Crinis Carbonisatus-Derived Carbon Dot Suspension Alleviates Temporal Lobe Epilepsy
Abstract
1. Introduction
2. Results and Discussion
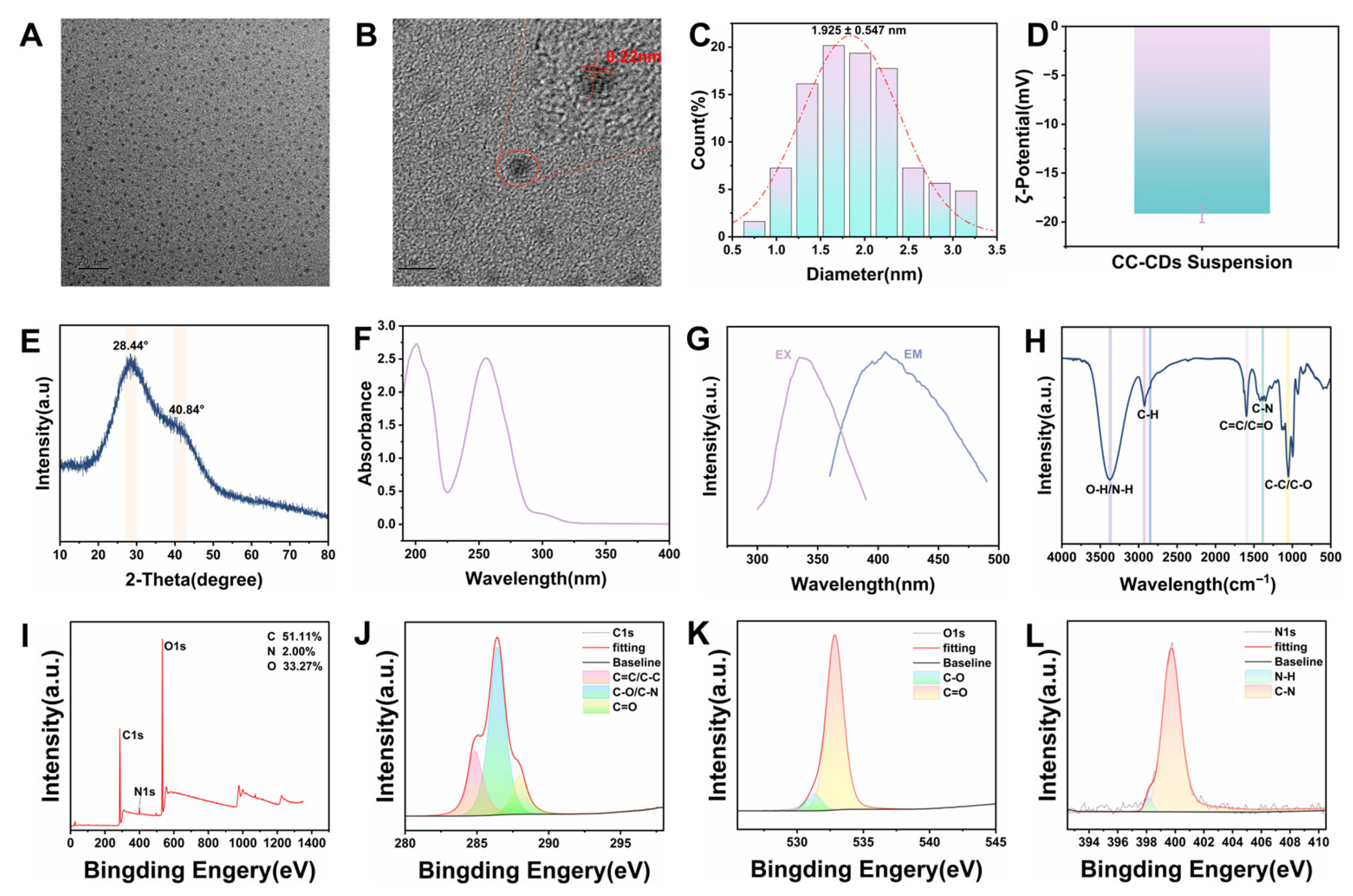
2.1. Characterization Findings of CC-CD Suspension
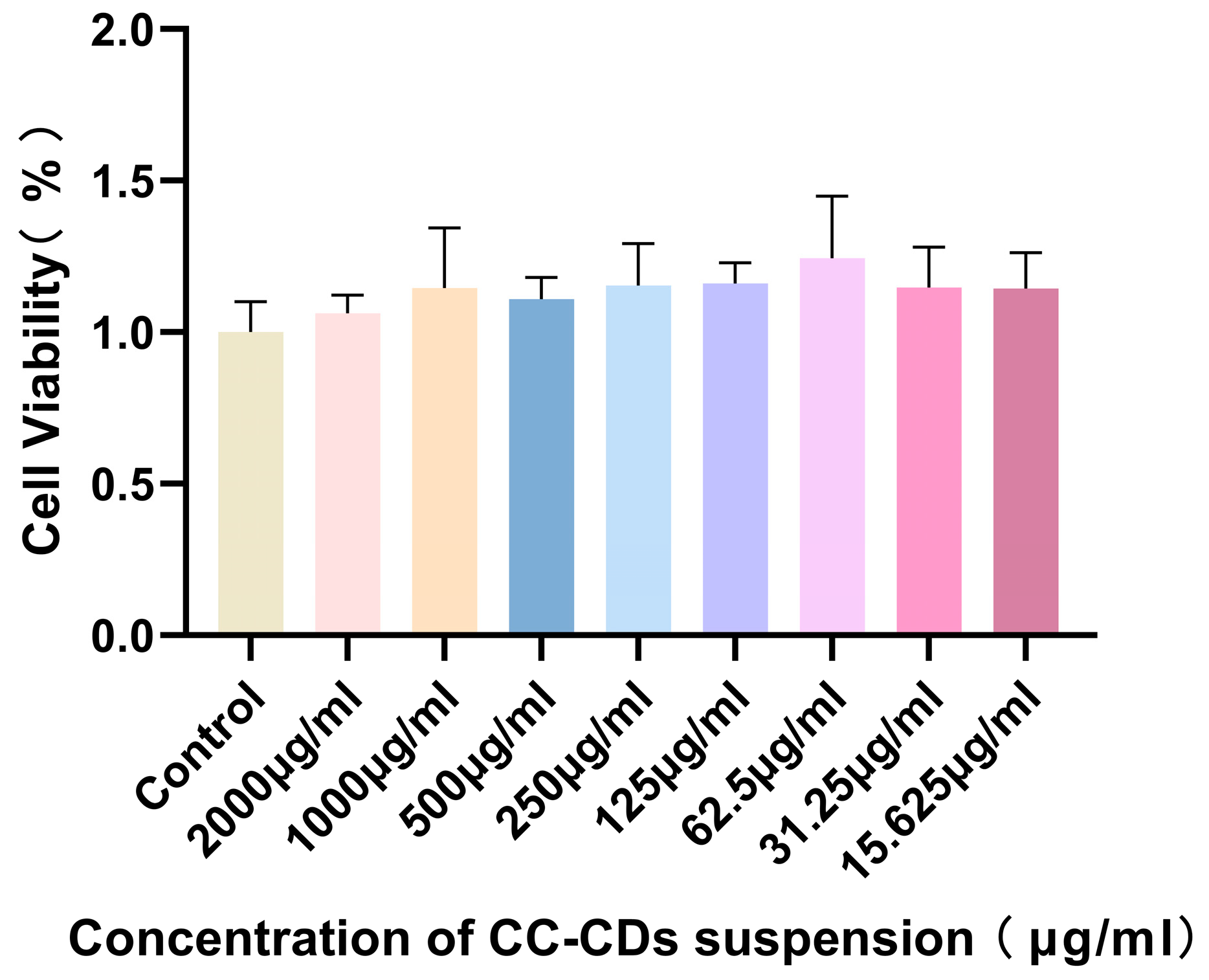
2.2. Cytotoxicity Detection
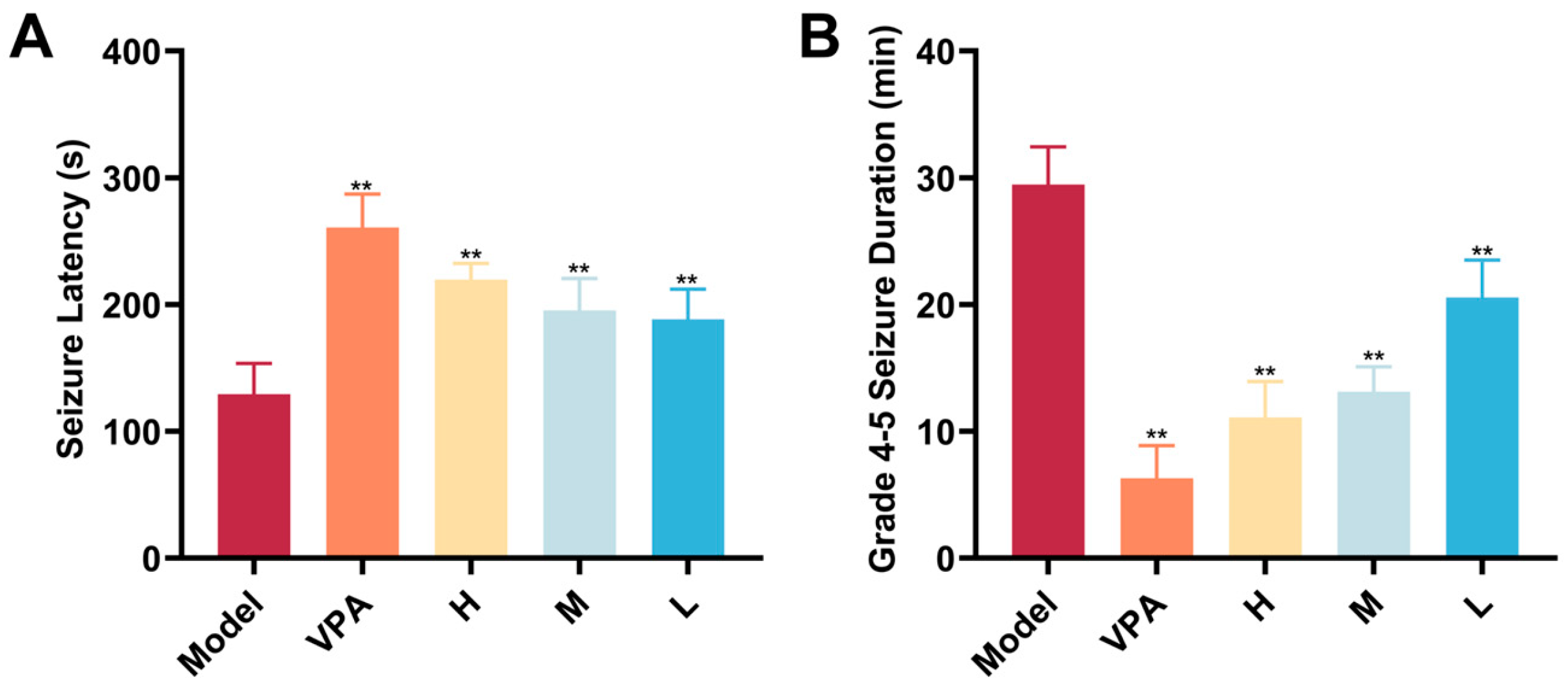
2.3. Seizure-Related Endpoint Detection
2.4. Behavioral Assessment Findings
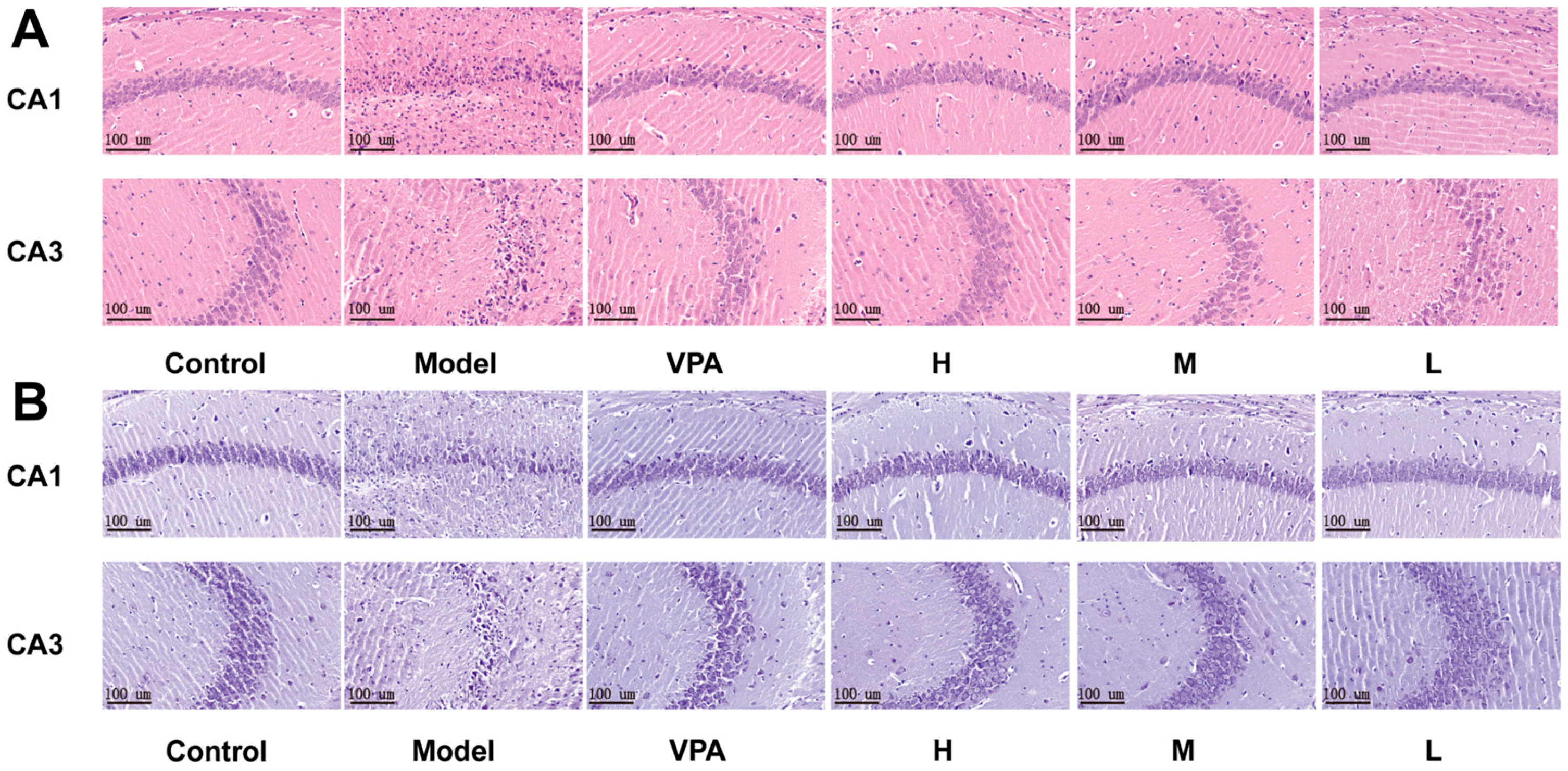
2.5. Histological Evaluation of Brain Tissue
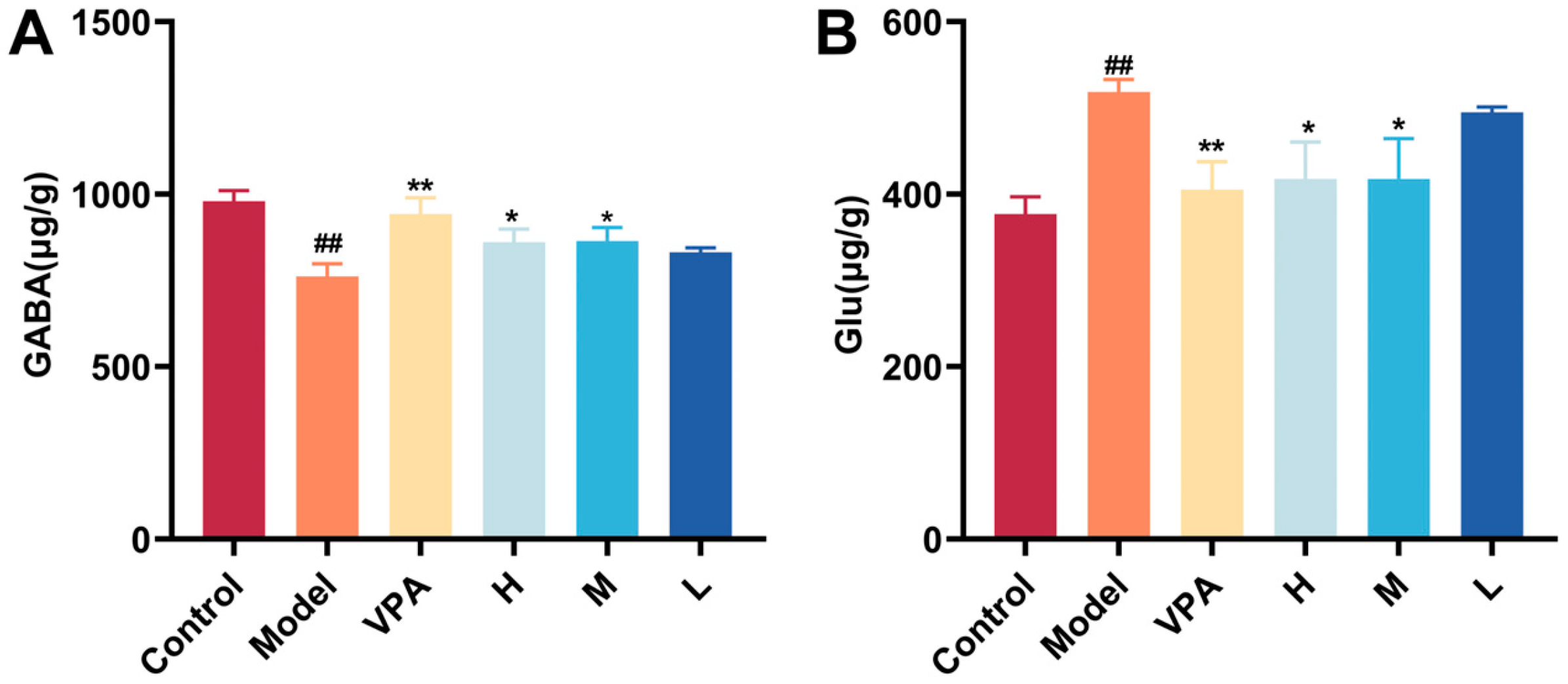
2.6. The Effect of CC-CD Suspension on γ-Aminobutyric Acid (GABA) and Glutamate (Glu)
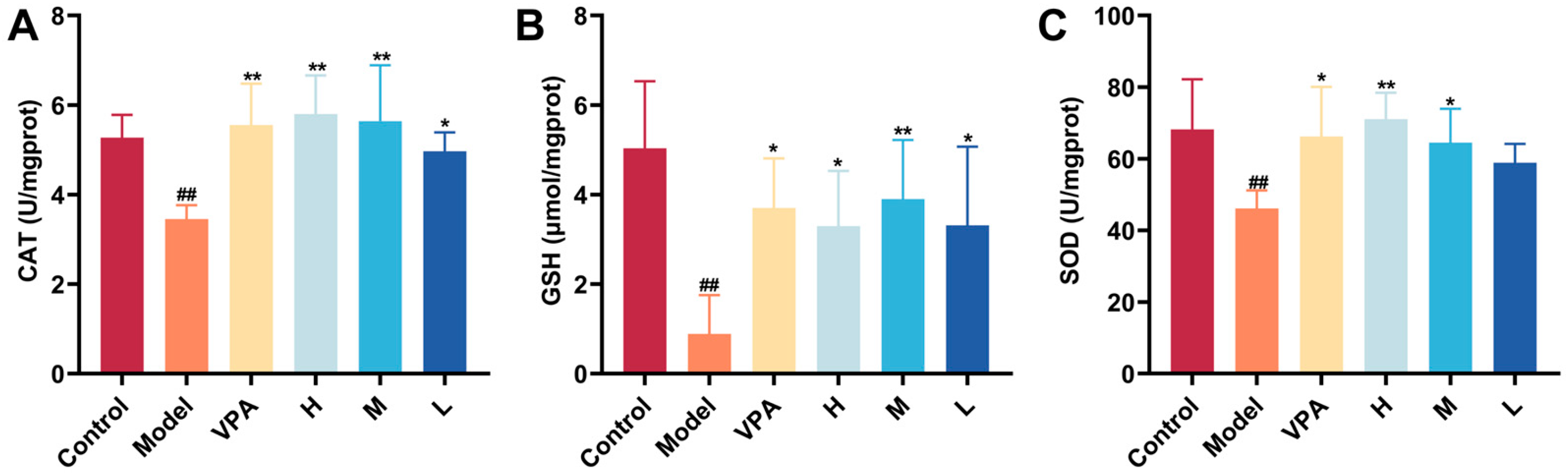
2.7. The Effect of CC-CD Suspension on Oxidative Stress Levels in TLE Mice
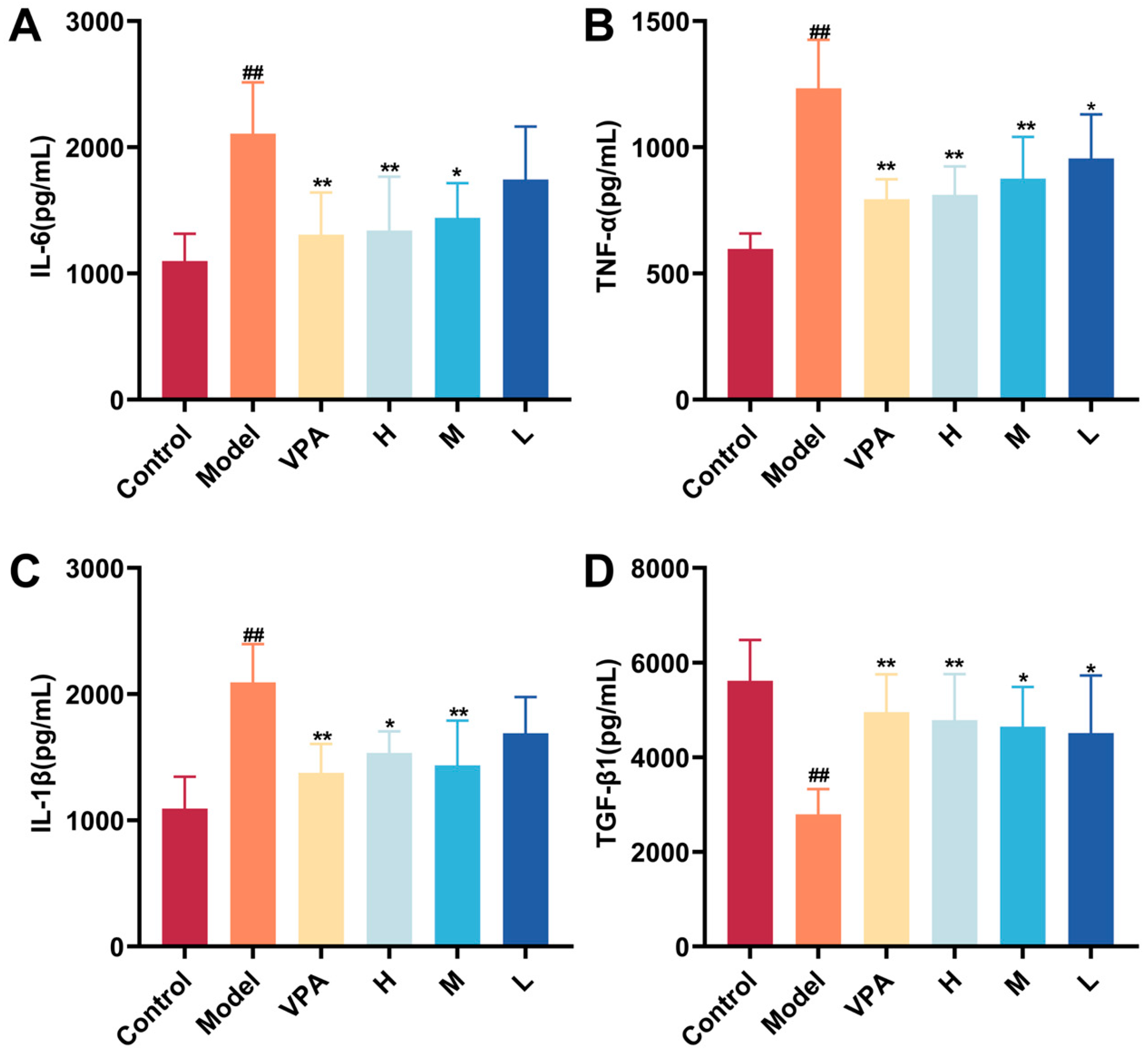
2.8. The Effect of CC-CD Suspension on Inflammation Cytokine Levels in TLE Mice
2.9. The Effect of CC-CD Suspension on MAPK Pathway-Related Protein Expression in TLE Mice
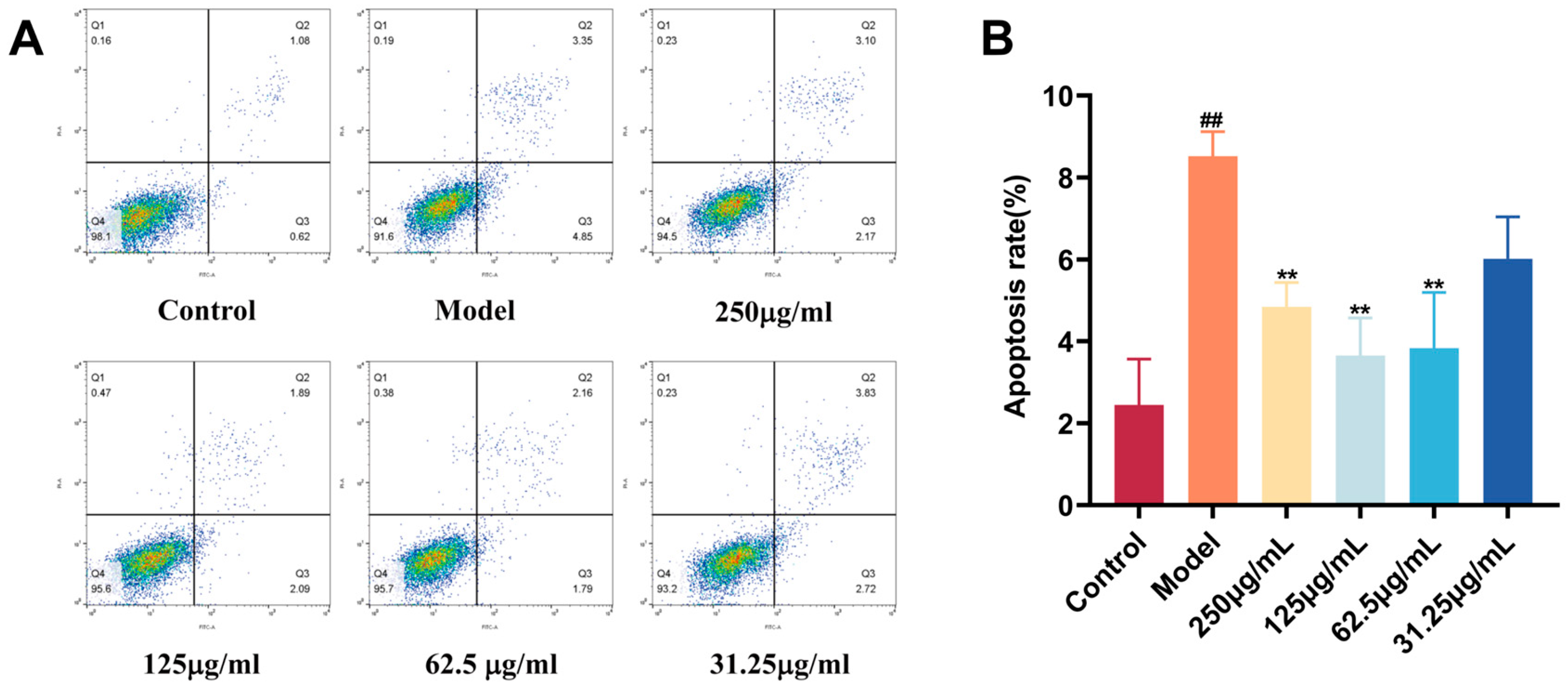
2.10. Protective Effect of CC-CD Suspension on BV2 Cells
2.11. Study Limitations
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Preparation and Characterization of CC-CD Suspension
3.3.1. Preparation of CC-CDs
3.3.2. Formulation of CC-CD Suspension
3.3.3. Characterization of CC-CD Suspension
3.4. Biocompatibility of CC-CD Suspension
3.5. TLE Model Establishment and Drug Administration
3.6. Behavioral Observations
3.6.1. Open Field Test
3.6.2. Novel Object Recognition
3.6.3. Y Maze Test
3.7. Histopathological Analysis
3.8. Detection of Brain Tissue Neurotransmitters
3.9. Enzyme Linked Immunosorbent Assay
3.10. Western Blot
3.11. Flow Cytometry
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, Z.; Liang, Z.; Zhang, J.; Liu, S. Role of Glycerophospholipid Metabolism in Epilepsy. Curr. Neuropharmacol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Ravikumar, M.; Durairaj, B.; Uvarajan, D. Necrostatin-1 as a Potential Anticonvulsant: Insights from Zebrafish Larvae Model of PTZ-Induced Seizures. Mol. Neurobiol. 2025, 62, 4534–4544. [Google Scholar] [CrossRef]
- Albrecht, J.; Czuczwar, S.J.; Zielińska, M.; Miziak, B. Methionine Sulfoximine as a Tool for Studying Temporal Lobe Epilepsy: Initiator, Developer, Attenuator. Neurochem. Res. 2025, 50, 84. [Google Scholar] [CrossRef]
- Fan, J.; Dong, X.; Tang, Y.; Wang, X.; Lin, D.; Gong, L.; Chen, C.; Jiang, J.; Shen, W.; Xu, A.; et al. Preferential pruning of inhibitory synapses by microglia contributes to alteration of the balance between excitatory and inhibitory synapses in the hippocampus in temporal lobe epilepsy. CNS Neurosci. Ther. 2023, 29, 2884–2900. [Google Scholar] [CrossRef]
- Egbenya, D.L.; Hussain, S.; Lai, Y.C.; Anderson, A.E.; Davanger, S. Synapse-specific changes in Arc and BDNF in rat hippocampus following chronic temporal lobe epilepsy. Neurosci. Res. 2023, 191, 1–12. [Google Scholar] [CrossRef]
- Bell, B.; Lin, J.J.; Seidenberg, M.; Hermann, B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat. Rev. Neurol. 2011, 7, 154–164. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, R.; Pan, L.; Li, Y.; Xu, Y.; Li, Y.; Guo, A.; Huang, W.; Tan, T.; Li, P.; et al. Edaravone dexborneol exerts anti-epileptic effects on rodent temporal lobe epilepsy by promoting NMDAR deactivation and inhibiting oxidative stress. Phytomedicine 2025, 140, 156558. [Google Scholar] [CrossRef]
- Feng, S.; Qiao, W.; Xia, L.; Yu, L.; Lang, Y.; Jin, J.; Liu, Y.; Chen, F.; Feng, W.; Chen, Y. Nanoengineered, ultrasmall and catalytic potassium calcium hexacyanoferrate for neuroprotection and temporal lobe epilepsy treatment. Sci. Bull. 2025, 70, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S. Editorial: Nano-Biomaterials for the Delivery of Therapeutic and Biological Cues for Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 832487. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.K.; Verma, K.K.; Kumar, R.; Arora, V. A Concise Review of Carbon Dots and their Pharmaceutical and Biomedical Applications. Recent. Adv. Drug Deliv. Formul. 2023, 17, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Fernandez, G.; Cigales Canga, J.; Soldado, A.; Ruiz Encinar, J.; Costa-Fernandez, J.M. Functionalized heteroatom-doped carbon dots for biomedical applications: A review. Anal. Chim. Acta 2023, 1284, 341874. [Google Scholar] [CrossRef]
- Singh, P.; Bhankar, V.; Kumar, S.; Kumar, K. Biomass-derived carbon dots as significant biological tools in the medicinal field: A review. Adv. Colloid Interface Sci. 2024, 328, 103182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Kong, H.; Cheng, J.; Zhang, M.; Sun, Z.; Wang, S.; Liu, J.; Qu, H.; Zhao, Y. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif. Cells Nanomed. Biotechnol. 2020, 48, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Luo, J.; Zhang, M.; Wu, J.; Zhang, Y.; Kong, H.; Qu, H.; Cheng, G.; Zhao, Y. Protective Effects of Radix Sophorae Flavescentis Carbonisata-Based Carbon Dots Against Ethanol-Induced Acute Gastric Ulcer in Rats: Anti-Inflammatory and Antioxidant Activities. Int. J. Nanomed. 2021, 16, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, D.; Wang, G.; Zhang, M.; Sun, Y.; Ding, J. Antibacterial carbon dots. Mater. Today Bio 2025, 30, 101383. [Google Scholar] [CrossRef]
- Fibriani, A.; Taharuddin, A.A.P.; Stephanie, R.; Yamahoki, N.; Laurelia, J.; Wisnuwardhani, P.H.; Agustiyanti, D.F.; Angelina, M.; Rubiyana, Y.; Ningrum, R.A.; et al. Curcumin-derived carbon-dots as a potential COVID-19 antiviral drug. Heliyon 2023, 9, e20089. [Google Scholar] [CrossRef]
- Kong, H.; Zhao, Y.; Cao, P.; Luo, J.; Liu, Y.; Qu, H.; Zhang, Y.; Zhao, Y. The Bioactivity of Scutellariae Radix Carbonisata-Derived Carbon Dots: Antiallergic Effect. J. Biomed. Nanotechnol. 2021, 17, 2485–2494. [Google Scholar] [CrossRef]
- Liu, K.; Liu, X.; Wen, L.; Zhai, W.; Ye, R.; Zhang, B.; Xie, W.; Zhang, X.; Zhang, W.; Li, H.; et al. Blocking Metallothionein-2 Expression by Copper-Doped Carbon Dots Induces Cellular Antioxidant System Collapse for Antitumor Therapy. Nano Lett. 2024, 24, 10699–10709. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, D.; Li, Z.; Luan, X.; Yang, J.; Tang, S.; Song, Y. A Nanoinhibitor Targeting cGAS-STING Pathway to Reverse the Homeostatic Imbalance of Inflammation in Psoriasis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316007. [Google Scholar] [CrossRef]
- Mahawar, S.; Rakshit, D.; Patel, I.; Gore, S.K.; Sen, S.; Ranjan, O.P.; Mishra, A. Fisetin-loaded chitosan nanoparticles ameliorate pilocarpine-induced temporal lobe epilepsy and associated neurobehavioral alterations in mice: Role of ROS/TNF-α-NLRP3 inflammasomes pathway. Nanomedicine 2024, 59, 102752. [Google Scholar] [CrossRef]
- Wu, T.; Li, M.; Li, T.; Zhao, Y.; Yuan, J.; Zhao, Y.; Tian, X.; Kong, R.; Zhao, Y.; Kong, H.; et al. Natural biomass-derived carbon dots as a potent solubilizer with high biocompatibility and enhanced antioxidant activity. Front. Mol. Biosci. 2023, 10, 1284599. [Google Scholar] [CrossRef]
- Wang, X.; Wu, T.; Yang, Y.; Zhou, L.; Wang, S.; Liu, J.; Zhao, Y.; Zhang, M.; Zhao, Y.; Qu, H.; et al. Ultrasmall and highly biocompatible carbon dots derived from natural plant with amelioration against acute kidney injury. J. Nanobiotechnology 2023, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Qiang, R.; Huang, H.; Chen, J.; Shi, X.; Fan, Z.; Xu, G.; Qiu, H. Carbon Quantum Dots Derived from Herbal Medicine as Therapeutic Nanoagents for Rheumatoid Arthritis with Ultrahigh Lubrication and Anti-inflammation. ACS Appl. Mater. Interfaces 2023, 15, 38653–38664. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.K.; Zhang, L.L.; Yang, Z.Y.; Guo, X.H.; Wu, Y.; Zhang, W.; Luo, J.K.; Tang, T.; Wang, Y. Herbal medicine derived carbon dots: Synthesis and applications in therapeutics, bioimaging and sensing. J. Nanobiotechnology 2021, 19, 320. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, M.; Peng, Y.; Dong, B.; Jiang, Y.; Hu, C.; Zhu, P.; Xing, W.; Yu, L.; Xu, R.; et al. Research progress on the treatment of epilepsy with traditional Chinese medicine. Phytomedicine 2023, 120, 155022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, Y.; Li, T.; Zhao, Y.; Yuan, J.; He, C.; Huang, Y.; Ma, J.; Zhang, Y.; Lu, F.; et al. Green carbon dots derived from Zingiberis Rhizoma Carbonisatum alleviate ovalbumin-induced allergic rhinitis. Front. Immunol. 2024, 15, 1492181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Lu, F.; Zhang, M.; Kong, H.; Cheng, J.; Luo, J.; Zhao, Y.; Qu, H. The neuroprotective effect of pretreatment with carbon dots from Crinis Carbonisatus (carbonized human hair) against cerebral ischemia reperfusion injury. J. Nanobiotechnology 2021, 19, 257. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Cao, Q.; Xu, R.; Li, B. Regulation of Janus Kinase-signal Transducers and Activators of Transcription Pathways in Psoriatic Mice by Chinese Herbal Compound and Efficacy of Chinese Traditional Medicine under Nano-suspension Technology. Cell. Mol. Biol. 2022, 68, 149–157. [Google Scholar] [CrossRef]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhang, M.; Zhang, J.; Li, Y.; Jia, P.; Zhang, H.; Duan, L.; Li, Y.; Li, Y.; et al. Carbon Dots as a Potential Therapeutic Agent for the Treatment of Cancer-Related Anemia. Adv. Mater. 2022, 34, e2200905. [Google Scholar] [CrossRef]
- Thota, S.P.; Kurdekar, A.; Vadlani, P.V.; Kumar, B.S. Green Synthesis of Highly Fluorescent Carbon Dots from Groundnut Shell Biomass for Bioimaging Applications. J. Fluoresc. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.H.; Pourmadadi, M.; Abdouss, M.; Kalaee, M.R.; Moradi, O.; Rahdar, A.; Díez-Pascual, A.M. Novel chitosan/γ-alumina/carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int. J. Biol. Macromol. 2023, 251, 126280. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, M.; Zheng, N.; Liu, B.; Tang, J.; Tang, J.; Zhang, F.; Chen, G. Green synthesis of carbon dots and their application as fluorescent probes for rutin detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 337, 126084. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.J.; Vasimalai, N.; John, S.A.; Sethuraman, M.G. On-Off-On Fluorometric Detection of Hg(II) and L-Cysteine Using Red Emissive Nitrogen-Doped Carbon Dots for Environmental and Clinical Sample Analysis. J. Fluoresc. 2025, 35, 1139–1150. [Google Scholar] [CrossRef]
- Architha, N.; Ragupathi, M.; Shobana, C.; Selvankumar, T.; Kumar, P.; Lee, Y.S.; Kalai Selvan, R. Microwave-assisted green synthesis of fluorescent carbon quantum dots from Mexican Mint extract for Fe(3+) detection and bio-imaging applications. Environ. Res. 2021, 199, 111263. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Pu, J.; Cheng, G.; Liu, Y.; Xiao, S.; Cao, J. Epigynum auritum-Derived Near-Infrared Carbon Dots for Bioimaging and Antimicrobial Applications. Molecules 2025, 30, 422. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, Y.; Wang, Y.; Hou, Y.; Zhang, R.; Zong, M.; Sun, L.; Liu, Y.; Qi, J.; Wu, X.; et al. The Potential of Novel Synthesized Carbon Dots Derived Resveratrol Using One-Pot Green Method in Accelerating in vivo Wound Healing. Int. J. Nanomed. 2023, 18, 6813–6828. [Google Scholar] [CrossRef]
- Abdulsatar Esmail, L.; Sanaan Jabbar, H. Violuric acid carbon dots as a highly fluorescence probe for ultrasensitive determination of Zn (II) in tomato paste. Food Chem. 2023, 413, 135638. [Google Scholar] [CrossRef]
- Sakthi Velu, K.; Jegatheeswaran, S.; Akhtar, M.S.; Khan, M.R.; Mohandoss, S.; Ahmad, N. Formulation and Characterization of β-Cyclodextrins-Nitazoxanide Inclusion Complexes: Enhanced Solubility, In Vitro Drug Release, and Antiviral Activity in Vero Cells. Pharmaceutics 2024, 16, 1494. [Google Scholar] [CrossRef]
- Koparde, S.V.; Nille, O.S.; Kolekar, A.G.; Bote, P.P.; Gaikwad, K.V.; Anbhule, P.V.; Pawar, S.P.; Kolekar, G.B. Okra peel-derived nitrogen-doped carbon dots: Eco-friendly synthesis and multi-functional applications in heavy metal ion sensing, nitro compound detection and environmental remediation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 321, 124659. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, S.; Wang, Y.; Tong, M.; Sun, H.; Dong, M.; Lu, Y.; Niu, W.; Wang, L. Yam Carbon Dots Promote Bone Defect Repair by Modulating Histone Demethylase 4B. Int. J. Nanomed. 2024, 19, 10415–10434. [Google Scholar] [CrossRef]
- Durmus, M.T.; Bozkurt, E. Green synthesis of carbon dot structures from Rheum Ribes and Schottky diode fabrication. Beilstein J. Nanotechnol. 2024, 15, 1369–1375. [Google Scholar] [CrossRef]
- Abinaya, K.; Raja, K.; Raja, K.; Sathya Moorthy, P.; Senthil, A.; Chandrakumar, K. Impact of green carbon dot nanoparticles on seedling emergence, crop growth and seed yield in blackgram (Vigna mungo L. Hepper). Sci. Rep. 2024, 14, 23783. [Google Scholar] [CrossRef]
- Joshi, S.; Kapur, J. Status epilepticus: Updates on mechanisms and treatments. Epilepsia Open 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Adali, N. Factors influencing quality of life in patients with temporal lobe epilepsy. Arq. Neuropsiquiatr. 2025, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trend, C.; Puntambekar, I.; Baxendale, S. Subjective memory complaints in people with epilepsy: Are there “signature” complaints associated with anxiety and depression? Epilepsia Open 2025, 10, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.I.; Gupta, A.K.; Singh, R.; Gupta, N.K.; Dodd, H.; Musmar, B.; Singh, A.; George, D.D.; LoPresti, M.A.; Wensel, A.M. Trigeminal nerve stimulation in drug-resistant epilepsy: A systematic review. Clin. Neurol. Neurosurg. 2025, 251, 108834. [Google Scholar] [CrossRef]
- Zhao, K.; Xiang, L.; Yang, S.; Chen, X.; Yang, X.; Dong, J.; Wu, S.; Yang, S.; Zhang, M.; Hu, W. 11,12-Diacetyl-Carnosol Ameliorates Depression-Like Behaviors and Memory Dysfunction in CUMS Mouse Model via Inhibiting HMGB1-Mediated Neuroinflammation. CNS Neurosci. Ther. 2025, 31, e70406. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Siraj, F.; Yadav, R.; Kumari, S.; Tiwari, A.K.; Tiwari, M. The anti-epileptogenic and cognition enhancing effect of novel 1-[4-(4-benzo [1, 3] dioxol-5-ylmethyl-piperazin-1-yl)-phenyl]-3-phenyl-urea (BPPU) in pentylenetetrazole induced chronic rat model of epilepsy. Biomed. Pharmacother. 2018, 105, 470–480. [Google Scholar] [CrossRef]
- Golderman, V.; Ben-Shimon, M.; Maggio, N.; Dori, A.; Gofrit, S.G.; Berkowitz, S.; Qassim, L.; Artan-Furman, A.; Zeimer, T.; Chapman, J.; et al. Factor VII, EPCR, aPC Modulators: Novel treatment for neuroinflammation. J. Neuroinflammation 2022, 19, 138. [Google Scholar] [CrossRef]
- Zhao, T.; Cui, X.; Zhang, X.; Zhao, M.; Rastegar-Kashkooli, Y.; Wang, J.; Li, Q.; Jiang, C.; Li, N.; Xing, F.; et al. Hippocampal sclerosis: A review on current research status and its mechanisms. Ageing Res. Rev. 2025, 108, 102716. [Google Scholar] [CrossRef]
- Lin, J.; Lin, X.; Fu, Y.J.; Li, X.; Li, X.; Wang, Y.; Zhao, L.; Yu, S.; Piao, Y.S. Activation of the Reelin/GSK-3β/p-Tau Signaling Pathway in the Hippocampus of Patients with Temporal Lobe Epilepsy. Neuropsychiatr. Dis. Treat. 2025, 21, 409–419. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Takemiya, T.; Sugiura, H.; Yamagata, K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediators Inflamm. 2014, 2014, 901902. [Google Scholar] [CrossRef] [PubMed]
- Balosso, S.; Liu, J.; Bianchi, M.E.; Vezzani, A. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid. Redox Signal 2014, 21, 1726–1740. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Beppu, K.; Kosai, Y.; Kido, M.A.; Akimoto, N.; Mori, Y.; Kojima, Y.; Fujita, K.; Okuno, Y.; Yamakawa, Y.; Ifuku, M.; et al. Expression, subunit composition, and function of AMPA-type glutamate receptors are changed in activated microglia; possible contribution of GluA2 (GluR-B)-deficiency under pathological conditions. Glia 2013, 61, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Li, Z.; He, T.; Li, N.; Xu, X.; Yu, P.; Lu, X.; Nie, J.; Liu, D.; Cai, Q.; et al. Astrocyte-selective STAT3 knockdown rescues methamphetamine withdrawal-disrupted spatial memory in mice via restoring the astrocytic capacity of glutamate clearance in dCA1. Glia 2021, 69, 2404–2418. [Google Scholar] [CrossRef]
- Morales, I.; Rodriguez, M. Self-induced accumulation of glutamate in striatal astrocytes and basal ganglia excitotoxicity. Glia 2012, 60, 1481–1494. [Google Scholar] [CrossRef]
- Puttachary, S.; Sharma, S.; Stark, S.; Thippeswamy, T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed. Res. Int. 2015, 2015, 745613. [Google Scholar] [CrossRef]
- Kovac, S.; Dinkova Kostova, A.T.; Herrmann, A.M.; Melzer, N.; Meuth, S.G.; Gorji, A. Metabolic and Homeostatic Changes in Seizures and Acquired Epilepsy-Mitochondria, Calcium Dynamics and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 1935. [Google Scholar] [CrossRef]
- Tipton, A.E.; Russek, S.J. Regulation of Inhibitory Signaling at the Receptor and Cellular Level; Advances in Our Understanding of GABAergic Neurotransmission and the Mechanisms by Which It Is Disrupted in Epilepsy. Front. Synaptic Neurosci. 2022, 14, 914374. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, B.F.; Schonwald, A.; Boyko, M.; Zlotnik, A. The Role of Glutamate and Blood-Brain Barrier Disruption as a Mechanistic Link between Epilepsy and Depression. Cells 2024, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Dyomina, A.V.; Zubareva, O.E.; Smolensky, I.V.; Vasilev, D.S.; Zakharova, M.V.; Kovalenko, A.A.; Schwarz, A.P.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Reduces Epileptogenesis, Provides Neuroprotection, and Attenuates Behavioral Impairments in Rats in the Lithium-Pilocarpine Model of Epilepsy. Pharmaceuticals 2020, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Vongthip, W.; Sillapachaiyaporn, C.; Kim, K.W.; Sukprasansap, M.; Tencomnao, T. Thunbergia laurifolia Leaf Extract Inhibits Glutamate-Induced Neurotoxicity and Cell Death through Mitophagy Signaling. Antioxidants 2021, 10, 1678. [Google Scholar] [CrossRef]
- Wu, C.; Xie, J.; Yao, Q.; Song, Y.; Yang, G.; Zhao, J.; Zhang, R.; Wang, T.; Jiang, X.; Cai, X.; et al. Intrahippocampal Supramolecular Assemblies Directed Bioorthogonal Liberation of Neurotransmitters to Suppress Seizures in Freely Moving Mice. Adv. Mater. 2024, 36, e2314310. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Gao, X.; Chen, F.; Xu, X.; Liu, J.; Dong, F.; Liu, Y. Ro25-6981 alleviates neuronal damage and improves cognitive deficits by attenuating oxidative stress via the Nrf2/ARE pathway in ischemia/reperfusion rats. J. Stroke Cerebrovasc. Dis. 2023, 32, 106971. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Singh, S.; Singh, T.G. Pharmacological modulation of cytokines correlating neuroinflammatory cascades in epileptogenesis. Mol. Biol. Rep. 2022, 49, 1437–1452. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, D.; Stefan, H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: Uncontrolled inflammation drives disease progression? J. Neurol. Sci. 2010, 296, 1–6. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Tuo, J.; Zhang, L.; Zhang, J.; Yu, C.; Xu, Z. Levodopa-induced dyskinesia: Interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation. Front. Immunol. 2023, 14, 1253273. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X.; Li, Y.; Chen, S.; Liu, S.; Yu, Z.; Wang, W. Transforming growth factor-β1 protects against LPC-induced cognitive deficit by attenuating pyroptosis of microglia via NF-κB/ERK1/2 pathways. J. Neuroinflammation 2022, 19, 194. [Google Scholar] [CrossRef]
- Li, L.Y.; Li, J.L.; Zhang, H.M.; Yang, W.M.; Wang, K.; Fang, Y.; Wang, Y. TGFβ1 treatment reduces hippocampal damage, spontaneous recurrent seizures, and learning memory deficits in pilocarpine-treated rats. J. Mol. Neurosci. 2013, 50, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Zhao, K.; Chen, B.; Sun, Z. Natural products modulating MAPK for CRC treatment: A promising strategy. Front. Pharmacol. 2025, 16, 1514486. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wu, Z.; Zhang, C.; Gao, T.; Ling, X.; Xu, M.; Wang, W.; Jin, X.; Li, K.; Chen, L.; et al. Jujuboside A Exhibits an Antiepileptogenic Effect in the Rat Model via Protection against Traumatic Epilepsy-Induced Oxidative Stress and Inflammatory Responses. Evid. Based Complement. Altern. Med. 2022, 2022, 7792791. [Google Scholar] [CrossRef]
- Pflüger, P.; Viau, C.M.; Coelho, V.R.; Berwig, N.A.; Staub, R.B.; Pereira, P.; Saffi, J. Gamma-decanolactone inhibits iNOS and TNF-alpha production by lipopolysaccharide-activated microglia in N9 cells. Eur. J. Pharmacol. 2016, 780, 38–45. [Google Scholar] [CrossRef]
- Nix, J.S.; Blakeley, J.; Rodriguez, F.J. An update on the central nervous system manifestations of neurofibromatosis type 1. Acta Neuropathol. 2020, 139, 625–641. [Google Scholar] [CrossRef]
- Nateri, A.S.; Raivich, G.; Gebhardt, C.; Da Costa, C.; Naumann, H.; Vreugdenhil, M.; Makwana, M.; Brandner, S.; Adams, R.H.; Jefferys, J.G.; et al. ERK activation causes epilepsy by stimulating NMDA receptor activity. Embo J. 2007, 26, 4891–4901. [Google Scholar] [CrossRef]
- Han, H.J.; Hyun, C.G. Anti-Inflammatory Effects and Human Skin Safety of the Eastern Traditional Herb Mosla japonica. Life 2025, 15, 418. [Google Scholar] [CrossRef]
- Xi, L.; Ji, X.; Ji, W.; Yang, Y.; Zhang, Y.; Long, H. Jing-an oral liquid alleviates Tourette syndrome via the NMDAR/MAPK/CREB pathway in vivo and in vitro. Pharm. Biol. 2022, 60, 1790–1800. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.F.; Fiadeiro, M.B.; Bernardino, L.I.; Fonseca, C.S.P.; Baltazar, G.M.F.; Cristóvão, A.C.B. “Lipopolysaccharide-induced animal models for neuroinflammation—An overview”. J. Neuroimmunol. 2024, 387, 578273. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, X.; Shi, T.; Yang, L.; Zhang, B.; Shang, B.; He, R.; Yi, S.; He, J.; Hu, J.; et al. Paeoniflorin Alleviates Lipopolysaccharide-Induced Neuroinflammation and Depression Through the Keap1/Nrf2/HO-1 Signaling Pathway. Antioxidants 2025, 14, 585. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, A.; Dong, J.; Yao, Y.; Zhu, M.; Xu, K.; Al Hamda, M.H. MicroRNA-23a contributes to hippocampal neuronal injuries and spatial memory impairment in an experimental model of temporal lobe epilepsy. Brain Res. Bull. 2019, 152, 175–183. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Shi, Y.; Guan, L.; Li, H.; Li, S.; Li, Y.; Zhang, Y.; Lin, J. Abnormal neuronal damage and inflammation in the hippocampus of kainic acid-induced epilepsy mice. Cell Biochem. Funct. 2021, 39, 791–801. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Lü, Y.; Chen, X.; Cheng, L.; Mi, X.; Xu, X.; Deng, W.; Zhang, Y.; Wang, N.; et al. Effects of JIP3 on epileptic seizures: Evidence from temporal lobe epilepsy patients, kainic-induced acute seizures and pentylenetetrazole-induced kindled seizures. Neuroscience 2015, 300, 314–324. [Google Scholar] [CrossRef]
- Hashemi, P.; Moloudi, M.R.; Vahabzadeh, Z.; Izadpanah, E. Anticonvulsant Effects of Royal Jelly in Kainic Acid-Induced Animal Model of Temporal Lobe Epilepsy Through Antioxidant Activity. Neurochem. Res. 2023, 48, 2187–2195. [Google Scholar] [CrossRef]
- Ma, M.; Fan, M.; Xu, S.; Zheng, Q.; Wang, C.; Chen, P.; Wei, Y.; Ge, J. Melatonin alleviates retinal injury induced by vigabatrin and partially enhances its antiepileptic effects. Int. Immunopharmacol. 2025, 153, 114516. [Google Scholar] [CrossRef]


| Grade | Corresponding Behavioral Characteristics |
|---|---|
| 0 Grade | Normal activity, no stress response. |
| 1 Grade | Facial muscle twitching, blinking, chewing, salivation, etc., reduced activity. |
| 2 Grade | Rhythmic nodding or wet—dog shakes visible. |
| 3 Grade | Unilateral forelimb twitching and spasm visible, no hindlimb spasm, upright posture. |
| 4 Grade | Bilateral forelimb twitching, spasm, falling, and hindlimbs upright. |
| 5 Grade | Generalized tonic spasm, forced standing or falling, death. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, M.; Dong, L.; He, C.; Zou, P.; Xia, M.; Jin, B.; Wang, S.; Lu, Z.; Qu, H.; et al. Crinis Carbonisatus-Derived Carbon Dot Suspension Alleviates Temporal Lobe Epilepsy. Pharmaceuticals 2025, 18, 1481. https://doi.org/10.3390/ph18101481
Huang Y, Li M, Dong L, He C, Zou P, Xia M, Jin B, Wang S, Lu Z, Qu H, et al. Crinis Carbonisatus-Derived Carbon Dot Suspension Alleviates Temporal Lobe Epilepsy. Pharmaceuticals. 2025; 18(10):1481. https://doi.org/10.3390/ph18101481
Chicago/Turabian StyleHuang, Yan, Menghan Li, Liyang Dong, Chenxin He, Peng Zou, Minlong Xia, Bilin Jin, Siqi Wang, Zixuan Lu, Huihua Qu, and et al. 2025. "Crinis Carbonisatus-Derived Carbon Dot Suspension Alleviates Temporal Lobe Epilepsy" Pharmaceuticals 18, no. 10: 1481. https://doi.org/10.3390/ph18101481
APA StyleHuang, Y., Li, M., Dong, L., He, C., Zou, P., Xia, M., Jin, B., Wang, S., Lu, Z., Qu, H., Zhang, Y., & Kong, H. (2025). Crinis Carbonisatus-Derived Carbon Dot Suspension Alleviates Temporal Lobe Epilepsy. Pharmaceuticals, 18(10), 1481. https://doi.org/10.3390/ph18101481






