Flavonoid-Based Combination Therapies and Nano-Formulations: An Emerging Frontier in Breast Cancer Treatment
Abstract
1. Introduction
2. Flavonoids as Natural Therapeutics: Mechanisms and Applications
2.1. Structural Insights into the Chemistry of Flavonoids
2.2. Nature’s Reservoir: Sources of Flavonoids
2.3. Overcoming the Challenges: Bioavailability and Metabolism
3. Epidemiological Studies: Role of Flavonoids in Breast Cancer Prevention
4. Unlocking the Chemotherapeutic Potential of Flavonoids in Breast Cancer Treatment
| Flavonoid | In Vitro/In Vivo | Breast Cancer Cell Lines | Effects | References |
|---|---|---|---|---|
| Hesperidin; Apigenin; Quercetin (Propolis) | In vitro | MCF-7 | Accumulation in G0/G1 phase, cell cycle, proliferation, apoptosis | [115,116] |
| Quercetin | In vitro | MCF-7Ca/TAM-R | Increase in ERα and inhibition of HER2 | [117] |
| Nude/MCF-7 | MCF-7 | Inhibition of von Willebrand Factor (vWF); suppression of calcineurin activity; tumor microvessel density modulation; decrease in VEGF/VEGFR2 signaling and NFAT activation. | [118] | |
| Hesperidin | In vitro | MCF-7 | Increase apoptosis via G0/G1 phase arrest, caspase-3 and caspase-9 upregulation, increase BAX activation, and inhibit BCL-2 expression. | [119] |
| Luteolin | In vitro | MCF-7 | Increase apoptosis via G0/G1 phase arrest, caspase-8 and caspase-9 upregulation and inhibit BCL-2, pAKT, pIGF-1R, Erα expression. | [119] |
| Nude/T47D | T47D | VEGF secretion and mRNA expression, tumor microvessel density, tumor-specific VEGF expression, and BAX levels | [120,121] | |
| Nobiletin | In vitro | MCF-7 | Increase in CYP1 enzyme activity, elevation of CYP1A1 protein expression, upregulation of CYP1B1 mRNA levels, and G1 cell cycle arrest. | [122] |
| Eupatorin | In vitro | MCF-7 | Increased apoptosis in G2/M phase; enhanced activity of BAX, caspase-9, and caspase-8; modulation of RAF-1 and inhibition of VEGFA, BCL2L11, CHK1, CHK2, HIF1A, and AKT | [123] |
| Xanthohumol | In vitro | MCF-7 | Enhance apoptosis during G1 phase arrest while reducing the levels of pAKT (S473); pMAPK (T202/Y204); and phosphorylated ERα at multiple sites (S104/S106, S118, S167, S305, Y537). | [124] |
| Silibinin | In vitro | MCF-7, T47D | Enhanced BAX expression; mitochondrial cytochrome c release; nuclear translocation of AIF; induction of autophagy; activation of caspase-8, and reduced BCL-2 expression; along with modulation of ERα and ERβ activity. | [125,126] |
| Kaempferol | Nude/MCF-7 | MCF-7 | Increase cleaved PARP; BAX and downregulation of BCL-2, pAKT, pMEK1/2, pERK1/2 and pIRS-1. | [127,128] |
| Chalcone; Licochalcone A | In vitro | MCF-7 | Induce plasma membrane damage; BAX upregulation; cleaved PARP, and CIDEA; downregulate G2/M and S cell cycle phases, cyclin-D1, and BCL-2. | [129] |
| LW-214 (flavone) | Nude/MCF-7 | MCF-7 | Enhance BAX expression; cleaved PARP, caspase-9, ROS generation; mitochondrial cytochrome c release; nuclear translocation of AIF, pJNK, and pASK1 levels; reducing BCL-2 and TRX-1 expression. | [130] |
| NSC 686288 (flavone) | In vitro | MCF-7 | Increase cleaved PARP, caspase-9, and ROS levels while decreasing AhR signaling along with the expression of CYP1A1 and CYP1B1. | [131] |
| 2′-Nitroflavone | In vitro | MCF-7 | Cytotoxicity | [132] |
| Pentamethoxylated-flavone | In vitro | MCF-7 | Alters the expression of the BCL-2 protein and promotes cell death. | [133] |
| Puerarin | In vitro | MCF-7, LPS | Decrease NF-κB p65, MMP-9; MMP-2; CCR7; CXCR4; VCAM-1; ICAM-1; TNFα; IL-6; pNF-κB p65; pIκBα; pERK1/2 Downregulate NF-κB MMP-9, p65, CCR7, MMP-2, VCAM-1, TNFα, CXCR4, ICAM-1, pIκBα, p65, IL-6, pNF-κB and pERK1/2. | [134] |
| Calycosin | In vitro | MCF-7, T47D | Decrease FOXP3; MMP-9; VEGF, MMP-9; | [135] |
| Orientin | In vitro | MCF-7, TPA | Reduce IL-8 levels; PKCα membrane translocation; pERK activation, and nuclear translocation of c-JUN, c-FOS, and STAT3. | [136] |
| Corylin | In vitro | MCF-7 | Increase miR-34c and decrease LINC00963 mRNAMMP-9; cytotoxicity | [137] |
| Hinokiflavone | In vitro | MCF-7 | Decrease MMP-9; cytotoxicity | [138] |
| 3,6-Dihydroxy flavone | In vitro | MCF-7 | Upregulation of E-cadherin with downregulation of SNAIL, TWIST, SLUG, N-cadherin, NOTCH1, and NICD. | [139] |
| LFG-500 (Flavone) | MMTV-PyMT transgenic mice | MCF-7 | Upregulation ZO-1; E-cadherin; pYAP; pMST1/2; pLATS1/2 and reduction in N-cadherin; vimentin; SLUG; SNAIL; YAP; ILK | [140] |
| Hispidulin | In vitro | MCF-7 | Upregulation of E-cadherin and downregulation of occludin; pSMAD2/3 | [141] |
| Calycosin | In vitro | Nude/T47D | Upregulation of E-cadherin and downregulation of N-cadherin, vimentin, CD147, MMP-2, MMP-9, and BATF. | [142] |
| 2′-Hydroxy flavanone | Nude/MCF-7 | MCF-7 | Upregulation of E-cadherin and downregulation of vimentin, along with modulation of RLIP76 and ERα expression. | [143,144] |
| Kaempferol | In vitro | MCF-7 | Upregulation of E-cadherin and downregulation of N-cadherin, SNAIL, SLUG, cathepsin D, MMP-9, and MMP-2. | [127,128] |
| Wogonoside | Nude/MCF-7 | MCF-7 | Downregulation of VEGF expression; inhibition of VEGF promoter activity; suppression of endothelial cell (EC) migration; reduction in EC invasion, and impairment of tubulogenesis. | [145] |
| Jaceidin | Swiss albino /Ehrlich Ascites Carcinoma cells | MCF-7 | serum VEGF | [146] |
5. Synergizing Flavonoids with Synthetic Drugs in Combination Therapy
6. Advancement in Flavonoid Nano-Formulations and Codelivery Strategies in Breast Cancer Prevention
7. Safety, Toxicity, and Regulatory Aspects of Flavonoid-Based Nanoformulations
8. Conclusion and Future Perspectives: Advancing Flavonoid Research in Cancer Therapy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 22 October 2024).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: Singapore, 2020; ISBN 978-0-429-39903-9. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, M. Mutual Interactions between Flavonoids and Enzymatic and Transporter Elements Responsible for Flavonoid Disposition via Phase II Metabolic Pathways. RSC Adv. 2012, 2, 7948–7963. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. Flavonoids in Cancer Prevention. Available online: http://www.eurekaselect.com (accessed on 10 November 2024).
- Hazafa, A.; Rehman, K.-U.-; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Luqman, S.; Meena, A. Research Progress in Flavonoids as Potential Anticancer Drug Including Synergy with Other Approaches. Curr. Top. Med. Chem. 2020, 20, 1791–1809. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA Nanoparticles Containing Various Anticancer Agents and Tumour Delivery by EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Bhatia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–93. ISBN 978-3-319-41129-3. [Google Scholar]
- Mohapatra, P.; Singh, P.; Singh, D.; Sahoo, S.; Sahoo, S.K. Phytochemical based nanomedicine: A panacea for cancer treatment, present status and future prospective. OpenNano 2022, 7, 100055. [Google Scholar] [CrossRef]
- Mohapatra, P.; Singh, P.; Sahoo, S.K. Phytonanomedicine: A novel avenue to treat recurrent cancer by targeting cancer stem cells. Drug Discov. Today 2020, 25, 1307–1321. [Google Scholar] [CrossRef]
- Sharma, T.; Singh, D.; Mahapatra, A.; Mohapatra, P.; Sahoo, S.; Sahoo, S.K. Advancements in clinical translation of flavonoid nanoparticles for cancer treatment. OpenNano 2022, 8, 100074. [Google Scholar] [CrossRef]
- Morsy, H.M.; Zaky, M.Y.; Yassin, N.Y.S.; Khalifa, A.Y.Z. Nanoparticle-based flavonoid therapeutics: Pioneering biomedical applications in antioxidants, cancer treatment, cardiovascular health, neuroprotection, and cosmeceuticals. Int. J. Pharm. 2025, 670, 125135. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pallauf, K.; Duckstein, N.; Rimbach, G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 2017, 76, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Lakshman, K.; Lakshman, K.; Lakshman, K. Role of Polyphenols and Flavonoids as Anti-Cancer Drug Candidates: A Review. Pharmacogn. Res. 2023, 15, 206–216. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Jucá, M.M.; Cysne Filho, F.M.; de Almeida, J.C.; Mesquita, D.D.; Barriga, J.R.; Dias, K.C.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Product Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Azad, A.K.; Dayoob, M.; Zohera, F.T. Anticancer Activity of Flavonoids: Past, Present, and Future. In Advances in Medical Diagnosis, Treatment, and Care; Roy, A., Ed.; IGI Global: Hershey, PA, USA, 2024; pp. 1–21. ISBN 9798369316467. [Google Scholar]
- Tovar-Pérez, E.G.; Aguilera-Aguirre, S.; López-García, U.; Valdez-Morales, M.; Ibarra-Zurita, A.K.; Ortiz-Basurto, R.I.; Chacón-López, A. Effect of ultrasound treatment on the quality and contents of polyphenols, lycopene and rutin in tomato fruits. Czech J. Food Sci. 2020, 38, 20–27. [Google Scholar] [CrossRef]
- Wang, R.-S.; Dong, P.-H.; Shuai, X.-X.; Chen, M.-S. Evaluation of Different Black Mulberry Fruits (Morus nigra L.) Based on Phenolic Compounds and Antioxidant Activity. Foods 2022, 11, 1252. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Dettmann, J.; Nimalaratne, C.; Schieber, A. Characterization and Quantification of Polyphenols in Amazon Grape (Pourouma Cecropiifolia Martius). Molecules 2010, 15, 8543–8552. [Google Scholar] [CrossRef]
- Shafi, W.; Mansoor, S.; Jan, S.; Singh, D.B.; Kazi, M.; Raish, M.; Alwadei, M.; Mir, J.I.; Ahmad, P. Variability in Catechin and Rutin Contents and Their Antioxidant Potential in Diverse Apple Genotypes. Molecules 2019, 24, 943. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Patel, D.K. Chapter 26—The Beneficial Role of Rutin, A Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. ISBN 978-0-12-813820-5. [Google Scholar]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Mazzucato, A.; Willems, D.; Bernini, R.; Picarella, M.E.; Santangelo, E.; Ruiu, F.; Tilesi, F.; Soressi, G.P. Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Physiol. Biochem. 2013, 72, 125–133. [Google Scholar] [CrossRef]
- De Araújo, M.E.M.B.; Moreira Franco, Y.E.; Alberto, T.G.; Sobreiro, M.A.; Conrado, M.A.; Priolli, D.G.; Frankland Sawaya, A.C.H.; Ruiz, A.L.T.G.; de Carvalho, J.E.; de Oliveira Carvalho, P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of Rutin to Antiproliferative Quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H. Synergistic Effect of Apple Extracts and Quercetin 3-β-d-Glucoside Combination on Antiproliferative Activity in MCF-7 Human Breast Cancer Cells in Vitro. J. Agric. Food Chem. 2009, 57, 8581–8586. [Google Scholar] [CrossRef]
- Khatri, P.; Chen, L.; Rajcan, I.; Dhaubhadel, S. Functional characterization of Cinnamate 4-hydroxylase gene family in soybean (Glycine max). PLoS ONE 2023, 18, e0285698. [Google Scholar] [CrossRef]
- Davies, K.M. Genetic modification of plant metabolism for human health benefits. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 622, 122–137. [Google Scholar] [CrossRef]
- Ai, J.; Bao, B.; Battino, M.; Giampieri, F.; Chen, C.; You, L.; Cespedes-Acuña, C.L.; Ognyanov, M.; Tian, L.; Bai, W. Recent advances on bioactive polysaccharides from mulberry. Food Funct. 2021, 12, 5219–5235. [Google Scholar] [CrossRef]
- Peng, C.-H.; Lin, H.-T.; Chung, D.-J.; Huang, C.-N.; Wang, C.-J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018, 26, 778–787. [Google Scholar] [CrossRef]
- Demir, S.; Turan, I.; Aliyazicioglu, Y.; Kilinc, K.; Yaman, S.O.; Ayazoglu Demir, E.; Arslan, A.; Mentese, A.; Deger, O. Morus Rubra Extract Induces Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells Through Endoplasmic Reticulum Stress and Telomerase. Nutr. Cancer 2017, 69, 74–83. [Google Scholar] [CrossRef]
- Shang, A.; Luo, M.; Gan, R.-Y.; Xu, X.-Y.; Xia, Y.; Guo, H.; Liu, Y.; Li, H.-B. Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.). Antioxidants 2020, 9, 678. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Liang, J.; Ai, J.-Y.; Cui, J.-L.; Huang, W.-D.; You, Y.-L.; Zhan, J.-C. Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway. Molecules 2022, 27, 4266. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Zhang, X.-H.; Seo, J.-S. Suppression of oxidative stress by grape seed supplementation in rats. Nutr. Res. Pract. 2012, 6, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Kaynar, L.; Koçyiğit, I.; Hacioğlu, S.K.; Saraymen, R.; Oztürk, A.; Orhan, O.; Sağdiç, O. The effect of grape seed extract on radiation-induced oxidative stress in the rat liver. Turk. J. Gastroenterol. 2008, 19, 92–98. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.-W. Quercetin-3-Glucoside Extracted from Apple Pomace Induces Cell Cycle Arrest and Apoptosis by Increasing Intracellular ROS Levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef]
- Pandey, J.; Bastola, T.; Tripathi, J.; Tripathi, M.; Rokaya, R.K.; Dhakal, B.; DC, R.; Bhandari, R.; Poudel, A. Estimation of Total Quercetin and Rutin Content in Malus domestica of Nepalese Origin by HPLC Method and Determination of Their Antioxidative Activity. J. Food Qual. 2020, 2020, 8853426. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, X.; Long, C.; Du, Y.; Li, J.; Yue, J.; Pan, S. Variations of Flavonoid Composition and Antioxidant Properties among Different Cultivars, Fruit Tissues and Developmental Stages of Citrus Fruits. Chem. Biodivers. 2020, 17, e1900690. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- Yao, Q.; Lin, M.-T.; Zhu, Y.-D.; Xu, H.-L.; Zhao, Y.-Z. Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin. Molecules 2018, 23, 2547. [Google Scholar] [CrossRef]
- Moustafa, A.M.Y.; Ahmed, S.H.; Nabil, Z.I.; Hussein, A.A.; Omran, M.A. Extraction and phytochemical investigation of Calotropis procera: Effect of plant extracts on the activity of diverse muscles. Pharm. Biol. 2010, 48, 1080–1190. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Hassanpour, H.; Alijanpour, A. Evaluation of hackberry (Celtis australis L.) fruits as sources of bioactive compounds. Sci. Rep. 2023, 13, 12233. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Masand, N. Chapter 12—Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 401–430. [Google Scholar]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Tanaka, S.; Shinoki, A.; Hara, H. Melibiose, a Nondigestible Disaccharide, Promotes Absorption of Quercetin Glycosides in Rat Small Intestine. J. Agric. Food Chem. 2016, 64, 9335–9341. [Google Scholar] [CrossRef]
- Khodzhaieva, R.S.; Gladkov, E.S.; Kyrychenko, A.; Roshal, A.D. Progress and Achievements in Glycosylation of Flavonoids. Front. Chem. 2021, 9, 637994. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.-Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef]
- Deng, R.; Liu, Y.; Wu, X.; Zhao, N.; Deng, J.; Pan, T.; Cao, L.; Zhan, F.; Qiao, X. Probing the interaction of hesperidin showing antiproliferative activity in colorectal cancer cells and human hemoglobin. Int. J. Biol. Macromol. 2024, 281, 136078. [Google Scholar] [CrossRef]
- Qadir Nanakali, N.M.; Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Sharifi, M.; Asemi, R.; Yousefi, B. The role of dietary polyphenols in alternating DNA methylation in cancer. Crit. Rev. Food Sci. Nutr. 2023, 63, 12256–12269. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, Y.; Pu, F.; Yang, C.; Xiao, X.; Du, H.; He, J.; Lu, S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chem. 2024, 430, 137115. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Ye, Z.; Gao, N.; Xu, X.; Weng, Q.; Xu, R.; Ye, L. Effects of Dietary Flavonoids on the Metabolism of Vortioxetine andits Potential Mechanism. CMC 2024, 31, 3624–3630. [Google Scholar] [CrossRef]
- Lian, X.; Shi, M.; Liang, Y.; Lin, Q.; Zhang, L. The Effects of Unconventional Feed Fermentation on Intestinal Oxidative Stress in Animals. Antioxidants 2024, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gu, K.; Zhou, F. Dietary Flavonoid Intake and Cancer Mortality: A Population-Based Cohort Study. Nutrients 2023, 15, 976. [Google Scholar] [CrossRef]
- Li, M.; Cai, Q.; Gao, Y.-T.; Franke, A.A.; Zhang, X.; Zhao, Y.; Wen, W.; Lan, Q.; Rothman, N.; Shyr, Y.; et al. Phytoestrogens and lung cancer risk: A nested case-control study in never-smoking Chinese women. Am. J. Clin. Nutr. 2022, 115, 643–651. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Wang, X.; Peng, Y.; Wang, P.; Si, C.; Gong, J.; Zhou, H.; Zhang, M.; Chen, L.; et al. Dietary flavonoid intake and risk of hormone-related cancers: A population-based prospective cohort study. Phytomedicine 2024, 133, 155950. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Liu, K.; Wang, Y.; Jiang, Y.; Zhang, C. Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women. Nutrients 2023, 15, 3253. [Google Scholar] [CrossRef]
- Yang, J.; Shen, H.; Mi, M.; Qin, Y. Isoflavone Consumption and Risk of Breast Cancer: An Updated Systematic Review with Meta-Analysis of Observational Studies. Nutrients 2023, 15, 2402. [Google Scholar] [CrossRef]
- Song, S.; Cheun, J.-H.; Moon, H.-G.; Noh, D.-Y.; Jung, S.-Y.; Lee, E.S.; Kim, Z.; Youn, H.J.; Cho, J.; Yoo, Y.B.; et al. Dietary Isoflavone Intake and Breast Cancer Prognosis: A Prospective Analysis and Meta-Analysis. Nutr. Cancer 2024, 76, 42–54. [Google Scholar] [CrossRef]
- Naghshi, S.; Tutunchi, H.; Yousefi, M.; Naeini, F.; Mobarak, S.; Asadi, M.; Sadeghi, O. Soy isoflavone intake and risk of cardiovascular disease in adults: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2024, 64, 6087–6101. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef]
- Feng, X.-L.; Ho, S.C.; Mo, X.-F.; Lin, F.-Y.; Zhang, N.-Q.; Luo, H.; Zhang, X.; Zhang, C.-X. Association between flavonoids, flavonoid subclasses intake and breast cancer risk: A case-control study in China. Eur. J. Cancer Prev. 2020, 29, 493. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Yeo, W.; Goggins, W.; Kwok, C.; Cheng, A.; Chong, M.; Lee, R.; Cheung, K.L. Pre-diagnosis and early post-diagnosis dietary soy isoflavone intake and survival outcomes: A prospective cohort study of early stage breast cancer survivors. Cancer Treat. Res. Commun. 2021, 27, 100350. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Kushwaha, S.; Luqman, S.; Meena, A. Flavonoids as Prospective Aromatase Inhibitors in Breast Cancer Prevention/Therapy. Curr. Mol. Pharmacol. 2021, 14, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Boniface, P.K.; Elizabeth, F.I. Flavones as a Privileged Scaffold in Drug Discovery: Current Developments. Curr. Org. Synth. 2019, 16, 968–1001. [Google Scholar] [CrossRef]
- Nakai, S.; Fujita, M.; Kamei, Y. Health Promotion Effects of Soy Isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef]
- Srinivasan, A.; Thangavel, C.; Liu, Y.; Shoyele, S.; Den, R.B.; Selvakumar, P.; Lakshmikuttyamma, A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 2016, 55, 743–756. [Google Scholar] [CrossRef]
- Mawalizadeh, F.; Mohammadzadeh, G.; Khedri, A.; Rashidi, M. Quercetin potentiates the chemosensitivity of MCF-7 breast cancer cells to 5-fluorouracil. Mol. Biol. Rep. 2021, 48, 7733–7742. [Google Scholar] [CrossRef]
- Widyananda, M.H.; Pratama, S.K.; Ansori, A.N.M.; Antonius, Y.; Kharisma, V.D.; Murtadlo, A.A.A.; Jakhmola, V.; Rebezov, M.; Khayrullin, M.; Derkho, M.; et al. Quercetin as an anticancer candidate for glioblastoma multiforme by targeting AKT1, MMP9, ABCB1, and VEGFA: An in silico study. Karbala Int. J. Mod. Sci. 2023, 9, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Teng, F.; Wu, T.; Li, S.; Li, K. Quercetin inhibits chronic stress-mediated progression of triple-negative breast cancer by blocking β2-AR/ERK1/2 pathway. Biomed. Pharmacother. 2024, 177, 116985. [Google Scholar] [CrossRef]
- Chen, W.-J.; Tsai, J.-H.; Hsu, L.-S.; Lin, C.-L.; Hong, H.-M.; Pan, M.-H. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting IGF1/IGF1R-mediated EMT program. J. Food Drug Anal. 2021, 29, 98. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, F.; Samadi, N.; Mostafavi-Pour, Z. Sequential Therapy of Breast Cancer Cell Lines with Vitamin C and Quercetin Improves the Efficacy of Chemotherapeutic Drugs. Nutr. Cancer 2017, 69, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Pampori, A.; Droutsa, E.; Laskari, M.; Karakostas, P.; Tsalikis, L.; Barmpalexis, P.; Dordas, C.; Assimopoulou, A.N. Occurrence of Luteolin in the Greek Flora, Isolation of Luteolin and Its Action for the Treatment of Periodontal Diseases. Molecules 2023, 28, 7720. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Huang, L.; Yang, F.; Liu, A.; Zhang, J. Combination of lapatinib and luteolin enhances the therapeutic efficacy of lapatinib on human breast cancer through the FOXO3a/NQO1 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 364–371. [Google Scholar] [CrossRef]
- Sato, Y.; Sasaki, N.; Saito, M.; Endo, N.; Kugawa, F.; Ueno, A. Luteolin Attenuates Doxorubicin-Induced Cytotoxicity to MCF-7 Human Breast Cancer Cells. Biol. Pharm. Bull. 2015, 38, 703–709. [Google Scholar] [CrossRef]
- Qin, W.; Guo, J.; Gou, W.; Wu, S.; Guo, N.; Zhao, Y.; Hou, W. Molecular mechanisms of isoflavone puerarin against cardiovascular diseases: What we know and where we go. Chin. Herbal. Med. 2022, 14, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Honglei, Y.; Yun, W.; Sheng, D.; Yun, H.; Anhua, Z.; Na, F.; Min, L.; Dandan, S.; Jing, W.; et al. Puerarin ameliorates myocardial remodeling of spontaneously hypertensive rats through inhibiting TRPC6-CaN-NFATc3 pathway. Eur. J. Pharmacol. 2022, 933, 175254. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Z.; Zhang, Z.; Zhao, C.; Li, J.; Jiang, J.; Huang, B.; Qin, Y. Puerarin inhibits EMT induced by oxaliplatin via targeting carbonic anhydrase XII. Front. Pharmacol. 2022, 13, 969422. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Dou, X.; Zhou, Z.; Ren, R.; Xu, M. Apigenin, flavonoid component isolated from Gentiana veitchiorum flower suppresses the oxidative stress through LDLR-LCAT signaling pathway. Biomed. Pharmacother. 2020, 128, 110298. [Google Scholar] [CrossRef]
- Maashi, M.S.; Al-Mualm, M.; Al-Awsi, G.R.L.; Opulencia, M.J.C.; Al-Gazally, M.E.; Abdullaev, B.; Abdelbasset, W.K.; Ansari, M.J.; Jalil, A.T.; Alsaikhan, F.; et al. Apigenin alleviates resistance to doxorubicin in breast cancer cells by acting on the JAK/STAT signaling pathway. Mol. Biol. Rep. 2022, 49, 8777–8784. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lin, J.; Liu, Y.-E.; Chen, H.-F.; Hsu, K.-W.; Lin, S.-H.; Peng, K.-Y.; Lin, K.-J.; Hsieh, C.-C.; Chen, D.-R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Lin, P.-H.; Chiang, Y.-F.; Shieh, T.-M.; Chen, H.-Y.; Shih, C.-K.; Wang, T.-H.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Li, S.-C.; et al. Dietary Compound Isoliquiritigenin, an Antioxidant from Licorice, Suppresses Triple-Negative Breast Tumor Growth via Apoptotic Death Program Activation in Cell and Xenograft Animal Models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef]
- Zheng, H.; Li, Y.; Wang, Y.; Zhao, H.; Zhang, J.; Chai, H.; Tang, T.; Yue, J.; Guo, A.M.; Yang, J. Downregulation of COX-2 and CYP 4A signaling by isoliquiritigenin inhibits human breast cancer metastasis through preventing anoikis resistance, migration and invasion. Toxicol. Appl. Pharmacol. 2014, 280, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Zhang, Z.-Y.; Li, Y.-P.; Li, D.; Huang, S.-L.; Gu, L.-Q.; Xu, J.; Huang, Z.-S. Syntheses and evaluation of novel isoliquiritigenin derivatives as potential dual inhibitors for amyloid-beta aggregation and 5-lipoxygenase. Eur. J. Med. Chem. 2013, 66, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the Role of Curcumin in Tumour Growth and Angiogenesis in Mouse Model of Human Breast Cancer. BioMed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef] [PubMed]
- Guneydas, G.; Topcul, M.R. Antiproliferative Effects of Curcumin Different Types of Breast Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 911–917. [Google Scholar] [CrossRef]
- Falah, R.R.; Talib, W.H.; Shbailat, S.J. Combination of metformin and curcumin targets breast cancer in mice by angiogenesis inhibition, immune system modulation and induction of p53 independent apoptosis. Ther. Adv. Med. Oncol. 2017, 9, 235–252. [Google Scholar] [CrossRef]
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent developments in formulation design for improving oral bioavailability of curcumin: A review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- KabaÅ‚a-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7—A comparative study. Cell Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Tao, L.; Qi, K.; Zhang, H.; Feng, D.; Wei, W.; Kong, H.; Chen, T.; Lin, Q. Quercetin reverses tamoxifen resistance in breast cancer cells. J. BUON 2015, 20, 707–713. [Google Scholar]
- Zhao, X.; Wang, Q.; Yang, S.; Chen, C.; Li, X.; Liu, J.; Zou, Z.; Cai, D. Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur. J. Pharmacol. 2016, 781, 60–68. [Google Scholar] [CrossRef]
- Lei, H.; Luo, J.; Tong, L.; Peng, L.; Qi, Y.; Jia, Z.; Wei, Q. Quercetin binds to calcineurin at a similar region to cyclosporin A and tacrolimus. Food Chem. 2011, 127, 1169–1174. [Google Scholar] [CrossRef]
- Magura, J.; Moodley, R.; Mackraj, I. The effect of hesperidin and luteolin isolated from Eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2022, 40, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Goyette, S.; Mafuvadze, B.; Hyder, S.M. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. SpringerPlus 2015, 4, 444. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer Targets Ther. 2018, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Androutsopoulos, V.P.; Sifakis, S.; Koutala, E.; Tsatsakis, A.; Arroo, R.R.J.; Boarder, M.R. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem. Toxicol. 2012, 50, 3320–3328. [Google Scholar] [CrossRef]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W.; Alitheen, N.B.; Long, K.; Yeap, S.K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019, 9, 1514. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Komatsu, M.; Tashiro, E.; Imoto, M.; Osada, H.; Miyoshi, Y.; Honda, J.; Sasa, M.; Katagiri, T. Xanthohumol suppresses oestrogen-signalling in breast cancer through the inhibition of BIG3-PHB2 interactions. Sci. Rep. 2014, 4, 7355. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, A.; Balakrishnan, S.; Kushwaha, H.S.; Mishra, K.P. Silibinin-Induced Apoptosis in MCF7 and T47D Human Breast Carcinoma Cells Involves Caspase-8 Activation and Mitochondrial Pathway. Cancer Investig. 2011, 29, 12–20. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Sun, Y.; Si, L.; Fu, J.; Hayashi, T.; Onodera, S.; Ikejima, T. Estrogen receptors participate in silibinin-caused nuclear translocation of apoptosis-inducing factor in human breast cancer MCF-7 cells. Arch. Biochem. Biophys. 2020, 689, 108458. [Google Scholar] [CrossRef]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in MCF-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hwang, K.-A.; Choi, K.-C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef]
- Bortolotto, L.F.B.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef]
- Pan, D.; Li, W.; Miao, H.; Yao, J.; Li, Z.; Wei, L.; Zhao, L.; Guo, Q. LW-214, a newly synthesized flavonoid, induces intrinsic apoptosis pathway by down-regulating Trx-1 in MCF-7 human breast cells. Biochem. Pharmacol. 2014, 87, 598–610. [Google Scholar] [CrossRef]
- Loaiza-Pérez, A.I.; Kenney, S.; Boswell, J.; Hollingshead, M.; Alley, M.C.; Hose, C.; Ciolino, H.P.; Yeh, G.C.; Trepel, J.B.; Vistica, D.T.; et al. Aryl hydrocarbon receptor activation of an antitumor aminoflavone: Basis of selective toxicity for MCF-7 breast tumor cells. Mol. Cancer Ther. 2004, 3, 715–725. [Google Scholar] [CrossRef]
- Cárdenas, M.; Marder, M.; Blank, V.C.; Roguin, L.P. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorganic Med. Chem. 2006, 14, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Eugenio, G.; Carcache, P.; Ninh, T.; Ren, Y.; Soejarto, D.; Kinghorn, A. A pentamethoxylated flavone from Glycosmis ovoidea promotes apoptosis through the intrinsic pathway and inhibits migration of MCF-7 breast cancer cells. Phytother. Res. 2020, 35, 1634–1645. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Wang, W.; Lin, S.; Yang, L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed. Pharmacother. 2017, 92, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Feng, C.; Wu, G.; Ye, Y.; Tian, J. Calycosin Inhibits the Migration and Invasion of Human Breast Cancer Cells by Down-Regulation of Foxp3 Expression. Cell. Physiol. Biochem. 2017, 44, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Pham, T.-H.; Bak, Y.; Ryu, H.-W.; Oh, S.-R.; Yoon, D.-Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 2018, 50, 35–42. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zhang, R. Corylin suppresses metastasis of breast cancer cells by modulating miR-34c/LINC00963 target. Libyan J. Med. 2021, 16, 1883224. [Google Scholar] [CrossRef]
- Kalva, S.; Azhagiya Singam, E.R.; Rajapandian, V.; Saleena, L.M.; Subramanian, V. Discovery of potent inhibitor for matrix metalloproteinase-9 by pharmacophore based modeling and dynamics simulation studies. J. Mol. Graph. Model. 2014, 49, 25–37. [Google Scholar] [CrossRef]
- Chen, J.; Chang, H.; Peng, X.; Gu, Y.; Yi, L.; Zhang, Q.-Y.; Zhu, J.; Mi, M. 3,6-dihydroxyflavone suppresses the epithelial-mesenchymal transition in breast cancer cells by inhibiting the Notch signaling pathway. Sci. Rep. 2016, 6, 28858. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Gong, S.; Huang, M.; Li, R.; Xiong, G.; Wang, F.; Zou, Q.; Qi, Q.; Yin, X. Targeting the ILK/YAP axis by LFG-500 blocks epithelial–mesenchymal transition and metastasis. Acta Pharmacol. Sin. 2021, 42, 1847–1859. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J. Hispidulin modulates epithelial-mesenchymal transition in breast cancer cells. Oncol. Lett. 2020, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, M.; Wang, J.; Yang, F.; Yang, P.; Liu, Y.; Chen, Z.; Zheng, Y. Calycosin Inhibits Breast Cancer Cell Migration and Invasion by suppressing EMT via BATF/TGF-β1. Aging 2021, 13, 16009. [Google Scholar] [CrossRef] [PubMed]
- Singhal, J.; Nagaprashantha, L.; Chikara, S.; Awasthi, S.; Horne, D.; Singhal, S. 2’-Hydroxyflavanone: A novel strategy for targeting breast cancer. Oncotarget 2017, 8, 75025–75037. [Google Scholar] [CrossRef]
- Singhal, J.; Chikara, S.; Horne, D.; Salgia, R.; Awasthi, S.; Singhal, S.S. 2′-Hydroxyflavanone inhibits in vitro and in vivo growth of breast cancer cells by targeting RLIP76. Mol. Carcinog. 2018, 57, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, K.; Hu, Y.; Zhou, Y.; Luo, X.; Li, X.; Wei, L.; Li, Z.; You, Q.; Guo, Q.; et al. Wogonoside inhibits angiogenesis in breast cancer via suppressing Wnt/β-catenin pathway. Mol. Carcinog. 2016, 55, 1598–1612. [Google Scholar] [CrossRef]
- Elhady, S.S.; Eltamany, E.E.; Shaaban, A.E.; Bagalagel, A.A.; Muhammad, Y.A.; El-Sayed, N.M.; Ayyad, S.N.; Ahmed, A.A.M.; Elgawish, M.S.; Ahmed, S.A. Jaceidin Flavonoid Isolated from Chiliadenus montanus Attenuates Tumor Progression in Mice via VEGF Inhibition: In Vivo and In Silico Studies. Plants 2020, 9, 1031. [Google Scholar] [CrossRef]
- Wang, R.; Deng, Z.; Zhu, Z.; Wang, J.; Yang, X.; Xu, M.; Wang, X.; Tang, Q.; Zhou, Q.; Wan, X.; et al. Kaempferol promotes non-small cell lung cancer cell autophagy via restricting Met pathway. Phytomedicine 2023, 121, 155090. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Zabaleta, M.E.; Forbes-Hernández, T.Y.; Simal-Gandara, J.; Quiles, J.L.; Cianciosi, D.; Bullon, B.; Giampieri, F.; Battino, M. Effect of polyphenols on HER2-positive breast cancer and related miRNAs: Epigenomic regulation. Food Res. Int. 2020, 137, 109623. [Google Scholar] [CrossRef]
- Gu, H.-F.; Mao, X.-Y.; Du, M. Prevention of breast cancer by dietary polyphenols—Role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2020, 60, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Bender, O.; Atalay, A. Chapter 28—Polyphenol Chlorogenic Acid, Antioxidant Profile, and Breast Cancer. In Cancer, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 311–321. ISBN 978-0-12-819547-5. [Google Scholar]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; Mackenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Activities of Epigallocatechin Gallate in Signaling Pathways in Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Wang, K.-L.; Chen, H.-Y.; Chiang, Y.-F.; Hsia, S.-M. Protective Effects of Epigallocatechin Gallate (EGCG) on Endometrial, Breast, and Ovarian Cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Háková, M.; Havlíková, L.C.; Švec, F.; Solich, P.; Erben, J.; Chvojka, J.; Šatínský, D. Novel nanofibrous sorbents for the extraction and determination of resveratrol in wine. Talanta 2020, 206, 120181. [Google Scholar] [CrossRef]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Venkatadri, R.; Muni, T.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, X.; Xing, K.; Wang, Y.; Li, F.; Yao, L. Resveratrol-induced cell inhibition of growth and apoptosis in MCF7 human breast cancer cells are associated with modulation of phosphorylated akt and caspase-9. Appl. Biochem. Biotechnol. 2006, 135, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.A.; Aslam, R.M.N.; Shakeel, A.; Shiza; Waqar, M.; Jmail, A.; Mehmood, M.H.; Gul, H. Cyanidin as potential anticancer agent targeting various proliferative pathways. Chem. Biol. Drug Des. 2023, 101, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Capocasa, F.; Sabbadini, S.; Alvarez-Suarez, J.M.; Afrin, S.; Rosati, C.; Pandolfini, T.; et al. Overexpression of the Anthocyanidin Synthase Gene in Strawberry Enhances Antioxidant Capacity and Cytotoxic Effects on Human Hepatic Cancer Cells. J. Agric. Food Chem. 2018, 66, 581–592. [Google Scholar] [CrossRef]
- Li, W.; Peng, C.; Zhaojie, L.; Wei, W. Chemopreventive and therapeutic properties of anthocyanins in breast cancer: A comprehensive review. Nutr. Res. 2022, 107, 48–64. [Google Scholar] [CrossRef]
- Paramanantham, A.; Kim, M.J.; Jung, E.J.; Kim, H.J.; Chang, S.-H.; Jung, J.-M.; Hong, S.C.; Shin, S.C.; Kim, G.S.; Lee, W.S. Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-κB Activation. Molecules 2020, 25, 3623. [Google Scholar] [CrossRef]
- Li, Z.-L.; Mi, J.; Lu, L.; Luo, Q.; Liu, X.; Yan, Y.-M.; Jin, B.; Cao, Y.-L.; Zeng, X.-X.; Ran, L.-W. The main anthocyanin monomer of Lycium ruthenicum Murray induces apoptosis through the ROS/PTEN/PI3K/Akt/caspase 3 signaling pathway in prostate cancer DU-145 cells. Food Funct. 2021, 12, 1818–1828. [Google Scholar] [CrossRef]
- Rabelo, A.C.S.; Guerreiro, C.D.A.; Shinzato, V.I.; Ong, T.P.; Noratto, G. Anthocyanins Reduce Cell Invasion and Migration through Akt/mTOR Downregulation and Apoptosis Activation in Triple-Negative Breast Cancer Cells: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2300. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, Q.; Deng, X.; Shi, K.; Zhang, W.; Jiang, Y.; Ma, X.; Zeng, J.; Wang, X. Old wine in new bottles: Kaempferol is a promising agent for treating the trilogy of liver diseases. Pharmacol. Res. 2022, 175, 106005. [Google Scholar] [CrossRef]
- He, W.; Zhang, J.; Ju, J.; Wu, Y.; Zhang, Y.; Zhan, L.; Li, C.; Wang, Y. Preparation, characterization, and evaluation of the antitumor effect of kaempferol nanosuspensions. Drug Deliv. Transl. Res. 2023, 13, 2885–2902. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Currie, V.E.; Seidman, A.D.; Bach, A.M.; Panageas, K.S.; Theodoulou, M.; Moasser, M.M.; D’Andrea, G.M.; Lake, D.E.; Choi, J.; et al. A phase II trial of gemcitabine in patients with metastatic breast cancer previously treated with an anthracycline and taxane. Clin. Breast Cancer 2005, 6, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bundred, N. Preclinical and clinical experience with fulvestrant (Faslodex) in postmenopausal women with hormone receptor-positive advanced breast cancer. Cancer Investig. 2005, 23, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Fandino, M.; Morales, S.; Cortés-Salgado, A.; Manso, L.; Apala, J.V.; Muñoz, M.; Gasol Cudos, A.; Salla Fortuny, J.; Gion, M.; Lopez-Alonso, A.; et al. Randomized Phase 0/I Trial of the Mitochondrial Inhibitor ME-344 or Placebo Added to Bevacizumab in Early HER2-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 35–45. [Google Scholar] [CrossRef]
- Ravasco, P. Does Watercress Intake Have an Impact on Cancer Patients Outcomes: A Randomized Longitudinal Trial. 2015. Available online: https://clinicaltrials.gov/study/NCT02468882 (accessed on 28 September 2025).
- Lathrop, K.; Kaklamani, V.; Brenner, A.; Li, R.; Nazarullah, A.; Hackman, S.; Thomas, C.; Rodriguez, M.; Elledge, R. Novel estrogen receptor beta agonist S-equol decreases tumor proliferation in patients with triple negative breast cancer (TNBC). J. Clin. Oncol. 2020, 38, 560. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Jakobušić Brala, C.; Karković Marković, A.; Kugić, A.; Torić, J.; Barbarić, M. Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies—An Update Overview. Molecules 2023, 28, 3746. [Google Scholar] [CrossRef]
- Lotha, R.; Sivasubramanian, A. Flavonoids nutraceuticals in prevention and treatment of cancer: A review. Asian J. Pharm. Clin. Res. 2018, 11, 42–47. [Google Scholar] [CrossRef][Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Dagur, H.S.; Khan, M.; Malik, N.; Alam, M.; Mushtaque, M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. Eur. J. Med. Chem. Rep. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, C.; Dong, S.-L.; Ou, C.-S.; Lu, J.-L.; Ye, J.-H.; Liang, Y.-R.; Zheng, X.-Q. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Wu, H.-T.; Liu, Y.-E.; Hsu, K.-W.; Wang, Y.-F.; Chan, Y.-C.; Chen, Y.; Chen, D.-R. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/mTOR Pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef]
- Safi, A.; Heidarian, E.; Ahmadi, R. Quercetin Synergistically Enhances the Anticancer Efficacy of Docetaxel through Induction of Apoptosis and Modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 Signaling Pathways in MDA-MB-231 Breast Cancer Cell Line. Int. J. Mol. Cell Med. 2021, 10, 11. [Google Scholar] [CrossRef]
- Roshanazadeh, M.; Rezaei, H.B.; Rashidi, M. Quercetin synergistically potentiates the anti-metastatic effect of 5-fluorouracil on the MDA-MB-231 breast cancer cell line. Iran. J. Basic Med. Sci. 2021, 24, 928. [Google Scholar] [CrossRef]
- Xing, Y.; Shen, W.; Sun, C.; Wang, R.; Fan, B.; Liang, G. Genistein-Chitosan Derivative Nanoparticles for Targeting and Enhancing the Anti-Breast Cancer Effect of Tamoxifen In Vitro. J. Pharm. Sci. 2024, 113, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, X.; Min, J.; Chen, X.; Liu, R.; Cui, X.; Cheng, J.; Xie, M.; Diel, P.; Hu, X. Genistein interferes with antitumor effects of cisplatin in an ovariectomized breast cancer xenograft tumor model. Toxicol. Lett. 2022, 355, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Dian, C.; Qian, Z.; Ran, M.; Yan, X.; Dian, L. Co-Delivery of Docetaxel and Curcumin Functionalized Mixed Micelles for the Treatment of Drug-Resistant Breast Cancer by Oral Administration. Int. J. Nanomed. 2024, 19, 8603–8620. [Google Scholar] [CrossRef] [PubMed]
- Effat, H.; El Houseini, M.E.; Abohashem, R.S. The Combined Impact of Curcumin: Piperine and Sorafenib on microRNAs and Different Pathways in Breast Cancer Cells. Ind. J. Clin. Biochem. 2024, 40, 32–45. [Google Scholar] [CrossRef]
- Sarkar, E.; Kotiya, A.; Khan, A.; Bhuyan, R.; Raza, S.T.; Misra, A.; Mahdi, A.A. The combination of Curcumin and Doxorubicin on targeting PI3K/AKT/mTOR signaling pathway: An in vitro and molecular docking study for inhibiting the survival of MDA-MB-231. Silico Pharmacol. 2024, 12, 58. [Google Scholar] [CrossRef]
- Sarkar, E.; Khan, A.; Ahmad, R.; Misra, A.; Dua, K.; Mahdi, A.A.; Raza, T. Synergism of Curcumin and Doxorubicin Proves an Effective Anticancer Therapeutics Against Breast Cancer: An in vitro Study. Res. Sq. 2024, 16, e75047. [Google Scholar]
- Deswal, B.; Bagchi, U.; Kapoor, S. Curcumin Suppresses M2 Macrophage-derived Paclitaxel Chemoresistance throughInhibition of PI3K-AKT/STAT3 Signaling. Anti-Cancer Agents Med. Chem. Anti-Cancer Agents 2024, 24, 146–156. [Google Scholar] [CrossRef]
- Wang, G.; Duan, P.; Wei, Z.; Liu, F. Curcumin sensitizes carboplatin treatment in triple negative breast cancer through reactive oxygen species induced DNA repair pathway. Mol. Biol. Rep. 2022, 49, 3259–3270. [Google Scholar] [CrossRef]
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide against MDA-MB-231 Breast Cancer Cells Via Targeting the STAT3 Signaling Pathway. Iran. J. Pharm. Res. IJPR 2020, 19, 122. [Google Scholar] [CrossRef]
- Pateliya, B.; Burade, V.; Goswami, S. Combining naringenin and metformin with doxorubicin enhances anticancer activity against triple-negative breast cancer in vitro and in vivo. Eur. J. Pharmacol. 2021, 891, 173725. [Google Scholar] [CrossRef]
- Alshamrani, S.; Kumar, A.; Aldughaim, M.S.; Alghamdi, K.M.; Hussain, M.D.; Alanazi, F.K.; Kazi, M. Development of Polymeric Micelles for Combined Delivery of Luteolin and Doxorubicin for Cancer Therapy. J. Cancer 2024, 15, 4717–4730. [Google Scholar] [CrossRef]
- Tamanna, S.; Perumal, E.; Rajanathadurai, J. Enhanced Apoptotic Effects in MDA-MB-231 Triple-Negative Breast Cancer Cells Through a Synergistic Action of Luteolin and Paclitaxel. Cureus 2024, 16, e65159. [Google Scholar] [CrossRef] [PubMed]
- Çilesiz, Y.; Çevik, Ö. Anticancer effect of the letrozole-quercetin combination mediated by FOXOs and estrogen receptors in breast cancer cells. J. Res. Pharm. 2021, 25, 479–489. [Google Scholar] [CrossRef]
- Almohammad Aljabr, B.; Zihlif, M.; Abu-Dahab, R.; Zalloum, H. Effect of quercetin on doxorubicin cytotoxicity in sensitive and resistant human MCF7 breast cancer cell lines. Biomed. Rep. 2024, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Roshanazadeh, M.; Babaahmadi Rezaei, H.; Rashidi, M. Quercetin Enhances the Suppressive Effects of Doxorubicin on the Migration of MDA-MB-231 Breast Cancer Cell Line. Int. J. Cancer Manag. 2021, 14, e119049. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, P.; Kawish, S.M.; Ahmad, S.; Iqbal, Z.; Vohora, D.; Kohli, K. Novel Chitosan-Coated Liposomes Coloaded with Exemestane and Genistein for an Effective Breast Cancer Therapy. ACS Omega 2024, 9, 9735–9752. [Google Scholar] [CrossRef]
- Synergistic Anti-Cancer Effects of ERB-041 and Genistein Through Estrogen Receptor Suppression-Mediated PI3K/AKT Pathway Downregulation in Canine Mammary Gland Tumor Cells. Available online: https://www.mdpi.com/1422-0067/25/5/2466 (accessed on 1 December 2024).
- Kaushik, S.; Shyam, H.; Sharma, R.; Balapure, A. Genistein synergizes centchroman action in human breast cancer cells. Indian J. Pharmacol. 2016, 48, 637. [Google Scholar] [CrossRef]
- Farhoudi Sefidan Jadid, M.; Jahangirzadehd, G.; Behroozi, J. Anti-proliferation effects of Apatinib in combination with Curcumin in breast cancer cells. Horm. Mol. Biol. Clin. Investig. 2023, 44, 27–32. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Chidambaram, K.; Pino-Figueroa, A.; Chandrasekaran, B.; Dhanaraj, P.; Venkatesan, K. Curcumin-Celecoxib: A synergistic and rationale combination chemotherapy for breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1916–1927. [Google Scholar]
- Ziasarabi, P.; Sahebkar, A.; Ghasemi, F. Evaluation of the Effects of Nanomicellar Curcumin, Berberine, and Their Combination with 5-Fluorouracil on Breast Cancer Cells. In Natural Products and Human Diseases; Sahebkar, A., Sathyapalan, T., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1328, pp. 21–35. ISBN 978-3-030-73233-2. [Google Scholar]
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2016, 22, 36. [Google Scholar] [CrossRef]
- Kang, Y.; Park, M.-A.; Heo, S.-W.; Park, S.-Y.; Kang, K.W.; Park, P.-H.; Kim, J.-A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim. Biophys. Acta (BBA) General Subj. 2013, 1830, 2638–2648. [Google Scholar] [CrossRef]
- Tu, S.-H.; Chiou, Y.-S.; Kalyanam, N.; Ho, C.-T.; Chen, L.-C.; Pan, M.-H. Garcinol sensitizes breast cancer cells to Taxol through the suppression of caspase-3/iPLA2 and NF-κB/Twist1 signaling pathways in a mouse 4T1 breast tumor model. Food Funct. 2017, 8, 1067–1079. [Google Scholar] [CrossRef]
- Pateliya, B.; Burade, V.; Goswami, S. Enhanced antitumor activity of doxorubicin by naringenin and metformin in breast carcinoma: An experimental study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Junedi, S.; Hermawan, A.; Fitriasari, A.; Setiawati, A.; Susidarti, R.A.; Meiyanto, E. The Doxorubicin-Induced G2/M Arrest in Breast Cancer Cells Modulated by Natural Compounds Naringenin and Hesperidin. Indones. J. Cancer Chemoprev. 2021, 12, 83–89. [Google Scholar] [CrossRef]
- Soltannezhad, S.; Jouni, F.J.; Osgoei, L.T. The Combined Therapeutic Effect of Capecitabine and Naringin on HER2+ (SK-BR-3) and HER2- (MCF-7) Human Breast Cancer Cells Lines. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Effat, H.; Abosharaf, H.A.; Radwan, A.M. Combined effects of naringin and doxorubicin on the JAK/STAT signaling pathway reduce the development and spread of breast cancer cells. Sci. Rep. 2024, 14, 2824. [Google Scholar] [CrossRef]
- Muthusamy, T.; Yadav, L.R.; Ramalingam, S.; Ramachandran, I.; Nagarajan, P. Synergistic Effect of 5-Fluorouracil Combined with Naringin in MDA-MB-231 Human Breast Cancer Cells. Int. J. Oncol. 2020, 3, 104–118. [Google Scholar]
- Louisa, M.; Mirawati, T.; Suyatna, F. in vitro Modulation of P-glycoprotein, MRP-1 and BCRP Expression by Mangiferin in Doxorubicin-Treated MCF-7 Cells. Asian Pac. J. Cancer Prev. 2014, 15, 1639–1642. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Fan, Y.; Wu, J.; Wang, Z.; Chen, Y.; Zhang, Y. The Synergistic Effect of Proanthocyanidin and HDAC Inhibitor Inhibit Breast Cancer Cell Growth and Promote Apoptosis. Int. J. Mol. Sci. 2023, 24, 10476. [Google Scholar] [CrossRef]
- Chen, X.-X.; Leung, G.P.-H.; Zhang, Z.-J.; Xiao, J.-B.; Lao, L.-X.; Feng, F.; Mak, J.C.-W.; Wang, Y.; Sze, S.C.-W.; Zhang, K.Y.-B. Proanthocyanidins from Uncaria rhynchophylla induced apoptosis in MDA-MB-231 breast cancer cells while enhancing cytotoxic effects of 5-fluorouracil. Food Chem. Toxicol. 2017, 107, 248–260. [Google Scholar] [CrossRef]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and Hesperidin Downregulate DNA Repair Genes in MCF-7 Breast Cancer Cells and Augment Doxorubicin Toxicity. Molecules 2020, 25, 4421. [Google Scholar] [CrossRef]
- Nimal, S.; Kumbhar, N.; Saruchi; Rathore, S.; Naik, N.; Paymal, S.; Gacche, R.N. Apigenin and its combination with Vorinostat induces apoptotic-mediated cell death in TNBC by modulating the epigenetic and apoptotic regulators and related miRNAs. Sci. Rep. 2024, 14, 9540. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Riahirad, H.; Pabarja, A.; Karimzadeh, M.R.; Saeidi, K. Kaempferol and docetaxel diminish side population and down-regulate some cancer stem cell markers in breast cancer cell line MCF-7. Biocell 2017, 41, 33. [Google Scholar] [CrossRef]
- Kusharyanti, I.; Larasati, L.; Susidarti, R.A.; Meiyanto, E. Hesperidin Increase Cytotoxic Activity of Doxorubicin on Hela Cell Line Through Cell Cycle Modulation and Apoptotis Induction. Indones. J. Cancer Chemoprev. 2011, 2, 267. [Google Scholar] [CrossRef]
- Nurhayati, I.P.; Khumaira, A.; Ilmawati, G.P.N.; Meiyanto, E.; Hermawan, A. Cytotoxic and Antimetastatic Activity of Hesperetin and Doxorubicin Combination Toward Her2 Expressing Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2020, 21, 1259–1267. [Google Scholar] [CrossRef]
- Febriansah, R.; Dyaningtyas, D.P.P.; Sarmoko; Nurulita, N.A.; Meiyanto, E.; Nugroho, A.E. Hesperidin as a preventive resistance agent in MCF–7 breast cancer cells line resistance to doxorubicin. Asian Pac. J. Trop. Biomed. 2014, 4, 228–233. [Google Scholar] [CrossRef]
- Amalina, N.D.; Salsabila, I.A.; Zulfin, U.M.; Jenie, R.I.; Meiyanto, E. In vitro synergistic effect of hesperidin and doxorubicin downregulates epithelial-mesenchymal transition in highly metastatic breast cancer cells. J. Egypt. Natl. Cancer Inst. 2023, 35, 6. [Google Scholar] [CrossRef]
- Khamis, A.A.; Ali, E.M.M.; Salim, E.I.; El-Moneim, M.A.A. Synergistic effects of bee venom, hesperidin, and piperine with tamoxifen on apoptotic and angiogenesis biomarker molecules against xerographic MCF-7 injected rats. Sci. Rep. 2024, 14, 1510. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Xiong, Q.; Wang, Y.; Wan, J.; Yuan, P.; Chen, H.; Zhang, L. Facile preparation of hyaluronic acid-based quercetin nanoformulation for targeted tumor therapy. Int. J. Biol. Macromol. 2020, 147, 937–945. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Sharma, G.; Park, J.; Sharma, A.R.; Jung, J.-S.; Kim, H.; Chakraborty, C.; Song, D.-K.; Lee, S.-S.; Nam, J.-S. Methoxy Poly(ethylene glycol)-Poly(lactide) Nanoparticles Encapsulating Quercetin Act as an Effective Anticancer Agent by Inducing Apoptosis in Breast Cancer. Pharm. Res. 2015, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim. Biophys. Acta (BBA) General Subj. 2016, 1860, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 45521. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Patel, G.; Thakur, N.S.; Kushwah, V.; Patil, M.D.; Nile, S.H.; Jain, S.; Kai, G.; Banerjee, U.C. Mycophenolate co-administration with quercetin via lipid-polymer hybrid nanoparticles for enhanced breast cancer management. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102147. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Pooja, D.; Kulhari, H.; Gudem, S.; Ravuri, H.G.; Bhargava, S.; Ramakrishna, S. Bombesin conjugated solid lipid nanoparticles for improved delivery of epigallocatechin gallate for breast cancer treatment. Chem. Phys. Lipids 2019, 224, 104770. [Google Scholar] [CrossRef]
- Naderi, E.; Aghajanzadeh, M.; Zamani, M.; Hashiri, A.; Sharafi, A.; Kamalianfar, A.; Naseri, M.; Danafar, H. Improving the anti-cancer activity of quercetin-loaded AgFeO2 through UV irradiation: Synthesis, characterization, and in vivo and in vitro biocompatibility study. J. Drug Deliv. Sci. Technol. 2020, 57, 101645. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR -2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Karimi, E.; Oskoueian, E.; Es-Haghi, A.; Yazdi, M.E.T. Comparative Study on the Biological Effects of Sodium Citrate-Based and Apigenin-Based Synthesized Silver Nanoparticles. Nutr. Cancer 2021, 73, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Chakraborty, A.; Mukherjee, B.; Gupta, S. Aptamer-Conjugated Apigenin Nanoparticles to Target Colorectal Carcinoma: A Promising Safe Alternative of Colorectal Cancer Chemotherapy. ACS Appl. Bio Mater. 2018, 1, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol. Lett. 2013, 223, 124–138. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef]
- Pourgholi, A.; Dadashpour, M.; Mousapour, A.; Firouzi Amandi, A.; Zarghami, N. Anticancer potential of silibinin loaded polymeric nanoparticles against breast cancer cells: Insight into the apoptotic genes targets. Asian Pac. J. Cancer Prev. 2021, 22, 2587–2596. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Song, X.; Wang, P.; Li, Y. Cholate-modified polymer-lipid hybrid nanoparticles for oral delivery of quercetin to potentiate the antileukemic effect. Int. J. Nanomed. 2019, 14, 4045–4057. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Wei, T.; Ma, X.; Cheng, Q.; Huo, S.; Zhang, C.; Zhang, Y.; Duan, X.; Liang, X.-J. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale 2016, 8, 5126–5138. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M.S.; Patere, S.; Dhawan, V.; Gude, R.P.; Hassan, P.A.; Aswal, V.; Steiniger, F.; Thamm, J.; Fahr, A. Lecithin-Based Novel Cationic Nanocarriers (Leciplex) II: Improving Therapeutic Efficacy of Quercetin on Oral Administration. Mol. Pharm. 2011, 8, 716–726. [Google Scholar] [CrossRef]
- Dora, C.L.; Costa Silva, L.F.; Mazzarino, L.; Siqueira, J.M.; Fernandes, D.; Pacheco, L.K.; Maioral, M.F.; Santos-Silva, M.C.; Muccillo Baisch, A.L.; Assreuy, J.; et al. Oral Delivery of a High Quercetin Payload Nanosized Emulsion: In Vitro and In Vivo Activity Against B16-F10 Melanoma. J. Nanosci. Nanotechnol. 2016, 16, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, M.; Ma, B.; Niu, R.; Zhang, H.; Kun, L. Antitumor activity and safety evaluation of nanaparticle-based delivery of quercetin through intravenous administration in mice. Mater. Sci. Eng. C 2017, 77, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-Y.; Chiu, G.N.C. Simultaneous liposomal delivery of quercetin and vincristine for enhanced estrogen-receptor-negative breast cancer treatment. Anti-Cancer Drugs 2010, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-Y.; Chiu, G.N.C. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 834–840. [Google Scholar] [CrossRef]
- Yu, J.; Chen, H.; Jiang, L.; Wang, J.; Dai, J.; Wang, J. Codelivery of Adriamycin and P-gp Inhibitor Quercetin Using PEGylated Liposomes to Overcome Cancer Drug Resistance. J. Pharm. Sci. 2019, 108, 1788–1799. [Google Scholar] [CrossRef]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1399–1406. [Google Scholar] [CrossRef]
- Xu, P.; Yin, Q.; Shen, J.; Chen, L.; Yu, H.; Zhang, Z.; Li, Y. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 2013, 454, 21–30. [Google Scholar] [CrossRef]
- Ghosh, P.; Bag, S.; Roy, A.S.; Subramani, E.; Chaudhury, K.; Dasgupta, S. Solubility enhancement of morin and epicatechin through encapsulation in an albumin based nanoparticulate system and their anticancer activity against the MDA-MB-468 breast cancer cell line. RSC Adv. 2016, 6, 101415–101429. [Google Scholar] [CrossRef]
- Rameshthangam, P.; Chitra, J.P. Synergistic anticancer effect of green synthesized nickel nanoparticles and quercetin extracted from Ocimum sanctum leaf extract. J. Mater. Sci. Technol. 2018, 34, 508–522. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; He, K.; Qiu, T.; Sun, J.; Liu, Q.; Zhang, X.; Zheng, H. Tumor-targeted delivery of silibinin and IPI-549 synergistically inhibit breast cancer by remodeling the microenvironment. Int. J. Pharm. 2020, 581, 119239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, X.; Hou, X.; Shen, J.; Shi, J.; Chen, H.; He, Y.; Wang, Z.; Feng, N. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J. Nanobiotechnol. 2020, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Davaran, S.; Fazeli, H.; Ghamkhari, A.; Rahimi, F.; Molavi, O.; Anzabi, M.; Salehi, R. Synthesis and characterization of novel P(HEMA-LA-MADQUAT) micelles for co-delivery of methotrexate and Chrysin in combination cancer chemotherapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1265–1286. [Google Scholar] [CrossRef]
- Rasouli, S.; Montazeri, M.; Mashayekhi, S.; Sadeghi-Soureh, S.; Dadashpour, M.; Mousazadeh, H.; Nobakht, A.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of Curcumin and Chrysin: Possible application in prevention of breast cancer local recurrence. J. Drug Deliv. Sci. Technol. 2020, 55, 101402. [Google Scholar] [CrossRef]
- Dahiya, S.; Rani, R.; Dhingra, D.; Kumar, S.; Dilbaghi, N. Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: Implication on antioxidant and anticancer potential. Adv. Nat. Sci Nanosci. Nanotechnol. 2018, 9, 035011. [Google Scholar] [CrossRef]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Kilicay, E.; Alpaslan, P.; Hazer, B.; Baki Denkbas, E. Enhanced antitumor activity of epigallocatechin gallate–conjugated dual-drug-loaded polystyrene–polysoyaoil–diethanol amine nanoparticles for breast cancer therapy. J. Bioact. Compat. Polym. 2018, 33, 38–62. [Google Scholar] [CrossRef]
- Meng, L.; Xia, X.; Yang, Y.; Ye, J.; Dong, W.; Ma, P.; Jin, Y.; Liu, Y. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Int. J. Pharm. 2016, 513, 8–16. [Google Scholar] [CrossRef]
- Jain, A.K.; Thanki, K.; Jain, S. Co-encapsulation of Tamoxifen and Quercetin in Polymeric Nanoparticles: Implications on Oral Bioavailability, Antitumor Efficacy, and Drug-Induced Toxicity. Mol. Pharm. 2013, 10, 3459–3474. [Google Scholar] [CrossRef]
- Sandhu, P.S.; Kumar, R.; Beg, S.; Jain, S.; Kushwah, V.; Katare, O.P.; Singh, B. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: Systematic approach for improved breast cancer therapeutics. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1703–1713. [Google Scholar] [CrossRef]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907–912. [Google Scholar] [CrossRef]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Sci. Rep. 2016, 6, 34053. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Q.; Wan, J.; Yu, D.-G.; Tan, F.; Yang, X. Improved synergistic anticancer action of quercetin and tamoxifen citrate supported by an electrospun complex nanostructure. Mater. Des. 2024, 238, 112657. [Google Scholar] [CrossRef]
- Alamri, A.H.; Debnath, S.; Alqahtani, T.; Alqahtani, A.; Alshehri, S.A.; Ghosh, A. Enhancing plant-derived smart nano inhibitor in targeting mammalian target of rapamycin (mTOR) in breast cancer using Curcuma longa-derived compound curcumin. Environ. Sci. Pollut. Res. 2023, 31, 46462–46469. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, M.; Pourmadadi, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M.; Rahdar, A.; Ferreira, L.F.R. Improving Curcumin Constraints with pH-Responsive Chitosan Based Graphene Oxide/Montmorillonite Nanohybrid Modified Agarose in Breast Cancer Therapy. BioNanoScience 2024, 14, 1613–1626. [Google Scholar] [CrossRef]
- Nurjis, F.; Sarwar, U.; Ali, J.S.; Fayyaz, M. Doxorubicin and Curcumin-Loaded Nanomicelles Targeting Multidrug Resistance in Cancer. BioNanoScience 2024, 14, 2159–2169. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Bai, D. Reversal of drug resistance in breast cancer cells by photodynamic action mediated by curcumin/Ko143/PLGA nanoparticles. AIP Adv. 2024, 14, 045341. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Q.; Du, S.; Guan, Y.; Qiu, J.; Chen, X.; Yuan, D.; Chen, T. Nanoparticles for co-delivery of paclitaxel and curcumin to overcome chemoresistance against breast cancer. J. Drug Deliv. Sci. Technol. 2023, 79, 104050. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Zarepour, A.; Nasri, N.; Cordani, M.; Zarrabi, A. A comparison study between doxorubicin and curcumin co-administration and co-loading in a smart niosomal formulation for MCF-7 breast cancer therapy. Eur. J. Pharm. Sci. 2023, 191, 106600. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Kasabri, V.; Sunoqrot, S.; Shalabi, D.; Alkhateeb, R.; Alhiari, Y. Preparation and Characterization of Rutin-Encapsulated Polymeric Micelles and Studies of Synergism with Bioactive Benzoic Acids and Triazolofluoroquinolones as Anticancer Nanomedicines. Asian Pac. J. Cancer Prev. 2023, 24, 977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J. Facile fabrication of combination delivery of kaempferol and sorafenib with PEGylated gold nanoparticles delivery to breast cancer: Investigation of cytotoxicity and apoptosis induction. J. Macromol. Sci. 2023, 60, 628–639. [Google Scholar] [CrossRef]
- Pingale, P.; Kendre, P.; Pardeshi, K.; Rajput, A. An emerging era in manufacturing of drug delivery systems: Nanofabrication techniques. Heliyon 2023, 9, e14247. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2005, 24, 67–75. [Google Scholar] [CrossRef]
- Javani, R.; Hashemi, F.S.; Ghanbarzadeh, B.; Hamishehkar, H. Quercetin-loaded niosomal nanoparticles prepared by the thin-layer hydration method: Formulation development, colloidal stability, and structural properties. LWT 2021, 141, 110865. [Google Scholar] [CrossRef]
- Halevas, E.G.; Avgoulas, D.I.; Katsipis, G.; Pantazaki, A.A. Flavonoid-liposomes formulations: Physico-chemical characteristics, biological activities and therapeutic applications. Eur. J. Med. Chem. Rep. 2022, 5, 100059. [Google Scholar] [CrossRef]
- Hoa, L.T.M.; Chi, N.T.; Nguyen, L.H.; Chien, D.M. Preparation and characterisation of nanoparticles containing ketoprofen and acrylic polymers prepared by emulsion solvent evaporation method. J. Exp. Nanosci. 2012, 7, 189–197. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, H.; Zhang, L.; Yang, W. Sonication-Assisted Electrochemical Synthesis of Silver Nanoparticles. Langmuir 2024, 40, 22455–22461. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hoang, Y.H.; Thai, N.T.-T.; Trinh, G.T.-N. Synthesis of copper nanoparticles by a sonication-mediated method using Malpighia glabra fruit extract and their applications. RSC Adv. 2024, 14, 34119–34134. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Seck, F.; Fonte, P. An insight on lipid nanoparticles for therapeutic proteins delivery. J. Drug Deliv. Sci. Technol. 2022, 77, 103839. [Google Scholar] [CrossRef]
- Ewii, U.E.; Attama, A.A.; Olorunsola, E.O.; Onugwu, A.L.; Nwakpa, F.U.; Anyiam, C.; Chijioke, C.; Ogbulie, T. Nanoparticles for drug delivery: Insight into in vitro and in vivo drug release from nanomedicines. Nano TransMed 2025, 4, 100083. [Google Scholar] [CrossRef]
- Anwar Ul Alam, M.; Kassu, A.; Kassama, L. Effect of sonication time and surfactant concentration on improving the bio-accessibility of lycopene synthesized in poly-lactic co-glycolic acid nanoparticles. arXiv 2023, arXiv:2301.10850. [Google Scholar]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Webster, T.J.; Kia, P.; Kalantari, K.; Misran, M.; Rasouli, E.; Maghareh Esfahan, Z.; Shameli, K. Nanoemulsions Based Therapeutic Strategies: Enhancing Targeted Drug Delivery against Breast Cancer Cells. Int. J. Nanomed. 2025, 20, 6133–6162. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Chhabra, V.; Virmani, R.; Pathak, K.; Akhtar, M.S.; Hussain Asim, M.; Arshad, S.; Siddique, F.; Fonte, P. Transforming cancer treatment: The potential of nanonutraceuticals. Int. J. Pharm. 2024, 667, 124919. [Google Scholar] [CrossRef]
- Talib, W.H.; Abuawad, A.; Thiab, S.; Alshweiat, A.; Mahmod, A.I. Flavonoid-based nanomedicines to target tumor microenvironment. OpenNano 2022, 8, 100081. [Google Scholar] [CrossRef]
- Abdi Syahputra, R.; Dalimunthe, A.; Utari, Z.D.; Halim, P.; Sukarno, M.A.; Zainalabidin, S.; Salim, E.; Gunawan, M.; Nurkolis, F.; Park, M.N.; et al. Nanotechnology and flavonoids: Current research and future perspectives on cardiovascular health. J. Funct. Foods 2024, 120, 106355. [Google Scholar] [CrossRef]
- Stevens Barrón, J.C.; Chapa González, C.; Álvarez Parrilla, E.; De la Rosa, L.A. Nanoparticle-Mediated Delivery of Flavonoids: Impact on Proinflammatory Cytokine Production: A Systematic Review. Biomolecules 2023, 13, 1158. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Mall, J.; Naseem, N.; Haider, M.F.; Rahman, M.A.; Khan, S.; Siddiqui, S.N. Nanostructured lipid carriers as a drug delivery system: A comprehensive review with therapeutic applications. Intell. Pharm. 2025, 3, 243–255. [Google Scholar] [CrossRef]
- Hashim, M.; Mujahid, H.; Hassan, S.; Bukhari, S.; Anjum, I.; Hano, C.; Abbasi, B.H.; Anjum, S. Implication of Nanoparticles to Combat Chronic Liver and Kidney Diseases: Progress and Perspectives. Biomolecules 2022, 12, 1337. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Bhardwaj, S.; Sikarwar, M.; Sriwastaw, S.; Sharma, G.; Gupta, M. Nanotoxicity unveiled: Evaluating exposure risks and assessing the impact of nanoparticles on human health. J. Trace Elem. Miner. 2025, 13, 100252. [Google Scholar] [CrossRef]
- Vignesh, A.; Menaka, C.; Amal, T.C.; Selvakumar, S.; Vasanth, K. Exploring the dual impact of nanoparticles on human well-being: A comprehensive review of risks and benefits. Next Nanotechnol. 2025, 8, 100223. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef]
- Gefen, T.; Vaya, J.; Khatib, S.; Harkevich, N.; Artoul, F.; Heller, E.D.; Pitcovski, J.; Aizenshtein, E. The impact of PEGylation on protein immunogenicity. Int. Immunopharmacol. 2013, 15, 254–259. [Google Scholar] [CrossRef]
- Simberg, D.; Barenholz, Y.; Roffler, S.R.; Landfester, K.; Kabanov, A.V.; Moghimi, S.M. PEGylation technology: Addressing concerns, moving forward. Drug Deliv. 2025, 32, 2494775. [Google Scholar] [CrossRef]
- Office of the Commissioner of the FDA’s Approach to Regulation of Nanotechnology Products. 2019. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products (accessed on 1 January 2020).
- Drug Products, Including Biological Products That Contain Nanomaterials—Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 24 September 2025).
- European Medicines Agency (EMA). Joint MHLW-EMA Reflection Paper on the Development of Block Copolymer Micelle Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/joint-mhlwema-reflection-paper-development-block-copolymer-micelle-medicinal-products_en.pdf (accessed on 28 September 2025).
- European Medicines Agency (EMA). Data Requirements for Intravenous Liposomal Products Developed with Reference to an Innovator Liposomal Product—Scientific Guideline. 2013. Available online: https://www.ema.europa.eu/en/data-requirements-intravenous-liposomal-products-developed-reference-innovator-liposomal-product-scientific-guideline (accessed on 24 September 2025).
- Committee for Medicinal Products for Human Use. Reflection Paper on Surface Coatings: General Issues for Consideration Regarding Parenteral Administration of Coated Nanomedicine Products; European Medicines Agency, Committee for Medicinal Products for Human Use: London, UK, 2013. [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guidelines. 2024. Available online: https://www.gov.uk/guidance/international-council-for-harmonisation-of-technical-requirements-for-pharmaceuticals-for-human-use-guidelines (accessed on 24 September 2025).
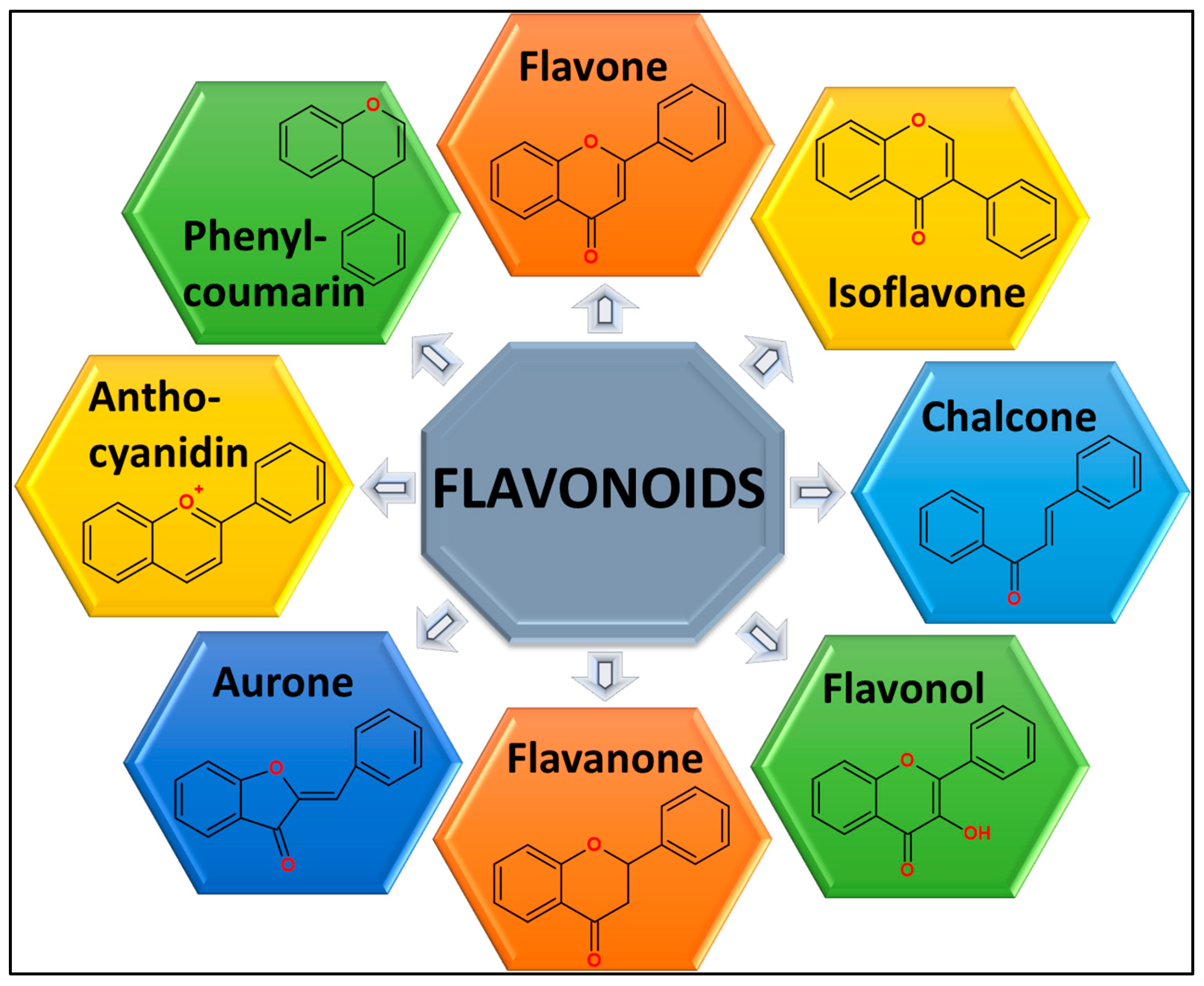
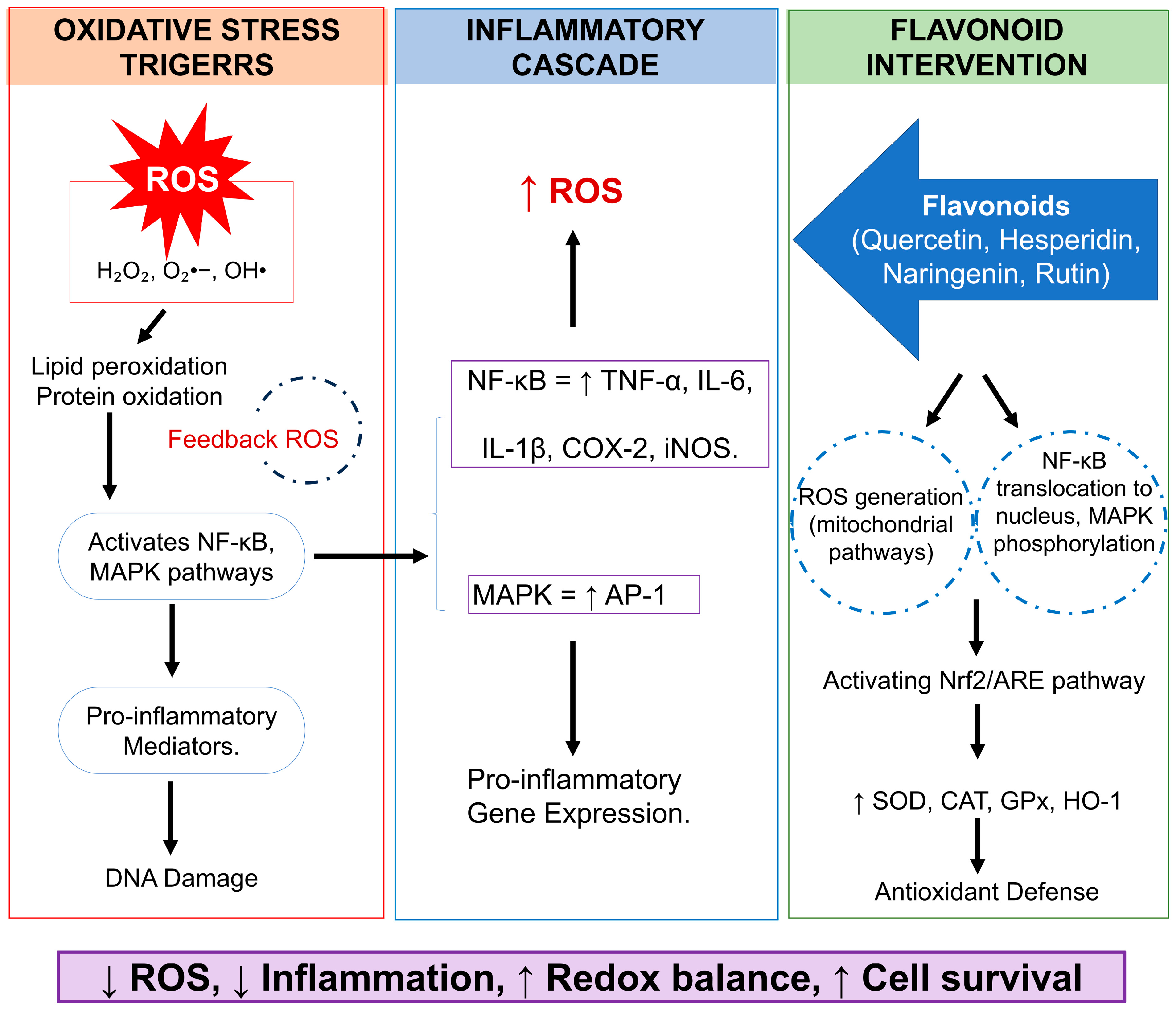
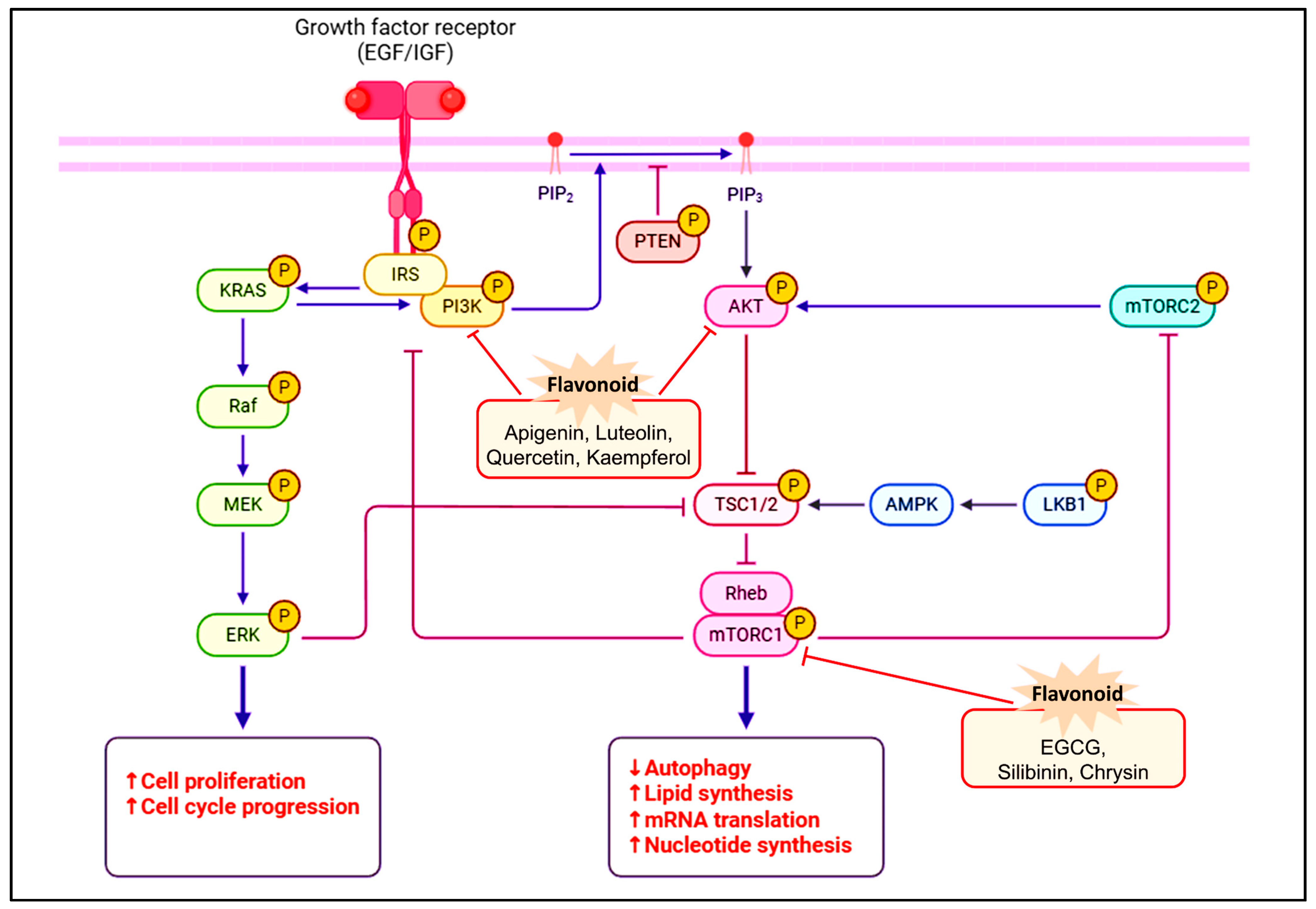
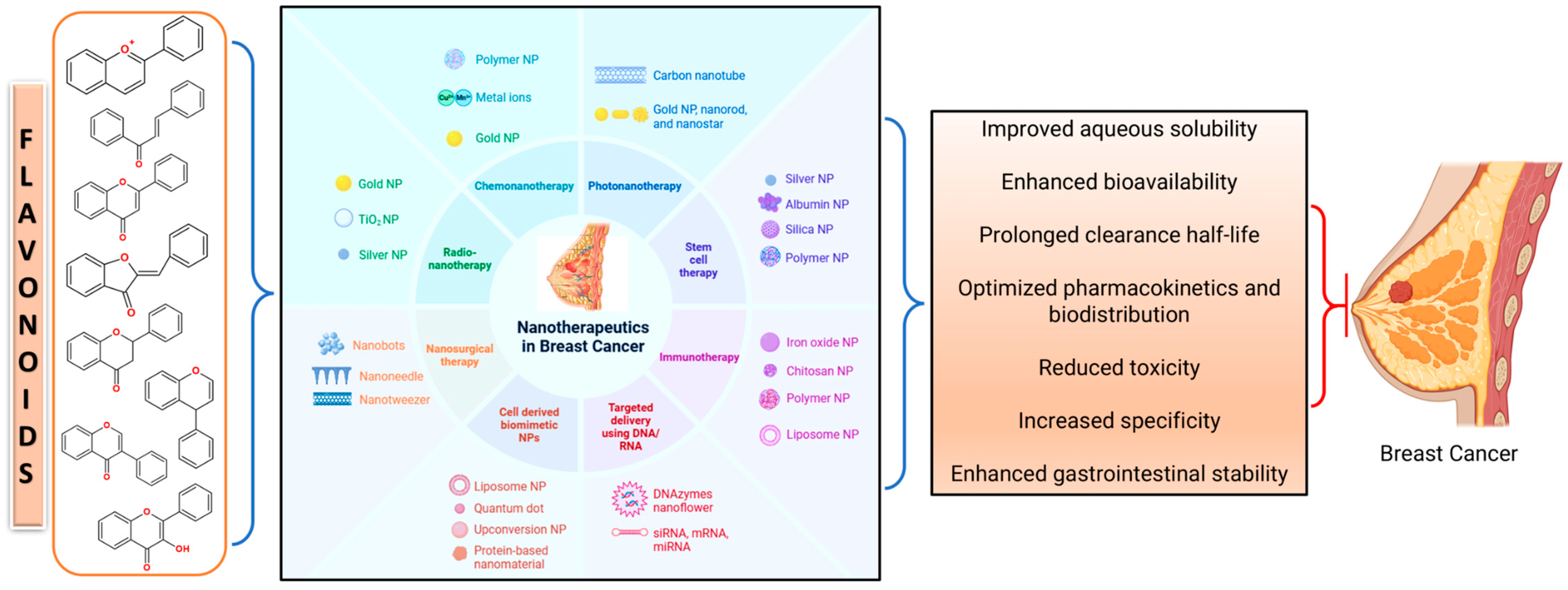
| Flavonoid | Clinical Trial | Objective of the Study | Clinical Trail Phase | NCT Number | Current Status/Year |
|---|---|---|---|---|---|
| Genistein | Biomarker Assays for a Phase 2 Trial of gemcitabine and genistein in Women with Metastatic Breast Cancer | Explore a target outcome among women with breast cancer at stage IV. The treatment included genistein and gemcitabine hydrochloride. | Phase 2 | NCT00244933 | Completed/2023 |
| The Role of Genistein in Breast Cancer Prevention in Women at elevated risk | Evaluate the role of genistein on the growth of breast epithelial cells isolated from women at elevated risk for breast cancer using a small-needle approach. | Phase 2 | NCT00290758 | Completed/2017 | |
| The Function of Genistein in the Prevention of Breast and Endometrial Cancer in Healthy Postmenopausal Women | Assess the efficacy of genistein in reducing DNA damage and facilitating apoptosis for preventing the development of breast and endometrial cancer in normal postmenopausal women. | Phase 1 | NCT00099008 | Completed/2013 | |
| AFP464, Aminoflavone | Examination of AFP464 and Faslodex in Oestrogen Receptor-Positive Breast Carcinoma | The response to clinical advantage | Phase 2 | NCT01233947 | Terminated/2012 |
| AFP464 in the Management of Patients with Solid tumours That Are Metastatic or Refractory and Unsuitable for Surgical Removal | Examine the disadvantages and ideal dose of AFP464 for patients with solid tumours that are metastatic or resistant to radiation therapy. | Phase 1 | NCT00348699 | Completed/2014 | |
| ME-344 (synthetic molecule having isoflavan ring structure) | ME-344 (57) has anti-angiogenic properties that change mitochondrial metabolism in early HER2-negative breast cancer. | When the mitochondrial phenotype has been produced, determine if adding ME-344 (57) to anti-angiogenic drugs improves anti-tumor action. | Early Phase 1 | NCT02806817 | Completed/2019 |
| Plant extracts | The Function of Plant-Based Dietary Supplements in Intravenous Chemotherapy | Analyze the quantitative effects of dietary supplement consumption on breast cancer patients receiving intravenous infusions of neo-adjuvant and/or adjuvant chemotherapy. | - | NCT03959618 | Completed/2021 |
| Effect of Watercress Consumption on Cancer Patient Results: A Longitudinal Analysis | Examine the possible advantages of therapeutic diets enhanced with watercress-derived nutraceuticals, giving particular attention to how they affect DNA damage and their greater impact on the prognosis of diseases worldwide. | Phase 3 | NCT02468882 | Unknown status/2015 | |
| S-Equol | A Pre-Surgical Clinical Trial Assessing the Efficacy of S-Equol Therapy in Women with Triple-Negative Breast Cancer (TNBC) | To assess the effectiveness of the ERβ agonist S-equol in preventing triple-negative breast cancer (TNBC) cells from proliferating. | Phase 1 | NCT02352025 | Completed/2020 |
| Flavonoids | Effect of Amino Acids and Flavonoids Containing FSMP on Chemotherapy Toxicity, Nutritional Status and Quality of life in Breast Cancer patients | To assess whether an amino acid- and flavonoid-based FSMP with nutritional counseling improves nutritional status, quality of life, and reduces chemotherapy toxicity in breast cancer patients compared to counseling alone. | Not Applicable | NCT05968677 | Recruiting/2025 |
| Hesperidin and Diosmin | The Effect of Oral Administration of Hesperidin and Diosmin in Reducing Paclitaxel-induced Peripheral Neuropathy in Breast Cancer Patients | To evaluate the neuroprotective potential of oral hesperidin and diosmin in reducing paclitaxel-induced peripheral neuropathy in breast cancer patients. | Phase 3 | NCT06811220 | Recruiting/2025 |
| Quercetin | Dasatinib Combined With Quercetin to Reverse Chemo Resistance in Triple-Negative Breast Cancer | To evaluate the efficacy and safety of dasatinib and quercetin in combination with chemotherapy in mTNBC patients who have progressed on prior chemotherapy. | Phase 2 | NCT06355037 | Recruiting/2024 |
| Flavonoids (s) | Synthetic Drug(s)/Combination Agents | Key Patent Claims & Features | Status | Patent Number/Title |
|---|---|---|---|---|
| Isoflavonoids, hydroxyphenyl flavonoids | Kinase inhibitors (nintedanib, dovitinib, regorafenib) | Combination therapies for breast cancer using flavonoids and kinase inhibitors; various dosage forms including oral/IV/topical | Patent granted | ES2877712T3 |
| Luteolin, quercetin, kaempferol | Docetaxel, cisplatin, enzalutamide, radiotherapy | Fixed molar ratio flavonoid blends combined with synthetic chemo/hormonal/radiotherapy; oral and parenteral forms | Patent published | US20170087125A1 |
| Catechins, anthocyanidins, and isoflavones. | Chemotherapeutic agents | Compositions including flavonoids plus chemotherapeutics to enhance anti-cancer effects | Patent granted | US20120213842A1 |
| Flavonoids/antioxidants | Cyclin-dependent kinase (CDK) inhibitors | Therapeutic combinations of flavonoids with CDK inhibitors targeting cancer progression | Patent granted | US10555931B2 |
| Anti-oncogenic flavonoids | Cisplatin, doxorubicin | Novel anti-oncogenic phytochemical combinations with synthetic drugs for cancer treatment | Patent granted | US20240009163A1 |
| Flavonoid mixtures (quercetin, vitamin C, Blueberry Extract) | Methantheline | Flavonoid compositions combined with chemotherapy drugs; methods of use patent | Patent published | WO2017053583A1 |
| Flavonoids/Phenolic Compounds | Anti-Cancer Drug | In Vitro/In Vivo | Cancer Cell Lines/Animal Study | Effect | References |
|---|---|---|---|---|---|
| Luteolin (Flavone) | Tamoxifen | In vitro | MCF-7 | Suppresses Ras expression to cause apoptosis in tamoxifen-resistant ER-positive breast cancer cells. | [187] |
| Doxorubicin | In vitro | MCF-7 | The viability of cells decreased. | [200] | |
| Paclitaxel | In vitro | MDA-MB-231 | Downregulation of BCL-2, mRNA expression | [201] | |
| Quercetin (Flavone) | Docetaxel | In vitro | MDA-MB-231 | Significantly higher BAX levels and upregulated p53 are coupled with decreased expression of the proteins BCL2, pERK1/2, AKT, and STAT3. | [188] |
| 5-fluorouracil | In vitro | MDA-MB-231 | Decreased MMP-2 and MMP-9 gene expression levels and a major decline in migration rate. | [189] | |
| In vitro | MCF-7 | Raising p53, caspase-9 activity, and Bax expression while lowering Bcl2 expression | [90] | ||
| Letrozole | In vitro | MCF-7 | Caused apoptosis produced by the mitochondria and suppressed cellular growth. | [202] | |
| MDA-MB-231 | |||||
| Doxorubicin | In vitro | Doxorubicin MCF-7-resistant | Decreased levels of important genes, such as SNAI2, PLAU, and CSF1, which reverses doxorubicin resistance in breast cancer cells. | [203] | |
| MDA-MB-231 | The MMP-2 and MMP-9 genes showed decreased migration and expression. | [204] | |||
| Genistein (Isoflavone) | Exemestane | In vitro | MCF-7 | Enhanced cytotoxicity | [205] |
| Tamoxifen | In vitro | MCF-7 | Potential to enhance TAM therapy | [190] | |
| Cisplatin | In vitro | Ovariectomized nude mouse breast cancer xenograft model | Enhanced the development of apoptosis and inhibited cell growth. | [191] | |
| ERB-041 | In vitro | CMT-U27; CF41.Mg | Downregulated the expression of ERα, which in turn decreased the regulation of the PI3K/AKT pathway. | [206] | |
| Centchroman | In vitro | MDA-MB-468 MDA-MB-231 MCF-7 | Synergistic anti-cancer potential | [207] | |
| Curcumin (Natural phenol) | Docetaxel | In vitro | MCF-7 | Maximum rates of cytotoxicity, cell apoptosis induction, and cellular uptake | [192] |
| Sorafenib | In vitro | MCF-7 | Apoptotic cell death is induced when vimentin, IL-6, STAT3, and MMP-9 levels are decreased and E-cadherin protein expression is increased. | [193] | |
| Doxorubicin | In vitro | MDA-MB-231 | Indicated that the AKT/mTOR pathway is being suppressed. | [194] | |
| In vitro | MDA-MB-231 MCF-7 | G0/G1 and S-phase cell cycle arrest were associated with enhanced mRNA expressions of the TP53, BRCA1, BRCA2, ATM, and CHEK2 genes (Ct-value). | [195] | ||
| Paclitaxel | In vitro | MCF-7 MDA-MB-231 | Curcumin decreased capacity of M2 TAMs to generate chemoresistance. | [196] | |
| Apatinib | In vitro | MCF-7 | Reduced proliferation and survival | [208] | |
| Celecoxib | In vitro | MDA-MB-231 | Suppressing the COX-2 pathways. | [209] | |
| 5-Fluorouracil | In vitro | MCF-7 | Pro-apoptotic, anti-metastatic, and anti-proliferative. | [210] | |
| Carboplatin | In vitro | CAL-51, CAL-51-R, MDA-MB-231 cells | By enhancing ROS-induced DNA damage, curcumin makes TNBC more susceptible to the anti-cancer effects of carboplatin, offering an efficient combined therapy approach for TNBC. | [197] | |
| Xanthohumol (Prenylflavonoid) | Doxorubicin | In vitro | Doxorubicin-resistant breast cancer cells MCF-7/ADR | Decreased the viability of tumor cells, caused apoptosis, slowed the cell cycle, improved the effects of doxorubicin, decreased stemness, elevated c-H2AX, inhibited STAT3 and EGFR, decreased Bcl-2 and pro-caspase 3, and improved Bax expression. | [211] |
| Doxorubicin-resistant breast cancer cells MCF-7/ADR | Reduced protein expression via inhibiting STAT3 and EGFR, which in turn controlled apoptosis resistance; reduced the expression of MDR1 but not BCRP. | [212] | |||
| Garcinol (Polyphenol) | Paclitaxel | In vivo | 4T1-Luc female Balb/c mice (5–6-weeks-old) | Downregulation of caspase-3, iPLA2, Cyclin A2, Cyclin B1, Cdc25A, Cdc2, Bcl-2, and COX-2, inhibition of paclitaxel-induced NF-κB/Twist1-regulated premetastatic signalling, and lower MMP-2 and MMP-9 activity were observed. | [213] |
| Naringenin | Cyclophosphamide | In vitro | MDA-MB-231 | BAX expression increased whereas Bcl-2 expression decreased. Caspases 3 and 9 were stimulated. | [198] |
| Doxorubicin | In vitro | Breast cancer mouse model | Combination therapy improves anticancer activity in vivo, reducing tumour volume and weight. | [214] | |
| In vitro and In vivo | MDA-MB-231 and 4T1 cells Xenograft mouse model | Increased cytokines (TNF-α and IL-1β) and decreased dose-related body weight loss | [199] | ||
| T47D and MCF-7 cells | In p53-deficient T47D cells, naringenin and hesperidin increased doxorubicin-induced G2/M arrest through a p53-independent pathway. whereas, in p53 wild-type MCF-7 cells, they inhibited G2/M arrest, showing that their anticancer activity is not dependent on cell cycle arrest. | [215] | |||
| Naringin | Capecitabine | In vitro | MCF-7 and SK-BR-3 | Elevation in the Bax/Bcl-2 ratio | [216] |
| Doxorubicin | In vitro | MCF-7 | Significantly raised the level of Bax while inhibiting the expression of STAT3, JAK1, Bcl-2, Survivin, and VEGF. | [217] | |
| 5-Fluorouracil | In vitro | MDA-MB-231 | Reduced cytological toxicity of 5-Fluorouracil | [218] | |
| Mangiferin (Polyphenolic C-glycoside) | Doxorubicin | In vitro | MCF-7 had already received short-term Doxorubicin | Suppressed P-gp expression | [219] |
| Proanthocyanidins (Polyphenols) | Histone deacetylase inhibitor Chidamide | In vitro | T47D and MDA-MB-231 | Suppress the growth and proliferation, while promoting apoptosis | [220] |
| 5-Fluorouracil | MDA-MB-231 | Cell viability and migration declined, apoptosis was induced, cell cycle was arrested in the G2/M phase, generated ROS was increased, mitochondrial membrane potential reduced, the Bax/Bcl-2 ratio was increased, caspase-3 was cleaved, and there was synergistic cytotoxicity with 5-fluorouracil. | [221] | ||
| Apigenin | Doxorubicin | In vitro | DOX resistant MCF-7 cells and MCF-7R | Reduced the phosphorylation and induction of JAK2 and STAT3 proteins. | [105] |
| MCF-7 | Interact with DNA, thus blocking the transcription process | [222] | |||
| Vorinostat | MDA-MB-231 | Increased HAT activity while suppressing DNMT and HDAC enzymatic activity. | [223] | ||
| Kaempferol | Docetaxel | In vitro | MCF-7 | Anti-CSCs agent (decreasing the markers linked to CSCs, such as aldh1a1 and abcb1) | [224] |
| Hesperidin | Doxorubicin | In vitro | Hela Cell Line | Enhanced the expression of Bax and reduced the level of Bcl-2 | [225] |
| MCF-7/HER2 cells | Reduced HER2, Rac1, MMP9 expression, apoptosis, and cell migration after inducing cell cycle arrest | [226] | |||
| MCF-7 resistant doxorubicin cells | Reduced level of Pgp expressions | [227] | |||
| 4T1 | Inhibition of Rac-1 and metalloproteinase-9 expression | [228] | |||
| Tamoxifen | In vivo | xerographic MCF-7 injected rats | Apoptotic genes (Bax, Casp3) are upregulated, while antiapoptotic genes (Bcl-2) and angiogenesis genes VEGF are downregulated. | [229] |
| Nano-Formulation | Flavonoid/Phenolic Compounds | Size (nm) | Effect | References |
|---|---|---|---|---|
| Polymeric nanoformulation | Quercetinhyaluronic acid | 230–480 nm | Prevent tumour development in tumour-developing mice using the passive EPR effect selective targeting mediated by CD44 receptor. | [233] |
| Quercetin–chitosan | <200 | Elevated serum SOD levels | [234] | |
| Quercetin–MPEG-PLA | 155 ± 3 | Induction of cell apoptosis | [235] | |
| Quercetin–Mesoporous silica | <200 | Apoptosis and cell cycle arrest by controlling the AKT and Bax signalling pathways-1. | [236] | |
| EGCG-PEG | 140 ± 7 to 182 ± 13 | Increased expression of p21, PTEN, and Bax, while inhibition of p-AKT, p-PDK1, Bcl-2 and Cyclin D1, reduced cancer cell migration. | [237] | |
| Fisetin–PLA | 225 ± 4 | Enhance the bioavailability and anti-cancer activity of fisetin. | [238] | |
| Lipid –polymer nanoparticles | Quercetin–Mycophenolic acid | 135 ± 10 to 175 ± 30 | Improved pharmacokinetics/pharmacodynamics, reduced first-pass metabolism and displayed additive/synergistic pharmacodynamics. | [239] |
| Solid-lipid nanoparticles | EGCG–Bombesin | 164 ± 2 | Both intrinsic and extrinsic routes can cause apoptosis, which reduces the amount of nutrients that cancer cells receive and prevents migration and angiogenesis. | [240] |
| Gold Nanoparticle | Quercetin–AgFeO2 | 19 | Under UV light, photodynamic therapy reduces cell growth. | [241] |
| Quercetin | 5 | Decreased expression of Vimentin, N-cadherin, Snail, Slug, Twist, MMP-2, MMP-9, p-EGFR, VEGFR-2, p-PI3K, Akt, and p-GSK3β, along with increased expression of E-cadherin protein, substantially decreased epithelial–mesenchymal transition, angiogenesis, and metastasis. | [242] | |
| Hesperidin | 40 | prevent cytokine secretion, including IL-1β, IL-6, IL-8, NO, and TNF-α, causing a rise in ROS. | [243] | |
| Silver Nanoparticles | Apigenin | 94 | Enhance caspase-3 protein, causing apoptosis in tumour cells. | [244] |
| Nano-Formulation | Flavonoid/Phenolic Compounds | Size (nm) | Effect | References |
|---|---|---|---|---|
| Liposomes | Quercetin and vincristine | 130–200 | Co-encapsulated medicines improved quercetin solubility, resulting in synergistic effects and controlled release. | [255] |
| Quercetin and vincristine | 130 | Successful reduction of tumour development in the JIMT-1 patient breast carcinoma xenograft model | [256] | |
| PEGylated Liposomes | Quercetin–Adriamycin (AMD) and doxorubicin | 85 | The combination prevents AMD-induced myelosuppression, elevates white blood cell levels, decreases myocardial cell death due to AMD, and promotes toxicity against resistant tumour cells. | [257] |
| Liposomes | Quercetin and doxorubicin (DOX) | 85 ± 2 | Reduced levels of Nrf2 and its related cytoprotective enzymes, including NQO1 and the drug transporter MRP1, is linked to apoptosis induction. | [258] |
| PLGA-Casein Nanoparticle | EGCG and paclitaxel (PTX) | 190 ± 12 | PTX triggered apoptosis while also suppressing essential genes required for tumour survival. | [259] |
| Lipid Nanoformulation | Silibinin–TPGS | 45 | Reduced levels of MMP-9 and Snails | [260] |
| Albumin based nanoparticles | Epicatechin and morin | 170 ± 6 | Prevent the successful proliferation of cancer cells, leading to cell death. | [261] |
| Nanoparticles based on polyethylene glycol. | Quercetin and Nickel | 50–700 | Reduced mitochondrial membrane potential and increased oxidative stress brought on by an excess of ROS. | [262] |
| Zein-lactoferrin micelles (Lipid nanoparticles) | Rapamycin and wagonin | 277 | Reduced levels of the PI3K-AKT and MAPK pathways, together with anti-angiogenic effects. | [263] |
| AEEAA-PEG-PCL Nanoparticle | Silibinin and IPI-549 | 35–37 | Inhibition of collagen production, angiogenesis, and anti-fibrotic effects in cancerous tissue | [264] |
| pW lipid Nano-particles | Silibinin and cryptotanshinone | 250 | Suppress CD31, TGF-β1, and MMP-9. | [265] |
| Polymeric Nanoparticle | Chyrisin and methotrexate | 198 | Enhanced disruption and cell wall shrinking causes apoptosis. | [266] |
| Chyrisin and curcumin | 400–500 | Upregulation of Cyclin D1, hTERT, Bax/Bcl-2, p53, caspase-3 & 7. | [267] | |
| Zein protein Nanocarrier | EGCG and piperine | 34–80 | The drug is beneficial in NPS because of its higher cellular intake and extended release. | [268] |
| Gold Nanoparticles | EGCG and citrate | 25 | It efficiently controls NF-κB, increasing apoptotic proteins like caspase-3, caspase-7, and Bax and decreasing anti-apoptotic proteins like Bcl2. | [269] |
| Polymeric Nanoparticles | EGCG and curcumin and alpha-tocopheryl succinate | 200–300 | Modified with transferrin and folate, two ligands unique to cancer that support antiproliferative activity. | [270] |
| Nanoemulsion | Baicalein–paclitaxel | 171 ± 6.2 | Decreased glutathione synthesis, and initiated apoptosis | [271] |
| Polymeric Nanoparticles | Quercetin–tamoxifen | >200 | Reduced MMP-2 and MMP-9, enhanced oxidative stress, and decreased GSH caused by lipid peroxidation | [272] |
| Nanoemulsion | Naringenin–tamoxifen | <701 | Reduced P-gp efflux leads to increased anticancer activity. | [273] |
| Nanomicelles | EGCG–Herceptin1 | 100–500 | Extended blood half-life and decreased cancer cell growth. | [274] |
| Polymeric nanoparticles | Quercetin–doxorubicin | 105 | P-glycoprotein expression and function are diminished. | [275] |
| Polymeric Nanoparticles | Quercetin–topotecan | <200 | Cell death occurs when there are significant modifications in the endoplasmic reticulum, mitochondria, and cell wall. | [276] |
| Nanofibers | Quercetin and tamoxifen citrate | Uniform size | The slow process of tumour development | [277] |
| Polymeric Nanoparticles | Genistein–Chitosan–Tamoxifen | 299.8 | The slow process of tumor development | [190] |
| Nanomicelles | Docetaxel and Curcumin | ~64 | The greatest levels of cellular absorption, cytotoxicity, cell apoptosis induction, and reactive oxygen species (ROS) production | [192] |
| Liposomes | Rapamycin–curcumin | Uniform size | Target mTOR to inhibit breast cancer development. | [278] |
| nanocomposites | chitosan/agarose/graphene oxide/montmorillonite–curcumin | Extended release of curcumin to inhibit MCF7 breast cancer cells | [279] | |
| Nanomicelles | Doxorubicin and Curcumin | 187 ± 1.36 | Activated apoptosis and reduced multidrug resistance (MDR) in breast cancer | [280] |
| Nanoparticles | curcumin/Ko143/PLGA | 232.32 ± 10.60 | Promote the intracellular level of the photosensitiser Curcumin (Cur) and improve photodynamic effectiveness by inhibiting the function of efflux pump. | [281] |
| Nanoparticles | Paclitaxel and curcumin | 85.8 ± 0.21 | Showed significant tumour suppression; prevent the formation of P-glycoprotein. | [282] |
| Niosome | Doxorubicin and curcumin | 200 | Regulation of PI3K/Akt/mTOR | [283] |
| Polymeric Micelles | Rutin/Benzoic Acids/Triazolofluoroquinolones | 18 ± 2 | Interaction with the DNA-Topoisomerase I enzyme complex. | [284] |
| Gold nanoparticle | Kaempferol and sorafenib | Uniform size | Enhanced apoptosis rate. | [285] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uniyal, P.; Akhtar, A.; Rawat, R. Flavonoid-Based Combination Therapies and Nano-Formulations: An Emerging Frontier in Breast Cancer Treatment. Pharmaceuticals 2025, 18, 1486. https://doi.org/10.3390/ph18101486
Uniyal P, Akhtar A, Rawat R. Flavonoid-Based Combination Therapies and Nano-Formulations: An Emerging Frontier in Breast Cancer Treatment. Pharmaceuticals. 2025; 18(10):1486. https://doi.org/10.3390/ph18101486
Chicago/Turabian StyleUniyal, Priyanka, Ansab Akhtar, and Ravi Rawat. 2025. "Flavonoid-Based Combination Therapies and Nano-Formulations: An Emerging Frontier in Breast Cancer Treatment" Pharmaceuticals 18, no. 10: 1486. https://doi.org/10.3390/ph18101486
APA StyleUniyal, P., Akhtar, A., & Rawat, R. (2025). Flavonoid-Based Combination Therapies and Nano-Formulations: An Emerging Frontier in Breast Cancer Treatment. Pharmaceuticals, 18(10), 1486. https://doi.org/10.3390/ph18101486








