Tacrolimus-Associated Tremor in Renal Transplant Patients: Potential Impact of the Galenic Formulation
Abstract
1. Introduction
2. Results
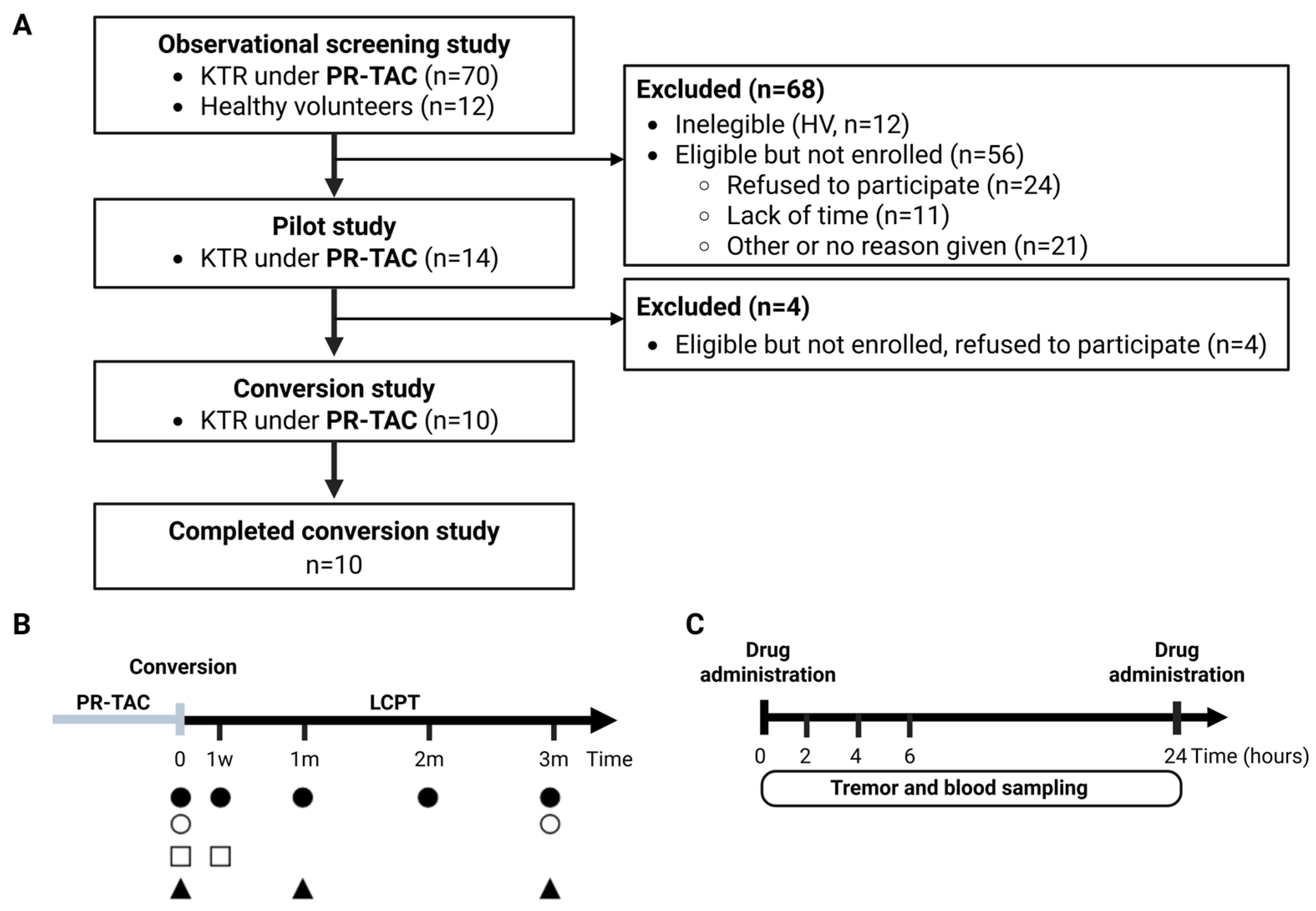
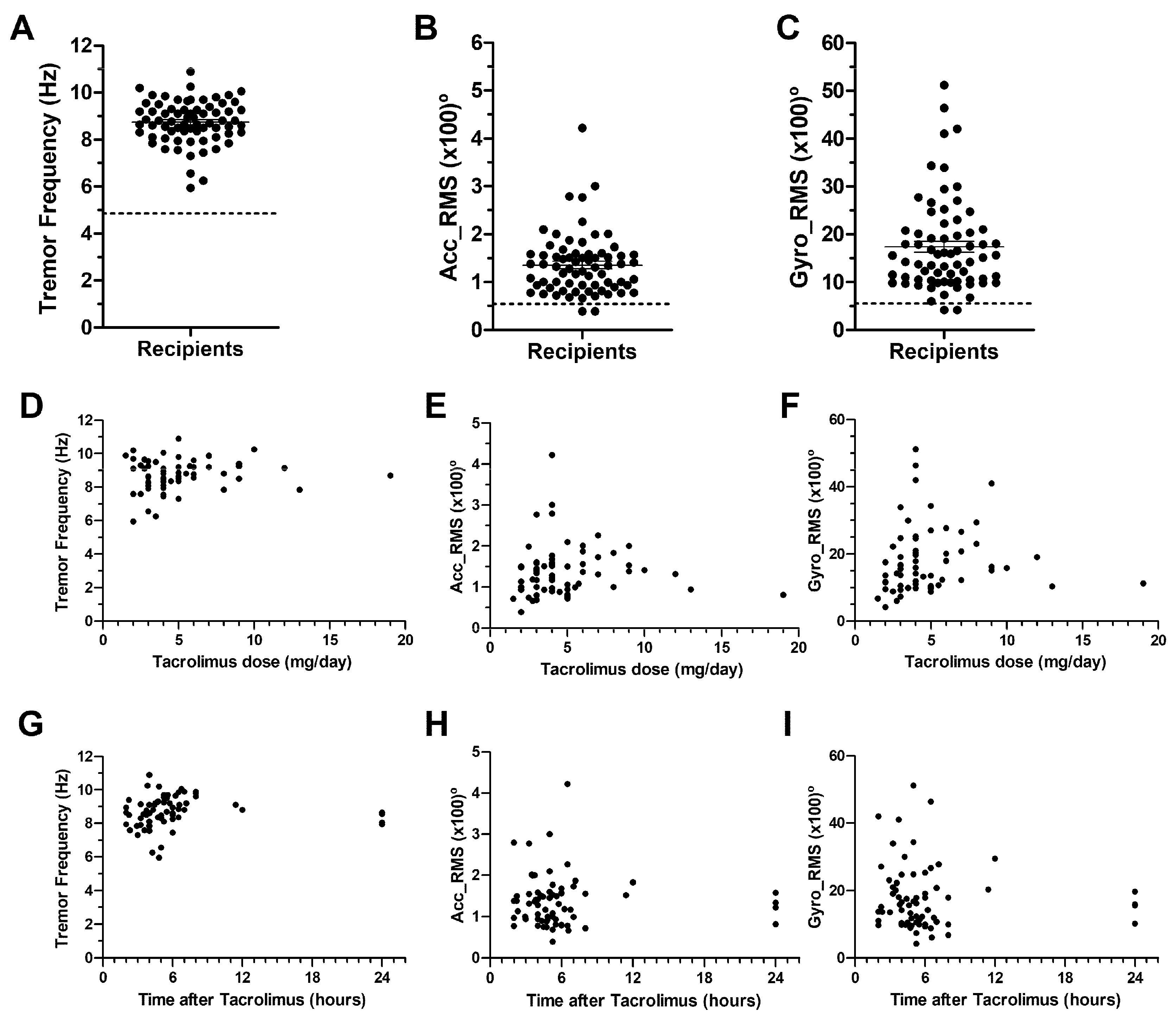
2.1. Observational Screening Study
2.2. Pilot Study. Tremor, and Tacrolimus Blood Level Correlations
2.3. Conversion Study
2.3.1. Patient Characteristics
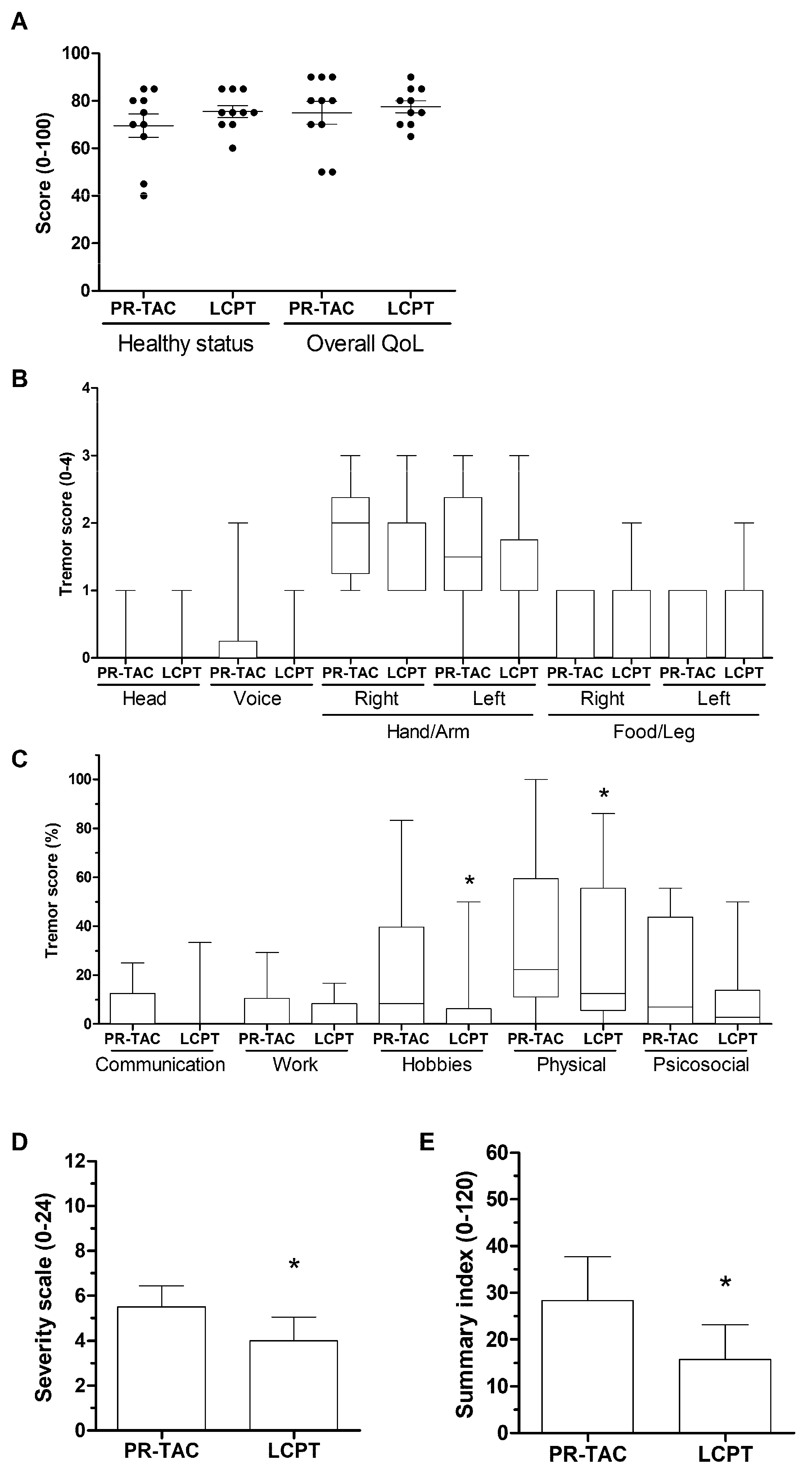
2.3.2. Impact of Conversion on Tremor
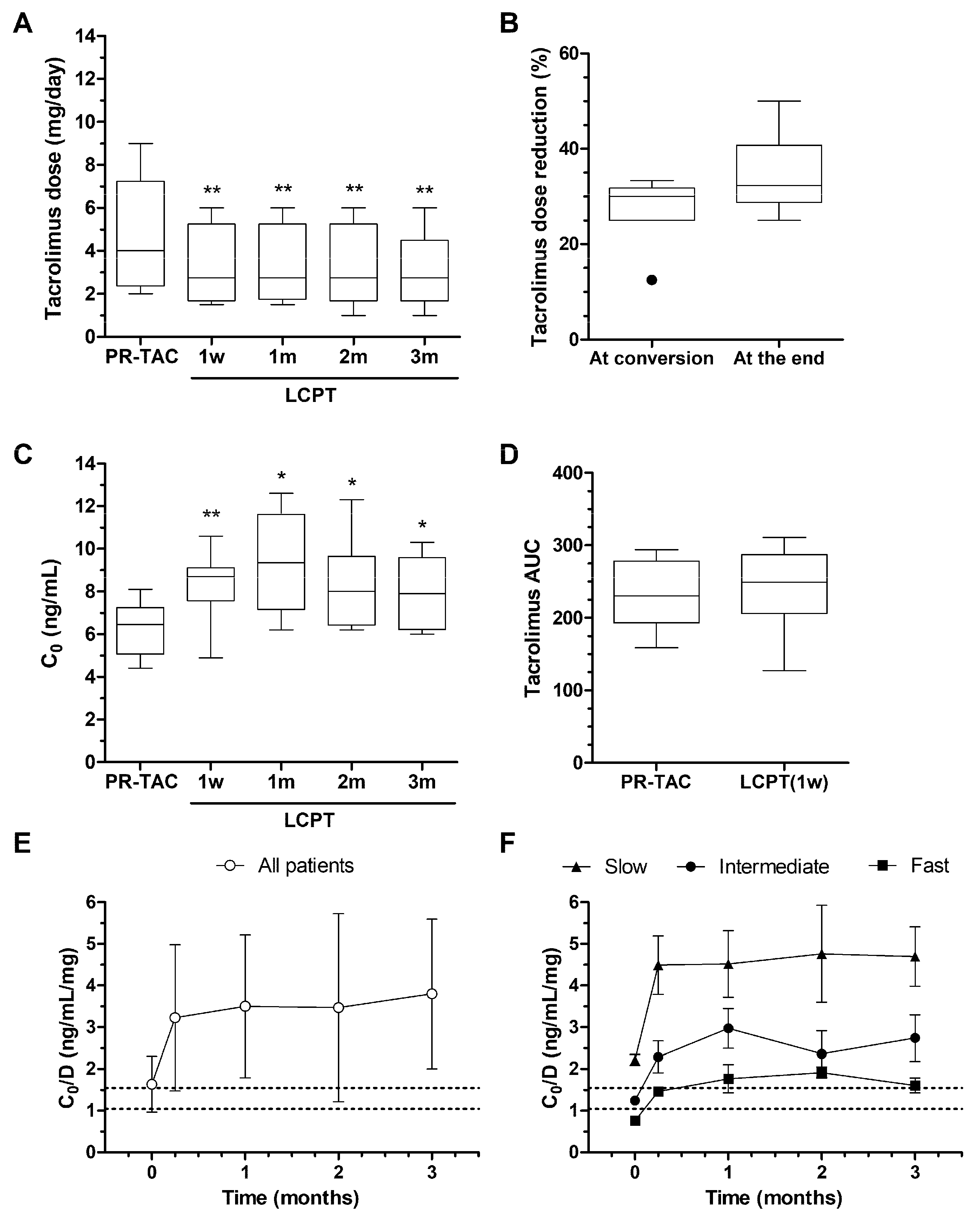
2.3.3. Impact of Conversion on Tacrolimus Dose, Blood Levels, and AUC
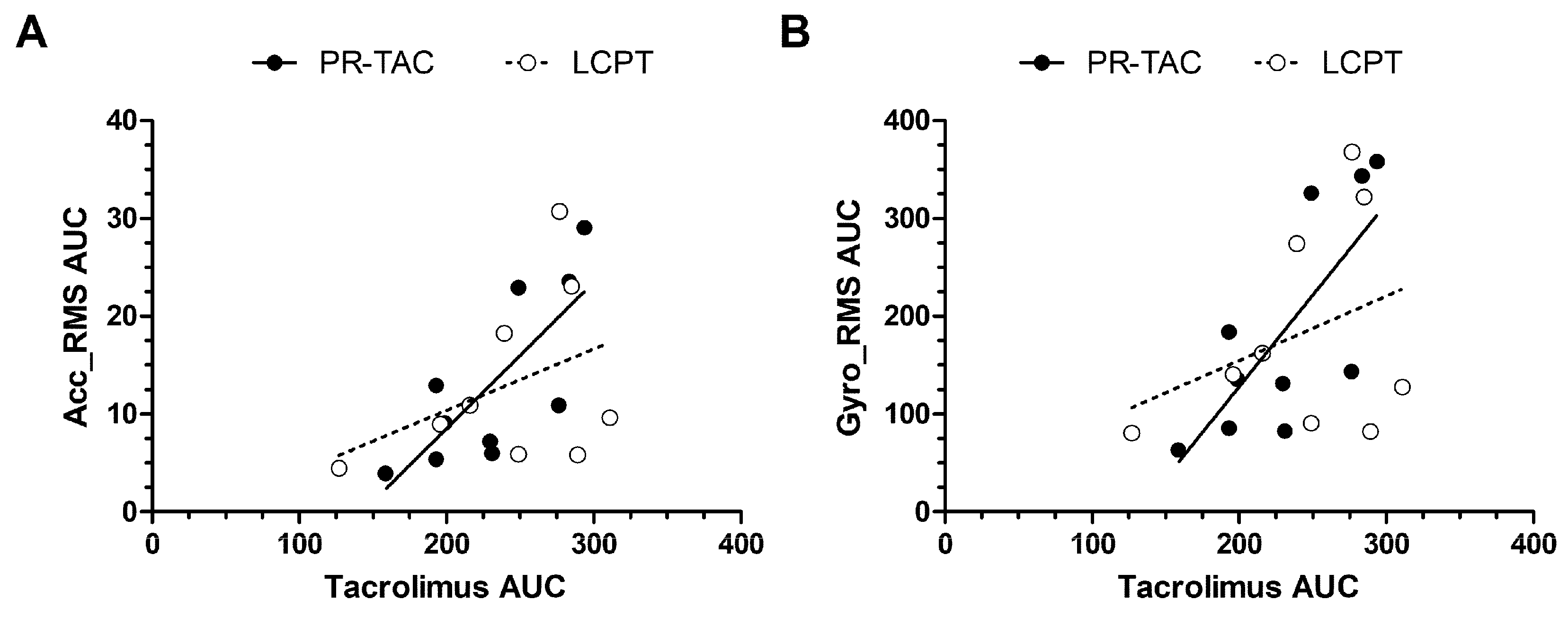
2.3.4. Impact of Conversion on Correlations Between Tacrolimus AUC and Tremor AUC
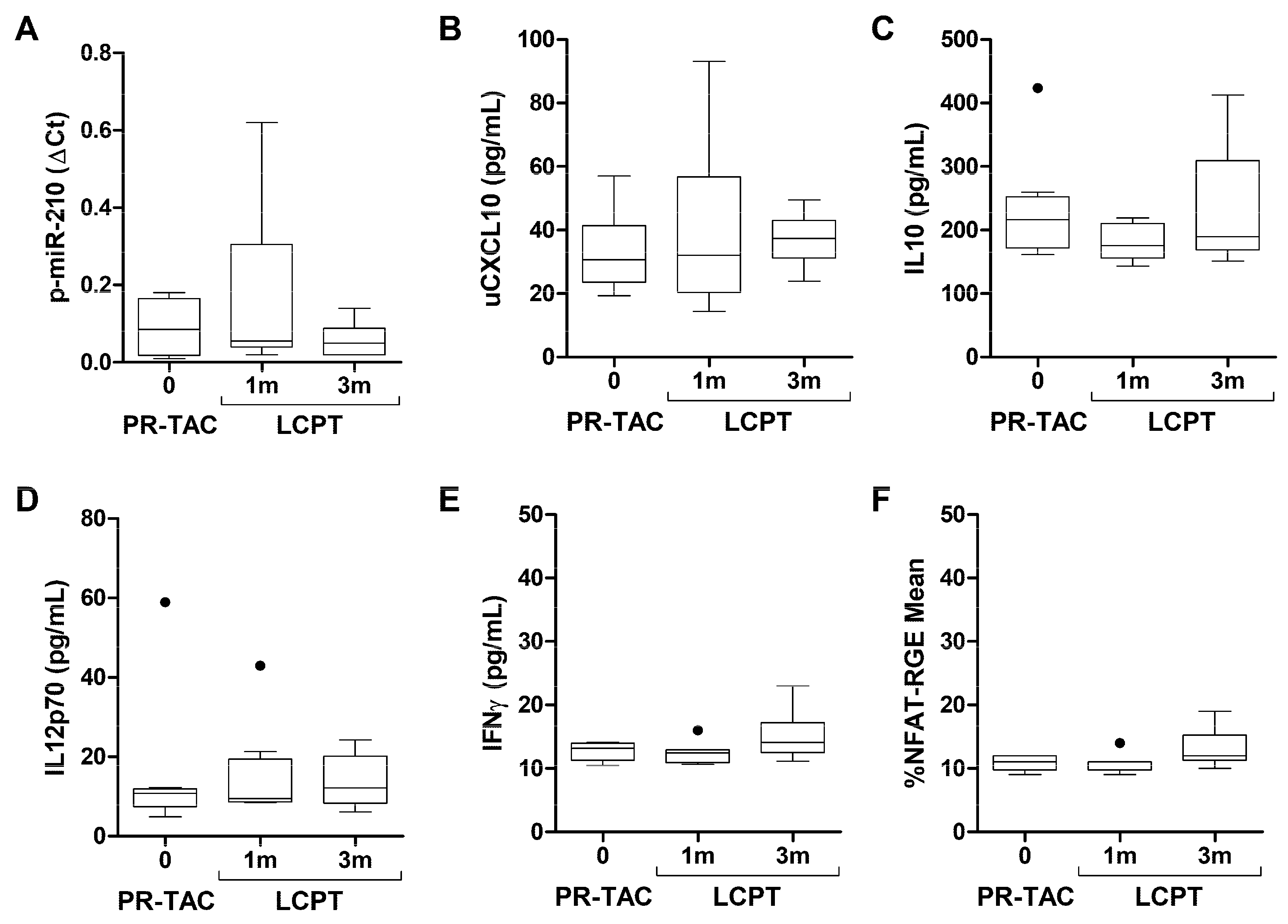
2.4. Biomarkers
3. Discussion
4. Methods
4.1. Patients and Procedures for the Screening Study
4.2. Patients and Procedures from the Conversion Study
4.3. Tremor Quantification Using DyCare Device
4.4. Quality of Life Measures
4.5. Blood Sample Collection
4.6. Tacrolimus AUC
4.7. Biomarkers Analysis
4.7.1. Plasmatic Expression of miRNA-210-3p
4.7.2. Urinary CXCL-10 Analysis
4.7.3. Soluble Cytokine Production
4.7.4. NFAT-Regulated Gene Expression
4.7.5. Quantitative Analysis of IL-2, IFNγ and GM-CSF Gene Expressions
4.8. Statistics
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, A.C.; Woodroffe, R.C.; Taylor, R.S.; Chapman, J.R.; Craig, J.C. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. Bmj 2005, 331, 810. [Google Scholar] [CrossRef] [PubMed]
- Bajon, A.; Sikora, J.; Siwek, M.; Wiecanowska, J.; Miedziaszczyk, M.; Idasiak-Piechocka, I.; Mania, A. Neurotoxicity of Calcineurin Inhibitors. Ann. Indian Acad. Neurol. 2025, 28, 505. [Google Scholar] [CrossRef] [PubMed]
- Miedziaszczyk, M.; Idasiak-Piechocka, I. Safety analysis of co-administering tacrolimus and omeprazole in renal transplant recipients - A review. Biomed. Pharmacother. 2023, 166, 115149. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Nigro, V.; Weinberg, J.; Woodle, E.S.; Alloway, R.R. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am. J. Transplant. 2017, 17, 432. [Google Scholar] [CrossRef]
- Gaber, A.O.; Alloway, R.R.; Bodziak, K.; Kaplan, B.; Bunnapradist, S. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): A phase 2 trial of stable renal transplant recipients. Transplantation 2013, 96, 191. [Google Scholar] [CrossRef]
- Bunnapradist, S.; Ciechanowski, K.; West-Thielke, P.; Mulgaonkar, S.; Rostaing, L.; Vasudev, B.; Budde, K. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): The phase III randomized MELT trial. Am. J. Transplant. 2013, 13, 760. [Google Scholar] [CrossRef]
- Budde, K.; Rostaing, L.; Maggiore, U.; Piotti, G.; Surace, D.; Geraci, S.; Procaccianti, C.; Nicolini, G.; Witzke, O.; Kamar, N.; et al. Prolonged-Release Once-Daily Formulation of Tacrolimus Versus Standard-of-Care Tacrolimus in de novo Kidney Transplant Patients Across Europe. Transpl. Int. 2022, 35, 10225. [Google Scholar] [CrossRef]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Le Meur, Y.; Marquet, P.; Oellerich, M.; et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther. Drug Monit. 2009, 31, 139. [Google Scholar] [CrossRef]
- Abouljoud, M.S.; Kumar, M.S.; Brayman, K.L.; Emre, S.; Bynon, J.S. Neoral rescue therapy in transplant patients with intolerance to tacrolimus. Clin. Transplant. 2002, 16, 168. [Google Scholar] [CrossRef]
- Tholking, G.; Fortmann, C.; Koch, R.; Gerth, H.U.; Pabst, D.; Pavenstädt, H.; Kabar, I.; Hüsing, A.; Wolters, H.; Reuter, S.; et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS ONE. 2014, 9, e111128. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Asberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261. [Google Scholar] [CrossRef] [PubMed]
- Bechstein, W.O. Neurotoxicity of calcineurin inhibitors: Impact and clinical management. Rev. Transpl. Int. 2000, 13, 313–326. [Google Scholar] [CrossRef]
- Bulatova, N.; Yousef, A.M.; Al-Khayyat, G.; Qosa, H. Adverse effects of tacrolimus in renal transplant patients from living donors. Curr. Drug Saf. 2011, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Kugler, C.; Fischer, S.; Gottlieb, J.; Tegtbur, U.; Welte, T.; Goerler, H.; Simon, A.; Haverich, A.; Strueber, M. Symptom experience after lung transplantation: Impact on quality of life and adherence. Clin. Transplant. 2007, 21, 590. [Google Scholar] [CrossRef]
- Kugler, C.; Geyer, S.; Gottlieb, J.; Simon, A.; Haverich, A.; Dracup, K. Symptom experience after solid organ transplantation. J. Psychosom. Res. 2009, 66, 101. [Google Scholar] [CrossRef]
- de Barros, C.T.; Cabrita, J. Self-report of symptom frequency and symptom distress in kidney transplant recipients. Pharmacoepidemiol. Drug Saf. 1999, 8, 395–403. [Google Scholar] [CrossRef]
- Drent, G.; De Geest, S.; Dobbels, F.; Kleibeuker, J.H.; Haagsma, E.B. Symptom experience, nonadherence and quality of life in adult liver transplant recipients. Neth. J. Med. 2009, 67, 161. [Google Scholar] [PubMed]
- Langone, A.; Steinberg, S.M.; Gedaly, R.; Chan, L.K.; Shah, T.; Sethi, K.D.; Nigro, V.; Morgan, J.C. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): An open-label, multicenter, prospective phase 3b study. Clin. Transplant. 2015, 29, 796. [Google Scholar] [CrossRef]
- Giral, M.; Grimbert, P.; Morin, B.; Bouvier, N.; Buchler, M.; Dantal, J.; Garrigue, V.; Bertrand, D.; Kamar, N.; Malvezzi, P.; et al. Impact of Switching from Immediate- or Prolonged-Release to Once-Daily Extended-Release Tacrolimus (LCPT) on Tremor in Stable Kidney Transplant Recipients: The Observational ELIT Study. Transpl. Int. 2024, 37, 11571. [Google Scholar] [CrossRef]
- Brunet, M.; Shipkova, M.; van Gelder, T.; Wieland, E.; Sommerer, C.; Budde, K.; Haufroid, V.; Christians, U.; López-Hoyos, M.; Barten, M.J.; et al. Barcelona Consensus on Biomarker-Based Immunosuppressive Drugs Management in Solid Organ Transplantation. Ther. Drug Monit. 2016, 38 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Wijdicks, E.F. Neurotoxicity of immunosuppressive drugs. Review. Liver Transpl. 2001, 7, 937. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, B.H.; Abu-Elmagd, K.; Wilson, J.; Fung, J.J.; Alessiani, M.; Jain, A.; Takaya, S.; Todo, S.N.; Tzakis, A.; Van Thiel, D.; et al. Neurologic complications of FK 506. Transplant. Proc. 1991, 23, 3175. [Google Scholar] [PubMed]
- Appignani, B.A.; Bhadelia, R.A.; Blacklow, S.C.; Wang, A.K.; Roland, S.F.; Freeman, R.B., Jr. Neuroimaging findings in patients on immunosuppressive therapy: Experience with tacrolimus toxicity. AJR Am. J. Roentgenol. 1996, 166, 683. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Bain, P.; Brin, M. Consensus statement of the Movement Disorder Society on Tremor. Mov. Disord. 1998, 13 (Suppl. 3), 2. [Google Scholar] [CrossRef]
- Paul, F.; Muller, J.; Christe, W.; Steinmuller, T.; Poewe, W.; Wissel, J. Postural hand tremor before and following liver transplantation and immunosuppression with cyclosporine or tacrolimus in patients without clinical signs of hepatic encephalopathy. Clin. Transplant. 2004, 18, 429. [Google Scholar] [CrossRef]
- Riemersma, N.L.; Kremer, D.; Knobbe, T.J.; Gan, C.T.; Nolte, S.; Gomes-Neto, A.W.; Blokzijl, H.; de Meijer, V.E.; Damman, K.; Eisenga, M.F.; et al. Tremor, Daily Functioning, and Health-Related Quality of Life in Solid Organ Transplant Recipients. Clin. Trial. Transpl. Int. 2023, 36, 10951. [Google Scholar] [CrossRef]
- Heits, N.; Keserovic, D.; Mund, N.; Ehmke, N.; Bernsmeier, A.; Hendricks, A.; Gunther, R.; Witt, K.; Becker, T.; Braun, F. Cognitive Evaluation in Liver Transplant Patients Under Calcineurin Inhibitor Maintenance Therapy. Transplant. Direct. 2017, 3, e146. [Google Scholar] [CrossRef]
- Campagne, O.; Mager, D.E.; Brazeau, D.; Venuto, R.C.; Tornatore, K.M. The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br. J. Clin. Pharmacol. 2019, 85, 516. [Google Scholar] [CrossRef]
- King, C.P.; Cossart, A.R.; Isbel, N.M.; Campbell, S.B.; Staatz, C.E. The association between tacrolimus exposure and tremor, headache and insomnia in adult kidney transplant recipients: A systematic review. Transplant Rev. 2024, 38, 100815. [Google Scholar] [CrossRef]
- Bunthof, K.L.W.; Al-Hassany, L.; Nakshbandi, G.; Hesselink, D.A.; van Schaik, R.H.N.; Dam, M.A.G.J.T.; Baas, M.C.; Hilbrands, L.B.; van Gelder, T. A randomized crossover study comparing different tacrolimus formulations to reduce intrapatient variability in tacrolimus exposure in kidney transplant recipients. Clin. Transl. Sci. 2022, 15, 930. [Google Scholar] [CrossRef]
- Millán, O.; Ruiz, P.; Fortuna, V.; Navasa, M.; Brunet, M. Nuclear factor of activated T cells as potential pharmacodynamic biomarker for the risk of acute and subclinical rejection in de novo liver recipients. Liver Int. 2020, 40, 931. [Google Scholar] [CrossRef]
- Sommerer, C.; Brunet, M.; Budde, K.; Millán, O.; Guirado Perich, L.; Glander, P.; Meuer, S.; Zeier, M.; Giese, T. Monitoring of gene expression in tacrolimus-treated de novo renal allograft recipients facilitates individualized immunosuppression: Results of the IMAGEN study. Br. J. Clin. Pharmacol. 2021, 87, 3851. [Google Scholar] [CrossRef]
- Millán, O.; Budde, K.; Sommerer, C.; Aliart, I.; Rissling, O.; Bardaji, B.; Matz, M.; Zeier, M.; Silva, I.; Guirado, L.; et al. Urinary miR-155-5p and CXCL10 as prognostic and predictive biomarkers of rejection, graft outcome and treatment response in kidney transplantation. Br. J. Clin. Pharmacol. 2017, 83, 2636. [Google Scholar] [CrossRef] [PubMed]
- Troster, A.I.; Pahwa, R.; Fields, J.A.; Tanner, C.M.; Lyons, K.E. Quality of life in Essential Tremor Questionnaire (QUEST): Development and initial validation. Park. Relat. Disord. 2005, 11, 367. [Google Scholar] [CrossRef]
- Martínez-Martín, P.; Jiménez-Jiménez, F.J.; Carroza Garcia, E.; Alonso-Navarro, H.; Rubio, L.; Calleja, P.; Díaz-Sánchez, M.; Benito-León, J. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. J. Clin. Epidemiol. 2010, 63, 767. [Google Scholar] [CrossRef]
- Millán, O.; Ruiz, P.; Orts, L.; Ferré, P.; Crespo, G.; Santana, M.; Fortuna, V.; Quintairos, L.; Navasa, M.; Brunet, M. Monitoring of miR-181a-5p and miR-155-5p Plasmatic Expression as Prognostic Biomarkers for Acute and Subclinical Rejection in de novo Adult Liver Transplant Recipients. Front. Immunol. 2019, 10, 873. [Google Scholar] [CrossRef]
- Giese, T.; Zeier, M.; Schemmer, P.; Uhl, W.; Schoels, M.; Dengler, T.; Buechler, M.; Meuer, S. Monitoring of NFAT-regulated gene expression in the peripheral blood of allograft recipients: A novel perspective toward individually optimized drug doses of cyclosporine A. Transplantation 2004, 77, 339. [Google Scholar] [CrossRef]
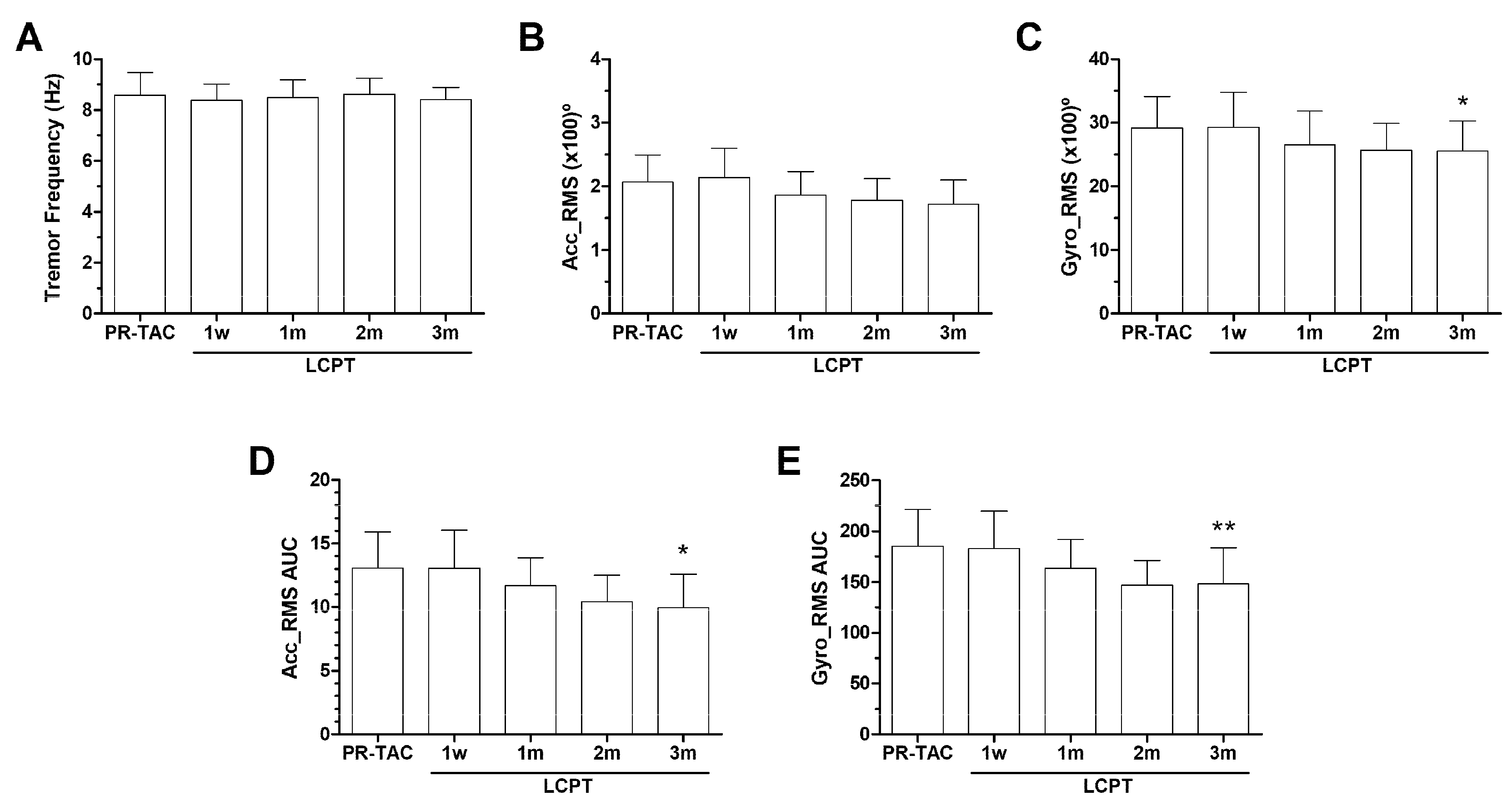
| Healthy Volunteers | KTx Recipients | p Value | |
|---|---|---|---|
| Number | 12 | 70 | |
| Tacrolimus dose (mg/day) | 0 | 4.84 (4.15–5.53) | |
| Time after last dose (h) | NA | 6.48 (5.15–7.82) | |
| Frequency (Hz) | 4.87 ± 1.06 | 8.74 ± 0.11 | 0.0163 |
| Accelerometer RMS (×100)° | 0.54 ± 0.05 | 1.36 ± 0.08 | <0.001 |
| Gyroscope RMS (×100)° | 5.57 ± 0.46 | 17.38 ± 1.16 | <0.001 |
| Blood Parameters | Baseline | At the End | Significance |
|---|---|---|---|
| Creatinine mg/dL | 1.51 ± 0.13 | 1.68 ± 0.11 | ns |
| eGFR ml/min/1.73 m2 | 49.9 ± 5.97 | 41.0 ± 4.33 | ns |
| Na mEq/L | 141.5 ± 4.2 | 143.4 ± 1.6 | ns |
| K mEq/L | 4.24 ± 0.13 | 4.40 ± 0.12 | ns |
| Glucose | 100.5 ± 9.46 | 97.4 ± 6.34 | ns |
| AST U/L | 20.10 ± 1.92 | 18.88 ± 2.54 | ns |
| ALT U/L | 20.90 ± 2.51 | 22.50 ± 3.21 | ns |
| Total Cholesterol | 178.4 ± 14.35 | 166.4 ± 14.40 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovira, J.; Millán, O.; Ventura-Aguiar, P.; Brunet, M.; Diekmann, F. Tacrolimus-Associated Tremor in Renal Transplant Patients: Potential Impact of the Galenic Formulation. Pharmaceuticals 2025, 18, 1488. https://doi.org/10.3390/ph18101488
Rovira J, Millán O, Ventura-Aguiar P, Brunet M, Diekmann F. Tacrolimus-Associated Tremor in Renal Transplant Patients: Potential Impact of the Galenic Formulation. Pharmaceuticals. 2025; 18(10):1488. https://doi.org/10.3390/ph18101488
Chicago/Turabian StyleRovira, Jordi, Olga Millán, Pedro Ventura-Aguiar, Mercè Brunet, and Fritz Diekmann. 2025. "Tacrolimus-Associated Tremor in Renal Transplant Patients: Potential Impact of the Galenic Formulation" Pharmaceuticals 18, no. 10: 1488. https://doi.org/10.3390/ph18101488
APA StyleRovira, J., Millán, O., Ventura-Aguiar, P., Brunet, M., & Diekmann, F. (2025). Tacrolimus-Associated Tremor in Renal Transplant Patients: Potential Impact of the Galenic Formulation. Pharmaceuticals, 18(10), 1488. https://doi.org/10.3390/ph18101488






