez-Fernandez, J.M.; Trepat, X.; et al. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. USA 2018, 115, 1925–1930. [Google Scholar] [CrossRef]
- Jo, M.H.; Li, J.; Jaumouille, V.; Hao, Y.; Coppola, J.; Yan, J.; Waterman, C.M.; Springer, T.A.; Ha, T. Single-molecule characterization of subtype-specific β1 integrin mechanics. Nat. Commun. 2022, 13, 7471. [Google Scholar] [CrossRef]
- De Belly, H.; Yan, S.; da Rocha, H.B.; Ichbiah, S.; Town, J.P.; Zager, P.J.; Estrada, D.C.; Meyer, K.; Turlier, H.; Bustamante, C.; et al. Cell protrusions and contractions generate long-range membrane tension propagation. Cell 2023, 186, 3049–3061. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, H.; Hou, Y.; Meng, D.; Jiang, J.; Lee, E.-B.; Fu, Y.; Zhang, X.; Chen, R.; Wang, Y. Heterogeneous focal adhesion cytoskeleton nanoarchitectures from microengineered interfacial curvature to oversee nuclear remodeling and mechanotransduction of mesenchymal stem cells. Cell. Mol. Biol. Lett. 2025, 30, 10. [Google Scholar] [CrossRef]
- Dupont, S.; Wickstrom, S.A. Mechanical regulation of chromatin and transcription. Nat. Rev. Genet. 2022, 23, 624–643. [Google Scholar] [CrossRef]
- Stephens, R.K.; Miroshnikova, Y.A. Nuclear periphery and its mechanical regulation in cell fate transitions. Curr. Opin. Struct. Biol. 2024, 87, 102867. [Google Scholar] [CrossRef]
- Tschumperlin, D.J.; Lagares, D. Mechano-therapeutics: Targeting Mechanical Signaling in Fibrosis and Tumor Stroma. Pharmacol. Ther. 2020, 212, 107575. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Thanh, H.; Lee, S.-J.; et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.E.; Collins, J.M.; Dawahare, J.H.; Trung Dung, N.; Lin, Y.; Voytik-Harbin, S.L.; Zorlutuna, P.; Yoder, M.C.; Boerckel, J.D. YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. J. Cell Biol. 2019, 218, 1369–1389. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Szeto, S.G.; Narimatsu, M.; Lu, M.; He, X.; Sidiqi, A.M.; Tolosa, M.F.; Chan, L.; De Freitas, K.; Bialik, J.F.; Majunnder, S.; et al. YAP/TAZ Are Mechanoregulators of TGF-β-Smad Signaling and Renal Fibrogenesis. J. Am. Soc. Nephrol. 2016, 27, 3117–3128. [Google Scholar] [CrossRef]
- Astudillo, P. Extracellular matrix stiffness and Wnt/β-catenin signaling in physiology and disease. Biochem. Soc. Trans. 2020, 48, 1187–1198. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Dabaghi, M.; Singer, R.; Noble, A.; Arizpe Tafoya, A.V.; Gonzalez-Martinez, D.A.; Gupta, T.; Formosa-Dague, C.; Rosas, I.O.; Kolb, M.R.; Shargall, Y.; et al. Influence of lung extracellular matrix from non-IPF and IPF donors on primary human lung fibroblast biology. Biomater. Sci. 2025, 13, 1721–1741. [Google Scholar] [CrossRef]
- Burgess, J.K.; Gosens, R. Mechanotransduction and the extracellular matrix: Key drivers of lung pathologies and drug responsiveness. Biochem. Pharmacol. 2024, 228, 116255. [Google Scholar] [CrossRef]
- Jandl, K.; Radic, N.; Zeder, K.; Kovacs, G.; Kwapiszewska, G. Pulmonary vascular fibrosis in pulmonary hypertension-The role of the extracellular matrix as a therapeutic target. Pharmacol. Ther. 2023, 247, 108438. [Google Scholar] [CrossRef] [PubMed]
- Matera, D.L.; DiLillo, K.M.; Smith, M.R.; Davidson, C.D.; Parikh, R.; Said, M.; Wilke, C.A.; Lombaert, I.M.; Arnold, K.B.; Moore, B.B.; et al. Microengineered 3D pulmonary interstitial mimetics highlight a critical role for matrix degradation in myofibroblast differentiation. Sci. Adv. 2020, 6, eabb5069. [Google Scholar] [CrossRef]
- Yanagihara, T.; Guignabert, C.; Kolb, M.R.J. Endothelial cells in pulmonary fibrosis: More than a bystander. Eur. Respir. J. 2023, 61, 2300407. [Google Scholar] [CrossRef]
- Balestrini, J.L.; Chaudhry, S.; Sarrazy, V.; Koehler, A.; Hinz, B. The mechanical memory of lung myofibroblasts. Integr. Biol. 2012, 4, 410–421. [Google Scholar] [CrossRef]
- Barcena-Varela, M.; Paish, H.; Alvarez, L.; Uriarte, I.; Latasa, M.U.; Santamaria, E.; Recalde, M.; Garate, M.; Claveria, A.; Colyn, L.; et al. Epigenetic mechanisms and metabolic reprogramming in fibrogenesis: Dual targeting of G9a and DNMT1 for the inhibition of liver fibrosis. Gut 2021, 70, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Lee, J.-H.; Ahn, K.S.; Shim, H.W.; Yoon, J.-Y.; Hyun, J.; Lee, J.H.; Jang, S.; Yoo, K.H.; Jang, Y.-K.; et al. Cyclic Stretch Promotes Cellular Reprogramming Process through Cytoskeletal-Nuclear Mechano-Coupling and Epigenetic Modification. Adv. Sci. 2023, 10, e2303395. [Google Scholar] [CrossRef] [PubMed]
- Barcena-Varela, M.; Caruso, S.; Llerena, S.; Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Urtasun, R.; Rebouissou, S.; Alvarez, L.; Jimenez, M.; et al. Dual Targeting of Histone Methyltransferase G9a and DNA-Methyltransferase 1 for the Treatment of Experimental Hepatocellular Carcinoma. Hepatology 2019, 69, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Li, J.Y.-S.; Nguyen, P.; Norwich, G.; Wang, Y.; Teng, D.; Shiu, Y.-T.; Shyy, J.Y.J.; Chien, S. Pulsatile flow induces chromatin interaction with lamin-associated proteins to enrich H3K9 methylation in endothelial cells. Proc. Natl. Acad. Sci. USA 2025, 122, e2424566122. [Google Scholar] [CrossRef]
- Song, Y.; Soto, J.; Li, S. Mechanical regulation of histone modifications and cell plasticity. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100872. [Google Scholar] [CrossRef]
- Alisafaei, F.; Jokhun, D.S.; Shivashankar, G.V.; Shenoy, V.B. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl. Acad. Sci. USA 2019, 116, 13200–13209. [Google Scholar] [CrossRef]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.-I.; Taylor, C.J.; Jetter, C.; et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-B.; Chen, Y.-P.; Tan, M.; Zhao, L.; Zhai, Y.-Y.; Sun, Y.-L.; Gong, Y.; Feng, X.-Q.; Du, J.; Fan, Y.-B. Extracellular Matrix Stiffness Regulates DNA Methylatioan by PKCα-Dependent Nuclear Transport of DNMT3L. Adv. Healthc. Mater. 2021, 10, 2100821. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Luo, Y.; Liu, Y.; Jiang, M.; Li, J.; Zhang, Q.; Bai, J. Hypermethylation of PPARG-encoding gene promoter mediates fine particulate matter-induced pulmonary fibrosis by regulating the HMGB1/NLRP3 axis. Ecotoxicol. Environ. Saf. 2024, 272, 116068. [Google Scholar] [CrossRef]
- Ting, L.; Feng, Y.; Zhou, Y.; Tong, Z.; Dong, Z. IL-27 induces autophagy through regulation of the DNMT1/lncRNA MEG3/ERK/p38 axis to reduce pulmonary fibrosis. Respir. Res. 2023, 24, 67. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Lai, J.; Tan, S.; Wang, M. Structure and Properties of Gelatin Methacryloyl (GelMA) Synthesized in Different Reaction Systems. Biomacromolecules 2023, 24, 2928–2941. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Z.; Wei, Q.; Ma, K.; Hu, W.; Huang, Q.; Su, J.; Li, H.; Zhang, C.; Fu, X. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1a-me diate d enhancement of angiogenesis. Acta Biomater. 2022, 147, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Wang, J.; Liu, F.; Yuan, L.; Ding, M.J.; Chen, L.D.; Yuan, J.L.; Yang, K.; Qian, J.; Lu, W.J. Non-inflammatory emphysema induced by NO2 chronic exposure and intervention with demethylation 5-Azacytidine. Life Sci. 2019, 221, 121–129. [Google Scholar] [CrossRef]
- Brembilla, N.-C.; El-Harane, S.; Durual, S.; Krause, K.-H.; Preynat-Seauve, O. Adipose-Derived Stromal Cells Exposed to RGD Motifs Enter an Angiogenic Stage Regulating Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 867. [Google Scholar] [CrossRef]
- Kim, H.J.; You, S.J.; Yang, D.H.; Chun, H.J.; Kim, M.S. Preparation of novel RGD-conjugated thermosensitive mPEG-PCL composite hydrogels and in vitro investigation of their impacts on adhesion-dependent cellular behavior. J. Ind. Eng. Chem. 2020, 84, 226–235. [Google Scholar] [CrossRef]
- Avsec, Z.; Weilert, M.; Shrikumar, A.; Krueger, S.; Alexandari, A.; Dalal, K.; Fropf, R.; McAnany, C.; Gagneur, J.; Kundaje, A.; et al. Base-resolution models of transcription-factor binding reveal soft motif syntax. Nat. Genet. 2021, 53, 354–366. [Google Scholar] [CrossRef]
- Ruan, H.; Hu, Q.; Wen, D.; Chen, Q.; Chen, G.; Lu, Y.; Wang, J.; Cheng, H.; Lu, W.; Gu, Z. A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Adv. Mater. 2019, 31, e1806957. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, W.; Chen, X.; He, Y.; Jiang, H.; Zhang, X.; Pan, L.; Ni, B.; Yang, F.; Xu, Y.; et al. An Injectable Epigenetic Autophagic Modulatory Hydrogel for Boosting Umbilical Cord Blood NK Cell Therapy Prevents Postsurgical Relapse of Triple-Negative Breast Cancer. Adv. Sci. 2022, 9, e2201271. [Google Scholar] [CrossRef]
- Chakraborty, A.; Pacelli, S.; Alexander, S.; Huayamares, S.; Rosenkrans, Z.; Vergel, F.E.; Wu, Y.; Chakravorty, A.; Paul, A. Nanoparticle-Reinforced Tough Hydrogel as a Versatile Platform for Pharmaceutical Drug Delivery: Preparation and in Vitro Characterization. Mol. Pharm. 2023, 20, 767–774. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, C.; Liu, N.; Zhao, M.; Chen, Z.; Liu, J.; Li, G.; Huang, H.; Guo, H.; Sun, T.; et al. Injectable conductive gelatin methacrylate/oxidized dextran hydrogel encapsulating umbilical cord mesenchymal stem cells for myocardial infarction treatment. Bioact. Mater. 2022, 13, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, D.; Tassey, J.; Li, J.; Liu, J.; Li, G.; Sun, Y.; Zhao, X.; Wang, T.; Zhang, Y.; et al. Complex hydrogel for cartilage regeneration and anti-inflammation. Compos. Part B Eng. 2024, 280, 111481. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Lin, Z.Y.; Wong, K.K.Y.; Lin, M.; Yildirimer, L.; Zhao, X. Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov. Today 2017, 22, 1351–1366. [Google Scholar] [CrossRef]
- Xing, L.; Chang, X.; Shen, L.; Zhang, C.; Fan, Y.; Cho, C.; Zhang, Z.; Jiang, H. Progress in drug delivery system for fibrosis therapy. Asian J. Pharm. Sci. 2021, 16, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Miszuk, J.; Liang, Z.; Hu, J.; Sanyour, H.; Hong, Z.; Fong, H.; Sun, H. Elastic Mineralized 3D Electrospun PCL Nanofibrous Scaffold for Drug Release and Bone Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, C.; Jiao, D.; Li, J.; Li, Y.; Zhou, X.; Zhao, H.; Zhao, Y.; Han, X. Synergistic effects of silver nanoparticles and cisplatin in combating inflammation and hyperplasia of airway stents. Bioact. Mater. 2022, 9, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W.; Jiao, D.; Tian, C.; Xu, K.; Zhu, H.; Han, X. All-in-one properties of an anticancer-covered airway stent for the prevention of malignant central airway obstruction. APL Bioeng. 2023, 7, 036116. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Gao, W.; Shi, M.; Tang, F.; Fu, X.; Chen, X. Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. J. Mater. Chem. B 2021, 9, 1395–1405. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Mancino, S.; Boraso, M.; Galmozzi, A.; Serafini, M.M.; De Fabiani, E.; Crestani, M.; Viviani, B. Dose-dependent dual effects of HDAC inhibitors on glial inflammatory response. Sci. Rep. 2025, 15, 12262. [Google Scholar] [CrossRef]
- Dushanan, R.; Weerasinghe, S.; Dissanayake, D.P.; Senthilnithy, R. Driving the new generation histone deacetylase inhibitors in cancer therapy; manipulation of the histone abbreviation at the epigenetic level: An in-silico approach. Can. J. Chem. 2022, 100, 880–890. [Google Scholar] [CrossRef]
- Hebbel, R.P.; Vercellotti, G.M.; Pace, B.S.; Solovey, A.N.; Kollander, R.; Abanonu, C.F.; Nguyen, J.; Vineyard, J.V.; Belcher, J.D.; Abdulla, F.; et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood 2010, 115, 2483–2490. [Google Scholar] [CrossRef]
- Hassan, A.A.; Radwan, H.A.; Abdelaal, S.A.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.R.; Hady, M.A. Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: Morphology, cell adhesion, and antibacterial activity. Int. J. Pharm. 2021, 593, 120143. [Google Scholar] [CrossRef]
- Aly, A.A.; Ahmed, M.K. Fibrous scaffolds of Ag/Fe co-doped hydroxyapatite encapsulated into polycaprolactone: Morphology, mechanical and in vitro cell adhesion. Int. J. Pharm. 2021, 601, 120557. [Google Scholar] [CrossRef]
- Baltes, F.; Caspers, J.; Henze, S.; Schlesinger, M.; Bendas, G. Targeting Discoidin Domain Receptor 1 (DDR1) Signaling and Its Crosstalk with β1-Integrin Emerges as a Key Factor for Breast Cancer Chemosensitization upon Collagen Type 1 Binding. Int. J. Mol. Sci. 2020, 21, 4956. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, J.; Ishihara, A.; Miura, R.; Mansurov, A.; Fukunaga, K.; Hubbell, J.A. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy. Sci. Adv. 2019, 5, eaaw6081. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Han, F.; Zhu, Z.; Yu, Q.; Liu, C.; Bao, Y.; Li, B.; Zhou, F. Sustained release of basic fibroblast growth factor in micro/nanofibrous scaffolds promotes annulus fibrosus regeneration. Acta Biomater. 2023, 166, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Li, S.; Xi, K.; Tang, J.; Shen, X.; Liu, Y.; Guo, R.; Zhang, N.; Gu, Y.; Xu, Y.; et al. ECM-engineered electrospun fibers with an immune cascade effect for inhibiting tissue fibrosis. Acta Biomater. 2023, 171, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Kim, H.S.; Chen, Y.; Li, Y.; LaMere, M.W.; Chen, C.; Wang, H.; Gong, J.; Palumbo, C.D.; Ashton, J.M.; et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci. Adv. 2021, 7, eabg3217. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; Wu, S.; Wu, J.; Zhang, J.; Li, J.; Lei, S.; Duan, X.; Men, K. Injectable and Photocurable Gene Scaffold Facilitates Efficient Repair of Spinal Cord Injury. ACS Appl. Mater. Interfaces 2024, 16, 4375–4394. [Google Scholar] [CrossRef]
- Seasock, M.J.; Shafiquzzaman, M.; Ruiz-Echartea, M.E.; Kanchi, R.S.; Tran, B.T.; Simon, L.M.; Meyer, M.D.; Erice, P.A.; Lotlikar, S.L.; Wenlock, S.C.; et al. Let-7 restrains an oncogenic circuit in AT2 cells to prevent fibrogenic cell intermediates in pulmonary fibrosis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Elliot, S.; Periera-Simon, S.; Xia, X.; Catanuto, P.; Rubio, G.; Shahzeidi, S.; El Salem, F.; Shapiro, J.; Briegel, K.; Korach, K.S.; et al. MicroRNA let-7 Downregulates Ligand-Independent Estrogen Receptor-mediated Male-Predominant Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1246–1257. [Google Scholar] [CrossRef]
- Chelladurai, P.; Kuenne, C.; Bourgeois, A.; Guenther, S.; Valasarajan, C.; Cherian, A.V.; Rottier, R.J.; Romanet, C.; Weigert, A.; Boucherat, O.; et al. Epigenetic reactivation of transcriptional programs orchestrating fetal lung development in human pulmonary hypertension. Sci. Transl. Med. 2022, 14, eabe5407. [Google Scholar] [CrossRef]
- Jolly, A.J.; Lu, S.; Dubner, A.M.; Strand, K.A.; Mutryn, M.F.; Pilotti-Riley, A.; Danis, E.P.; Nemenoff, R.A.; Moulton, K.S.; Majesky, M.W.; et al. Redistribution of the chromatin remodeler Brg1 directs smooth muscle-derived adventitial progenitor-to-myofibroblast differentiation and vascular fibrosis. JCI Insight 2023, 8, e164862. [Google Scholar] [CrossRef]
- Sha, J.-M.; Zhang, R.-Q.; Wang, X.-C.; Zhou, Y.; Song, K.; Sun, H.; Tu, B.; Tao, H. Epigenetic reader MeCP2 repressed WIF1 boosts lung fibroblast proliferation, migration and pulmonary fibrosis. Toxicol. Lett. 2023, 381, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Oakley, F.; Akiboye, F.; Elsharkawy, A.; Thorne, A.W.; Mann, D.A. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: Implications for wound healing and fibrogenesis. Cell Death Differ. 2007, 14, 275–285. [Google Scholar] [CrossRef]
- Pinese, C.; Lin, J.; Milbreta, U.; Li, M.; Wang, Y.; Leong, K.W.; Chew, S.Y. Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 2018, 76, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Tang, S.; Tang, W.; Mosley, D.D.; Yu, A.; Sil, D.; Romanova, S.; Bailey, K.L.; Knoell, D.L.; Wyatt, T.A.; et al. Perfluorocarbon Nanoemulsions Enhance Therapeutic siRNA Delivery in the Treatment of Pulmonary Fibrosis. Adv. Sci. 2022, 9, 2103676. [Google Scholar] [CrossRef]
- Qu, D.; Zhu, J.P.; Childs, H.R.; Lu, H.H. Nanofiber-based transforming growth factor-β3 release induces fibrochondrogenic differentiation of stem cells. Acta Biomater. 2019, 93, 111–122. [Google Scholar] [CrossRef]
- Wang, Z.; Song, X.; Cui, Y.; Cheng, K.; Tian, X.; Dong, M.; Liu, L. Silk fibroin H-fibroin/poly(ε-caprolactone) core-shell nanofibers with enhanced mechanical property and long-term drug release. J. Colloid Interface Sci. 2021, 593, 142–151. [Google Scholar] [CrossRef]
- Killaars, A.R.; Grim, J.C.; Walker, C.J.; Hushka, E.A.; Brown, T.E.; Anseth, K.S. Extended Exposure to Stiff Microenvironments Leads to Persistent Chromatin Remodeling in Human Mesenchymal Stem Cells. Adv. Sci. 2019, 6, 1801483. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, T. Histone demethylase KDM3B mediates matrix stiffness-induced osteogenic differentiation of adipose-derived stem cells. Arch. Biochem. Biophys. 2024, 757, 110028. [Google Scholar] [CrossRef]
- Muniyandi, P.; Palaninathan, V.; Mizuki, T.; Mohamed, M.S.; Hanajiri, T.; Maekawa, T. Scaffold mediated delivery of dual miRNAs to transdifferentiate cardiac fibroblasts. Mater. Sci. Eng. C 2021, 128, 112323. [Google Scholar] [CrossRef]
- Yuan, T.; Wu, M.; Zhu, C.; Yu, H.; Pham, M.D.; Bottermann, K.; Mao, Y.; Wang, Y.; Langner, M.; Peitzsch, M.; et al. Targeting miRNA-1a and miRNA-15b: A Novel Combinatorial Strategy to Drive Adult Cardiac Regeneration. Adv. Sci. 2025, 12, e2414455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; Chen, J.; Li, Q.; Gao, J.; Tan, H.; Zhu, Y.; Wang, Z.; Li, M.; Yang, H.; et al. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef]
- Acharya, N.; Kandel, R.; Roy, P.; Warraich, I.; Singh, K.P. Epigenetic therapeutics attenuate kidney injury and fibrosis by restoring the expression of epigenetically reprogrammed fibrogenic genes and signaling pathways. Eur. J. Pharm. Sci. 2025, 204, 106977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, W.; Guo, C.; Wu, J.; Zhang, S.; Shi, H.; Kwon, S.; Chen, J.; Dong, Z. Hypermethylation leads to the loss of HOXA5, resulting in JAG1 expression and NOTCH signaling contributing to kidney fibrosis. Kidney Int. 2024, 106, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Blagitko-Dorfs, N.; Schlosser, P.; Greve, G.; Pfeifer, D.; Meier, R.; Baude, A.; Brocks, D.; Plass, C.; Luebbert, M. Combination treatment of acute myeloid leukemia cells with DNMT and HDAC inhibitors: Predominant synergistic gene downregulation associated with gene body demethylation. Leukemia 2019, 33, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Brocks, D.; Schmidt, C.R.; Daskalakis, M.; Jang, H.S.; Shah, N.M.; Li, D.; Li, J.; Zhang, B.; Hou, Y.; Laudato, S.; et al. DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats. Nat. Genet. 2017, 49, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sun, Q.; Li, D.; Miao, S.; Chen, S.; Song, L.; Gao, C.; Chen, Y.; Tan, C.; Jiang, Y. Design, synthesis and anticancer potential of NSC-319745 hydroxamic acid derivatives as DNMT and HDAC inhibitors. Eur. J. Med. Chem. 2017, 134, 281–292. [Google Scholar] [CrossRef]
- Wang, Y.; Bruggeman, K.F.; Franks, S.; Gautam, V.; Hodgetts, S.I.; Harvey, A.R.; Williams, R.J.; Nisbet, D.R. Is Viral Vector Gene Delivery More Effective Using Biomaterials? Adv. Healthc. Mater. 2021, 10, e2001238. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhong, Z.; Yao, E.J.; Kiratitanaporn, W.; Suy, M.T.; Chen, S. 3D bioprinting of gene delivery scaffolds with controlled release. Bioprinting 2023, 31, e00270. [Google Scholar] [CrossRef]
- Eltaher, H.M.; Yang, J.; Shakesheff, K.M.; Dixon, J.E. Highly efficient intracellular transduction in three-dimensional gradients for programming cell fate. Acta Biomater. 2016, 41, 181–192. [Google Scholar] [CrossRef]
- Santorelli, M.; Bhamidipati, P.S.; Courte, J.; Swedlund, B.; Jain, N.; Poon, K.; Schildknecht, D.; Kavanagh, A.; Mackrell, V.A.; Sondkar, T.; et al. Control of spatio-temporal patterning via cell growth in a multicellular synthetic gene circuit. Nat. Commun. 2024, 15, 9867. [Google Scholar] [CrossRef]
- Chan, C.J.; Heisenberg, C.-P.; Hiiragi, T. Coordination of Morphogenesis and Cell-Fate Specification in Development. Curr. Biol. 2017, 27, R1024–R1035. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Upagupta, C.; Vierhout, M.; Ayaub, E.; Bellaye, P.S.; Gauldie, J.; Shimbori, C.; Inman, M.; Ask, K.; Kolb, M.R.J. The importance of interventional timing in the bleomycin model of pulmonary fibrosis. Eur. Respir. J. 2020, 55, 1901105. [Google Scholar] [CrossRef]
- Zheng, Q.; Tong, M.; Ou, B.; Liu, C.; Hu, C.; Yang, Y. Isorhamnetin protects against bleomycin-induced pulmonary fibrosis by inhibiting endoplasmic reticulum stress and epithelial-mesenchymal transition. Int. J. Mol. Med. 2019, 43, 117–126. [Google Scholar] [CrossRef]
- Uhl, F.E.; Zhang, F.; Pouliot, R.A.; Uriarte, J.J.; Enes, S.R.; Han, X.; Ouyang, Y.; Xia, K.; Westergren-Thorsson, G.; Malmstrom, A.; et al. Functional role of glycosaminoglycans in decellularized lung extracellular matrix. Acta Biomater. 2020, 102, 231–246. [Google Scholar] [CrossRef]
- Antczak, L.-A.M.; Moore, K.N.; Hendrick, T.E.; Heise, R.L. Binary fabrication of decellularized lung extracellular matrix hybridgels for in vitro chronic obstructive pulmonary disease modeling. Acta Biomater. 2024, 185, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, J.L.; Gard, A.L.; Gerhold, K.A.; Wilcox, E.C.; Liu, A.; Schwan, J.; Le, A.V.; Baevova, P.; Dimitrievska, S.; Zhao, L.; et al. Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine. Biomaterials 2016, 102, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Li, Y.; Zhou, L.; Dan, N.; Min, J.; Chen, Y.; Wang, Y. Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: A comprehensive review. Int. J. Biol. Macromol. 2023, 246, 125672. [Google Scholar] [CrossRef]
- Bilodeau, C.; Shojaie, S.; Goltsis, O.; Wang, J.; Luo, D.; Ackerley, C.; Rogers, I.; Cox, B.; Post, M. TP63 basal cells are indispensable during endoderm differentiation into proximal airway cells on acellular lung scaffolds. NPJ Regen. Med. 2021, 6, 12. [Google Scholar] [CrossRef]
- Evangelista-Leite, D.; Carreira, A.C.O.; Nishiyama, M.Y.; Gilpin, S.E.; Miglino, M.A. The molecular mechanisms of extracellular matrix-derived hydrogel therapy in idiopathic pulmonary fibrosis models. Biomaterials 2023, 302, 122338. [Google Scholar] [CrossRef]
- Savitri, C.; Ha, S.S.; Kwon, J.W.; Kim, S.H.; Kim, Y.-M.; Park, H.M.; Kwon, H.; Ji, M.J.; Park, K. Human Fibroblast-Derived Matrix Hydrogel Accelerates Regenerative Wound Remodeling Through the Interactions with Macrophages. Adv. Sci. 2024, 11, e2305852. [Google Scholar] [CrossRef]
- Zhou, Z.; Bu, Z.; Wang, S.; Yu, J.; Liu, W.; Huang, J.; Hu, J.; Xu, S.; Wu, P. Extracellular matrix hydrogels with fibroblast growth factor 2 containing exosomes for reconstructing skin microstructures. J. Nanobiotechnol. 2024, 22, 438. [Google Scholar] [CrossRef]
- Rosin, N.L.; Winstone, T.M.L.; Kelley, M.; Biernaskie, J.; Dufour, A.; Orton, D.J. Targeted proteomic approach for quantification of collagen type I and type III in formalin-fixed paraffin-embedded tissue. Sci. Rep. 2024, 14, 17769. [Google Scholar] [CrossRef]
- Lipton, A.; Leitzel, K.; Ali, S.M.; Polimera, H.V.; Nagabhairu, V.; Marks, E.; Richardson, A.E.; Krecko, L.; Ali, A.; Koestler, W.; et al. High turnover of extracellular matrix reflected by specific protein fragments measured in serum is associated with poor outcomes in two metastatic breast cancer cohorts. Int. J. Cancer 2018, 143, 3027–3034. [Google Scholar] [CrossRef]
- Milman Krentsis, I.; Zheng, Y.; Rosen, C.; Shin, S.Y.; Blagdon, C.; Shoshan, E.; Qi, Y.; Wang, J.; Yadav, S.K.; Bachar Lustig, E.; et al. Lung cell transplantation for pulmonary fibrosis. Sci. Adv. 2024, 10, eadk2524. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.-Q. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 2022, 8, eabn7162. [Google Scholar] [CrossRef]
- Duran, P.; Sesillo, F.B.; Cook, M.; Burnett, L.; Menefee, S.A.; Do, E.; French, S.; Zazueta-Damian, G.; Dzieciatkowska, M.; Saviola, A.J.; et al. Proregenerative extracellular matrix hydrogel mitigates pathological alterations of pelvic skeletal muscles after birth injury. Sci. Transl. Med. 2023, 15, eabj3138. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Katsura, H.; Fujimura, T.; Ogata, A.; Baba, S.; Yamaoka, A.; Kihara, M.; Abe, T.; Nishimura, O.; Kadota, M.; et al. Autocrine TGF-β-positive feedback in profibrotic AT2-lineage cells plays a crucial role in non-inflammatory lung fibrogenesis. Nat. Commun. 2023, 14, 4956. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; Guo, J.L.; Griffin, M.; Berry, C.E.; Wan, D.C.; Longaker, M.T. Modelling and targeting mechanical forces in organ fibrosis. Nat. Rev. Bioeng. 2024, 2, 305–323. [Google Scholar] [CrossRef]
- Jones, D.L.; Hallstroem, G.F.; Jiang, X.; Locke, R.C.; Evans, M.K.; Bonnevie, E.D.; Srikumar, A.; Leahy, T.P.; Nijsure, M.P.; Boerckel, J.D.; et al. Mechanoepigenetic regulation of extracellular matrix homeostasis via Yap and Taz. Proc. Natl. Acad. Sci. USA 2023, 120, e2211947120. [Google Scholar] [CrossRef]
- Wagner, D.E.; Alsafadi, H.N.; Mitash, N.; Justet, A.; Hu, Q.; Pineda, R.; Staab-Weijnitz, C.; Korfei, M.; Gvazava, N.; Wannemo, K.; et al. Inhibition of epithelial cell YAP-TEAD/LOX signaling attenuates pulmonary fibrosis in preclinical models. Nat. Commun. 2025, 16, 7099. [Google Scholar] [CrossRef]
- Lang, N.J.; Gote-Schniering, J.; Porras-Gonzalez, D.; Yang, L.; De Sadeleer, L.J.; Jentzsch, R.C.; Shitov, V.A.; Zhou, S.; Ansari, M.; Agami, A.; et al. Ex vivo tissue perturbations coupled to single-cell RNA-seq reveal multilineage cell circuit dynamics in human lung fibrogenesis. Sci. Transl. Med. 2023, 15, eadh0908. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, Y.; Wan, Z.; Chiu, M.C.; Huang, J.; Zhang, S.; Zhu, X.; Lan, Q.; Deng, Y.; Zhou, Y.; et al. Human respiratory organoids sustained reproducible propagation of human rhinovirus C and elucidation of virus-host interaction. Nat. Commun. 2024, 15, 10772. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.; Kannan, P.; Snowball, J.; Kofron, M.; Wayman, J.A.; Bridges, J.P.; Miraldi, E.R.; Swarr, D.; Zacharias, W.J. Alveolar epithelial progenitor cells require Nkx2-1 to maintain progenitor-specific epigenomic state during lung homeostasis and regeneration. Nat. Commun. 2023, 14, 8452. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.R.; Youngblood, R.L.; Oakes, R.S.; Kasputis, T.; Clough, D.W.; Spence, J.R.; Shea, L.D. Human lung organoids develop into adult airway-like structures directed by physico-chemical biomaterial properties. Biomaterials 2020, 234, 119757. [Google Scholar] [CrossRef]
- Chan, L.L.Y.; Anderson, D.E.; Cheng, H.S.; Ivan, F.X.; Chen, S.; Kang, A.E.Z.; Foo, R.; Gamage, A.M.; Tiew, P.Y.; Koh, M.S.; et al. The establishment of COPD organoids to study host-pathogen interaction reveals enhanced viral fitness of SARS-CoV-2 in bronchi. Nat. Commun. 2022, 13, 7635. [Google Scholar] [CrossRef]
- Ritzau-Reid, K.I.; Callens, S.J.P.; Xie, R.; Cihova, M.; Reumann, D.; Grigsby, C.L.; Prados-Martin, L.; Wang, R.; Moore, A.C.; Armstrong, J.P.K.; et al. Microfibrous Scaffolds Guide Stem Cell Lumenogenesis and Brain Organoid Engineering. Adv. Mater. 2023, 35, e2300305. [Google Scholar] [CrossRef]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef]
- Brassard, J.A.; Nikolaev, M.; Huebscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Chen, N.; Fan, Y.; Xu, Y.; Xu, X. Applications of lung cancer organoids in precision medicine: From bench to bedside. Cell Commun. Signal. 2023, 21, 350. [Google Scholar] [CrossRef]
- Lehmann, M.; Krishnan, R.; Sucre, J.; Kulkarni, H.S.; Pineda, R.H.; Anderson, C.; Banovich, N.E.; Behrsing, H.P.; Dean, C.H.; Haak, A.; et al. Precision-Cut Lung Slices: Emerging Tools for Preclinical and Translational Lung Research An Official American Thoracic Society Workshop Report. Am. J. Respir. Cell Mol. Biol. 2025, 72, 16–31. [Google Scholar] [CrossRef]
- Eom, S.; Lee, S.Y.; Park, J.T.; Choi, I. Alveoli-Like Multifunctional Scaffolds for Optical and Electrochemical In Situ Monitoring of Cellular Responses from Type II Pneumocytes. Adv. Sci. 2023, 10, 2301395. [Google Scholar] [CrossRef]
- Gao, H.; Peng, W.; Zhou, Y.; Ding, Z.; Su, M.; Wu, Z.; Yu, C. Flexible and multi-functional three-dimensional scaffold based on enokitake-like Au nanowires for real-time monitoring of endothelial mechanotransduction. Biosens. Bioelectron. 2024, 263, 116610. [Google Scholar] [CrossRef]
- Di Gregorio, E.; Bitonto, V.; Baroni, S.; Stefania, R.; Aime, S.; Broche, L.M.; Senn, N.; Ross, P.J.; Lurie, D.J.; Geninatti Crich, S. Monitoring tissue implants by field-cycling 1H-MRI via the detection of changes in the 14N-quadrupolar-peak from imidazole moieties incorporated in a “smart” scaffold material. J. Mater. Chem. B 2021, 9, 4863–4872. [Google Scholar] [CrossRef]
- Huang, Q.; Qu, Y.; Tang, M.; Lan, K.; Zhang, Y.; Chen, S.; Li, W.; Gu, L. ROS-responsive hydrogel for bone regeneration: Controlled dimethyl fumarate release to reduce inflammation and enhance osteogenesis. Acta Biomater. 2025, 195, 183–200. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Xiao, J.; Yang, R.; Feng, L.; Xu, H.; Xu, L.; Xing, Y. ROS-responsive injectable hydrogels loaded with exosomes carrying miR-4500 reverse liver fibrosis. Biomaterials 2025, 314, 122887. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kim, J. ROS-Scavenging Therapeutic Hydrogels for Modulation of the Inflammatory Response. ACS Appl. Mater. Interfaces 2022, 14, 23002–23021. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Z.; Liu, Y.; Zhan, Z.; Yang, L.; Wang, C.; Jiang, Q.; Ran, H.; Li, P.; Wang, Z. ROS-responsive liposomes as an inhaled drug delivery nanoplatform for idiopathic pulmonary fibrosis treatment via Nrf2 signaling. J. Nanobiotechnol. 2022, 20, 213. [Google Scholar] [CrossRef]
- Wang, B.; Gao, Y.; Sun, L.; Xue, M.; Wang, M.; Zhang, Z.; Zhang, L.; Zhang, H. Inhaled pulmonary surfactant biomimetic liposomes for reversing idiopathic pulmonary fibrosis through synergistic therapeutic strategy. Biomaterials 2023, 303, 122404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Yu, C.; Bao, H.; Cheng, S.; Huang, J.; Zhang, Z. ROS-Responsive Janus Au/Mesoporous Silica Core/Shell Nanoparticles for Drug Delivery and Long-Term CT Imaging Tracking of MSCs in Pulmonary Fibrosis Treatment. ACS Nano 2023, 17, 6387–6399. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, e2000713. [Google Scholar] [CrossRef]
- Lai, S.-M.; Jiang, S.-Y.F.; Chou, H.-C.; Lin, T.-Y.; Wei, Y.-E.; Yu, B.-Y. Novel two-way multiple shape memory effects of olefin block copolymer (OBC)/polycaprolactone (PCL) blends. Polym. Test. 2021, 102, 107333. [Google Scholar] [CrossRef]
- Gopinath, S.; Adarsh, N.N.; Nair, P.R.; Mathew, S. Shape-Memory Polymer Nanocomposites of Poly(ε-caprolactone) with the Polystyrene-block-polybutadiene-block-polystyrene-tri-block Copolymer Encapsulated with Metal Oxides. ACS Omega 2021, 6, 6261–6273. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pan, C.; Zhang, L.; Yue, D. Bio-Based, Self-Healing, Recyclable, Reconfigurable Multifunctional Polymers with Both One-Way and Two-Way Shape Memory Properties. ACS Appl. Mater. Interfaces 2023, 15, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yue, H.; Huang, M.; Hao, C.; He, S.; Liu, H.; Liu, W.; Zhu, C.; Dong, X.; Wang, D. Arbitrarily Reconfigurable and Thermadapt Reversible Two-Way Shape Memory Poly(thiourethane) Accomplished by Multiple Dynamic Covalent Bonds. ACS Appl. Mater. Interfaces 2021, 13, 43426–43437. [Google Scholar] [CrossRef]
- Chen, G.; Abdeen, A.A.; Wang, Y.; Shahi, P.K.; Robertson, S.; Xie, R.; Suzuki, M.; Pattnaik, B.R.; Saha, K.; Gong, S. A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nat. Nanotechnol. 2019, 14, 974–980. [Google Scholar] [CrossRef]
- Wang, C.; Vazquez-Gonzalez, M.; Fadeev, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Thermoplasmonic-Triggered Release of Loads from DNA-Modified Hydrogel Microcapsules Functionalized with Au Nanoparticles or Au Nanorods. Small 2020, 16, e2000880. [Google Scholar] [CrossRef]
- Metzger, J.M.; Wang, Y.; Neuman, S.S.; Snow, K.J.; Murray, S.A.; Lutz, C.M.; Bondarenko, V.; Felton, J.; Gimse, K.; Xie, R.; et al. Efficient in vivo neuronal genome editing in the mouse brain using nanocapsules containing CRISPR-Cas9 ribonucleoproteins. Biomaterials 2023, 293, 121959. [Google Scholar] [CrossRef]
- Seem, K.; Kaur, S.; Kumar, S.; Mohapatra, T. Epigenome editing for targeted DNA (de)methylation: A new perspective in modulating gene expression. Crit. Rev. Biochem. Mol. Biol. 2024, 59, 69–98. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.K.; Yim, H. CRISPR-mediated promoter de/methylation technologies for gene regulation. Arch. Pharmacal Res. 2020, 43, 705–713. [Google Scholar] [CrossRef]
- O’Geen, H.; Tomkova, M.; Combs, J.A.; Tilley, E.K.; Segal, D.J. Determinants of heritable gene silencing for KRAB-dCas9+DNMT3 and Ezh2-dCas9+DNMT3 hit-and-run epigenome editing. Nucleic Acids Res. 2022, 50, 3239–3253. [Google Scholar] [CrossRef]
- Policarpi, C.; Munafo, M.; Tsagkris, S.; Carlini, V.; Hackett, J.A. Systematic epigenome editing captures the context-dependent instructive function of chromatin modifications. Nat. Genet. 2024, 56, 1168–1180. [Google Scholar] [CrossRef]
- McCutcheon, S.R.; Rohm, D.; Iglesias, N.; Gersbach, C.A. Epigenome editing technologies for discovery and medicine. Nat. Biotechnol. 2024, 42, 1199–1217. [Google Scholar] [CrossRef]
- Roth, G.V.; Gengaro, I.R.; Qi, L.S. Precision epigenetic editing: Technological advances, enduring challenges, and therapeutic applications. Cell Chem. Biol. 2024, 31, 1422–1446. [Google Scholar] [CrossRef]
- Li, M.; Chen, F.; Yang, Q.; Tang, Q.; Xiao, Z.; Tong, X.; Zhang, Y.; Lei, L.; Li, S. Biomaterial-Based CRISPR/Cas9 Delivery Systems for Tumor Treatment. Biomater. Res. 2024, 28, 0023. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 systems: Delivery technologies and biomedical applications. Asian J. Pharm. Sci. 2023, 18, 100854. [Google Scholar] [CrossRef]
- Sun, L.; Fan, M.; Huang, D.; Li, B.; Xu, R.; Gao, F.; Chen, Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials 2021, 271, 120761. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zhang, X.; Zhou, D.; Xie, R.; Cai, Y.; Zhang, K.; Sun, X. Inhaled nano-based therapeutics for pulmonary fibrosis: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 215. [Google Scholar] [CrossRef]
- Chang, X.; Xing, L.; Wang, Y.; Yang, C.-X.; He, Y.-J.; Zhou, T.-J.; Gao, X.-D.; Li, L.; Hao, H.-P.; Jiang, H.L. Monocyte-derived multipotent cell delivered programmed therapeutics to reverse idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba3167. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Abrahamse, H. Optimizing CRISPR/Cas9 precision: Mitigating off-target effects for safe integration with photodynamic and stem cell therapies in cancer treatment. Biomed. Pharmacother. 2024, 180, 117516. [Google Scholar] [CrossRef]
- McDonald, S.M.; Augustine, E.K.; Lanners, Q.; Rudin, C.; Brinson, L.C.; Becker, M.L. Applied machine learning as a driver for polymeric biomaterials design. Nat. Commun. 2023, 14, 4838. [Google Scholar] [CrossRef]
- Suwardi, A.; Wang, F.K.; Xue, K.; Han, M.Y.; Teo, P.L.; Wang, P.; Wang, S.J.; Liu, Y.; Ye, E.Y.; Li, Z.B.; et al. Machine Learning-Driven Biomaterials Evolution. Adv. Mater. 2022, 34, 2102703. [Google Scholar] [CrossRef]
- Webb, M.A.; Patel, R.A. Data-Driven Design of Polymer-Based Biomaterials: High-throughput Simulation, Experimentation, and Machine Learning. ACS Appl. Bio Mater. 2023, 7, 510–527. [Google Scholar]
- Kappe, K.; Hoschke, K.; Riedel, W.; Hiermaier, S. Multi-objective optimization of additive manufactured functionally graded lattice structures under impact. Int. J. Impact Eng. 2024, 183, 104789. [Google Scholar] [CrossRef]
- Peng, B.; Wei, Y.; Qin, Y.; Dai, J.B.; Li, Y.; Liu, A.B.; Tian, Y.; Han, L.L.; Zheng, Y.F.; Wen, P. Machine learning-enabled constrained multi-objective design of architected materials. Nat. Commun. 2023, 14, 6630. [Google Scholar] [CrossRef]
- Reel, P.S.; Reel, S.; Pearson, E.; Trucco, E.; Jefferson, E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol. Adv. 2021, 49, 107739. [Google Scholar] [CrossRef]
- Briscik, M.; Tazza, G.; Vidács, L.; Dillies, M.A.; Déjean, S. Supervised multiple kernel learning approaches for multi-omics data integration. BioData Min. 2024, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Virijevicc, K.; Zivanovic, M.N.; Nikolic, D.; Milivojevic, N.; Pavic, J.; Moric, I.; Scenerovic, L.; Dragacevic, L.; Thurner, P.J.; Rufin, M.; et al. AI-Driven Optimization of PCL/PEG Electrospun Scaffolds for Enhanced In Vivo Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 22989–23002. [Google Scholar]
- Rafieyan, S.; Ansari, E.; Vasheghani-Farahani, E. A practical machine learning approach for predicting the quality of 3D (bio)printed scaffolds. Biofabrication 2024, 16, 045014. [Google Scholar] [CrossRef]
- Koudstaal, T.; Funke-Chambour, M.; Kreuter, M.; Molyneaux, P.L.; Wijsenbeek, M.S. Pulmonary fibrosis: From pathogenesis to clinical decision-making. Trends Mol. Med. 2023, 29, 1076–1087. [Google Scholar] [CrossRef]
- Chianese, M.; Screm, G.; Salton, F.; Confalonieri, P.; Trotta, L.; Barbieri, M.; Ruggero, L.; Mari, M.; Reccardini, N.; Geri, P.; et al. Pirfenidone and Nintedanib in Pulmonary Fibrosis: Lights and Shadows. Pharmaceuticals 2024, 17, 709. [Google Scholar] [CrossRef]
- Vancheri, C.; Kreuter, M.; Richeldi, L.; Ryerson, C.J.; Valeyre, D.; Grutters, J.C.; Wiebe, S.; Stansen, W.; Quaresma, M.; Stowasser, S.; et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis Results of the INJOURNEY Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 356–363. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Lei, B. Multifunctional MXene-Based Bioactive Materials for Integrated Regeneration Therapy. ACS Nano 2023, 17, 19526–19549. [Google Scholar] [CrossRef] [PubMed]
- Doryab, A.; Schmid, O. Bioactive Cell-Derived ECM Scaffold Forms a Unique Cellular Microenvironment for Lung Tissue Engineering. Biomedicine 2022, 10, 1791. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-T.; Liu, Y.-S.; Lee, Y.-h.; Rimando, M.G.; Lin, K.-h.; Lee, O.K. Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells. Acta Biomater. 2016, 32, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Walsh, D.P.; Ferreras, L.B.; Castano, I.M.; Chen, G.; LeMoine, M.; Osman, G.; Shakesheff, K.M.; Dixon, J.E.; O’Brien, F.J. Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: From development to application in tissue engineering. Biomaterials 2019, 216, 119277. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Tu, T.; Schmull, S.; Han, Y.; Wang, W.; Li, H. Dual inhibition of HDAC and tyrosine kinase signaling pathways with CUDC-907 attenuates TGFβ1 induced lung and tumor fibrosis. Cell Death Dis. 2020, 11, 765. [Google Scholar] [CrossRef]
- Liang, H.; Wang, Q.; Wang, D.; Zheng, H.; Kalvakolanu, D.V.; Lu, H.; Wen, N.; Chen, X.; Xu, L.; Ren, J.; et al. RGFP966, a histone deacetylase 3 inhibitor, promotes glioma stem cell differentiation by blocking TGF-β signaling via SMAD7 (vol 180, 114118, 2020). Biochem. Pharmacol. 2020, 182, 114223. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lee, H.-S.; Liou, J.-P.; Hua, H.-S.; Cheng, W.-H.; Yuliani, F.S.; Chen, B.-C.; Lin, C.-H. MPT0E028, a novel pan-HDAC inhibitor, prevents pulmonary fibrosis through inhibition of TGF-β-induced CTGF expression in human lung fibroblasts: Involvement of MKP-1 activation. Eur. J. Pharmacol. 2024, 977, 176711. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Sumo, S.; Bayrak, E.S.; Brey, E.M.; Cinar, A. Three-dimensional modeling of angiogenesis in porous biomaterial scaffolds. Biomaterials 2013, 34, 2875–2887. [Google Scholar] [CrossRef]
- Pupiute, A.; Ciuzas, D.; Baniukaitiene, O.; Tichonovas, M.; Martuzevicius, D.; Petrikaite, V.; Krugly, E. Development of two layer fibrous scaffolds for 3D in vitro modelling: Effects of morphology and surface properties on cell proliferation, adhesion and drug sensitivity. J. Drug Deliv. Sci. Technol. 2024, 101, 106213. [Google Scholar] [CrossRef]
- Jing, L.; Wang, X.; Leng, B.; Zhan, N.; Liu, H.; Wang, S.; Lu, Y.; Sun, J.; Huang, D. Engineered Nanotopography on the Microfibers of 3D-Printed PCL Scaffolds to Modulate Cellular Responses and Establish an In Vitro Tumor Model. ACS Appl. Bio Mater. 2021, 4, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.; McCarthy, H.O.; Buchanan, P.; Levingstone, T.J.; Dunne, N.J. Development and characterisation of 3D collagen-gelatin based scaffolds for breast cancer research. Biomater. Adv. 2022, 142, 213157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, A. Mechanotransduction-Epigenetic Coupling in Pulmonary Regeneration: Multifunctional Bioscaffolds as Emerging Tools. Pharmaceuticals 2025, 18, 1487. https://doi.org/10.3390/ph18101487
Wang J, Xu A. Mechanotransduction-Epigenetic Coupling in Pulmonary Regeneration: Multifunctional Bioscaffolds as Emerging Tools. Pharmaceuticals. 2025; 18(10):1487. https://doi.org/10.3390/ph18101487
Chicago/Turabian StyleWang, Jing, and Anmin Xu. 2025. "Mechanotransduction-Epigenetic Coupling in Pulmonary Regeneration: Multifunctional Bioscaffolds as Emerging Tools" Pharmaceuticals 18, no. 10: 1487. https://doi.org/10.3390/ph18101487
APA StyleWang, J., & Xu, A. (2025). Mechanotransduction-Epigenetic Coupling in Pulmonary Regeneration: Multifunctional Bioscaffolds as Emerging Tools. Pharmaceuticals, 18(10), 1487. https://doi.org/10.3390/ph18101487





